
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 26 July 2024
Sec. Sleep Disorders
Volume 15 - 2024 | https://doi.org/10.3389/fpsyt.2024.1256116
Background: Alterations gastrointestinal diseases (GDs) were reported in individuals with obstructive sleep apnea (OSA), however, the genetic background between OSA and GDs is still unclear.
Methods: This investigation employed Mendelian randomization (MR) analyses to evaluate the causal effect between OSA and 19 types of GDs (gastroesophageal reflux disease (GERD), ulcerative colitis, celiac disease, Crohn’s disease, chronic gastritis, irritable bowel syndrome, primary biliary cholangitis, diverticular disease, gastroduodenal ulcer, acute pancreatitis, non-alcoholic fatty liver disease, primary sclerosing cholangitis, cirrhosis, calculus of bile duct, calculus of gallbladder, pancreatic cancer, gastric cancer, colorectal cancer, and esophageal cancer). The inverse-variance weighted (IVW) method was used to evaluate the main effects model of causality.
Results: This MR study suggests that OSA may play a causal role inflammation-related GDs (GERD, PIVW=5.94×10-9; gastroduodenal ulcer, PIVW=1×10-4; chronic gastritis, PIVW=0.0214; ulcerative colitis, PIVW=0.0296), and gallstones (calculi of the gallbladder, PIVW=0.0429; calculi of the bile duct, PIVW=0.0068). After accounting for obesity, type 2 diabetes, smoking, and alcohol consumption, the multivariate MR (MVMR) analysis identified that OSA is an independent risk factor for GERD, gastroduodenal ulcer, and calculus of the bile duct. The reverse MVMR analysis showed a causal effect of GERD on OSA. Besides, we did not find that the predisposition to OSA was associated with 4 cancers.
Conclusion: This MR analysis provides compelling evidence of an independent causal relationship between genetically predicted OSA and an elevated risk of inflammation-related GDs. Besides, no causal association was observed between OSA and cancers. Further studies should be carried out to verify our findings.
Obstructive sleep apnea (OSA) is the most prevalent form of sleep-disordered breathing with a global prevalence rate of 9–38% (1, 2). OSA can lead to intermittent hypoxemia, sleep fragmentation, sympathetic hyperactivity, variations in intrathoracic pressure, and disturbances in physiological balance (3). These symptoms greatly decrease one’s quality of life and increase morbidity of other diseases (4, 5).
The quality of sleep and sleep-related breathing disorders constitute significant factors contributing to the development of Gastrointestinal diseases (GDs) (6). Observational cohort studies have consistently indicated a higher prevalence of GDs among individuals with OSA compared to those without, encompassing conditions such as gallstones (7), non-alcoholic fatty liver disease (NAFLD) (8), inflammatory bowel disease (IBD) (9) and gastroesophageal reflux disease (GERD) (10–12). A retrospective study involving 586,377 adults in the USA revealed an independent association between OSA and GERD, accounting for multiple potential confounding variables (13). Continuous positive airway pressure (CPAP) therapy has demonstrated efficacy in alleviating gastroesophageal reflux symptoms and stabilizing NAFLD progression in patients with OSA (14, 15). However, these findings have encountered scrutiny in recent research (16–18). At present, there is limited evidence of associations between OSA and other common gastrointestinal disorders. In summary, the causal link between OSA and GDs remains contentious, with debates centering on potential confounders and reverse causation biases. A comprehensive evaluation of this causal relationship is crucial to unraveling the mechanisms underlying disease co-occurrence and identifying therapeutic strategies that benefit both conditions.
Mendelian randomization (MR) provides a more precise evaluation of causality for exposure–outcome associations by leveraging genetic variations as instrumental variables (IVs) (19–21). By generating genotypes through randomly assigning parental alleles during conception, MR minimizes the possibility of potential residual confounders and reverse causalities that could be influenced by environmental and self-imposed factors (22). MR has been widely used to predict causal pathways in studies examining the effects of OSA on atrial fibrillation (23), interleukin levels (24), and neurodegenerative disease (25). However, the effects of OSA on a broad range of gastrointestinal outcomes have not been investigated.
We performed a two-sample MR analysis and multivariate Mendelian randomization (MVMR) to evaluate whether OSA and common GDs are causally related. In addition, we used the same MR analysis methods in the reverse direction to determine whether GDs increased susceptibility to OSA.
We performed a bidirectional two-sample MR analysis to explore the causal relationships between OSA and the risk of developing one of 19 GDs. A flowchart of the study design is presented in Figure 1. The validity of genetic instruments is based on three critical principles. First, genetic variants employed as IVs should exhibit high confidence in their association with risk factors. Second, the chosen IVs should be independent of confounders that can influence the outcome. Third, IVs should not affect the outcome directly but only through their respective exposure traits (26, 27).

Figure 1 Study flow diagram. (A) The flow of Two-Sample MR analysis. (B) The flow of MVMR analysis. The dashed lines represent possible pleiotropic or direct causal effects between variables that may violate the MR assumptions. IVW, inverse-variance weighted; WM, weighted median; MR, Mendelian randomization; MR-PRESSO, MR pleiotropy residual sum and outlier; OSA, Obstructive sleep apnea; GDs, gastrointestinal diseases; IVs, instrumental variables; MVMR, Multivariate Mendelian randomization; LASSO, least absolute shrinkage and selection operator.
The analysis used summary-level data from publicly available GWAS projects and consortia to investigate the potential correlation between OSA and 19 GDs. No overlapping data on exposures and outcomes were permitted, minimizing the likelihood of statistical bias. Each GWAS used in the study obtained approval from the relevant ethics committees. Table 1 provides an overview of the source information for the GWAS data incorporated in this study.
The statistical GWAS data for OSA were obtained from the FinnGen project (DATA FREEZE 8, https://www.finngen.fi/en), the largest independent GWAS project. The FinnGen data was preferred because it minimized the confounding bias caused by overlapping populations. Therefore, GWAS summary statistics obtained from 33,423 patients with OSA and 307,648 controls were included in this FinnGen study. The criteria for the diagnosis of OSA were an apnea–hypopnea index ≥5/h or respiratory event index ≥5/h (28).
A total of 19 common gastrointestinal diseases were included in this study. The GWAS summary-level data for the relationships between genetic variants and 18 types of GDs were obtained from the Integrative Epidemiology Unit (IEU) open GWAS project (https://gwas.mrcieu.ac.uk/). The GDs included GERD, gastroduodenal ulcer, gastric cancer, Crohn’s disease, ulcerative colitis, colorectal cancer, cirrhosis, primary sclerosing cholangitis, primary biliary cholangitis, chronic gastritis, esophageal cancer, calculus of the gallbladder, calculus of the bile duct, irritable bowel syndrome, celiac disease, diverticular disease, and acute pancreatitis. The summary-level data from the GWAS on NAFLD were obtained from the study by Anstee et al. (29).
The GWAS summary data for OSA utilized in this research was derived from the Finngen Project, an independent GWAS endeavor (28). This guarantees no sample overlap between the OSA GWAS summary statistics and the GDs-related GWAS statistics, thus ensuring the strength of the MR results.
For global screening of IVs, genome-wide significance level (p < 1×10-05) was used as a threshold for screening IVs significantly associated with exposure factors. This threshold ensures a significant association between IVs and exposure factors while ensuring that as many IVs as possible are obtained for subsequent analysis (30). A minor allele frequency threshold of 0.3 was permitted for palindromic single nucleotide polymorphisms (SNPs). SNPs with low linkage disequilibrium were excluded with a strict r2 cutoff of 0.001 and a clumping window greater than 10,000 kb. Detailed information on the SNPs employed as IVs is provided in Supplementary Table S1. As shown in Supplementary Table S1, the genome-wide significance p value between all IVs included in the analysis and the outcome phenotype was greater than 1×10-05. This ensured that there was no direct correlation between IVs and outcome phenotypes. To prevent weak instrument bias, the statistical strength of IVs was evaluated by calculating the F-statistic. When the F-statistic is greater than 10, IV is considered to have good statistical performance (31, 32):
In this context, R2 denotes the fraction of variability in exposure that can be ascribed to genetic variants. The variable n denotes the sample size of the exposed phenotype, and k represents the total number of IVs included in each MR analysis.
All statistical analyses were performed using R version 4.1.1 (R Foundation for Statistical Computing; Vienna, Austria). The two-sample MR analysis was performed utilizing the TwoSampleMR package (version 0.5.6); the MVMR analysis was performed using the MVMR package (version 0.3).
The principal method adopted in this research for determining the causal association between OSA and GDs was the IVW approach using a random-effects model. To ensure the reliability of the results and identify potential horizontal pleiotropy, multiple sensitivity analyses were carried out, inclusive of the weighted median, MR-Egger, weighted mode, and simple mode methods. Each of these analyses relies on distinct assumptions necessary for making valid inferences. The IVW method computes the effect as the IVW average of the ratio estimates of SNPs, with first-order weights based on each SNP serving as a valid IV (26, 33). The weighted-median method requires >50% of the chosen genetic instruments to be valid; the weighted mode produces reliable outcomes when the percentage of invalid instruments exceeds 50% (34). The MR-Egger method can yield a valid estimate of causal effects even if all SNPs are invalid instruments because it employs weighted regression without a restricted intercept (35). The weighted mode method is also resilient against invalid or pleiotropic SNPs, taking the IVW empirical density function mode as an effect estimate (36). The reliability of the causal inference conclusions is enhanced when the findings of all the methods are broadly consistent. However, causality inferred from other methods may have greater confidence intervals and standard errors than those derived from IVW (32, 37).
This study’s causal effect estimates of genetically predicted OSA on GDs are expressed as ORs with corresponding 95% CIs. The Bonferroni correction for multiple testing was conducted to correct P values. A P value less than 1.32 × 10−03 (0.05/19/2; 2 denotes both forward and reverse MR tests) was considered as strong evidence of a causal association. A value of p<0.05 was considered suggestive of a correlation.
Because obesity, T2D, smoking, and alcohol consumption are common risk factors for most GDs and OSA, MVMR analysis was used to estimate the risk effect of OSA on GDs after adjusting for these risk factors (38, 39). By integrating several risk exposures into the MR Analysis concurrently, MVMR elucidates whether each discrete risk exposure has an independent causal effect on the outcome when multiple risk exposures co-exist (40). In this research, OSA was amalgamated with the aforementioned four common risk factors as an exposure variable, and an MVMR model was assembled to assess whether OSA exposure had a direct association with GDs outcomes. Given that collinearity bias may be introduced if excessive exposure variables are incorporated in the MVMR model simultaneously, this study used each common risk factor individually with OSA exposure to construct an MVMR model. MVMR-Robust, MVMR-IVW, and the least absolute shrinkage and selection operator (LASSO) were used to determine the independent effects of OSA on GDs (41). Table 1 provides an overview of the source information for the risk-factors-related GWAS data used in MVMR analysis incorporated in this study. Reverse MVMR analysis using the same strategy determined the independent effect of GDs exposure on the risk of OSA outcomes.
Cochran’s Q-test of the IVW and MR-Egger methods was used to assess heterogeneity. Pleiotropy was evaluated using the intercept of the MR-Egger analysis. The Mendelian randomization pleiotropy residual sum and outlier (MR-PRESSO) approach was used to identify potential outlier SNPs through a global test, repeating the analysis after eliminating the outlying SNPs. This method provides a distortion test that can compare differences before and after removing outliers (37). Funnel plots were used to show the discrete relationships between SNPs and as a complementary basis for removing potential outliers. The Steiger directionality test was utilized to ascertain the causal direction between the assumed exposure and potential outcomes. This test can determine whether the genetic variant used as an IV for the exposure trait is also causally related to the outcome or whether it influences the outcome through the exposure trait (42). Leave-one-out analysis was performed by omitting each SNP to evaluate whether a single SNP influenced or resulted in remarkable MR results (43).
For this investigation, the globally significant threshold for IVs screening was set at p<1×10-5, r2 = 0.001, and a clumping window >10,000 kb. Based on these criteria, 747 SNPs were selected as IVs to evaluate causal relationships linking OSA to all 19 GDs, using OSA as the exposure factor. Unfortunately, when GDs were utilized as exposure factors during reverse MR analysis, the IVs that could be extracted from the genome-wide association study (GWAS) summary data on calculus of the bile duct and acute pancreatitis were insufficient. Consequently, no causal association between these two conditions and OSA was identified. The included IVs are detailed in Supplementary Table S1. Supplementary Table S2 presents the IVs featured in the MR analysis that identified a potential causal link between OSA and GDs.
Utilizing the two-sample MR method, with liability to OSA as the exposure and the risk of 19 GDs as outcomes, our analysis found that a genetic inclination towards OSA exhibited an association with 6 types of GDs risk (p < 0.05). According to IVW method, OSA exposure was significantly associated with an increased risk of GERD and gastroduodenal ulcer (p < 1.32 × 10−03). Genetically predicted OSA was significantly associated with a 1.1669 greater odds of GERD (95% confidence interval [CI]: 1.1078–1.2292, p=5.94×10-9; Figure 2A), 1.0019 greater odds of gastroduodenal ulcer (95% CI: 1.0010–1.0029; p=1×10-4). And OSA shows a suggestive correlation with 1.0008 greater odds of chronic gastritis (95% CI: 1.0001–1.0015; p=0.0214). The effect estimates from the sensitivity analysis methods (weighted median, MR-Egger, weighted mode, and simple mode methods) were consistent with IVW method.
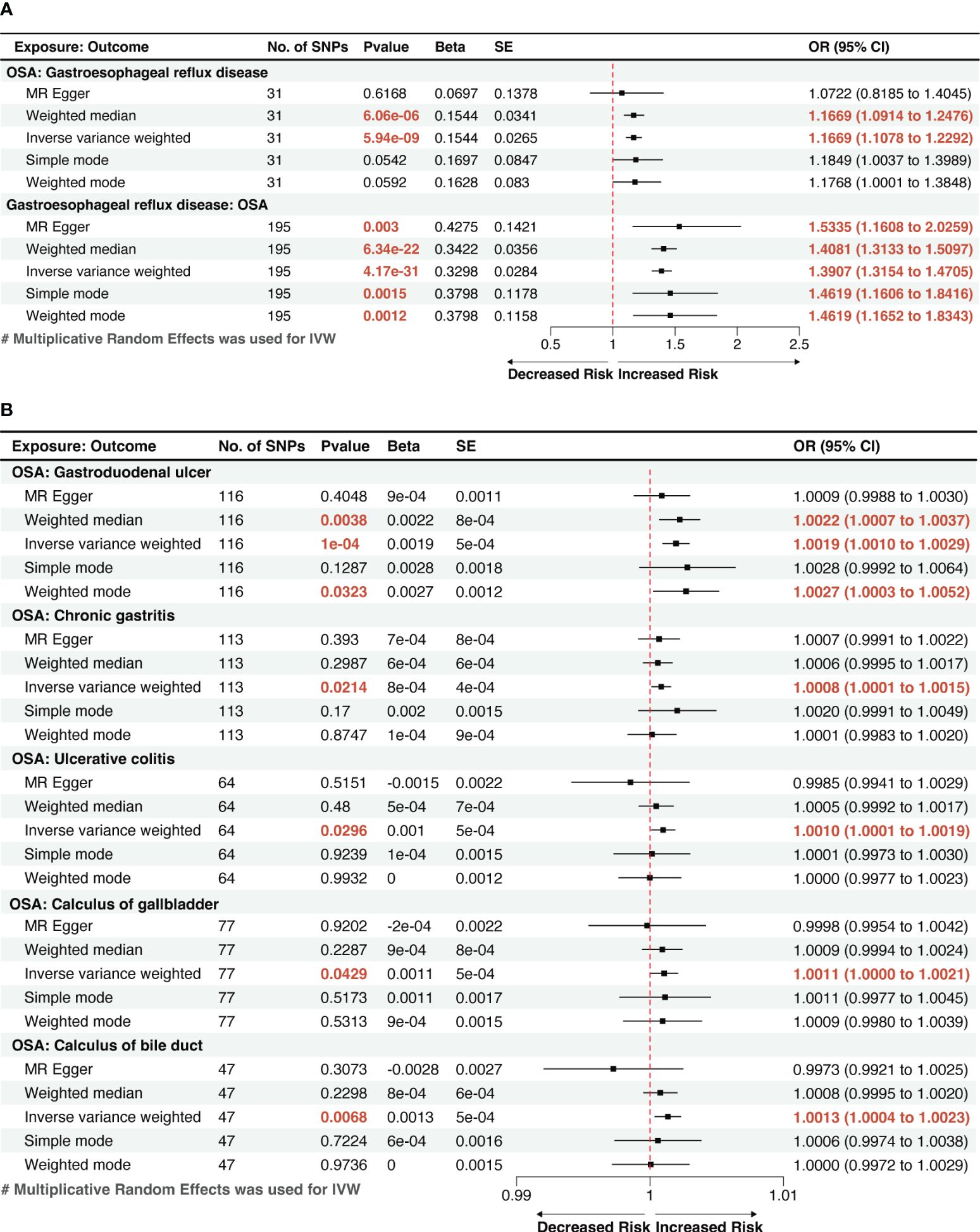
Figure 2 A forest plot of the potential causal relationship between OSA and GDs. (A) The forest plot of the potential causal relationship between OSA and GERD; (B) The forest plot of the potential causal relationship between OSA and other GDs. OSA, Obstructive sleep apnea; GERD, gastroesophageal reflux disease; GDs, gastrointestinal diseases; SNP, single nucleotide polymorphism; OR, odds ratio; SE, standard error; CI, confidence interval; IVW, inverse-variance weighted.
We also observed suggestive causal associations between genetically predicted OSA with ulcerative colitis (Odds ratio [OR]IVW =1.0010, 95% CI: 1.0001–1.0019, p=0.0296) and gallstones, including calculi of the gallbladder (ORIVW =1.0011, 95% CI: 1.0000–1.0021, p=0.0429) and calculi of the bile duct (ORIVW =1.0013, 95% CI: 1.0004–1.0023, p=0.0068). However, these causal associations were weakened in MR-Egger method, but effect estimates from other sensitivity analysis methods (weighted median, weighted mode, and simple mode methods) were still consistent with IVW method. (Figure 2B). The risk of the other 13 types of GDs was not significantly associated with genetically predicted OSA according to the MR analysis.
MVMR analysis indicated that OSA was an independent risk factor for GERD, gastroduodenal ulcer, and calculus of bile duct (Figures 3, 4). However, the effects of OSA on chronic gastritis (adjusted ORIVW =1.0007, p=0.0970), ulcerative colitis (adjusted ORIVW =1.0007, p=0.0970), and gallbladder calculus (adjusted ORIVW =1.0019, p=0.3621) were no longer significant in MVMR analysis after considering obesity (Figure 4).
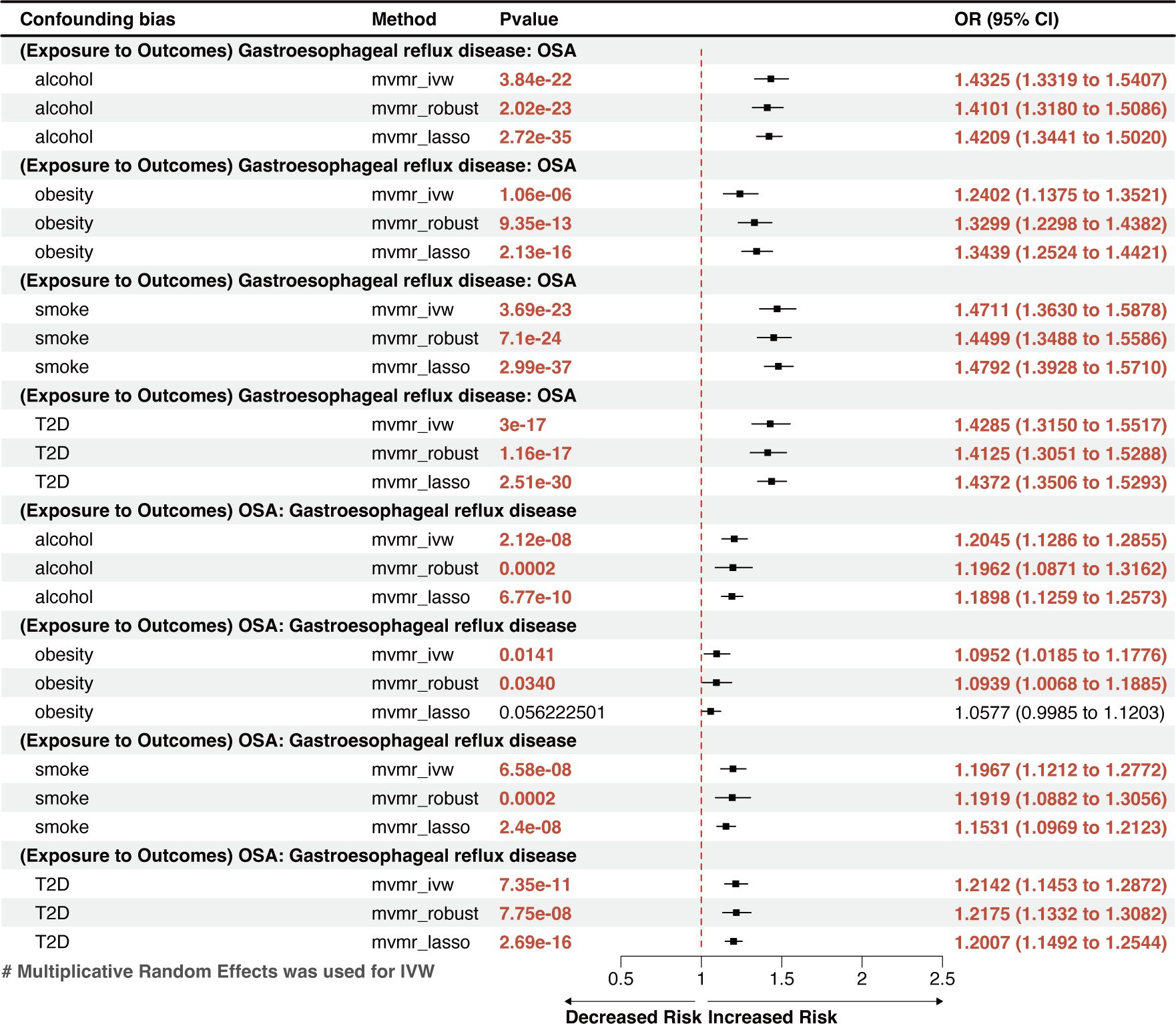
Figure 3 A forest plot of the MVMR results between OSA and GERD. OSA, Obstructive sleep apnea; GERD, gastroesophageal reflux disease; OR, odds ratio; CI, confidence interval; IVW, inverse-variance weighted; LASSO, least absolute shrinkage and selection operator; MVMR, multivariate Mendelian randomization.
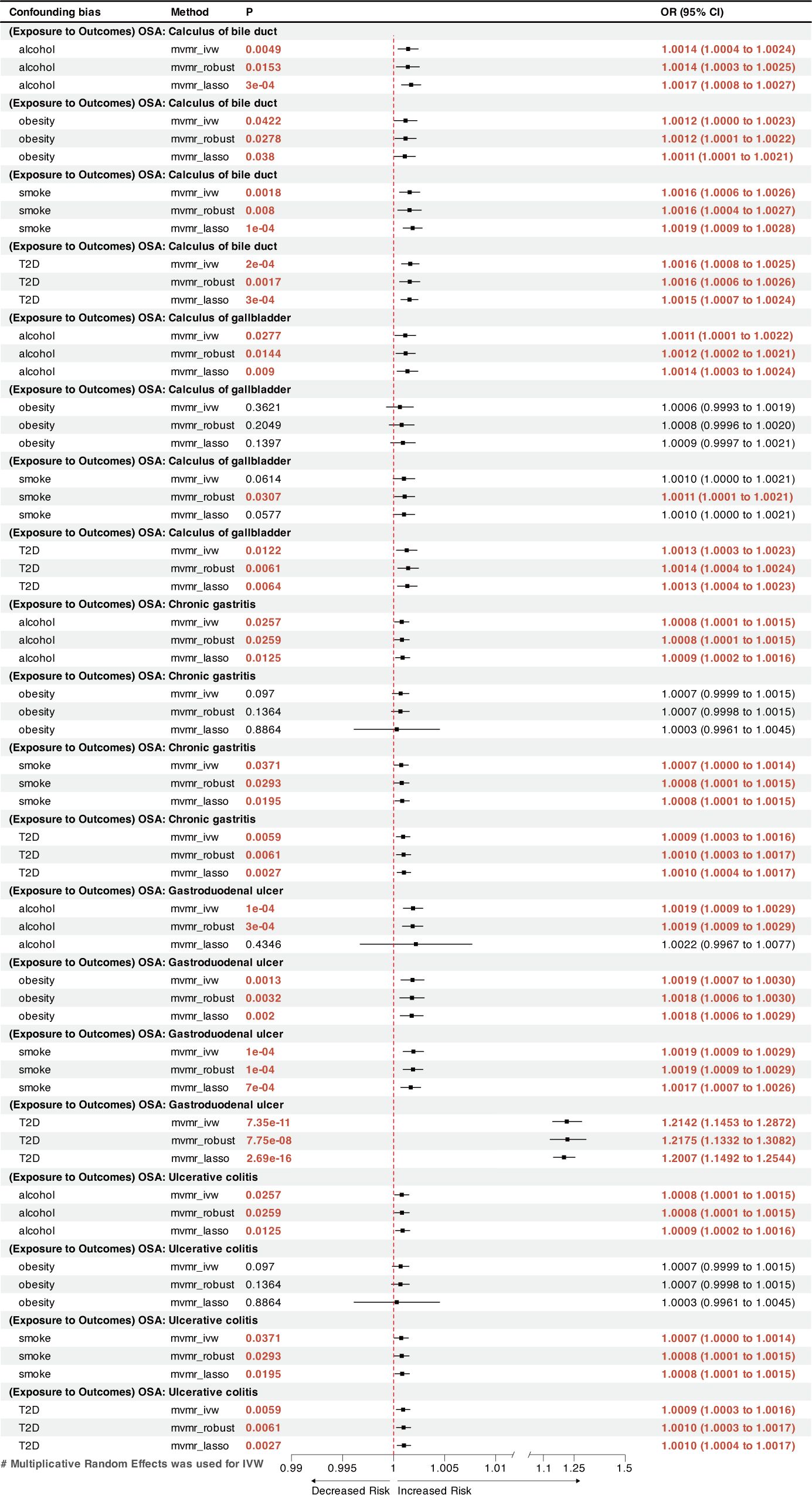
Figure 4 A forest plot of the MVMR results between OSA and other GDs. OSA, Obstructive sleep apnea; GDs, gastrointestinal diseases; OR, odds ratio; CI, confidence interval; IVW, inverse-variance weighted; LASSO, least absolute shrinkage and selection operator; MVMR, multivariate Mendelian randomization.
In the reversed-direction MR study, the potential causal effects of genetically predicted liability for each GD’s exposure on OSA were estimated using the factors found to be statistically significant (p<0.05). Acute pancreatitis and calculus of the bile duct were excluded from the MR analysis because of insufficient IVs (IVs < 3).
The reverse MR analysis showed that GERD had a remarkable causal effect on OSA, demonstrated by an effect estimate of 1.3907 (95% CI: 1.3154–1.4705, p=4.17×10-31) for GERD using the IVW method (Figure 2A). This result is in line with the outcomes from other methods, including MR-Egger (OR=1.5335, 95% CI: 1.1608–2.0259, p=0.0029) and the weighted-median method (OR=1.4081, 95% CI: 1.3033–1.5097, p=6.34×10-22), which showed significant associations with greater confidence intervals. Genetically predicted GERD remained associated with OSA after accounting for obesity, type 2 diabetes (T2D), smoking, and alcohol consumption in the MVMR analysis (Figure 3). Other genetically determined GDs were not observed to be significantly associated with OSA risk. The detailed reverse MR results are presented in Supplementary Table S3.
A total of six types of GDs were found to be causally correlated with OSA by Two-sample MR analysis. A total of 331 IVs were used in those MR analysis, corresponding to F- statistic ranging from 137.26 to 1228.67 (F-statistic > 10), which ensured that there was no weak instrument bias in the included IVs (Supplementary Table S2). A variety of methods were used to evaluate the dispersion and heterogeneity among IVs. The scatter plots (Supplementary Figure S1) and funnel plot (Supplementary Figure S2) shows the distribution of IVs in the potential causal relationship between OSA and 6 types of GDs. The leave-one-out analysis showed that single IVs had little effect on the causal association between OSA and GDs (Supplementary Figures S3-S9).
The Cochran’s Q test was used to identify potential heterogeneity. Supplementary Table S4 shows Cochran’s Q test results. In the significant causal association between OSA exposure and GDs outcomes, no heterogeneity was found between IVs (PCochran’s Q > 0.05). When GERD is an exposure factor, the potential causal association with OSA outcome may be influenced by heterogeneity (PCochran’s Q < 0.05). Therefore, the random-effects IVW method was used in this study to eliminate the bias caused by possible heterogeneity.
The MR-Egger intercept analysis was used for the potential horizontal pleiotropy of MR Analysis between OSA and GDs. On the whole, horizontal pleiotropy was not discovered by MR-Egger intercept analysis. (P > 0.05, Supplementary Table S5). MR-PRESSO was used to identify potential outlier IVs and to supplement the evaluation of level pleiotropy. Four potential outliers were detected in the MR-PRESSO analysis of the effect of OSA on GERD. After those IVs removal, horizontal pleiotropy was eliminated. However, in the reverse-MR analysis, possible underlying heterogeneity for the effect of GERD on OSA persisted after eliminating the two SNP outliers (MR-PRESSO Globle Test Pvalue < 0.05, Supplementary Table S6). The MR Steiger’s test did not find a potential reverse causal association affecting the robustness of the results (Table 2).
This MR analysis is the first to systematically evaluate the cause-and-effect relationship between OSA and digestive disorders. Two-sample MR analysis found a strong correlation between OSA and the incidence of gastroduodenal ulcer and GERD. A nominal causal association was found between OSA and increased risk of calculus of bile duct, calculus of gallbladder, chronic gastritis, and ulcerative colitis. The MVMR analysis found a reciprocal causal link between OSA and GERD. After adjusting for four risk factors (obesity, T2D, smoking, and alcohol consumption) in MVMR analysis, OSA increases the risk of gastroduodenal ulcer and calculus of the bile duct independently.
Numerous epidemiological studies have provided compelling evidence linking several gastrointestinal disorders to sleep disorders such as OSA (6, 10, 44). OSA can induce intermittent hypoxemia, sleep fragmentation, oxidative stress, systemic inflammatory response, and disrupt intestinal flora, potentially contributing to the onset of gastrointestinal diseases. Conversely, nocturnal awakenings, sleep deprivation and sleep fragmentation due to gastrointestinal diseases may reciprocally influence the occurrence of OSA. However, the causal relationship remains unclear due to limitations in temporal sequencing in existing observational studies. To address this gap, we conducted bidirectional MR and MVMR analyses to robustly evaluate the causal connections between OSA and common GDs, providing robust evidence of their causal associations. This findings underscores the importance of early screening for these disorders in OSA-diagnosed patients.
Global population–based studies observed an 8–33% incidence rate of GERD (11). However, individuals with OSA have much greater rates, ranging from 40% to 60% (45, 46). A retrospective study of 586,377 adults in the USA discovered an independent link between OSA and GERD while considering multiple potential confounders (13). However, another multicenter, cross-sectional, observational study conducted in Turkey (n=1104) found that the severity of OSA did not independently predict the occurrence of GERD (17). Recently, an MR study discovered an implied bidirectional relationship between OSA and GERD (47); however, it was unclear whether the results were affected by shared risk factors, particularly obesity. Our findings, which were based on MR techniques that accounted for obesity, smoking, alcohol use, and T2D, indicated a bidirectional, independent causal link between GERD and OSA. This finding can benefit future research on the comorbidity of the two diseases and the optimization of a joint treatment plan.
The supine position itself increases the occurrence of nocturnal GER (48). GER events can be caused by declining intrathoracic negative pressure and elevating abdominal pressure during apneic episodes in OSA patients. This pressure fluctuation also relaxes and opens the lower esophageal sphincter, worsening GERD symptoms (10, 49). Common symptoms of GERD include heartburn or regurgitation, non-cardiac chest pain, chronic cough, and sore throat (50). Nocturnal uncomfortable symptoms can lead to heightened sleep arousal and diminished sleep duration by influencing the interaction of various neural pathways (51). The gastric content of reflux can irritate edema and cause spasms in the upper airway and larynx, thereby contributing to the collapse of the upper airway (10). Additionally, reflux can stimulate the vagus nerve, cause bronchial constriction (52), and affect normal systolic function of the respiratory muscles (53), potentially leading to sleep apnea. The complex pathophysiology underlying their causal relationship warrants further investigation.
The present investigation found previously unrecognized causal links between a genetic predisposition to OSA and a greater incidence of gastroduodenal ulcer and chronic gastritis. An earlier study conducted by Wu et al. found that OSA could affect duodenal morphology via increased oxidative stress and activation of transcription factors, which can lead to the disruption of intestinal tight junctions, culminating in intestinal damage (54). This disruption may lead to the development of gastroduodenal ulcer. Furthermore, multiple cross-sectional studies have shown that shorter sleep durations and poor sleep quality increase the likelihood of gastroduodenal ulcer recurrence (55, 56). The MVMR analysis showed that OSA’s effect on chronic gastritis was diminished after accounting for obesity, possibly indicating that OSA and obesity synergistically contribute to chronic gastritis.
Inflammatory bowel diseases (IBD), including Crohn’s disease and ulcerative colitis, represent chronic inflammatory condition of the gastrointestinal tract. The pathogenesis of intestinal lesions in IBD remains elusive. The bidirectional interactions between sleep disorders and IBD have recently received considerable attention (57, 58). OSA causes intermittent hypoxia, which results in periodic reductions in the oxygen partial pressure gradient within the lumen, promoting intestinal dysbiosis and inflammation (59). Disruption of the circadian rhythm can impair normal intestinal immune system activity, leading to an increased release of inflammatory factors and exacerbating intestinal inflammation (60–62). A retrospective study using US-wide diagnostic coding data independently linked IBD to a higher prevalence of OSA (63), consistent with the findings of this MR analysis. The potential causal relationship between genetically predicted OSA and ulcerative colitis identified in this study may provide new perspectives to explore the pathogenesis and treatment of IBD.
Moreover, we observed a positive link between OSA and gallstones, including calculi in the gallbladder and bile duct. This outcome is consistent with the finding from a cross-sectional study conducted by Chen et al. on 3,827 patients (7). OSA may cause gallstones by impeding gallbladder contraction through stimulating sympathetic nerves and enhancing bile supersaturation through inflammation associated with chronic intermittent hypoxia (64, 65). A previous Mendelian analysis showed that 1−standard deviation increase in the body mass index resulted 1.631 times increased odds of developing gallstones (66). However, the current MVMR analysis found that the causal relationship between OSA and gallbladder calculi was no longer significant after considering obesity; the causal relationship with bile duct calculi remained. This result suggests that OSA may affect gallbladder calculi, but not an independent risk factor.
Recent research has revealed a connection between OSA and the initiation and progression of NAFLD (67), indicating that patients with OSA face poor prognoses and are susceptible to other liver conditions (68, 69). However, MR results did not reveal a significant causal association between genetic predisposition to OSA and the development of NAFLD or cirrhosis. In addition, separate epidemiological studies by Nieto et al. (70) and Marshall et al. (71) reported significantly higher cancer mortality rates among patients with sleep apnea. However, Kendzerska et al. (72) concluded that OSA severity does not increase cancer incidence. Our study found no correlations between genetically predicted OSA and gastrointestinal cancers, including esophageal, gastric, or colorectal cancers. These findings suggest that the observed epidemiological relationships may stem from shared genetic components or unmeasured confounding factors.
This study had several advantages. First, this study is the first to comprehensively assess the cause-and-effect relationship underlying OSA with major GDs. We utilized two-sample MR based on SNPs from large sample populations (33,423 cases and 307,648 controls for OSA; an average of 9,530 cases and 299,113 controls for each GD); the sample sizes for exposure and outcomes did not overlap. Second, multiple MR statistical methods and multivariate MR analyses were applied to minimize confounding bias and guarantee the reliability of the findings, including adjustments for obesity, T2D, smoking, and alcohol consumption.
This study had limitations. First, the summary-level data from the GWASs included trans-ancestry populations among the source populations, as shown in Table 1. This inclusion could bias results because of the possibility of population mixture confounding. Second, MVMR analysis was performed for common risk factors for OSA and GDs, but other potential confounders may have a potential impact on the independence of causal association. Third, completely excluding the possibility that the SNPs linked to GERD affect OSA via additional causative pathways (i.e., horizontal pleiotropy) was challenging.
This MR analysis provides compelling evidence of an independent causal relationship between genetically predicted OSA and an elevated risk of multiple inflammation-related GDs. In summary, this study provides strong evidence that OSA and GERD are independent risk factors for each other. In addition, a novel independent causal association was found between genetically predicted OSA and gastroduodenal ulcer. The increased risk of chronic gastritis, ulcerative colitis, and gallstones (including calculus in the gallbladder and bile duct) may also be correlated directly with OSA. Understanding the biological pathways and mechanisms underlying these correlations will aid physicians and researchers in developing preventive and therapeutic strategies for patients with OSA presenting with gastrointestinal symptoms.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
WY: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – original draft. JZ: Data curation, Investigation, Validation, Writing – original draft. MJ: Conceptualization, Supervision, Validation, Writing – review & editing. YK: Data curation, Formal analysis, Writing – original draft. HQ: Data curation, Visualization, Writing – original draft. YQ: Data curation, Formal analysis, Writing – original draft. SW: Conceptualization, Investigation, Supervision, Validation, Writing – review & editing. JT: Conceptualization, Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from Public service development and reform pilot project of Beijing Medical Research Institute (BMR2021-3) National natural science foundation of China (82271193), Capital's Funds for Health Improvement and Research (2022-2-1132), Beijing Hospitals Authority’s Ascent Plan (DFL20221102), Beijing Hospitals Authority clinical medicine development project (YGLX202335).
The authors wish to acknowledge all the participants and investigators for contributing to and sharing summary-level data on the GWAS.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1256116/full#supplementary-material
Supplementary Figure 1 | Scatter plot of the effect of the potential causal relationship between OSA and GDs. Figures (A–G) show the MR results in the significant causal relationship between OSA and GDs. The slope equals the b-value calculated using the five methods, representing the causal effect. The positive slope indicates that exposure is a risk factor, whereas a negative slope is the opposite. OSA, Obstructive sleep apnea; GDs, gastrointestinal diseases; MR, Mendelian randomization; SNP, single nucleotide polymorphisms; IVs, instrumental variables.
Supplementary Figure 2 | Funnel plot of the effect of the potential causal relationship between OSA and GDs. Figures (A–G) show the distribution of IVs in the significant causal relationship between OSA and GDs, respectively. Red boxes indicate potential outliers that were eliminated from the analysis. OSA, Obstructive sleep apnea; GDs, gastrointestinal diseases; MR, Mendelian randomization; SNP, single nucleotide polymorphisms; IVs, instrumental variables; SE, standard error.
Supplementary Figure 3 | MR leave-one-out sensitivity analysis for ‘Gastroesophageal reflux disease’ on ‘OSA’. MR, Mendelian randomization; OSA, Obstructive sleep apnea.
Supplementary Figure 4 | MR leave-one-out sensitivity analysis for ‘OSA’ on ‘Gastroduodenal ulcer’. MR, Mendelian randomization; OSA, Obstructive sleep apnea.
Supplementary Figure 5 | MR leave-one-out sensitivity analysis for ‘OSA’ on ‘Chronic gastritis’. MR, Mendelian randomization; OSA, Obstructive sleep apnea.
Supplementary Figure 6 | MR leave-one-out sensitivity analysis for ‘OSA’ on ‘Ulcerative colitis’. MR, Mendelian randomization; OSA, Obstructive sleep apnea.
Supplementary Figure 7 | MR leave-one-out sensitivity analysis for ‘OSA’ on ‘Calculus of gallbladder’. MR, Mendelian randomization; OSA, Obstructive sleep apnea.
Supplementary Figure 8 | MR leave-one-out sensitivity analysis for ‘OSA’ on ‘Calculus of bile duct’. MR, Mendelian randomization; OSA, Obstructive sleep apnea.
Supplementary Figure 9 | MR leave-one-out sensitivity analysis for ‘Gastroesophageal reflux disease’ on ‘OSA’. MR, Mendelian randomization; OSA, Obstructive sleep apnea.
1. Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, et al. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev. (2017) 34:70–81. doi: 10.1016/j.smrv.2016.07.002
2. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. (2019) 7:687–98. doi: 10.1016/S2213-2600(19)30198-5
3. Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: A review. JAMA. (2020) 323:1389–400. doi: 10.1001/jama.2020.3514
4. Brodie KD, Goldberg AN. Obstructive sleep apnea: A surgeon's perspective. Med Clin North Am. (2021) 105:885–900. doi: 10.1016/j.mcna.2021.05.010
5. Yeghiazarians Y, Jneid H, Tietjens JR, Redline S, Brown DL, El-Sherif N, et al. Obstructive sleep apnea and cardiovascular disease: A scientific statement from the american heart association. Circulation. (2021) 144:e56–67. doi: 10.1161/CIR.0000000000000988
6. Orr WC, Fass R, Sundaram SS, Scheimann AO. The effect of sleep on gastrointestinal functioning in common digestive diseases. Lancet Gastroenterol Hepatol. (2020) 5:616–24. doi: 10.1016/S2468-1253(19)30412-1
7. Chen CH, Lin CL, Hsu CY, Kao CH. Risk of gallstones in patients with obstructive sleep apnea: a nationwide observational cohort study. Sleep Breath. (2019) 23:355–62. doi: 10.1007/s11325-018-1696-5
8. Mesarwi OA, Loomba R, Malhotra A. Obstructive sleep apnea, hypoxia, and nonalcoholic fatty liver disease. Am J Respir Crit Care Med. (2019) 199:830–41. doi: 10.1164/rccm.201806-1109TR
9. Valentin S, Renel B, Manneville F, Caron B, Choukour M, Guillaumot A, et al. Prevalence of and factors associated with respiratory symptoms among patients with inflammatory bowel disease: A prospective study. Inflamm Bowel Dis. (2023) 29:207–16. doi: 10.1093/ibd/izac062
10. Lim KG, Morgenthaler TI, Katzka DA. Sleep and nocturnal gastroesophageal reflux: an update. Chest. (2018) 154:963–71. doi: 10.1016/j.chest.2018.05.030
11. El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. (2014) 63:871–80. doi: 10.1136/gutjnl-2012-304269
12. Pardak P, Filip R, Wolinski J, Krzaczek M. Associations of obstructive sleep apnea, obestatin, leptin, and ghrelin with gastroesophageal reflux. J Clin Med. (2021) 10:5195. doi: 10.3390/jcm10215195
13. Gilani S, Quan SF, Pynnonen MA, Shin JJ. Obstructive sleep apnea and gastroesophageal reflux: A multivariate population-level analysis. Otolaryngol Head Neck Surg. (2016) 154:390–5. doi: 10.1177/0194599815621557
14. Wang L, Han H, Wang G, Liu H, Sun Z, Li B, et al. Relationship between reflux diseases and obstructive sleep apnea together with continuous positive airway pressure treatment efficiency analysis. Sleep Med. (2020) 75:151–5. doi: 10.1016/j.sleep.2020.07.024
15. Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism. (2016) 65:1124–35. doi: 10.1016/j.metabol.2016.05.004
16. Shepherd K, Orr W. Mechanism of gastroesophageal reflux in obstructive sleep apnea: airway obstruction or obesity? J Clin Sleep Med. (2016) 12:87–94. doi: 10.5664/jcsm.5402
17. Basoglu OK, Vardar R, Tasbakan MS, Ucar ZZ, Ayik S, Kose T, et al. Obstructive sleep apnea syndrome and gastroesophageal reflux disease: the importance of obesity and gender. Sleep Breath. (2015) 19:585–92. doi: 10.1007/s11325-014-1051-4
18. El Hage Chehade N, Fu Y, Ghoneim S, Shah S, Song G, Fass R. Association between obstructive sleep apnea and gastroesophageal reflux disease: A systematic review and meta-analysis. J Gastroenterol Hepatol. (2023) 38:1244–51. doi: 10.1111/jgh.16245
19. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. (2008) 27:1133–63. doi: 10.1002/sim.3034
20. Swerdlow DI, Kuchenbaecker KB, Shah S, Sofat R, Holmes MV, White J, et al. Selecting instruments for Mendelian randomization in the wake of genome-wide association studies. Int J Epidemiol. (2016) 45:1600–16. doi: 10.1093/ije/dyw088
21. Burgess S, Small DS, Thompson SG. A review of instrumental variable estimators for Mendelian randomization. Stat Methods Med Res. (2017) 26:2333–55. doi: 10.1177/0962280215597579
22. Burgess S, Timpson NJ, Ebrahim S, Davey Smith G. Mendelian randomization: where are we now and where are we going? Int J Epidemiol. (2015) 44:379–88. doi: 10.1093/ije/dyv108
23. Chen W, Cai X, Yan H, Pan Y. Causal effect of obstructive sleep apnea on atrial fibrillation: A mendelian randomization study. J Am Heart Assoc. (2021) 10:e022560. doi: 10.1161/JAHA.121.022560
24. Yi M, Zhao W, Fei Q, Tan Y, Liu K, Chen Z, et al. Causal analysis between altered levels of interleukins and obstructive sleep apnea. Front Immunol. (2022) 13:888644. doi: 10.3389/fimmu.2022.888644
25. Li J, Zhao L, Ding X, Cui X, Qi L, Chen Y. Obstructive sleep apnea and the risk of Alzheimer's disease and Parkinson disease: A Mendelian randomization study OSA, Alzheimer's disease and Parkinson disease. Sleep Med. (2022) 97:55–63. doi: 10.1016/j.sleep.2022.06.004
26. Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. (2019) 4:186. doi: 10.12688/wellcomeopenres
27. Benn M, Nordestgaard BG. From genome-wide association studies to Mendelian randomization: novel opportunities for understanding cardiovascular disease causality, pathogenesis, prevention, and treatment. Cardiovasc Res. (2018) 114:1192–208. doi: 10.1093/cvr/cvy045
28. Strausz S, Ruotsalainen S, Ollila HM, Karjalainen J, Kiiskinen T, Reeve M, et al. Genetic analysis of obstructive sleep apnoea discovers a strong association with cardiometabolic health. Eur Respir J. (2021) 57:2003091. doi: 10.1183/13993003.03091-2020
29. Anstee QM, Darlay R, Cockell S, Meroni M, Govaere O, Tiniakos D, et al. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically characterised cohort(☆). J Hepatol. (2020) 73:505–15. doi: 10.1016/j.jhep.2020.04.003
30. Li P, Wang H, Guo L, Gou X, Chen G, Lin D, et al. Association between gut microbiota and preeclampsia-eclampsia: a two-sample Mendelian randomization study. BMC Med. (2022) 20:443. doi: 10.1186/s12916-022-02657-x
31. Palmer TM, Lawlor DA, Harbord RM, Sheehan NA, Tobias JH, Timpson NJ, et al. Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res. (2012) 21:223–42. doi: 10.1177/0962280210394459
32. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x
33. Burgess S, Bowden J, Fall T, Ingelsson EThompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology. (2017) 28:30–42. doi: 10.1097/EDE.0000000000000559
34. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965
35. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080
36. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46:1985–98. doi: 10.1093/ije/dyx102
37. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
38. Luo Q, Li N, Zhu Q, Yao X, Wang M, Heizhati M, et al. Non-dipping blood pressure pattern is associated with higher risk of new-onset diabetes in hypertensive patients with obstructive sleep apnea: UROSAH data. Front Endocrinol (Lausanne). (2023) 14:1083179. doi: 10.3389/fendo.2023.1083179
39. Cai X, Song S, Hu J, Zhu Q, Yang W, Hong J, et al. Body roundness index improves the predictive value of cardiovascular disease risk in hypertensive patients with obstructive sleep apnea: a cohort study. Clin Exp Hypertens. (2023) 45:2259132. doi: 10.1080/10641963.2023.2259132
40. Burgess S, Thompson SG. Multivariable Mendelian randomization: the use of pleiotropic genetic variants to estimate causal effects. Am J Epidemiol. (2015) 181:251–60. doi: 10.1093/aje/kwu283
41. Grant AJ, Burgess S. Pleiotropy robust methods for multivariable Mendelian randomization. Stat Med. (2021) 40:5813–30. doi: 10.1002/sim.9156
42. Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PloS Genet. (2017) 13:e1007081. doi: 10.1371/journal.pgen.1007081
43. Hu J, Song J, Chen Z, Yang J, Shi Q, Jin F, et al. Reverse causal relationship between periodontitis and shortened telomere length: Bidirectional two-sample Mendelian random analysis. Front Immunol. (2022) 13:1057602. doi: 10.3389/fimmu.2022.1057602
44. Vernia F, Di Ruscio M, Ciccone A, Viscido A, Frieri G, Stefanelli G, et al. Sleep disorders related to nutrition and digestive diseases: a neglected clinical condition. Int J Med Sci. (2021) 18:593–603. doi: 10.7150/ijms.45512
45. Chen MJ, Wu MS, Lin JT, Chang KY, Chiu HM, Liao WC, et al. Gastroesophageal reflux disease and sleep quality in a Chinese population. J Formos Med Assoc. (2009) 108:53–60. doi: 10.1016/S0929-6646(09)60032-2
46. Sabate JM, Jouet P, Merrouche M, Pouzoulet J, Maillard D, Harnois F, et al. Gastroesophageal reflux in patients with morbid obesity: a role of obstructive sleep apnea syndrome? Obes Surg. (2008) 18:1479–84. doi: 10.1007/s11695-008-9508-9
47. Zhu Q, Hua L, Chen L, Mu T, Dong D, Xu J, et al. Causal association between obstructive sleep apnea and gastroesophageal reflux disease: A bidirectional two-sample Mendelian randomization study. Front Genet. (2023) 14:1111144. doi: 10.3389/fgene.2023.1111144
48. Schuitenmaker JM, van Dijk M, Oude Nijhuis RAB, Smout A, Bredenoord AJ. Associations between sleep position and nocturnal gastroesophageal reflux: A study using concurrent monitoring of sleep position and esophageal pH and impedance. Am J Gastroenterol. (2022) 117:346–51. doi: 10.14309/ajg.0000000000001588
49. Kuribayashi S, Kusano M, Kawamura O, Shimoyama Y, Maeda M, Hisada T, et al. Mechanism of gastroesophageal reflux in patients with obstructive sleep apnea syndrome. Neurogastroenterol Motil. (2010) 22:611–e172. doi: 10.1111/nmo.2010.22.issue-6
50. Yadlapati R, Gyawali CP, Pandolfino JE. AGA clinical practice update on the personalized approach to the evaluation and management of GERD: expert review. Clin Gastroenterol Hepatol. (2022) 20:984–994.e1. doi: 10.1016/j.cgh.2022.01.025
51. Qin S, Wang C, Wang X, Wu W, Liu C. Causal association of gastroesophageal reflux disease with obstructive sleep apnea and sleep-related phenotypes: a bidirectional two-sample Mendelian randomization study. Front Neurol. (2023) 14:1283286. doi: 10.3389/fneur.2023.1283286
52. Cazzola M, Calzetta L, Matera MG. Long-acting muscarinic antagonists and small airways in asthma: Which link? Allergy. (2021) 76:1990–2001. doi: 10.1111/all.14766
53. Giordano F, Zicca A, Barba C, Guerrini R, Genitori L. Vagus nerve stimulation: Surgical technique of implantation and revision and related morbidity. Epilepsia. (2017) 58 Suppl 1:85–90. doi: 10.1111/epi.13678
54. Wu J, Sun X, Wu Q, Li H, Li L, Feng J, et al. Disrupted intestinal structure in a rat model of intermittent hypoxia. Mol Med Rep. (2016) 13:4407–13. doi: 10.3892/mmr.2016.5068
55. Fang B, Liu H, Yang S, Xu R, Chen G. Sleep duration, depression, and peptic ulcer recurrence in older patients with mild cognitive impairment. Health Psychol. (2020) 39:77–87. doi: 10.1037/hea0000814
56. Fang B, Liu H, Yang S, Xu R, Chen G. Effect of subjective and objective sleep quality on subsequent peptic ulcer recurrence in older adults. J Am Geriatr Soc. (2019) 67:1454–60. doi: 10.1111/jgs.15871
57. Qazi T, Farraye FA. Sleep and inflammatory bowel disease: an important bi-directional relationship. Inflamm Bowel Dis. (2019) 25:843–52. doi: 10.1093/ibd/izy334
58. Ballesio A, Zagaria A, Baccini F, Micheli F, Di Nardo G, Lombardo C. A meta-analysis on sleep quality in inflammatory bowel disease. Sleep Med Rev. (2021) 60:101518. doi: 10.1016/j.smrv.2021.101518
59. Hatamnejad MR, Baradaran Ghavami S, Shirvani M, Asghari Ahmadabad M, Shahrokh S, Farmani M, et al. Selective serotonin reuptake inhibitors and inflammatory bowel disease; Beneficial or malpractice. Front Immunol. (2022) 13:980189. doi: 10.3389/fimmu.2022.980189
60. Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. (2013) 13:190–8. doi: 10.1038/nri3386
61. Parekh PJ, Oldfield Iv EC, Challapallisri V, Ware JC, Johnson DA. Sleep disorders and inflammatory disease activity: chicken or the egg? Am J Gastroenterol. (2015) 110:484–8. doi: 10.1038/ajg.2014.247
62. Gombert M, Carrasco-Luna J, Pin-Arboledas G, Codoñer-Franch P. The connection of circadian rhythm to inflammatory bowel disease. Transl Res. (2019) 206:107–18. doi: 10.1016/j.trsl.2018.12.001
63. Hoffman K, Mansoor E, Panhwar MS, Regueiro M, Cooper G, Qazi T. Prevalence of obstructive sleep apnea is increased in patients with inflammatory bowel disease: A large, multi-network study. Crohns Colitis 360. (2022) 4:otac026. doi: 10.1093/crocol/otac026
64. Lammert F, Gurusamy K, Ko CW, Miquel JF, Mendez-Sanchez N, Portincasa P, et al. Gallstones. Nat Rev Dis Primers. (2016) 2:16024. doi: 10.1038/nrdp.2016.24
65. Unnikrishnan D, Jun J, Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord. (2015) 16:25–34. doi: 10.1007/s11154-014-9304-x
66. Chen L, Yang H, Li H, He C, Yang L, Lv G. Insights into modifiable risk factors of cholelithiasis: A Mendelian randomization study. Hepatology. (2022) 75:785–96. doi: 10.1002/hep.32183
67. Umbro I, Fabiani V, Fabiani M, Angelico F, Del Ben M. Association between non-alcoholic fatty liver disease and obstructive sleep apnea. World J Gastroenterol. (2020) 26:2669–81. doi: 10.3748/wjg.v26.i20.2669
68. Chou TC, Liang WM, Wang CB, Wu TN, Hang LW. Obstructive sleep apnea is associated with liver disease: a population-based cohort study. Sleep Med. (2015) 16:955–60. doi: 10.1016/j.sleep.2015.02.542
69. Trzepizur W, Boursier J, Le Vaillant M, Ducluzeau PH, Dubois S, Henni S, et al. Increased liver stiffness in patients with severe sleep apnoea and metabolic comorbidities. Eur Respir J. (2018) 51:1800601. doi: 10.1183/13993003.00601-2018
70. Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farre R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. (2012) 186:190–4. doi: 10.1164/rccm.201201-0130OC
71. Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. (2014) 10:355–62. doi: 10.5664/jcsm.3600
Keywords: obstructive sleep apnea, gastrointestinal disease, Mendelian randomization, gastroesophageal reflux, causal relationship
Citation: Yan W, Zhou J, Jiang M, Kong Y, Qin H, Qi Y, Wang S and Tai J (2024) Obstructive sleep apnea and 19 gastrointestinal diseases: a Mendelian randomization study. Front. Psychiatry 15:1256116. doi: 10.3389/fpsyt.2024.1256116
Received: 10 July 2023; Accepted: 09 July 2024;
Published: 26 July 2024.
Edited by:
Georgia Trakada, National and Kapodistrian University of Athens, GreeceReviewed by:
Mangala Hegde, Indian Institute of Technology Guwahati, IndiaCopyright © 2024 Yan, Zhou, Jiang, Kong, Qin, Qi, Wang and Tai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shan Wang, d3NhcXVhcml1c0BzaW5hLmNvbQ==; Jun Tai, dHJlbnR0akAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.