- 1Department of Psychiatry, University of Hong Kong, Hong Kong SAR, China
- 2Janssen Medical Affairs Asia Pacific, Singapore, Singapore
- 3Department of Psychiatry, Depression Center, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea
- 4Department of Psychiatry & Behavioral Science, Juntendo University Graduate School of Medicine, Tokyo, Japan
- 5The Melbourne Clinic, Department of Psychiatry, University of Melbourne, Melbourne, VIC, Australia
- 6Department of Psychiatry, National Cheng Kung University Hospital, Tainan, Taiwan
- 7National Clinical Research Center for Mental Disorders & Mood Disorders Center, Beijing Anding Hospital, Capital Medical University, Beijing, China
Background: Anhedonia, a core diagnostic feature for major depressive disorder (MDD), is defined as the loss of pleasure and interest in daily activities. Its prevalence in MDD patients vary from 35 to 70%. Anhedonia in MDD negatively impacts functioning and is associated with treatment resistance and poorer prognosis for various clinical outcomes. Owing to its complexity, there remains considerable heterogeneity in the conceptualization, diagnosis and clinical management of anhedonia in MDD.
Methods: This modified Delphi panel was conducted to elicit expert opinion and establish consensus on concepts relating to clinical features, diagnosis and treatment of MDD with anhedonia (MDDwA) amongst psychiatrists in the Asia-Pacific region. Seven themes were covered. A three-stage process was adopted for consensus generation (two online survey rounds, followed by a moderated consensus meeting). Statements were developed based on a literature review and input from a steering committee of six regional experts. The panel included 12 psychiatrists practicing in Australia, China, Hong Kong, Japan, South Korea and Taiwan with ≥5 years of specialist clinical experience, including assessment or management of patients with MDDwA.
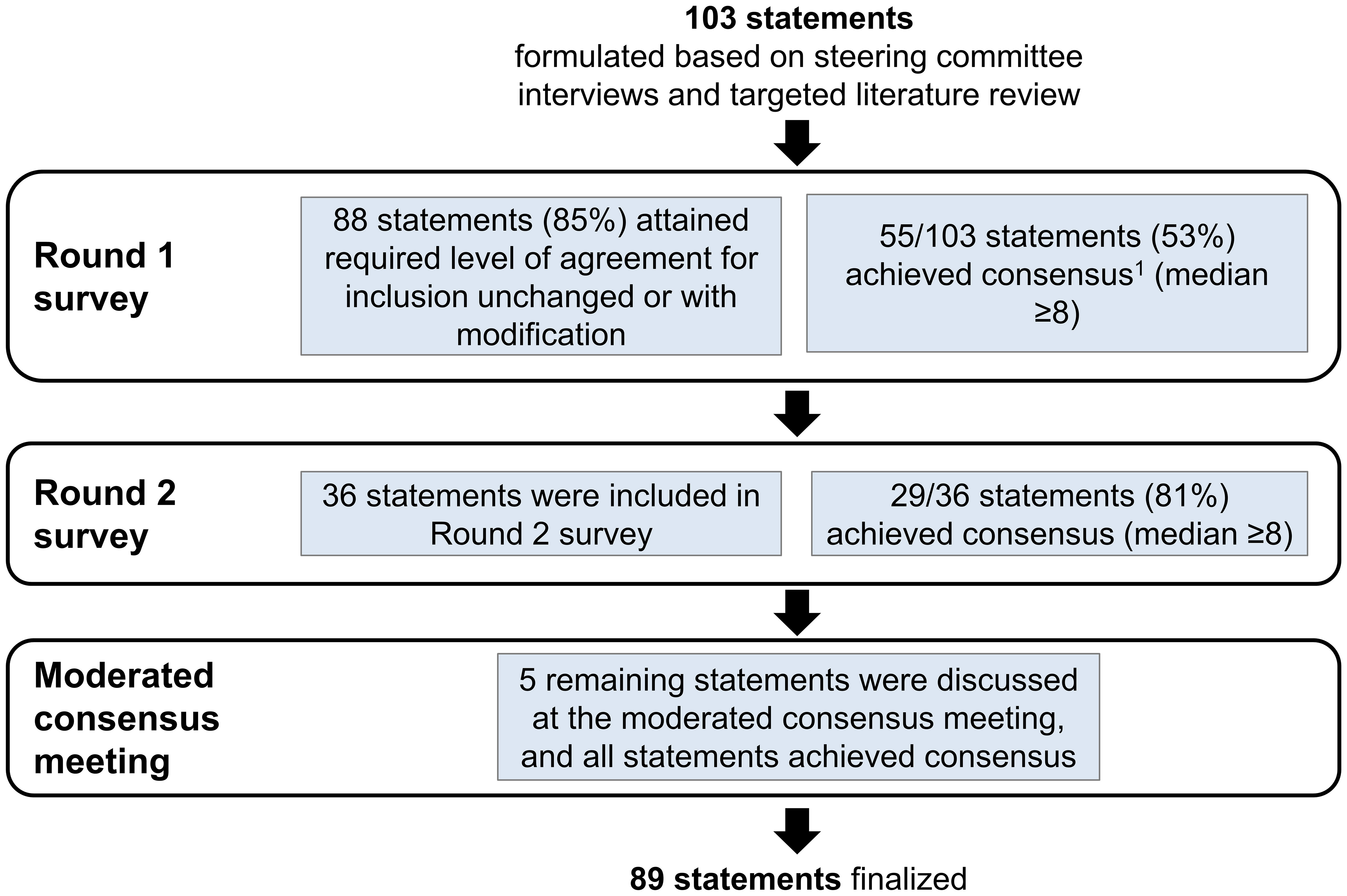
Results: Overall, consensus was achieved (median ≥8) on 89/103 statements (86%). About half of the statements (55/103, 53%) achieved consensus in Round 1, and 29/36 modified statements achieved consensus in Round 2. At the moderated consensus meeting, five modified statements were discussed by the steering committee and consensus was achieved on all statements (5/5). The findings highlighted a lack of clear and practical methods in clinical practice for assessing anhedonia in MDD patients and limited physician awareness of anhedonia in Asia-Pacific.
Conclusion: Insights from this Delphi consensus provide a reference point for psychiatrists in Asia-Pacific to optimize their strategies for personalized diagnosis and management of patients with MDDwA. Identification of distinct and clinically relevant subtypes in MDD may be valuable for guiding personalized diagnosis and management approaches, including type-specific therapies.
1 Introduction
Major depressive disorder (MDD) affects a substantial and growing number of individuals worldwide (estimated 280 million people) and is a leading cause of morbidity and disability (1–3). MDD imposes a substantial burden on individuals, adversely affecting their daily functioning and quality of life (4–6). According to the Diagnostic and Statistical Manual of Mental Disorders (5th edition; DSM-5), a diagnosis of MDD requires at least five depressive symptoms, one of which must be depressed mood or anhedonia (the loss of pleasure or interest in daily activities), associated with a change from previous functioning (7). Although anhedonia is considered a core diagnostic feature of MDD, there is considerable heterogeneity in how the concept is defined and operationalized and consequently in how it is diagnosed and managed (8, 9). Anhedonia in MDD has been described as a symptom complex that encompasses overlapping concepts and various deficits in reward processing and positive affect. This presents notable challenges for diagnosis; indeed, estimates in the literature of anhedonia prevalence in MDD patients range from 35–70% (10, 11).
The impact on patients and clinical burden associated with anhedonia in MDD are substantial but often overlooked. Notably, anhedonia adversely affects psychosocial functioning, which can reduce the likelihood of achieving remission in MDD patients (12). In addition, anhedonia is a predictor of poor antidepressant treatment outcomes (13) and poorer prognosis for various clinical outcomes, regardless of depression severity (9). Moreover, a recent study found that in patients with mood disorders, including MDD, anhedonia was strongly correlated with quality of life (QoL): more severe anhedonia was associated with poorer physical and mental health-related QoL, and reduced life enjoyment and satisfaction (14). However, anhedonia in MDD often proves difficult to treat, as most conventional MDD pharmacotherapies do not adequately address anhedonia symptoms. Some commonly used first-line selective serotonin reuptake inhibitors (SSRIs) may even have a negative impact as they are known to blunt responses to rewarding stimuli (15).
Given the importance of anhedonia in MDD diagnosis and treatment, there has been increased interest and progress in reconceptualizing and assessing anhedonia, with the aim of addressing practical diagnostic and management challenges. Even so, findings from preclinical and clinical studies have been inconsistent, hindering translation to clinical practice (8, 16). Current anhedonia-specific assessment tools (symptom scales and behavioral tasks) reflect a variety of conceptualizations [reviewed in (8)]. For example, the Snaith-Hamilton Pleasure Scale (SHAPS), Fawcett-Clark Pleasure Capacity Scale, and Dimensional Anhedonia Rating Scale (DARS) assess different dimensions of anhedonia, and not all are specific to depression (17–19). Other depression-specific instruments for assessing symptom severity, such as the Hamilton Depression Rating Scale (HAM-D), include only a limited number of items for anhedonia. It has been proposed that the use of symptom scales alongside behavioral methods and biomarkers (if these can be identified) would enable a comprehensive assessment of the range of possible deficits associated with anhedonia in MDD (8, 16). On the other hand, it remains unclear how these insights are best applied in clinical settings. Nevertheless, defining distinct subtypes of MDD (e.g., based on biological variables and neuroimaging alongside clinical symptoms) is viewed as a promising strategy to improve diagnosis and management, as well as to predict the disease course (20, 21).
Issues such as diagnostic and symptom heterogeneity, along with the paucity of suitable clinical tools and specific guidelines, may contribute to substantial regional variability in the diagnosis and management of MDD with anhedonia (MDDwA). A modified Delphi panel study was therefore conducted to elicit expert opinion amongst psychiatrists in the Asia-Pacific region and establish consensus on concepts relating to clinical features, diagnosis, and treatment of MDDwA.
2 Methods
2.1 Modified Delphi process for consensus generation
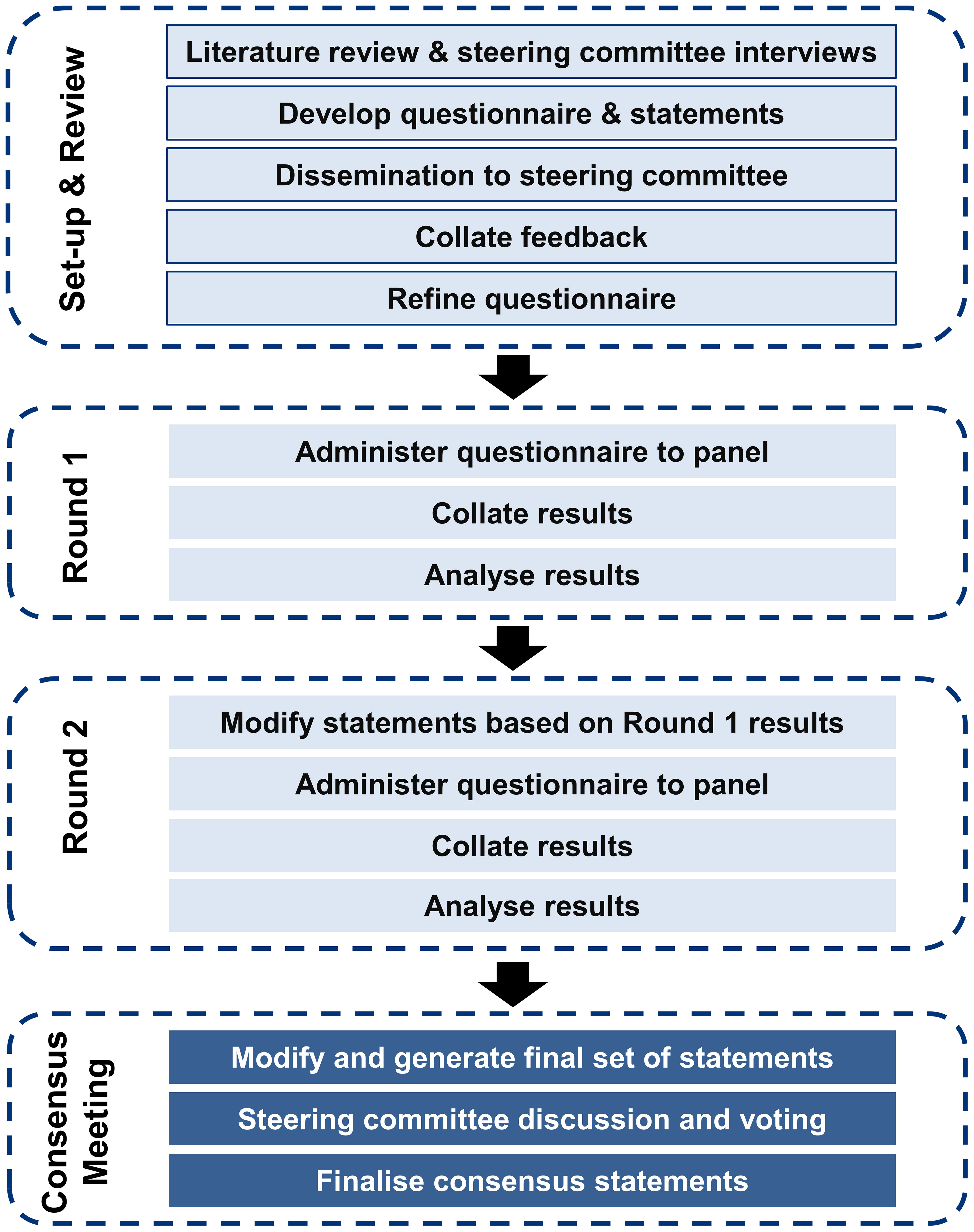
The Delphi method is a systematic and structured approach for identifying the consensus views of a group of experts in areas where the available evidence is limited or conflicting. An iterative process is followed, involving multiple rounds of surveys completed independently by a panel of experts. After each round, the experts review the collated anonymized responses and may revise their responses until consensus is reached based on pre-defined criteria. This method has been used to generate consensus among experts on topics such as the definition and assessment of treatment-resistant depression and culturally-relevant adaptation of mental health first-aid guidelines (22, 23). A modified Delphi panel study was conducted to generate consensus on research questions related to anhedonia in MDD among psychiatrists practicing in the Asia-Pacific region. The study involved four phases: panel identification and recruitment, questionnaire development, survey data collection and analysis (two rounds), followed by a moderated meeting to finalize the consensus statements. Figure 1 illustrates the modified Delphi process for consensus generation. A steering committee of six regional experts (the authors of this work) provided oversight and input on study design, questionnaire development, and panel eligibility criteria and/or nomination. Details of the modified Delphi process are provided in the Supplementary Methods.
2.2 Identification and recruitment of Delphi panel participants
The panel comprised 12 experienced psychiatrists currently practicing in Australia, China, Hong Kong, Japan, South Korea, or Taiwan. Psychiatrists meeting the following criteria were identified: currently involved in diagnosis, treatment and management of MDD patients with anhedonia; at least five years of experience and expertise in diagnosing, treating and managing MDD patients with anhedonia; average monthly caseload of at least 20 patients suspected or confirmed to have MDD; average monthly caseload of at least ten patients suspected or confirmed to have MDD with anhedonia; possess experience of journal/conference authorship, academic, guideline development or clinical leadership related to MDDwA. Of 293 potential participants identified across the six countries/territories according to the eligibility criteria, 18 were found to meet these criteria. Only participants who were able and willing to complete both rounds of the study were recruited. The panel included 12 psychiatrists (two per country/territory) in total.
2.3 Survey questionnaire development and consensus classification
A targeted literature review was conducted on the epidemiology and best-practice recommendations for the diagnosis and management of MDDwA in the Asia-Pacific region. The literature search strategy (Supplementary Table 1) was designed to identify publications on these topics: current opinion on the definition of anhedonia in MDD or diagnosis of patients with MDDwA; current treatment options for patients with MDDwA; opinions on the diagnosis and treatment of patients with MDDwA; clinical guidelines specific to MDDwA. The information retrieved and literature gaps identified (Supplementary Table 2) were used to guide questionnaire development for the Round 1 survey.
A set of statements for the Round 1 survey were developed based on the literature review and input from the steering committee on clinical accuracy, comprehensibility, and feasibility for use in the online survey. The statements covered themes related to MDDwA, including: prevalence; risk factors; clinical definitions; diagnosis; treatment and management; disease impact and physician perspectives on novel therapy options. Each statement was to be scored by panel participants according to a 9-point Likert scale, which quantifies how strongly the participant agrees/disagrees with the statement (1–3, strongly disagree; 4–6, neither agree or disagree; 7–9, strongly agree). A free-text response/rationale was requested whenever a participant gave a statement a rating below 7 (neither agree or disagree; strongly disagree). For multiple-option questions, participants were required to select any options they considered applicable. For two such options, ‘None of the above (please provide rationale)’ and ‘Others (please specify)’, participants were required to provide input as free text. Responses to open-ended questions were also provided as free text. The free-text responses collected in both survey rounds were used to guide edits to improve clarity, merge or modify statements, and formulate additional statements or questions for consideration.
2.4 Data collection and analysis
The process of consensus generation is summarized in Figure 1. After providing consent to participate in the study, each panelist received a personalized email link to a password-protected electronic survey platform. Panelists were asked to complete the surveys independently and remained blinded to the identities of other panelists and the steering committee throughout the process. For both Rounds 1 and 2, panelists were asked to provide their responses within one week, with email reminders as needed. Responses from all 12 panelists were required in order to proceed with Round 2.
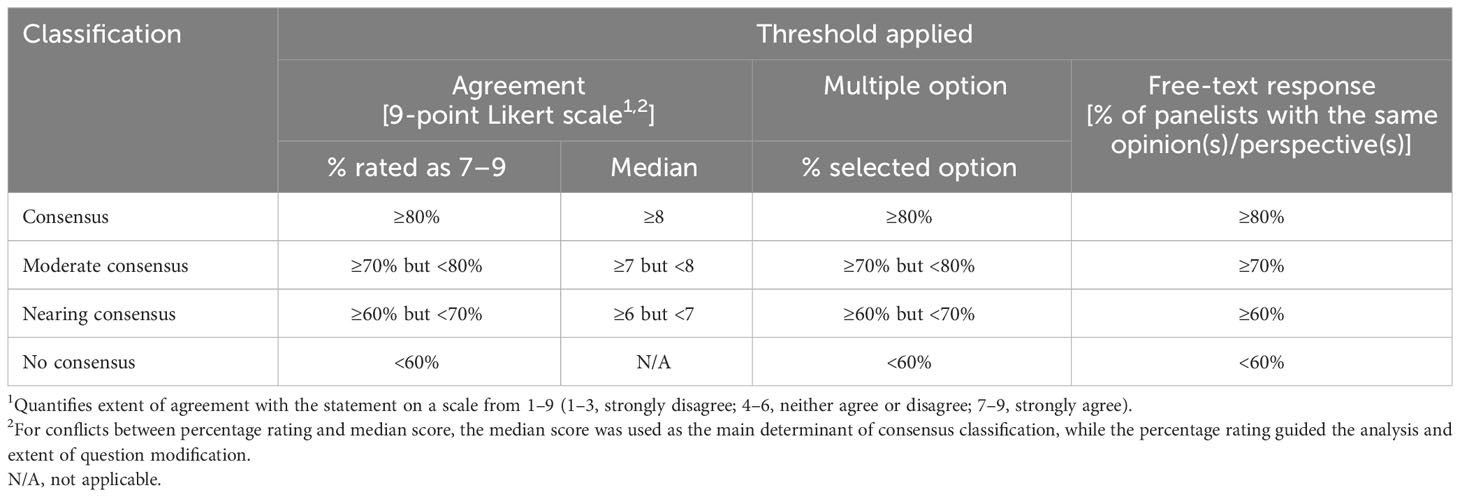
Descriptive statistics were used to summarize and analyze the response data (e.g., mean, median, standard deviation [SD] and interquartile range [IQR] of statement ratings, counts and proportions of participants selecting each response option). The consensus classification for each statement was determined according to the pre-defined criteria in Table 1.
In Round 1, statements that achieved consensus (median score ≥8) were included in the final list of consensus statements. Statements with a median rating of ≥6 but <8 were modified for presentation in Round 2 based on input from the panel, which was subsequently validated by the steering committee. Statements that did not achieve consensus (median score <6) were removed from the set of consensus statements. However, depending on clinical importance, selected few statements were retained as key insights for discussion during the moderated consensus meeting. In the Round 2 survey, panelists were shown the anonymized and consolidated Round 1 results and were asked to score the modified statements derived from Round 1.
2.5 Moderated consensus meeting
After both survey rounds were completed, a moderated virtual meeting was held to facilitate interactive discussion within the steering committee to ratify the findings and identify areas of consensus and other issues of clinical importance (Figure 1). Consolidated responses and analyses from Rounds 1 and 2 were presented to allow the steering committee to vote on statements that did not achieve consensus in Round 2. Voting utilized real-time anonymous electronic polling functionalities. A final report summarizing the objectives, methodology, final consensus statements, and conclusions was developed and disseminated to the steering committee and the expert panel.
3 Results and discussion
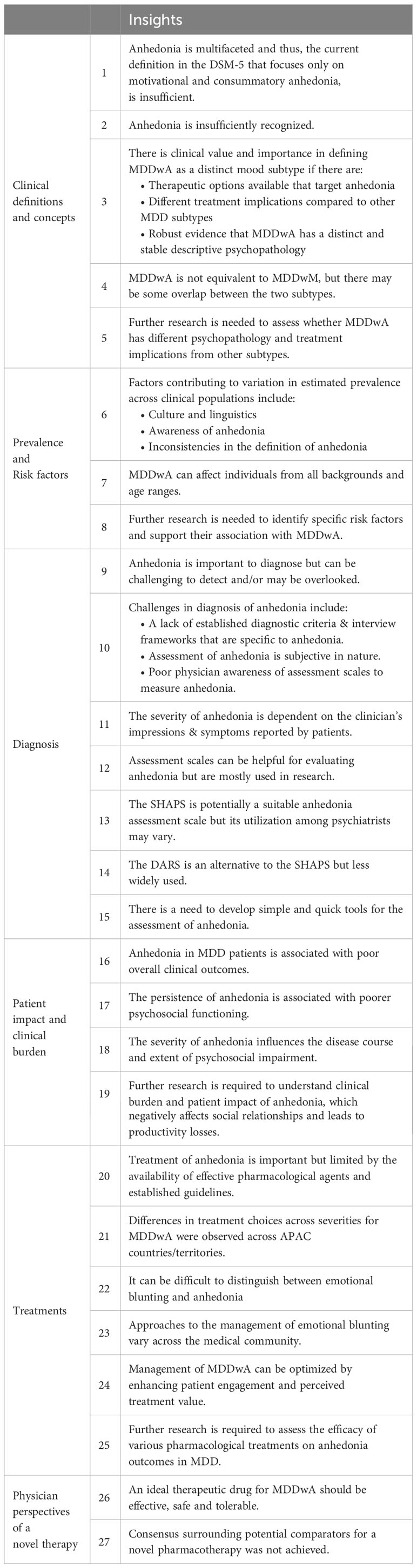
A total of 103 statements were presented to the panelists for scoring. Overall, consensus was achieved (median ≥8) on 89 statements (86%). About half of the statements (55/103, 53%) achieved consensus in Round 1 (Figure 2). In Round 2, a further 29 statements achieved consensus, out of 36 modified statements presented to the panelists for scoring (29/36). At the moderated consensus meeting, five modified statements were discussed by the steering committee and consensus was achieved on all statements (5/5). The statements are summarized according to the following thematic areas: clinical definitions and concepts (Supplementary Table 3), prevalence (Supplementary Table 4), risk factors (Supplementary Table 5), patient impact and clinical burden (Supplementary Table 6), diagnosis (Supplementary Table 7), treatment (Supplementary Table 8), and physician perspectives on novel therapies (Supplementary Table 9). The key insights from this study are presented in Table 2.
3.1 Clinical definitions and concepts
The statements that achieved consensus in this thematic area are summarized in Supplementary Table 3. Most panelists agreed with the existing DSM-5 definition of anhedonia (second criteria A-2) as markedly diminished interest or pleasure in daily activities (7), and considered anhedonia a core feature of MDD (S1 and S2). However, the panelists concurred that anhedonia is multifaceted and there are still inconsistencies in the definition of anhedonia in MDD used amongst the medical community (S3). The panelists highlighted that the definition in the DSM-5 insufficiently reflects advances in the understanding of anhedonia (9, 10, 24). The panel also agreed that the definition of anhedonia in MDD should be expanded to encompass reduction in hedonic function including loss of interest and satisfaction in activities previously considered pleasurable and diminished motivation and desire to pursue pleasure or activities that generate pleasure (S4). Other facets of hedonic function were presented for consideration, but these did not achieve consensus (i.e., only half of the panelists [50%] selected the options of ‘paralysis of the sense of wellbeing’ and ‘paralysis of feeling’). Notably, the panelists agree that anhedonia is insufficiently recognized due to a lack of physician awareness of the nature, importance and impact of anhedonia (S5). One expert pointed out that although anhedonia is listed in the DSM-5 as a core diagnostic criterion, the lack of explicit mention of anhedonia symptoms could contribute to its insufficient recognition relative to other symptoms that are clearly listed.
3.2 Prevalence and risk factors
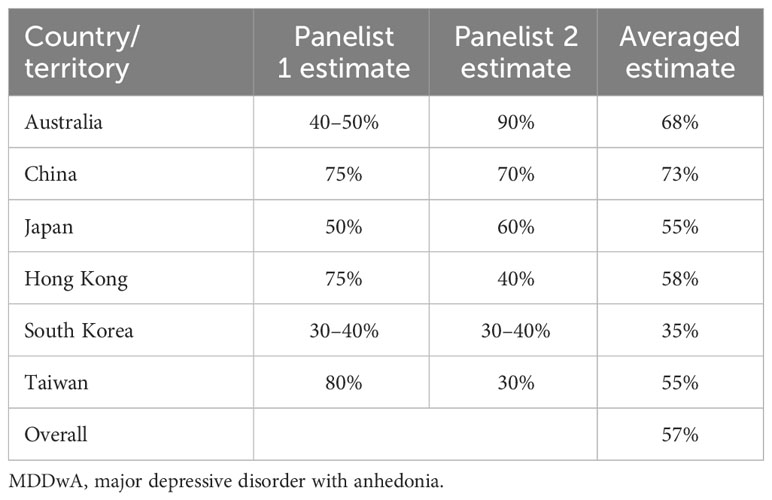
The statements that achieved consensus in these thematic areas are summarized in Supplementary Tables 4 and 5. There was consensus that anhedonia is one of the most frequent symptoms of depression (S16) and most panelists estimated that at least 40% of their MDD patients present with anhedonia, albeit with a wide range of estimates (S17; Table 3). This is consistent with published literature wherein estimates range from around 35–70% (10, 11). In the consensus meeting, one proposed explanation for the wide variation across countries/territories in estimated anhedonia prevalence was differences in the level of awareness and understanding of anhedonia, which could be in part due to differences in language and culture. For example, in Japan, the lack of a direct translation of the term “anhedonia” in Japanese and connotations of sexual anhedonia may result in under-reporting by patients. It was suggested that, if physicians in general were more aware of anhedonia in MDD, they would routinely ask their patients targeted questions specific to the various facets of anhedonia during consultations. The contribution of insufficient patient awareness to under-reporting of incidence of anhedonia in MDD patients was also discussed. For example, in Hong Kong, local mental health promotion activities largely focus on low mood, suicidal ideation and functional performance in MDD, overlooking anhedonia; this can lead to under-reporting due to low awareness of anhedonia. In summary, inconsistencies in definitions and diagnostic criteria for anhedonia may contribute to the variable prevalence of anhedonia estimated within the Asia-Pacific region.
In the consensus meeting, it was agreed that MDDwA affects individuals from all backgrounds and age ranges, and risk factors can include family history of depression, chronic health stressors and life events (S18, S19 and S21). However, it was noted that many of these are general risk factors for MDD and literature on identifying specific risk factors with MDDwA is scarce; thus, it was concluded that further research is needed to identify risk factors specific to MDDwA (S22).
3.3 Clinical impact and burden
The statements that achieved consensus in this thematic area are summarized in Supplementary Table 6. The panelists concurred that anhedonia is associated with poor overall clinical outcomes in MDD patients (S49 to S52). The panelists also agreed that anhedonia severity influences the disease course and extent of psychosocial impairment (S53, S59, and S61). This is in line with reported literature that more severe anhedonia is associated with the extent of depressive symptoms, illness chronicity and reduced psychosocial functioning (9, 11–13, 25). MDD patients with anhedonia may have an inherently higher likelihood of treatment non-adherence, due to deficits in motivation to engage in activities such as treatments and to anticipate positive outcomes [S54] (13). The panelists agreed that patients with anhedonia were more likely to stop their treatments prematurely.
3.4 Diagnosis
The statements that achieved consensus in this thematic area are summarized in Supplementary Table 7. Panelists agreed that anhedonia is challenging to detect and/or may be overlooked during diagnosis (S23 and S25). Several barriers to accurate diagnosis were highlighted.
First, established diagnostic criteria and interview frameworks that are specific to anhedonia are lacking (S24). The panel highlighted that although the DSM-5 provides some guidance, this is markedly limited and establishing well-defined criteria can guide diagnosis of anhedonia in clinical practice. A targeted literature review conducted as part of the study identified no clinical guidelines specific to anhedonia in MDD (Supplementary Table 1).
Second, anhedonia assessment is subjective, depending on both the physician’s skill and awareness, and the patient’s ability to articulate their feelings and/or experiences. Panelists agreed that diagnosis is based on good patient history taking by the physician, asking specific and targeted questions about anhedonia symptoms, and being perceptive to notice discrepancies between patients’ reported symptoms and behavior/signs elicited during interviews (S37 and S39). For patients, their ability to articulate their feelings and/or subjective experiences strongly influences whether anhedonia is identified during consultation (S40), as well as the physician’s impressions of anhedonia severity (S43).
Third, although a number of anhedonia assessment scales are available (e.g., SHAPS, DARS), the panelists agreed that the use of these is mostly confined to research (S28 to S30). They are not often used clinically, and physician awareness of these scales is poor. The SHAPS (a 14-item, self-reported questionnaire specifically assessing hedonic capacity) has been considered the gold standard for assessment of anhedonia (17). The SHAPS assesses anhedonia across four consummatory pleasure domains (hobbies/pastimes, food/drinks, social activities, and sensory experiences) using examples, but lacks assessment of anticipatory pleasure (S48). The DARS is a ‘second generation’ self-report scale designed to assess anhedonia across additional dimensions to pleasure (reward, interest, motivation, effort and pleasure) identified through principal component analysis (18). The 17 items include the same four pleasure domains as SHAPS but were designed to increase generalizability by asking respondents to provide their own examples for rating. Although the DARS is a more comprehensive scale to measure anhedonia, most of the panelists (up to 92%) were insufficiently familiar with it, and it is less widely used in clinical practice than the SHAPS (S45 to S47). Notably, despite high generalizability reported within western populations (18, 26–29), the SHAPS and DARS may lack cultural validity, particularly for Asian populations. In view of the significant variation in the understanding and expression of emotions across different cultures (30), non-English versions of the DARS (Chinese, German, Polish and Spanish) have been constructed and validated for use (31–34). The challenges described above may be compounded by the varied clinical definitions of anhedonia in use (8, 35).
A major limitation of the SHAPS and DARS highlighted by the panelists is the time taken for completion. Since anhedonia is a transdiagnostic symptom dimension present in multiple conditions (e.g., MDD, schizophrenia, bipolar disorder), comprehensive assessment scales such as the HAM-D (Hamilton Depression Rating Scale) and MADRS (Montgomery-Åsberg Depression Rating Scale) were seen as offering more time-effective alternatives as they measure a wider range of symptoms within a single test (S31). Overwhelmingly, panelists reported that they currently use MADRS (92%) and HAM-D (83%) when diagnosing anhedonia in MDD; only 42% use SHAPS and 25% use DARS. These findings emphasize the need to develop simple and quick tools for anhedonia assessment for clinical practice (S26) that measure the different facets of anhedonia, distinguish anhedonia from related constructs, and are generalizable for use across different cultures and populations (S27).
3.5 Treatment and clinical management
The statements that achieved consensus in this thematic area are summarized in Supplementary Table 8. Although the panelists agreed that it is important to treat anhedonia (S67), one of the challenges in treatment is the limited availability of effective pharmacotherapies and the lack of standardized, established management guidelines specific to anhedonia in MDD patients (S68 and S69). There are no pharmacotherapies specifically approved for treatment of anhedonia in MDD patients, although some agents (e.g., vortioxetine, agomelatine, bupropion) have been used (S71). In the consensus meeting, it was highlighted that selecting appropriate treatments based on the mechanism of action of various facets of anhedonia present in the patient can aid in predicting and improving treatment outcomes. Interestingly, a recent systematic review of 17 clinical studies concluded that most pharmacotherapeutic agents for MDD were associated with improvement in measures of anhedonia to varying degrees, except for escitalopram/riluzole combination treatment (36). However, due to the small number of studies and the heterogeneity of study designs and samples across the studies, further research is required to confirm these findings.
The panel’s responses indicate that treatment options in use across the Asia-Pacific region vary considerably, especially with respect to anhedonia severity. This is because prescribing choices are determined by local regulatory approval status and availability, cost/reimbursement policies, and national or regional MDD management guidelines and habits in clinical practice to some degree (37). Consensus was not achieved on potential treatment options for MDD patients with mild anhedonia, although these may include bupropion (150–300 mg), if available, and adjunctive cognitive behavioral therapy (S82). However, consensus was reached on potential treatment options for moderate and severe anhedonia in MDD patients. The panel concurred that treatment options for moderate anhedonia in MDD may include adjunctive aripiprazole (5–10 mg), adjunctive CBT and adjunctive rTMS (S83); and may include adjunctive aripiprazole, adjunctive mood stabilizers (e.g., lithium), adjunctive CBT and adjunctive rTMS for treatment of severe anhedonia in MDD (S84). Additionally, the panel agreed that third-line management options to treat severe anhedonia in MDD include adjunctive ECT (S85). Although a general consensus on treatment options for anhedonia in MDD patients was not achieved, it was agreed that management of MDDwA can be optimized through patient engagement and enhancing patients’ perception of the value of treatment (S86 to S89). Further research is required to assess the efficacy of various pharmacological treatments for anhedonia in MDD patients (S72 and S73).
Emotional blunting, defined as the inability to experience both positive and negative emotions, is present in several psychiatric disorders, and is described as a common side effect of antidepressants, particularly SSRIs, in MDD patients. Approximately half of MDD patients taking monoaminergic antidepressants reported experiencing emotional blunting [S76 (38–41);]. Over half of the panelists (58%) estimated that 30–49% of patients treated with SSRIs/SNRIs report some degree of emotional numbness or blunting. There was consensus that chronic SSRI administration may induce emotional blunting as a side effect (S80). Notably, this has implications for evaluating the effectiveness of SSRIs for treating MDD if anhedonia is present. It may be difficult to distinguish between emotional blunting as a side effect and residual anhedonia after treatment, and additional time and evaluation may be required to differentiate between the two (S74 and S75). As some patients may experience emotional blunting with SSRIs, whereas others may benefit from treatment without experiencing this side effect, there is still debate about the efficacy of SSRIs in treating anhedonia in MDD (S76). For patients experiencing emotional blunting on SSRI therapy, switching to a different agent may be an option, as observed in a recent study in MDD patients that showed positive effects on emotional blunting and other MDD symptoms after switching to vortioxetine (42).
Patients with MDD often have one or more physical or psychiatric comorbidities, such as cardiometabolic disorders, chronic pain-related conditions, or substance addiction, which may be associated with greater MDD severity and worse treatment outcomes [reviewed in (43–45)]. For example, MDD patients with higher insulin resistance showed more pronounced depressive symptomatology, including anhedonia, than those with lower insulin resistance (46). For several conditions, there is also considerable evidence of bidirectional effects [reviewed in (47)], underscoring the importance of managing both MDD/anhedonia and any comorbidities. The presence of comorbid conditions also increases the complexity and challenges of clinical management, such as communication/coordination between treating physicians and teams to manage each condition, and greater attention to drug-drug interactions and adverse side effects from polypharmacy.
3.6 Physician perspectives on novel therapies for MDDwA
The statements that achieved consensus in this thematic area are summarized in Supplementary Table 9. An ideal novel pharmacotherapy for MDDwA should effectively target anhedonia alongside other MDD symptoms, support physical, social, cognitive, and functional recovery, and should have a good safety and tolerability profile (S90). Although consensus was not achieved on suitable comparators for head-to-head trials of novel pharmacotherapy agents, a number of commonly prescribed agents (e.g., monotherapy escitalopram, adjunctive intranasal esketamine, monotherapy agomelatine, monotherapy vortioxetine, adjunctive aripiprazole and adjunctive bupropion) were mentioned as potentially useful comparators (S92). For example, a recent pooled analysis reported that agomelatine and vortioxetine showed significant short-term efficacy for anhedonia (48). The experts’ feedback emphasized that novel pharmacotherapies should demonstrate superiority for anhedonia and similar efficacy for MDD overall, with respect to existing treatment options (S92).
3.7 MDDwA as a distinct subtype of depression
Importantly, there was clearly perceived clinical value in establishing MDDwA as a distinct MDD subtype to optimize treatment/management and to develop targeted treatments that may increase response and remission rates (S8). Establishing MDDwA as a distinct subtype was considered reasonable if: there was robust evidence for a distinct and stable descriptive psychopathology; treatment implications differ from other MDD subtypes; and type-specific therapeutic options are available (S9).
Most of the panel (82%) supported the establishment of MDDwA as a distinct subtype to inform treatment decision-making and identification of subgroups, such as the choice of treatment if anhedonia is present (S12). The current challenge in delineating MDDwA as a distinct subtype may lie in distinguishing it from MDD with melancholia (MDDwM), since anhedonia has been proposed as one of the markers of MDDwM (49). On the other hand, recent data-driven approaches to define MDD subtypes using neurobiological and clinical features [reviewed in (50)] point to the relevance of anhedonia in defining MDD subtypes, besides being a core diagnostic feature. In one study, patterns of functional network connectivity and clinical symptoms were used to identify two putative subtypes, an insomnia-dominated subtype and an anhedonia-dominated subtype (21). Another study identified four putative subtypes with distinct patterns of functional connectivity, each associated with specific profiles of clinical symptoms. Of these four subtypes, two were characterized by increased anhedonia and psychomotor retardation, and two by increased anxiety and fatigue (51). The prevailing view among the panelists was that MDDwA differs from MDDwM in several ways (S13). MDDwM has a distinct and characteristic symptom cluster (i.e., generalized psychomotor retardation, weight loss/loss of appetite, early morning waking, excessive guilt, sense of hopelessness and diurnal variation (7)) that is absent in MDDwA. Patients with MDDwM tend to present with more severe affect and symptoms along with difficulty in mood elevation, compared with MDD patients with anhedonia. There are also reported differences in the brain areas involved, mainly the frontal area for MDDwM patients, and reward-related brain areas (e.g., insula, anterior cingulate cortex, orbitofrontal cortex) in MDD patients with anhedonia (S13).
3.8 Strengths and limitations of this work
The modified Delphi process provided a suitable means of exploring this complex topic, given the diverse models and conceptualizations of anhedonia in MDD, lack of gold-standard anhedonia-specific practice guidelines, and variability in clinical practice. A targeted literature research also revealed a paucity of research and quantitative data for the region, reinforcing the value of an expert panel for generating insights. Another strength of this study is expert representation from countries/territories across the Asia-Pacific region within both the steering committee and the panelists. This allowed variations in local diagnostic and treatment practice to be captured, as well as providing insights into potential reasons for the wide range of estimated prevalence of anhedonia across countries/territories.
One limitation of the study is the relatively small number of panelists (n=12) included. Researchers have proposed optimum panel sizes ranging from 10 to 50, while also acknowledging that this number depends on factors such as the target topic, degree of panel homogeneity, the expected amount of data generated per participant, and the resources available (52, 53). In addition to the data generated from the 12 expert panelists’ responses, it was considered that the involvement of a steering committee comprising six additional experts would enable adequate alignment on the consensus statements and non-consensus insights retained for discussion. We acknowledge that a larger and more diverse panel of practitioners, perhaps representing additional countries/territories within the Asia-Pacific, as well as a greater range of practice settings, would better capture the variation that exists in health systems.
3.9 Future work
The present work underscores the lack of clear and practical methods in clinical practice for assessing anhedonia in MDD patients, with implications for both diagnosis and management. Within the Asia-Pacific region, poor physician awareness of anhedonia was identified as a prominent unmet need. With a variable but likely underestimated prevalence of anhedonia in MDD patients (35–70% as estimated by panelists), attention and awareness among both physicians and patients is urgently warranted. The panel agreed that further research is needed to clarify and refine the definitions and concepts in use and distinguish anhedonia from other symptom domains in MDD. This would be complemented by ongoing efforts to elucidate the neurobiological mechanisms underlying anhedonia. Within the region, initiatives to assess anhedonia prevalence in a more standardized way and quantify its impact on treatment adherence and outcomes could help promote awareness among physicians and patients, and potentially reduce delays in diagnosis and treatment. One limitation is the lack of availability of quantitative data in the region and hence, future work should prioritize quantitative studies of the prevalence and impact of anhedonia in MDD patients across the region. We also note that, in a culturally diverse region such as the Asia Pacific, educational initiatives for physicians and patients should be developed with the local sociocultural context and patient population in mind. All of these initiatives must be supported by efforts to evaluate and develop treatment options with greater efficacy specifically for anhedonia than existing treatments but similar efficacy for MDD overall, and with less likelihood of side-effects such as emotional blunting.
4 Conclusion
Identification of distinct and clinically relevant subtypes in MDD may be valuable for guiding personalized diagnosis and management approaches, including type-specific therapies. Given the importance of anhedonia in MDD diagnosis, prognosis, and treatment decision-making, recognizing a distinct depressive subtype characterized by anhedonia could help improve clinical management and guide the development of new treatment options. While acknowledging that considerable conceptual and mechanistic research is still needed, the study highlighted a fundamental need for simple and accurate tools to assess anhedonia in routine practice settings. Insights from this Delphi consensus provide a reference point for psychiatrists in the Asia-Pacific to optimize their strategies for personalized diagnosis and management of patients with MDDwA.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
CC: Writing – review & editing, Conceptualization. KH: Writing – review & editing, Conceptualization, Methodology. HJ: Conceptualization, Writing – review & editing. TK: Conceptualization, Writing – review & editing. CN: Conceptualization, Writing – review & editing. YY: Conceptualization, Writing – review & editing. LZ: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The modified Delphi panel study was funded by Janssen Asia Pacific. Janssen Asia Pacific was not involved in conducting the study and did not influence the decisions or outcomes of the consensus.
Acknowledgments
The authors would like to acknowledge support for the modified Delphi panel study from Ainsley Chan and members of the Vista Health team. The modified Delphi panel study was conducted by an independent healthcare and life sciences consultancy (Vista Health Pte Ltd). Medical writing and editorial support to the authors for manuscript preparation was provided by Tech Observer Asia Pacific.
Conflict of interest
CN reports consultancy for Janssen-Cilag, Lundbeck, Grunbiotics, Servier and Eli Lilly, and speaker honoraria from Servier, Lundbeck, Eli Lilly, Janssen-Cilag, Astra-Zeneca, and Pfizer in the last 10 years, unrelated to this work. TK reports personal fees from Janssen Asia Pacific related to the submitted work. TK also reports grants and personal fees from Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Meiji Seika Pharma Co., Ltd., Shionogi & Co., Ltd., Takeda Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., and Eisai Co., Ltd., personal fees from Taisho Pharma Co., Ltd., Mochida Pharmaceutical Co., Janssen Pharmaceutical K.K., Janssen Asia Pacific, Yoshitomiyakuhin, MSD K.K., Kyowa Pharmaceutical Industry Co., Ltd., Taisho Pharmaceutical Co., Ltd., Teijin Pharma, Viatris, Mylan N.V., Lundbeck Japan K.K., EA Pharma Co., Ltd., Nihon Medi-physics Co., Ltd., Ono Pharmaceutical Co., Ltd., outside the submitted work. YY reports serving on the advisory board for Janssen-Cilag, Pfizer, Eli Lilly and Lundbeck, and reports grants from GlaxoSmithKline, Eli Lilly, Pfizer, Janssen-Cilag, Sanofi-Aventis, Wyeth, Otsuka, Astellas, Dai Nippon Sumitomo, Lundbeck, Roche, Mitsubishi Tanabe and Boehringer Ingelheim. KH is an employee of Janssen Pharmaceutical Companies of Johnson & Johnson, Asia Pacific.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1338063/full#supplementary-material
References
1. World Health Organization. Depression (2023). Available online at: https://www.who.int/health-topics/depression.
2. GBD Diseases & Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: A systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
3. Covid-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the Covid-19 pandemic. Lancet. (2021) 398:1700–12. doi: 10.1016/S0140-6736(21)02143-7
4. Berlim MT, Fleck MPA. Quality of life and major depression. In: Ritsner MS, Awad AG, editors. Quality of Life Impairment in Schizophrenia, Mood and Anxiety Disorders. Springer Netherlands, Dordrecht (2007). p. 241–52.
5. Trivedi MH, Morris DW, Wisniewski SR, Lesser I, Nierenberg AA, Daly E, et al. Increase in work productivity of depressed individuals with improvement in depressive symptom severity. Am J Psychiatry. (2013) 170:633–41. doi: 10.1176/appi.ajp.2012.12020250
6. Lin CH, Yen YC, Chen MC, Chen CC. Depression and pain impair daily functioning and quality of life in patients with major depressive disorder. J Affect Disord. (2014) 166:173–8. doi: 10.1016/j.jad.2014.03.039
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition Vol. xliv. . Arlington, VA, US: American Psychiatric Publishing, Inc (2013) p. 947–xliv. p.
8. Rizvi SJ, Pizzagalli DA, Sproule BA, Kennedy SH. Assessing Anhedonia in depression: potentials and pitfalls. Neurosci Biobehav Rev. (2016) 65:21–35. doi: 10.1016/j.neubiorev.2016.03.004
9. Wang S, Leri F, Rizvi SJ. Anhedonia as a central factor in depression: neural mechanisms revealed from preclinical to clinical evidence. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 110:110289. doi: 10.1016/j.pnpbp.2021.110289
10. Shankman SA, Katz AC, DeLizza AA, Sarapas C, Gorka SM, Campbell ML. The different facets of anhedonia and their associations with different psychopathologies. In: Ritsner MS, editor. Anhedonia: A Comprehensive Handbook Volume I. Springer Netherlands, Dordrecht (2014). p. 3–22.
11. Pelizza L, Ferrari A. Anhedonia in schizophrenia and major depression: state or trait? Ann Gen Psychiatry. (2009) 8:22. doi: 10.1186/1744-859X-8-22
12. Vinckier F, Gourion D, Mouchabac S. Anhedonia predicts poor psychosocial functioning: results from a large cohort of patients treated for major depressive disorder by general practitioners. Eur Psychiatry. (2017) 44:1–8. doi: 10.1016/j.eurpsy.2017.02.485
13. Uher R, Perlis RH, Henigsberg N, Zobel A, Rietschel M, Mors O, et al. Depression symptom dimensions as predictors of antidepressant treatment outcome: replicable evidence for interest-activity symptoms. Psychol Med. (2012) 42:967–80. doi: 10.1017/S0033291711001905
14. Whitton AE, Kumar P, Treadway MT, Rutherford AV, Ironside ML, Foti D, et al. Distinct profiles of Anhedonia and reward processing and their prospective associations with quality of life among individuals with mood disorders. Mol Psychiatry. (2023). doi: 10.1038/s41380-023-02165-1
15. McCabe C, Mishor Z, Cowen PJ, Harmer CJ. Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry. (2010) 67:439–45. doi: 10.1016/j.biopsych.2009.11.001
16. Lin J, Su Y, Rizvi SJ, Jagoda J, Li J, Wu Y, et al. Define and characterize the anhedonia in major depressive disorder: an explorative study. J Affect Disord. (2022) 313:235–42. doi: 10.1016/j.jad.2022.06.082
17. Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton pleasure scale. Br J Psychiatry. (1995) 167:99–103. doi: 10.1192/bjp.167.1.99
18. Rizvi SJ, Quilty LC, Sproule BA, Cyriac A, Michael Bagby R, Kennedy SH. Development and validation of the dimensional Anhedonia rating scale (Dars) in a community sample and individuals with major depression. Psychiatry Res. (2015) 229:109–19. doi: 10.1016/j.psychres.2015.07.062
19. Fawcett J, Clark DC, Scheftner WA, Gibbons RD. Assessing Anhedonia in psychiatric patients. Arch Gen Psychiatry. (1983) 40:79–84. doi: 10.1001/archpsyc.1983.01790010081010
20. Kraus C, Kadriu B, Lanzenberger R, Zarate CA Jr., Kasper S. Prognosis and improved outcomes in major depression: A review. Transl Psychiatry. (2019) 9:127. doi: 10.1038/s41398-019-0460-3
21. Wang Y, Tang S, Zhang L, Bu X, Lu L, Li H, et al. Data-driven clustering differentiates subtypes of major depressive disorder with distinct brain connectivity and symptom features. Br J Psychiatry. (2021) 219:606–13. doi: 10.1192/bjp.2021.103
22. Fernando M, Chandrasiri A, Dayabandara M, Reavley NJ. Cultural adaptation of mental health first aid guidelines for depression for Sri Lanka: A Delphi expert consensus study. BMC Psychiatry. (2021) 21:585. doi: 10.1186/s12888-021-03598-0
23. Rybak YE, Lai KSP, Ramasubbu R, Vila-Rodriguez F, Blumberger DM, Chan P, et al. Treatment-resistant major depressive disorder: Canadian expert consensus on definition and assessment. Depress Anxiety. (2021) 38:456–67. doi: 10.1002/da.23135
24. Treadway MT, Zald DH. Reconsidering Anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. (2011) 35:537–55. doi: 10.1016/j.neubiorev.2010.06.006
25. Gong L, Yin Y, He C, Ye Q, Bai F, Yuan Y, et al. Disrupted reward circuits is associated with cognitive deficits and depression severity in major depressive disorder. J Psychiatr Res. (2017) 84:9–17. doi: 10.1016/j.jpsychires.2016.09.016
26. Nakonezny PA, Morris DW, Greer TL, Byerly MJ, Carmody TJ, Grannemann BD, et al. Evaluation of anhedonia with the Snaith-Hamilton pleasure scale (Shaps) in adult outpatients with major depressive disorder. J Psychiatr Res. (2015) 65:124–30. doi: 10.1016/j.jpsychires.2015.03.010
27. Loas G, Dubal S, Perot P, Tirel F, Nowaczkowski P, Pierson A. Validation of the french version of the Snaith-Hamilton pleasure scale (Shaps, Snaith et al. 1995). Determination of the statistical parameters in 208 normal subjects and 103 hospitalized patients presenting with depression or schizophrenia. Encephale. (1997) 23:454–8.
28. Franz M, Lemke MR, Meyer T, Ulferts J, Puhl P, Snaith RP. German version of the Snaith-Hamilton-pleasure scale (Shaps-D). Anhedonia in schizophrenic and depressive patients. Fortschr Neurol Psychiatr. (1998) 66:407–13. doi: 10.1055/s-2007-995279
29. Franken IH, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: further validation of the Snaith-Hamilton pleasure scale (Shaps). J Affect Disord. (2007) 99:83–9. doi: 10.1016/j.jad.2006.08.020
30. Chentsova-Dutton YE, Choi E, Ryder AG, Reyes J. "I felt sad and did not enjoy life": cultural context and the associations between Anhedonia, depressed mood, and momentary emotions. Transcult Psychiatry. (2015) 52:616–35. doi: 10.1177/1363461514565850
31. Ching E-k, Chiang TP, Wong JOY, Siu BWM, Cheng KM, Lui SSY, et al. Validation of the Chinese dimensional anhedonia rating scale in depressed patients in Hong Kong. psychol Test Adaptation Dev. (2021) 2:72–9. doi: 10.1027/2698-1866/a000012
32. Arrua-Duarte E, Migoya-Borja M, Barrigon ML, Barahona I, Delgado-Gomez D, Courtet P, et al. Spanish adaptation of the dimensional anhedonia rating scale (Dars). J Affect Disord. (2019) 245:702–7. doi: 10.1016/j.jad.2018.11.040
33. Gorostowicz A, Rizvi SJ, Kennedy SH, Chrobak AA, Dudek D, Cyranka K, et al. Polish adaptation of the dimensional Anhedonia rating scale (Dars) - validation in the clinical sample. Front Psychiatry. (2023) 14:1268290. doi: 10.3389/fpsyt.2023.1268290
34. Wellan SA, Daniels A, Walter H. State anhedonia in young healthy adults: psychometric properties of the German dimensional anhedonia rating scale (Dars) and effects of the Covid-19 pandemic. Front Psychol. (2021) 12:682824. doi: 10.3389/fpsyg.2021.682824
35. Borsini A, Wallis ASJ, Zunszain P, Pariante CM, Kempton MJ. Characterizing Anhedonia: A systematic review of neuroimaging across the subtypes of reward processing deficits in depression. Cognit Affect Behav Neurosci. (2020) 20:816–41. doi: 10.3758/s13415-020-00804-6
36. Cao B, Zhu J, Zuckerman H, Rosenblat JD, Brietzke E, Pan Z, et al. Pharmacological interventions targeting Anhedonia in patients with major depressive disorder: A systematic review. Prog Neuropsychopharmacol Biol Psychiatry. (2019) 92:109–17. doi: 10.1016/j.pnpbp.2019.01.002
37. Lode H, Torres A, Cockle A. What drives our choices? Evidence, guidelines or habit? Int J Antimicrob Agents. (2007) 29 Suppl 1:S17–22. doi: 10.1016/S0924-8579(07)70006-7
38. Goodwin GM, Price J, De Bodinat C, Laredo J. Emotional blunting with antidepressant treatments: A survey among depressed patients. J Affect Disord. (2017) 221:31–5. doi: 10.1016/j.jad.2017.05.048
39. Ma H, Cai M, Wang H. Emotional blunting in patients with major depressive disorder: A brief non-systematic review of current research. Front Psychiatry. (2021) 12:792960. doi: 10.3389/fpsyt.2021.792960
40. Kajanoja J, Scheinin NM, Karukivi M, Karlsson L, Karlsson H. Is antidepressant use associated with difficulty identifying feelings? A brief report. Exp Clin Psychopharmacol. (2018) 26:2–5. doi: 10.1037/pha0000165
41. Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. (2014) 216:67–73. doi: 10.1016/j.psychres.2014.01.042
42. Fagiolini A, Florea I, Loft H, Christensen MC. Effectiveness of Vortioxetine on emotional blunting in patients with major depressive disorder with inadequate response to Ssri/Snri treatment. J Affect Disord. (2021) 283:472–9. doi: 10.1016/j.jad.2020.11.106
43. Almeida SS, Zizzi FB, Cattaneo A, Comandini A, Di Dato G, Lubrano E, et al. Management and treatment of patients with major depressive disorder and chronic diseases: A multidisciplinary approach. Front Psychol. (2020) 11:542444. doi: 10.3389/fpsyg.2020.542444
44. Berk M, Kohler-Forsberg O, Turner M, Penninx B, Wrobel A, Firth J, et al. Comorbidity between major depressive disorder and physical diseases: A comprehensive review of epidemiology, mechanisms and management. World Psychiatry. (2023) 22:366–87. doi: 10.1002/wps.21110
45. Destoop M, Morrens M, Coppens V, Dom G. Addiction, Anhedonia, and comorbid mood disorder. A Narrative Review Front Psychiatry. (2019) 10:311. doi: 10.3389/fpsyt.2019.00311
46. Krupa AJ, Dudek D, Siwek M. Consolidating evidence on the role of insulin resistance in major depressive disorder. Curr Opin Psychiatry. (2024) 37:23–8. doi: 10.1097/YCO.0000000000000905
47. Arnaud AM, Brister TS, Duckworth K, Foxworth P, Fulwider T, Suthoff ED, et al. Impact of major depressive disorder on comorbidities: A systematic literature review. J Clin Psychiatry (2022) 83(6):21r14328. doi: 10.4088/JCP.21r14328
48. McIntyre RS, Loft H, Christensen MC. Efficacy of vortioxetine on anhedonia: results from a pooled analysis of short-term studies in patients with major depressive disorder. Neuropsychiatr Dis Treat. (2021) 17:575–85. doi: 10.2147/NDT.S296451
49. Fletcher K, Parker G, Paterson A, Fava M, Iosifescu D, Pizzagalli DA. Anhedonia in melancholic and non-melancholic depressive disorders. J Affect Disord. (2015) 184:81–8. doi: 10.1016/j.jad.2015.05.028
50. Beijers L, Wardenaar KJ, van Loo HM, Schoevers RA. Data-driven biological subtypes of depression: systematic review of biological approaches to depression subtyping. Mol Psychiatry. (2019) 24:888–900. doi: 10.1038/s41380-019-0385-5
51. Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. (2017) 23:28–38. doi: 10.1038/nm.4246
52. Jorm AF. Using the delphi expert consensus method in mental health research. Aust N Z J Psychiatry. (2015) 49:887–97. doi: 10.1177/0004867415600891
Keywords: anhedonia, major depressive disorder, DSM-5, Delphi consensus, Asia Pacific
Citation: Cheng C, Herr K, Jeon HJ, Kato T, Ng CH, Yang YK and Zhang L (2024) A Delphi consensus on clinical features, diagnosis and treatment of major depressive disorder patients with anhedonia amongst psychiatrists in the Asia-Pacific. Front. Psychiatry 15:1338063. doi: 10.3389/fpsyt.2024.1338063
Received: 14 November 2023; Accepted: 01 February 2024;
Published: 23 February 2024.
Edited by:
Tasuku Hashimoto, International University of Health and Welfare (IUHW), JapanReviewed by:
Francesco Monaco, Azienda Sanitaria Locale Salerno, ItalyMarcin Siwek, Jagiellonian University, Poland
Copyright © 2024 Cheng, Herr, Jeon, Kato, Ng, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Keira Herr, S0hlcnIzQGl0cy5qbmouY29t
†These authors have contributed equally to this work
 Calvin Cheng
Calvin Cheng Keira Herr
Keira Herr Hong Jin Jeon
Hong Jin Jeon Tadafumi Kato4†
Tadafumi Kato4† Chee H. Ng
Chee H. Ng Yen Kuang Yang
Yen Kuang Yang Ling Zhang
Ling Zhang