
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Psychiatry , 22 May 2024
Sec. Molecular Psychiatry
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1350978
Purpose: This study aims to examine the potential influence of RS4680 (COMT), RS16965628 (SLC6A4), and RS1019385 (GRIN2B) polymorphisms on the therapeutic response to repetitive transcranial magnetic stimulation (rTMS) and selective serotonin reuptake inhibitors (SSRIs) in individuals with obsessive-compulsive disorder (OCD).
Patients and methods: Thirty-six untreated outpatients diagnosed with OCD were recruited and allocated to active or sham rTMS groups for two weeks. The mean age of the participants was 31.61, with 17 males (47.22%) and 19 females (52.78%). Peripheral blood samples (5 mL) were collected from each participant using ethylenediaminetetraacetic acid (EDTA) vacuum tubes for genotyping purposes, clinical evaluation was taken place at baseline and second week.
Results: The A allele of RS4680, C allele of RS16965628, and GG allele of RS1019385 were identified as potential bio-markers for predicting treatment response to OCD treatments (rTMS & SSRIs).
Conclusion: Those genes may serve as bio-markers for the combined treatment of rTMS and SSRIs in OCD. The finding hold promise for further research and the potential implementation of precision treatment of OCD.
Clinical trial registration: https://www.chictr.org.cn, identifier ChiCTR1900023641.
Obsessive-compulsive disorder (OCD) is a chronic psychiatric disorder, characterized by persistent distressing obsessions and/or compulsions that impair the quality of daily life, associated with severe functional impairment (1–3). Previous research studies have indicated that selective serotonin re-uptake inhibitors (SSRIs) and repetitive transcranial magnetic stimulation (rTMS) are effective treatments for OCD (4–9).
The therapeutic efficacy of SSRIs could be supported by the serotonergic hypothesis (10).
The hypothesis postulates the presence of an aberration, presumably a diminution of function, within the serotonergic system in OCD, or posits the implication of the serotonergic system in some capacity in the pathogenesis of OCD (11–13). Thus, the SSRIs could be effective in treating OCD might because they target on the serotonin system (6, 14). However, it could also be argued that the development of the serotonin hypothesis stemmed from the efficacy of SSRIs in treating OCD, there are still numerous other medicines and treatments that are beneficial for OCD patients. Nonetheless, SSRIs is still one of the effective treatment for OCD (15–17).
RTMS is a comparatively new treatment approach for OCD, which was designed for resistent OCD, and those who cannot afford the huge side effects of medicines (4, 18, 19). As a non-invasive neuromodulation technique, rTMS harnesses rapidly altering electromagnetic fields produced by a coil positioned on the scalp to modulate cortical and subcortical function (5, 20, 21). By adjusting stimulation parameters, rTMS can selectively diminish or enhance cortical excitability in specific regions; frequencies equal to or below 1 Hz typically inhibit activity (referred to as low-frequency rTMS), while frequencies at or above 5 Hz typically stimulate activity (referred to as high-frequency rTMS) (22–24). As several studies suggested, low-frequency rTMS administered over the (pre-) supplementary motor area (pre-SMA/SMA) has been observed to reinstate cortical inhibition in the motor cortex among OCD patients, correlating with amelioration in OCD symptoms (20, 25–27).
However, it has been observed that some patients exhibit inadequate response to these therapies. For instance, studies have shown that SSRIs are ineffective for 40-60% of OCD patients (18, 28). Similarly, some studies did not observe adequate therapeutic effect of rTMS on OCD (29–31), and there is insufficient evaluation of the therapeutic efficacy of rTMS treatment on OCD (20, 32). In light of this, the concept of candidate genes that may impact the therapeutic effect of OCD treatments has been proposed (33), they identified the LL allele of 5-HTTLPR (L/S) in the candidate gene SLC6A4 as a potential bio-marker for OCD treatment. Since there may be other bio-markers, attention has been directed towards genes within the neurotransmitter systems, including the serotonin transporter gene SLC6A4, the glutamate receptor gene GRIN2B, and the gene related to serotonin, dopamine, norepinephrine, and epinephrine (COMT) which have been previously identified as candidate genes for OCD (34–38).
As a gene that has already been identified as a potential candidate for OCD treatment, SLC6A4 holds significant value for further investigation (33). The SLC6A4 gene can encode the serotonin transporter (5-HTT) protein, which has the job to control the release of serotonin (5-HT) from the synaptic terminals remove that from the synaptic cleft (39–42). Due to the importance of serotonin in impacting OCD, it is understandable that SLC6A4 might be the potential bio-marker for OCD treatment (6, 14, 43). Except for the polymorphism of 5-HTTLPR (SLC6A4) that previous research had already done with (33), the C alleles of rs16965628 (SLC6A4) have also been suggested to potentially lead to alterations in the serotonergic system (44). Therefore, rs16965628 is suitable for investigation in the present study, as it could be considered as a highly possible candidate gene foe OCD (40).
Moreover, GRIN2B seem also deserve the investigation as a possible candidate gene for OCD treatment, due to the previous finding of the association between G allele and OCD (45). Previous studies suggested that there is a significant correlation between the rs1019385 polymorphism of the N-methyl-D-aspartate 2B glutamate receptor (GRIN2B) and reduced glutamatergic concentration (Glx) in the anterior cingulate cortex (ACC); Individuals with the GG genotype demonstrated reduced Glx compared to those carrying the T allele (45, 46). As the reduced Glx was suggested to be related with OCD (47), rs1019385 (GRIN2B) could also be a suitable gene for investigation in the present study.
Furthermore, as another possible candidate gene, Catechol-O-methyltransferase (COMT) serves as a pivotal enzyme in the metabolic deactivation of dopamine and norepinephrine catecholamines, facilitating the catabolism of dopamine (DA) (48, 49). The Met (A) allele of Val158Met (G to A), commonly referred to as RS4680 (COMT), is proposed to decrease enzyme activity, elevate cortical dopamine signaling, and potentially play a role in OCD development (50, 51). Hence, RS4680 (COMT) emerges as another pertinent gene for investigation in the current study.
Given the limited amount of research investigating RS4680 (COMT), RS16965628 (SLC6A4), and RS1019385 (GRIN2B) as candidate genes for OCD treatment and their strong correlation with OCD, the present study aimed to investigate whether the polymorphism of those three genes would influence the therapeutic effect of rTMS and SSRIs in individuals with OCD. To achieve the aim, a study with an experimental, between-subject, double-blind design was conducted, with the recruitment of 36 untreated outpatients diagnosed with OCD.
This study employed an experimental, between-subject, double-blind design. The participants consisted of 36 untreated outpatients diagnosed with OCD according to the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV) criteria (Figure 1). The mean age of the participants was 31.61, with 17 males (47.22%) and 19 females (52.78%). The study was granted ethical approval by the Ethics Committee of Wuxi Mental Health Center, China (clinical trials registry number: [ChiCTR1900023641]). Each participant has received informed consent before participating.
All participants were randomly assigned to two different groups for an 8-week therapy. However, as the drop-out rate was extremely high after the second week, this study only analyzed the data of the first two weeks. Peripheral blood samples (5 mL) were collected from each participant using ethylenediaminetetraacetic acid (EDTA) vacuum tubes for genotyping purposes, specifically examining the polymorphism of RS4680 (COMT), RS16965628 (SLC6A4), and RS1019385 (GRIN2B). In the active rTMS group, participants received 1-Hz rTMS over the pre-supplementary motor area (pre-SMA) once per day, 5 days per week, for 2 weeks. The pre-SMA was identified as being 15% of the distance from the inion to the nasion along the anterior plane to the Cz (vertex) on the sagittal midline. For the sham rTMS group, the Neurosoft sham coil was positioned at the same location. Both groups received selective serotonin reuptake inhibitors (SSRIs) as part of their drug therapy. Further details regarding the genotyping and therapy used in this study can be found elsewhere (33).
Clinical evaluations were conducted at baseline and 2 weeks following the treatment timeline using three measuring tools: the Chinese version of Yale–Brown obsessive-compulsive scale (Y-BOCS), the Hamilton Anxiety Rating Scale (HAMA), and the 17-item Hamilton Depression Rating Scale (HAMD). The Chinese version of Y-BOCS was found to be a dependable computerized cognitive test, noted for its repeatability, sensitivity, and strong validity and reliability (33). There were also previous research affirmed the validity and sensitivity of the Chinese editions of HAMD and HAMA, underscoring their suitability for gauging clinical severity in patients and endorsing their ongoing utilization in research settings (52).
Statistical analysis was performed using SPSS, employing the chi-square test, Related-Samples Friedman’s Two-Way Analysis of Variance, and the Scheirer-Ray-Hare Test. The Chi-square test was used to test the categories data such as gender. The Mann–Whitney test, Related-Samples Friedman’s Two-Way Analysis, and the Scheirer-Ray-Hare Test were used to test non-normally distributed data.
Table 1 presents descriptive and statistical comparisons of the active rTMS and sham rTMS groups. At baseline, there were no significant differences between the active and sham groups in terms of demographics or baseline clinical ratings (Table 1).
The result shows that both SSRIs and rTMS augmentation of SSRIs led to significant improvements in assessment scores. The Y-BOCS scores demonstrated a significant decrease over time. The Scheirer-Ray-Hare test analysis results (Table 2) for the two-week Y-BOCS assessment showed that the main effect of the therapy type was not significant (H = 2.91, p > 0.05), while the main effect of the therapy duration was significant (H = 12.19, p < 0.05, η2 = 0.12). Friedman test (Table 3) suggests that the assessment score had a significant change after therapy in both the active rTMS group (χ2 (2) = 17.44, p < 0.01, W = 0.55) and sham rTMS group (χ2 (2) = 9.58, p < 0.01, W = 0.24). Post hoc analysis with Wilcoxon signed-rank tests was conducted with a Bonferroni correction applied, resulting in a significance level set at p < 0.017. In the active rTMS group, Y-BOCS scores significantly decreased from baseline to week 1 (Z = -2.96, p < 0.017) and from baseline to week 2 (Z = -3.33, p < 0.017), while the decrease from week 1 to week 2 was not significant (Z = -1.74, p = 0.082). Similarly, in the sham rTMS group, Y-BOCS scores significantly decreased from baseline to week 2 (Z = -2.45, p<0.017), while the decrease from baseline to week 1 (Z = -2.12, p = 0.034) and from week 1 to week 2 (Z = -1.55, p = 0.120) was not significant (Table 3). HAMA scores exhibited significant changes after therapy in both the active group (χ2 (2) = 7.77, p < 0.05, W = 0.24) and sham group(χ2 (2) = 11.40, p < 0.01, W = 0.29) (Table 3). Besides, in the active rTMS group, a significant change in assessment scores was found both from baseline to week 1 (Z = -2.81, p < 0.017) and from baseline to week 2 (Z = -2.88, p < 0.017). In the sham rTMS groups, a significant change was only found between baseline and week 2 (Z = -2.66, p < 0.017) (Table 3). Additionally, the analysis of the two-week HAMD assessment showed that the main effect of the therapy duration was significant (H = 10.50, p < 0.05, η2 = 0.10) (Table 2). Patients in the active rTMS group demonstrated a significantly larger reduction rate at week 1 compared to the patients in the sham group (U = 93.50, p < 0.05, r = 0.354) (Figure 2). The HAMD scores showed a significant decrease over time in both the active rTMS (χ2 (2) = 16.00, p < 0.05, W = 0.50) and sham rTMS groups (χ2 (2) = 11.86, p < 0.05, W = 0.30) (Table 3).

Figure 2 Change in HAMA score’s reduction rate in patients with OCD during the study. Data are shown at the time of inclusion in the study (baseline) and after the period of active or sham stimulation (weeks 1 and 2). *The active rTMS group’s week 1 reduction rate is significantly larger compared with the sham rTMS group (p < 0.05).
The first significant difference was observed in the RS4680 genotype. No significant differences were found in general baseline characteristics between different genotypes. However, significant genotype-related effects on the intervention outcomes were identified (Table 4). Patients with the GG genotype showed a smaller improvement in Y-BOCS scores (H = 6.374, p < 0.05, η2 = 0.19); and greater variability in Y-BOCS score was found in Sham rTMS groups of GG genotype than in others. (Figure 3A). Additionally, an interaction between rTMS intervention and RS4680 genotype was found to have effects on HAMA (H = 4.645, p < 0.05, η2 = 0.14) and HAMD (H = 5.549, p < 0.05, η2 = 0.17) scores at week 2, suggesting that genotypes may influence the outcome of rTMS. Regarding the RS16965628 genotype, the analysis results (Table 2) showed that the main effect of rTMS (H = 4.514, p < 0.05, η2 = 0.15) was significant for the HAMD Week2 Reduction Rate. The rTMS intervention significantly improved HAMD scores to a greater extent in the active group, regardless of whether patients had the GC or GG genotype, compared to the sham group; and the improvement of the HAMD score of patients carrying the GC genotype are more concentrated than the improvement of the patients with GG genotype (Figure 3B). Furthermore, the RS16965628 genotype exerted a significant influence on the reduction of HAMD scores (H = 6.361, p < 0.05, η2 = 0.20), with patients carrying the GG genotype showing greater improvement compared to C allele carriers (Table 4 and Figure 3B). However, the interaction between intervention type and genotype did not impact the rate of HAMD score reduction (H = 0.024, p > 0.05) (Table 4). For RS1019385, polymorphism of the gene also demonstrated a significant influence on HAMA score reduction (H = 6.057, p < 0.05, η2 = 0.19). Specifically, individuals with the CC genotype exhibited greater improvement in HAMA scores compared to those with other genotypes; and the reduction rate of the HAMA score are found to be more volatility in AC genotype carrier (Figure 3C).
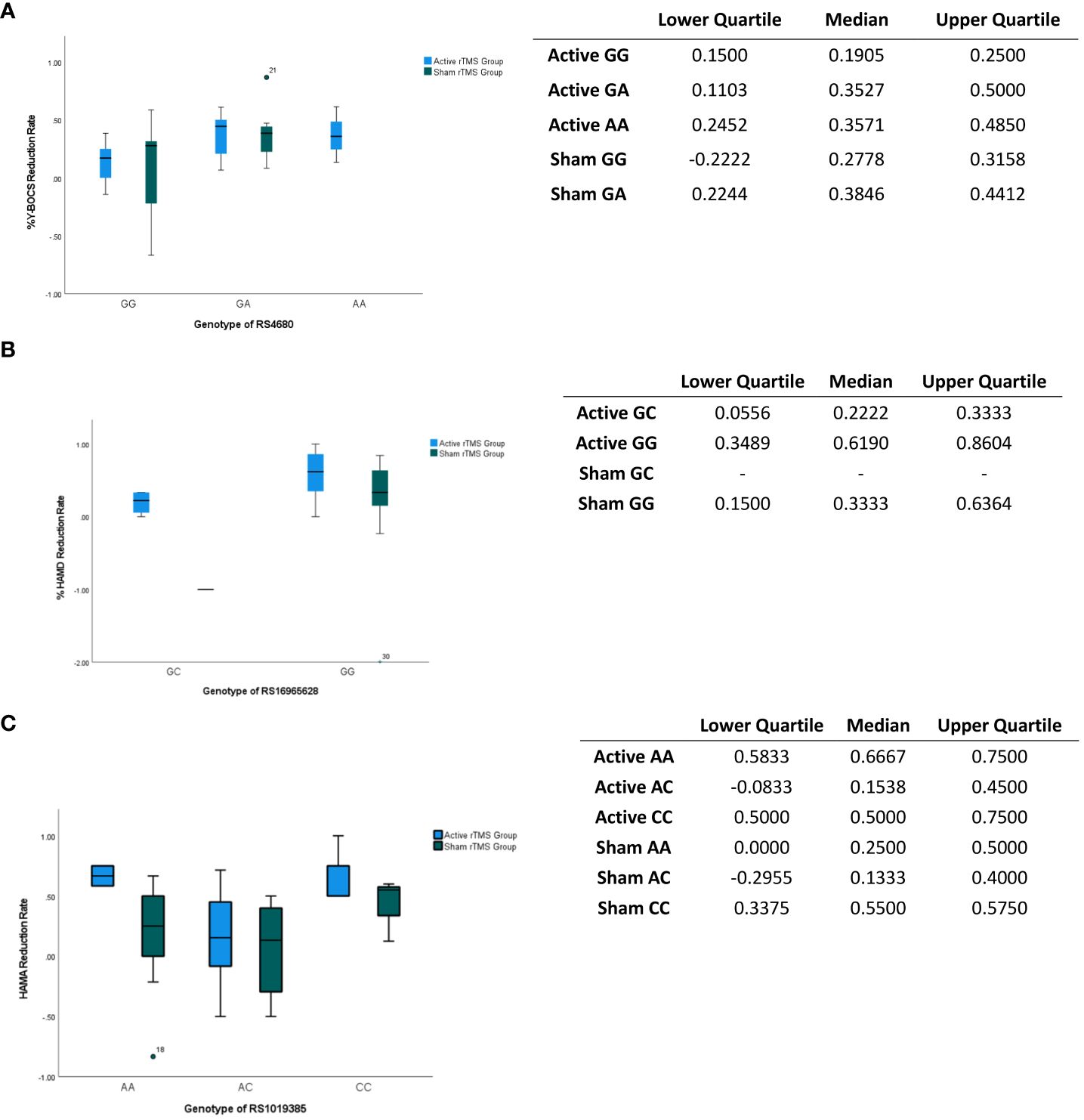
Figure 3 Impacts of gene locus on the assessments score. (A) Impacts of gene locus RS4680 on the Y-BOCS reduction at week 2. The gene locus RS4680 exerts a substantial impact on the Y-BOCS score, as evidenced by its statistical significance (p<0.05). The table shows the interquartile and median of the boxplot. (B) Impacts of gene locus RS16965628 on the HAMD reduction at week 2. The gene locus RS12965628 exerts a notable influence on the reduction of the HAMD score, as evidenced by its statistically significant effect (p<0.05). The table shows the interquartile and median of the boxplot. (C) Impacts of gene locus RS1019385 on the HAMA reduction at week 2. The gene locus RS1019385 exerts a notable influence on the reduction of the HAMD score, as evidenced by its statistically significant effect (p<0.05). The table shows the interquartile and median of the boxplot. The point shows the outlier.
The present study provides evidence that both SSRIs and rTMS augmentation of SSRIs led to significant improvements in OCD symptoms following a two-week intervention. Additionally, polymorphisms of RS4680, RS16965628, and RS1019385, were found to be associated with treatment response. The study reveals that individuals with the GG allele of RS4680 (COMT) exhibited a relatively poorer response in terms of reducing Y-BOCS scores, not only to SSRIs treatment but also to combined treatment involving SSRIs and rTMS. This finding may be attributed to the decreased susceptibility of patients with the GG allele (RS4680) to rTMS or the combination treatment. RS4680, also known as Val158Met (G to A), is closely linked to the risk of OCD (50, 51). It has been suggested that the Met (A) allele reduces the enzyme activity, enhances cortical dopamine signaling, and contributes to OCD. This explanation aligns with the current study’s finding that patients with the GG allele of RS4680 lack the A (Met) allele and are therefore less susceptible to both OCD and its treatments (50).
Regarding the serotonin transporter protein gene (SLC6A4), no significant association was observed between different genotypes and Y-BOCS scores. However, the C allele at RS16965628 (SLC6A4) predicted improvements in HAMD scores, indicating that patients with the C allele may be more responsive to rTMS augmentation therapy of SSRIs in reducing depressive symptoms. This result could be said to keep consistent with previous experimental findings on the association between the C allele and OCD patients (35, 44), highlighting the strong connection between serotonin and the depressive symptoms of OCD patients. The potential bio-marker of the C allele in RS16965628 in SLC6A4 further supports the notion proposed by Zhang (33) in 2019 that SLC6A4 could serve as a candidate gene for the combined treatment of SSRI and rTMS.
Furthermore, the study results indicate that GG allele in RS1019385 significantly benefits in reducing HAMA scores, implying that patients with this specific genotype may be more susceptible to rTMS augmentation therapy of SSRIs in alleviating anxiety symptoms. This finding might be seen as be supported by Arnold (45) who established a significant association between ACC Glx levels and the GRIN2B-RS1019385 polymorphism, with individuals carrying the GG genotype showing reduced Glx compared to T allele carriers. The G allele of RS1019385 represents a variant in the promoter region, potentially leading to decreased transcription and impacting glutamatergic neurotransmission, thereby possibly contributing to OCD (45–47).
There are several limitations of this study, including a small sample size, high drop-out rate and a short trial period of two weeks. These may account for the low effect size of some of the results. These limitations might have affected the reliability and validity of the current study. Therefore, future research should include a larger and more diverse sample size and conduct longer interventions, some better welfare might be provided to minimize withdrawal rates.
In conclusion, the A allele of COMT RS4680, the C allele of SLC6A4 RS16965628, and the GG allele of GRIN2B RS1019385 may serve as bio-markers for the combined treatment of rTMS and SSRIs in OCD, influencing Y-BOCS, HAMD, and HAMA scores, respectively. Due to limitations in sample size and trial duration, further research is needed to validate these findings. The findings of this study provide valuable insights for further investigation and the implementation of precision treatment for OCD.
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by Ethics Committee of Wuxi Mental Health Center, China (clinical trials registry number: [ChiCTR1900023641]). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
LC: Conceptualization, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YW: Conceptualization, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JY: Data curation, Methodology, Resources, Writing – review & editing. KZ: Data curation, Methodology, Resources, Writing – review & editing. YZ: Supervision, Validation, Writing – review & editing. XF: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Supervision, Validation, Writing – review & editing. GW: Project administration, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Development Fund of Shanghai Pudong New Area [PKJ2020-Y34]; Medical discipline Construction Project of Pudong Health Committee of Shanghai (Grant No.: PWYgy2021-02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
OCD, obsessive-compulsive disorder; Y-BOCS, Yale-Brown obsessive compulsive scale; HAMA, Hamilton anxiety rating scale; HAMD, Hamilton depression rating scale; rTMS, repetitive transcranial magnetic stimulation.
1. Agarwal V, Yaduvanshi R, Arya A, Gupta PK, Sitholey P. A study of phenomenology, psychiatric co-morbidities, social and adaptive functioning in children and adolescents with OCD. Asian J Psychiatry. (2016) 22:69–73. doi: 10.1016/j.ajp.2016.04.005
2. Shephard E, Batistuzzo MC, Hoexter MQ, Stern ER, Zuccolo PF, Ogawa CY, et al. Neurocircuit models of obsessive-compulsive disorder: limitations and future directions for research. Braz J Psychiatry. (2022) 44:187–200. doi: 10.1590/1516-4446-2020-1709
3. Markarian Y, Larson MJ, Aldea MA, Baldwin SA, Good D, Berkeljon A, et al. Multiple pathways to functional impairment in obsessive–compulsive disorder. Clin Psychol Rev. (2010) 30:78–88. doi: 10.1016/j.cpr.2009.09.005
4. Berlim MT, Neufeld NH, Van den Eynde F. Repetitive transcranial magnetic stimulation (rTMS) for obsessive–compulsive disorder (OCD): An exploratory meta-analysis of randomized and sham-controlled trials. J Psychiatr Res. (2013) 47:999–1006. doi: 10.1016/j.jpsychires.2013.03.022
5. George MS, Post RM. Daily left prefrontal repetitive transcranial magnetic stimulation for acute treatment of medication-resistant depression. Am J Psychiatry. (2011) 168:356–64. doi: 10.1176/appi.ajp.2010.10060864
6. Soomro GM, Altman DG, Rajagopal S, Oakley Browne M. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Systematic Rev. (2008) 1. doi: 10.1002/14651858.CD001765.pub3
7. Thamby A, Karthik S, Sharma L, Thimmashetty VH, Balachander S, Shivakumar V, et al. Transcranial direct current stimulation for treatment-resistant obsessive-compulsive disorder—A large case series. Asian J Psychiatry. (2021) 60:102625–5. doi: 10.1016/j.ajp.2021.102625
8. Rodrigues da Silva D, Maia A, Cotovio G, Oliveira J, Oliveira-Maia AJ, Barahona-Corrêa JB. Motor cortical inhibitory deficits in patients with obsessive-compulsive disorder–A systematic review and meta-analysis of transcranial magnetic stimulation literature. Front Psychiatry. (2022) 13:1050480. doi: 10.3389/fpsyt.2022.1050480
9. Zou J, Wu S, Yuan X, Hu Z, Tang J, Hu M. Effects of acceptance and commitment therapy and repetitive transcranial magnetic stimulation on obsessive–compulsive disorder. Front Psychiatry. (2022) 12:720518. doi: 10.3389/fpsyt.2021.720518
10. Barr LC, Goodman WK, Price LE. The serotonin hypothesis of obsessive compulsive disorder. Int Clin Psychopharmacol. (1993) 8 Suppl 2:79–82. doi: 10.1097/00004850-199311002-00011
11. Zohar J, Chopra M, Sasson Y, Amiaz R, Amital D. Obsessive compulsive disorder: serotonin and beyond. World J Biol Psychiatry. (2000) 1:92–100. doi: 10.3109/15622970009150571
12. Tobias J, Neziroglu F, Bhagavan HN. Obsessive-compulsive disorders: A serotonergic hypothesis. Neuro-Psychopharmacology. (1979), 117–25. doi: 10.1016/b978-0-08-023089-4.50019-3
13. Morgan WP. Physical activity and mental health. Taylor Francis. (2013). doi: 10.4324/9780203782361
14. Stahl SM. Mechanism of action of serotonin selective reuptake inhibitors. J Affect Disord. (1998) 51:215–35. doi: 10.1016/s0165-0327(98)00221-3
15. Kotapati VP, Khan AM, Dar S, Begum G, Bachu R, Adnan M, et al. The effectiveness of selective serotonin reuptake inhibitors for treatment of obsessive-compulsive disorder in adolescents and children: A systematic review and meta-analysis. Front Psychiatry. (2019) 10:523. doi: 10.3389/fpsyt.2019.00523
16. Cartwright C, Hollander E. SSRIs in the Treatment of obsessive-compulsive disorder. Depression Anxiety. (1998) 8:105–13. doi: 10.1002/(sici)1520-6394(1998)8:1+%3C105::aid-da16%3E3.0.co;2-t
17. Bloch MH, McGuire J, Landeros-Weisenberger A, Leckman JF, Pittenger C. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. (2009) 15:850–5. doi: 10.1038/mp.2009.50
18. Pallanti S, Quercioli L. Treatment-refractory obsessive-compulsive disorder: Methodological issues, operational definitions and therapeutic lines. Prog Neuropsychopharmacol Biol Psychiatry. (2006) 30:400–12. doi: 10.1016/j.pnpbp.2005.11.028
19. Simpson HB, Huppert JD, Petkova E, Foa EB, Liebowitz MR. Response versus remission in obsessive-compulsive disorder. J Clin Psychiatry. (2006) 67:269–76. doi: 10.4088/jcp.v67n0214
20. Rehn S, Eslick GD, Brakoulias V. A meta-analysis of the effectiveness of different cortical targets used in repetitive transcranial magnetic stimulation (rTMS) for the treatment of obsessive-compulsive disorder (OCD). Psychiatr Q. (2018) 89:645–65. doi: 10.1007/s11126-018-9566-7
21. Mantovani A, Lisanby SH, Pieraccini F, Ulivelli M, Castrogiovanni P, Rossi S. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS). Int J Neuropsychopharmacol. (2006) 9:95–100. doi: 10.1017/S1461145705005729
22. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Neuropsychopharmacology. (2011) 37:102–16. doi: 10.1038/npp.2011.225
23. Conca A, Di Pauli J, Beraus W, Hausmann A, PesChina W, Schneider H, et al. Combining high and low frequencies in rTMS antidepressive treatment: preliminary results. Hum Psychopharmacology: Clin Exp. (2002) 17:353–6. doi: 10.1002/hup.422
24. Cao X, Deng C, Su X, Guo Y. Response and remission rates following high-frequency vs. Low-frequency repetitive transcranial magnetic stimulation (rTMS) over right DLPFC for treating major depressive disorder (MDD): A meta-analysis of randomized, double-blind trials. Front Psychiatry. (2018) 9:413. doi: 10.3389/fpsyt.2018.00413
25. Mantovani A, Rossi S, Bassi BD, Simpson HB, Fallon BA, Lisanby SH. Modulation of motor cortex excitability in obsessive-compulsive disorder: An exploratory study on the relations of neurophysiology measures with clinical outcome. Psychiatry Res. (2013) 210:1026–32. doi: 10.1016/j.psychres.2013.08.054
26. Russo M, Naro A, Mastroeni C, Morgante F, Terranova C, Muscatello MR, et al. Obsessive-compulsive disorder: A “sensory-motor” problem? Int J Psychophysiol. (2014) 92:74–8. doi: 10.1016/j.ijpsycho.2014.02.007
27. Lusicic A, Schruers KR, Pallanti S, Castle DJ. Transcranial magnetic stimulation in the treatment of obsessive–compulsive disorder: current perspectives. Neuropsychiatr Dis Treat. (2018) 14:1721–36. doi: 10.2147/ndt.s121140
28. Sina M, Ahmadiani A, Asadi S, Shams J. Association of serotonin receptor 2a haplotypes with obsessive–compulsive disorder and its treatment response in Iranian patients: a genetic and pharmacogenetic study. Neuropsychiatr Dis Treat. (2018) 14:1199–209. doi: 10.2147/ndt.s163946
29. Mansur C, Cabral S, Myczkowsky M, Sartorelli M, Rosa M, Pridmore S, et al. rTMS for resistant OCD (Interim analysis). J ECT. (2007) 23:55–6. doi: 10.1097/01.yct.0000264363.43870.7a
30. Gomes PVO, Brasil-Neto JP, Allam N, Rodrigues de Souza E. A randomized, double-blind trial of repetitive transcranial magnetic stimulation in obsessive-compulsive disorder with three-month follow-up. J Neuropsychiatry Clin Neurosci. (2012) 24:437–43. doi: 10.1176/appi.neuropsych.11100242
31. Pelissolo A, Harika-Germaneau G, Rachid F, Gaudeau-Bosma C, Tanguy M-L, BenAdhira R, et al. Repetitive transcranial magnetic stimulation to supplementary motor area in refractory obsessive-compulsive disorder treatment: a sham-controlled trial. Int J Neuropsychopharmacol. (2016) 19:pyw025. doi: 10.1093/ijnp/pyw025
32. Rostami R, Kazemi R, Jabbari A, Madani AS, Rostami H, Taherpour MA, et al. Efficacy and clinical predictors of response to rTMS treatment in pharmacoresistant obsessive-compulsive disorder (OCD): a retrospective study. BMC Psychiatry. (2020) 20:372. doi: 10.1186/s12888-020-02769-9
33. Zhang K, Fan X, Yuan J, Yin J, Su H, Hashimoto K, et al. Impact of serotonin transporter gene on rTMS augmentation of SSRIs for obsessive compulsive disorder. Neuropsychiatr Dis Treat. (2019) 15:1771–9. doi: 10.2147/ndt.s209319
34. Rashidi F, Ahmadipour E, Shiravand S, Ahmadiani A, Asadi S, Shams J. Association of the functional serotonin transporter haplotype with familial form of obsessive compulsive disorder in Iranian patients. Int J Psychiatry Clin Pract. (2017) 22:47–53. doi: 10.1080/13651501.2017.1353634
35. Grünblatt E, Marinova Z, Roth A, Gardini E, Ball J, Geissler J, et al. Combining genetic and epigenetic parameters of the serotonin transporter gene in obsessive-compulsive disorder. J Psychiatr Res. (2018) 96:209–17. doi: 10.1016/j.jpsychires.2017.10.010
36. Kohlrausch FB, Giori IG, Melo-Felippe FB, Vieira-Fonseca T, Velarde LGC, de Salles Andrade JB, et al. Association of GRIN2B gene polymorphism and Obsessive Compulsive disorder and symptom dimensions: A pilot study. Psychiatry Res. (2016) 243:152–5. doi: 10.1016/j.psychres.2016.06.027
37. Sinopoli VM, Burton CL, Kronenberg S, Arnold PD. A review of the role of serotonin system genes in obsessive-compulsive disorder. Neurosci Biobehav Rev. (2017) 80:372–81. doi: 10.1016/j.neubiorev.2017.05.029
38. Vulink N, Westenberg HGM, Van Nieuwerburgh F, Deforce D, Fluitman SBAHA, Meinardi JSC, et al. Catechol-O-methyltranferase gene expression is associated with response to citalopram in obsessive-compulsive disorder. Int J Psychiatry Clin Pract. (2012) 16:277–83. doi: 10.3109/13651501.2011.653375
39. Oz MD, Baskak B, Uckun Z, Artun NY, Ozdemir H, Ozel TK, et al. Association between serotonin 2A receptor (HTR2A), serotonin transporter (SLC6A4) and brain-derived neurotrophic factor (BDNF) gene polymorphisms and citalopram/sertraline induced sexual dysfunction in MDD patients. Pharmacogenomics J. (2019) 20:443–50. doi: 10.1038/s41397-019-0127-8
40. Cengiz M, Okutan SN, Bayoglu B, Sakalli Kani A, Bayar R, Kocabasoglu N. Genetic polymorphism of the serotonin transporter gene, SLC6A4 rs16965628, is associated with obsessive compulsive disorder. Genet Testing Mol Biomarkers. (2015) 19:228–34. doi: 10.1089/gtmb.2014.0319
41. Hildonen M, Levy AM, Dahl C, Bjerregaard VA, Birk Møller L, Guldberg P, et al. Elevated Expression of SLC6A4 Encoding the Serotonin Transporter (SERT) in Gilles de la Tourette Syndrome. Genes. (2021) 12:86. doi: 10.3390/genes12010086
42. Reigstad CS, Linden DR, Szurszewski JH, Sonnenburg JL, Farrugia G, Kashyap PC. Correlated gene expression encoding serotonin (5-HT) receptor 4 and 5-HT transporter in proximal colonic segments of mice across different colonization states and sexes. Neurogastroenterol motility/Neurogastroenterology Motil. (2016) 28:1443–8. doi: 10.1111/nmo.12840
43. Baumgarten HG, Grozdanovic Z. Role of serotonin in obsessive-compulsive disorder. Br J Psychiatry. (1998) 173:13–20. doi: 10.1192/s0007125000297857
44. Lindholm Carlstrom E, Saetre P, Rosengren A, Thygesen JH, Djurovic S, Melle I, et al. Association between a genetic variant in the serotonin transporter gene (SLC6A4) and suicidal behavior in patients with schizophrenia. Behav Brain Functions. (2012) 8:24. doi: 10.1186/1744-9081-8-24
45. Arnold PD, MacMaster FP, Richter MA, Hanna GL, Sicard T, Burroughs E, et al. Glutamate receptor gene (GRIN2B) associated with reduced anterior cingulate glutamatergic concentration in pediatric obsessive–compulsive disorder. Psychiatry Research: Neuroimaging. (2009) 172:136–9. doi: 10.1016/j.pscychresns.2009.02.005
46. Szeszko PR, MacMillan S, McMeniman M, Chen S, Baribault K, Lim KO, et al. Brain structural abnormalities in psychotropic drug-naive pediatric patients with obsessive-compulsive disorder. Am J Psychiatry. (2004) 161:1049–56. doi: 10.1176/appi.ajp.161.6.1049
47. Batistuzzo MC, Sottili BA, Shavitt RG, Lopes AntônioC, Cappi C, Alice M, et al. Lower ventromedial prefrontal cortex glutamate levels in patients with obsessive–compulsive disorder. Front Psychiatry. (2021) 12:668304. doi: 10.3389/fpsyt.2021.668304
48. Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci. (1998) 95:9991–6. doi: 10.1073/pnas.95.17.9991
49. Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Mannisto PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. J Neurosci. (2007) 27:10196–209. doi: 10.1523/jneurosci.0665-07.2007
50. Kumar P, Rai V. Catechol-O-methyltransferase gene Val158Met polymorphism and obsessive compulsive disorder susceptibility: a meta-analysis. Metab Brain Dis. (2019) 35:241–51. doi: 10.1007/s11011-019-00495-0
51. hamidian s, Pourshahbaz A, moradkhani a, Dolatshahi B, Ananloo ES, Ohadi M. A case-control association study of COMT rs4680 Polymorphism with Obsessive-Compulsive Disorder and Cognitive Functions: The role of sex. Res Square (Research Square). (2020). doi: 10.21203/rs.3.rs-34240/v1
Keywords: OCD, COMT, SLC6A4, GRIN2B, repetitive transcranial magnetic stimulation
Citation: Chu L, Wu Y, Yin J, Zhang K, Zhong Y, Fan X and Wang G (2024) Neurotransmitter system gene variants as biomarkers for the therapeutic efficacy of rTMS and SSRIs in obsessive-compulsive disorder. Front. Psychiatry 15:1350978. doi: 10.3389/fpsyt.2024.1350978
Received: 26 January 2024; Accepted: 06 May 2024;
Published: 22 May 2024.
Edited by:
Aye-Mu Myint, Maastricht University, NetherlandsReviewed by:
Jianmin Yuan, Nanjing Medical University, ChinaCopyright © 2024 Chu, Wu, Yin, Zhang, Zhong, Fan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiwang Fan, ZmFueGl3YW5nMjAyMEAxNjMuY29t; Guoqiang Wang, d3V4aW1oY2d1b3FpYW5nQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.