- 1Psychiatry Department, Faculty of Medicine, University of Geneva, Geneva, Switzerland
- 2Psychiatry Department, Geneva University Hospital, Geneva, Switzerland
- 3Interdisciplinary Center for Health Sciences, Scuola Superiore Sant’Anna, Pisa, Italy
- 4Cardiology Division, Fondazione Toscana Gabriele Monasterio, Pisa, Italy
Introduction: People with psychosis spectrum disorders (PSD) face an elevated risk of metabolic syndrome (MetS), which may reduce their life expectancy by nearly 20%. Pinpointing the shared and specific characteristics and clinical implications of MetS in PSD is crucial for designing interventions to reduce this risk, but an up-to-date review on MetS across the psychosis spectrum is lacking.
Methods: This narrative review fills this gap by examining the clinical literature on characteristics and implications of MetS in both distinct PSD and transdiagnostically, i.e., across traditional categorical diagnoses, with a focus on psychiatric and cardio-metabolic management.
Results: We discuss common and specific characteristics of MetS in PSD, as well as factors contributing to MetS development in PSD patients, including unhealthy lifestyle factors, genetic predisposition, pro-inflammatory state, drugs consumption, antipsychotic medication, and psychotic symptoms. We highlight the importance of early identification and management of cardio-metabolic risk in PSD patients, as well as the existing gaps in the literature, for instance in the screening for MetS in younger PSD patients. We compare hypotheses-generating clinical associations and characteristics of MetS in different PSD, concluding by reviewing the existing recommendations and challenges in screening, monitoring, and managing MetS in PSD.
Conclusion: Early identification and management of MetS are crucial to mitigate the long-term cardio-metabolic toll in PSD patients. Interventions should focus on healthy lifestyle and appropriate pharmacological and behavioral interventions. Further translational and clinical research is needed to develop targeted interventions and personalized treatment approaches for this vulnerable population, aiming at improving physical health and overall well-being.
1 Introduction: a transdiagnostic approach to metabolic syndrome in psychosis
Metabolic syndrome (MetS), also known as syndrome X, is a cluster of interconnected physiological, clinical, and metabolic risk factors associated with an elevated risk for cardiovascular disease, type 2 diabetes mellitus (T2DM), and overall mortality (1). Its defining criteria, often based on the International Diabetes Federation guidelines, encompass components such as obesity, hypertension, dyslipidemia, and insulin resistance (1), although other definitions exist.
Growing evidence has highlighted a bidirectional relationship between MetS and the prevalence and severity of various psychiatric disorders (2), particularly those falling within the psychosis spectrum (1–3). Psychosis-spectrum disorders (PSD) encompass serious and common psychiatric conditions such as schizophrenia, schizoaffective disorder, and bipolar disorder (BD) (3). Individuals affected by these disorders often exhibit an increased vulnerability to cardiometabolic comorbidities, including obesity, insulin resistance, hypertension, diabetes, and ultimately, MetS (4–7).
Not only does MetS contribute to substantial morbidity, elevated costs for public health systems, and premature mortality, thereby underscoring the need for tailored and comprehensive treatment strategies that address both psychiatric and metabolic concerns, but it also reveals a critical imperative for a holistic and integrated approach to healthcare that transcends traditional boundaries between mental and physical health domains.
While existing literature has predominantly focused on MetS in individual psychiatric disorders, there remains a notable gap in scholarship pertaining to a comprehensive evaluation of both shared characteristics and distinctive aspects specifically within PSD.
This gap persists, despite the growing body of evidence that underscores the clinical and research advantages derived from adopting a transdiagnostic and dimensional perspective (8). This innovative framework places a heightened emphasis on investigating features that transcend specific psychiatric diagnoses (9, 10), such as MetS, thereby fostering an integrative perspective that surpasses traditional reductionistic paradigms. This approach is supported by the significant overlap across psychiatric disorders in symptoms (11, 12), genetics (13), and high comorbidity (14), suggesting that traditional categorical classifications may not be the most appropriate for investigating features of psychiatric disorders.
Therefore, the present narrative review examines the existing literature on common and distinct clinical implications and characteristics of MetS across the spectrum of psychotic disorders.
2 Shared and distinct epidemiological characteristics of psychosis spectrum disorders
In the general population, MetS prevalence ranges from 10 to 20% in adults and 0-19% in children (15, 16); it is most common in South Asians (17) and African Americans (18), while it is lowest in Europeans (17).
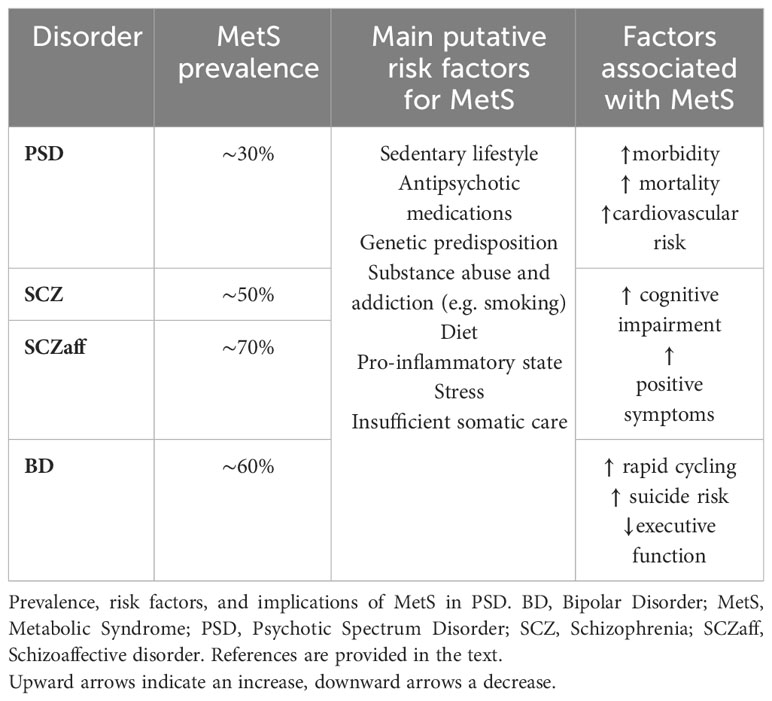
MetS prevalence in PSD has been estimated to be as high as 30% (19), while, as a comparison, it was found to be around 20% in BPD patients (16). A meta-analysis on 1,009 treatment naïve patients with first-episode psychosis (FEP) found a MetS prevalence of 13% (20), while another metanalysis founds MetS in 20% of the 800 untreated schizophrenia patients (19). Males and Asian patients seem to have the highest prevalence of MetS (20).
However, these rates are difficult to compare with the general population, since FEP patients are typically relatively young. Interestingly, a case-control study found a similar MetS prevalence between 303 drug-naïve FEP patients and 153 controls. Despite this, 61% of FEP patients displayed at least one MetS component compared to 37% of controls, along with more prevalent alterations in other cardiovascular risk factors, suggesting an increased risk in this populations (21).
Among specific diagnostic groups of PSD patients, the highest prevalence of MetS seems to be found among patients with BD or schizoaffective disorder (22, 23), with prevalences as high as 67% in BD patients (22, 24, 25), and up to 70% among schizoaffective disorder ones (26).
MetS prevalence in schizophrenia ranges from 10% to 50% (19, 22, 27). Interestingly, a metanalysis on 7616 patients highlighted a higher risk for MetS in schizoaffective disorder compared to schizophrenia (26). Conclusive evidence on the prevalence of MetS in other PSD is lacking.
The wide heterogeneity in prevalence reporting may depend on numerous factors, such as the aforementioned geographical, genetic, and gender differences, potential discrepancies in diagnostic criteria for MetS and specific PSD, and, importantly, on differences in comorbidity and medications, as discussed in the next section.
3 Pathogenesis and pathophysiology of metabolic syndrome in psychosis-spectrum disorders
The pathogenesis and pathophysiology of MetS in patients with PSD is complex and multifaceted, with multiple contributing factors. The relationship between MetS and PSD remains enigmatic, with different viewpoints surrounding whether MetS acts as a comorbidity, a consequence, or a confounding factor in the pathophysiology of PSD. While MetS pathogenesis and pathophysiology is beyond the scope of this review and will only be briefly reviewed, we will highlight in the following sections the pathophysiological aspects that are most relevant to PSD patients.
3.1 Antipsychotics increase the risk of metabolic syndrome
Antipsychotic medications have gained attention for their increased risk of MetS. Indeed, the prevalence of antipsychotic-related MetS ranges from 23 to 50 percent depending on the sample, but prolonged use of almost all antipsychotics, and especially second-generation ones, is associated with MetS and weight gain to varying degrees (28–30), even in younger populations (31). Indeed, the rate of MetS was highest for clozapine (51.9%), olanzapine (28.2%) and risperidone (27.9%) in the aforementioned metanalysis on 25,692 schizophrenia patients (19). Figure 1 classifies the main antipsychotics based on their risk of metabolic syndrome (32).
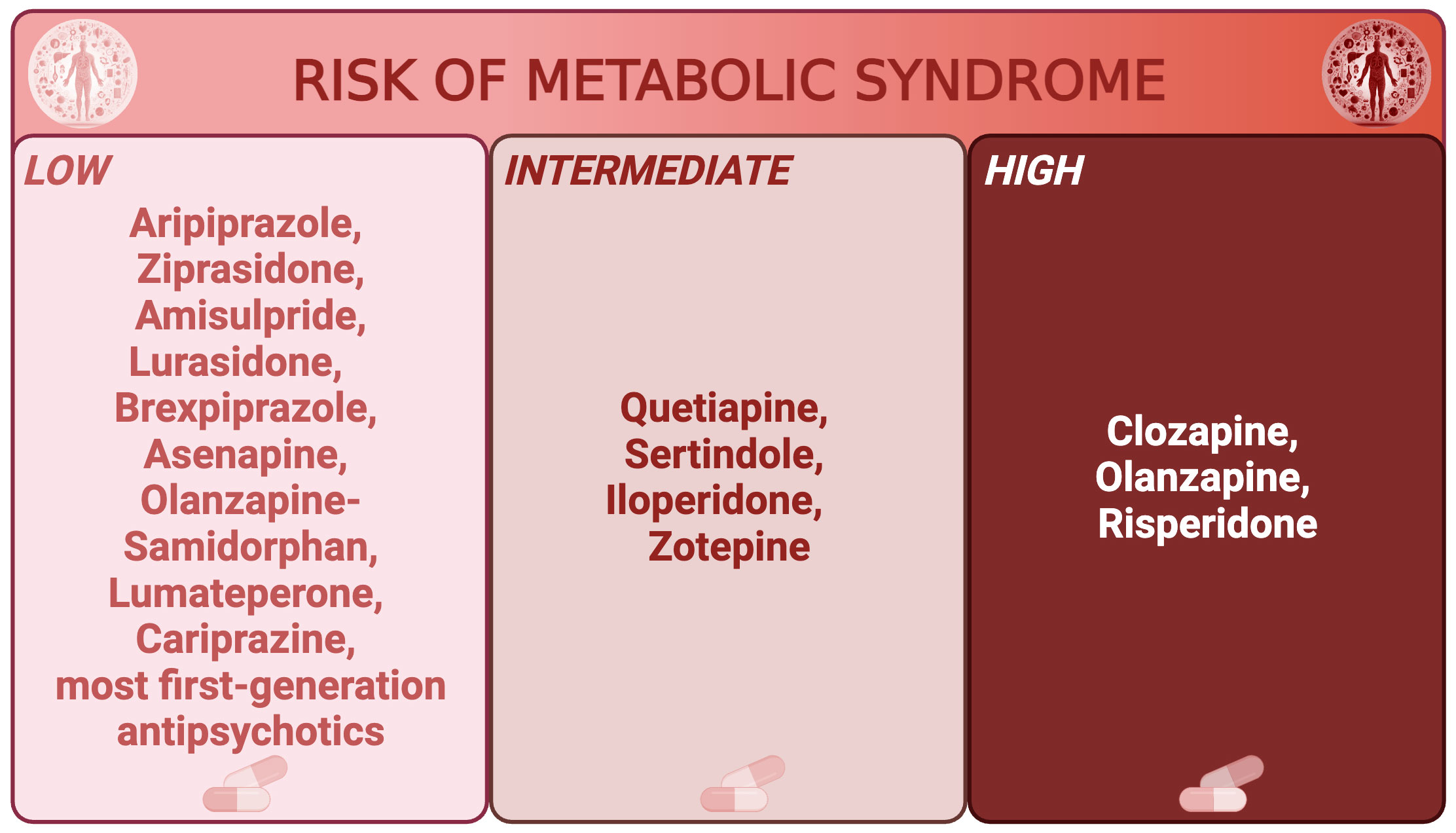
Figure 1 Main antipsychotics classified by risk of metabolic syndrome. Main antipsychotics are divided in low, intermediate, and high risk of metabolic syndrome.
3.2 Metabolic syndrome may precede antipsychotic treatment in PSD patients
Evidence from antipsychotic-naïve PSD patients suggest a direct link between PSD and MetS that could be, at least partly, independent of antipsychotic usage. For instance, antipsychotic-naïve patients with schizophrenia and schizoaffective disorders exhibit higher insulin resistance compared with matched controls (33), and specific dysregulations of metabolic parameters, including high-density lipoprotein cholesterol and homocysteine, have been identified in treatment-naive FEP patients (34). Similarly, a meta-analysis of 23 studies found that waist-to-hip ratio was increased in antipsychotic-naive and minimally treated PSD patients compared to healthy controls (35).
Another interesting hypothesis involve the fact that early-life stress (ELS) and adversities have been found to increase the risk for developing PSD in a meta-analysis of prospective and cross-sectional case-control studies on almost eighty thousands subjects (36). This finding supported by significant gene-environment correlation between polygenetic risk scores for schizophrenia and childhood adversity, highlighted in another meta-analysis (37). Similarly, severe stress during adolescence has been suggested to contribute to risk of greater waist circumference in early psychosis (38). ELS is associated with a pro-inflammatory state in the general population (39) as well as in psychiatric and PSD patients (40, 41). In fact, not only is inflammation a pivotal component of MetS (42, 43), but it is also closely related with PSD pathophysiology (40, 44–46), even in FEP patients (47). Together, these findings suggest that PSD patients may present a specific vulnerability to MetS due to ELS and to the associated pro-inflammatory state, which both precede psychiatric diagnoses and antipsychotic treatments.
3.3 Metabolic syndrome and inflammation are interrelated
The interplay between chronic inflammation, MetS and obesity is established through various studies (42, 43). MetS is characterized by an elevated inflammatory state [heightened levels of inflammatory molecules such as C-reactive protein, tumor necrosis factor-alpha, and interleukins 6 and 18 (43)] independent of obesity (48). In fact, adiponectin, an anti-inflammatory adipokine, is decreased in MetS, and its decline parallels the increase in the number of MetS components an individual exhibits, coinciding with elevated inflammatory markers (48, 49). Visceral adipose tissue functions as both a paracrine and an endocrine organ, secreting proinflammatory and atherogenic adipokines such as leptin and tumor necrosis factor-alpha, along with anti-inflammatory adipokines like adiponectin (50). Dysregulation of adipokine secretion, coupled with infiltration of macrophages into adipose tissue, leads to chronic low-grade inflammation associated with insulin resistance and type 2 diabetes (51). Insulin resistance is intimately tied to hypertension through various, multifactorial mechanisms, involving endothelial dysfunction stemming from free fatty acid-induced generation of reactive oxygen species, as well as hyperinsulinemia-induced activation of the sympathetic nervous system and inhibition of nitric oxide synthase, alongside effects mediated by adipose tissue-derived cytokines (52, 53). Obesity is accompanied by hyperactivity of the renin-angiotensin-aldosterone system, further compounding hypertension (54). Indeed, excessive visceral fat is associated with heightened insulin resistance (53), a pivotal factor in MetS pathophysiology (1). The increased volume of expanded adipose tissue results in an augmented release of free fatty acids into the portal circulation, which are then transported to the liver and stored as triglycerides, substantiating the “portal theory” of MetS (53, 55). Insulin resistance disrupts the regulation of lipolysis, causing an elevated release of free fatty acids into the bloodstream (1). Hepatic insulin action is impaired due to the greater influx of free fatty acids, leading to elevated gluconeogenesis and subsequent hyperglycemia (56) (53). Peripheral insulin resistance in muscle tissue contributes to reduced glucose disposal (57). Over time, the pancreatic beta cells face increased demands to counteract resistance, ultimately resulting in increased risk of type 2 diabetes mellitus. As suggested in the previous section, these mechanisms are particularly relevant in the context of PSD, but consistent evidence supports the relationship between MetS, inflammation, and severe mental illnesses in general (58, 59).
3.4 Common and specific environmental factors modulate metabolic syndrome risk in PSD patients
In addition to these mechanisms, PSD patients exhibit distinct lifestyle patterns that contribute to the heightened risk of MetS within this population. More in detail, research has consistently shown that individuals with PSD tend to have dietary habits characterized by high consumption of processed foods, saturated fats, and refined sugars (60). These dietary choices not only contribute to obesity, a core component of MetS but also lead to dyslipidemia and insulin resistance. It is crucial for healthcare providers to assess and educate PSD patients about the importance of balanced nutrition, emphasizing whole foods, fruits, vegetables, and lean proteins, while reducing the intake of processed and sugary items.
Smoking, a prevalent risk factor for MetS, is of particular concern in PSD patients. Studies have shown that individuals with PSD have higher smoking rates than the general population. This not only contributes to an increased risk of MetS but also intensifies the cardiovascular risk inherent to both smoking and MetS. It is imperative for clinicians to prioritize smoking cessation interventions among PSD patients to mitigate these compounding risks. Notably, Mendelian randomization studies suggested a link between cigarette smoking and BD or schizophrenia (45), and individuals with these disorders tend to consume a greater number of cigarettes per day compared to the general population (61) and up to 75% of schizophrenic patients are smokers as opposed to 25% in the general population (62). It has been reported that individuals with schizophrenia who smoke engage in less physical exercise compared to nonsmokers, further exacerbating the risk of cardiovascular disease (61). Finally, tobacco users with PSD are more inclined to consume alcohol and caffeine on a daily basis, while also being less likely to avoid high salt and saturated fat intake (61).
Sedentary behaviors are another significant contributor to the development of MetS in PSD patients. Many individuals with PSD lead relatively inactive lives due to symptoms of their disorder, medication side effects, or lack of motivation. The consequences of sedentary lifestyles extend beyond weight gain and include decreased insulin sensitivity, muscle mass loss, and worsening of MetS components (60). Interventions that promote physical activity tailored to the needs and abilities of PSD patients are crucial. Collaborative efforts between mental health and physical health professionals can develop exercise programs that not only improve metabolic health but also positively impact the overall well-being and symptom management of individuals with PSD (60). Overall, sedentary behavior, unhealthy diet, and smoking thus contribute to MetS and cardiovascular risk among PSD (61–63). Recognizing and addressing these lifestyle factors are essential steps in reducing the prevalence and severity of MetS in this vulnerable population, as discussed in Sections 4.2. and 5.
4 Clinical implications
MetS in patients with PSD carries significant clinical implications, encompassing both shared and distinctive features compared to the general population.
In general, MetS carries a heightened cardiovascular and type 2 diabetes mellitus risk, and the combined presence of MetS components may exacerbate these risks beyond the individual risk factors (64–67). Other conditions associated with MetS are obstructive sleep apneas (68, 69), hypogonadism (70, 71), chronic kidney disease (72), cancers (such as colon, pancreas, kidney, prostate, endometrial, and breast cancer) (73), Nonalcoholic Fatty Liver Disease (NAFLD) (74), hyperuricemia and gout (75), and Polycystic Ovarian Syndrome (PCOS) (76). In particular, PCOS is associated to a higher prevalence of PSD, such as BD (77, 78) or schizophrenia (79). Additionally, multiple components of MetS, including hypertension, hyperlipidemia, and diabetes, associate with an elevated risk of cognitive decline and dementia, especially when accompanied by high inflammation levels (80).
Several factors complicate the management of these risk factors in PSD patients with MetS. These include, first of all, the well-documented stigma and lower quality of somatic care offered to psychiatric patients (81), particularly those with PSD (82).
Secondly, psychiatric symptoms, and especially psychotic ones, may hinder the long-term monitoring of somatic comorbidities and MetS. Even during non-acute phases, residual symptoms may disrupt proper follow-up, with schizophrenia and schizoaffective disorders potentially harboring residual psychotic symptoms (such as persecutory delusions involving healthcare professionals). Similar challenges are present for residual cognitive or depressive symptoms in BD, which could lead patients to miss medical appointments.
Finally, the environmental and idiosyncratic factors, outlined in the previous section, render PSD patients uniquely vulnerable to MetS risks, exemplified by their nearly twofold elevated risk of early cardiovascular-related mortality (83).
4.1 The deleterious impact of metabolic syndrome on psychiatric outcomes in specific PSD
MetS itself might exert clinical repercussions on psychiatric prognosis and symptoms in PSD patients. For instance, its influence on the course of BD results in more adverse outcomes, including reduced quality of life, heightened complexity of the disease, rapid cycling, increased suicide risk, functional impairment, and suboptimal treatment response (84). On the same line, obesity has been associated with various adverse outcomes in individuals with BD, including an elevated frequency of manic and depressive episodes over the course of their lives, more challenging-to-treat index mood episodes, a heightened recurrence rate of mood episodes, a greater tendency towards depressive episodes, and a shorter duration until relapse, as compared to their non-obese counterparts (85, 86). Consequently, physical comorbidity and MetS has been identified as a significant risk factor for psychiatric readmissions and other unfavorable outcomes in BD (87).
Similarly, a positive correlation has been established between body-mass index (BMI) and the severity of positive symptoms in drug-naïve FEP schizophrenia patients (88), and there is emerging evidence linking cognitive impairment in schizophrenia to metabolic dysfunction (89). Indeed, a recent meta-analysis highlighted that individuals with schizophrenia who also have MetS or diabetes mellitus tend to experience more severe cognitive deficits, and that there is a significant relationship between cognitive impairment in schizophrenia and the individual components of MetS (including hypertension, dyslipidemia, abdominal obesity and diabetes), suggesting that MetS may contribute to functional decline in these patients (90). Similarly, preliminary evidence suggests that also in individuals with BD MetS is associated with a higher prevalence of impaired executive function (encompassing critical aspects like action planning, inhibition, and impulse control) (91). Hence, interventions targeting obesity and cardio-metabolic risk could have dual benefits on both cardiovascular health and cognitive and functional disability related to PSD.
4.2 Managing metabolic syndrome in patients with psychosis spectrum disorders
From what has been discussed above, it is clear that managing MetS in patients with PSD presents unique challenges and considerations that differentiate their care from the general population. Despite this complexity, the following paragraph will summarize the main pillars to guide clinical care of this life-threatening syndrome.
First of all, to mitigate the risk of developing MetS, it is crucial to avoid, when possible, antipsychotic medications known to increase the risk of weight-gain and metabolic complications, such as clozapine and olanzapine (32), in patients experiencing a first psychotic episode. When clinical arguments support a role of antipsychotic medication in MetS development, adaptation of pharmacological treatment should be considered (28, 92, 93). Dose reduction of antipsychotics should be explored with caution, considering the patient’s history, clinical status, and potential for symptom exacerbation. Switching to antipsychotic drugs with lower weight gain and dyslipidemia risks, such as aripiprazole or ziprasidone, might promote weight loss and improve lipid profiles (28, 92, 93). Close clinical monitoring during antipsychotic switching is crucial. Gradual cross titration and a monitoring period of two to three months are recommended to assess efficacy. Interestingly, a recent dose-response metanalysis highlighted antipsychotic-specific metabolic signatures at specific dosages, which should be considered when adjusting antipsychotic dosages in PSD patients (94).
Lifestyle interventions customized for PSD patients can aid in weight loss and metabolic improvement (95). However, their efficacy remains variable (95). Structured interventions focusing on health education, physical activity, and active monitoring may yield better outcomes.
As in the general population, hyperglycemia/diabetes, dyslipidemia and blood pressure necessitate vigilant monitoring and patients with new-onset diabetes should be referred to primary care or an endocrinologist depending on the context (96).
Tobacco smoking cessation in PSD patients emphasizes varenicline and nicotine replacement therapy (97). Varenicline exhibits greater efficacy compared to nicotine replacement therapy or bupropion (97). Patient-specific factors should guide medication selection (98–104). For instance, antidepressants may improve negative symptoms in schizophrenia and schizoaffective disorder, but their use must be approached cautiously, especially considering the risk of iatrogenic mood episodes.
As a second-line intervention, pharmacological treatment of metabolic alterations and weight loss treatment in PSD patients may be considered and is discussed in detail elsewhere (96, 105–108) and in Section 4.4. Concerning non-pharmacological and mixed interventions for metabolic dysregulation in PSD, a systematic review of 23 randomized controlled trials suggested that cognitive/behavioral interventions and pharmacological adjuncts both showed modest but significant effects in preventing weight gain. In terms of weight loss treatments, cognitive/behavioral interventions were more effective than standard care. However, the findings were limited by the small number of studies, small sample sizes, short study durations, and variability in interventions (109).
Finally, it should be noted that, due to the aforementioned potential challenges with patient follow-up and referrals, some PSD patients receive clinical care solely in mental health settings (110). Hence, collaborative approaches involving healthcare providers with expertise in metabolic abnormalities, internal medicine, endocrinology, and care management professionals can optimize treatment (110). These professionals facilitate clinician collaboration and patient navigation within complex healthcare systems (110).
4.3 Challenges in metabolic and cardiovascular risk assessment for early-stage PSD patients
It is important to highlight that metabolic and cardiovascular risk should be assessed and monitored also in PSD patients who do not have MetS. This aligns with the findings from the aforementioned meta-analysis, which revealed comparable MetS prevalence between drug-naïve FEP patients and controls. However, the FEP group demonstrated markedly higher prevalence of MetS components and cardiovascular risk factors (21). This implies that MetS might not effectively predict early cardiovascular risk in young psychosis patients, and we are lacking instrument to assess the early metabolic and cardiovascular risk in this population (21). Indeed, other cardiovascular risk predictors, such as the Framingham score for coronary cardiovascular disease risk or the SCORE, lack applicability for young psychosis patients, being primarily tailored for individuals aged 45 and above, and would underestimate cardiovascular risk in youths (21). One alternative algorithm for predicting cardiovascular risk in schizophrenia is PRIMROSE, validated in chronic schizophrenia patients, but also unsuitable for young individuals in early psychosis stages (111, 112). Indeed, while notable metabolic changes (e.g. triglyceride concentration, waist circumference, and high-density lipoprotein-cholesterol concentration) may be present in FEP individuals, and worsen over the course of the first following year, no conclusive predictors of MetS or of antipsychotic-induced weight gain (113) have been identified in this population, yet (114), and polygenetic risk scores-based predictions showed preliminary early promise but remain to be validated (115). Thus, individualized, longitudinal, and comprehensive surveillance of clinical and biological markers of metabolic and cardiovascular risk is necessary in PSD patients, and in FEP ones, who may be a higher risk of weight-gain and may benefit the most from preventive interventions.
This comprehensive risk profile should consider medical factors like obesity, dyslipidemia, hypertension, hyperglycemia, and established diabetes, as well as behavioral factors such as poor diet and smoking (19). This risk profile can guide ongoing monitoring, treatment selection, and management. Among the components of MetS, increased waist size, or abdominal obesity, appears to be a strong predictor of MetS in PSD. Waist size is closely associated with factors like hyperinsulinemia, dyslipidemia, and impaired glucose tolerance. Some evidence supports using waist size or BMI as a simple screening tool for MetS in schizophrenia and schizoaffective disorder, although specificity was found to be lower than sensitivity (55% and 92% respectively) (116). Indeed, to ensure effective monitoring, guidelines recommend measuring waist circumference and BMI regularly (at least quarterly or annually on the long term, depending on the guidelines) as minimum monitoring, especially for patients receiving atypical antipsychotics (19). While measuring waist circumference and BMI may seem straightforward, affordable, and relatively simple, it’s concerning that approximately 50% of patients in routine care lack recorded BMI measurements (76), and roughly 60% of inpatients may not undergo a comprehensive physical examination (19, 117).
4.4 Cardiovascular risk in MetS and management in PSD patients
Patients with MetS are known to have a higher risk of cardiovascular disease (CVD) (118–120). A large systematic review and meta-analysis on about 950,000 patients established that MetS is associated with a 2-fold increase in fatal and non-fatal CV outcomes, and a 1.5-fold increase in all-cause mortality (120). It is currently unknown whether the risk associated with MetS exceeds the risk associated with the sum of its individual components (121), but the higher the number of individual components of MetS, the greater the risk of CVD (122). MetS patients not only are at higher risk of developing myocardial infarction (MI) (122), but they also tend to have larger infarct size and more in-hospital complications (123). Of all the different components of MetS, hyperglicemia and BMI ≥28.0 kg/m2 are associated with major adverse cardiovascular events (MACE) in patients with MI aged <45 year (124). Moreover, MetS in acute coronary syndrome (ACS) is significantly more present in women (55.9%-66.3%) than in men (40.2%-47.3%) (125, 126).
PSD patients have a reduced life expectancy of 15–25 years as compared to the general population, mostly due to increased CV deaths (127). In a recent multicentric French study, 4,424 patients with schizophrenia were shown to develop CVD requiring hospitalization, such as MI, heart failure (HF) and stroke, at an early age, around ten years earlier than the general population (128). Importantly, these CV events are associated with a high percentage of preventable and treatable risk factors, such as hypertension (11.3%), obesity (9.7%), and diabetes (7.8%) (128). CVD in PSD patients may be caused by comorbidities, the adoption of unhealthy lifestyles in patients with inadequately treated PSD, and the use of antipsychotic drugs (129, 130).
Appropriate CV risk assessment and management is therefore crucial in patients with PSD. CV risk assessment may be estimated through different methods that generally show strong disagreement and may significantly underpredict CVD in PSD patients (131). However, based on current evidence, the assessment of MetS should be preferred as CV risk-estimator in mental healthcare as it is relatively easy and fast to use, and it is not restricted to older individuals (131). In particular, patients are considered to have MetS if they fulfil three or more of the following criteria (132): 1) waist circumference ≥ 88/102 cm (female/male); 2) systolic blood pressure ≥130 mmHg, or diastolic blood pressure ≥ 85 mmHg, or antihypertensive pharmacological treatment; 3) HDL-cholesterol <1.30/1.03 mmol/L (female/male) or receiving lipid-lowering drugs; 4) triglycerides ≥1.7 mmol/L or receiving lipid-lowering drugs; and 5) fasting glucose ≥6.1 mmol/L or receiving antidiabetic medications.
Shared guidelines for CVD risk management in PSD patients should be introduced in the daily clinical practice of in- and out-patient psychiatric services, such as the Lester Cardiometabolic Health Resource (133), summarized in Figure 2.
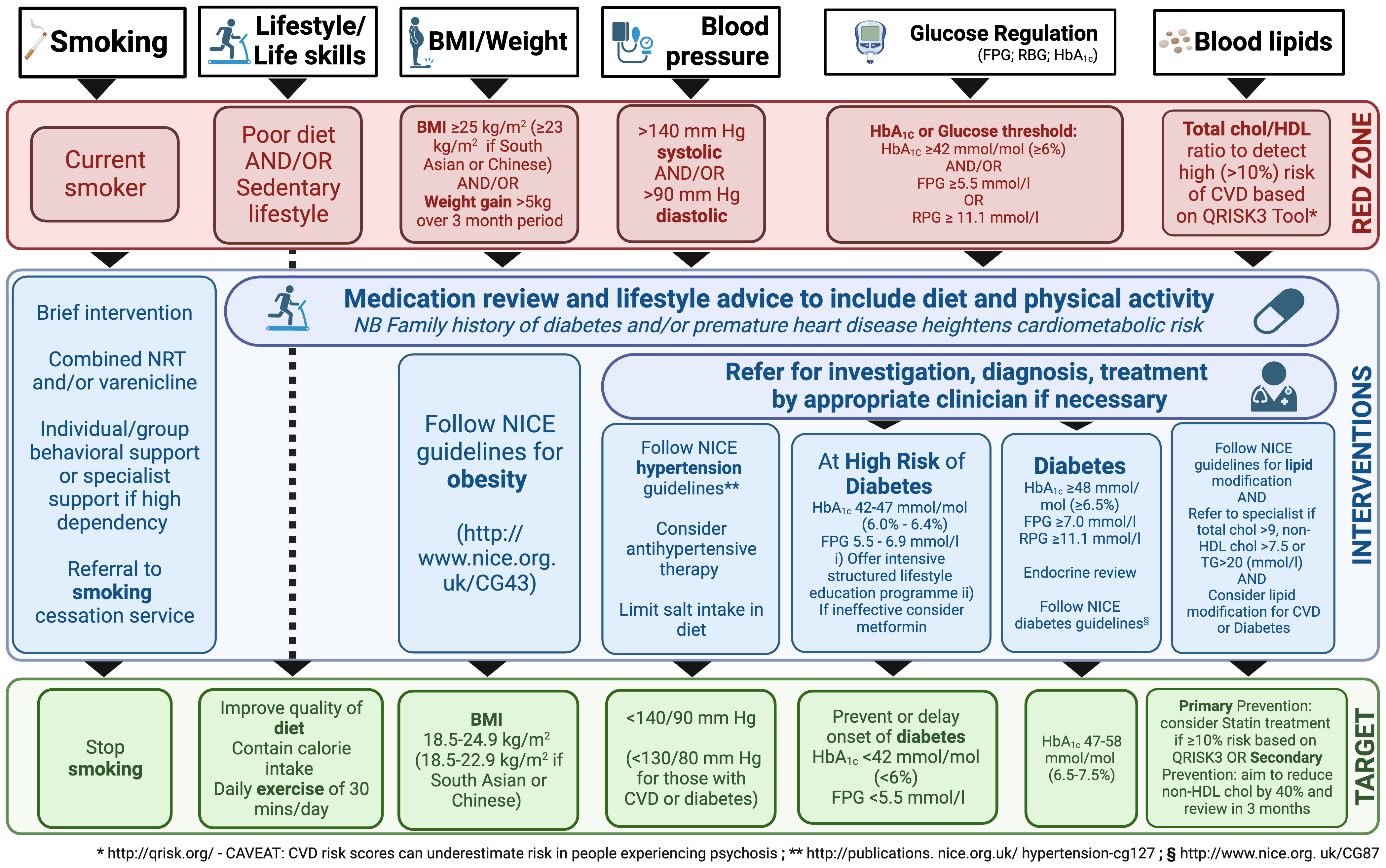
Figure 2 Resource for Promoting Cardiometabolic Wellness: A Framework for Interventions in Patients Taking Antipsychotic Medication. BMI, body mass index; chol, cholesterol; CVD, cardiovascular disease; FPG, fasting plasma glucose; HDL, high-density lipoprotein; NICE, National Institute for Health and Care Excellence; NRT, nicotine replacement therapy; RPG, random plasma glucose; TG, triglycerides. The figure is inspired from the Lester Cardiometabolic Health Resource guidelines (133).
At baseline, the specialist should look for a history of substantial weight gain (e.g., 5 kg), especially if rapid; assess smoking activity, exercise, and diet; and characterize family history (diabetes, obesity, CVD in first degree). At examination, weight, BMI, blood pressure (BP), and pulse should be recorded, whereas laboratory tests should include fasting estimates of plasma glucose (FPG), HbA1c, and lipid profile (total cholesterol, non-HDL, HDL, triglycerides).
As mentioned, negative symptoms (apathy, avolition, social withdrawal) need to be targeted and smoking cessation, a heart-healthy diet, physical activity (at least 30 minutes per day), and regular sleep routines need to be actively promoted (127, 134). Smoking, in particular, not only increases CV risk, but also complicates treatment, since the hydrocarbons in cigarette smoke accelerate the metabolism of dibenzodiazepines, clozapine and olanzapine (135). Weight should be assessed weekly in the first 6 weeks of taking a new antipsychotic, as rapid early weight gain may predict severe weight gain in the longer term.
If the patient has not successfully reached their targets after 3 months, pharmacological interventions such as anti-hypertensive, lipid-lowering, and diabetes therapy should be considered, according to current recommendations (96, 105–108). For instance, potential approaches may include metformin, topiramate, aripiprazole augmentation for clozapine patients, or liraglutide, if necessary (96, 105–108). Metformin is often preferred for its tolerability and effectiveness (105). Indeed, a systematic review of 17 randomized controlled trials involving 1,388 participants, various medications, including metformin, H2 antagonists, and monoamine modulators, showed potential for preventing weight gain and BMI increase in people with limited certainty of evidence, while topiramate did not appear effective; however, further research is needed to confirm these findings (89). As always, careful consideration of efficacy, side effects, dosing, and contraindications is required when choosing medication. Notably, some antipsychotics (i.e. clozapine, loxapine, haloperidol, melperone, risperidone, and olanzapine) are known to cause significant creatine kinase (CK) elevations (136). Therefore, it is prudent to check CK levels in PSD patients before adding a statin to antipsychotic treatment.
5 Conclusions, strengths, limitations, and perspectives
This narrative review provides a comprehensive overview of the current state of knowledge regarding MetS in individuals with PSD (Table 1). Fundamental concepts surrounding MetS in PSD revolve around the importance of early identification and management to mitigate the long-term cardiovascular and metabolic consequences. While there is consensus on the need for early intervention, questions persist about the most effective strategies for achieving this goal. BMI and waist circumference emerge as reasonable and simple screening measures, but their specificity may be low and more personalized and extensive monitoring may be needed depending on the populations (19). Depending on the clinical context, assessments should also include weight history, smoking, exercise, diet, family history, blood pressure, pulse, and laboratory tests (at least FPG, HbA1c, and lipid profile). The role of healthcare professionals in multidisciplinary teams, including psychiatrists, physicians, nurses, and other specialists, is vital in educating and motivating PSD patients to make lifestyle improvements. Nonetheless, the challenges of implementing and sustaining the aforementioned behavioral interventions, such as smoking cessation, dietary enhancements, and exercise regimens, must be considered. While the aforementioned Lester Cardiometabolic Health Resource (133) provides useful guidelines for managing cardiovascular and metabolic risk in PSD patients, there are ongoing debates regarding the monitoring instruments for MetS risk and the ideal balance between lifestyle modifications and pharmacological interventions in managing MetS in PSD.
The transdiagnostic approach of this review opens avenues for future research MetS in PSD, which have relevant clinical implications for monitoring and interventions. For instance, by recognizing common vulnerabilities that transcend diagnostic boundaries, a transdiagnostic perspective allows for the identification of shared risk factors and early indicators in youths at high risk for PSD, that could evolve towards distinct PSD (e.g. BD or schizoaffective disorder). Hence, by addressing shared risk factors transdiagnostically, clinicians and researchers can pave the way for more effective and personalized early interventions, ultimately shaping a more nuanced and proactive approach to the diverse trajectories that high-risk youth may traverse within the spectrum of PSD. In this sense, a transdiagnostic approach may potentially guide tailored early or preventive interventions targeting metabolic dysregulation across different PSD, aiming at mitigating the long-term metabolic and psychiatric outcomes. Indeed, this review highlights the importance of early identification and management of MetS in PSD patients, emphasizing the need for a multidisciplinary approach and providing a holistic view of the topic. Inclusion of recent research findings ensures that the information is up-to-date and relevant. However, limitations must be acknowledged. Firstly, as a narrative review, the article lacks the systematic rigor of a meta-analysis or systematic review, and no quantitative synthesis was performed. Furthermore, the examined literature on MetS in PSD presents itself some limitations. These include the lack of conclusive studies on MetS in various PSD subtypes, such as delusional disorder, schizotypal personality disorder, schizophreniform disorder, brief psychotic disorder, and psychosis associated with substance use or other medical conditions. While some evidence exists on schizoaffective disorder, it is less studied than schizophrenia and bipolar disorder. This lack of research hinders our understanding of how MetS manifests and progresses in different PSD populations, impeding the development of tailored interventions for these specific groups. Additionally, some studies employ cross-sectional designs, hindering the establishment of causation and the determination of directional relationships. Variability in diagnostic criteria for both MetS and PSD further complicates comparisons between studies. Confounding factors such as medication use, lifestyle, and socioeconomic status may not always be adequately addressed. Publication bias can distort the overall understanding of this relationship. The biological mechanisms linking MetS and PSD remain incompletely understood, and more research is needed in this area. Future research should address these limitations to advance our understanding in this field. Similarly, further research into a suitable scoring system for assessing MetS risk in young FEP patients is desperately needed, as this population may benefit the most from early interventions and seems particularly vulnerable to MetS.
In light of these challenges and gaps, potential developments in the field of MetS in PSD emerge. Researchers and healthcare professionals should collaborate to explore novel, PSD-specific interventions and community resources that can effectively address MetS and its associated risks (96). Ongoing research into non-pharmacological interventions for MetS should be encouraged and expanded (137). The present transdiagnostic approach aims at paving the way to further research investigating for instance the role of shared biological or psychosocial factors, such as stress and lifestyle behaviors, in the development of MetS across PSD, and to randomized controlled trials to assess the efficacy of transdiagnostic interventions, such as lifestyle modifications and psychosocial therapies, for reducing MetS risk and improving overall well-being in individuals with PSD.
Indeed, the recognition of commonalities among PSD underscores the importance of shared interventions to address factors that cut across specific disorders. However, it’s equally crucial to emphasize the development of personalized treatment approaches that take into account individual and disorder-specific differences based on genetic, environmental, and pharmacological factors. The future of research in this field should aim to enhance and add specificities to transdiagnostic interventions. By tailoring treatments to the unique characteristics and needs of each patient, we can potentially improve treatment outcomes and the overall well-being of individuals with PSD.
Author contributions
LS: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Software, Visualization. AA: Supervision, Writing – original draft, Writing – review & editing. GP: Writing – original draft. OS: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Open access funding by University of Geneva.
Acknowledgments
The authors would like to thank Noël Suzanne Harris for her valuable help in proofreading the manuscript. Figures were prepared using Biorender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. (2005) 365:1415–28. doi: 10.1016/S0140-6736(05)66378-7.
2. Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. (2015) 14:339–47. doi: 10.1002/wps.20252.
3. Moreno-Küstner B, Martín C, Pastor L. Prevalence of psychotic disorders and its association with methodological issues. A systematic Rev meta-analyses. PloS One. (2018) 13:e0195687. doi: 10.1371/journal.pone.0195687.
4. Ganguli R, Strassnig M. Prevention of metabolic syndrome in serious mental illness. Psychiatr Clin North Am. (2011) 34:109–25. doi: 10.1016/j.psc.2010.11.004.
5. Meyer JM, Nasrallah HA, McEvoy JP, Goff DC, Davis SM, Chakos M, et al. The Clinical Antipsychotic Trials Of Intervention Effectiveness (CATIE) Schizophrenia Trial: clinical comparison of subgroups with and without the metabolic syndrome. Schizophr Res. (2005) 80:9–18. doi: 10.1016/j.schres.2005.07.015.
6. Tirupati S, Chua LE. Obesity and metabolic syndrome in a psychiatric rehabilitation service. Aust N Z J Psychiatry. (2007) 41:606–10. doi: 10.1080/00048670701392841.
7. Sokal J, Messias E, Dickerson FB, Kreyenbuhl J, Brown CH, Goldberg RW, et al. Comorbidity of medical illnesses among adults with serious mental illness who are receiving community psychiatric services. J Nerv Ment Dis. (2004) 192:421–7. doi: 10.1097/01.nmd.0000130135.78017.96.
8. Mansell W, Harvey A, Watkins E, Shafran R. Conceptual foundations of the transdiagnostic approach to CBT. J Cogn Psychotherapy. (2009) 23:6–19. doi: 10.1891/0889-8391.23.1.6.
9. McGorry PD, Hartmann JA, Spooner R, Nelson B. Beyond the “at risk mental state” concept: transitioning to transdiagnostic psychiatry. World Psychiatry. (2018) 17:133–42. doi: 10.1002/wps.20514.
10. van Os J, Guloksuz S. A critique of the “ultra-high risk” and “transition” paradigm. World Psychiatry (2017) 16(2):200–6. doi: 10.1002/wps.20423
11. Russo M, Levine SZ, Demjaha A, Forti MD, Bonaccorso S, Fearon P, et al. Association between symptom dimensions and categorical diagnoses of psychosis: A cross-sectional and longitudinal investigation. Schizophr Bulletin. (2014) 40:111–9. doi: 10.1093/schbul/sbt055.
12. Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, et al. Clinical phenotypes of psychosis in the bipolar-schizophrenia network on intermediate phenotypes (B-SNIP). Am J Psychiatry. (2013) 170:1263–74. doi: 10.1176/appi.ajp.2013.12101339.
13. Pettersson E, Larsson H, Lichtenstein P. Common psychiatric disorders share the same genetic origin: A multivariate sibling study of the Swedish population. Mol Psychiatry. (2016) 21:717–21. doi: 10.1038/mp.2015.116.
14. Kessler RC, Ormel J, Petukhova M, McLaughlin KA, Green JG, Russo LJ, et al. Development of lifetime comorbidity in the world health organization world mental health surveys. Arch Gen Psychiatry. (2011) 68:90–100. doi: 10.1001/archgenpsychiatry.2010.180.
15. Belete R, Ataro Z, Abdu A, Sheleme M. Global prevalence of metabolic syndrome among patients with type I diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndrome. (2021) 13:25. doi: 10.1186/s13098-021-00641-8.
16. Kahl KG, Greggersen W, Schweiger U, Cordes J, Correll CU, Frieling H, et al. Prevalence of the metabolic syndrome in patients with borderline personality disorder: results from a cross-sectional study. Eur Arch Psychiatry Clin Neurosci. (2013) 263:205–13. doi: 10.1007/s00406-012-0339-2.
17. Tillin T, Forouhi N, Johnston DG, McKeigue PM, Chaturvedi N, Godsland IF. Metabolic syndrome and coronary heart disease in South Asians, African-Caribbeans and white Europeans: a UK population-based cross-sectional study. Diabetologia. (2005) 48:649–56. doi: 10.1007/s00125-005-1689-3.
18. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adultsFindings from the third national health and nutrition examination survey. JAMA. (2002) 287:356–9. doi: 10.1001/jama.287.3.356.
19. Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—A systematic review and meta-analysis. Schizophr Bull. (2013) 39:306–18. doi: 10.1093/schbul/sbr148.
20. Garrido-Torres N, Rocha-Gonzalez I, Alameda L, Rodriguez-Gangoso A, Vilches A, Canal-Rivero M, et al. Metabolic syndrome in antipsychotic-naïve patients with first-episode psychosis: a systematic review and meta-analysis. psychol Med. (2021) 51:2307–20. doi: 10.1017/S0033291721002853.
21. Garrido-Torres N, Ruiz-Veguilla M, Alameda L, Canal-Rivero M, Ruiz MJ, Gómez-Revuelta M, et al. Prevalence of metabolic syndrome and related factors in a large sample of antipsychotic naïve patients with first-episode psychosis: Baseline results from the PAFIP cohort. Schizophr Res. (2022) 246:277–85. doi: 10.1016/j.schres.2022.07.007.
22. John AP, Koloth R, Dragovic M, Lim SCB. Prevalence of metabolic syndrome among Australians with severe mental illness. Med J Aust. (2009) 190:176–9. doi: 10.5694/j.1326-5377.2009.tb02342.x.
23. van Winkel R, van Os J, Celic I, Van Eyck D, Wampers M, Scheen A, et al. Psychiatric diagnosis as an independent risk factor for metabolic disturbances: results from a comprehensive, naturalistic screening program. J Clin Psychiatry. (2008) 69:1319–27. doi: 10.4088/JCP.v69n0817.
24. Babić D, Maslov B, Martinac M, Nikolić K, Uzun S, Kozumplik O. Bipolar disorder and metabolic syndrome: comorbidity or side effects of treatment of bipolar disorder. Psychiatr Danub. (2010) 22:75–8.
25. Ho CSH, Zhang MWB, Mak A, Ho RCM. Metabolic syndrome in psychiatry: advances in understanding and management. Adv Psychiatr Treat 2018/01/02 Ed. (2014) 20:101–12. doi: 10.1192/apt.bp.113.011619.
26. Bartoli F, Crocamo C, Caslini M, Clerici M, Carrà G. Schizoaffective disorder and metabolic syndrome: A meta-analytic comparison with schizophrenia and other non-affective psychoses. J Psychiatr Res. (2015) 66–67:127–34.
27. Shojaeimotlagh V, Hashiehbaf A, Karami M, Monjazebi F, Gheshlagh RG. Prevalence of metabolic syndrome in Iranian patients with schizophrenia: A systematic review and meta-analysis. Diabetes Metab Syndr. (2019) 13:143–7. doi: 10.1016/j.dsx.2018.08.014.
28. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. (2004) 27:596–601. doi: 10.2337/diacare.27.2.596.
29. Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. (2005) 353:1209–23. doi: 10.1056/NEJMoa051688.
30. Newcomer JW, Haupt DW, Fucetola R, Melson AK, Schweiger JA, Cooper BP, et al. Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry. (2002) 59:337–45. doi: 10.1001/archpsyc.59.4.337.
31. McIntyre RS, Jerrell JM. Metabolic and cardiovascular adverse events associated with antipsychotic treatment in children and adolescents. Arch Pediatr Adolesc Med. (2008) 162:929–35. doi: 10.1001/archpedi.162.10.929.
32. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis.
33. Cohn TA, Remington G, Zipursky RB, Azad A, Connolly P, Wolever TMS. Insulin resistance and adiponectin levels in drug-free patients with schizophrenia: A preliminary report. Can J Psychiatry. (2006) 51:382–6. doi: 10.1177/070674370605100608.
34. Enez Darcin A, Yalcin Cavus S, Dilbaz N, Kaya H, Dogan E. Metabolic syndrome in drug-naïve and drug-free patients with schizophrenia and in their siblings. Schizophr Res. (2015) 166:201–6. doi: 10.1016/j.schres.2015.05.004.
35. Shah P, Iwata Y, Caravaggio F, Plitman E, Brown EE, Kim J, et al. Alterations in body mass index and waist-to-hip ratio in never and minimally treated patients with psychosis: A systematic review and meta-analysis. Schizophr Res. (2019) 208:420–9. doi: 10.1016/j.schres.2019.01.005.
36. Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. (2012) 38:661–71. doi: 10.1093/schbul/sbs050.
37. Woolway GE, Smart SE, Lynham AJ, Lloyd JL, Owen MJ, Jones IR, et al. Schizophrenia polygenic risk and experiences of childhood adversity: A systematic review and meta-analysis. Schizophr Bull. (2022) 48:967–80. doi: 10.1093/schbul/sbac049.
38. Alameda L, Levier A, Gholam-Rezaee M, Golay P, Vandenberghe F, Delacretaz A, et al. Psychological trauma occurring during adolescence is associated with an increased risk of greater waist circumference in Early Psychosis patients treated with psychotropic medication. PloS One. (2020) 15:e0242569. doi: 10.1371/journal.pone.0242569.
39. Kuhlman KR, Horn SR, Chiang JJ, Bower JE. Early life adversity exposure and circulating markers of inflammation in children and adolescents: A systematic review and meta-analysis. Brain Behav Immun. (2020) 86:30–42. doi: 10.1016/j.bbi.2019.04.028.
40. Quidé Y, Bortolasci CC, Spolding B, Kidnapillai S, Watkeys OJ, Cohen-Woods S, et al. Association between childhood trauma exposure and pro-inflammatory cytokines in schizophrenia and bipolar-I disorder. psychol Med. (2019) 49:2736–44. doi: 10.1017/S0033291718003690.
41. Petruso F, Giff AE, Milano BA, Menduni De Rossi M, Saccaro LF. Inflammation and emotion regulation: a narrative review of evidence and mechanisms in emotion dysregulation disorders. Neuronal Signaling. (2023), NS20220077. doi: 10.1042/NS20220077
42. Klöting N, Blüher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. (2014) 15:277–87. doi: 10.1007/s11154-014-9301-0.
43. Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. (2010) 2010:289645. doi: 10.1155/2010/289645.
44. Saccaro LF, Schilliger Z, Dayer A, Perroud N, Piguet C. Inflammation, anxiety, and stress in bipolar disorder and borderline personality disorder: A narrative review. Neurosci Biobehav Rev. (2021) 127:184–92. doi: 10.1016/j.neubiorev.2021.04.017.
45. Saccaro LF, Gasparini S, Rutigliano G. Applications of Mendelian randomization in psychiatry: a comprehensive systematic review. Psychiatr Genet. (2022) 32:199–213. doi: 10.1097/YPG.0000000000000327.
46. Saccaro LF, Crokaert J, Perroud N, Piguet C. Structural and functional MRI correlates of inflammation in bipolar disorder: A systematic review. J Affect Disord. (2023) 325:83–92. doi: 10.1016/j.jad.2022.12.162.
47. Fraguas D, Díaz-Caneja CM, Ayora M, Hernández-Álvarez F, Rodríguez-Quiroga A, Recio S, et al. Oxidative stress and inflammation in first-episode psychosis: A systematic review and meta-analysis. Schizophr Bull. (2019) 45:742–51. doi: 10.1093/schbul/sby125.
48. Hung J, McQuillan BM, Chapman CML, Thompson PL, Beilby JP. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol. (2005) 25:1268–73. doi: 10.1161/01.ATV.0000163843.70369.12.
49. Xydakis AM, Case CC, Jones PH, Hoogeveen RC, Liu MY, Smith EO, et al. Adiponectin, inflammation, and the expression of the metabolic syndrome in obese individuals: the impact of rapid weight loss through caloric restriction. J Clin Endocrinol Metab. (2004) 89:2697–703. doi: 10.1210/jc.2003-031826.
50. Kucerova J, Babinska Z, Horska K, Kotolova H. The common pathophysiology underlying the metabolic syndrome, schizophrenia and depression. A review. BioMed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2015) 159:208–14. doi: 10.5507/bp.2014.060.
51. Kahn R. Metabolic syndrome: is it a syndrome? Does it matter? Circulation. (2007) 115:1806–10; discussion 1811. doi: 10.1161/CIRCULATIONAHA.106.658336.
52. Andreassi MG. Metabolic syndrome, diabetes and atherosclerosis: influence of gene-environment interaction. Mutat Res. (2009) 667:35–43. doi: 10.1016/j.mrfmmm.2008.10.018.
53. Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, et al. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. (2007) 120:S3–8; discussion S29-32. doi: 10.1016/j.amjmed.2006.11.012.
54. Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd-Jones D, et al. Obesity-related hypertension: pathogenesis, cardiovascular risk, and treatment–a position paper of the The Obesity Society and The American Society of Hypertension. Obes (Silver Spring). (2013) 21:8–24. doi: 10.1002/oby.20181.
55. Klop B, Elte JWF, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. (2013) 5:1218–40. doi: 10.3390/nu5041218.
56. Nikolic D, Katsiki N, Montalto G, Isenovic ER, Mikhailidis DP, Rizzo M. Lipoprotein subfractions in metabolic syndrome and obesity: clinical significance and therapeutic approaches. Nutrients. (2013) 5:928–48. doi: 10.3390/nu5030928.
57. Samson SL, Garber AJ. Metabolic syndrome. Endocrinol Metab Clin North Am. (2014) 43:1–23. doi: 10.1016/j.ecl.2013.09.009.
58. Agarwal SM, Vernon AC, Venkatasubramanian G, Hahn MK. Editorial: cardiovascular and physical health in severe mental illness. Front Psychiatry. (2021) 12:760250. doi: 10.3389/fpsyt.2021.760250.
59. Reponen EJ, Dieset I, Tesli M, Mørch RH, Aas M, Vedal TSJ, et al. Atherogenic lipid ratios related to myeloperoxidase and C-reactive protein levels in psychotic disorders. Front Psychiatry. (2020) 11:672. doi: 10.3389/fpsyt.2020.00672.
60. Manzanares N, Monseny R, Ortega L, Montalvo I, Franch J, Gutiérrez-Zotes A, et al. Unhealthy lifestyle in early psychoses: the role of life stress and the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. (2014) 39:1–10. doi: 10.1016/j.psyneuen.2013.09.023.
61. Bobes J, Arango C, Garcia-Garcia M, Rejas J. Healthy lifestyle habits and 10-year cardiovascular risk in schizophrenia spectrum disorders: an analysis of the impact of smoking tobacco in the CLAMORS schizophrenia cohort. Schizophr Res. (2010) 119:101–9. doi: 10.1016/j.schres.2010.02.1030.
62. Hennekens CH, Hennekens AR, Hollar D, Casey DE. Schizophrenia and increased risks of cardiovascular disease. Am Heart J. (2005) 150:1115–21. doi: 10.1016/j.ahj.2005.02.007.
63. Bergman BC, Perreault L, Hunerdosse D, Kerege A, Playdon M, Samek AM, et al. Novel and reversible mechanisms of smoking-induced insulin resistance in humans. Diabetes. (2012) 61:3156–66. doi: 10.2337/db12-0418.
64. Hu G, Qiao Q, Tuomilehto J, Balkau B, Borch-Johnsen K, Pyorala K, et al. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med. (2004) 164:1066–76. doi: 10.1001/archinte.164.10.1066.
65. Jeppesen J, Hansen TW, Rasmussen S, Ibsen H, Torp-Pedersen C, Madsbad S. Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease: a population-based study. J Am Coll Cardiol. (2007) 49:2112–9. doi: 10.1016/j.jacc.2007.01.088.
66. Eberly LE, Prineas R, Cohen JD, Vazquez G, Zhi X, Neaton JD, et al. Metabolic syndrome: risk factor distribution and 18-year mortality in the multiple risk factor intervention trial. Diabetes Care. (2006) 29:123–30. doi: 10.2337/diacare.29.1.123.
67. Sattar N, McConnachie A, Shaper AG, Blauw GJ, Buckley BM, de Craen AJ, et al. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. (2008) 371:1927–35. doi: 10.1016/S0140-6736(08)60602-9.
68. Parish JM, Adam T, Facchiano L. Relationship of metabolic syndrome and obstructive sleep apnea. J Clin Sleep Med. (2007) 3:467–72. doi: 10.5664/jcsm.26910.
69. Gruber A, Horwood F, Sithole J, Ali NJ, Idris I. Obstructive sleep apnoea is independently associated with the metabolic syndrome but not insulin resistance state. Cardiovasc Diabetol. (2006) 5:22. doi: 10.1186/1475-2840-5-22.
70. Corona G, Mannucci E, Petrone L, Balercia G, Paggi F, Fisher AD, et al. ATPIII-defined metabolic syndrome, type 2 diabetes mellitus, and prevalence of hypogonadism in male patients with sexual dysfunction. J Sex Med. (2007) 4:1038–45. doi: 10.1111/j.1743-6109.2007.00529.x.
71. Chen RYT, Wittert GA, Andrews GR. Relative androgen deficiency in relation to obesity and metabolic status in older men. Diabetes Obes Metab. (2006) 8:429–35. doi: 10.1111/j.1463-1326.2005.00532.x.
72. Rashidi A, Ghanbarian A, Azizi F. Are patients who have metabolic syndrome without diabetes at risk for developing chronic kidney disease? Evidence based on data from a large cohort screening population. Clin J Am Soc Nephrol. (2007) 2:976–83. doi: 10.2215/CJN.01020207.
73. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. (2003) 348:1625–38. doi: 10.1056/NEJMoa021423.
74. Smits MM, Ioannou GN, Boyko EJ, Utzschneider KM. Non-alcoholic fatty liver disease as an independent manifestation of the metabolic syndrome: results of a US national survey in three ethnic groups. J Gastroenterol Hepatol. (2013) 28:664–70. doi: 10.1111/jgh.12106.
75. Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med. (2007) 120:442–7. doi: 10.1016/j.amjmed.2006.06.040.
76. Ehrmann DA, Liljenquist DR, Kasza K, Azziz R, Legro RS, Ghazzi MN, et al. Prevalence and predictors of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2006) 91:48–53. doi: 10.1210/jc.2005-1329.
77. Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol. (2013) 6:1–13. doi: 10.2147/CLEP.
78. Krępuła K, Bidzińska-Speichert B, Lenarcik A, Tworowska-Bardzińska U. Psychiatric disorders related to polycystic ovary syndrome. Endokrynol Pol. (2012) 63:488–91.
79. Matevosyan NR. Schizophrenia and Stein-Leventhal syndrome: comorbidity features. Arch Gynecol Obstet. (2011) 284:1035–41. doi: 10.1007/s00404-011-1963-1.
80. Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. (2004) 292:2237–42. doi: 10.1001/jama.292.18.2237.
81. Sølvhøj IN, Kusier AO, Pedersen PV, Nielsen MBD. Somatic health care professionals’ stigmatization of patients with mental disorder: a scoping review. BMC Psychiatry. (2021) 21:443. doi: 10.1186/s12888-021-03415-8
82. Irwin KE, Henderson DC, Knight HP, Pirl WF. Cancer care for individuals with schizophrenia. Cancer. (2014) 120:323–34. doi: 10.1002/cncr.28431.
83. De Hert M, van Eyck D, De Nayer A. Metabolic abnormalities associated with second generation antipsychotics: fact or fiction? Development of guidelines for screening and monitoring. Int Clin Psychopharmacol. (2006) 21 Suppl 2:S11–15. doi: 10.1016/j.eurpsy.2005.05.011.
84. Giménez-Palomo A, Gomes-da-Costa S, Dodd S, Pachiarotti I, Verdolini N, Vieta E, et al. Does metabolic syndrome or its component factors alter the course of bipolar disorder? A systematic review. Neurosci Biobehav Rev. (2022) 132:142–53. doi: 10.1016/j.neubiorev.2021.11.026.
85. Fagiolini A, Kupfer DJ, Houck PR, Novick DM, Frank E. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry. (2003) 160:112–7. doi: 10.1176/appi.ajp.160.1.112.
86. Lackner N, Bengesser SA, Birner A, Painold A, Fellendorf FT, Platzer M, et al. Abdominal obesity is associated with impaired cognitive function in euthymic bipolar individuals. World J Biol Psychiatry. (2016) 17:535–46. doi: 10.3109/15622975.2015.1046917.
87. Šprah L, Dernovšek MZ, Wahlbeck K, Haaramo P. Psychiatric readmissions and their association with physical comorbidity: a systematic literature review. BMC Psychiatry. (2017) 17:2. doi: 10.1186/s12888-016-1172-3.
88. Tian Y, Wang D, Wei G, Wang J, Zhou H, Xu H, et al. Prevalence of obesity and clinical and metabolic correlates in first-episode schizophrenia relative to healthy controls. Psychopharmacology. (2021) 238:745–53. doi: 10.1007/s00213-020-05727-1.
89. Agarwal SM, Stogios N, Ahsan ZA, Lockwood JT, Duncan MJ, Takeuchi H, et al. Pharmacological interventions for prevention of weight gain in people with schizophrenia. Cochrane Database Syst Rev. (2022) 10:CD013337. doi: 10.1002/14651858.CD013337.pub2
90. Bora E, Akdede BB, Alptekin K. The relationship between cognitive impairment in schizophrenia and metabolic syndrome: a systematic review and meta-analysis. psychol Med. (2017) 47:1030–40. doi: 10.1017/S0033291716003366.
91. Dalkner N, Bengesser SA, Birner A, Fellendorf FT, Fleischmann E, Großschädl K, et al. Metabolic syndrome impairs executive function in bipolar disorder. Front Neurosci. (2021) 15:717824. doi: 10.3389/fnins.2021.717824
92. Baptista T, Kin NMKNY, Beaulieu S, de Baptista EA. Obesity and related metabolic abnormalities during antipsychotic drug administration: mechanisms, management and research perspectives. Pharmacopsychiatry. (2002) 35:205–19. doi: 10.1055/s-2002-36391.
93. Stroup TS, McEvoy JP, Ring KD, Hamer RH, LaVange LM, Swartz MS, et al. A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry. (2011) 168:947–56. doi: 10.1176/appi.ajp.2011.10111609.
94. Sabé M, Pallis K, Solmi M, Crippa A, Sentissi O, Kaiser S. Comparative effects of 11 antipsychotics on weight gain and metabolic function in patients with acute schizophrenia: A dose-response meta-analysis. J Clin Psychiatry. (2023) 84(2):45463. doi: 10.4088/JCP.22r14490.
95. Bonfioli E, Berti L, Goss C, Muraro F, Burti L. Health promotion lifestyle interventions for weight management in psychosis: a systematic review and meta-analysis of randomised controlled trials. BMC Psychiatry. (2012) 12:78. doi: 10.1186/1471-244X-12-78.
96. Larsen JR, Vedtofte L, Jakobsen MSL, Jespersen HR, Jakobsen MI, Svensson CK, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: A randomized clinical trial. JAMA Psychiatry. (2017) 74:719–28. doi: 10.1001/jamapsychiatry.2017.1220.
97. Zawertailo L. Safety of smoking cessation drugs for mentally ill patients. Lancet. (2016) 387:2481–2. doi: 10.1016/S0140-6736(16)30294-X.
98. Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. (2016) 387:2507–20. doi: 10.1016/S0140-6736(16)30272-0.
99. Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev. (2013) 2013:CD007253. doi: 10.1002/14651858.CD007253.pub3.
100. Evins AE, Cather C, Pratt SA, Pachas GN, Hoeppner SS, Goff DC, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. (2014) 311:145–54. doi: 10.1001/jama.2013.285113.
101. Chengappa KNR, Perkins KA, Brar JS, Schlicht PJ, Turkin SR, Hetrick ML, et al. Varenicline for smoking cessation in bipolar disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. (2014) 75:765–72. doi: 10.4088/JCP.13m08756.
102. Anthenelli RM, Morris C, Ramey TS, Dubrava SJ, Tsilkos K, Russ C, et al. Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: a randomized trial. Ann Intern Med. (2013) 159:390–400. doi: 10.7326/0003-4819-159-6-201309170-00005.
103. Evins AE, Cather C, Culhane MA, Birnbaum A, Horowitz J, Hsieh E, et al. A 12-week double-blind, placebo-controlled study of bupropion sr added to high-dose dual nicotine replacement therapy for smoking cessation or reduction in schizophrenia. J Clin Psychopharmacol. (2007) 27:380–6. doi: 10.1097/01.jcp.0b013e3180ca86fa.
104. West R, Evins AE, Benowitz NL, Russ C, McRae T, Lawrence D, et al. Factors associated with the efficacy of smoking cessation treatments and predictors of smoking abstinence in EAGLES. Addiction. (2018) 113:1507–16. doi: 10.1111/add.14208.
105. Kahn RS, Kane JM, Correll CU, Arevalo C, Simmons A, Graham C, et al. Olanzapine/samidorphan in young adults with schizophrenia, schizophreniform disorder, or bipolar I disorder who are early in their illness: results of the randomized, controlled ENLIGHTEN-early study. J Clin Psychiatry. (2023) 84:22m14674. doi: 10.4088/JCP.22m14674.
106. Mizuno Y, Suzuki T, Nakagawa A, Yoshida K, Mimura M, Fleischhacker WW, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. (2014) 40:1385–403. doi: 10.1093/schbul/sbu030.
107. Correll CU, Newcomer JW, Silverman B, DiPetrillo L, Graham C, Jiang Y, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: A 24-week phase 3 study. Am J Psychiatry. (2020) 177:1168–78. doi: 10.1176/appi.ajp.2020.19121279.
108. Fiedorowicz JG, Miller DD, Bishop JR, Calarge CA, Ellingrod VL, Haynes WG. Systematic review and meta-analysis of pharmacological interventions for weight gain from antipsychotics and mood stabilizers. Curr Psychiatry Rev. (2012) 8:25–36. doi: 10.2174/157340012798994867.
109. Faulkner G, Cohn T, Remington G. Interventions to reduce weight gain in schizophrenia. Cochrane Database Syst Rev. (2007) 1):CD005148. doi: 10.1002/14651858.CD005148.pub2.
110. Vanderlip ER, Raney LE, Druss BG. A framework for extending psychiatrists’ Roles in treating general health conditions. Am J Psychiatry. (2016) 173:658–63. doi: 10.1176/appi.ajp.2015.15070950.
111. Osborn DPJ, Hardoon S, Omar RZ, Holt RIG, King M, Larsen J, et al. Cardiovascular risk prediction models for people with severe mental illness: results from the prediction and management of cardiovascular risk in people with severe mental illnesses (PRIMROSE) research program. JAMA Psychiatry. (2015) 72:143–51. doi: 10.1001/jamapsychiatry.2014.2133.
112. Perry BI, Upthegrove R, Crawford O, Jang S, Lau E, McGill I, et al. Cardiometabolic risk prediction algorithms for young people with psychosis: a systematic review and exploratory analysis. Acta Psychiatrica Scandinavica. (2020) 142:215–32. doi: 10.1111/acps.13212.
113. Fitzgerald I, Sahm LJ, Byrne A, O’Connell J, Ensor J, Ní Dhubhlaing C, et al. Predicting antipsychotic-induced weight gain in first episode psychosis – A field-wide systematic review and meta-analysis of non-genetic prognostic factors. Eur Psychiatry. (2023) 66:e42. doi: 10.1192/j.eurpsy.2023.2417.
114. Coentre R, Levy P, Góis C, Figueira ML. Metabolic syndrome following a first episode of psychosis: results of a 1-year longitudinal study conducted in metropolitan Lisbon, Portugal. J Int Med Res. (2022) 50:03000605221106703. doi: 10.1177/03000605221106703.
115. Muntané G, Vázquez-Bourgon J, Sada E, Martorell L, Papiol S, Bosch E, et al. Polygenic risk scores enhance prediction of body mass index increase in individuals with a first episode of psychosis. Eur Psychiatry. (2023) 66:e28. doi: 10.1192/j.eurpsy.2023.9.
116. Tirupati S, Chua LE. Body mass index as a screening test for metabolic syndrome in schizophrenia and schizoaffective disorders. Australas Psychiatry. (2007) 15:470–3. doi: 10.1080/10398560701636906.
117. Hodgson R, Adeyemo O. Physical examination performed by psychiatrists. Int J Psychiatry Clin Pract. (2004) 8:57–60. doi: 10.1080/13651500310004830.
118. Kazlauskienė L, Butnorienė J, Norkus A. Metabolic syndrome related to cardiovascular events in a 10-year prospective study. Diabetol Metab Syndrome. (2015) 7:102. doi: 10.1186/s13098-015-0096-2.
119. McNeill AM, Rosamond WD, Girman CJ, Golden SH, Schmidt MI, East HE, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. (2005) 28:385–90. doi: 10.2337/diacare.28.2.385.
120. Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. (2007) 49:403–14. doi: 10.1016/j.jacc.2006.09.032.
121. Inchiostro S, Fadini GP, de Kreutzenberg SV, Citroni N, Avogaro A. Is the metabolic syndrome a cardiovascular risk factor beyond its specific components? J Am Coll Cardiol. (2007) 49:2465; author reply 2465–2466. doi: 10.1016/j.jacc.2007.04.019.
122. Barbalho YG de S, Stival MM, de Lima LR, da Silva ICR, Silva AdeO, da Costa MVG, et al. Impact of metabolic syndrome components in high-risk cardiovascular disease development in older adults. CIA. (2020) 15:1691–700. doi: 10.2147/CIA.S252589.
123. Clavijo LC, Pinto TL, Kuchulakanti PK, Torguson R, Chu WW, Satler LF, et al. Metabolic syndrome in patients with acute myocardial infarction is associated with increased infarct size and in-hospital complications. Cardiovasc Revascularization Med. (2006) 7:7–11. doi: 10.1016/j.carrev.2005.10.007.
124. Gao J, Wang Y, Yang YN, Wu XY, Cui Y, Zou ZH, et al. Impact of metabolic syndrome and its components on clinical severity and long-term prognosis in patients with premature myocardial infarction. Front Endocrinol. (2022) 13:920470. doi: 10.3389/fendo.2022.920470
125. Macut D, Ognjanović S, Ašanin M, Krljanać G, Milenković T. Metabolic syndrome and myocardial infarction in women. Curr Pharm Des. (2021) 27:3786–94. doi: 10.2174/1381612827666210610114029.
126. Mente A, Yusuf S, Islam S, McQueen MJ, Tanomsup S, Onen CL, et al. Metabolic syndrome and risk of acute myocardial infarction: A case-control study of 26,903 subjects from 52 countries. J Am Coll Cardiol. (2010) 55:2390–8. doi: 10.1016/j.jacc.2009.12.053.
127. Gardner-Sood P, Lally J, Smith S, Atakan Z, Ismail K, Greenwood KE, et al. Cardiovascular risk factors and metabolic syndrome in people with established psychotic illnesses: baseline data from the IMPaCT randomized controlled trial. Psychol Med. (2015) 45:2619–29. doi: 10.1017/S0033291715000562.
128. Marche JC, Bannay A, Baillot S, Dauriac-Le Masson V, Leveque P, Schmitt C, et al. Prevalence of severe cardiovascular disease in patients with schizophrenia. L’Encéphale. (2022) 48:125–31. doi: 10.1016/j.encep.2021.02.008.
129. Sanchez-Martinez V, Romero-Rubio D, Abad-Perez MJ, Descalzo-Cabades MA, Alonso-Gutierrez S, Salazar-Fraile J, et al. Metabolic syndrome and cardiovascular risk in people treated with long-acting injectable antipsychotics. Endocr Metab Immune Disord Drug Targets. (2018) 18:379–87. doi: 10.2174/1871530317666171120151201.
130. Salvi V, Aguglia A, Barone-Adesi F, Bianchi D, DonFrancesco C, Dragogna F, et al. Cardiovascular risk in patients with severe mental illness in Italy. Eur Psychiatry. (2020) 63:e96. doi: 10.1192/j.eurpsy.2020.94.
131. Quadackers D, Liemburg E, Bos F, Doornbos B, Risselada A, Bartels-Velthuis A, et al. Cardiovascular risk assessment methods yield unequal risk predictions: a large cross-sectional study in psychiatric secondary care outpatients. BMC Psychiatry. (2023) 23:536. doi: 10.1186/s12888-023-05022-1.
132. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404.
133. Shiers DE, Rafi I, Cooper SJ, Holt RIG. Positive Cardiometabolic Health Resource: an intervention framework for patients with psychosis and schizophrenia. 2014 update. Royal College of Psychiatrists. (2014).
134. González-Rodríguez A, Seeman MV, Guàrdia A, Natividad M, Román E, Izquierdo E, et al. A review of cardiovascular risk factors in women with psychosis. Women. (2023) 3:200–13. doi: 10.3390/women3020016.
135. Rostami-Hodjegan A, Amin AM, Spencer EP, Lennard MS, Tucker GT, Flanagan RJ. Influence of dose, cigarette smoking, age, sex, and metabolic activity on plasma clozapine concentrations: a predictive model and nomograms to aid clozapine dose adjustment and to assess compliance in individual patients. J Clin Psychopharmacol. (2004) 24:70–8. doi: 10.1097/01.jcp.0000106221.36344.4d.
136. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. (1996) 15:395–405. doi: 10.1016/0893-133X(95)00276-J.
Keywords: psychosis, bipolar disorder, schizophrenia, schizoaffective disorder, psychiatry, cardiology, BMI, weight
Citation: Saccaro LF, Aimo A, Panichella G and Sentissi O (2024) Shared and unique characteristics of metabolic syndrome in psychotic disorders: a review. Front. Psychiatry 15:1343427. doi: 10.3389/fpsyt.2024.1343427
Received: 23 November 2023; Accepted: 15 February 2024;
Published: 04 March 2024.
Edited by:
Wing Chung Chang, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Annarita Vignapiano, Department of Mental Health, ItalyEdwin Lee, The University of Hong Kong, Hong Kong SAR, China
Copyright © 2024 Saccaro, Aimo, Panichella and Sentissi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luigi F. Saccaro, THVpZ2lGcmFuY2VzY28uU2FjY2Fyb0B1bmlnZS5jaA==
 Luigi F. Saccaro
Luigi F. Saccaro Alberto Aimo
Alberto Aimo Giorgia Panichella
Giorgia Panichella Othman Sentissi
Othman Sentissi