- 1Department of Psychiatry, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
- 2Department of Psychiatry College of Medicine and Health Science, Wollo University, Dessie, Ethiopia
- 3Department of General Midwifery, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
- 4Department of Reproductive Health, Institute of Public Health, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
- 5Department of Internal Medicine, University of Gondar College of Medicine and Health Science, Comprehensive Specialized Hospital, Gondar, Ethiopia
Background: Clinical research and epidemiological studies have shown that many women experience physical and behavioral symptoms that begin during the luteal phase of the menstrual cycle and terminate around the onset of menses; this is called premenstrual syndrome. The reviews stated that the pooled prevalence of premenstrual syndrome was around 50 percent. However, there has been no review done on premenstrual syndrome in Africa. Therefore, the aim of this systematic review and meta-analysis was to summarize the most recent data evidence on the pooled prevalence of premenstrual syndrome and its pooled effect of associated factors in Africa.
Method: We used an appropriate guideline for systematic reviews and meta-analyses reports, which is the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). This review protocol was registered in PROSPERO (CRD42023414021). The publications were identified from PubMed/Medline, EMBASE, Scopus databases, and other grey searches. The included papers were the original data that reported the prevalence of premenstrual syndrome and associated factors published, in English, and papers available online from January 1, 2000, to May 30, 2023. The data was extracted in Microsoft Excel, and then it would be imported into STATA 11.0 for analysis.
Results: We have included 16 studies conducted in African countries with 6530 study participants. In this meta-analysis, the pooled prevalence of premenstrual syndrome among the reproductive-age participants in Africa was 46.98 (95% CI: 28.9–65.06%). Further, in subgroup analysis, the pooled prevalence of premenstrual syndrome was 57.32% in Nigeria, 43.8% in Ethiopia, and 38.6% among university students and 66.04% among secondary school students. Among associated factors, the early age of menarche was significantly related to premenstrual syndrome.
Conclusion: In this review, the pooled prevalence of premenstrual syndrome in Africa was high. Among factors, the early age of menarche was a risk factor for premenstrual syndrome. This finding might help the stakeholders (mental health policy makers, administrators, and mental health professionals) to address prevention, early screening, and management of PMS among reproductive-age women, and to give attention to more vulnerable bodies.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42023414021.
Introduction
Clinical research and epidemiological studies have shown that many women experience recurrent physical, behavioral, and/or psychological signs and symptoms that begin during the luteal phase of the menstrual cycle and terminate around the onset of menses or shortly thereafter; this is called premenstrual syndrome and/or premenstrual dysphoric disorder (1–3). These signs and symptoms are characterized as; Behavioural and/or psychological signs and symptoms: affective lability (feeling suddenly sad or tearful, or increased sensitivity to rejection), irritability or anger, depressed mood, feelings of hopelessness, anxiety, tension, restlessness, decreased interest in usual activities, lethargy, difficulty in concentration, changes in appetite, and hypersomnia or insomnia. Physical signs and symptoms include breast tenderness or swelling, joint or muscle pain, a sensation of “bloating” or weight gain, headaches or migraines, and abdominal cramps (2, 3).
The severe form of premenstrual syndrome is called premenstrual dysphoric disorder (PMDD) (4). Premenstrual dysphoric disorder was under depressive disorder not otherwise specified in the Diagnostic and Statistical Manual of Mental Disorder (DSM-IV) (5), but after careful scientific review of the evidence, PMDD is a new diagnosis in the Diagnostic and Statistical Manual of Mental Disorder-5 (DSM-5) (6). Almost 20 years of additional research on this condition has confirmed a specific and treatment-responsive form of depressive disorder that begins sometime following ovulation, remits within a few days of menses, and has a marked impact on functioning (6).
The scholars were also strengthened that PMS is a number of signs and symptoms that are characterized by physical symptoms and emotional and behavioral disturbances in the luteal phase of the menstrual cycle in the reproductive age women (7–10). Reproductive age in women (from menarche to menopause) has a different range, but mostly it falls between 15 and 49 years (11, 12). The study showed that an estimated 90% of women of reproductive age suffered from mild to acute premenstrual symptoms. Among them, about 20 to 40 percent encounter moderate to severe symptoms (PMS), while 2 to 8 percent experience severe PMS/PMDD (13). Another study revealed that 95% of women experience PMS; of them, 5% suffered severe PMS (14). In the United States of America (USA), the 12-month prevalence of PMDD is between 1.8% and 5.8% of menstruating women (15). And also, the systematic review and meta-analysis studies revealed that the prevalence of PMS was 47.8% (7) in the global population, PMS (43%), and PMDD (8%) (16) in India, whereas in Ethiopia, more than half (53%) (17) and (54.5%) (18) of the reproductive-age women had PMS and PMDD, respectively. However, there has been no review done on PMS and/or PMDD in Africa. Indeed, PMS and/or PMDD status has been the topic of a large number of studies, with a large variation in reported prevalence rates from 6.1% (19) to 94.8% (20).
The precise cause of PMS is unknown, but research has indicated that changing hormone levels such as those of oestrogen, progesterone, testosterone, prolactin, and serotonin synthesis in the brain also appear to play a major part in PMS (3, 15) (21). Stress, a history of interpersonal trauma, seasonal variations, and sociocultural features of female sexual behavior in general and female gender role in particular are environmental factors linked to the expression of premenstrual dysphoric disorder (6). Premenstrual dysphoric disorder’s heritability in the United States is uncertain. Premenstrual symptoms, on the other hand, have heritability estimates ranging from 30% to 80%, with the most stable component considered to be roughly 50% heritable (6). And also, the review stated that alcohol intake was significantly associated with PMS (22). Whereas, other reviews showed that PMS/PMDD was associated with a higher risk of post-partum depression (PPD) (23), and suicidality (24, 25). Women with PMS or PMDD suffered distress and impairment in interpersonal or workplace functioning. These decreased productivity levels at work and school affect quality of life, increase health care utilization, and can lead to at least 2 days per month of absenteeism at work and an increase in medical appointments (26–28).
Identifying risk factors and the pooled prevalence of MPS could help health care professionals and policymakers plan health promotion, prevention, and early intervention programs. However, per our search, there has been no systematic review or meta-analysis of the epidemiology of PMS in Africa. So, in response to this research gap, the aim of this study is to summarize the most recent data evidence from January 2000 to May 2023 for reproductive-age women in Africa. What is the pooled prevalence of PMS and the pooled effects of associated factors?
Materials and methods
Study design
In this study, we followed an appropriate guideline for systematic reviews and meta-analyses reports: of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (29) (Supplementary File 1). This review protocol was registered in PROSPERO (CRD42023414021).
Searching strategy
The publications were searched by PubMed/Medline, EMBASE, Cochrane Library, Scopus, HINARI, PsycINFO, African Journals Online (AJOL), and also the grey literature was searched by Google Scholar and World Health Organization (WHO) reports. We used the following search terms (“prevalence” OR “epidemiology” OR “magnitude” AND “premenstrual syndrome” OR “premenstrual dysphoric disorder” AND “associated factors” OR “risk factors” AND “women” AND “Africa”) (Supplementary File 2).
Eligibility criteria
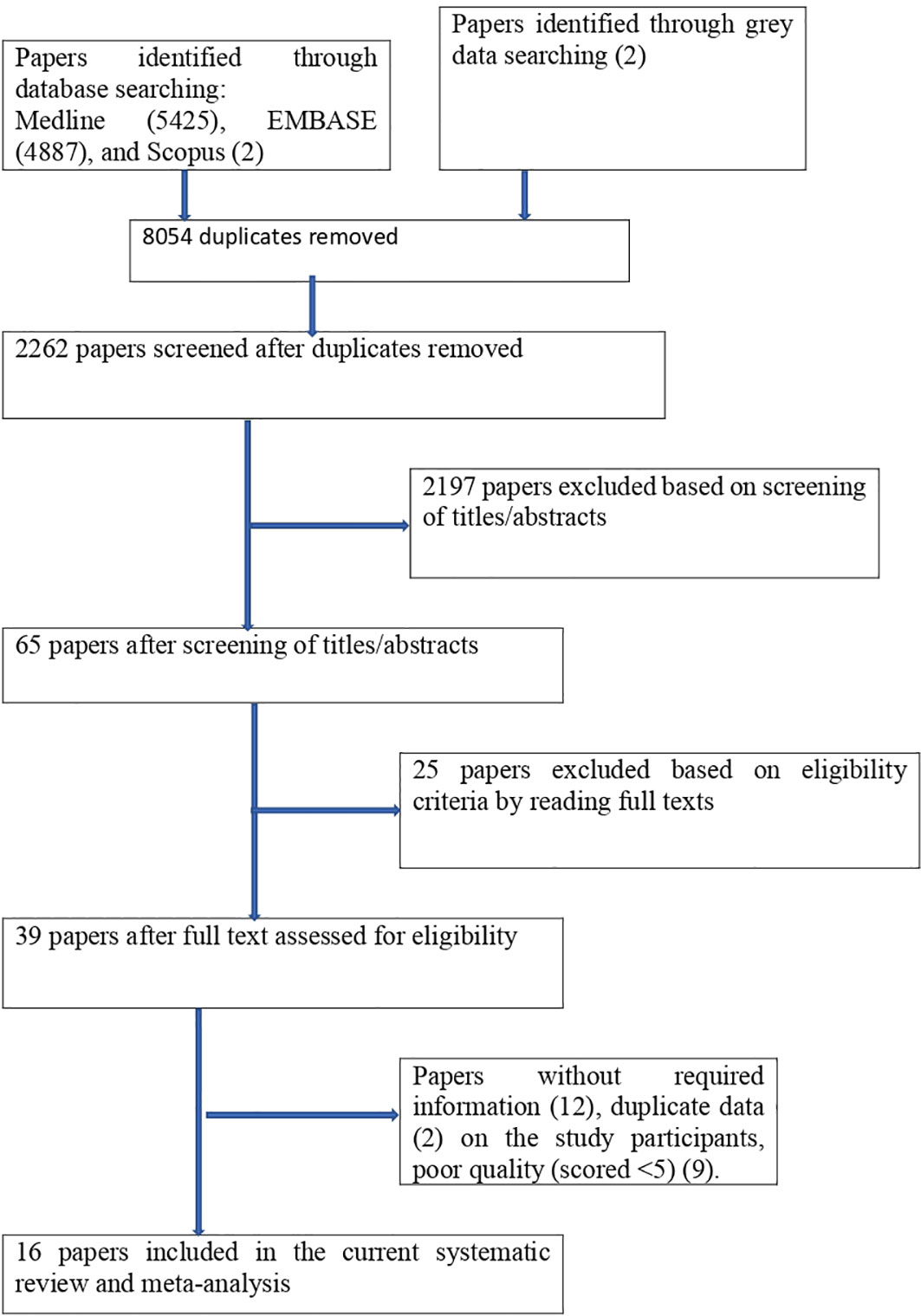
The studies had the following criteria: they were done in African countries and were all relevant observational studies (cross-sectional). And also, published in English, published and unpublished articles were considered. Searching was performed from 1 February 2023 to 30 May 2023, and articles available online from 1 January 2000 to 30 May 2023 were considered, but studies that could not be fully accessed (conference abstracts) were excluded. We looked over each study’s abstract and title before adding those to our meta-analysis. Following the selection of pertinent research, the entire text was examined. As seen in Figure 1, we did not include any article with no interesting variables in our study.
Data extraction
MM and GN independently extracted all the necessary data from the articles using a standardized data extraction format. The data extraction format included the following items: the first author’s name, publication year, country where the study was conducted, a screening tool used to examine PMS, number of participants, prevalence of PMS, and associated factors with PMS. The data extraction format was in the form of a two-by-two table. A cross-check was done by MM and GM authors. If contrasting results occurred between the two authors during data extraction, they were discussing ways to achieve consensus and double extraction with other authors.
Tools and validity
Among the sixteen studies reporting estimates of the prevalence of PMS, six were assessed using the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV). Three studies were assessed using the Premenstrual Syndrome Scale (PMSS), with an inter-rater reliability between 0.81 and 0.97 (30). The PMSS consists of 40 items on a 5-point Likert-type scale (as “never” was scored as “1”, rarely as “2”, sometimes as “3”, very often as “4”, and always as “5” points). The scale score is 40 to 200. A total score estimated at 80 points or above indicates PMS, and an increase in the scores indicates an increase in PMS severity (30, 31).
The American College of Obstetricians and Gynecologist diagnostic criteria (ACOG) were used to assess PMS in two studies (32), where the following criteria were followed:
1. The patient reports one or more of the following affective and somatic symptoms five days before menses in each of the three prior menstrual cycles: affective, depression, angry outbursts, anxiety, irritability, confusion, social withdrawal, somatic, Breast tenderness, abdominal bloating, headache, swelling of the extremities.
2. Symptoms are relieved within 4 days of menses onset without recurrence until at least cycle day 13.
3. Symptoms present in the absence of any pharmacologic therapy, hormone ingestion, or drug or alcohol abuse.
4. Symptoms occur reproducibly during two cycles of prospective recording.
5. The patient suffers from an identifiable dysfunction in social or economic performance.
Another two studies were assessed using the International Classification of Diseases, Tenth Edition (ICD-10). The remaining three studies were measured using a different tool, as shown here: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) (6). The premenstrual symptoms screening tool (PSST) was used to assess PMDD, which includes a list of premenstrual psychiatric and physical symptoms and a measure of functional impairment in accordance with DSM-5 criteria (33). And the Calendar of Premenstrual Experiences (COPE) is a valid and reliable tool for the diagnosis of PMS (31, 34, 35). The COPE scoring for each symptom is a 3-point rating scale: absent = 0, present = 1, severe = 2 (31).
Quality appraisal
The quality of the research reports included in this review was assessed using the Joanna Briggs Institute (JBI) for cross-sectional study quality assessment (36). For this quality assessment tool, it included the following components: the methodological quality of the study, the comparability of the included studies, and the quality of the original articles with respect to statistical analysis. All authors independently evaluated the quality of the original research using JBI. The tool has a total of 9 scores, and articles with medium and high quality (articles that score 5 and above out of a 9-point scale) were included in this review for analysis. Also, some papers were excluded due to low quality scores (that scored less than 5). If there were any discrepancies between authors during the quality assessment of the included studies, they were solved by the involvement of other authors.
Data processing and analysis
The data was extracted in Microsoft Excel, and then it will be imported into STATA 11.0 for analysis. The extracted data were shown using texts, tables, and forest plots. The standard error of prevalence for each primary or original study was analyzed using the binomial distribution. The prevalence of the original studies was checked for heterogeneity using a heterogeneity I-squire (I2) test (37). We used a random-effects meta-analysis model to estimate Der Simonian and Laird’s pooled effect of PMS. And we made a leave-one-out sensitivity analysis to identify the possible source of heterogeneity in the pooled meta-analysis of the prevalence of PMS among women in African countries. Publication bias was detected using funnel plot analysis (38) and Egger - weighted regression tests (39). A p value of less than 5% significance in the Egger test was considered to have statistically significant publication bias (39), Subgroup analysis was done to identify the impact of factors in a particular group for the prediction of the pooled prevalence of PMS.
Results
Study identification
There were 10314 publications identified in database searches, and another 2 records were added through grey searches. Among them, 8054 were removed due to duplicates; 2197 papers were excluded based on screening of titles/abstracts; 25 papers were excluded based on eligibility criteria by reading full texts; papers were excluded due to (without required information (12), duplicate data (2), and poor quality (9). Finally, a total of 16 studies were involved in this systematic review and meta-analysis (Figure 1).
Characteristics of included studies
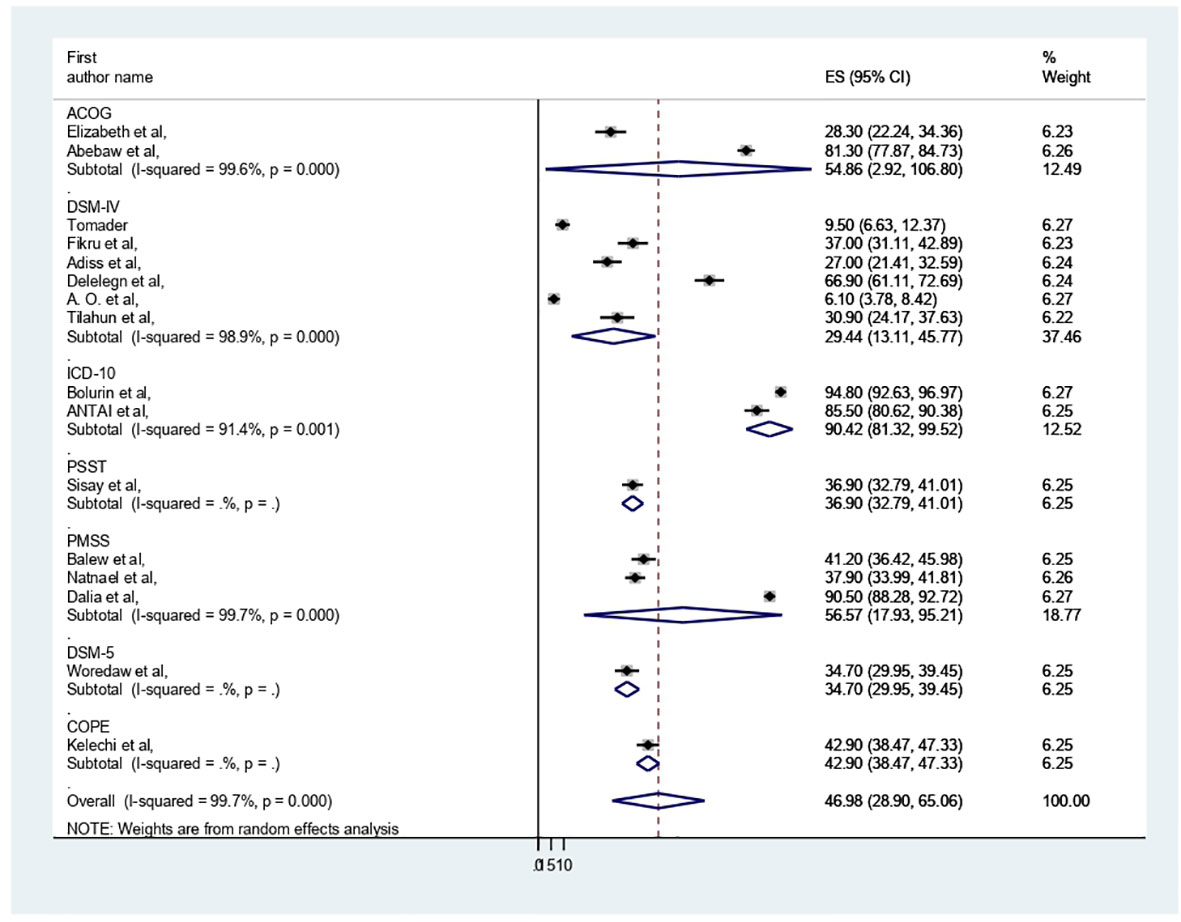
A total of 16 studies conducted in African countries, including 6530 study participants, were included in this review. Among the 16 studies included, nine were from Ethiopia (40–48), four from Nigeria (19, 20, 49, 50), and one from Uganda (51), Sudan (52) and Egypt (53). All of them were followed cross-sectional study designs. Regarding assessment tools, most of them were assessed by DSM-IV (six studies) and PMSS (three studies) (Table 1).
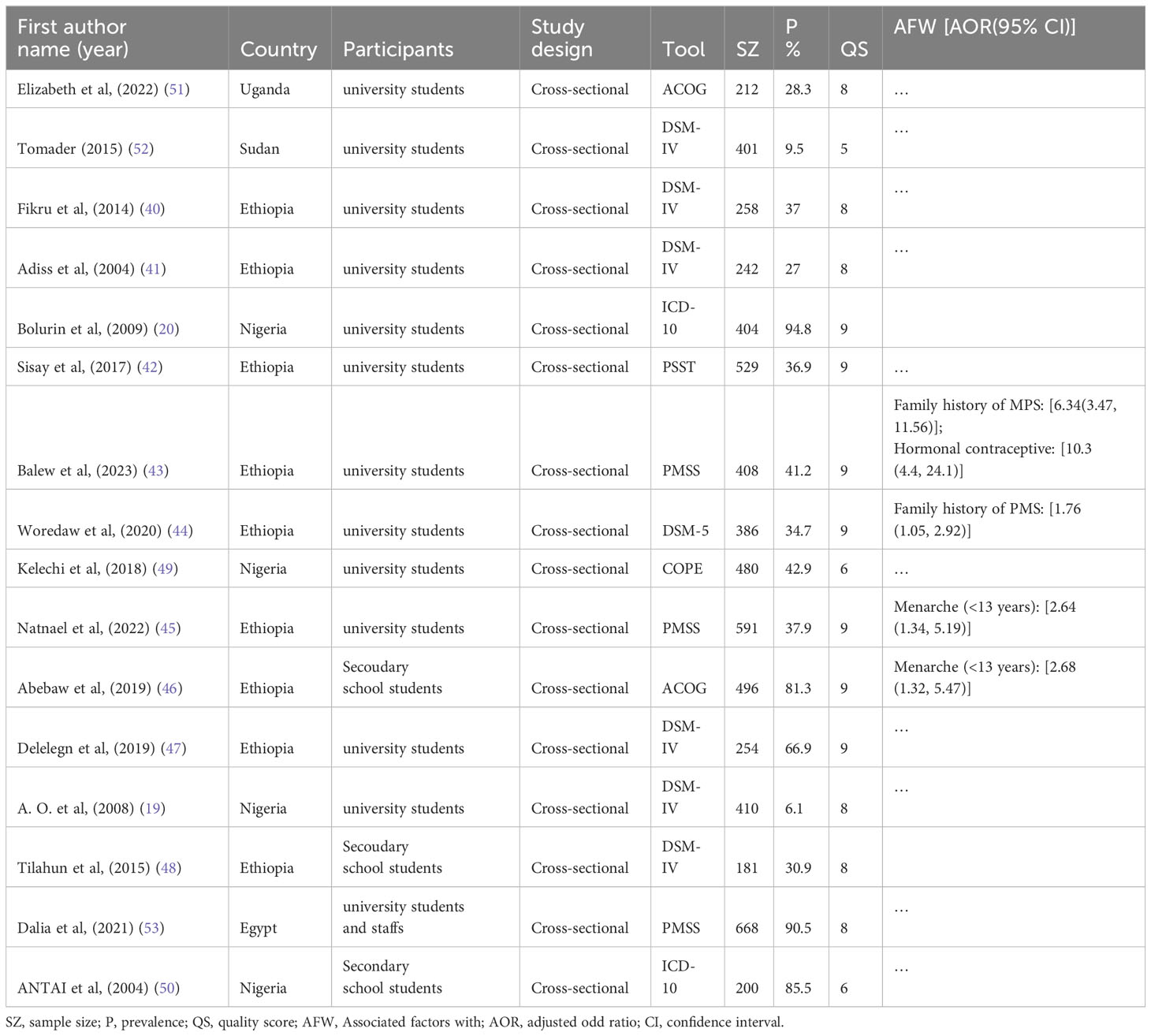
Table 1 Characteristics of studies included in the systematic review and meta-analysis on prevalence of premenstrual syndrome and associated in Africa.
Study quality appraisals
To assess the quality of the studies we used the Joanna Briggs Institute (JBI) quality assessment criteria. All the studies involved in this review have good quality (JBI score >=5) (Supplementary File 3).
Meta- analysis
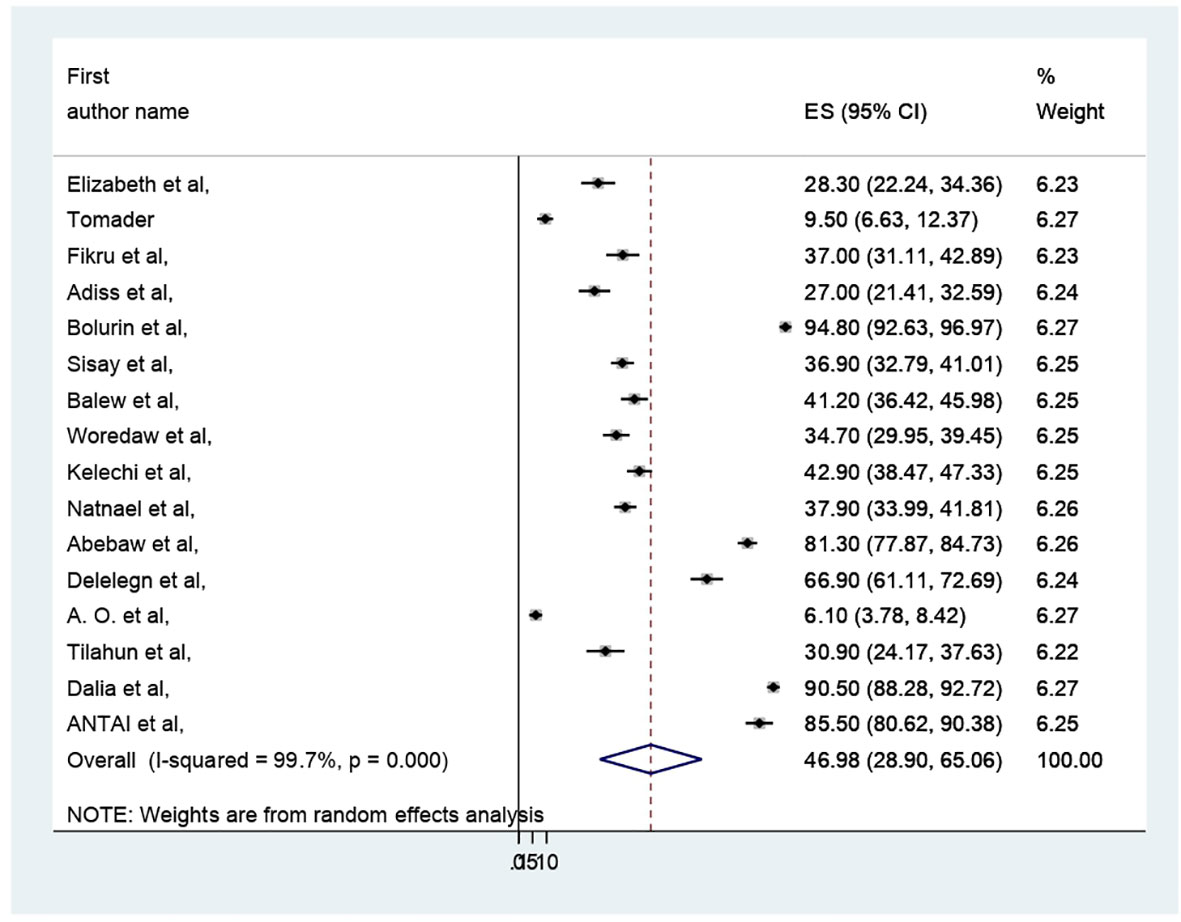
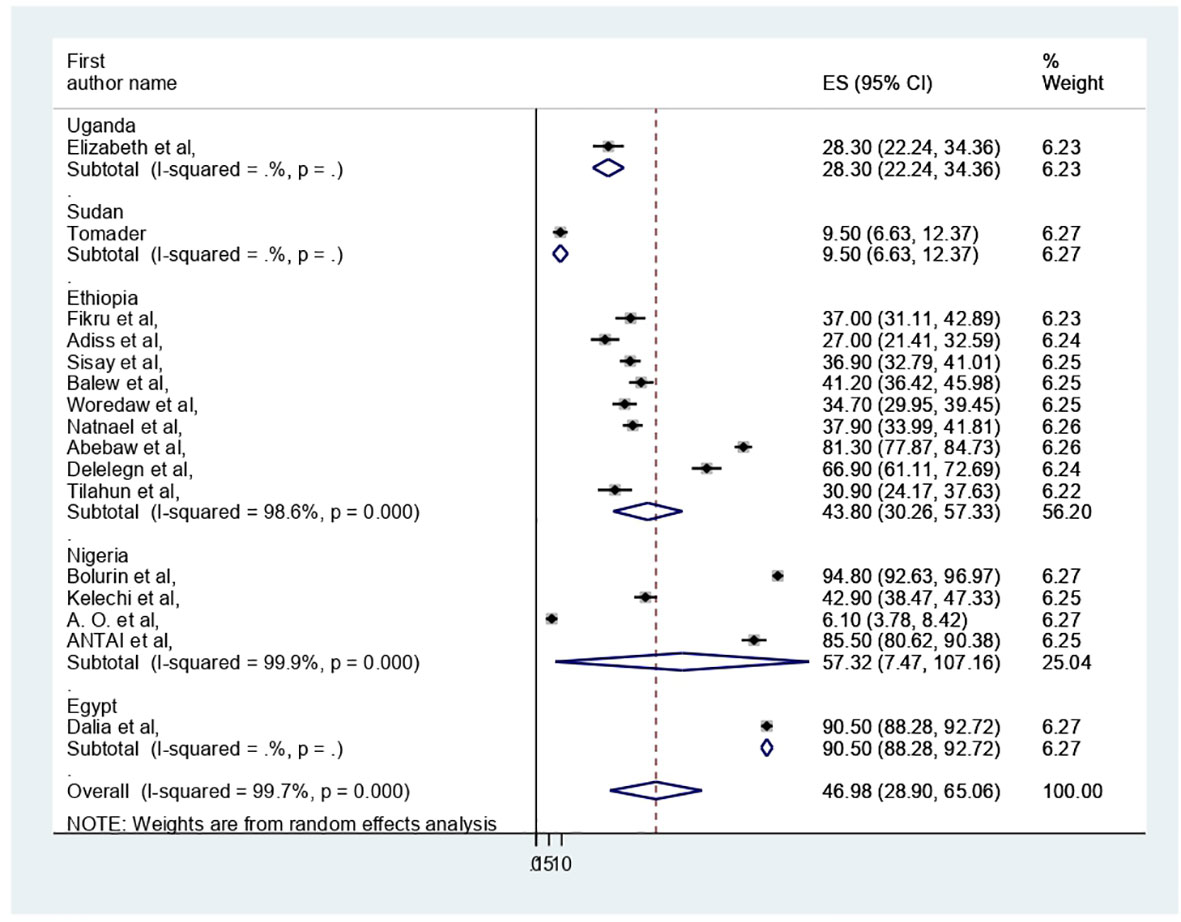
The pooled prevalence of PMS in Africa was 46.98 (95% CI: 28.9–65.06%) (Figure 2). Due to apparent heterogeneity across the studies, we have used a random effect model while conducting a meta-analysis (I2 = 99.7%, p < 0.000).
Publication bias
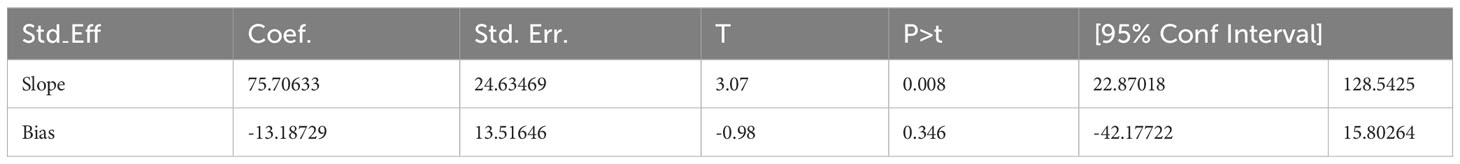
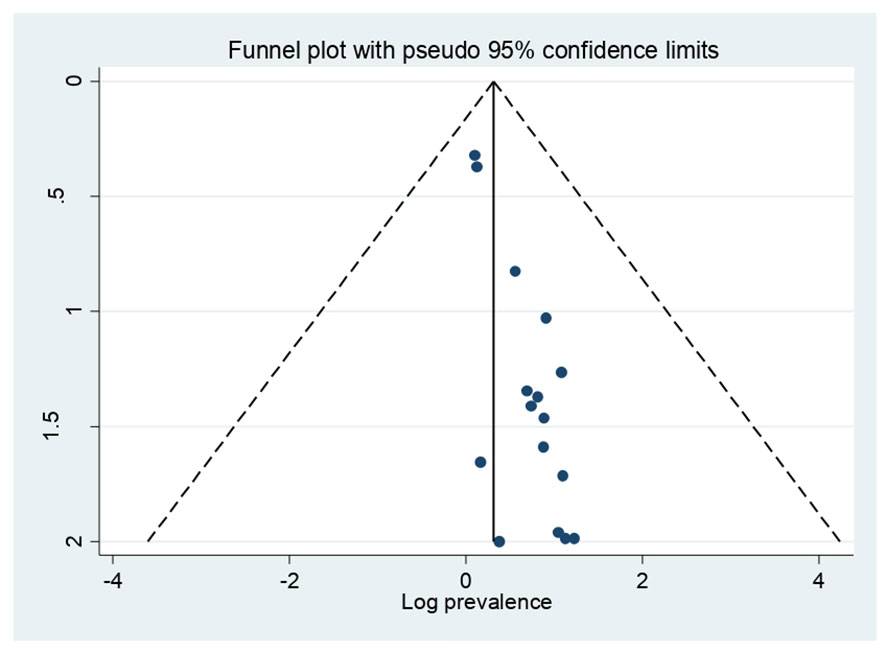
In this study, a funnel plot falls inside the triangle, which indicates the absence of publication bias (Figure 3), and Egger’s regression test (P = 0.346) strengthened it (Table 2).
Subgroup analysis
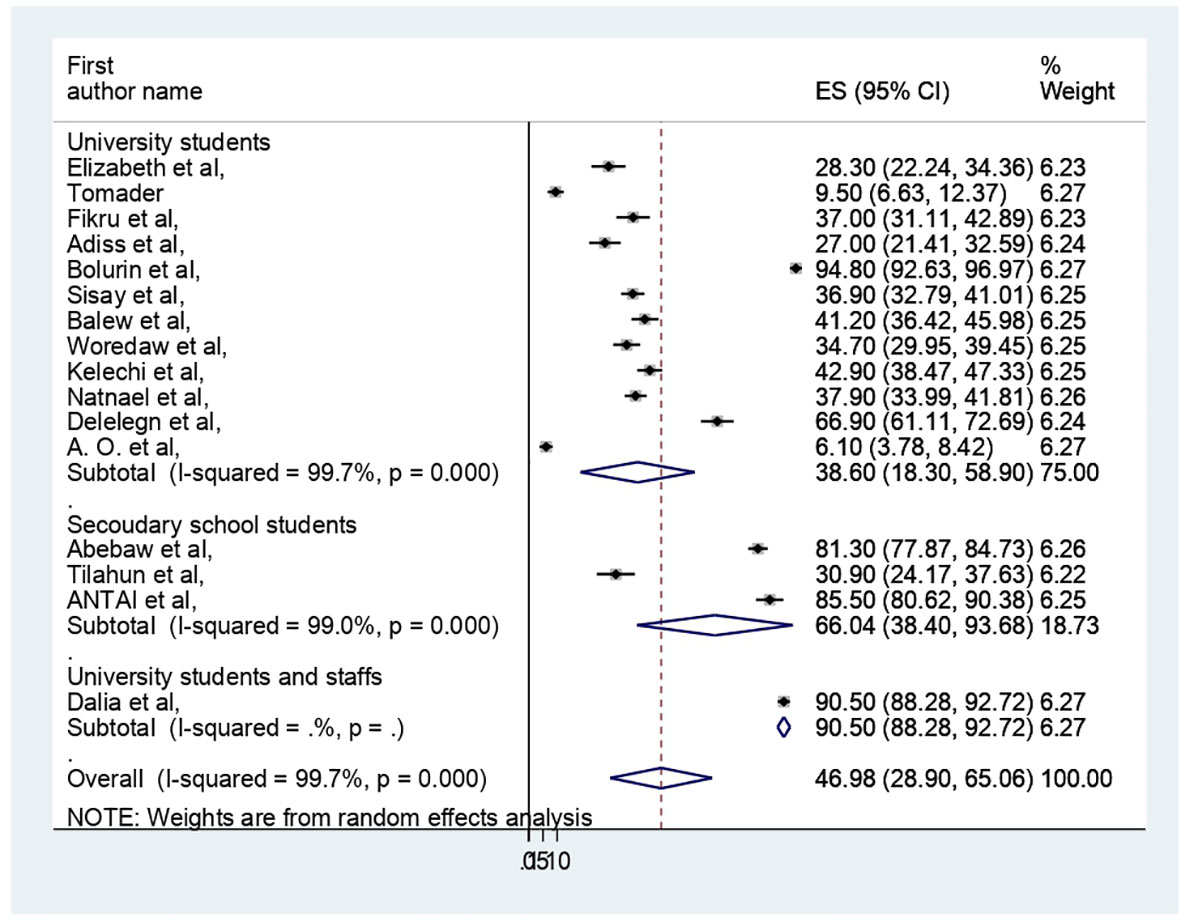
The presence of heterogeneity was confirmed (I2 = 99.7%, p < 0.000). So, subgroup analysis was conducted based on study country, type of participants, and assessment tool. A higher pooled prevalence of PMS was found in Nigeria (57.32%, I2 = 99.9%, p = 0.000), followed by Ethiopia (43.8%) (I2 = 99.6%, p = 0.000). The study participants’ pooled prevalence of PMS among university students and secondary school students was 38.6% (I2 = 99.7%, p = 0.000) and 66.04% (I2 = 99%, p = 0.000), respectively. Regarding the assessment tool, the pooled prevalence of PMS was 29.44% by DSM-IV and 56.57% by PMSS. Therefore, this result showed there was high heterogeneity among subgroups, as indicated by I2 (91.4 and above) and P = 0.001 (Figures 4–6), which indicate the need to conduct the sensitivity test.
A leave-out-one sensitivity analysis
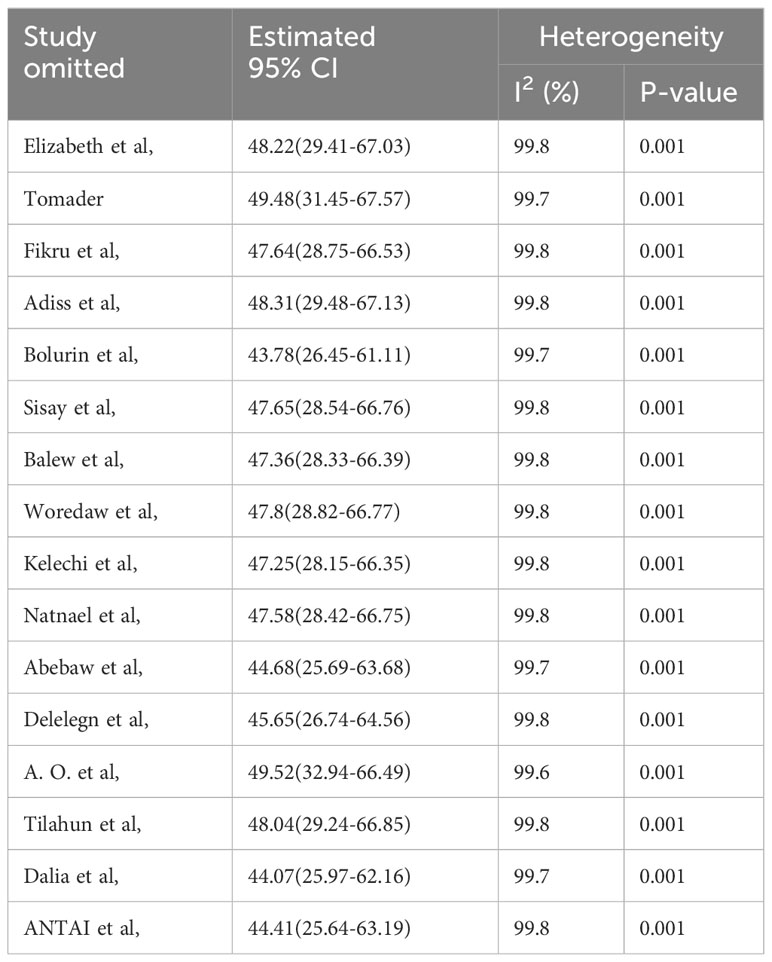
The sensitivity analysis was done to check the heterogeneity of those studies by omitting one author or one study step by step to check the effect of each study on the overall prevalence of PMS in this systematic review and meta-analysis study. As evidenced by the results, all the values are within the estimated 95% confidence-interval (CI) ranged 43.78% to 49.52%, which indicates the omission of a single study had no significant difference in the prevalence of this systematic review and meta-analysis (Table 3).
Narrative analysis
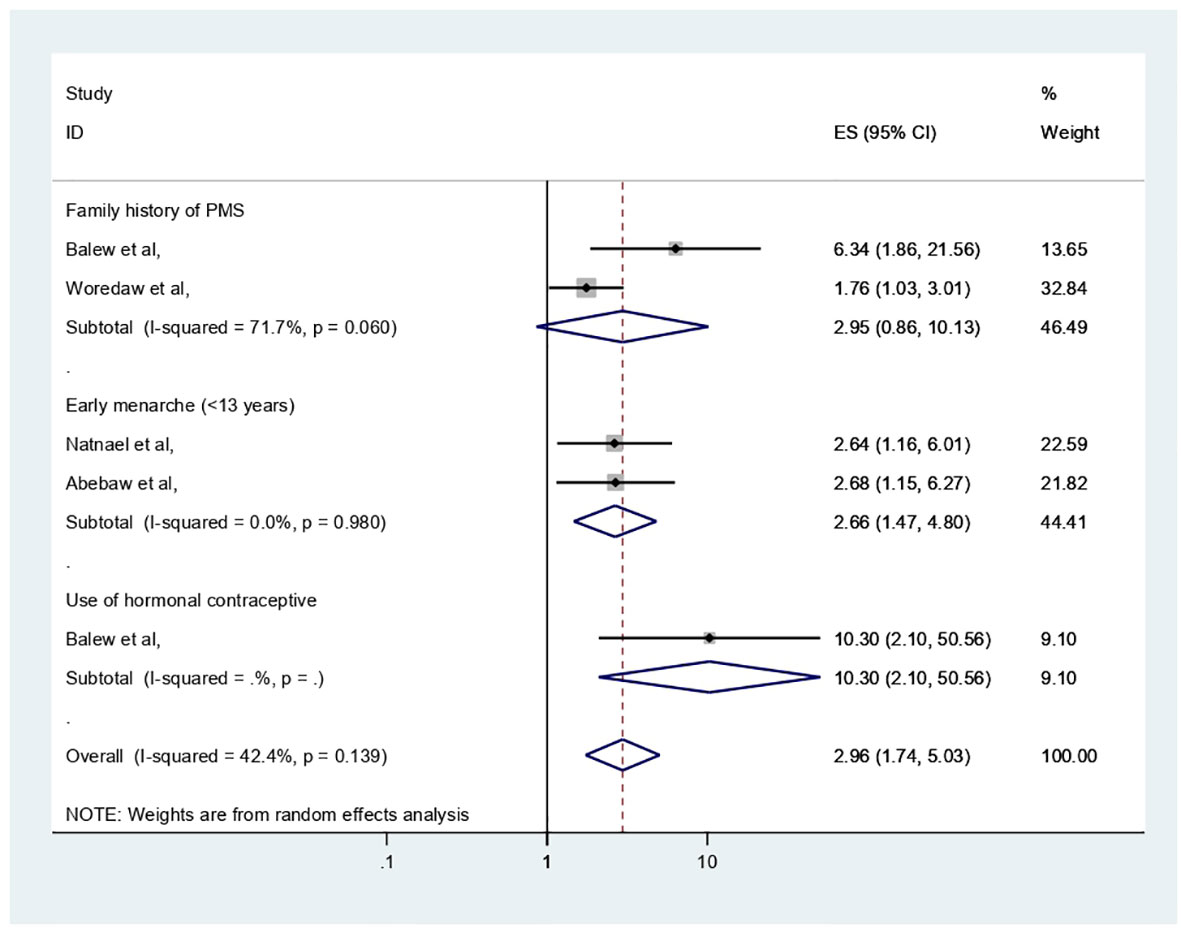
The extracted important factors associated with PMS among participants of reproductive age, the individual study, in Africa were provided in Table 1 with reference to the studies analyzed in logistic regression with adjusted odd ratio. This section has narrated significant factors associated with PMS in Africa. The following results were obtained from the pooled analysis for these factors and in cases where two or more publications were present: age of menarche (<13 years) (adjusted odd ratio (AOR) = 2.66, 95% CI: 1.47–4.8) with two previous studies and within this review was significantly associated with PMS, whereas perceived family history of PMS (AOR = 2.96, 95% CI: 0.86–10.13) with two previous studies, was not significantly associated with PMS (Figure 7).
Discussion
In our systematic review and meta-analysis, we synthesized 16 studies investigating the prevalence and associated factors of PMS among 6530 reproductive age participants in Africa, of whom 3068 had been screened for PMS. In this review, the pooled prevalence of PMS in Africa was 46.98 (95% CI: 28.9–65.06%). This result was in line with the previous studies of systematic reviews and meta-analyses done in Ethiopia (17, 18), India (16) and Global (7). On the other hand, it was less than the Iranian study (54). This disparity may be attributed to the study participants’ residency in Iran, but this review encompassed five nations; in Iran, 24 articles with a sample size of 9147 women of reproductive age were included. Additionally, additional assessment instruments (such as the American Psychiatric Association, Premenstrual Assessment Scale, Daily Record of Severity of Problems Chart, Researcher Made Questionnaire, Premenstrual Assessment Scale, and Hallbridge et al. questionnaire) were utilized in Iran, which were not included in this review. Further, in this analysis, DSM-IV was dominantly used for PMS assessment. This could result from differences in premenstrual syndrome screening and assessment methods’ specificity and sensitivity.
In this review, regarding subgroup analysis, the pooled prevalence of PMS among participants of reproductive age was higher in Nigeria (57.32%) compared with Ethiopia (43.8%). This finding may vary depending on the number of studies and participant sample size. For example, four research with 1494 participants were conducted in Nigeria, while nine studies with 3345 participants were conducted in Ethiopia. It could also be due to variations in research regions and assessment tools along with sociocultural differences. Additionally, we used the study participant type; among secondary school and university students, the pooled prevalence of PMS was 66.04% and 38.6%, respectively. The menarche age and participant age may be the cause, as shown by the results of the earlier Iranian review (54).
In terms of factors associated with PMS, they were extracted in the individual study in Africa with reference to the studies analyzed in logistic regression with an adjusted odd ratio. However, most studies were analyzed by ANOVA, and some of them involved linear logistic regression.
The important variables linked to PMS have been discussed in this section, and in cases where two or more publications were present, our review identified that age at menarche and family history of PMS were positively and negatively associated with PMS. According to this finding, early menarche (<13 years) (AOR = 2.66, 95% CI: 1.47–4.8) was a risk factor for PMS. The results of this study could be explained by the earlier menarche age being linked to the early development of ovarian functions and ovulation with steroid hormone fluctuations at such a young age with less physical and psychological maturity, which may cause PMS manifestations; however, the earlier Ethiopian meta-analysis did not support this theory (17). In contrast, perceived family history of PMS (AOR = 2.96, 95% CI: 0.86–10.13) was not significantly associated with PMS in this analysis. The severe level of premenstrual dysphoric disorder’s heritability in the United States is unknown. However, Premenstrual symptoms have heritability estimates ranging from 30% to 80% (6).
Regarding this study, all participants were secondary school and university students. This is a productive age group, so the ministry of education should incorporate the module into the curriculum and provide training about premenstrual symptoms and their management. We recommend more representative samples, or rather, a cross-sectional study design, be used in future research that concentrates on a more precise diagnosis.
Limitation
The limitation of this review is that it included only studies published in English that were cross-sectional studies since there were no studies conducted with other study designs and a small number of articles were included.
Conclusion
In this review, the pooled prevalence of PMS in Africa was high. Among factors, the early age of menarche was a risk factor for PMS. This finding might help the stakeholders (mental health policy makers, administrators, and mental health professionals) to address prevention, early screening, and management of PMS among reproductive-age women and to give attention to more vulnerable bodies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
FA: Conceptualization, Data curation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. MM: Data curation, Writing – review & editing. GiM: Data curation, Writing – review & editing. GN: Writing – review & editing. TT: Writing – review & editing. SF: Writing – review & editing. GR: Writing – review & editing. JS: Writing – review & editing. GeM: Writing – review & editing. DA: Writing – review & editing. TN: Writing – review & editing.
Funding
The authors declared that there was no financial support received for the research, authorship and/ or publication of this article.
Acknowledgments
We would like to thank the authors of the primary articles.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1338304/full#supplementary-material
References
1. Cowen P, Harrison P, Burns T. Shorter Oxford textbook of psychiatry. USA: Oxford University Press (2012).
2. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 5th edn. Washington, DC: American American Psychiatric Association (2013).
4. Steiner M, Peer M, Palova E, Freeman EW, Macdougall M, Soares CN. The Premenstrual Symptoms Screening Tool revised for adolescents (PSST-A): prevalence of severe PMS and premenstrual dysphoric disorder in adolescents. Arch Womens Ment Health (2011) 14:77–81. doi: 10.1007/s00737-010-0202-2
5. Sadock BJ. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry (1998). Lippincott Williams & Wilkins. Copyright© 2007.
6. Guha M. Diagnostic and statistical manual of mental disorders: DSM-5 (5th edition). (2014) 28(3):36–7. doi: 10.1108/RR-10-2013-0256
7. Direkvand-Moghadam A, Sayehmiri K, Delpisheh A, Sattar KJ. Epidemiology of Premenstrual syndrome (PMS)-A systematic review and meta-analysis study. J Clin Diagn Res (2014) 8 (2):106–9. doi: 10.7860/JCDR/2014/8024.4021
8. Kleinstäuber M, Schmelzer K, Ditzen B, Andersson G, Hiller W, Weise C. Psychosocial profile of women with premenstrual syndrome and healthy controls: a comparative study. Int J Behav Med (2016) 23:752–63. doi: 10.1007/s12529-016-9564-9
9. Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician (2016) 94:236–40.
10. Gudipally PR, Sharma GK. Premenstrual syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2023).
11. Golshiri P, Akbari M, Abdollahzadeh MR. Age at natural menopause and related factors in Isfahan, Iran. J Menopausal Med (2016) 22:87–93. doi: 10.6118/jmm.2016.22.2.87
12. Bahrami N, Soleimani MA, Chan YH, Ghojazadeh M, Mirmiran P. Menarche age in Iran: A meta-analysis. Iran J Nurs Midwifery Res (2014) 19(5):444.
13. Matsumoto T, Asakura H, Hayashi T. Biopsychosocial aspects of premenstrual syndrome and premenstrual dysphoric disorder. J Gynaecol Endocrinol (2013) 29(1):67–73. doi: 10.3109/09513590.2012.705383
14. O’Brien PM. Helping women with premenstrual syndrome. BMJ: Brit Med J (1993) 307(6917):1471. doi: 10.1136/bmj.307.6917.1471
15. Sadock BJ, Sadock VA, Ruiz P. Kaplan & Sadock’s synopsis of psychiatry: behavioral sciences/clinical psychiatry (Eleventh ed.). Philadelphia, PA: Wolters Kluwer (2015) p. 438.
16. Dutta A, Sharma A. Prevalence of premenstrual syndrome and premenstrual dysphoric disorder in India: A systematic review and meta-analysis. Health Promot Perspect (2021) 11(2):161. doi: 10.34172/hpp.2021.20
17. Geta TG, Woldeamanuel GG, Dassa TT. Prevalence and associated factors of premenstrual syndrome among women of the reproductive age group in Ethiopia: Systematic review and meta-analysis. PLoS One (2020) 15(11):e0241702. doi: 10.1371/journal.pone.0241702
18. Duko B, Mekuriaw B, Molla A, Ayano G. The prevalence of premenstrual dysphoric disorder among adolescents in Ethiopia: a systematic review and meta-analysis. Ir J Med Sci (1971-) (2021) 190:419–27. doi: 10.1007/s11845-020-02275-7
19. Adewuya AO, Loto OM, Adewumi TA. Premenstrual dysphoric disorder amongst Nigerian university students: prevalence, comorbid conditions, and correlates. Arch Womens Ment Health (2008) 11:13–8. doi: 10.1007/s00737-008-0213-4
20. Ehalaiye B, Eigbefoh J, Eifediyi RA, Omorogbe F, Isabu PA, Ugiagbe OA, et al. Premenstrual syndrome: prevalence, pattern and severity among female university students in Ekpoma, Nigeria. Trop J Obstet Gynaecol (2009) 26:142–50.
21. Wood SH, Mortola JF, Chan Y-F, Moossazadeh F, Yen SS. Treatment of premenstrual syndrome with fluoxetine: a double-blind, placebo-controlled, crossover study. Obstet Gynecol (1992) 80(3 Part 2):339–44.
22. del Mar Fernández M, Saulyte J, Inskip HM, Takkouche B. Premenstrual syndrome and alcohol consumption: a systematic review and meta-analysis. BMJ Open (2018) 8(3):e019490. doi: 10.1136/bmjopen-2017-019490
23. Cao S, Jones M, Tooth L, Mishra GD. History of premenstrual syndrome and development of postpartum depression: a systematic review and meta-analysis. J Psychiatr Res (2020) 121:82–90. doi: 10.1016/j.jpsychires.2019.11.010
24. Prasad D, Wollenhaupt-Aguiar B, Kidd KN, de Azevedo Cardoso T, Frey BN. Suicidal risk in women with premenstrual syndrome and premenstrual dysphoric disorder: a systematic review and meta-analysis. J Womens Health (2021) 30(12):1693–707. doi: 10.1089/jwh.2021.0185
25. Osborn E, Brooks J, O’Brien P, Wittkowski A. Suicidality in women with Premenstrual Dysphoric Disorder: a systematic literature review. Arch Womens Ment Health (2021) 24:173–84. doi: 10.1007/s00737-020-01054-8
26. Rapkin AJ, Winer SA. Premenstrual syndrome and premenstrual dysphoric disorder: quality of life and burden of illness. Expert Rev Pharmacoecon Outcomes Res (2009) 9(2):157–70. doi: 10.1586/erp.09.14
27. Borenstein JE, Dean BB, Endicott J, Wong J, Brown C, Dickerson V, et al. Health and economic impact of the premenstrual syndrome. J Reprod Med (2003) 48(7):515–24.
28. Bhuvaneswari K, Rabindran P, Bharadwaj B. Prevalence of premenstrual syndrome and its impact on quality of life among selected college students in Puducherry. Natl Med J India (2019) 32(1):17–9.
29. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
30. Padmavathi P, Sankar R, Kokilavani N, Dhanapal K, Ashok B. Validity and reliability study of premenstrual syndrome scale (PMSS). Int J Adv Nurs Manag (2014) 2(1):04–5.
31. Oo HH, Sein MT, Mar O, Aung AJ. Assessment of premenstrual syndrome among reproductive aged Myanmar women. Asian J Med Sci (2016) 7(4):39–43. doi: 10.3126/ajms.v7i4.13298
32. Yildirim N, Guzel D, Akdemir A. Prophylactic surgery for genetic predisposition of female organs. In: Dilek O.N., Uranues S., Latifi R. (eds) Prophylactic surgery (2021) Springer, Cham. doi: 10.1007/978-3-030-66853-2_27
33. Câmara R, Köhler CA, Frey BN, Hyphantis TN, Carvalho AF. Validation of the Brazilian Portuguese version of the premenstrual symptoms screening tool (PSST) and association of PSST scores with health-related quality of life. Braz J Psychiatry (2016) 39:140–6. doi: 10.1590/1516-4446-2016-1953
34. Steiner M. Premenstrual syndrome and premenstrual dysphoric disorder: guidelines for management. J Psychiatry Neurosci (2000) 25:459–68.
35. Feuerstein M, Shaw WS. Measurement properties of the calendar of premenstrual experience in patients with premenstrual syndrome. J Reprod Med (2002) 47:279–89.
36. Munn Z, Moola S, Lisy K, Riitano D, Tufanaru CJ. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid-Based Healthc. (2015) 13:147–53. doi: 10.1097/XEB.0000000000000054
37. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med (2002) 21:1539–58. doi: 10.1002/sim.1186
38. Sterne JA, Egger MJ. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol (2001) 54:1046–55. doi: 10.1016/S0895-4356(01)00377-8
39. Egger M, Smith GD, Schneider M, Minder CJB. Bias in meta-analysis detected by a simple. graphical test (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
40. Tolossa FW, Bekele ML. Prevalence, impacts and medical managements of premenstrual syndrome among female students: cross-sectional study in college of health sciences, Mekelle University, Mekelle, Northern Ethiopia. BMC Women’s Health (2014) 14:1–9. doi: 10.1186/1472-6874-14-52
41. Tenkir A, Fisseha N, Ayele B. Premenstrual syndrom: prevalence and effect on academic and social performances of students in Jimma University, Ethiopia. Ethiop J Health Dev (2003) 17:181–8.
42. Alemu SM, Habtewold TD, Haile YG. Mental and reproductive health correlates of academic performance among Debre Berhan university female students, Ethiopia: the case of premenstrual dysphoric disorder. Biomed Res Int (2017) 2017:8. doi: 10.1155/2017/9348159
43. Zeleke B, Workineh Y, Melese A, Semachew A, Yigizaw M. Premenstrual syndrome, life style & behavioral coping mechanisms and associated factors among public high school regular female students at Bahir Dar City, Northwest, Ethiopia. Research Square [preprint] (2023). doi: 10.21203/rs.3.rs-2418487/v1
44. Minichil W, Eskindir E, Demilew D, Mirkena Y. Magnitude of premenstrual dysphoric disorder and its correlation with academic performance among female medical and health science students at University of Gondar, Ethiopia, 2019: a cross-sectional study. BMJ Open (2020) 10:e034166. doi: 10.1136/bmjopen-2019-034166
45. Eshetu N, Abebe H, Fikadu E, Getaye S, Jemal S, Geze S, et al. Premenstrual syndrome, coping mechanisms and associated factors among Wolkite university female regular students, Ethiopia. BMC Womens Health (2022) 22:22:88. doi: 10.1186/s12905-022-01658-5
46. Abeje A, Berhanu Z. Premenstrual syndrome and factors associated with it among secondary and preparatory school students in Debremarkos town, North-west Ethiopia. BMC Res Notes (2016) 2019:12:1–5.
47. Tsegaye D, Getachew Y. Premenstrual dysphoric disorder and associated factors among female health science students in Wollo University, Ethiopia, 2017/18. Matern Health Neonatol Perinatol (2019) 5:1–8. doi: 10.1186/s40748-019-0102-z
48. Mossie TB, Tesfaye YB, Metekiya WM, Tegegne MT. Magnitude of premenstrual dysphoric disorder and associated factors among high school girls, Mekelle, North Ethiopia. (2015) 29.
49. Nworie KM, Aluh DO, Onyekwum CA. Assessment of premenstrual syndrome among female students in Southeast Nigeria. J Obstet Gynecol Investig (2018) 1:55–61. doi: 10.5114/jogi.2018.79426
50. Antai AB, Udezi AW, Ekanem EE, Okon UJ, Umoiyoho AU. Premenstrual syndrome: prevalence in students of the University of Calabar, Nigeria. Afr J Biomed Res (2004) 7(2). doi: 10.4314/ajbr.v7i2.54067
51. Atim E, Okecho FN, Ndagire R, Nassozi CL. Prevalence and severity of premenstrual syndrome among female university students in central Uganda: A cross-sectional study. Stud J Health Res Afr (2022) 3:10–0.
53. Kamel DM, Tantawy SA, Alsayed N, Bekhet AH, Elbkery N, Khairy A. The relationship between premenstrual syndrome and the quality of sleep among Egyptian women: An observational study. (2021) 56:172–8. doi: 10.31688/ABMU.2021.56.2.05
Keywords: Africa, prevalence, meta-analysis, premenstrual syndrome, PMS, systematic review
Citation: Andualem F, Melkam M, Takelle GM, Nakie G, Tinsae T, Fentahun S, Rtbey G, Seid J, Gedef GM, Bitew DA and Godana TN (2024) Prevalence of premenstrual syndrome and its associated factors in Africa: a systematic review and meta-analysis. Front. Psychiatry 15:1338304. doi: 10.3389/fpsyt.2024.1338304
Received: 14 November 2023; Accepted: 09 January 2024;
Published: 31 January 2024.
Edited by:
Karen Tabb, University of Illinois at Urbana-Champaign, United StatesReviewed by:
Alfredo Briones-Aranda, Autonomous University of Chiapas, MexicoSarya Swed, University of Aleppo, Syria
Copyright © 2024 Andualem, Melkam, Takelle, Nakie, Tinsae, Fentahun, Rtbey, Seid, Gedef, Bitew and Godana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fantahun Andualem, ZmFudGFhbmR1MjdAZ21haWwuY29t
 Fantahun Andualem
Fantahun Andualem Mamaru Melkam
Mamaru Melkam Girmaw Medfu Takelle
Girmaw Medfu Takelle Girum Nakie
Girum Nakie Techilo Tinsae
Techilo Tinsae Setegn Fentahun
Setegn Fentahun Gidey Rtbey
Gidey Rtbey Jemal Seid2
Jemal Seid2 Getachew Muluye Gedef
Getachew Muluye Gedef Tilahun Nega Godana
Tilahun Nega Godana