- 1Unit for Paediatric and Adolescent Psychiatry, Division of Paediatrics, University Medical Centre Maribor, Maribor, Slovenia
- 2Laboratory for Digital Signal Processing, Faculty of Electrical Engineering and Computer Science, University of Maribor, Maribor, Slovenia
- 3Department of Psychiatry, Faculty of Medicine University of Maribor, Maribor, Slovenia
- 4Department of Psychology, Faculty of Arts, University of Maribor, Maribor, Slovenia
Borderline Personality Disorder (BPD), impacting approximately 2% of adults worldwide, presents a formidable challenge in psychiatric diagnostics. Often underdiagnosed or misdiagnosed, BPD is associated with high morbidity and mortality. This scoping review embarks on a comprehensive exploration of observable cues in BPD, encompassing language patterns, speech nuances, facial expressions, nonverbal communication, and physiological measurements. The findings unveil distinctive features within the BPD population, including language patterns emphasizing external viewpoints and future tense, specific linguistic characteristics, and unique nonverbal behaviors. Physiological measurements contribute to this exploration, shedding light on emotional responses and physiological arousal in individuals with BPD. These cues offer the potential to enhance diagnostic accuracy and complement existing diagnostic methods, enabling early identification and management in response to the urgent need for precise psychiatric care in the digital era. By serving as possible digital biomarkers, they could provide objective, accessible, and stress-reducing assessments, representing a significant leap towards improved psychiatric assessments and an invaluable contribution to the field of precision psychiatry.
Introduction
Psychiatric conditions cause disability, premature mortality, and strain on society due to costly and prolonged treatment. While research has made strides, there remains an urgent need for faster progress in identifying and managing these disorders (1). The need for improvement in understanding and managing psychiatric disorders, while relevant to many conditions, is particularly urgent for borderline personality disorder (BPD). BPD is complex, making its diagnosis and treatment unique challenges. It affects around 2% of adults globally, and leads to adverse outcomes like education and career difficulties, shorter relationships, conflicts, risky behaviors, limited social support, reduced life satisfaction, and increased healthcare use. Individuals with BPD grapple with emotional regulation, an unstable self-concept, and relationship problems. BPD symptoms are broad and can change over a lifetime, often involving maladaptive behaviors such as occasional aggression, heightened rejection sensitivity, self-harm, and suicidal thoughts (2). In fact, individuals with BPD face an increased risk of premature death (3). Unfortunately, up to 10% succumb to suicide (4). This heightened mortality risk translates to an estimated loss of 5·0-9·3 years of life expectancy (5).
Despite several structured and semi-structured interviews, mental health diagnosis and treatment heavily depend on unstructured psychiatric interviews and subjective assessments. This reliance results in the underdetection of BPD, with over 40% of patients misdiagnosed as depressed. The complex overlap of BPD and depression symptoms underscores the critical need for precise diagnosis (6, 7). Accurate diagnosis is a crucial step for most individuals with BPD, providing significant relief and enabling them to comprehend their behaviors and past experiences. It precedes vital psychoeducation and treatment (2).
Artificial intelligence (AI) has advanced notably in healthcare, particularly in oncology, radiology, and dermatology. However, uptake of AI in psychiatry is notably slower and more challenging compared to other fields of medicine (8). This phenomenon can be attributed to several factors, such as, subjectivity of symptoms and diagnosis (1, 9), complexity of symptoms and disorders (9, 10), lack of uniform, objective biomarkers and diverse DSM-5 diagnostic criteria (11). Psychiatry’s complex data processing and clinical decision-making surpass the challenges faced in tasks like tumor identification in medical images where AI has excelled (12). Moreover, the use of AI requires access to large amounts of patient data, which often includes sensitive information shared during therapy sessions (13). The complexity of mental disorders also requires large, diverse, and high-quality datasets to train AI models effectively. However, most current datasets are small, lack diversity, and may not accurately represent the broad spectrum of psychiatric conditions (9). Thus, AI models in psychiatry often suffer from the limitation of insufficient and biased training data. AI has the potential to transform the field of psychiatry, including those working with BPD patients, however, not as a classifier generating diagnosis, but rather by providing tools that can enhance treatment efficacy and diagnostic accuracy. For instance, AI-driven tools can assist in developing personalized treatment plans by analyzing individual patient data and by predicting which treatments are most likely to be effective for individual patients, based on their unique data profiles (14).
AI has significant potential to enhance psychiatric care, but ethical and practical concerns must be addressed. Key issues include ensuring privacy and data security (15) as digital biomarkers and especially video recordings can reveal sensitive information (16, 17) and raise concerns about misuse, re-identification, and harm to the patient-therapist relationship (15, 17). Psychiatric patients may worry about stigma and discrimination, which can impact their willingness to participate in research (16), especially when their images and behaviors are recorded (17). Implementing AI methods into clinical psychiatric practice also introduces challenges, including ensuring informed consent, especially for those with cognitive impairments, and validating digital biomarkers to prevent harm from false results. Additionally, efforts must be made to reduce bias in AI models, as biased algorithms could exacerbate existing disparities in mental health care. AI’s potential to worsen health disparities due to unequal access and cultural differences, alongside data bias affecting algorithm effectiveness, adds complexity (15). Historically, mental health research has suffered from biases, and without addressing these issues, AI could reinforce existing inequities (18). Furthermore, transparency and explainability of AI-driven decisions are crucial for building trust between clinicians and patients and ensuring that AI is used as a supportive tool rather than a replacement for human. The integration of AI into clinical practice must also contend with regulatory gaps that allow unproven products to enter the market, raising safety and exploitation concerns. Addressing these issues, particularly in the context of video-based AI tools, is crucial for the ethical deployment of AI in mental health (15).
Despite these challenges, AI holds considerable potential for improving the monitoring and screening of patients. Using natural language processing could be integrated into mainstream therapies for BPD like dialectical-behavioral therapy and mentalization therapy. AI could also help patients re-author their self-narratives into more coherent sequences, promoting mentalization and insight into causal connections and self-agency (14). The most significant potential of AI in psychiatry, however, lies in the monitoring/screening of patients (19, 20). Namely, when used to extract digital biomarkers, AI is leveraged for delivering real-time insights and comprehensive contextual analysis, rather than for classifying disorders. In this area AI has demonstrated strong reliability (high accuracy and efficiency) in extracting relevant features, such as discrete and categorical data, from various datasets (21). While, when it comes to classification tasks, AI often encounters challenges related to data quality and inherent biases and explainability, which can significantly affect its performance. As such, AI can be employed to develop automated screening and assessment tools that quickly identify individuals at risk for mental health disorders (22, 23). A growing area of interest explores the connections between observable cues like language patterns, speech nuances, and facial expressions and the psychological characteristics of those who display them (24). These observable cues appear naturally, spontaneously and are less susceptible to cognitive biases and social acceptability concerns, enhancing the objectivity of psychiatric assessments (25). Digital biomarkers, using AI and observable cues such as language patterns and facial expressions, can advance the management of psychiatric disorders, including complex ones like BPD. They provide objective, accessible, and stress-reducing assessments, empowering patients and identifying high-risk individuals, aligning with precision medicine. This integration addresses the urgent need for precise psychiatric care and promises to revolutionize mental health management in the digital era (26).
This scoping review aims to inveil concealed observable features of BPD in conversations that can be harnessed by AI methods. By extensively summarizing literature results on cues like language, speech, facial expressions, nonverbal communication, and physiological measurements, our research provides valuable insights into distinct BPD characteristics. This knowledge could contribute to the development of sensing technology and machine learning algorithms, potentially supporting and refining existing psychiatric methods for earlier diagnosis and more personalized treatment of this often-overlooked disorder.
Methods
Overview
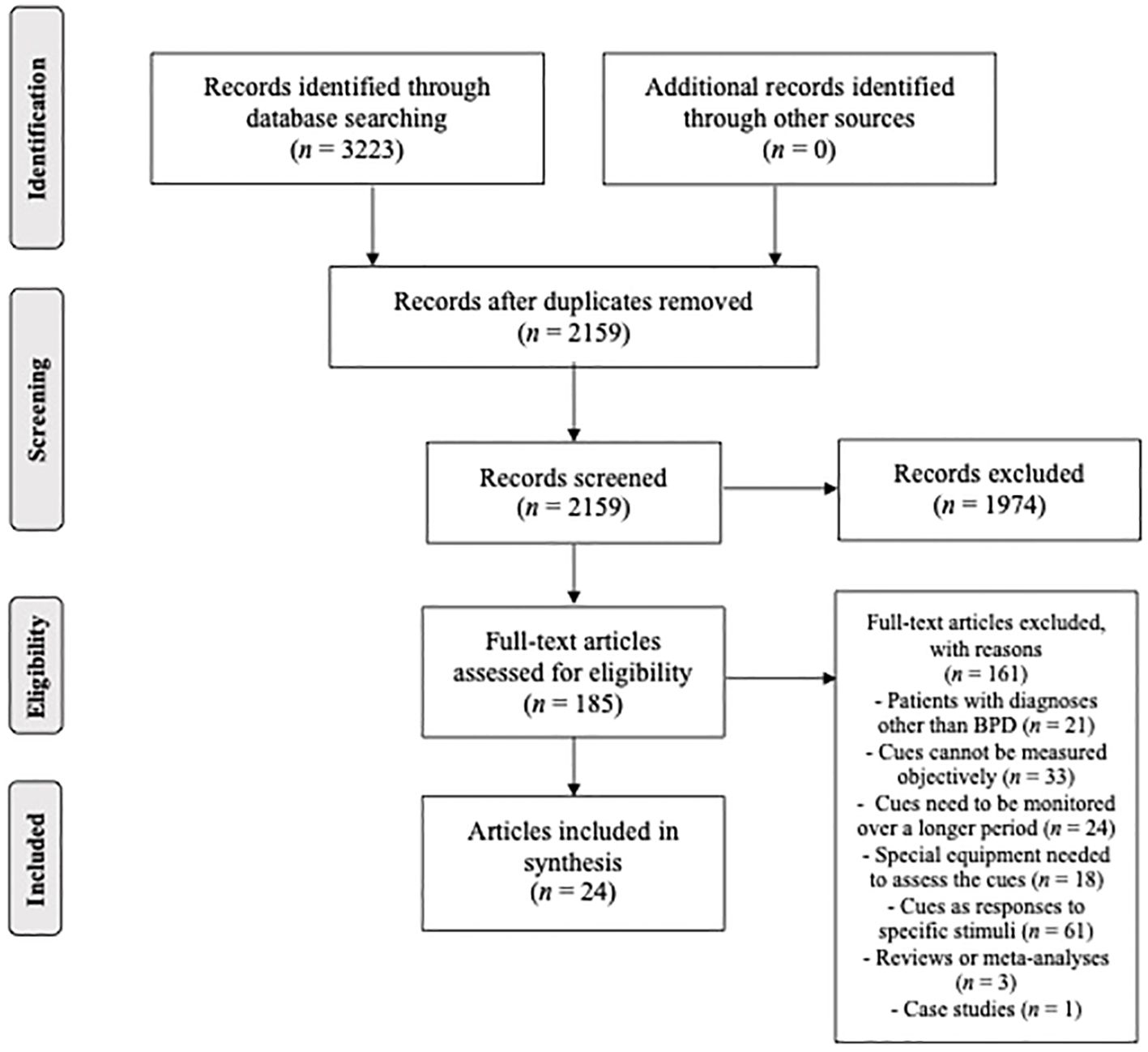
A methodological framework for conducting scoping reviews by Arksey and O’Malley and Levac and colleagues was followed in the preparation of this study (27, 28). Therefore, we (1) identified the research questions, (2) identified relevant studies, (3) selected final studies to be included in the review, (4) charted the data, and (5) collated, summarized, and reported results. Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping reviews (PRISMA-ScR, see Figure 1) was followed to ensure that the process was systematic, complete, and transparent (29).
Identifying the research question
Our research question aimed to identify which observable cues, spontaneously expressed by individuals with BPD, can be objectively measured during a clinical interview or in a home setting. We focused on identifying cues such as language use, speech patterns, facial expressions, other forms of nonverbal communication, and physiological measurements. To guide this investigation, we established specific inclusion and exclusion criteria, which are explained in the following section.
Identifying relevant studies
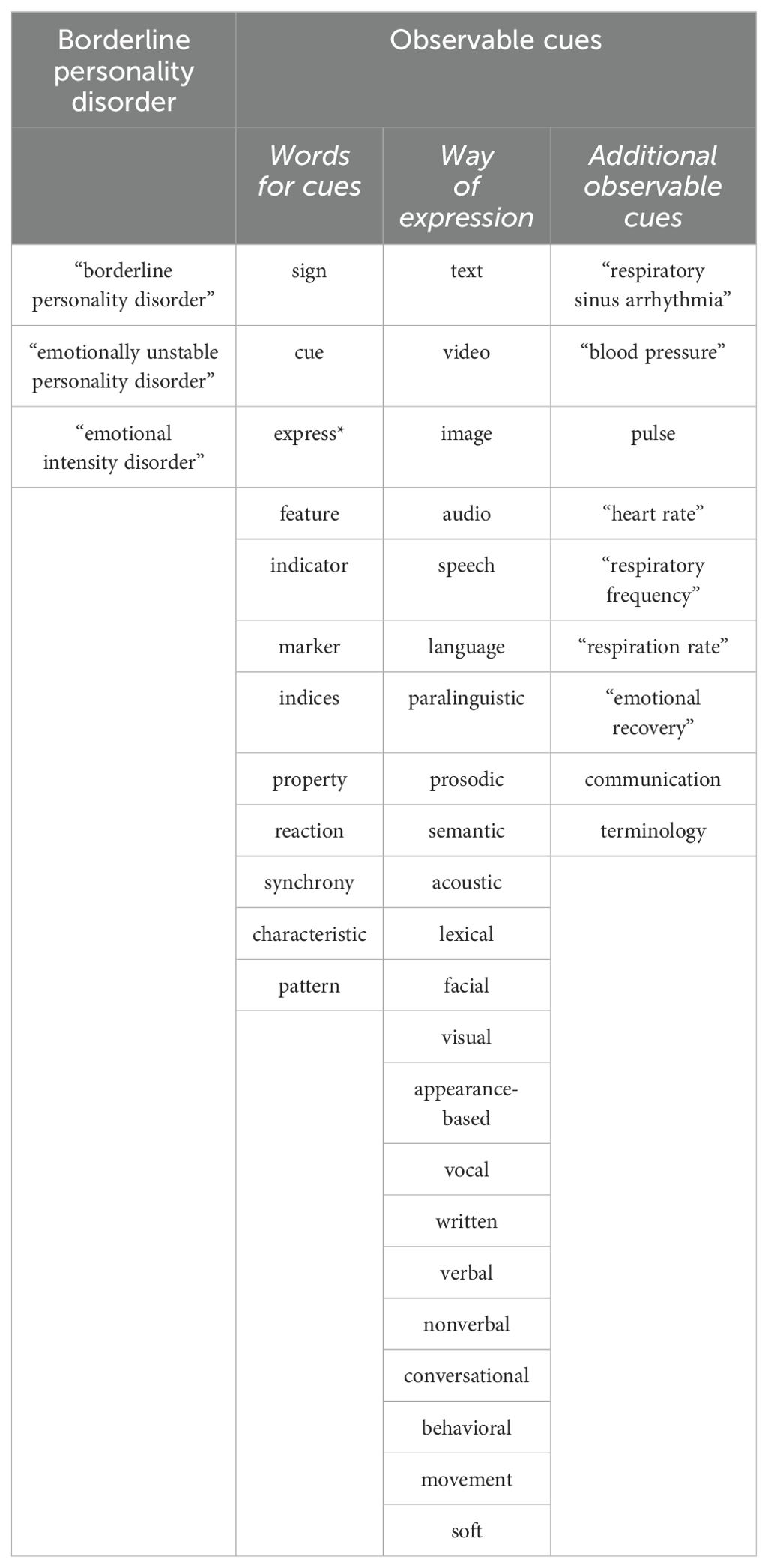
Scopus and Web of Science, two large and extensively used databases were used to identify the relevant papers (30). These databases complement each other well (31), and are known for their large overlap with other sources, such as MEDLINE and EMBASE (32). After several rounds of preliminary searches in both databases, which helped us refine our search strategy, we carried out the main search on March 31, 2023. The final version of our search strategy combined different terms related to borderline personality disorder and observable cues (Table 1). All terms were searched for in their singular and plural forms, as well as British and American English. The terms were later combined into a nested format using Boolean operators (AND, OR) and searched for in titles, abstracts, and keywords. The exact search string was (“borderline personality disorder” OR “emotionally unstable personality disorder” OR “emotional intensity disorder”) AND [((sign OR cue OR express* OR feature OR indicator OR marker OR indices OR property OR reaction OR synchrony OR characteristic OR pattern] AND [text OR video OR image OR audio OR speech OR language OR paralinguistic OR prosodic OR semantic OR acoustic OR lexical OR facial OR visual OR appearance-based OR vocal OR written OR verbal OR nonverbal OR conversational OR behavioral OR behavioural OR movement OR soft)] OR [“respiratory sinus arrhythmia” OR “blood pressure” OR pulse OR “heart rate” OR “respiratory frequency” OR “respiration rate” OR “emotional recovery” OR communication OR terminology)].”
To be considered for inclusion, the records had to: 1) be available in English and 2) be published in scientific journals or conference proceedings. Records were then excluded if: 1) they employed samples of patients with disorders other than BPD (e.g., depression), 2) focused on cues that cannot be measured objectively (e.g., reliance on self-report), 3) focused on cues that need to be monitored over a longer period (e.g., monitoring patients for 24 hours), 4) focused on cues that need specialized equipment to be assessed (e.g., brain imaging techniques), 5) focused on cues that are responses to specific stimuli (e.g., recording eye movements during cognitively demanding tasks), 6) they were reviews or meta-analyses, and 7) if they were single case studies (i.e., with N = 1). The methodological quality of studies was not a reason for exclusion.
Study selection
The main search, performed in SCOPUS and Web of Science, resulted in 3223 English language articles (SCOPUS: 1570, Web of Science: 1653) published in scientific journals and conference proceedings. An additional search in Google Scholar was also performed to ensure we didn’t miss any relevant records, but it did not lead to the identification of any previously unidentified articles. The two chosen databases overlapped considerably, which led to the removal of 1064 records. After screening the titles and abstracts, 185 records (8·6% of identified unique records) still fit the criteria. However, further assessment revealed that only 24 articles (1·1% of identified unique records) met the inclusion criteria. These articles were included in the final synthesis.
Charting the data
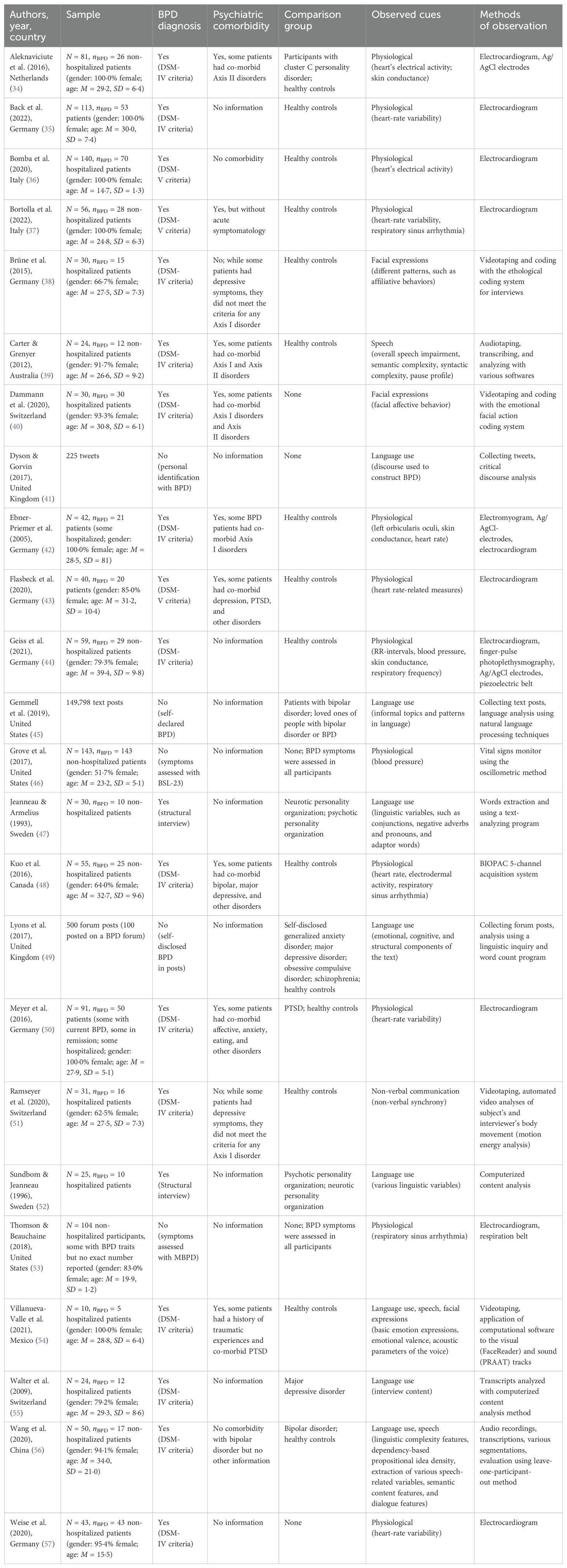
Following the research question, a spreadsheet form was developed and used for the extraction of the following information from the reviewed papers: (1) authors, (2) year of publication, (3) country where the study was carried out, (4) sample characteristics, (5) BPD diagnosis, (6) (potential) psychiatric comorbidity, (7) comparison group, (8) observed cues, and (9) methods of observation. The data were extracted from the reviewed papers by two researchers (SM and (NP)) and refined in an iterative process while reviewing the papers.
Collating, summarizing, and reporting results
We followed the aim of scoping reviews, i.e., mapping of the existing findings and providing their overview in a descriptive manner (27). The results were analyzed by three authors (SM, HGK and NP) via thematic analysis (33). Main categories of cues of borderline personality disorders were predefined, and included features related to (1) language use, (2) speech, (3) facial expressions, (4) other features of nonverbal communication, and (5) features related to physiological measurements. The process of collating and summarizing results was reviewed by two authors (NP and US).
Results
Characteristics of reviewed studies
While the publication year ranged from 1993 to 2022, more than 75·0% of included studies (n = 19; 79·2%) were published in the last ten years, and precisely 50·0% (n = 12) were published in the last five years. Geographically, most of the studies (n = 17; 70·8%) were conducted in Europe, followed by studies conducted in North America (n = 5; 20·8%), Australia (n = 1; 4·2%), and Asia (n = 1; 4·2%).
The reviewed studies involving human participants had sample sizes ranging from 10 to 143 (average 58·1, SD = 39·0), but BPD patients were only a subset of the sample. Additionally, the three studies without human participants analyzed from 225 to 149,798 text posts. In studies reporting demographic data, the samples were entirely or predominantly female, whereas the average age of participants was mostly (73·9%) in the 25-35 range. Most studies used DSM (either DSM-IV or DSM-V) criteria to diagnose BPD (n = 17; 70·8%), while others used different ways of diagnosing BPD (n = 2; 8·3%) or did not attempt a diagnosis (n = 5; 20·8%). Nearly half (47·4%) reported significant comorbidity in their samples.
Regarding observed cues, half of the studies (n = 12; 50·0%) focused on physiological cues like heart-rate variability, blood pressure, skin conductance, and respiratory sinus arrhythmia. The other studies primarily examined language use (n = 6; 31·6%), facial expressions (n = 2; 8·3%), speech (n = 1; 4·2%), and non-verbal communication (n = 1; 4·2%). Two studies (n = 2; 8·3%) observed cues from multiple categories. Various methods, including electrocardiogram, Ag/AgCl electrodes, electromyogram, videotaping, audio recording, and coding systems, were used to measure these cues objectively. Patient data were compared to healthy controls in 45·8% of studies (n = 11), other disorder patients in 12·5% (n = 3), both healthy controls and other disorder patients in 20·8% (n = 5), and in the remaining 20·8% (n = 5), there was no comparison group. For more details, refer to Table 2.
Features related to Language use
Patients with BPD within borderline personality organization (BPO) tend to favor language that highlights external viewpoints and maintains an impersonal tone. This language conveys ideas neutrally, avoiding personal attribution. Multiple studies support the prevalence of third person singular pronoun use among patients with BPD (47, 49). Notably, Jeanneau and Armelius highlight the prominence of the pronoun “they” as characteristic of BPD (47). Singular pronoun use, including “you” is heightened in BPD (49). Another distinct feature is the frequent use of future tense (47, 52).
Numerous other formal grammatical features are distinct in BPD, including adaptor words (like “so”), time-space adverbs (like “then”), conjunctions (such as “because”), intent expressions (like “I mean”), capability expressions (“I can”), negative verbs, nouns, and adjectives (like “awful”, “disgusting”, “unhappy”, “crazy”), as well as frequent negation (“not”) (47, 52). Another observed linguistic feature is the combination of nonfluencies with conjunctions. A preference for absolute words, coupled with common adverbs and article words (such as “a”, “an”, and “the”), is also noticeable. Conjunctions combined with nonfluencies, and the pairing of “you” with verbs and nonfluencies, also emerge as notable markers (56). While patients with BPD use adjectives frequently, interjections are less common (54).
When comparing bipolar disorder (BD) and BPD, several distinctions encompassing quantitative measures like Brunet’s index (BI), moving average type-to-token ratio (MATTR), and mean length sentence (MLS) emerged in Wang et al., but they do not clarify further on the actual differences between both conditions. Other notable differences include “we” with prepositions and a mix of absolute words, common adverbs, and negations. Thematic content focuses on social processes and drives. The study emphasizes the significance of linguistic complexity in differentiating BPD from BD and controls. Linguistic features play a key role, while content features have limited impact on classification. Findings underscore linguistic features’ importance, the drawback of omitting linguistic and dialogue aspects, and the relative dispensability of dialogue and content features (56).
Dyson and Gorvin unveiled discourse dynamics about BPD, depicting a spectrum from BPD as a source of tension to its depiction as a distinct and unique existence. Each of these narrative repertoires exhibited its own set of linguistic patterns and frequently employed words and phrases that actively contributed to shaping these portrayals. The “BPD as a source of tension” repertoire highlights several preferred themes, encompassing struggles for control, reductionist and deterministic terminology, expressions of powerlessness, and a biomedical standpoint. Specific linguistic patterns and commonly used words/phrases, like “disorder”, “do everything right”, “disorder takes over”, “out of nowhere disorder takes over”, “stupid things”, etc. reinforce this view. In contrast, the “BPD as a different existence” repertoire emphasizes themes of acceptance, positive self-comparisons, and openness. Using linguistic strategies describing BPD as just one of many ways to engage with the world, and the consistent use of words and expressions like “passion”, “specialness”, “embrace”, “unstable”, “abnormal”, and “different”, a coherent alternative narrative emerged (41).
In BPO language use, words often carry aggressive and depressive connotations (52). Individuals with BPD show limited negativity in interviews (55). On the other hand, their writing contains numerous negative emotion words (49). This is consistent with heightened negative emotional valence (45). Further exploration of their online conversations revealed distinctive characteristics, including an emphasis on mood-related vocabulary, a distinct focus on dating experiences (both positive and negative), a notable intertwining of work-related concerns with discussions about medication and diagnoses, and an overall optimistic outlook regarding the efficacy of treatment through medication, despite acknowledged challenges (54).
No significant differences were found between patients with BPD and healthy controls in terms of the complexity of sentence structure, or the complexity of the content being communicated (39).
Features related to speech
Although there were no significant overall speech differences between BPD patients and healthy controls, individuals with BPD displayed notably higher pause frequency during neutral speech conditions (39). Additionally, a distinctive dialogue-related aspect emerges, encompassing number of words per second, pause duration, and relative floor control (56). Compared to the control group, patients show more frequent correlations, especially positive ones, between elements of prosody and facial expressions of certain negative emotions. Specifically, patients exhibited a positive correlation between facial expressions of disgust and anger and the acoustical parameters of adjectives and interjections, both in terms of decibels and fundamental frequency, which was absent in controls. Additionally, some correlations exhibit opposite patterns: negative in controls and positive in patients, notably for disgust and adjectives (f0-dB), anger and adjectives (dB), and anger and interjections (f0). Vocal characteristics of adjectives and interjections showed no significant differences between patients with BPD and controls (54).
Features related to facial expressions
In contrast to healthy controls, individuals with BPD exhibited reduced affiliative behavior, concerning patterns of behavior that invite and positively reassure social interaction, including “head to side” movements; “bob”, a sharp upward movement of the head, similar to an inverted nod; “flash”, a quick raising and lowering of the eyebrows; “raise”, a movement in which the eyebrows are raised and kept up for some time; and “smile”, in which the lip corners are typically drawn back and up. Both groups demonstrated comparable inclinations to engage in flight behaviors, expressing the avoidance of social interaction and comprising behavioral features that lead to cutting off communication (38).
Regarding facial affective behavior, individuals with BPD demonstrated prominent negative emotions like disgust and contempt. However, they also exhibited social smiles, indicative of positive social interactions (40). Patients with BPD displayed less than half the amount of sadness compared to the control group, indicating a complex blend of emotional expressions (54). Cluster analysis divided patients into two groups: Cluster 1 showed higher overall facial activity and intense negative emotions (anger, contempt, disgust), combined with significant social smiles; Cluster 2 displayed lower levels of specific negative emotions while maintaining notable social smiles (40).
Other features of nonverbal communication
Nonverbal synchrony between patients with BPD and interviewers was significantly higher than chance (pseudosynchrony). Patients with BPD often led in nonverbal interactions, with interviewers mirroring their cues more than the opposite (51).
Features related to physiological measurements
Several studies found no significant heart rate differences between individuals with BPD and control groups at baseline (37, 42, 50). On the contrary, some studies revealed patients with BPD having elevated rates (37, 48, 57).
In terms of ECG measurements encompassing various parameters such as respiratory sinus arrhythmia (RSA), square root of the mean squared differences of successive NN intervals (RMSSD), standard deviation of normal-to-normal intervals (SDNN), and vagal-mediated heart rate variability (HRV), Bortolla et al. found no significant differences in baseline measurements between individuals with BPD and control groups (37). Likewise, no substantial differences in ECG amplitudes were observed between patients with BPD and healthy controls (43). Patients with BPD exhibited reduced RSA levels with a detected negative correlation between BPD symptom severity and RSA, indicating decreased RSA as symptoms intensify (48, 53). Females with BPD demonstrated lower mean RMSSD values compared to healthy controls (35). Regarding HRV, heightened BPD symptom severity was linked to reduced HRV during rest (57). In another study individuals with BPD showed notable heart rate fluctuations compared to other groups, without significant distinctions in HRV measures (50). The study by Flasbeck et al. unveiled significant connections among cardiovascular measures, symptom severity, and the parasympathetic nervous system (PNS) index (43). Additionally, individuals with BPD displayed longer QTcd duration than controls, aligning with findings from Bomba et al. showing a mild yet statistically significant positive correlation between QTc and QTcd measurements within the BPD group (36).
Patients with BPD showed higher systolic blood pressure (BPsys) than controls, particularly during rest. Additionally, patients with BPD consistently displayed elevated diastolic blood pressure (BPdia) in comparison to controls (44). Notably, BPD symptomatology did not predict cardiovascular reactivity (CVR), computed from BPsys and BPdia values (46).
Resting skin conductance levels (SCL) in patients with BPD resembled those of healthy controls (42). Yet Aleknaviciute et al. revealed heightened overall SCL in patients with BPD, indicating intensified physiological responses associated with emotional experiences (34).
Patients with BPD showed significantly lower mean resting scores in left orbicularis oculi EMG compared to controls (42).
Discussion
Our study revealed numerous unique characteristics of BPD in language, nonverbal cues, and physiology. These findings provide a comprehensive understanding of BPD with potential implications for diagnosis and treatment.
Language patterns in BPD patients offer insights into their emotional regulation and cognitive processes. They often use impersonal language, including third-person pronouns like “they”, possibly linked to insecure attachments rooted in early life traumas (47, 49). Their increased use of future tense may stem from heightened uncertainty and anxiety related to emotional fluctuations and a desire for change, possibly even as an unconscious way of navigating the future (47). Unique linguistic characteristics, including vague adaptor words, adverbs, conjunctions, and negation, provide insights into coping with emotional difficulties, self-reflection, and identity integration (47). Discourse dynamics in the study uncovers two distinct narratives surrounding BPD. They often portray BPD as an existence of tension, allowing them to distance themselves from personal agency over their behaviors and the stigma attached to the diagnosis. On the other hand, some individuals construct BPD as a different existence, embracing their uniqueness and challenging prevailing conceptualizations. This narrative offers more positive subject positions and may alleviate some of the negativity associated with BPD. However, it can also lead to unintended comparisons with others, potentially hindering access to care and support (41). Concealment of intense negative emotions in interviews, in contrast to open expression in writing, reflects their greater comfort with written self-expression. This divergence in communication styles may pose interpersonal challenges, as suppressed spoken emotions hinder effective communication. Identity issues in BPD can contribute to this struggle, leading to primitive defense mechanisms like splitting that manifest in non-verbal behaviors (55).
Analyzing nonverbal behavior in BPD patients during clinical interactions offers valuable insights into their emotions, feelings, and the therapeutic relationship quality, surpassing verbal communication analysis alone (38). Speech-related findings, notably increased pause frequency during neutral speech, distinguish BPD patients from healthy controls. These pauses may result from developmental disruptions in brain networks linked to early psychological trauma, affecting the corpus callosum’s development (39). Correlations between prosody and facial expressions, particularly related to disgust and anger, reveal a strong connection between speech and nonverbal cues, with diagnostic potential. These correlations may be tied to emotional hyperreactivity and heightened sympathetic tone, facilitating intense social interactions, even with negative emotions. These intensified emotional signals may serve as a call for help, particularly during initial clinical interviews (54).
Individuals with BPD exhibit distinct social behavior patterns, including reduced affiliative behaviors and inclinations toward avoidance of social interactions. This underscores their interpersonal challenges, emphasizing the need to address emotional regulation, coping mechanisms, and social skill development in therapy. Patients with BPD show a complex interplay of emotions across different modes of expression. In spoken interviews, they display limited negativity, possibly reflecting a more neutral emotional valence, although context and potential emotion suppression need consideration. However, in written communication, BPD patients use numerous negative emotion words, indicating heightened negative emotional valence in this modality. This contrast between spoken and written expressions highlights the nuanced nature of emotional dynamics in BPD, influenced by emotional suppression, contextual factors, and alexithymia. Negative facial expressions like disgust and contempt contribute to interpersonal challenges, fostering emotional aggression and social withdrawal. The use of social smiles may serve as a defense mechanism to limit interpersonal contact while maintaining emotional distance, illustrating the multifaceted nature of emotional regulation in individuals with BPD (40). Concealing sadness and displaying anger, disgust, and contempt may result from feeling threatened, particularly in interactions with male interviewers, given their history of trauma and abuse (45, 49, 52, 54, 55).
Patients with BPD exhibit significantly elevated nonverbal synchrony with interviewers, surpassing chance levels, indicating complex behavioral and emotional interactions. This heightened synchrony possibly reflects the intense emotional experiences and interpersonal challenges often associated with BPD, illustrating emotional contagion where their feelings influence those around them. Additionally, patients with BPD tend to display fewer prosocial behaviors and engage less in social interactions, partly driven by their negatively biased facial emotional displays. This sensitive measure of nonverbal synchrony offers insights into these subtle changes in coordinated movement (51).
Physiological measurements in individuals with BPD might provide insights into their emotional responses and physiological arousal (58–61). Some studies found no significant baseline heart rate differences between BPD and control groups (37, 42, 50), while others indicated elevated heart rates in BPD patients (37, 48, 57), suggesting varying physiological arousal. The inconsistency may be due to several factors: the inclusion of participants taking medications and those with additional anxiety disorders. These factors, especially anxiety disorders associated with hyperarousal, could affect psychophysiological measures, potentially skewing the results and making them less representative of the primary condition being studied (59). ECG measurements revealed reduced respiratory sinus arrhythmia (RSA) levels in BPD patients (48, 53), which is an index of heart rate variability mediated by the vagus nerve. Reduced basal vagal activity is considered a sign of susceptibility to negative emotional states and is associated with adverse clinical outcomes such as panic, anger, and hostility (62). This suggests that individuals with BPD may have deficiencies in their baseline emotional functioning, characterized by heightened emotional intensity and vulnerability. This implies that the primary emotional issues in BPD may not originate from emotional responses but rather from abnormalities in their overall emotional resting state (48). This diminished vagal tone could imply an increased predisposition to fight-or-flight responses, even during resting periods, possibly contributing to the inner tension experienced by individuals with BPD (44). In BPD, symptom severity correlates with reduced heart rate variability (HRV) during rest, linked to heightened susceptibility to negative emotions and adverse clinical outcomes. While BPD individuals exhibit heart rate fluctuations, their HRV remains stable. Additionally, BPD patients often have longer corrected QT dispersion (QTcd) durations, indicating potential cardiac abnormalities or altered autonomic nervous system functioning. They tend to show elevated systolic blood pressure (BPsys), particularly at rest, and consistently heightened diastolic blood pressure (BPdia). Notably, BPD symptom severity doesn’t predict cardiovascular reactivity, indicating consistent differences in their cardiovascular system responses. Varying skin conductance levels (SCL) suggest potential disparities in emotional reactivity. These findings collectively suggest underlying dysregulation in autonomic systems in individuals with BPD, which may contribute to emotional instability and clinical manifestations (44). Furthermore, lower resting scores in left orbicularis oculi electromyography (EMG) in BPD patients may reflect distinct facial muscle activity patterns (42).
Study limitations
The findings in this review should be considered in light of certain limitations observed in the included articles. Most studies primarily focused on female participants, potentially limiting generalizability, despite more recent research challenging the perception that BPD mainly affects females. Diagnostic bias toward females results from their greater likelihood to seek early mental health assistance, while males may delay diagnosis due to substance abuse and incarceration. Diverse gender representation in BPD research is crucial (63, 64). Additionally, the concentration of studies on young adults aged 25-35 neglects other age groups with BPD. Geographic concentration in Europe could introduce regional biases. Sample sizes varied significantly, some being very small, and some studies lacked comparisons with healthy controls or other groups, making it difficult to draw clear inferences. Nearly half of the studies had comorbidity within their samples, potentially confounding observed cues specific to BPD. Diagnostic heterogeneity arose from varying diagnostic methods. Most studies investigated physiological cues, while only a few explored language use, facial expressions, speech, and non-verbal communication. Our review is limited to English language articles, potentially introducing publication bias. We acknowledge the limitation of not being able to include articles in other languages, which could have offered valuable insights into this subject. Similarly, some of our findings may be biased due to the fact that we included conference proceedings, which are not always refereed, and the fact that we did not assess the methodological quality of the included articles. Mentioned limitations underscore the need for further research addressing these constraints and exploring a broader range of observable cues in diverse BPD populations. Future studies should consider comorbid conditions and the impact of medication on individuals with BPD, as well as recognize diagnostic variations. Addressing these factors can offer a more nuanced and accurate portrayal of BPD, facilitating improved assessment and treatment strategies for this complex disorder.
Clinical implications
Our in-depth study of BPD characteristics uncovers innovative possibilities for precision psychiatry using digital biomarkers. We identify distinct language patterns in individuals with BPD, emphasizing external viewpoints and an increased use of the future tense. Additionally, specific linguistic characteristics unique to individuals with BPD shed light on their thought processes and emotional experiences, enhancing diagnostic precision. These linguistic cues serve as essential building blocks for the development of precise diagnostic algorithms.
In the realm of nonverbal behaviors, our investigation unveils intriguing insights, including an increased frequency of pauses in BPD individuals’ speech. These findings suggest unique communication tendencies and emotional regulation challenges. Moreover, our research highlights significant correlations between prosody (the rhythm and melody of speech) and facial expressions, providing valuable information about emotional states and interpersonal dynamics. These nonverbal cues enhance our ability to recognize and understand the complexities of the disorder.
The integration of physiological measurements into our study represents a significant advance. By analyzing emotional responses and physiological arousal in individuals with BPD, we gain deeper insights into the psychophysiological aspects of the disorder. This knowledge not only aids in early identification but also opens the door to personalized interventions that address the unique needs of each patient.
Our approach aims to complement existing BPD management methods, ushering in an era of precision and customization in mental healthcare. By incorporating machine learning insights into the diagnostic process, we intend to improve the accuracy of BPD assessments and empower clinicians to make more informed decisions. These groundbreaking tools represent a transformative shift in BPD diagnosis and treatment, offering renewed hope for those dealing with this condition. As our research advances, we anticipate improved diagnostic precision and highly personalized treatment strategies, ultimately creating a brighter future for those facing the challenges of BPD. Digital biomarkers play a central role in this transformative journey.
Conclusion
In conclusion, this review underscores the pressing need for improved BPD diagnosis and management. The integration of AI and observable cues, including language patterns and nonverbal behaviors, charts a promising course towards enhancing diagnostic precision and personalized treatments for individuals with BPD. These unique cues, harnessed by AI-driven machine learning algorithms, stand as a beacon for the potential transformation of BPD management, facilitating early identification and timely interventions. However, this promising future must be approached with caution, addressing significant ethical concerns such as privacy, data security, and bias. Ensuring transparency and preventing the exacerbation of existing disparities are essential to realizing AI’s transformative potential in mental health care. By balancing innovation with ethical considerations, we can pave the way for more equitable and effective solutions, ultimately improving outcomes and quality of life for those contending with BPD.
Author contributions
SM: Conceptualization, Data curation, Investigation, Project administration, Writing – original draft, Writing – review & editing. US: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – review & editing. IM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. GM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. HK: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing. NP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work has been funded by the European Union within the project SMILE, Grant Agreement No 101080923. The content of this paper does not reflect the official opinion of the European Union or any other institution. Responsibility for the information and views expressed herein lies entirely with the authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fakhoury M. Artificial intelligence in psychiatry. In: Kim YK, editor. Frontiers in Psychiatry. Springer Singapore, Singapore (2019). p. 119–25. Available at: http://link.springer.com/10.1007/978-981-32-9721-0_6 (accessed March 31, 2023).
2. Bohus M, Stoffers-Winterling J, Sharp C, Krause-Utz A, Schmahl C, Lieb K. Borderline personality disorder. Lancet. (2021) 398:1528–40. doi: 10.1016/S0140-6736(21)00476-1
3. Temes CM, Frankenburg FR, Fitzmaurice GM, Zanarini MC. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. (2019) 80. https://www.psychiatrist.com/JCP/article/Pages/2019/v80/18m12436.aspx (accessed March 31, 2023).
4. Paris J. Suicidality in borderline personality disorder. Medicina (Mex). (2019) 55:223. doi: 10.3390/medicina55060223
5. Jacobi F, Grafiadeli R, Volkmann H, Schneider I. Krankheitslast der Borderline-Persönlichkeitsstörung: Krankheitskosten, somatische Komorbidität und Mortalität. Nervenarzt.>. (2021) 92:660–9. doi: 10.1007/s00115-021-01139-4
6. Ruggero CJ, Zimmerman M, Chelminski I, Young D. Borderline personality disorder and the misdiagnosis of bipolar disorder. J Psychiatr Res. (2010) 44:405–8. doi: 10.1016/j.jpsychires.2009.09.011
7. Wilf TJ. Diagnosing borderline personality disorder: Avoid these pitfalls. Curr Psychiatry. (2023) 22. https://www.mdedge.com/psychiatry/article/264397/personality-disorders/diagnosing-borderline-personality-disorder-avoid (accessed March 31, 2023).
8. Zhang M, Scandiffio J, Younus S, Jeyakumar T, Karsan I, Charow R. The adoption of AI in mental health care–perspectives from mental health professionals: qualitative descriptive study. JMIR Form Res. (2023) 7:e47847. doi: 10.2196/47847
9. Yan WJ, Ruan QN, Jiang K. Challenges for artificial intelligence in recognizing mental disorders. Diagnostics. (2022) 13:2. doi: 10.3390/diagnostics13010002
10. Avula VCR, Amalakanti S. Artificial intelligence in psychiatry, present trends, and challenges: An updated review. Arch Ment Health. (2024) 25(1):85–90. doi: 10.4103/amh.amh_167_23
11. Allsopp K, Read J, Corcoran R, Kinderman P. Heterogeneity in psychiatric diagnostic classification. Psychiatry Res september. (2019) 279:15–22. doi: 10.1016/j.psychres.2019.07.005
12. Lee EE, Torous J, De Choudhury M, Depp CA, Graham SA, Kim HC. Artificial intelligence for mental health care: clinical applications, barriers, facilitators, and artificial wisdom. Biol Psychiatry Cognit Neurosci Neuroimaging. (2021) 6:856–64. doi: 10.1016/j.bpsc.2021.02.001
13. McCradden M, Hui K, Buchman DZ. Evidence, ethics and the promise of artificial intelligence in psychiatry. J Med Ethics. avgust. (2023) 49:573–9. doi: 10.1136/jme-2022-108447
14. Szalai J. The potential use of artificial intelligence in the therapy of borderline personality disorder. J Eval Clin Pract junij. (2021) 27:491–6. doi: 10.1111/jep.13530
15. Andreoletti M, Haller L, Vayena E, Blasimme A. Mapping the ethical landscape of digital biomarkers: A scoping review. PLoS Digit Health. (2024) 3:e0000519. doi: 10.1371/journal.pdig.0000519
16. Parziale A, Mascalzoni D. Digital biomarkers in psychiatric research: data protection qualifications in a complex ecosystem. Front Psychiatry. (2022) 13:873392. doi: 10.3389/fpsyt.2022.873392
17. Funkenstein AB, Kessler KA, Schen CR. Learning through the lens: ethical considerations in videotaping psychotherapy. Harv Rev Psychiatry. (2014) 22:316–22. doi: 10.1097/HRP.0000000000000024
18. Timmons AC, Duong JB, Simo Fiallo N, Lee T, Vo HPQ, Ahle MW. A call to action on assessing and mitigating bias in artificial intelligence applications for mental health. Perspect Psychol Sci. (2023) 18:1062–96. doi: 10.1177/17456916221134490
19. Thakkar A, Gupta A, De Sousa A. Artificial intelligence in positive mental health: a narrative review. Front Digit Health. (2024) 6:1280235. doi: 10.3389/fdgth.2024.1280235
20. Abd-alrazaq A, Alhuwail D, Schneider J, Toro CT, Ahmed A, Alzubaidi M. The performance of artificial intelligence-driven technologies in diagnosing mental disorders: an umbrella review. NPJ Digit Med. (2022) 5:87. doi: 10.1038/s41746-022-00631-8
21. Gougherty AV, Clipp HL. Testing the reliability of an AI-based large language model to extract ecological information from the scientific literature. NPJ Biodivers. (2024) 3:13. doi: 10.1038/s44185-024-00043-9
22. Terra M, Baklola M, Ali S, El-Bastawisy K. Opportunities, applications, challenges and ethical implications of artificial intelligence in psychiatry: a narrative review. Egypt J Neurol Psychiatry Neurosurg. (2023) 59:80. doi: 10.1186/s41983-023-00681-z
23. Pham KT, Nabizadeh A, Selek S. Artificial intelligence and chatbots in psychiatry. Psychiatr Q. (2022) 93:249–53. doi: 10.1007/s11126-022-09973-8
24. Liang Y, Zheng X, Zeng DD. A survey on big data-driven digital phenotyping of mental health. Inf Fusion. (2019) 52:290–307. doi: 10.1016/j.inffus.2019.04.001
25. Low DM, Bentley KH, Ghosh SS. Automated assessment of psychiatric disorders using speech: A systematic review. Laryngoscope Investig Otolaryngol. (2020) 5:96–116. doi: 10.1002/lio2.v5.1
26. Blom JMC, Benatti C, Mascalzoni D, Tascedda F, Pani L. Editorial: Digital biomarkers in testing the safety and efficacy of new drugs in mental health: A collaborative effort of patients, clinicians, researchers, and regulators. Front Psychiatry. (2023) 13:1107037. doi: 10.3389/fpsyt.2022.1107037
27. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. doi: 10.1080/1364557032000119616
28. Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci december. (2010) 5:69. doi: 10.1186/1748-5908-5-69
29. Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D. PRISMA extension for scoping reviews (PRISMA-scR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. doi: 10.7326/M18-0850
30. Zhu J, Liu W. A tale of two databases: the use of Web of Science and Scopus in academic papers. Scientometrics.> . (2020) 123:321–35. doi: 10.1007/s11192-020-03387-8
31. Burnham JF. Scopus database: a review. BioMed Digit Libr. (2006) 3:1. doi: 10.1186/1742-5581-3-1
32. Gavel Y, Iselid L. Web of Science and Scopus: a journal title overlap study. Online Inf Rev. (2008) 32:8–21. doi: 10.1108/14684520810865958
33. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. (2006) 3:77–101. doi: 10.1191/1478088706qp063oa
34. Aleknaviciute J, Tulen JHM, Kamperman AM, De Rijke YB, Kooiman CG, Kushner SA. Borderline and cluster C personality disorders manifest distinct physiological responses to psychosocial stress. Psychoneuroendocrinology. (2016) 72:131–8. doi: 10.1016/j.psyneuen.2016.06.010
35. Back SN, Schmitz M, Koenig J, Zettl M, Kleindienst N, Herpertz SC. Reduced vagal activity in borderline personality disorder is unaffected by intranasal oxytocin administration, but predicted by the interaction between childhood trauma and attachment insecurity. J Neural Transm april. (2022) 129:409–19. doi: 10.1007/s00702-022-02482-9
36. Bomba M, Nicosia F, Riva A, Corbetta F, Conti E, Lanfranconi F. QTc dispersion and interval changes in drug-free borderline personality disorder adolescents. Eur Child Adolesc Psychiatry. (2020) 29:199–203. doi: 10.1007/s00787-019-01343-3
37. Bortolla R, Spada GE, Galli M, Visitini R, Talè C, Maffei C. Psychophysiological aspects of Borderline Personality Disorder reactivity to interpersonal stimuli: associations to components of childhood abuse. Mediterr J Clin Psychol. (2022) 10. doi: 10.13129/2282-1619/mjcp-3435
38. Brüne M, Kolb M, Ebert A, Roser P, Edel MA. Nonverbal communication of patients with borderline personality disorder during clinical interviews: A double-blind placebo-controlled study using intranasal oxytocin. J Nerv Ment Dis. (2015) 203:107–11. doi: 10.1097/NMD.0000000000000240
39. Carter PE, Grenyer BFS. The effect of trauma on expressive language impairment in borderline personality disorder: Trauma and expressive language in BPD. Pers Ment Health. (2012) 6:183–95. doi: 10.1002/pmh.1177
40. Dammann G, Rudaz M, Benecke C, Riemenschneider A, Walter M, Pfaltz MC. Facial affective behavior in borderline personality disorder indicating two different clusters and their influence on inpatient treatment outcome: A preliminary study. Front Psychol. (2020) 11:1658. doi: 10.3389/fpsyg.2020.01658
41. Dyson H, Gorvin L. How is a label of borderline personality disorder constructed on twitter: A critical discourse analysis. Issues Ment Health Nurs. (2017) 38:780–90. doi: 10.1080/01612840.2017.1354105
42. Ebner-Priemer UW, Badeck S, Beckmann C, Wagner A, Feige B, Weiss I. Affective dysregulation and dissociative experience in female patients with borderline personality disorder: a startle response study. J Psychiatr Res. (2005) 39:85–92. doi: 10.1016/j.jpsychires.2004.05.001
43. Flasbeck V, Popkirov S, Ebert A, Brüne M. Altered interoception in patients with borderline personality disorder: a study using heartbeat-evoked potentials. Borderline Pers Disord Emot Dysregulation. (2020) 7:24. doi: 10.1186/s40479-020-00139-1
44. Geiss L, Beck B, Hitzl W, Hillemacher T, Hösl KM. Cardiovascular autonomic modulation during metronomic breathing and stress exposure in patients with borderline personality disorder. Neuropsychobiology.> . (2021) 80:359–73. doi: 10.1159/000511543
45. Gemmell J, Isenegger K, Dong Y, Glaser E, Morain A. Comparing automatically extracted topics from online mental health disorder forums. In: V: 2019 International Conference on Computational Science and Computational Intelligence (CSCI). IEEE, Las Vegas, NV, USA (2019). p. 1347–52. Available at: https://ieeexplore.ieee.org/document/9071370/ (accessed March 31, 2023).
46. Grove JL, Smith TW, Crowell SE, Williams PG, Jordan KD. Borderline personality features, interpersonal correlates, and blood pressure response to social stressors: Implications for cardiovascular risk. Pers Individ Differ julij. (2017) 113:38–47. doi: 10.1016/j.paid.2017.03.005
47. Jeanneau M, Armelius BÅ. Linguistic characteristics of neurotic, borderline and psychotic personality organization. Scand J Psychol. (1993) 34:64–75. doi: 10.1111/j.1467-9450.1993.tb01101.x
48. Kuo JR, Fitzpatrick S, Metcalfe RK, McMain S. A multi-method laboratory investigation of emotional reactivity and emotion regulation abilities in borderline personality disorder. J Behav Ther Exp Psychiatry. (2016) 50:52–60. doi: 10.1016/j.jbtep.2015.05.002
49. Lyons M, Aksayli ND, Brewer G. Mental distress and language use: Linguistic analysis of discussion forum posts. Comput Hum Behav. (2018) 87:207–11. doi: 10.1016/j.chb.2018.05.035
50. Meyer PW, Müller LE, Zastrow A, Schmidinger I, Bohus M, Herpertz SC. Heart rate variability in patients with post-traumatic stress disorder or borderline personality disorder: relationship to early life maltreatment. J Neural Transm. (2016) 123:1107–18. doi: 10.1007/s00702-016-1584-8
51. Ramseyer F, Ebert A, Roser P, Edel M, Tschacher W, Brüne M. Exploring nonverbal synchrony in borderline personality disorder: A double-blind placebo-controlled study using oxytocin. Br J Clin Psychol. (2020) 59:186–207. doi: 10.1111/bjc.12240
52. Sundbom E, Jeanneau M. Multivariate modelling and personality organization: A comparative study of the Defense Mechanism Test and linguistic expressions. Scand J Psychol. (1996) 37:74–83. doi: 10.1111/j.1467-9450.1996.tb00640.x
53. Thomson ND, Beauchaine TP. Respiratory sinus arrhythmia mediates links between borderline personality disorder symptoms and both aggressive and violent behavior. J Pers Disord. (2019) 33:544–59. doi: 10.1521/pedi_2018_32_358
54. Villanueva-Valle J, Díaz JL, Jiménez S, Rodríguez-Delgado A, Arango De Montis I, León-Bernal A. Facial and vocal expressions during clinical interviews suggest an emotional modulation paradox in borderline personality disorder: an explorative study. Front Psychiatry. (2021) 12:628397. doi: 10.3389/fpsyt.2021.628397
55. Walter M, Berth H, Selinger J, Gerhard U, Küchenhoff J, Frommer J. The lack of negative affects as an indicator for identity disturbance in borderline personality disorder: A preliminary report. Psychopathology.> . (2009) 42:399–404. doi: 10.1159/000241196
56. Wang B, Wu Y, Taylor N, Lyons T, Liakata M, Nevado-Holgado AJ. Learning to Detect Bipolar Disorder and Borderline Personality Disorder with Language and Speech in Non-Clinical Interviews (2020). Available online at: https://arxiv.org/abs/2008.03408 (accessed March 31, 2023).
57. Weise S, Parzer P, Zimmermann R, Fürer L, Resch F, Kaess M. Emotion dysregulation and resting-state autonomic function in adolescent borderline personality disorder—A multimodal assessment approach. Pers Disord Theory Res Treat. (2020) 11:46–53. doi: 10.1037/per0000367
58. Baschnagel JS, Coffey SF, Hawk LW, Schumacher JA, Holloman G. Psychophysiological assessment of emotional processing in patients with borderline personality disorder with and without comorbid substance use. Pers Disord Theory Res Treat. (2013) 4:203–13. doi: 10.1037/a0029777
59. Cavazzi T, Becerra R. Psychophysiological research of borderline personality disorder: review and implications for biosocial theory. Eur J Psychol. (2014) 10:185–203. doi: 10.5964/ejop.v10i1.677
60. Kuo JR, Linehan MM. Disentangling emotion processes in borderline personality disorder: Physiological and self-reported assessment of biological vulnerability, baseline intensity, and reactivity to emotionally evocative stimuli. J Abnorm Psychol. (2009) 118:531–44. doi: 10.1037/a0016392
61. Rosenthal MZ, Gratz KL, Kosson DS, Cheavens JS, Lejuez CW, Lynch TR. Borderline personality disorder and emotional responding: A review of the research literature. Clin Psychol Rev. (2008) 28:75–91. doi: 10.1016/j.cpr.2007.04.001
62. Beauchaine T. Vagal tone, development, and Gray’s motivational theory: Toward an integratedmodel of autonomic nervous system functioning in psychopathology. Dev Psychopathol. (2001) 13:183–214. doi: 10.1017/S0954579401002012
63. Sansone RA, Sansone LA. Gender patterns in borderline personality disorder. Innov Clin Neurosci. (2011) 8:16–20.
Keywords: borderline personality disorder, review, digital biomarkers, observable cues, language use, speech, facial expression, physiological measurements
Citation: Močnik S, Smrke U, Mlakar I, Močnik G, Gregorič Kumperščak H and Plohl N (2024) Beyond clinical observations: a scoping review of AI-detectable observable cues in borderline personality disorder. Front. Psychiatry 15:1345916. doi: 10.3389/fpsyt.2024.1345916
Received: 28 November 2023; Accepted: 25 November 2024;
Published: 10 December 2024.
Edited by:
Alessandra Maria Passarotti, University of Illinois Chicago, United StatesReviewed by:
André Luiz Monezi Andrade, Pontifical Catholic University of Campinas, BrazilSylvia Martin, Uppsala University, Sweden
Copyright © 2024 Močnik, Smrke, Mlakar, Močnik, Gregorič Kumperščak and Plohl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Močnik, c2FyYS5tb2NuaWtAdW0uc2k=
 Sara Močnik
Sara Močnik Urška Smrke
Urška Smrke Izidor Mlakar
Izidor Mlakar Grega Močnik
Grega Močnik Hojka Gregorič Kumperščak
Hojka Gregorič Kumperščak Nejc Plohl
Nejc Plohl