- 1Department of Traditional Chinese Medicine, Chengdu Eighth People’s Hospital (Geriatric Hospital of Chengdu Medical College), Chengdu, China
- 2College of Rehabilitation and Health Preservation, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Department of Rehabilitation, Traditional Chinese Medicine Hospital of Longquanyi District, Chengdu, China
- 4Department of Medicine, Leshan Vocational and Technical College, Leshan, China
Introduction: Internet addiction disorder (IAD) has grown into public health concern of global proportions. Previous studies have indicated that individuals with IAD may exhibit altered levels of serotonin and dopamine, which are known to play crucial roles in depression, anxiety, impulsivity, and addiction. Therefore, polymorphisms in the receptors that mediate the effects of serotonin and dopamine and affect their functional states as well as their activities are suspect. In this study, we aimed to investigate the association between IAD and rs6313 (T102C) polymorphism in the serotonin 2A receptor (5-HT2A) gene, (HTR2A).
Methods: Twenty patients with IAD and twenty healthy controls (HCs) were included in this study. Young’s Internet Addiction Test (IAT), Self-Rating Anxiety Scale, Self-Rating Depression Scale, Yale-Brown Obsessive-Compulsive Scale (Y-BOCS), Barratt Impulse Scale, Pittsburgh Sleep Quality Index (PSQI), and Social Support Rating Scale (SSRS) were used to assess the severity of internet addiction, mental status, impulsive traits, sleep quality, and social support. Genotyping was performed to identify rs6313 polymorphisms in the HTR2A gene of all participants.
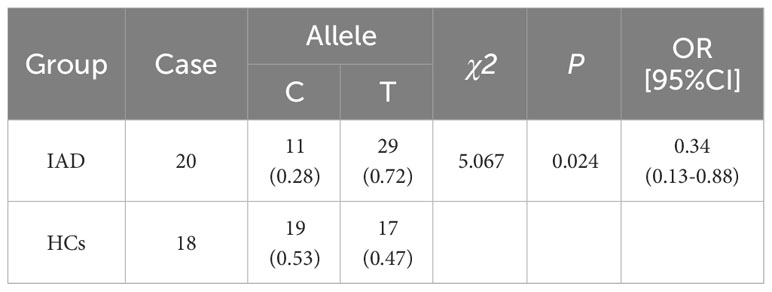
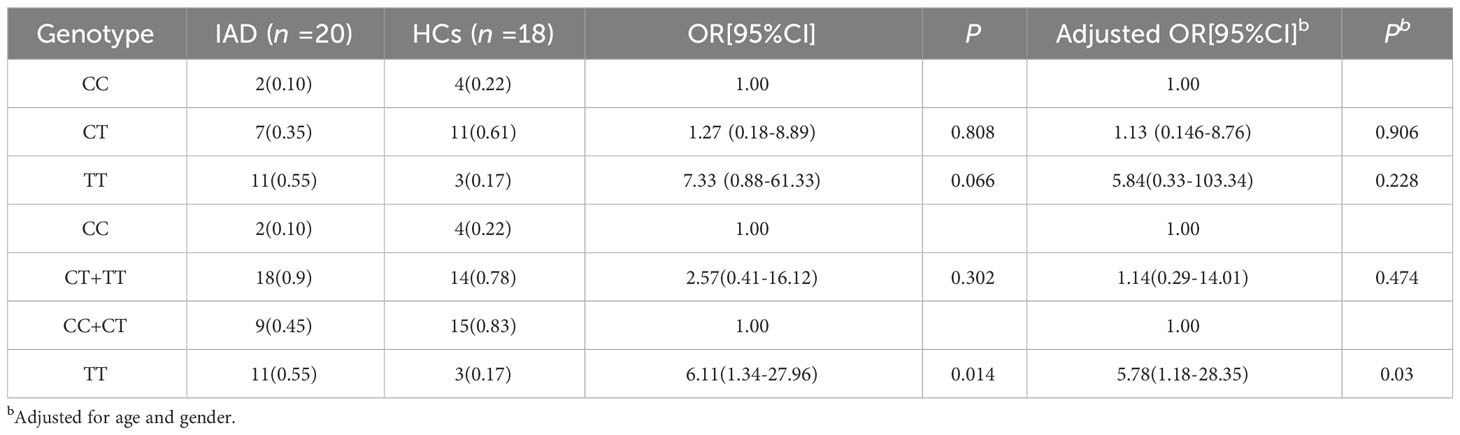
Results: The frequencies of the C and T alleles of HTR2A T102C were 28% and 72% in the IAD group and 53% and 47% in the HCs group, respectively, indicating that the differences between these two groups were significant. No significant difference was observed in the distribution of the CC, CT, and TT genotypes of HTR2A gene T102C between the IAD and the HCs groups. Additionally, there was no difference in the distribution of the frequencies of the HTR2A gene T102C CC and CT+TT genotypes between the two groups. However, the distribution between the TT and CC+CT genotypes showed an apparent statistical difference in the HTR2A gene T102C between the two groups. Correlation analysis indicated that the IAT score was positively correlated with the Y-BOCS and BIS scores for the CC+CT genotype in patients with IAD. Moreover, the IAT score was positively correlated with the PSQI score in patients with IAD carrying the TT genotype.
Conclusion: The present study demonstrates that rs6313 in HTR2A is associated with IAD, and that the T allele of rs6313 in HTR2A may be a risk factor for IAD.
1 Introduction
The advent of internet technology has made life, study, and work more convenient for young people. However, inappropriate internet usage may become addictive leading to periodic or chronic indulgence which may harm the physical and mental health of those involved, and weaken their interpersonal communication skills, and social relationships (1, 2). This condition has been termed as Internet addiction disorder (IAD) or Pathological internet use, defined as a behavioral addiction (3). Currently, IAD is considered as a serious public health concern due to its high prevalence worldwide (4–6).
Many scholars who investigated internet addiction from the perspectives of neuroimaging and neurobiology have suggested that IAD may be closely correlated with the dopaminergic and serotoninergic systems. Weinstein et al., reported that compulsive behavior and loss of control in individuals with IA were associated with low levels of dopamine D2 and 5-HT2A receptors in the orbitofrontal cortex (7). Moreover, a study conducted by Dresp–Langley revealed that dysregulation of the serotonin (5-HT) and dopamine (DA) neurotransmitter pathways may be the mechanism underlying IAD (8). Liu et al., reported a significant difference between the levels of dopamine in the peripheral blood of individuals with IAD and healthy participants (9), while Luo et al. (10), found that the levels of 5-HT in the platelets of individuals with IA were different from those of healthy controls. Additionally, Ariatama (11) and Hou (12) found that individuals with IA displayed lower dopamine transporter (DAT) concentrations.
Importantly, 5-HT and DA reportedly play crucial roles in depression, anxiety, impulsivity, and addiction (13–18). Therefore, genes involved in the dopaminergic or serotonergic systems may be regarded as potential candidate genes associated with IAD.
The effects of 5-HT are mediated by the corresponding receptor. Human 5-HT receptors (5-HTR) can be categorized into seven types: 5-HT1 ~ 7R. The serotonin 2A receptor (HTR2A) is a subtype of 5-HTR. Myers study reported that the HTR2A expression is regulated at the gene level (19). This indicates that receptor polymorphisms may affect the functional state of receptors, thereby influencing the activity of 5-HT. Current research on HTR2A gene polymorphism mainly focuses on two SNPs, rs6313 (T102C) and rs6311 (-1438A/G) (20–22). A meta-analysis by Cao et al. (23), suggested that rs6313 in HTR2A was associated with substance use disorders. Several studies have demonstrated that HTR2A is associated with depression, anxiety, sleep, impulsive actions, and cognitive control (24–30). Previous studies have confirmed that depression and anxiety are risk factors for the development of IA (31, 32), and that self-control plays an important role in the generative mechanism of IA (33, 34). Besides, sleep problems is also a common symptom of IA (35). Hence, we hypothesized that the polymorphism in HTR2A rs6313 may be associated with IAD as well as with related comorbidities, such as depression, anxiety, impulsivity, and sleep problems. In this study, we tested the above hypothesis by evaluating the following: 1. the relationship between IAD and rs6313 polymorphism in HTR2A; 2. the association between IAT scores and clinical scores of different genotypes in patients with IAD.
2 Materials and methods
2.1 Subjects
G*Power was used to evaluate sample size. Chi-square test, with w = 0.5, power = 0.85, and df = 1 was used to analyze results. G*Power indicated that a sample size of 36 would be appropriate. With due consideration given to the dropout rate, 40 participants were recruited from the University of Electronic Science and Technology, Chengdu University of Traditional Chinese Medicine, and the Sichuan Vocational and Technical College of Communication. The participants included 20 patients with IAD and 20 HCs. Beard’s Diagnostic Questionnaire was used to diagnose IAD (36). All participants in our study were right-handed, and none reported any other organic or mental illnesses. In addition, IAD individuals had not undergone any form of therapeutic intervention. This study was subjected to ethical scrutiny and approved by the Ethics Review Board of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (Permission number: 2016KL-005). Signed informed consent was obtained from all participants.
2.2 Clinical measures
2.2.1 Young’s internet addiction test
The IAT measures the degree of IAD and consists of 20 items (37). The possible scores range from 20 to 100. Moreover, IAT has been confirmed as having adequate validity and reliability. The Cronbach’s alpha was 0,93 (38).
2.2.2 Self-rating depression scale
The SDS consists of 20 self-report questions, which are used to assess depression symptoms (39). Previous studies have shown that the SDS has adequate reliability and validity (40, 41).
2.2.3 Self-rating anxiety scale
The SAS is widely used to ascertain the anxiety state of an individual (42). It consists of 20 items. The Cronbach’s alpha for this scale was 0,883 (43).
2.2.4 Yale-brown obsessive-compulsive scale
The Y-BOCS consists of 10 core items that test the severity of obsessive-compulsive symptoms (44). The Chinese version of the Y-BOCS was found to have adequate reliability in Chinese sample studies, with a Cronbach’s alpha of 0,83 (45).
2.2.5 Barratt impulsiveness scale
The BIS-11 is a self-reported questionnaire consisting of 30 items that evaluates the impulsivity of individuals (46). The Chinese version of the BIS-11 has previously been verified as reliable, with a Cronbach’s alpha of 0,80 (47).
2.2.6 Pittsburgh sleep quality index
The PSQI is widely used to assess the subjective sleep quality of individuals in clinical settings, and consists of 19 items (48). The Cronbach’s alpha for this scale was 0,81 (49).
2.2.7 Social support rating scale
The SSRS was developed by Xiao and consists of ten items (50). This scale is a brief measure of social support and is widely used in Chinese samples (51, 52).
2.3 Blood sample collection and preservation
The 5 mL of elbow venous blood was collected and stored in ethylenediaminetetraacetic acid (EDTA) anticoagulant tubes. Upon receipt, the blood samples were stored at −80°C until used.
2.4 Genome DNA extraction
Genomic DNA was extracted using the Blood Gene Mini Kit (200 µL, CWBIO, Beijing, China): (1) Approximately 10ˆ5 whole blood mononuclear cells were added to 200 µL Buffer GR, treated with 20 µL Protein K and mixed very gently; (2) next, 200 µL GL was added to the preparation, which was then mixed for 15 s, and incubated at 56°C for 10 min, and treated with 200 µL anhydrous ethanol; (3) the solution obtained via the previous steps was added to the adsorption column, and centrifuged at 12000 rpm for 1 min, following which the waste liquid was poured into a collection tube; (4) next, 500 µL of GW1 buffer was added to the adsorption column, which was then centrifuged at 12000 rpm for 1 min, the waste liquid being poured into the collection tube. Subsequently, 500 µL of GW2 buffer was added to the adsorption column, centrifuged at 12000 rpm for 1 min, and the waste liquid poured into the collection tube; (5) next, the solution in the adsorption column was centrifuged at 12000 rpm for 2 min. Immediately afterwards, the adsorption column was placed in a clean centrifuge tube, and let stand at room temperature for a few minutes until the residual liquid in the adsorption column evaporated; (6) approximately, 50-200 µL of GE buffer was added to the adsorption column, let stand at room temperature for 5 min, and centrifuged at 12000 rpm for 2 min. The DNA solution resulting was cryopreserved at −20°C; (7) DNA fragments were detected via 1% agarose gel electrophoresis.
2.5 PCR amplification
A reaction volume of 25 μL/tube (2*TaqMan Realtime PCR Mix 12.5 μL + PCR primer pair 1.2 μL + ddH2O 8.3 μL and cDNA template 3 μL) was used. PCR amplification was performed on an FTC-3000QPCR system (Funglyn Biotech, Toronto, Canada). The amplification conditions were as follows: pre-denaturation at 95°C for 10 min; denaturation at 95°C for 10 s; annealing at 55°C for 30 s; extended at 72°C for 30 s; repetition of cycle for 35 times; a final extension at 72°C for 5 min. The following primers were used: forward, CAGCCTCAGTGTTACAGAGT; and reverse, CAGCAATAGTTAGAATAATCACT. The PCR products so obtained were subjected to sequencing (Please refer to our Supplementary Materials for the relevant electrophoresis and sequencing diagrams).
2.6 Statistical analyses
Statistical analyses were conducted using SPSS 21.0 and GraphPad Prism 7.0 software. The Chi-square test was used to evaluate differences between the genotypes and allele frequencies of the two groups. An independent samples t-test or a non-parametric test, was used to detect differences in scale scores between the two groups. For correlation analysis, the data were tested for K-S normality. The Pearson correlation coefficient was used to analyze normal distribution, and the Spearman correlation coefficient was used to analyze non-normal distribution. P ≤ 0.05 was considered to be statistically significant.
3 Results
3.1 Demographic and clinical measures
Forty participants consented to take part in the study, of which 20 (100%) patients with IAD and 18 (90%) HCs had valid genetic data. The average age of the 20 patients with IAD (15 male and 5 female) was 20.00 ± 3.00 years, while that of the 18 HCs (14 male and 4 female) was 21.83 ± 2.43 years. There were no significant differences between the ages (P = 0.841, χ2 = 0.04) or sexes (P = 0.374, z = -0.888) of the two groups. As shown in Figure 1, compared with HCs, patients with IAD had higher IAT, SDS, SAS, Y-BOCS, BIS-11, and PSQI scores. However, the SSRS scores of patients with IAD were lower than those of HCs.
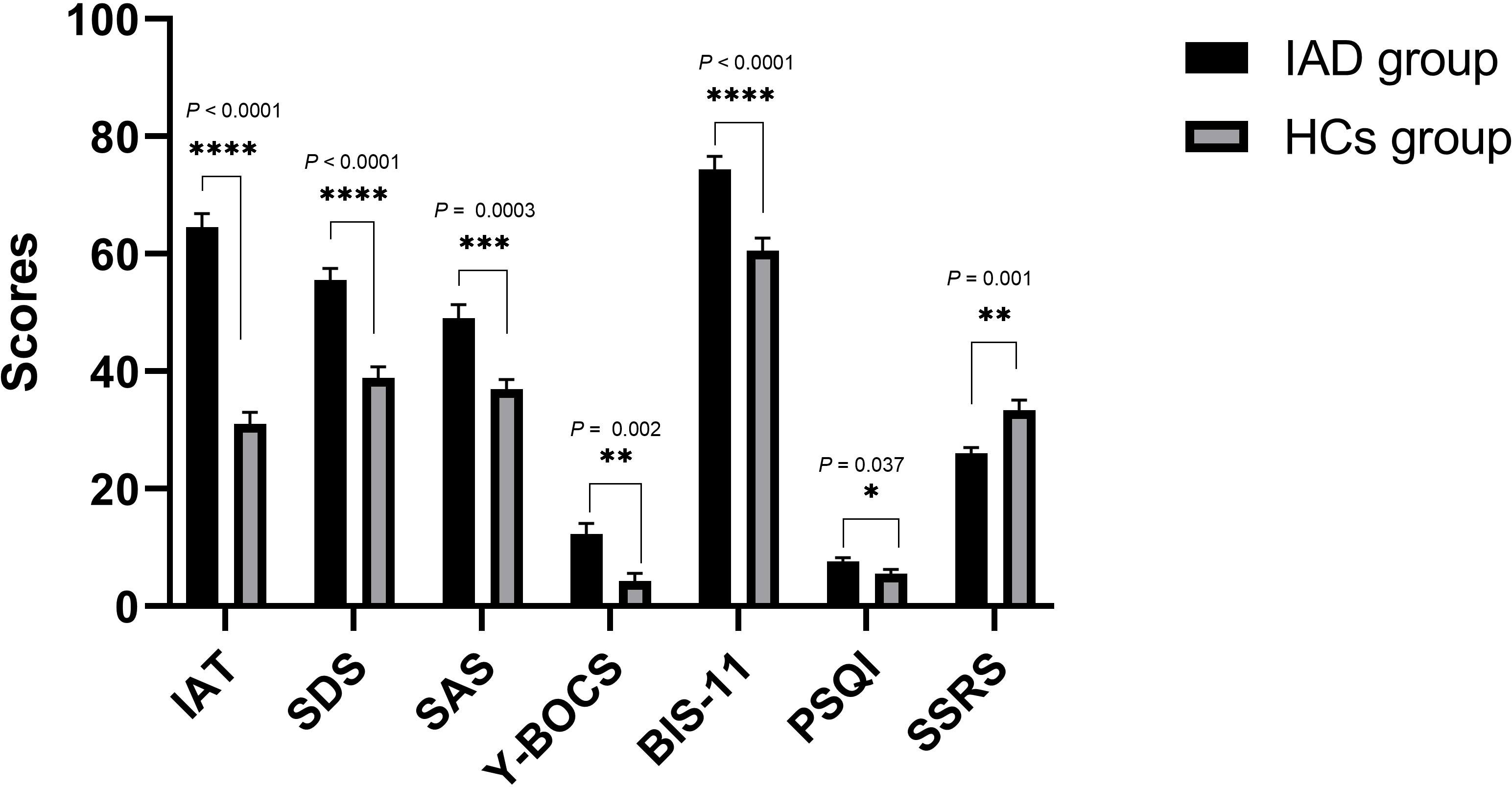
Figure 1 Clinical measures in the IAD group and the HCs group. Bars represent means, error bars represent SEM, triangles and circles represent individual data points. **** P < 0.0001, *** P < 0.0005, ** P < 0.01, * P < 0.05. IAT, Young’s Internet Addiction Test; SDS, Self-Rating Depression Scale; SAS, Self-Rating Anxiety Scale; Y-BOCS, Yale-Brown Obsessive-Compulsive Scale; BIS-11, Barratt Impulse Scale; PSQI, Pittsburgh Sleep Quality Index; SSRS, Social Support Rating Scale.
3.2 Allele frequency and genotype distribution
The genotype distributions of T102C of the HCs group followed the Hardy-Weinberg equilibrium (χ2 = 0.919; P = 0.337), and the genotype distributions of T102C of the IAD group also followed the Hardy-Weinberg equilibrium (χ2 = 0.298; P = 0.58). Table 1 shows that the C allele frequencies in the IAD group and the HCs group were 0.28 and 0.53, respectively, and the T allele frequencies in the IAD group and the HCs group were 0.72 and 0.47, respectively. There were differences between the allele distributions in the two groups (P ≤ 0.05). As shown in Table 2, there was no difference in the distribution of the CC, CT, and TT genotypes of T102C in HTR2A between the IAD and HCs groups (P > 0.05). Additionally, there was no difference in the distribution of the frequencies of CC and CT+TT genotypes of T102C in HTR2A in the two groups (P > 0.05). However, a statistical difference was observed in the frequency distributions of the TT and CC+CT genotypes of T102C in HTR2A between the IAD group and the HCs group (P ≤ 0.05).
3.3 Relationship between the IAT score and clinical scores in the CC+CT genotype or the TT genotype in patients with IAD
There was no correlation between the IAT scores and SDS (r = 0.553, P = 0.123), SAS (r = 0.662, P = 0.052), PSQI (r = 0.165, P = 0.672), or SSRS (r = -0.372, P = 0.324) scores of the CC+CT genotype in patients with IAD. Moreover, no significant association was observed between the IAT score and the SDS (r = 0.566, P = 0.069), SAS (r = 0.48, P = 0.135), Y-BOCS (r = 0.54, P = 0.087), BIS (r = 0.386, P = 0.241), or SSRS (r = 0.109, P = 0.75) scores of the TT genotype in patients with IAD. However, as shown in Figures 2A and B, the IAT score was positively correlated with the Y-BOCS (r = 0.758, P = 0.018) and BIS scores (r = 0.763, P = 0.017) of the CC+CT genotype in patients with IAD. Additionally, Figure 2C shows the IAT score was positively correlated with the PSQI score (r = 0.749, P = 0.008) of the TT genotype in patients with IAD.
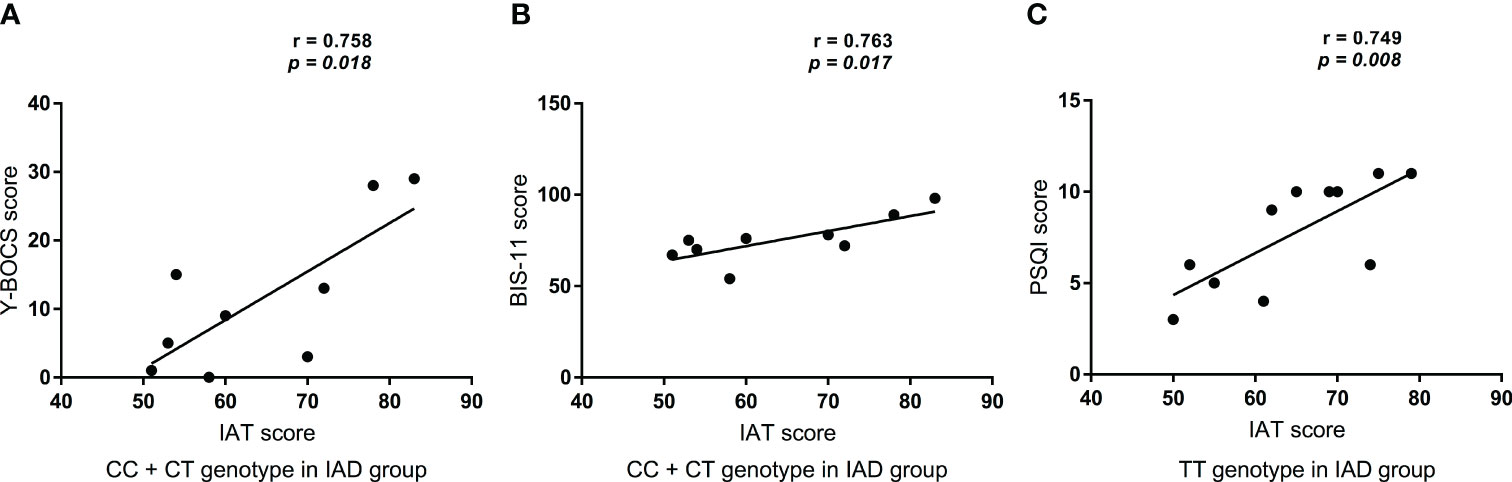
Figure 2 Correlations between IAT score and Clinical scores. (A) The relationship between IAT score and Y-BOCS score in CC+CT genotype in patients with IAD (n = 9); (B) The relationship between IAT score and BIS-11 score in CC+CT genotype in patients with IAD (n = 9); (C) The relationship between IAT score and PSQI score in TT genotype in patients with IAD (n = 11).
4 Discussion
IAD, also referred to as pathological internet use or overuse, has come to be considered as a serious global public health issue. Internet addiction often manifests as excessive use of the internet, or an inability to fully concentrate on daily activities, or to effectively control desires and behaviors related to internet use, thereby adversely affecting all aspects of life (53). Depression and anxiety are the most common psychological disorders that have been found to be associated with IAD (32). Individuals beset with negative emotions resort to the internet to cope with psychological issues. If internet use relieves their negative emotions even temporarily, they may become more prone to excessive internet use, with such interdependency quickly turning into a vicious cycle of negativity (54). The present study indicated that patients with IAD had higher SDS and SAS scores than HCs. A previous review revealed that the reward system plays a crucial role in IAD (7). Similarly, in our previous study, we found that IAD was associated with reward systems (55). Moreover, the 5-HT system participates in the regulation of brain reward function (56). Abnormal expression of the 5-HT system is often associated with impulsive behavior (57) and sleep problems (58), which are common symptoms of IAD. A recent animal experiment reported that 5-HT levels may affect social interactions during protracted opioid withdrawal in mice (59). Our study showed that patients with IAD had higher Y-BOCS, BIS-11, and PSQI scores than HCs. In addition, patients with IAD had lower SSRS scores than healthy participants.
Overall, the 5-HT system plays a crucial role in IA. Several genetic studies have explored IAD. According to one review, the main candidate genes involved in IAD included those encoding the dopamine D2 receptor, monoamine-oxidase-A, and serotonin transporter (5-HTT) (60). HTR2A is a subtype of 5-HTR, which affect 5-HTT function and serotonergic transmission (61, 62), and has thus turned into a research hotspot in substance addiction, aggressive behavior, and obsessive-compulsive disorder in recent years (63–65). Mounting evidence has implicated serotonin neurotransmission through the 5-HT2A receptor as a driver of relapse-related behavior in substance addiction (66, 67). HTR2A, which consists of two introns and three exons, is located on chromosome 13q14-q21. It encodes a G protein-coupled receptor associated with phospholipase, which is mainly distributed in the frontal cortex, hippocampus, amygdala, and peripheral blood. Recent research aimed at the association between HTR2A polymorphisms and addiction has mainly focused on rs6313 (polymorphism rs6313 is located in codon 102 of 5-HTR2A exon 1) (23, 68, 69).
A previous study on Mexican mestizos showed that the T allele carrier of the single nucleotide polymorphism rs6313 (102T>C) in HTR2A was associated with the risk of cigarette smoking (21). White et al., reported that the T102C TT genotype (Homozygotes for T102) was a significant predictive risk factor for cigarette smoking (68). In the present study, we conducted a genotyping analysis of T102C in patients with IAD and healthy participants, and found that the TT genotype of rs6313 in HTR2A was associated with an increased risk of IA.
Furthermore, to understand the relationship between different genotypes of rs6313 in HTR2A in patients with IAD and clinical scores, we conducted a correlation analysis and found that the IAT score was correlated with the BIS-11 and Y-BOCS scores in patients with IAD carrying the C allele of rs6313. The IAT score was only correlated with the PSQI score for the TT genotype of rs6313 in patients with IAD. Moreover, 5-HT activity is related to cognition and impulsive behavior (70), while HTR2A is known to regulate 5-HT neurotransmission (71). Polesskaya et al., reported that compared with the T allele, the presence of the C allele in rs6313 reduced the expression of HTR2A by 20% (72). This indicated that changes in the mRNA expression of HTR2A may modulate the function of 5-HT, in turn influencing its cognitive and impulsive effects. Previous studies have shown that the 5-HT2A receptors play a role in sleep (27). Elmenhorst et al., indicated that sleep deprivation increases HTR2A binding in the neocortex of healthy individuals (73). Notably, a recent study reported that preventing mice from sleeping resulted in a significant increase in HTR2A mRNA levels in their cortices (74). One Chinese study reported that although −1438G/A of HTR2A was associated with sleep quality in non-manual workers, T102C was not (26). Japanese researchers have reported that the C allele of HTR2A T102C was associated with sleep bruxism (75). Interestingly, the present study showed a positive correlation between the degree of internet addiction and sleep problems in the TT genotype of rs6313 in patients with IAD. These contradictory results may be attributed to factors such as differences in age and disease status.
This study had several limitations. Our results indicated that the T allele of rs6313 was associated with IAD. However, small sample size limited the generalizability of our current results. In addition, we did not consider the involvement of the SNPs in other genes linked to internet addiction. Therefore, future studies involving larger sample sizes and other genes that are likely to be linked to IAD are warranted.
To the best of our knowledge, this exploratory study is the first to focus on the association between genetic polymorphisms in HTR2A and IAD. Our results suggest that the T allele of rs6313 in HTR2A is a risk factor for IAD. Although our results are of a preliminary nature, they may provide new insights into the pathogenesis of IAD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study underwent ethical scrutiny and was approved by the Ethics Review Board of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (Permission number: 2016KL-005). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YD: Writing – original draft, Formal Analysis. CZ: Data curation, Writing – review & editing. LZ: Writing – review & editing. CW: Writing – review & editing. HL: Formal Analysis, Writing – review & editing. TZ: Conceptualization, Funding acquisition, Methodology, Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Natural Science Foundation of China (81072852 and 81574047), the Key Research and Development Projects of Sichuan Science and Technology Department (2019YFS0175), the Xinglin Scholars Scientific Research Promotion Program of Chengdu University of Traditional Chinese Medicine (XSGG2019007), and the Training Funds of Academic and Technical Leader in Sichuan Province.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1292877/full#supplementary-material
References
1. Shao YJ, Zheng T, Wang YQ, Liu L, Chen Y, Yao YS. Internet addiction detection rate among college students in the People's Republic of China: a meta-analysis. Child Adolesc Psychiatry Ment Health (2018) 12(1):25. doi: 10.1186/s13034-018-0231-6
2. Shen Y, Wang L, Huang C, Guo J, Zhang XY. Sex differences in prevalence, risk factors and clinical correlates of internet addiction among chinese college students. J Affect Disord (2021) 279:680–6. doi: 10.1016/j.jad.2020.10.054
3. Christakis DA, Moreno MM, Jelenchick L, Myaing MT, Zhou C. Problematic internet usage in US college students: a pilot study. BMC Med (2011) 9:77. doi: 10.1186/1741-7015-9-77
4. Chung TWH, Sum SMY, Chan MWL. Adolescent internet addiction in hong kong: prevalence, psychosocial correlates, and prevention. J Adolesc Health (2019) 64:S34–43. doi: 10.1016/j.jadohealth.2018.12.016
5. Tang J, Yu Y, Du Y, Ma Y, Zhang D, Wang J. Prevalence of internet addiction and its association with stressful life events and psychological symptoms among adolescent internet users. Addictive Behav (2014) 39(3):744–7. doi: 10.1016/j.addbeh.2013.12.010
6. Weinstein A, Lejoyeux M. Internet addiction or excessive internet use. Am J Drug Alchol Abuse. (2010) 36:277–83. doi: 10.3109/00952990.2010.491880
7. Weinstein A, Lejoyeux M. Neurobiological mechanisms underlying internet gaming disorder. Dialogues Clin Neurosci (2020) 22(2):113–26. doi: 10.31887/DCNS.2020.22.2/aweinstein
8. Dresp-Langley B. Children’s health in the digital age. Internet J Environ Res Public Health (2020) 17:3240. doi: 10.20944/preprints202003.0258.v1
9. Liu M, Luo J. Relationship between peripheral blood dopamine level and internet addiction disorder in adolescents: a pilot study. Int J Clin Exp Med (2015) 8(6):9943–8.
10. Luo JH, Wu HR, Meng H, Du YS, Lin ZG. Study of platelet serotonin in adolescents with internet addiction disorder. Chin J School Health (2011) 32:190–1. doi: 10.16835/j.cnki.1000-9817.2011.02.029
11. Ariatama B, Effendy E, Amin MM. Relationship between internet gaming disorder with depressive syndrome and dopamine transporter condition in online games player. Open Access Maced J Med Sci (2019) 7(16):2638–42. doi: 10.3889/oamjms.2019.476
12. Hou H, Jia S, Hu S, Fan R, Sun W, Sun T, et al. Reduced striatal dopamine transporters in people with Internet addiction disorder. J BioMed Biotechnol (2012) 2012:854524. doi: 10.1155/2012/854524
13. Dell'Osso L, Carmassi C, Mucci F, Marazziti D. Depression, serotonin and tryptophan. Curr Pharm Des (2016) 22(8):949–54. doi: 10.2174/1381612822666151214104826
14. Deurwaerdère PD, Giovanni GD. Serotonin in health and disease. Int J Mol Sci (2020) 21(10):3500. doi: 10.3390/ijms21103500
15. Olivier JDA, Olivier B. Translational studies in the complex role of neurotransmitter systems in anxiety and anxiety disorders. Adv Exp Med Biol (2020) 1191:121–40. doi: 10.1007/978-981-32-9705-0_8
16. Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience (2012) 215:42–58. doi: 10.1016/j.neuroscience.2012.03.065
17. Wise RA, Robble MA. Dopamine and addiction. Annu Rev Psychol (2020) 71:79–106. doi: 10.1146/annurev-psych-010418-103337
18. Li Y, Simmler LD, Zessen RV, Flakowski J, Wan JX, Deng F, et al. Synaptic mechanism underlying serotonin modulation of transition to cocaine addiction. Science (2021) 373(6560):1252–6. doi: 10.1126/science.abi9086
19. Myers RL, Airey DC, Manier DH, Shelton RC, Sanders-Bush E. Polymorphisms in the regulatory region of the human serotonin 5-HT2A receptor gene (HTR2A) influence gene expression. Biol Psychiatry (2007) 61(2):167–73. doi: 10.1016/j.biopsych.2005.12.018
20. Gong P, Liu J, Blue P, Li S, Zhou X. Serotonin receptor gene (HTR2A) T102C polymorphism modulates individuals' perspective taking ability and autistic-like traits. Front Hum Neurosci (2015) 9:575. doi: 10.3389/fnhum.2015.00575
21. Pérez-Rubio G, Ramírez-Venegas A, Díaz V, Gómez LG, Fabián KE, Carmona SG, et al. Polymorphisms in HTR2A and DRD4 predispose to smoking and smoking quantity. PloS One (2017) 12(1):e0170019. doi: 10.1371/journal.pone.0170019
22. Yeom JW, Jeong S, Seo JY, Jeon S, Lee HJ. Association of the serotonin 2A receptor rs6311 polymorphism with diurnal preference in Koreans. Psychiatry Investig (2020) 17(11):1137–42. doi: 10.30773/pi.2020.0358
23. Cao J, Liu XT, Han SZ, Zhang C, Liu Z, Li D. Association of the HTR2A gene with alcohol and heroin abuse. Hum Genet (2014) 133(3):357–65. doi: 10.1007/s00439-013-1388-y
24. Steinberg LJ, Underwood MD, Bakalian MJ, Kassir SA, Mann JJ, Arango V. 5-HT1A receptor, 5-HT2A receptor and serotonin transporter binding in the human auditory cortex in depression. J Psychiatry Neurosci (2019) 44(5):294–302. doi: 10.1503/jpn.180190
25. Xiang M, Jiang Y, Hu Z, Yang Y, Du X, Botchway BO, et al. Serotonin receptors 2A and 1A modulate anxiety-like behavior in post-traumatic stress disordered mice. Am J Transl Res (2019) 11(4):2288–303.
26. Wang J, Gao X, Gao P, Liu J. A cross-sectional study on the relationship among cytokines, 5-HT2A receptor polymorphisms, and sleep quality of non-manual workers in Xinjiang, China. Front Psychiatry (2022) 13:777566. doi: 10.3389/fpsyt.2022.777566
27. Landolt HP, Wehrle R. Antagonism of serotonergic 5-HT2A/2C receptors: mutual improvement of sleep, cognition and mood? Eur J Neurosci (2009) 29(9):1795–809. doi: 10.1111/j.1460-9568.2009.06718.x
28. Fink LH, Anastasio NC, Fox RG, Rice KC, Moeller FG, Cunningham KA. Individual differences in impulsive action reflect variation in the cortical serotonin 5-HT2A receptor system. Neuropsychopharmacology (2015) 40(8):1957–68. doi: 10.1038/npp.2015.46
29. Kojima K, Hirano S, Kimura Y, Seki C, Ikoma Y, Takhata K, et al. Brain 5-HT2A receptor binding and its neural network related to behavioral inhibition system. Brain Image Behav (2022) 16(3):1337–48. doi: 10.1007/s11682-021-00609-2
30. Carhart-Harris RL, Nutt DJ. Serotonin and brain function: A tale of two receptors. J Psychopharmacology. (2017) 31(9):1091–120. doi: 10.1177/0269881117725915
31. Yi X, Li G. The longitudinal relationship between internet addiction and depressive symptoms in adolescents: A random-intercept cross-lagged panel model. Int J Environ Res Public Health (2021) 18(24):12869. doi: 10.3390/ijerph182412869
32. Ho RC, Zhang MW, Tsang TY, Toh AH, Pan F, Lu Y, et al. The association between internet addiction and psychiatric co-morbidity: a meta-analysis. BMC Psychiatry (2014) 14:183. doi: 10.1186/1471-244X-14-183
33. Blachnio A, Prezepiorka A. Dysfunction of self-regulation and self-control in facebook addiction. Psychiatr Quart. (2016) 87:493–500. doi: 10.1007/s11126-015-9403-1
34. Kim EJ, Namkoong K, Ku T, Kim SJ. The relationship between online game addiction and aggression, self-control and narcissistic personality traits. Eur Psychiatry (2008) 23:212–8. doi: 10.1016/j.eurpsy.2007.10.010
35. Kawabe K, Horicuchi F, Oka Y, Ueno SL. Association between sleep habits and problems and internet addiction in adolescents. Psychiatry Investig (2019) 16(8):581–7. doi: 10.30773/pi.2019.03.21.2
36. Beard KW, Wolf EM. Modification in the proposed diagnostic criteria for Internet addiction. Cyberpsychol Behav (2001) 4:377–83. doi: 10.1089/109493101300210286
37. Young KS. Internet addiction: The emergence of a new clinical disorder. CyberPsychol Behav (1996) 1:237–44. doi: 10.1089/cpb.1998.1.237
38. Lai C-M, Mak K-K, Watanabe H, Ang RP, Pang JS, Ho RCM. Psychometric properties of the internet addiction test in Chinese adolescents. J Pediatr Psychol (2013) 38:794–807. doi: 10.1093/jpepsy/jst022
39. Zung WW. A self-rating depression scale. Arch Gen Psychiatry (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
40. Leung KK, Lue BH, Lee MB, Tang LY. Screening of depression in patients with chronic medical diseases in a primary care setting. Fam Pract (1998) 15:67–75. doi: 10.1093/fampra/15.1.67
41. Cheung SK. Reliability and factor structure of the Chinese version of the depression self-rating scale. Educ Psychol Meas. (1996) 56:142–54. doi: 10.1177/0013164496056001011
42. Zung WW. A rating instrument for anxiety disorders. Psychosomatics (1971) 12:371–9. doi: 10.1016/S0033-3182(71)71479-0
43. Shi M, Liu L, Wang ZY, Wang L. The mediating role of resilience in the relationship between big five personality and anxiety among Chinese medical students: A cross-sectional study. PloS One (2015) 10:e0119916. doi: 10.1371/journal.pone.0119916
44. Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The yale-brown obsessive compulsive scale. II. Validity. Arch Gen Psychiatry (1989) 46:1012–6. doi: 10.1001/archpsyc.1989.01810110054008
45. Tang HS, Huang CC, Chen KY, Chen CC. Reliability and validity of the Chinese version of the Yale Brown obsessive compulsive disorder scale (Y-BOCS). Taiwanese J Psychiatry (2006) 20:279–89.
46. Patton JH, Stanford MS, Barratt ES. Factor structure of the barratt impulsiveness scale. J Clin Psychol (1995) 51:768–74. doi: 10.1002/1097-4679(199511)51:63.0.co;2-1
47. Yao S, Yang H, Zhu X, Auerbach RP, Tong X. An examination of the psychometric properties of the chinese version of the barratt impulsiveness scale, 11th version in a sample of Chinese adolescents. Percept Mot Skills. (2007) 104:1169. doi: 10.2466/pms.104.4.1169-1182
48. Smyth C. The Pittsburgh sleep quality index (PSQI). J Gerontol Nurs. (1999) 25:10–1. doi: 10.3928/0098-9134-19991201-10
49. Tsai P-S, Wang S-Y, Wang M-Y, Su C-T, Yang T-T, Huang C-J, et al. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual Life Res (2005) 14:1943–52. doi: 10.1007/s11136-005-4346-x
50. Xiao S. Theoretical basis and research application of Social Support Scale. J Clin Psychiat. (1994) 4:98–100.
51. Lai S, Su C, Song S, Yan M, Tang C, Zhang Q, et al. Depression and deliberate self-harm among rural adolescents of Sichuan Province in Western China: A 2-year longitudinal study. Front Psychiatry (2021) 12:605785. doi: 10.3389/fpsyt.2021.605785
52. Shi L, Wang L, Jia X, Li Z, Mu H, Liu X, et al. Prevalence and correlates of symptoms of post-traumatic stress disorder among Chinese healthcare workers exposed to physical violence: a cross-sectional study. BMJ Open (2017) 7:e016810. doi: 10.1136/bmjopen-2017-016810
53. Weinstein A, Feder LC, Rosenberg KP, Dannon P. Internet addiction disorder: Overview and controversies. Behaviroral Addictions: Criteria, Evidence, and Treatment (New York: Academic Press) (2014). p. 99–117. doi: 10.1016/B978-0-12-407724-9.00005-7
54. Bozkurt H, Coskun M, Ayaydin H, Adak I. Prevalence and patterns of psychiatric disorders in referred adolescents with Internet addiction. Psychiatry Clin Neuriscience. (2013) 67(5):352–9. doi: 10.1111/pcn.12065
55. Wang Y, Qin Y, Li H, Yao DZ, Sun B, Li Z, et al. The modulation of reward and habit systems by acupuncture in adolescents with internet addiction. Neural Plasticity. (2020) 2020:7409417. doi: 10.1155/2020/7409417
56. McBride WJ. Role of serotonin in brain reward and regulation of alcohol drinking behavior. Handb Behav Neurosci (2010) 21:399–414. doi: 10.1016/S1569-7339(10)70092-8
57. Li Y, Zhong W, Wang D, Feng Q, Liu Z, Zhou J, et al. Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun (2016) 7:10503. doi: 10.1038/ncomms10503
58. Cespuglio R. Serotonin: its place today in sleep preparation, triggering or maintenance. Sleep Med (2018) 49:31–9. doi: 10.1016/j.sleep.2018.05.034
59. Pomrenze MB, Cardozo Pinto DF, Neumann PA, Llorach P, Tucciarone JM, Morishita W, et al. Modulation of 5-HT release by dynorphin mediates social deficits during opioid withdrawal. Neuron (2022) 110(24):4125–43. doi: 10.1016/j.neuron.2022.09.024
60. Werling AM, Grünblatt E. A review of the genetic basis of problematic Internet use. Curr Open Behav Sci (2022) 46:101149. doi: 10.1016/j.cobeha.2022.101149
61. Laje G, Cannon DM, Allen AS, Klaver JM, Peck SA, Liu X, et al. Genetic variation in HTR2A influences serotonin transporter binding potential as measured using PET and [11C] DASB. Int J NEUROPSYCHOPH. (2010) 13(6):715–24. doi: 10.1017/s1461145709991027
62. Falkenberg VR, Gurbaxani BM, Unger ER and Rajeevan MS. Functional genomics of serotonin receptor 2A (HTR2A): interaction of polymorphism, methylation, expression and disease association. Neuromol Med (2011) 13:66–76. doi: 10.1007/s12017-010-8138-2
63. Pérez-Rubio G, López-Flores LA, García-Carmona S, García-Gómez L, Noé-Díaz V, Ambrocio-Ortiz E, et al. Genetic variants as risk factors for cigarette smoking at an early age and relapse to smoking cessation treatment: A pilot study. Gene (2019) 694:93–6. doi: 10.1016/j.gene.2019.01.036
64. Erjavec GN, Tudor L, Perkovic MN, Podobnik J, Curkovic KD, Curkovic M, et al. Serotonin 5-HT2A receptor polymorphisms are associated with irritability and aggression in conduct disorder. Prog Neuro-Psychoph. (2022) 117:110542. doi: 10.1016/j.pnpbp.2022.110542
65. Mattina GF, Samaan Z, Hall GB, Steiner M. The association of HTR2A polymorphisms with obsessive-compulsive disorder and its subtypes: a meta-analysis. J Affect Disord (2020) 275:278–89. doi: 10.1016/j.jad.2020.06.016
66. Howell LL, Cunningham KA. Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev (2015) 67(1):176–97. doi: 10.1124/pr.114.009514
67. Sholler DJ, Stutz SJ, Fox RG, Boone EL, Wang Q, Rice KC, et al. The 5-HT2A receptor (5-HT2AR) regulates impulsive action and cocaine cue reactivity in male sprague-dawley rats. J Pharmacol Exp Ther (2019) 368(1):41–9. doi: 10.1124/jpet.118.251199
68. White MJ, Young RM, Morris CP, Lawford BR. Cigarette smoking in young adults: The influence of the HTR2A T102C polymorphism and punishment sensitivity. Drug Alcohol Dependence. (2011) 114(2-3):140–6. doi: 10.1016/j.drugalcdep.2010.08.014
69. Alrfooh A, Smith RM. Genetic and epigenetic analysis of the serotonin 2A receptor in the context of cocaine abuse. Epigenetics (2021) 17(10):1246–58. doi: 10.1080/15592294.2021.2005277
70. Bizot JC, Thiébot MH. Impulsivity as a confounding factor in certain animal tests of cognitive function. Cogn Brain Res (1996) 3(3-4):243–50. doi: 10.1016/0926-6410(96)00010-9
71. Boothman LJ, Allers KA, Rasmussen K, Sharp T. Evidence that central 5-HT2A and 5-HT2B/C receptors regulate 5-HT cell firing in the dorsal raphe nucleus of the anaesthetised rat. Br J Pharmacol (2003) 139:998–1004. doi: 10.1038/sj.bjp.0705328
72. Polesskaya OO, Sokolov BP. Differential expression of the “C” and “T” alleles of the 5-HT2A receptor gene in the temporal cortex of normal individuals and schizophrenics. J Neurosci Res (2002) 67(6):812–22. doi: 10.1002/jnr.10173
73. Elmenhorst D, Kroll T, Matusch A, Bauer A. Sleep deprivation increases cerebral serotonin 2A receptor binding in humans. Sleep (2012) 35(12):1615–23. doi: 10.5665/sleep.2230
74. Zhao X, Ozols AB, Meyers KT, Campbell J, McBride A, Marballi KK, et al. Acute sleep deprivation upregulates serotonin 2A receptors in the frontal cortex of mice via the immediate early gene Egr3. Mol Psychiatry (2022) 27(3):1599–610. doi: 10.1038/s41380-021-01390-w
Keywords: internet addiction disorder, 5-HT receptor, serotonin 2A receptor, rs6313 gene, gene polymorphism
Citation: Dai Y, Zhang C, Zhang L, Wen C, Li H and Zhu T (2024) Genetic polymorphism in HTR2A rs6313 is associated with internet addiction disorder. Front. Psychiatry 15:1292877. doi: 10.3389/fpsyt.2024.1292877
Received: 03 October 2023; Accepted: 29 January 2024;
Published: 14 February 2024.
Edited by:
Annagiulia Di Trana, National Institute of Health (ISS), ItalyReviewed by:
Ömer Faruk Akça, Meram Faculty of Medicine, TürkiyeAlessandro Di Giorgi, Marche Polytechnic University, Italy
Copyright © 2024 Dai, Zhang, Zhang, Wen, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianmin Zhu, dGlhbm1pbnpodUBjZHV0Y20uZWR1LmNu
 Yu Dai
Yu Dai Chenchen Zhang3
Chenchen Zhang3 Tianmin Zhu
Tianmin Zhu