- 1Department of Psychiatry, Washington University School of Medicine, St. Louis, MO, United States
- 2Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, MO, United States
Introduction: Structural brain connectivity abnormalities have been associated with several psychiatric disorders. Schizophrenia (SCZ) is a chronic disabling disorder associated with accelerated aging and increased risk of dementia, though brain findings in the disorder have rarely been directly compared to those that occur with aging.
Methods: We used an automated approach to reconstruct key white matter tracts and assessed tract integrity in five participant groups. We acquired one-hour-long high-directional diffusion MRI data from young control (CON, n =28), bipolar disorder (BPD, n =21), and SCZ (n =22) participants aged 18-30, and healthy elderly (ELD, n =15) and dementia (DEM, n =9) participants. Volume, fractional (FA), radial diffusivity (RD) and axial diffusivity (AD) of seven key white matter tracts (anterior thalamic radiation, ATR; dorsal and ventral cingulum bundle, CBD and CBV; corticospinal tract, CST; and the three superior longitudinal fasciculi: SLF-1, SLF-2 and SLF-3) were analyzed with TRACULA. Group comparisons in tract metrics were performed using multivariate and univariate analyses. Clinical relationships of tract metrics with recent and chronic symptoms were assessed in SCZ and BPD participants.
Results: A MANOVA showed group differences in FA (λ=0.5; p=0.0002) and RD (λ=0.35; p<0.0001) across the seven tracts, but no significant differences in tract AD and volume. Post-hoc analyses indicated lower tract FA and higher RD in ELD and DEM groups compared to CON, BPD and SCZ groups. Lower FA and higher RD in SCZ compared to CON did not meet statistical significance. In SCZ participants, a significant negative correlation was found between chronic psychosis severity and FA in the SLF-1 (r= -0.45; p=0.035), SLF-2 (r= -0.49; p=0.02) and SLF-3 (r= -0.44; p=0.042).
Discussion: Our results indicate impaired white matter tract integrity in elderly populations consistent with myelin damage. Impaired tract integrity in SCZ is most prominent in patients with advanced illness.
Introduction
Schizophrenia (SCZ) and bipolar disorder (BPD) are both chronic psychiatric illnesses, with some overlapping clinical features. SCZ consists primarily of psychotic symptoms, including delusions, hallucinations, and disorganized behaviors, and often involves cognitive impairment. BPD often involves psychotic features (1), but its core clinical symptoms involve mood abnormalities typically involving periods of mania as well as depression. Many studies have reported genetic overlap between these two disorders (2–4), with associated familial overlap (5). As a result of such findings, increasingly patients with those disorders are studied together.
Numerous studies have investigated white matter integrity in SCZ and BPD. Diffusion imaging studies in SCZ typically report lower fractional anisotropy (FA) in one or more white matter tracts, often regionally, however, the specificity of significant abnormalities has been heterogenous across studies (6, 7). The large, multi-site ENIGMA study involving 4,322 individuals found widespread FA reduction in SCZ, with the anterior corona radiata (d=0.40) and corpus callosum (d=0.39) showing the greatest effects (8). Abnormal diffusion imaging findings in BPD have been more variable and have shown less robust findings than in SCZ. One meta-analysis reported two clusters of decreased FA, both in the right hemisphere: one close to the parahippocampus and the other close to the anterior cingulate (9). Another voxel-based meta-analytic study reported three significant clusters of decreased FA in BPD: a right posterior temporoparietal cluster and two left cingulate clusters (10). More recently, a mega-analysis across 3,033 individuals collected through the ENIGMA network reported lower FA in 29 regions, most notably within the corpus callosum and cingulum (11). Fewer studies have compared white matter anisotropy across both disorders (12, 13). A recent meta-analysis of diffusion and structural imaging white matter abnormalities in SCZ and BPD found a shared decrease in corpus callosum volume and FA across disorders, as well as SCZ-specific white matter abnormalities involving the left cingulum and the right anterior limb of the internal capsule (ALIC) (13).
Cognitive impairment occurs less commonly in BPD than in SCZ (14), where deficits in a range of domains including attention, working memory, verbal memory, and executive functioning are core features of the disorder and are generally chronologically progressive (15–17). SCZ has also been associated with an increased risk of dementia later in life, including showing precocious onset among younger individuals with the disorder (18–20). SCZ has therefore been hypothesized to be a disorder of accelerated biological aging (18, 21, 22). Accelerated aging is supported by studies reporting shared genetics between SCZ and a variety of age-related diseases (23, 24). Measures of biological aging could prove valuable for assessing SCZ patients’ risk for physical and cognitive decline and for evaluating intervention effectiveness. Direct comparisons of the brains of SCZ patients with those with age-related brain changes using identical imaging methods have however rarely been done.
TRACULA (TRActs Constrained by UnderLying Anatomy) is a software package that uses global probabilistic tractography combined with anatomical priors informed by the individual’s own anatomy to accurately map known fiber tracts in the brain and can evaluate multiple diffusion tensor imaging (DTI) parameter values within these tracts (25). TRACULA has been used in multiple research studies to evaluate different neuropathologies such as temporal lobe epilepsy (26), multiple sclerosis (27), amyotrophic lateral sclerosis (28), and obsessive-compulsive disorder (29). Only one prior study has conducted tract-based analysis in SCZ with TRACULA and used diffusion imaging data collected on the same customized ‘Connectom’ scanner used for the Human Connectome Project’s (HCP) “Young Adult” study. This study found a trend towards lower tract FA in patients, most significantly in the left anterior thalamic radiation, but no significant FA abnormalities in bipolar disorder (12). Two studies have used TRACULA in BPD. One of these found reduced FA particularly in the parietal part of the superior longitudinal fasciculus (SLF) (30a), and most notably in the SLF, cingulum-cingulate gyrus bundles, and corticospinal tracts in another study (31).
Aging has been associated with a generalized reduction in white matter FA and increased radial diffusivity (RD), particularly in later age, attributed primarily to a degeneration of myelin sheaths and loss of myelinated fibers (32–34). In Alzheimer’s dementia, diffusion imaging results have been variable (35), but further decreases in white matter integrity (relative to age-matched controls) are generally reported (36–38). Using TRACULA, reduced tract FA and increased mean diffusivity (MD) has been previously been found with aging non-uniformly across most tracts (39). Furthermore, elderly individuals with Alzheimer’s dementia or mild cognitive impairment had increased MD in the cingulum bundles compared to age-related control subjects (40). While the tract abnormalities with dementia share similarities to that reported for SCZ, these populations have not been directly compared using tract-based methods.
In the current pilot study, we use TRACULA to investigate white matter tract integrity in young healthy controls and individuals with SCZ and BPD, as well as in healthy elderly and a dementia cohort, all collected using the same scanner and acquisition protocol. Based on the most notable abnormalities reported in prior TRACULA studies in psychiatric populations (12, 30, 31), we focus our investigations on seven white matter tracts: the anterior thalamic radiation; three superior longitudinal fasciculi, the dorsal and ventral cingulum bundles, and the corticospinal tract. We hypothesized that previous TRACULA findings in SCZ and BPD will be reproducible in these major fiber tract pathways. Secondly, we hypothesized that white matter abnormalities in aging and dementia will have similar abnormalities as those seen with SCZ, but greater in severity.
Methods
Subjects
The study recruited a younger and an older subject cohort, recruited through community advertisements and volunteer databases. The young subject cohort included three groups of 18 to 30-year-old individuals: 28 healthy young control (CON), 22 schizophrenia (SCZ) and 21 bipolar I disorder (BPD). Participants were diagnosed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV) (41). To minimize clinical heterogeneity within the BPD group, only participants with a history of euphoric mania (versus mania characterized by primarily irritable mood) were included in the study. Written informed consent was obtained prior to participation, and all study protocols were approved by the Institutional Review Board at the Washington University School of Medicine in St. Louis, MO.
The older cohort (53-84 yrs) included 15 elderly healthy individuals (ELD) and 9 individuals with dementia (DEM). DEM participant diagnoses were ascertained through a review of medical records and included 8 participants with Alzheimer’s disease and 1 participant with Frontotemporal Dementia.
All participants were excluded if they: (a) met DSM-IV criteria for substance dependence or severe/moderate abuse during the prior 3 months; (b) had a clinically unstable or severe general medical disorder; or (c) had a history of head injury with documented neurological sequelae or loss of consciousness.
Behavioral assessments
Recent symptoms (i.e., in the prior two weeks) were assessed using the Scale for the Assessment of Negative Symptoms (SANS), and the Scale for the Assessment of Positive Symptoms (SAPS) (42). Chronic symptoms (i.e., prior year) were assessed using the Washington Early Recognition Center Affectivity and Psychosis (WERCAP) Screen, both affective (aWERCAP) and psychosis (pWERCAP) components (43–46).
Image acquisition
Structural T1w MRI images were acquired on a 3T Siemens Prisma with a 32-channel head coil using a 3D MPRAGE sequence (47, 48) (0.8 mm isotropic voxels, TR/TI = 2400/1000 ms, TE = 2.2 ms, flip angle = 8°, FOV = 256 ×240×166 mm, matrix size = 320 ×300, 208 sagittal slices, in-plane (iPAT) acceleration factor of 2). T2w volumes were also acquired at the same spatial resolution using the variable-flip-angle turbo-spin-echo 3D SPACE sequence (49) (TR/TE=3200/564 ms; same FOV, matrix and in-plane acceleration). The dMRI acquisition protocol was substantially similar to our previous study collected on the HCP ‘Connectom’ scanner (12), but with some modifications necessitated by the lower gradient strength of the Prisma scanner (80 mT/m, vs. 100 mT/m for the ‘Connectom’ scanner). The dMRI scans used the multi-band (MB) sequences from the Center for Magnetic Resonance Research, with 1.25 isotropic voxels, TR = 5000 ms, TE = 104 ms, 6/8 partial Fourier, and MB factor = 4. A full dMRI session included 6 runs (each approximately 8.5 min), representing 3 different gradient tables, with each table acquired once with anterior-to-posterior and posterior-to-anterior phase encoding polarities, respectively. Each gradient table includes approximately 90 diffusion weighting directions plus 6 b = 0 acquisitions interspersed throughout each run. Diffusion weighting consisted of 3 shells of b = 1000, 2000, and 3000 s/mm2 interspersed with an approximately equal number of acquisitions on each shell within each run. The diffusion directions matched those used in the HCP “Young Adult” and our previous study (12).
Image preprocessing
The diffusion data were preprocessed using the “DiffusionPreprocessing” stream of the HCPpipelines (v4.3.0) (50, 51), using the QuNex container (v0.91.11). This pipeline includes intensity normalization, susceptibility distortion correction (via FSL’s ‘topup’) (52), and correction for eddy current distortions and motion via FSL’s ‘eddy’ tool (53). We used the advanced ‘eddy’ features of outlier replacement (54), slice-to-volume motion correction (55), and correction for susceptibility-by-movement interactions (56). The b-vectors were rotated to account for motion (57). Finally, the dMRI data was corrected for gradient nonlinearity distortion as part of resampling to the subject’s native T1w space from the HCP structural pipeline output (while maintaining the same 1.25 mm spatial resolution of the dMRI data). Following that preprocessing, processing continued using FSL’s ‘bedpostx’ (58, 59) to estimate the diffusion orientation distribution. Bedpostx was run outside of TRACULA, within the QuNex container (number of fibers per voxel = 3; deconvolution model = 3 (zeppelins); burnin = 3000; rician noise; gradient nonlinearities accounted for).
TRACULA
TRACULA (TRActs Contstrained by UnderLying Anatomy) is an automated method (25) for estimating global probabilistic tractography. This method uses a Bayesian framework for global tractography that determines the connection that best fits two selected endpoints based on the diffusion data. In addition, TRACULA also incorporates prior anatomical knowledge based on manually verified trajectories of tracts in a training set created by Yendiki et al. (25). For every individual, TRACULA reconstructs probabilistic distributions of 18 major white matter tracts. A sample participant’s estimation and identification of white matter tracts is shown in Figure 1. More specifically, TRACULA uses the endpoints established in the training set’s tracts, and transforms them into each individual’s native space. Then, TRACULA establishes probabilistic streamlines that are constrained by the relative positions of white-matter pathways to surrounding anatomical structures (obtained from the individual’s own FreeSurfer segmentation) and uses control points to control the allowed curvature of the tract. It does not presume exact tract spatial location or shape, so the trajectory of the tract is only restricted with respect to the surrounding anatomical structures. This allows for variation across individuals while still establishing the same tracts for across-individual comparison.
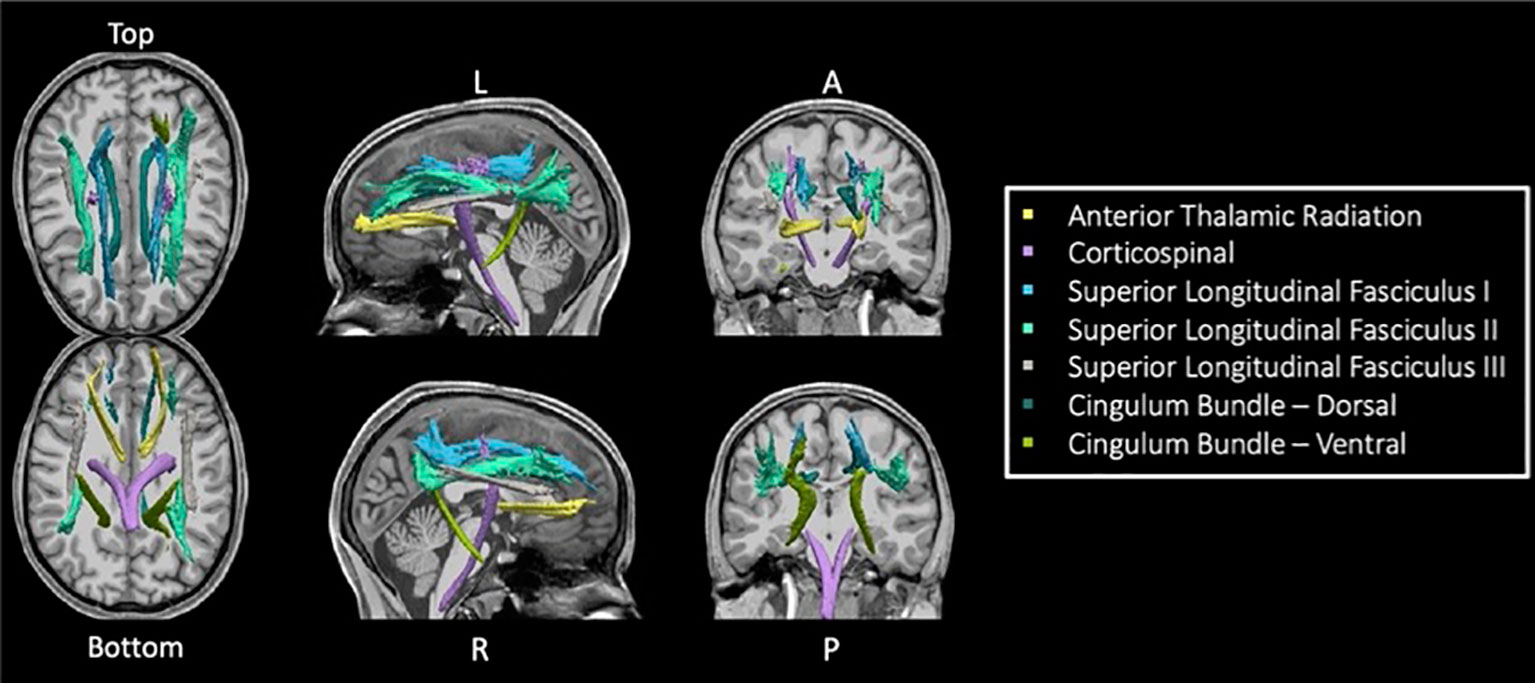
Figure 1 Seven TRACULA tracts assessed. Figure shows the seven reconstructed tracts from TRACULA used in the current analysis.
Given that preprocessing of the diffusion data was implemented using the HCPpipelines (to enable use of more advanced preprocessing features), we only used the TRACULA steps specifically necessary to generate the path distributions (i.e., “prep -prior” to estimate anatomical neighborhood prior for each pathway of interest, and the “path” step to generate the path distributions, using TRACULA from FreeSurfer v6.0). FSL’s “dtifit” was applied to perform least-squares tensor estimations, specifically eigenvectors, eigenvalues and DTI parameters (FA, AD, and RD), using just the b=0 and b=1000 s/mm2 shells, since the tensor model is not valid for high b-values. (Note however that all shells were used as input to ‘bedpostx’ and thus contributed to the estimation of the path distributions). FA is a commonly used DTI metric that establishes the directional asymmetry of water diffusion at each voxel (10, 30, 60, 61). Tract volumes, and average values for FA, RD, and AD within the 20% posterior distribution for each path (tract) of interest were computed as the final step of TRACULA (25).
Statistical analysis
All statistical analyses were done using SAS 9.4 (SAS Institute Inc., Cary, NC). To compare each tract metric (i.e., tract volume, FA, RD, and AD) across groups, multiple analyses of variance was used with the seven tracts as the dependent variables, group as an independent (“class”) variable, and sex as a covariate (i.e., MANCOVA). For tract volume comparisons, intracranial volume was also included as a covariate. Post-hoc pairwise analyses were done when the MANCOVA met statistical significance (p<0.05) for the diagnostic group effect, uncontrolled for multiple comparisons. Z-scores were generated using three younger groups only. Relationships between mean FA for each tract and clinical measures (i.e., SAPS – positive and disorganized, SANS, WERCAP-affectivity, and WERCAP-psychosis) and age were investigated using Pearson’s correlations.
Results
Demographic and clinical profiles
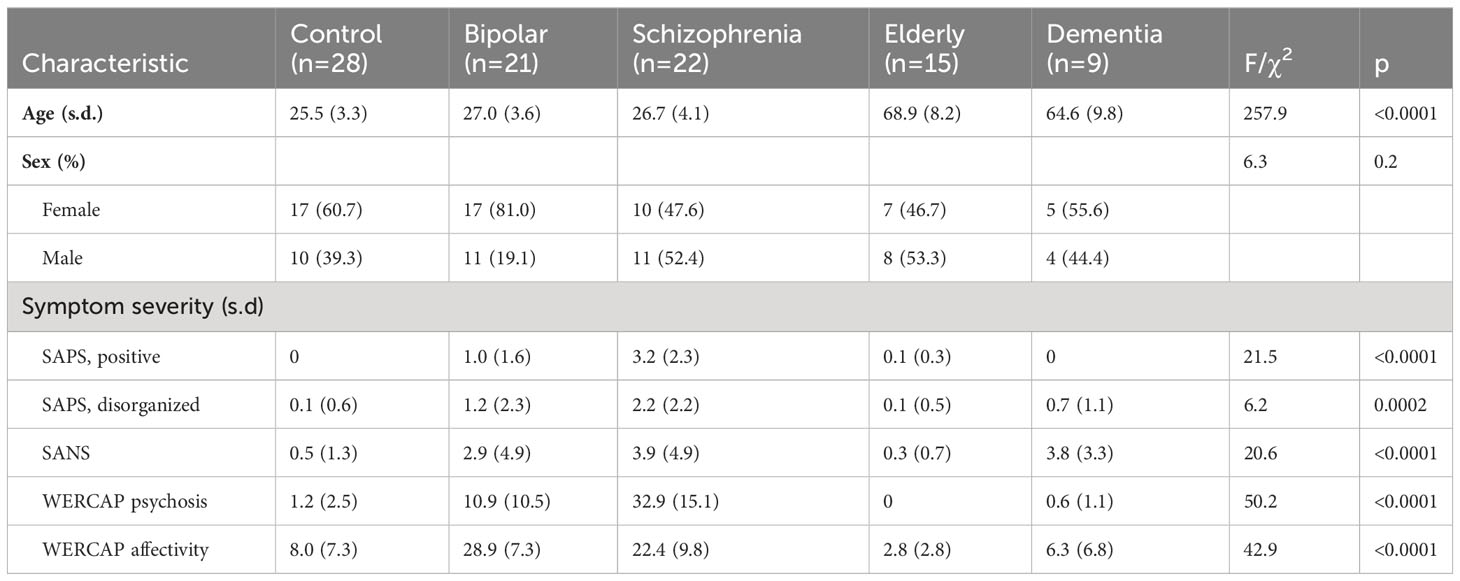
Table 1 shows demographic and clinical information across groups. Mean age was similar across the young participant groups, and similar across the two older groups. Sex was relatively evenly balanced across groups, other than in the BPD group which had substantially more females than males.
Age relationship with tract metrics
Correlations of age with tract volume and FA were assessed using the individuals from the young participant groups: CON, BPD and SCZ.
A Pearson correlation, partialling out group, did not show a significant age correlation with any tract volume. There were also no significant tract volume relationships with age, when correlations were done separately in each group. Tract FA relationships with age were significant only for the SLF-3 (r= -0.24; p=0.046), after partialling out diagnostic group. There were no significant FA relationships with age when correlations were done separately in each group.
Due to a relatively small number of ELD and DEM participants, age correlations were not done in these groups.
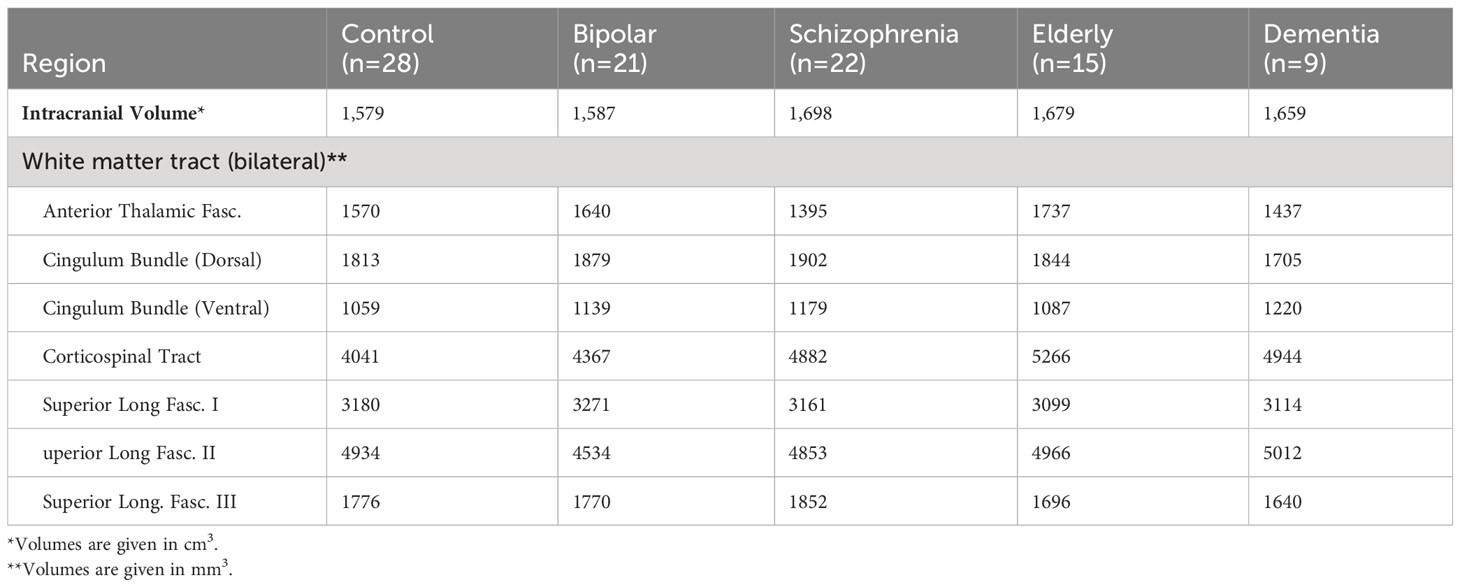
Intracranial and tract volumes
Intracranial and tract volume least-square means, controlled for sex, across groups, is shown in Table 2. Intracranial volumes controlled for sex did not differ across groups (F=2.1, p=0.09).
A MANCOVA comparing the seven tract volumes across all five participant groups did not meet statistical significance (Wilks’ Lambda = 0.7; p=0.5). MANCOVA results were similar when tract volumes were controlled for intracranial volume (p=0.5).
Figure 2 depicts average group z-scores of tract volumes divided by intracranial volumes to correct for brain size, and adjusted such that CON group z-scores are zero.
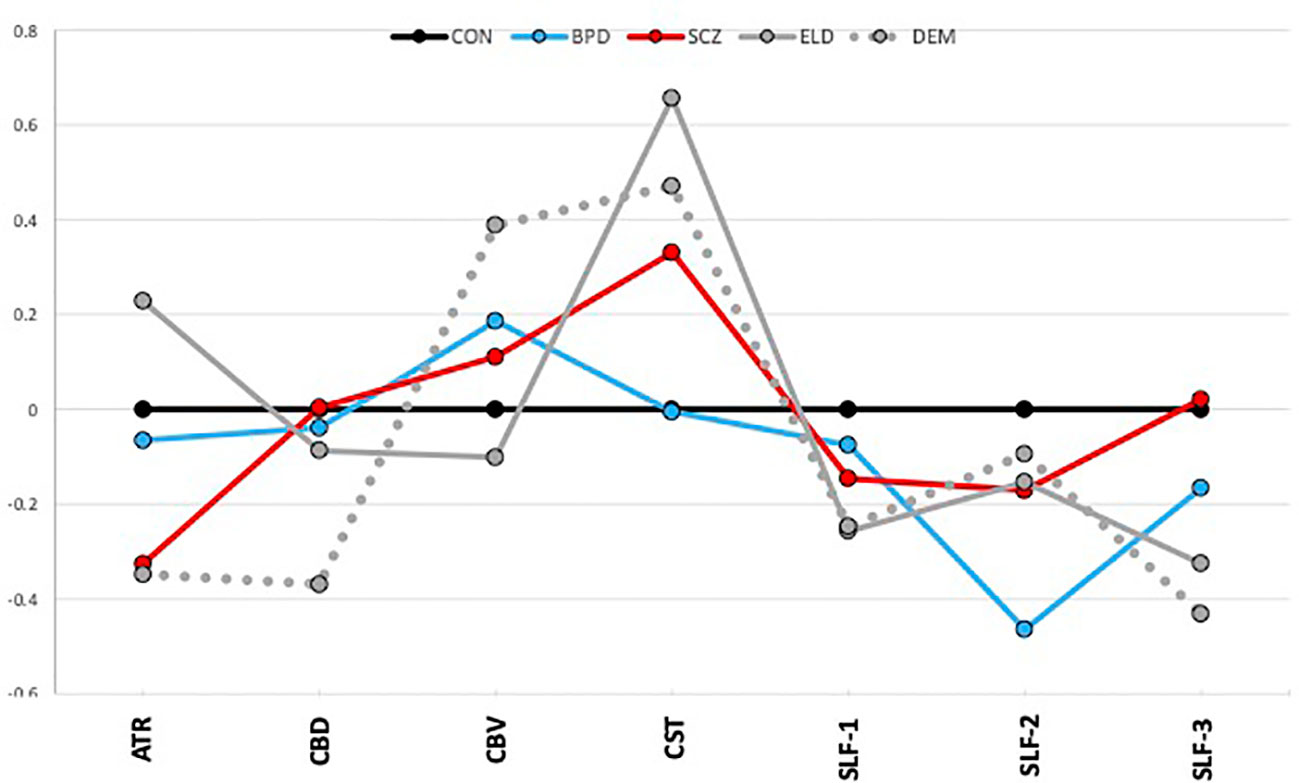
Figure 2 Tract volumes controlled for intracranial volume (z-scores). The graph shows z-scores of tract volumes (normalized to the mean of the healthy young control group). Black=young control; Blue=bipolar disorder; Red=schizophrenia; Gray, solid=elderly control; and Gray, dotted=dementia.
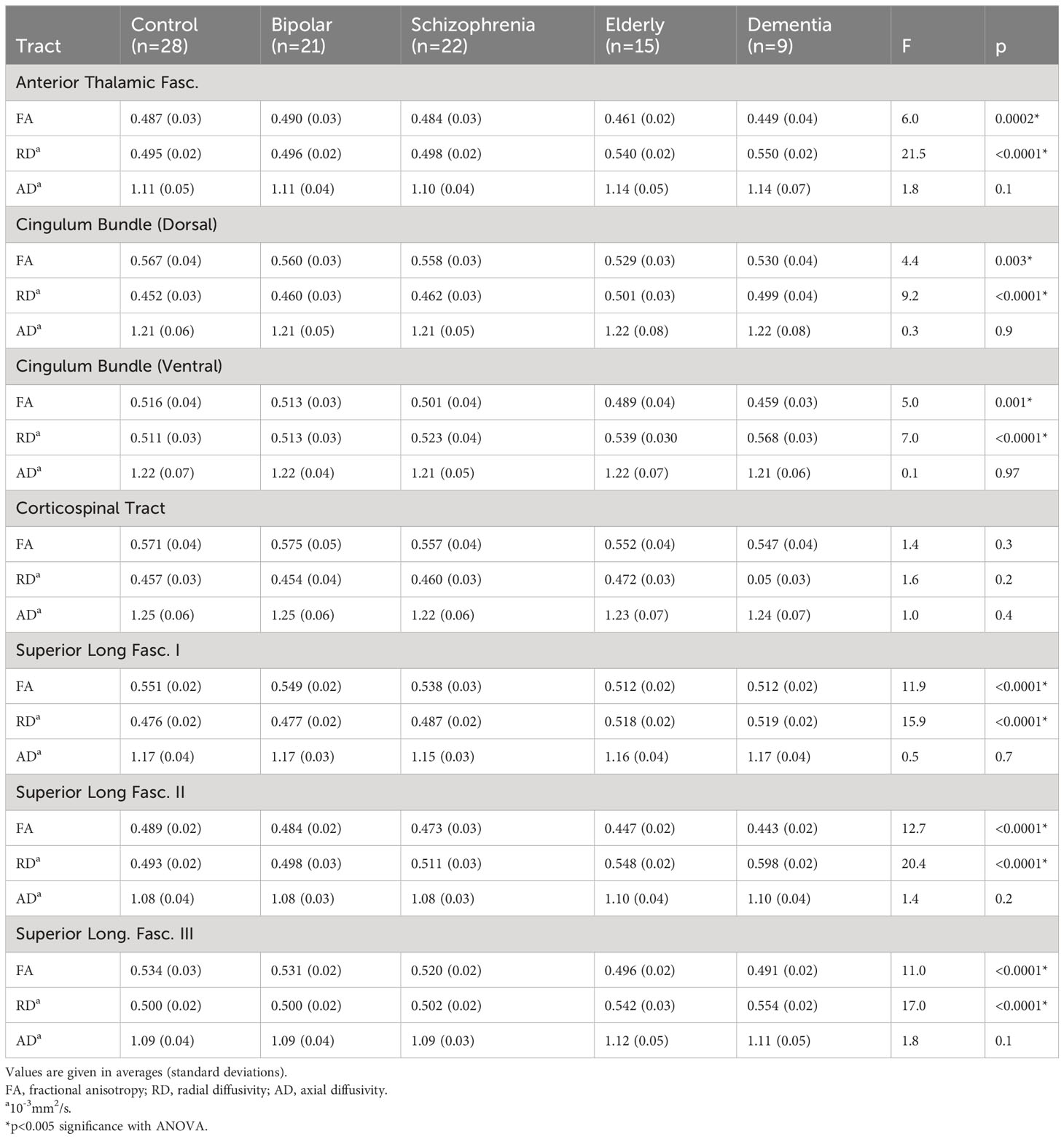
Tract FAs
A MANCOVA of all five groups’ tract FAs showed a significant effect (Wilks’ Lambda = 0.5; p=0.0002). Figure 3A depicts group z-scores (relative to CON) for each tract FA. Post-hoc pairwise comparisons (Student’s t-tests) showed statistically significant group effects for six tracts as shown in Table 3 – the ATR, CBD, CBV, SLF-1, SLF-2, and SLF-3. Only values for the CST were non-significant. As depicted in Figure 3A, post hoc results were driven by significant pairwise FA group differences between ELD or DEM and CON, BPD, and/or SCZ groups.
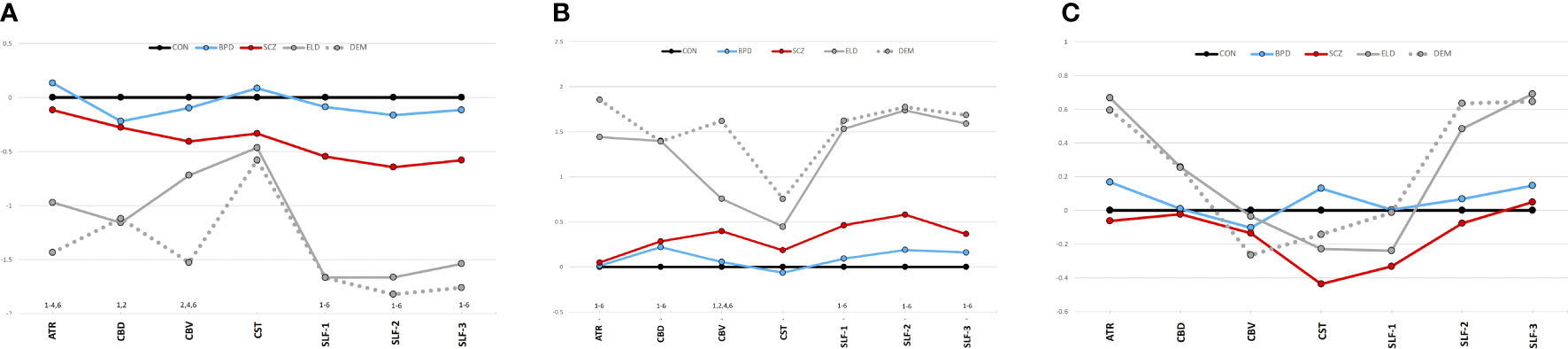
Figure 3 (A) Tract diffusion imaging metrics across groups (z-scores). (A) Fractional anisotropy. (B) Radial diffusivity. (C) Axial diffusivity. The graphs show z-scores of each diffusion metric (computed as in Figure 2). Black=young control; Blue=bipolar disorder; Red=schizophrenia; Gray, solid=elderly control; and Gray, dotted=dementia. 1Significant difference (p<0.05) between CON vs. ELD. 2Significant difference (p<0.05) between CON vs. DEM. 3Significant difference (p<0.05) between BPD vs. ELD. 4Significant difference (p<0.05) between BPD vs. DEM. 5Significant difference (p<0.05) between SCZ vs. ELD. 6Significant difference (p<0.05) between SCZ vs. DEM.
Significant pairwise group differences were not observed between any of the younger participant groups. The average FA of each tract was lower in the SCZ group compared to the CON group, particularly in the three SLF tracts (see Figure 3A), though none of these differences met statistical significance.
Tract RDs
Results of a MANOVA of all five groups’ tract RDs showed statistical significance (Wilks’ Lambda = 0.35; p<0.0001). Figure 3B depicts group z-scores for each tract for RD. Post-hoc analyses showed statistically significant group effects for six tracts as shown in Table 3 – the ATR, CBD, CBV, SLF-1, SLF-2, and the SLF-3. Only CST RD group differences with non-significant. As depicted in Figure 3B, results of post-hoc analyses were driven by significant pairwise RD group difference between ELD or DEM and CON, BPD and/or SCZ. Significant pairwise effects were not observed between any of the younger participant groups. The average RD of each tract was higher in the SCZ group compared to the CON group, particularly in the three SLF tracts (see Figure 3B), though none of these differences met statistical significance.
Tract ADs
Results of a MANOVA of all five groups’ tract ADs did not show significant group effects (Wilks’ Lambda = 0.74; p=0.5). Figure 3C depicts group z-scores for each tract for AD. Mean tract AD in each group is shown in Table 3.
Clinical relationship with tract volume
Pearson’s correlations were done to investigate relationships of tract volume with each of the five symptoms listed in Table 1. In SCZ subjects, a significant correlation was only found for ATR volume with SAPS positive symptoms (r=0.47; p=0.028), which remained even after partialling out sex (r=0.48; p=0.03). When both SCZ and BPD subjects were assessed together, no significant clinical correlations were observed.
Clinical relationship with tract FA
The correlation of tract FA with the same five symptom measures was also assessed. In SCZ subjects, a significant correlation was only found for WERCAP positive symptoms and FA in the SLF-1 (r= -0.45; p=0.035), SLF-2 (r= -0.49; p=0.02) and SLF-3 (r= -0.44; p=0.042). These relationships are shown in Figure 4. When both BPD and SCZ were included in the analyses, the strength of correlations decreased in the SLF-1 (r= -0.33; p=0.029), SLF-2 (r= -0.36; p=0.02) and SLF-3 (r= -0.29; p=0.059).
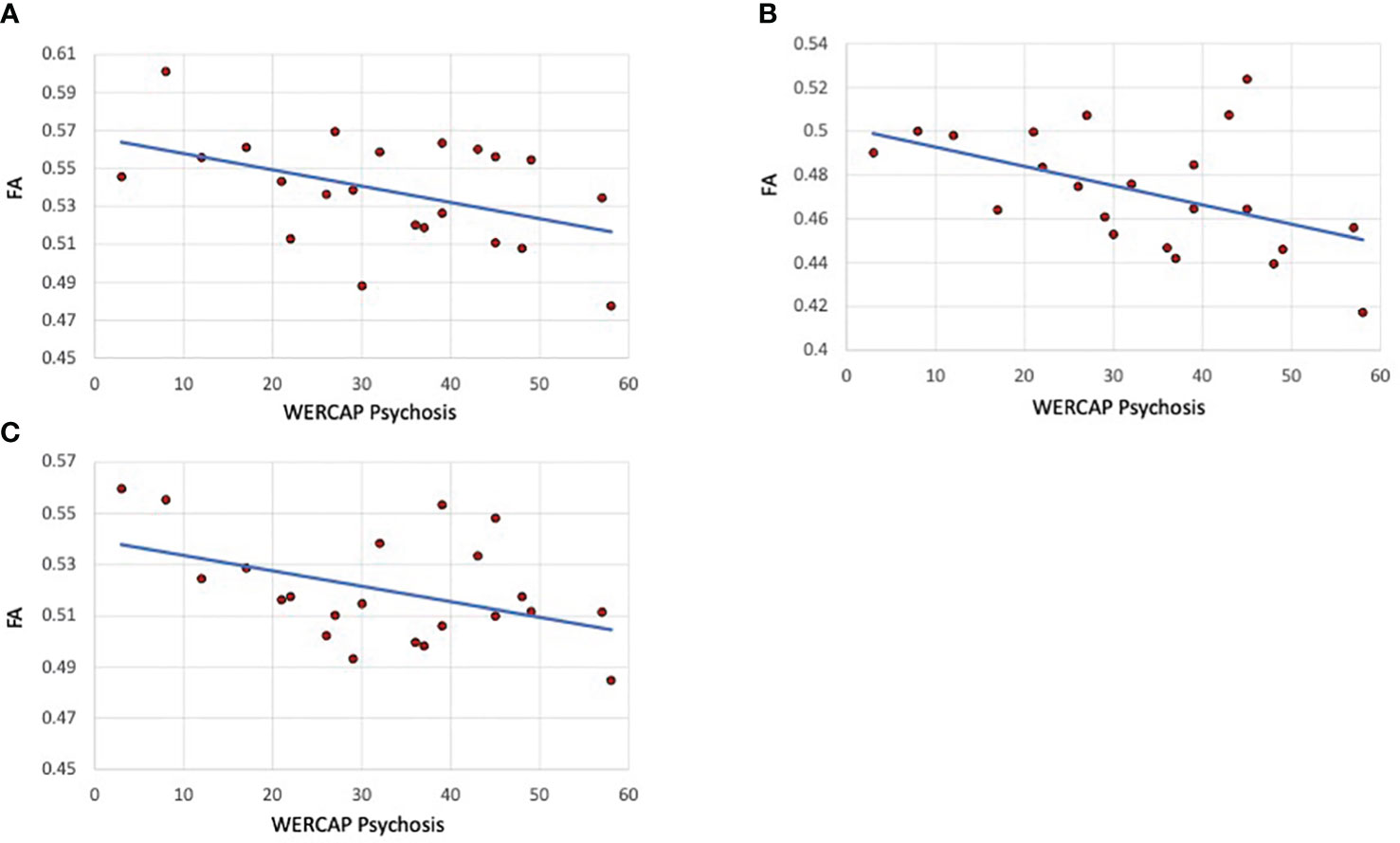
Figure 4 Psychosis correlations with SLF I, II and III fractional anisotropy in schizophrenia patients. (A) SLF I (B) SLF-II. (C) SLF-III. The graphs show scatterplots with regression line between fractional anisotropy within the specific tract and chronic psychosis scores (i.e., over last twelve months) based on the WERCAP screen in schizophrenia participants only.
Discussion
Our study provides preliminary data comparing diffusion imaging metrics across younger psychiatric populations and older cohorts using an automated tract-based analysis. We found that the volumes of the white matter tracts did not differ significantly across groups, whereas there were significant differences in tract fractional anisotropy across the various tracts studied. This contrasts with several other studies which have reported decreased white matter volumes, though results have been inconsistent and highly variable. SCZ has been associated with a reduction of prefrontal or frontal white matter (62–65), temporal white matter (62), or posterior brain white matter (66) volumes. Others have reported reduced white matter volume in regions corresponding to the inferior longitudinal fasciculus in first-episode SCZ (67) or to the left fronto-occipital fasciculus in prodromal individuals who converted to psychosis (68). Studies of aging and dementia have also been associated with reductions in white matter volume (34, 69–71). Notably, white matter volumes have been reported to gradually increase in the first 40 years of life and then rapidly decrease after 60 years (70–72). The absence of substantial tract volume differences between our younger and older cohorts in our study was therefore unexpected and suggests that tract-wide volume loss may not be observed until after age 70. As our study involved measures of entire tracts, minor white structural changes in disease are likely diluted by the inclusion of less affected parts of the tracts in the analyses, as has been shown by comparing tract-based analyses results to that of voxel-based analyses (12).
Our study found a substantially reduced FA in most white matter tracts in elderly and dementia participants compared to the younger cohorts, with findings in dementia not significantly lower than in the healthy elderly population. Similarly, we found an increase in RD in the older cohorts, indicating an increase in the water diffusion in the direction perpendicular to the direction of the white matter fiber. These findings are consistent with the generalized reduced white matter integrity reported with old age (32, 34), attributed primarily to the degeneration of myelin sheaths and loss of myelinated fibers (33).
We did not find significant diffusion abnormalities in SCZ or BPD participants. FA in SCZ trended towards lower values, and RD towards higher values, suggesting potential myelin degeneration in this population, albeit to a relatively mild degree. Here again, finding statistically significant diffusion metrics in a whole white matter tract would be less likely if there are regions across the tract that are unaffected or only minimally affected. The white matter regions affected in SCZ have been highly variable across studies, and rarely involve universal white matter abnormalities (6, 7). In general, findings have implicated prefrontal and temporal lobes and the fiber tracts connecting these regions. Analysis of data from the ENIGMA schizophrenia DTI work group of 2,359 healthy controls and 1,963 schizophrenia patients from 29 independent international studies, reported that FA reductions in SCZ are widespread, and involved all major WM fasciculi, with the anterior corona radiata and corpus callosum showing the greatest effects (8). An earlier meta-analytic study found significant FA reductions primarily in two regions: the left frontal deep white matter and the left temporal deep white matter in SCZ (6). Similarly, a meta-analytic study of first-episode psychosis reported FA reduction in the right limbic white matter and left temporal white matter (73). Regarding BPD, diffusion imaging abnormalities have generally been found to be less severe than that found in SCZ (9–11), consistent with the results of our current study.
Tract-based white matter assessments in psychiatric disorders, as described in this study, are relatively infrequent. Another study using TRACULA in twenty-four SCZ patients similarly did not find significant tract effects compared to thirty healthy controls. That study however found a trend toward lower FA in the ATR (12), a tract that was the least affected in our current study. This variability is consistent with the hypothesis that the specific white matter regions most affected in schizophrenia tend to vary widely across patients. A failure to achieve statistically significant findings in schizophrenia using TRACULA may thus be related to heterogeneity across individuals combined with the relatively low sample size. Two substantially larger TRACULA studies of bipolar disorder subjects (with sample sizes of 96 and 72) have shown significant findings across several tracts (30, 31), which was not observed in a smaller TRACULA study of thirty-three bipolar disorder subjects (12). This suggests that tract-based diffusion imaging studies require a larger sample size, compared to that required for voxel-based studies, to detect groupwise FA differences (12).
We however found a significant moderate correlation between chronic psychotic symptom severity and FA in each of the three SLF tracts, which was not observed with more recent symptoms. The WERCAP Screen estimates chronic symptoms over the last year and may therefore more accurately capture cumulative psychopathology compared to the SAPS which estimates recent symptoms (i.e. over the last two weeks), which may be more indicative of state-related changes. The SLF is the largest associative fiber bundle system in the brain, and connects the frontal, temporal, and parietal lobes within the same hemisphere (74, 75). The main functions supported by the regions connected by the SLF are visual and spatial cognition, attention processes, control of motor processes and executive functions, and language functions (76). SLF-I represents the dorsal division, connecting the superior parietal and superior frontal lobes, and appears to be involved with regulating motor behavior. The SLF II originates in the caudal-inferior parietal cortex and terminates in the dorsolateral prefrontal cortex and appears to be involved in visuospatial attention. The SLF-III is the most ventral, extending from the supramarginal gyrus, anterior to the angular gyrus, to the ventral premotor and prefrontal areas. Frontoparietal dysconnectivity has been attributed to SCZ etiopathogenesis (76), and impaired SLF has been found in SCZ in several studies (7, 8, 77–79) and associated with psychotic symptom severity (79, 80). Our results are consistent with these findings and suggest that decreased integrity of SLF tracts may indicate a more advanced symptom profile.
Our study and its interpretation have some limitations. Firstly, the sample size used in our study was only modest for each group (n=28 or fewer) and thus may not have had sufficient power to detect significant group differences, particularly since the white matter regions affected are highly variable across individuals. Nevertheless, our study showed strong trends towards lower FA values in schizophrenia, and FA in the SLF showed a moderate inverse correlation with psychotic symptom severity. In the future, larger tract-based studies of diffusion data acquired similarly are needed to identify significant group effects, as well as subtypes of patients with unique patterns of white matter impairment. Secondly, results from tract-based methods may miss white matter damage if it does not involve a substantial portion of the tract since the healthier parts of the tract could dilute the effect. Thus, combining tract-based methods with voxel-based methods would overcome the disadvantages of each method, facilitating the estimation of both general and regional tract integrity (12). Thirdly, our study did not account for potential confounders in our analyses, including substance and medication use which may have influenced the findings. Antipsychotic use, for example, has been reported to increase tract FA (81, 82), while others have reported a subtle loss of white matter integrity (83). Diffusion studies have also found poorer integrity of white matter in cannabis users compared to non-users (84, 85). In addition, potential protective factors for white matter abnormalities including psychotherapy (86, 87) or cognitive training (88) may have been a confounder in our study, and such data were unavailable from our participants for analyses.
In conclusion, using the automated tractography tool TRACULA, our study showed significantly impaired white matter integrity with aging, suggesting demyelination. The pattern of white matter abnormalities in schizophrenia was similar to that in aging, but was much lesser in severity and did not meet statistical significance. In the future, larger sample sizes and aggregation with other data sets are recommended to increase the power to detect group differences.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Washington University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DM wrote the original draft and conducted most of the statistical analyses. SC conducted neuroimaging processing and analyses. JS guided imaging processing and analyses. MH oversaw neuroimaging acquisition, processing, and analyses. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. National Institutes of Health, R01MH104414 and R21MH131962.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Coryell W, Leon AC, Turvey C, Akiskal HS, Mueller T, Endicott J. The significance of psychotic features in manic episodes: a report from the NIMH collaborative study. J Affect Disord (2001) 67:79–88. doi: 10.1016/S0165-0327(99)00024-5
2. Potash JB, Zandi PP, Willour VL, Lan TH, Huo Y, Avramopoulos D, et al. Suggestive linkage to chromosomal regions 13q31 and 22q12 in families with psychotic bipolar disorder. Am J Psychiatry (2003) 160:680–6. doi: 10.1176/appi.ajp.160.4.680
3. Park N, Juo SH, Cheng R, Liu J, Loth JE, Lilliston B, et al. Linkage analysis of psychosis in bipolar pedigrees suggests novel putative loci for bipolar disorder and shared susceptibility with schizophrenia. Mol Psychiatry (2004) 9:1091–9. doi: 10.1038/sj.mp.4001541
4. Goes FS, Zandi PP, Miao K, Mcmahon FJ, Steele J, Willour VL, et al. Mood-incongruent psychotic features in bipolar disorder: familial aggregation and suggestive linkage to 2p11-q14 and 13q21-33. Am J Psychiatry (2007) 164:236–47. doi: 10.1176/ajp.2007.164.2.236
5. Kendler KS, Mcguire M, Gruenberg AM, Spellman M, O'hare A, Walsh D. The Roscommon Family Study. II. The risk of nonschizophrenic nonaffective psychoses in relatives. Arch Gen Psychiatry (1993) 50:645–52. doi: 10.1001/archpsyc.1993.01820200059006
6. Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res (2009) 108:3–10. doi: 10.1016/j.schres.2008.11.021
7. Vitolo E, Tatu MK, Pignolo C, Cauda F, Costa T, Ando A, et al. White matter and schizophrenia: A meta-analysis of voxel-based morphometry and diffusion tensor imaging studies. Psychiatry Res Neuroimaging (2017) 270:8–21. doi: 10.1016/j.pscychresns.2017.09.014
8. Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry (2018) 23:1261–9. doi: 10.1038/mp.2017.170
9. Vederine FE, Wessa M, Leboyer M, Houenou J. A meta-analysis of whole-brain diffusion tensor imaging studies in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry (2011) 35:1820–6. doi: 10.1016/j.pnpbp.2011.05.009
10. Nortje G, Stein DJ, Radua J, Mataix-Cols D, Horn N. Systematic review and voxel-based meta-analysis of diffusion tensor imaging studies in bipolar disorder. J Affect Disord (2013) 150:192–200. doi: 10.1016/j.jad.2013.05.034
11. Favre P, Pauling M, Stout J, Hozer F, Sarrazin S, Abe C, et al. Widespread white matter microstructural abnormalities in bipolar disorder: evidence from mega- and meta-analyses across 3033 individuals. Neuropsychopharmacology (2019) 44:2285–93. doi: 10.1038/s41386-019-0485-6
12. Mamah D, Ji A, Rutlin J, Shimony JS. White matter integrity in schizophrenia and bipolar disorder: Tract- and voxel-based analyses of diffusion data from the Connectom scanner. NeuroImage Clin (2019) 21:101649. doi: 10.1016/j.nicl.2018.101649
13. Zhao G, Lau WKW, Wang C, Yan H, Zhang C, Lin K, et al. A comparative multimodal meta-analysis of anisotropy and volume abnormalities in white matter in people suffering from bipolar disorder or Schizophrenia. Schizophr Bull (2022) 48:69–79. doi: 10.1093/schbul/sbab093
14. Li W, Zhou FC, Zhang L, Ng CH, Ungvari GS, Li J, et al. Comparison of cognitive dysfunction between schizophrenia and bipolar disorder patients: A meta-analysis of comparative studies. J Affect Disord (2020) 274:652–61. doi: 10.1016/j.jad.2020.04.051
15. Bowie CR, Harvey PD. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr Dis Treat (2006) 2:531–6. doi: 10.2147/nedt.2006.2.4.531
16. Mamah D, Mutiso VN, Ndetei DM. Neurocognition in Kenyan youth at clinical high risk for psychosis. Schizophr Res Cognit (2021) 25:100198. doi: 10.1016/j.scog.2021.100198
17. Mccutcheon RA, Keefe RSE, Mcguire PK. Cognitive impairment in schizophrenia: aetiology, pathophysiology, and treatment. Mol Psychiatry (2023) 28:1902–18. doi: 10.1038/s41380-023-01949-9
18. Stroup TS, Olfson M, Huang C, Wall MM, Goldberg T, Devanand DP, et al. Age-specific prevalence and incidence of dementia diagnoses among older US adults with Schizophrenia. JAMA Psychiatry (2021) 78:632–41. doi: 10.1001/jamapsychiatry.2021.0042
19. Richmond-Rakerd LS, D'souza S, Milne BJ, Caspi A, Moffitt TE. Longitudinal associations of mental disorders with dementia: 30-year analysis of 1.7 million New Zealand citizens. JAMA Psychiatry (2022) 79:333–40. doi: 10.1001/jamapsychiatry.2021.4377
20. Caspi A, Shireby G, Mill J, Moffitt TE, Sugden K, Hannon E. Accelerated pace of aging in schizophrenia: five case-control studies. Biol Psychiatry (2023) S0006-3223(23)01693-1. doi: 10.1016/j.biopsych.2023.10.023
21. Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophr Bull (2008) 34:1024–32. doi: 10.1093/schbul/sbm140
22. Jeste DV, Wolkowitz OM, Palmer BW. Divergent trajectories of physical, cognitive, and psychosocial aging in schizophrenia. Schizophr Bull (2011) 37:451–5. doi: 10.1093/schbul/sbr026
23. Chen J, Bacanu SA, Yu H, Zhao Z, Jia P, Kendler KS, et al. Genetic relationship between schizophrenia and nicotine dependence. Sci Rep (2016) 6:25671. doi: 10.1038/srep25671
24. Pillinger T, Osimo EF, De Marvao A, Shah M, Francis C, Huang J, et al. Effect of polygenic risk for schizophrenia on cardiac structure and function: a UK Biobank observational study. Lancet Psychiatry (2023) 10:98–107. doi: 10.1016/S2215-0366(22)00403-5
25. Yendiki A, Panneck P, Srinivasan P, Stevens A, Zollei L, Augustinack J, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform (2011) 5:23. doi: 10.3389/fninf.2011.00023
26. Kreilkamp BA, Weber B, Richardson MP, Keller SS. Automated tractography in patients with temporal lobe epilepsy using TRActs Constrained by UnderLying Anatomy (TRACULA). NeuroImage Clin (2017) 14:67–76. doi: 10.1016/j.nicl.2017.01.003
27. Gharaylou Z, Sahraian MA, Hadjighassem M, Kohanpour M, Doosti R, Nahardani S, et al. Widespread disruptions of white matter in familial multiple sclerosis: DTI and NODDI study. Front Neurol (2021) 12:678245. doi: 10.3389/fneur.2021.678245
28. Sarica A, Cerasa A, Vasta R, Perrotta P, Valentino P, Mangone G, et al. Tractography in amyotrophic lateral sclerosis using a novel probabilistic tool: a study with tract-based reconstruction compared to voxel-based approach. J Neurosci Methods (2014) 224:79–87. doi: 10.1016/j.jneumeth.2013.12.014
29. Watanabe A, Nakamae T, Sakai Y, Nishida S, Abe Y, Yamada K, et al. The detection of white matter alterations in obsessive-compulsive disorder revealed by TRActs Constrained by UnderLying Anatomy (TRACULA). Neuropsychiatr Dis Treat (2018) 14:1635–43. doi: 10.2147/NDT.S164058
30. Sprooten E, Barrett J, Mckay DR, Knowles EE, Mathias SR, Winkler AM, et al. A comprehensive tractography study of patients with bipolar disorder and their unaffected siblings. Hum Brain Mapp (2016) 37:3474–85. doi: 10.1002/hbm.23253
31. Ji A, Godwin D, Rutlin J, Kandala S, Shimony JS, Mamah D. Tract-based analysis of white matter integrity in psychotic and nonpsychotic bipolar disorder. J Affect Disord (2017) 209:124–34. doi: 10.1016/j.jad.2016.11.038
32. Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med (2000) 44:259–68. doi: 10.1002/1522-2594(200008)44:2<259::AID-MRM13>3.0.CO;2-6
33. Peters A. The effects of normal aging on myelinated nerve fibers in monkey central nervous system. Front Neuroanat (2009) 3:11. doi: 10.3389/neuro.05.011.2009
34. Rathee R, Rallabandi VP, Roy PK. Age-related differences in white matter integrity in healthy human brain: evidence from structural MRI and diffusion tensor imaging. Magn Reson Insights (2016) 9:9–20. doi: 10.4137/MRI.S39666
35. Stebbins GT, Murphy CM. Diffusion tensor imaging in Alzheimer's disease and mild cognitive impairment. Behav Neurol (2009) 21:39–49. doi: 10.1155/2009/915041
36. Sugihara S, Kinoshita T, Matsusue E, Fujii S, Ogawa T. Usefulness of diffusion tensor imaging of white matter in Alzheimer disease and vascular dementia. Acta Radiol (2004) 45:658–63. doi: 10.1080/02841850410008388
37. Huang J, Friedland RP, Auchus AP. Diffusion tensor imaging of normal-appearing white matter in mild cognitive impairment and early Alzheimer disease: preliminary evidence of axonal degeneration in the temporal lobe. AJNR Am J Neuroradiol (2007) 28:1943–8. doi: 10.3174/ajnr.A0700
38. Mayo CD, Mazerolle EL, Ritchie L, Fisk JD, Gawryluk JR, Alzheimer's Disease Neuroimaging I. Longitudinal changes in microstructural white matter metrics in Alzheimer's disease. NeuroImage Clin (2017) 13:330–8. doi: 10.1016/j.nicl.2016.12.012
39. Storsve AB, Fjell AM, Yendiki A, Walhovd KB. Longitudinal changes in white matter tract integrity across the adult lifespan and its relation to cortical thinning. PloS One (2016) 11:e0156770. doi: 10.1371/journal.pone.0156770
40. Lee SH, Coutu JP, Wilkens P, Yendiki A, Rosas HD, Salat DH, et al. Tract-based analysis of white matter degeneration in Alzheimer's disease. Neuroscience (2015) 301:79–89. doi: 10.1016/j.neuroscience.2015.05.049
41. First MB, Spitzer RL, Gibbon M, Williams BW. Structured clinical interview for DSM-IV axis I disorders, clinical version (SCID-CV). Washington, DC: American Psychiatric Publishing (1996).
42. Andreasen NC. Methods for assessing positive and negative symptoms. Mod Probl Pharmacopsychiatry (1990) 24:73–88. doi: 10.1159/000418013
43. Mamah D, Owoso A, Sheffield JM, Bayer C. The WERCAP Screen and the WERC Stress Screen: psychometrics of self-rated instruments for assessing bipolar and psychotic disorder risk and perceived stress burden. Compr Psychiatry (2014) 55:1757–71. doi: 10.1016/j.comppsych.2014.07.004
44. Hsieh CJ, Godwin D, Mamah D. Utility of Washington early recognition center self-report screening questionnaires in the assessment of patients with schizophrenia and bipolar disorder. Front Psychiatry (2016) 7:149. doi: 10.3389/fpsyt.2016.00149
45. Mamah D, Mutiso VN, Ndetei DM. Psychotic-like experiences among 9,564 Kenyan adolescents and young adults. Psychiatry Res (2021) 302:113994. doi: 10.1016/j.psychres.2021.113994
46. Mamah D, Mutiso VN, Ndetei DM. Longitudinal and cross-sectional validation of the WERCAP screen for assessing psychosis risk and conversion. Schizophr Res (2022) 241:201–9. doi: 10.1016/j.schres.2022.01.031
47. Mugler JP 3rd, Brookeman JR. Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med (1990) 15:152–7. doi: 10.1002/mrm.1910150117
48. Van Der Kouwe AJW, Benner T, Salat DH, Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage (2008) 40:559–69. doi: 10.1016/j.neuroimage.2007.12.025
49. Mugler JP 3rd, Bao S, Mulkern RV, Guttmann CR, Robertson RL, Jolesz FA, et al. Optimized single-slab three-dimensional spin-echo MR imaging of the brain. Radiology (2000) 216:891–9. doi: 10.1148/radiology.216.3.r00au46891
50. Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage (2013) 80:105–24. doi: 10.1016/j.neuroimage.2013.04.127
51. Glasser MF, Smith SM, Marcus DS, Andersson JL, Auerbach EJ, Behrens TE, et al. The Human Connectome Project's neuroimaging approach. Nat Neurosci (2016) 19:1175–87. doi: 10.1038/nn.4361
52. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage (2003) 20:870–88. doi: 10.1016/S1053-8119(03)00336-7
53. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage (2016) 125:1063–78. doi: 10.1016/j.neuroimage.2015.10.019
54. Andersson JLR, Graham MS, Zsoldos E, Sotiropoulos SN. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage (2016) 141:556–72. doi: 10.1016/j.neuroimage.2016.06.058
55. Andersson JLR, Graham MS, Drobnjak I, Zhang H, Filippini N, Bastiani M. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: Within volume movement. Neuroimage (2017) 152:450–66. doi: 10.1016/j.neuroimage.2017.02.085
56. Andersson JLR, Graham MS, Drobnjak I, Zhang H, Campbell J. Susceptibility-induced distortion that varies due to motion: Correction in diffusion MR without acquiring additional data. Neuroimage (2018) 171:277–95. doi: 10.1016/j.neuroimage.2017.12.040
57. Sotiropoulos SN, Jbabdi S, Xu J, Andersson JL, Moeller S, Auerbach EJ, et al. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage (2013) 80:125–43. doi: 10.1016/j.neuroimage.2013.05.057
58. Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med (2003) 50:1077–88. doi: 10.1002/mrm.10609
59. Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage (2007) 34:144–55. doi: 10.1016/j.neuroimage.2006.09.018
60. Adler CM, Adams J, Delbello MP, Holland SK, Schmithorst V, Levine A, et al. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: A diffusion tensor imaging study. Am J Psychiatry (2006) 163:322–4. doi: 10.1176/appi.ajp.163.2.322
61. Cao H, Plichta MM, Schafer A, Haddad L, Grimm O, Schneider M, et al. Test-retest reliability of fMRI-based graph theoretical properties during working memory, emotion processing, and resting state. Neuroimage (2014) 84:888–900. doi: 10.1016/j.neuroimage.2013.09.013
62. Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F. Brain morphology and schizophrenia. A magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry (1992) 49:921–6. doi: 10.1001/archpsyc.1992.01820120009003
63. Buchanan RW, Vladar K, Barta PE, Pearlson GD. Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry (1998) 155:1049–55. doi: 10.1176/ajp.155.8.1049
64. Paillere-Martinot M, Caclin A, Artiges E, Poline JB, Joliot M, Mallet L, et al. Cerebral gray and white matter reductions and clinical correlates in patients with early onset schizophrenia. Schizophr Res (2001) 50:19–26. doi: 10.1016/S0920-9964(00)00137-7
65. Hulshoff Pol HE, Schnack HG, Bertens MG, Van Haren NE, van der Tweel I, Staal WG, et al. Volume changes in gray matter in patients with schizophrenia. Am J Psychiatry (2002) 159:244–50. doi: 10.1176/appi.ajp.159.2.244
66. Cannon TD, Van Erp TG, Huttunen M, Lonnqvist J, Salonen O, Valanne L, et al. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry (1998) 55:1084–91. doi: 10.1001/archpsyc.55.12.1084
67. Chan WY, Yang GL, Chia MY, Lau IY, Sitoh YY, Nowinski WL, et al. White matter abnormalities in first-episode schizophrenia: a combined structural MRI and DTI study. Schizophr Res (2010) 119:52–60. doi: 10.1016/j.schres.2009.12.012
68. Walterfang M, Mcguire PK, Yung AR, Phillips LJ, Velakoulis D, Wood SJ, et al. White matter volume changes in people who develop psychosis. Br J Psychiatry (2008) 193:210–5. doi: 10.1192/bjp.bp.107.043463
69. Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol (1999) 56:338–44. doi: 10.1001/archneur.56.3.338
70. Sexton CE, Walhovd KB, Storsve AB, Tamnes CK, Westlye LT, Johansen-Berg H, et al. Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. J Neurosci (2014) 34:15425–36. doi: 10.1523/JNEUROSCI.0203-14.2014
71. Liu H, Yang Y, Xia Y, Zhu W, Leak RK, Wei Z, et al. Aging of cerebral white matter. Ageing Res Rev (2017) 34:64–76. doi: 10.1016/j.arr.2016.11.006
72. Liu H, Wang L, Geng Z, Zhu Q, Song Z, Chang R, et al. A voxel-based morphometric study of age- and sex-related changes in white matter volume in the normal aging brain. Neuropsychiatr Dis Treat (2016) 12:453–65. doi: 10.2147/NDT.S90674
73. Yao L, Lui S, Liao Y, Du MY, Hu N, Thomas JA, et al. White matter deficits in first episode schizophrenia: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry (2013) 45:100–6. doi: 10.1016/j.pnpbp.2013.04.019
74. Nakajima R, Kinoshita M, Shinohara H, Nakada M. The superior longitudinal fascicle: reconsidering the fronto-parietal neural network based on anatomy and function. Brain Imaging Behav (2020) 14:2817–30. doi: 10.1007/s11682-019-00187-4
75. Janelle F, Iorio-Morin C, D'amour S, Fortin D. Superior longitudinal fasciculus: A review of the anatomical descriptions with functional correlates. Front Neurol (2022) 13:794618. doi: 10.3389/fneur.2022.794618
76. Podwalski P, Tyburski E, Szczygiel K, Rudkowski K, Waszczuk K, Andrusewicz W, et al. Psychopathology and integrity of the superior longitudinal fasciculus in deficit and nondeficit schizophrenia. Brain Sci (2022) 12(2):267. doi: 10.3390/brainsci12020267
77. Dong D, Wang Y, Chang X, Jiang Y, Klugah-Brown B, Luo C, et al. Shared abnormality of white matter integrity in schizophrenia and bipolar disorder: A comparative voxel-based meta-analysis. Schizophr Res (2017) 185:41–50. doi: 10.1016/j.schres.2017.01.005
78. Yang X, Cao D, Liang X, Zhao J. Schizophrenia symptomatic associations with diffusion tensor imaging measured fractional anisotropy of brain: a meta-analysis. Neuroradiology (2017) 59:699–708. doi: 10.1007/s00234-017-1844-9
79. Joo SW, Kim H, Jo YT, Ahn S, Choi YJ, Park S, et al. White matter impairments in patients with schizophrenia: A multisite diffusion MRI study. Prog Neuropsychopharmacol Biol Psychiatry (2021) 111:110381. doi: 10.1016/j.pnpbp.2021.110381
80. Chawla N, Deep R, Khandelwal SK, Garg A. Reduced integrity of superior longitudinal fasciculus and arcuate fasciculus as a marker for auditory hallucinations in schizophrenia: A DTI tractography study. Asian J Psychiatry (2019) 44:179–86. doi: 10.1016/j.ajp.2019.07.043
81. Ozcelik-Eroglu E, Ertugrul A, Oguz KK, Has AC, Karahan S, Yazici MK. Effect of clozapine on white matter integrity in patients with schizophrenia: a diffusion tensor imaging study. Psychiatry Res (2014) 223:226–35. doi: 10.1016/j.pscychresns.2014.06.001
82. Sagarwala R, Nasrallah HA. The effect of antipsychotic medications on white matter integrity in first-episode drug-naive patients with psychosis: A review of DTI studies. Asian J Psychiatr (2021) 61:102688. doi: 10.1016/j.ajp.2021.102688
83. Szeszko PR, Robinson DG, Ikuta T, Peters BD, Gallego JA, Kane J, et al. White matter changes associated with antipsychotic treatment in first-episode psychosis. Neuropsychopharmacology (2014) 39:1324–31. doi: 10.1038/npp.2013.288
84. Orr JM, Paschall CJ, Banich MT. Recreational marijuana use impacts white matter integrity and subcortical (but not cortical) morphometry. NeuroImage Clin (2016) 12:47–56. doi: 10.1016/j.nicl.2016.06.006
85. Robinson EA, Gleeson J, Arun AH, Clemente A, Gaillard A, Rossetti MG, et al. Measuring white matter microstructure in 1,457 cannabis users and 1,441 controls: A systematic review of diffusion-weighted MRI studies. Front Neuroimaging (2023) 2:1129587. doi: 10.3389/fnimg.2023.1129587
86. Kennis M, Van Rooij SJ, Tromp Do PM, Fox AS, Rademaker AR, Kahn RS, et al. Treatment outcome-related white matter differences in veterans with posttraumatic stress disorder. Neuropsychopharmacology (2015) 40:2434–42. doi: 10.1038/npp.2015.94
87. Zhong Z, Yang X, Cao R, Li P, Li Z, Lv L, et al. Abnormalities of white matter microstructure in unmedicated patients with obsessive-compulsive disorder: Changes after cognitive behavioral therapy. Brain Behav (2019) 9:e01201. doi: 10.1002/brb3.1201
Keywords: schizophrenia, bipolar disorder, dementia, diffusion, brain, connectivity, TRACULA
Citation: Mamah D, Chen S, Shimony JS and Harms MP (2024) Tract-based analyses of white matter in schizophrenia, bipolar disorder, aging, and dementia using high spatial and directional resolution diffusion imaging: a pilot study. Front. Psychiatry 15:1240502. doi: 10.3389/fpsyt.2024.1240502
Received: 15 June 2023; Accepted: 15 January 2024;
Published: 01 February 2024.
Edited by:
Wu Jeong Hwang, Seoul National University, Republic of KoreaReviewed by:
Marcella Bellani, University of Verona, ItalyMassimo Tusconi, University of Cagliari, Italy
Copyright © 2024 Mamah, Chen, Shimony and Harms. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Mamah, bWFtYWhkQHd1c3RsLmVkdQ==
 Daniel Mamah
Daniel Mamah ShingShiun Chen1
ShingShiun Chen1 Michael P. Harms
Michael P. Harms