
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 20 May 2024
Sec. Schizophrenia
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1309822
 Teng-Hung Yu1,2
Teng-Hung Yu1,2 Thung-Lip Lee1,3
Thung-Lip Lee1,3 Chin-Feng Hsuan1,2,4
Chin-Feng Hsuan1,2,4 Cheng-Ching Wu1,2,5
Cheng-Ching Wu1,2,5 Chao-Ping Wang1,3
Chao-Ping Wang1,3 Yung-Chuan Lu3,6
Yung-Chuan Lu3,6 Ching-Ting Wei7,8
Ching-Ting Wei7,8 Fu-Mei Chung1
Fu-Mei Chung1 Yau-Jiunn Lee9
Yau-Jiunn Lee9 I-Ting Tsai2,10*†
I-Ting Tsai2,10*† Wei-Hua Tang11,12*†
Wei-Hua Tang11,12*†Introduction: Of all psychiatric disorders, schizophrenia is associated with the highest risk of all-cause mortality. This study aimed to investigate independent risk factors for all-cause mortality in patients with chronic schizophrenia. In addition, the possible causal inter-relationships among these independent risk factors and all-cause mortality were also explored.
Methods: We conducted an analysis of 1,126 patients with chronic schizophrenia from our psychiatric department from April 2003 to August 2022, and retrospectively reviewed their medical records. The study endpoint was all-cause mortality. Baseline clinical characteristics including sociodemographic data, biochemical data, lifestyle factors, comorbidities and antipsychotic treatment were examined with Cox proportional hazards analysis.
Results: The all-cause mortality rate was 3.9% (44 patients). Multivariate Cox regression analysis revealed that several factors were independently associated with all-cause mortality, including diabetes mellitus (DM), hypertension, heart failure, gastroesophageal reflux disease (GERD), peptic ulcer disease, ileus, underweight, fasting glucose, triglycerides, albumin, and hemoglobin. Structural equation modeling (SEM) analysis revealed that several factors had statistically significant direct effects on all-cause mortality. Heart failure, hypertension, underweight, age at onset, and ileus showed positive direct effects, while albumin and hemoglobin demonstrated negative direct effects. In addition, several factors had indirect effects on all-cause mortality. GERD indirectly affected all-cause mortality through ileus, and peptic ulcer disease had indirect effects through albumin and ileus. Ileus, underweight, DM, and hypertension also exhibited indirect effects through various pathways involving albumin, hemoglobin, and heart failure. Overall, the final model, which included these factors, explained 13% of the variability in all-cause mortality.
Discussion: These results collectively suggest that the presence of DM, hypertension, heart failure, GERD, peptic ulcer disease, ileus, and underweight, along with lower levels of albumin or hemoglobin, were independently associated with all-cause mortality. The SEM analysis further revealed potential causal pathways and inter-relationships among these risk factors contributing to all-cause mortality in patients with chronic schizophrenia.
Schizophrenia is a multidimensional disorder that encompasses various subtypes, each with distinct neurobiological underpinnings (1–3). Recognized as a chronic and severe mental disorder, schizophrenia is a psychiatric syndrome characterized by positive, negative, and disorganized symptoms (4). While schizophrenia has an estimated heritability of 79%, it can also be influenced by various factors, including genetics, environment, exposure to viruses, infection, prenatal malnutrition, birth complications, psychosocial factors, migrant status, and urbanicity (5–7). The highest burden of schizophrenia is observed among individuals aged 25–54 years, encompassing their most productive years (8).
Schizophrenia is a global disorder (9), affecting an estimated 24 million people [~1 in 300 people (0.32%)] worldwide. Among adults, the prevalence of schizophrenia is estimated to be 1 in 222 people (0.45%) according to the Institute of Health Metrics and Evaluation (10). With a lifetime prevalence ranging from approximately 0.5% to 1%, schizophrenia is a significant global public health concern (11). According to the Global Burden of Disease 2019, the raw prevalence, incidence, and burden of schizophrenia have increased since 1990, and no reduction has been observed in age-adjusted estimates (12). Regional studies in the United States, China, India, and South Korea have suggested a steady increase in the annual incidence of schizophrenia (13–15). In Taiwan, the 1-year prevalence rate of schizophrenia is 3.34 per 1,000 people, with a 6-year (cumulative) prevalence rate of 6.42 per 1,000 people from 1996 to 2001 (16). Of all psychiatric disorders, schizophrenia is associated with one of the highest risks of mortality (17), with an all-cause mortality rate 2 to 3-fold higher than that in the general population, and a substantially shorter life expectancy (18–20). Consequently, studies on the risk factors affecting mortality in patients with chronic schizophrenia are warranted.
The modifiable risk factors associated with mortality in patients with schizophrenia include limited access to physical care, poor lifestyle behaviors, and whether or not antipsychotic medications are prescribed (21, 22). Schizophrenia has also been associated with elevated frequencies of comorbidities, with the majority of excess deaths being attributed to chronic diseases including type II diabetes mellitus (DM) (23), hypertension (24), cardiovascular diseases (25), respiratory diseases (26), stroke (27), and cancer (28), with unnatural causes such as suicide accounting for <15% (29). In addition, a previous study demonstrated a high cardiometabolic risk in patients with schizophrenia spectrum disorders. This underscores the importance of proper management, ranging from lifestyle modifications to addressing risk factors, and including the careful selection of antipsychotic drugs with a favorable cardiometabolic profile (30). However, few studies have investigated the effect of demographics, clinical characteristics, lifestyle factors, comorbid illnesses, and biochemical factors on all-cause mortality in patients with chronic schizophrenia. Moreover, Peritogiannis et al. demonstrated a complex interplay of factors that synergistically contribute to physical morbidity in patients with chronic schizophrenia, ultimately leading to increased mortality (31). Furthermore, another study reported that the association between schizophrenia and cardiometabolic risk factors is complex and influenced by an interplay of environmental factors, genetic vulnerability, and disease-related factors (32). Thus, we hypothesized that to comprehend the effects of inter-relationships and causal pathways of risk factors on all-cause mortality in patients with chronic schizophrenia, various aspects need to be considered.
To test this hypothesis, the aims of this study were: (1) to conduct a retrospective study assessing the associations among risk factors at baseline including demographics, clinical characteristics, lifestyle factors, comorbid illnesses [e.g. DM, chronic kidney disease (CKD), hypertension, gastrointestinal diseases, liver diseases, heart failure, anemia, cardiovascular diseases, cardiac arrhythmias, chronic obstruction pulmonary disease/asthma, cancer, peripheral arterial occlusion disease, obesity status], and biochemical factors with all-cause mortality in patients with chronic schizophrenia; and (2) use a structural equation model (SEM) to determine possible causal inter-relationships among the aforementioned risk factors and all-cause mortality. In addition, to emphasize the importance of regular screening for risk factors in patients with chronic schizophrenia.
In this retrospective study, we conducted an analysis of 1,684 consecutive patients using the electronic database of Taipei Veterans General Hospital, Yuli Branch, from April 2003 to August 2022. The entire dataset was de-identified, delinked, and encrypted before being made available for analysis. The inclusion criteria were patients with: (1) chronic schizophrenia who were >18 years of age at the diagnosis, (2) schizophrenia diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders IV, (3) complete clinical and follow-up data. The exclusion criteria were: (1) a diagnosis of major affective disorders (including mania, major depression, bipolar disorder and schizoaffective disorder), and (2) patients without complete clinical and follow-up data. In addition, as our psychiatric ward does not admit patients with substance and alcohol abuse or dependence, these patients were not included in this study. Moreover, patients with a poor treatment response and those with suicidal intention or behavior were also excluded from the study to mitigate the impact of these factors. Although some of the patients had a history of treatment resistance or suicidal behavior, those included in this study were in a stable and chronic condition. In our hospital, we use the Clinical Global Impression Severity (CGI-S) score (33) and Mini-Mental State Examination (MMSE) test to assess the psychological and intellectual clinical status of patients. The CGI-S is rated on a 7-point scale as follows: 1 = normal, not at all ill; 2 = borderline mentally ill; 3 = mildly ill; 4 = moderately ill; 5 = markedly ill; 6 = severely ill; 7 = among the most extremely ill patients. In general, none of the patients at our institution have a low CGI-S score, because all of our patients are chronic and have been transferred from other psychiatric wards in Taiwan. Those with higher CGI-S scores were initially admitted to the acute ward and subsequently excluded from our study. Consequently, the CGI-S scores of the patients included in this study at the time of inclusion ranged between 3 and 4, which is equivalent to positive and negative syndrome scale scores of 55 to 78. In addition, the median MMSE score (interquartile range) of the patients was 23.0 (17.0-27.0) (Table 1).
Clinical information collected for subsequent analysis included: (1) sociodemographic factors: age, sex, and age at onset; (2) lifestyle factors: former/current smokers compared to non-smokers [ceased smoking ≥1 year], and former/current alcohol drinkers compared to non-drinkers [ceased drinking ≥1 year]; (3) anthropometric variables: weight, height, body mass index (BMI); and blood pressure (after a 5-min rest); (4) comorbidities: DM, hypertension, hyperlipidemia, anemia, CKD, cardiovascular diseases, heart failure, cardiac arrhythmias, peripheral arterial occlusion disease, gastroesophageal reflux disease (GERD), peptic ulcer disease, Ileus, cancer, hepatitis B virus (HBV)/hepatitis C virus (HCV) infections, chronic obstruction pulmonary disease/asthma; (5) obesity status according to the Ministry of Health and Welfare, Taiwan, definitions (all values given as BMI in kg/m2) (34): severe obesity (≥35), moderate obesity (30≤-<35), mild obesity (27≤-<30), overweight (24≤-<27), normal weight (18.5≤-<24), underweight (<18.5); (6) antipsychotic treatment: (typical antipsychotics and atypical antipsychotics); and (7) biochemical data: HbA1C, fasting glucose, albumin, total protein, uric acid, lipid profile, liver and renal function parameters, total and differential leukocyte counts, and hematological parameters.
Detailed data were retrieved from Taipei Veterans General Hospital, Yuli Branch Psychiatric Database. Two research assistants (P.-L.L. and P.-Y.T.) collected the data, all of which were checked by a study author (F.-M.C.).
This study was conducted according to the principles of the Declaration of Helsinki and was approved by the Human Research Ethics Committee of Kaohsiung E-Da Hospital (EMRP61110N and EMRP66111N). To ensure the protection of any potentially identifiable personal data of the subjects, the entire dataset has been de-identified and encrypted before being made available for analysis. This process aligns with national legislation and institutional requirements. As such, written informed consent from the participants was not required.
Categorical variables are given as frequency (percentage), while continuous variables are given as mean (±standard deviation). Comparisons in baseline variables between survivors and non-survivors were performed with the Student's t test or χ2 test. We defined the outcome as the duration from diagnosis to death. Univariate and multivariate Cox proportional hazard analyses were performed to evaluate relationships between baseline biochemical and clinical risk factors with all-cause mortality, and the results are presented as hazard ratio (HR) with 95% confidence interval (CI). JMP (version 7.0, SAS Institute) was used for all other statistical analyses. Two-sided p < 0.05 were considered significant. In addition, we also used IBM SPSS AMOS version 24 (Amos Development Corporation, Meadville, PA, USA) to fit the path model and SEM. We used root mean square error of approximation <0.08, standardized root mean square residual <0.06, and comparative fit index (CFI) >0.90 to assess the fit of the data to the models (35). Furthermore, model fit was estimated using the maximum likelihood method. The findings are presented as standardized path coefficients along with the corresponding statistical significance.
Of the 1,684 patients with chronic schizophrenia initially screened from April 2003 to August 2022, 558 were excluded from the study (90 with higher CGI-S scores and 468 without complete clinical and follow-up data). The final study population included 1,126 consecutive patients (586 men and 540 women; mean age, 56.7 ± 11.7 years), and they were followed until October 31, 2022 (median follow-up, 26.3 months; range, 2–230 months). At the end of the study, 44 patients (3.9%) had died of all causes, of whom four were related to choking, three to coronavirus disease 2019 (COVID-19) infection, one to hepatocellular carcinoma, 21 to sepsis due to pneumonia and urinary tract infections, and 15 to sudden cardiac death.
At baseline, the median age of the patients was 57 (range, 23–94) years, 196 (17.4%) patients had DM, 209 (18.6%) had hyperlipidemia, and 410 (36.4%) had hypertension (Table 1). The non-survivors were older, had an older age at onset, and lower diastolic blood pressure and BMI values than the survivors. In addition, more of the non-survivors were female, and they had higher rates of DM, hypertension, anemia, heart failure, GERD, peptic ulcer disease, ileus, cancer, HBV and HCV infections, and underweight, and lower rates of overweight and mild obesity than the survivors (Table 1).
The non-survivors had higher baseline alkaline phosphatase and eosinophil count, and lower fasting glucose, total cholesterol, LDL-cholesterol, albumin, lymphocyte count, red blood cell count, hemoglobin, and hematocrit than the survivors (Table 2).
Univariate Cox regression analysis showed that age, age at onset, diastolic blood pressure, BMI, DM, hypertension, anemia, heart failure, GERD, peptic ulcer disease, ileus, HBV/HCV infections, and underweight were associated with all-cause mortality. Multivariate Cox regression analysis showed that DM [HR 4.02 (1.83–8.52), p = 0.001], hypertension [HR 3.45 (1.66–7.35), p = 0.001], heart failure [HR 4.31 (1.13–12.90), p = 0.035], GERD [HR 2.48 (1.13–5.19), p = 0.025], peptic ulcer disease [HR 3.18 (1.15–7.50), p = 0.028], ileus [HR 2.80 (1.19–6.01), p = 0.020], and underweight [HR 3.91 (1.10–18.46), p = 0.034] were independently associated with all-cause mortality (Table 3).
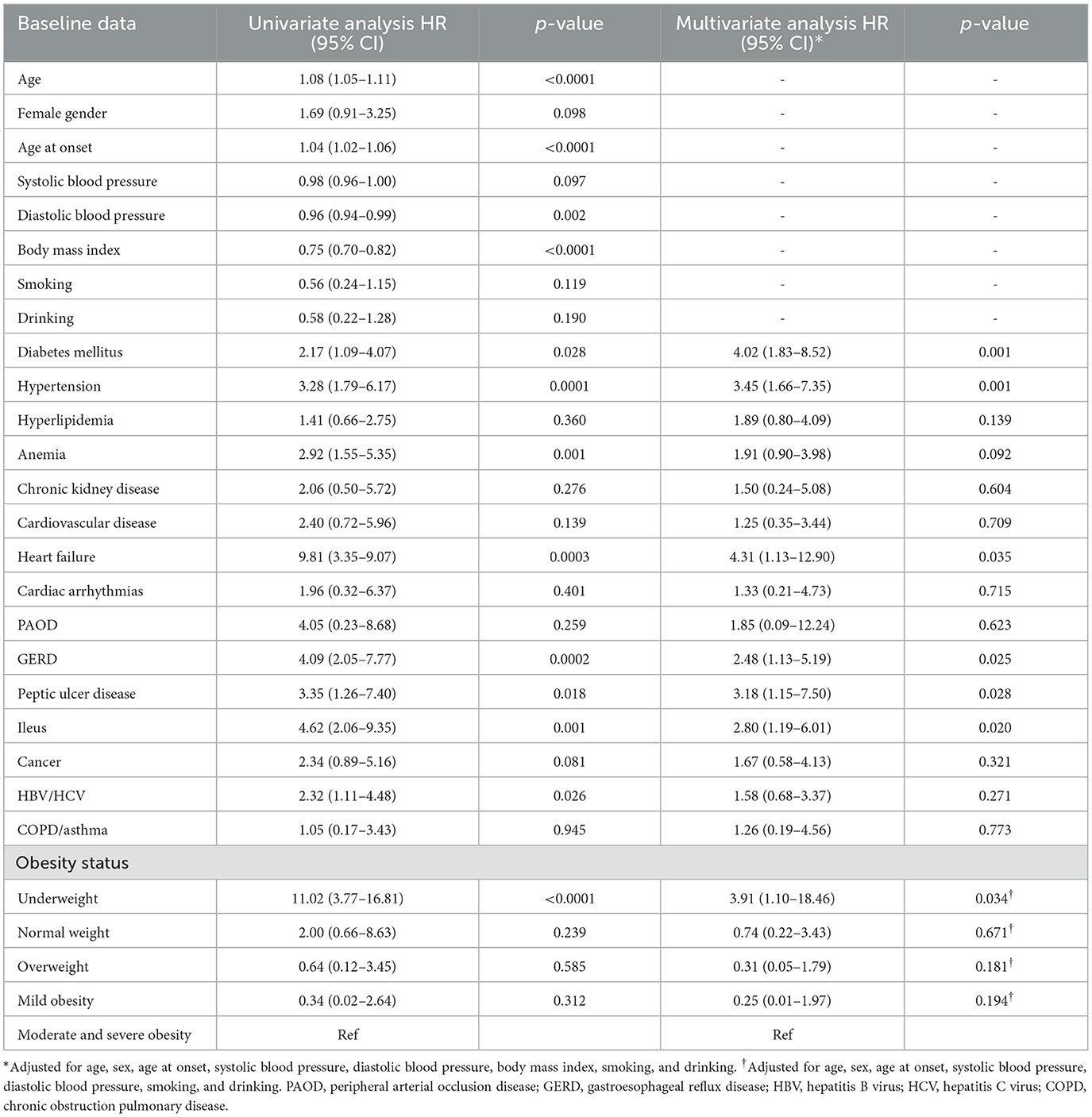
Table 3. Cox proportional hazard model of baseline clinical risk factors for the development of all-cause mortality in the whole cohort.
Univariate Cox regression analysis showed that fasting glucose, total cholesterol, LDL-cholesterol, albumin, lymphocyte count, red blood cell count, hemoglobin, and hematocrit were associated with all-cause mortality. Multivariate Cox regression analysis showed that fasting glucose [HR 0.96 (0.93–0.99), p = 0.044], triglycerides [HR 0.98 (0.96–0.99), p = 0.048], albumin [HR 0.10 (0.03–0.28), p < 0.0001], and hemoglobin [HR 0.33 (0.12–0.92), p = 0.035] were independently associated with all-cause mortality (Table 4).
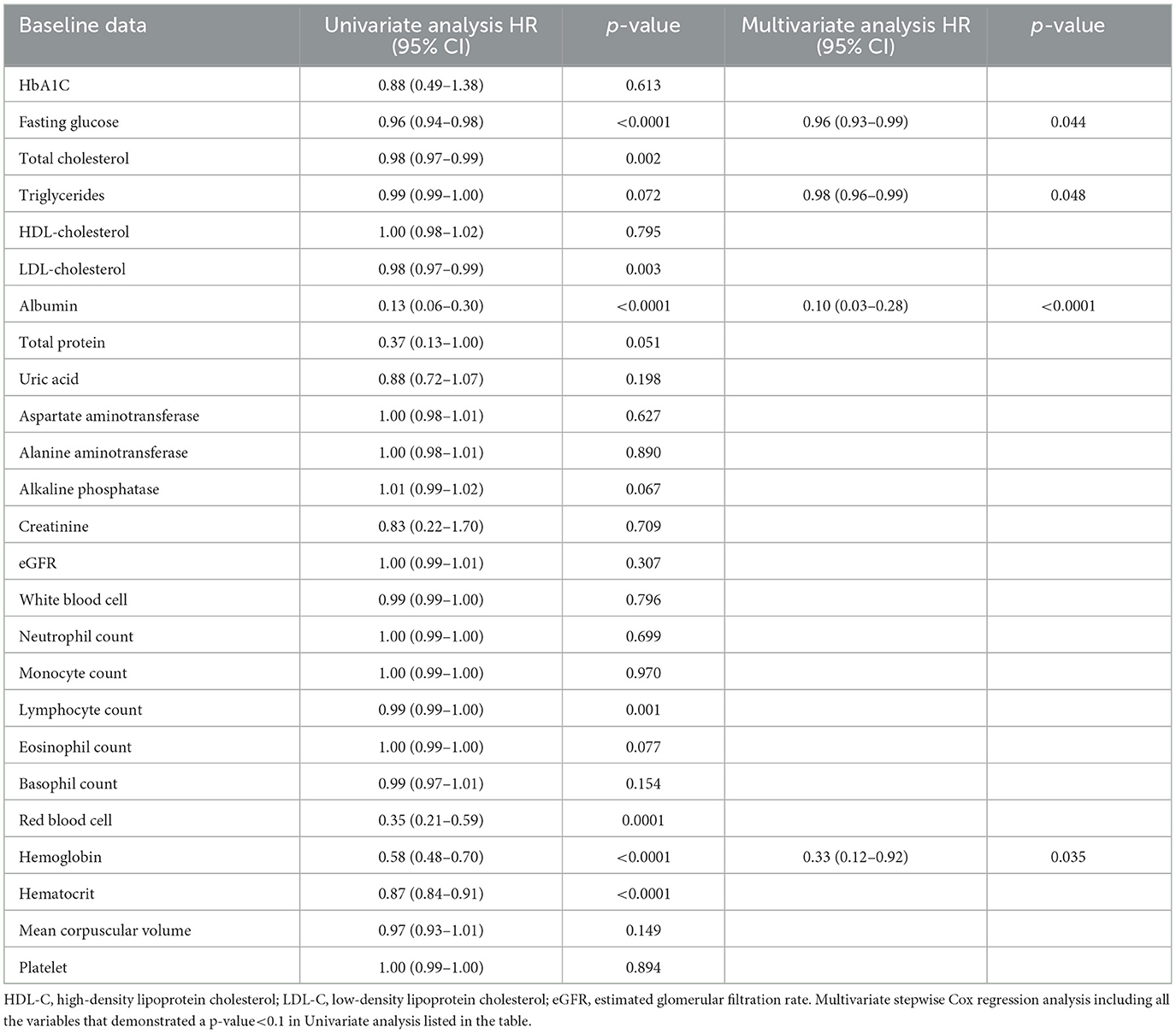
Table 4. Cox proportional hazard model of baseline biochemical risk factors for the development of all-cause mortality in the whole cohort.
As with the Cox proportional hazard model described above (Tables 3, 4), we designed an SEM model to assess the effects of DM, hypertension, heart failure, GERD, peptic ulcer disease, ileus, underweight, albumin, and hemoglobin on all-cause mortality. The results showed that the model fit the data well, with a CFI of 0.958, a root mean square error of approximation of 0.027, and a standardized root mean square residual of 0.030 (Figure 1). Heart failure (β = 0.079), hypertension (β = 0.066), underweight (β = 0.222), age at onset (β = 0.146), and ileus (β = 0.058) had statistically significant positive direct effects on all-cause mortality. In addition, albumin (β = −0.096) and hemoglobin (β = −0.070) had statistically significant negative direct effects on all-cause mortality. Moreover, GERD indirectly affected all-cause mortality through ileus (β = 0.113). Peptic ulcer disease indirectly affected all-cause mortality through albumin (β = −0.059) and ileus (β = 0.138). Ileus indirectly affected all-cause mortality through albumin (β = −0.092) and underweight (β = 0.142). Underweight indirectly affected all-cause mortality through albumin (β = −0.132) and hemoglobin (β = −0.131). DM indirectly affected all-cause mortality through heart failure (β = 0.071) and hemoglobin (β = −0.059). Age at onset indirectly affected all-cause mortality through albumin (β = −0.089), heart failure (β = 0.114), and hypertension (β = 0.114). Albumin indirectly affected all-cause mortality through hemoglobin (β = 0.217) and heart failure (β = −0.061). Hemoglobin indirectly affected all-cause mortality through heart failure (β = −0.073). Hypertension indirectly affected all-cause mortality through heart failure (β = 0.074). The model explained 13% of the variability in all-cause mortality (Figure 1).
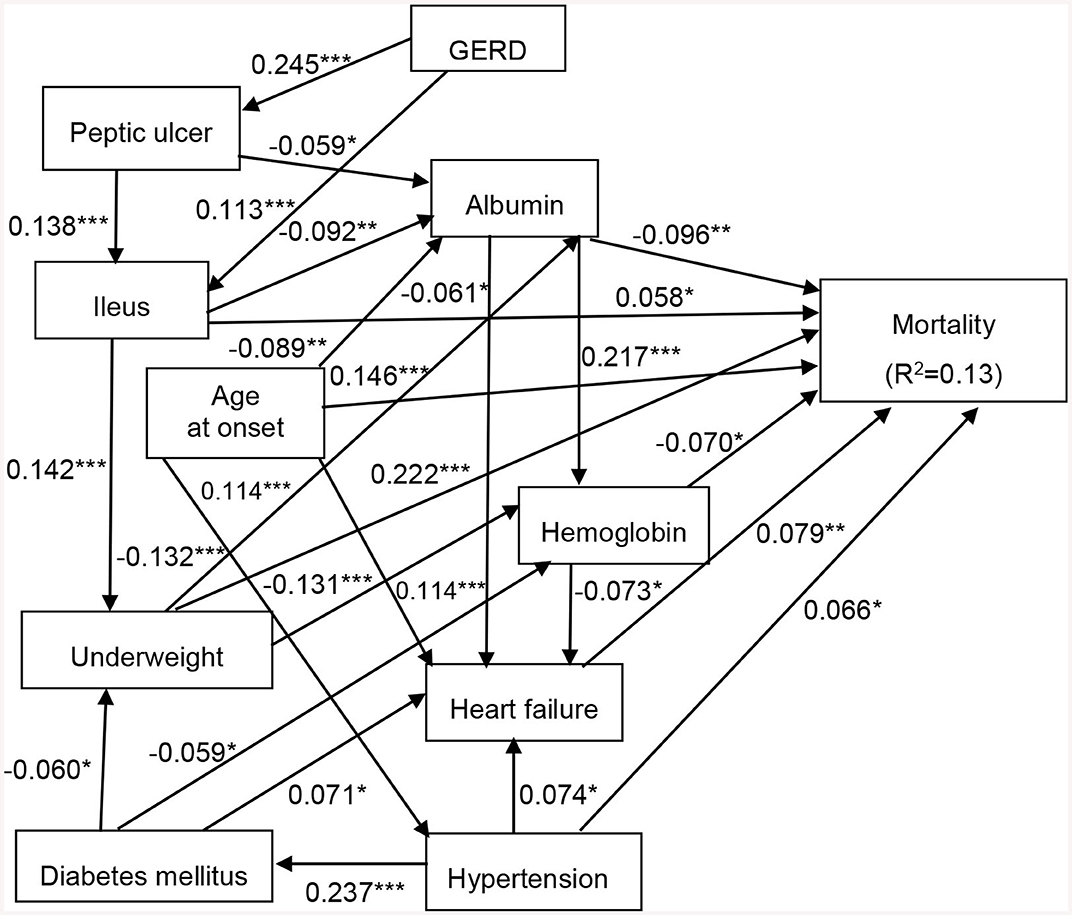
Figure 1. Structural equation model for all-cause mortality in patients with chronic schizophrenia. Comparative fit index (CFI), 0.958; goodness of fit index (GFI), 0.992; root mean square error of approximation (RMSEA), 0.027; standardized root mean square residual (SRMR), 0.030. *p < 0.05, **p < 0.01, and ***p < 0.001. Path loadings are standardized coefficients. GERD, gastroesophageal reflux disease.
In this study, we investigated associations among baseline risk factors with all-cause mortality in patients with chronic schizophrenia. There are two key findings in this study. First, multivariate Cox regression analysis showed that DM, hypertension, heart failure, GERD, peptic ulcer disease, ileus, underweight, fasting glucose, triglycerides, albumin, and hemoglobin all contributed to the risk of all-cause mortality in the enrolled patients with chronic schizophrenia. Second, the causal relationships of DM, hypertension, heart failure, GERD, peptic ulcer disease, ileus, underweight, albumin, and hemoglobin on all-cause mortality were confirmed by SEM analysis.
A previous study reported a 2.9-fold higher risk of all-cause mortality in patients with schizophrenia compared to the general population, and a 1.6-fold higher risk compared to controls matched for physical diseases (36). Although several risk factors including cardiovascular diseases (37), stroke (37, 38), smoking (39), physical fitness and inactivity (40), obesity (41), dyslipidemia (42), any cancer (43), and suicide (44) have been associated with all-cause mortality in patients with schizophrenia, the exact pathophysiological mechanisms have yet to be clarified.
We also found associations between all-cause mortality with DM, hypertension, heart failure, GERD, peptic ulcer disease, ileus, underweight, fasting glucose, triglycerides, albumin, and hemoglobin in our study cohort (Tables 3, 4). Previous studies have reported a 2 to 3-fold higher prevalence of type 2 DM in patients with schizophrenia compared to the general population, with a rate ranging from 6% to 21% (45, 46). Patients with schizophrenia have a high mortality rate, and metabolic abnormalities including type 2 DM are important causes. Possible etiologies of the development of diabetes in schizophrenic patients include: (a) inherited susceptibility to both schizophrenia and diabetes (47); (b) antipsychotic medications that affect dopaminergic, serotonergic, and histaminergic receptors, and hypothalamic regulation (48), and influence leptin resistance and pancreatic muscarinic receptors (48); (c) neuroendocrine pathways, elevated cortisol and hypothalamic axis dysregulation (49), and nutritional deficiency (50, 51); (d) many environmental factors, such as limited availability of quality food and poor diet (52), and insufficient physical activity due to social isolation and symptoms (52, 53), which are related to both diabetes and schizophrenia. However, we found that lower fasting glucose and triglycerides were associated with higher all-cause mortality in our study cohort. Consistent with our results, a previous study reported a significantly higher prevalence of hypotriglyceridemia in underweight schizophrenic patients (54). The poor diet in patients with schizophrenia could partially explain the higher prevalence of metabolic abnormalities (55).
A previous study reported a possible link between severe mental illnesses including schizophrenia with an increased risk of alterations in left ventricular function and structure due to the early onset of cardiovascular disease and other factors including smoking, obesity, hypertension, myocardial infarction and DM (56). These factors could lead to a greater decrease in left ventricular ejection fraction and more severe heart failure compared to the general population (57, 58). In addition, in a study of approximately 22,000 schizophrenic patients, Kilbourne et al. reported that hypertension was a major risk factor for cardiac death (59). These findings support the association between hypertension and heart failure with all-cause mortality in the present study (Table 3). Similarly, we also found that peptic ulcer disease, GERD, and ileus were related to all-cause mortality. This is in agreement with other studies which have reported an association between schizophrenia with peptic ulcer disease, GERD, and ileus (60–62). Liao et al. suggested that schizophrenic patients are at a slightly elevated risk of peptic ulcer disease compared to the general population (60). This may be due to higher rates of Helicobacter pylori infection, smoking, alcohol consumption, taking anxiolytics and hypnotics, anti-depressants, or non-steroidal anti-inflammatory drugs among these patients. Furthermore, Kasap et al. suggested that schizophrenic patients who smoke and drink alcohol may have a higher rate of reflux symptoms (61). Moreover, Nielsen et al. suggested that female sex, older age, treatment with high-potency first-generation antipsychotic drugs, clozapine, anticholinergics, tricyclic antidepressants, and opioids may be associated with a higher risk of ileus in patients with schizophrenia (62). In the present study, we also found that underweight status was related to all-cause mortality. This finding was also reported in a previous study, in which all-cause mortality was associated with underweight status compared with normal weight status (HR: 1.33, 95% CI: 1.01–1.76), potentially due to frailty in older age groups (41).
Multivariate Cox regression analysis of the biochemical risk factors in this study showed associations between albumin and hemoglobin with all-cause mortality (Table 4). Huang reported a significantly lower serum albumin level in Taiwanese schizophrenic patients during the acute phase compared to controls (63). In addition, serum albumin level has been proposed to be a prognostic indicator of mortality in older hospitalized patients (64), survival in women infected with immunodeficiency virus (65), and disease in patients with inflammation or injury (66). Regarding the association between hemoglobin and all-cause mortality, a previous study reported a higher prevalence of anemia among chronic psychiatric patients compared to the general population (67). This could be due to reasons including poor physical condition and lifestyle habits, drugs taken, and nutritional disorders. This may suggest that serum albumin and hemoglobin concentrations could also be used as markers of the clinical course in patients with schizophrenia.
To the best of our knowledge, this study is the first to investigate the causal relationships of DM, hypertension, heart failure, GERD, peptic ulcer disease, ileus, underweight, albumin, and hemoglobin with all-cause mortality in patients with schizophrenia. However, the exact mechanisms underlying the associations among these risk factors with all-cause mortality remain unclear. SEM analysis showed significant positive direct effects from heart failure, underweight, age at onset, ileus and hypertension on all-cause mortality. Furthermore, albumin and hemoglobin had significant negative direct effects on all-cause mortality. Previous studies have demonstrated associations between DM (36), hypertension (59), heart failure (68), and gastrointestinal diseases (e.g., GERD, peptic ulcer disease, and ileus) (36), underweight (41), age at onset (36), albumin (64), and hemoglobin (67) with all-cause mortality in patients with schizophrenia. We also found that GERD indirectly affected all-cause mortality through ileus, peptic ulcer disease indirectly affected all-cause mortality through low albumin and ileus, and that ileus indirectly affected all-cause mortality through low albumin and underweight. In addition, we found that underweight indirectly affected all-cause mortality through low albumin and low hemoglobin, DM and albumin indirectly affected all-cause mortality through heart failure and low hemoglobin, and that hemoglobin and hypertension indirectly affected all-cause mortality through heart failure. The most frequent clinical signs of ileus are a decrease in or no intestinal sounds and gastric reflux (69). Nielsen et al. showed that clozapine or anticholinergic treatment was associated with a higher risk of fatal ileus in patients with schizophrenia (62). Furthermore, emerging evidence has suggested relationships between a lower serum albumin level with peptic ulcer disease and bowel disease in patients with schizophrenia (63, 70). Notably, a previous study (63) reported that Taiwanese inpatients with schizophrenia had lower serum albumin levels, suggesting that patients in the acute phase of disease have similar systemic responses, as also shown in other studies (71, 72). Moreover, the combination of hypoalbuminemia and low BMI has been proposed to potentially be a useful marker of high mortality in older people (73). Kamruzzaman found that underweight women were more likely to be anemic (74). In addition, anemia has been shown to have a cumulative additive effect on left ventricular function and global strain in patients with type 2 DM (75). Furthermore, anemia is a common comorbidity in patients with heart failure, and is associated with poor outcomes (76). Chronic hypertension and cardiac structural and functional changes can predispose to the development of heart failure (77). Therefore, it is reasonable to suggest that GERD, peptic ulcer disease, ileus, underweight, DM, albumin, hemoglobin, and hypertension may be involved in common pathways contributing to all-cause mortality in patients with chronic schizophrenia.
The limitations of this study include the relatively few cases of all-cause mortality. In addition, the length of follow-up (median, 26.3 months) may be insufficient to reveal cases of late all-cause mortality. Moreover, the single-center nature of this retrospective study may limit the application of our results to other Taiwanese patients with schizophrenia. Further larger-scale studies with patients of different ethnicity and longer follow-up periods are needed to verify our findings. In addition, there was a long duration between the age at onset and the start of follow-up. This extended duration raises the possibility that factors influencing mortality patterns along the treatment trajectory in individuals with schizophrenia may not align with the highest risk period for suicide in this population, and this may have influenced our findings. Finally, only patients with stable and chronic schizophrenia were included into this study. This limitation may affect the generalizability of our results to other subtypes of schizophrenia or to patients with acute conditions and high CGI-S scores.
In summary, we found that DM, hypertension, heart failure, GERD, peptic ulcer disease, ileus, underweight, lower circulating albumin and hemoglobin levels were associated with all-cause mortality in patients with chronic schizophrenia. In addition, SEM delineated inter-relationships of the risk factors and potential pathways that may contribute to all-cause mortality in patients with chronic schizophrenia. These findings provide valuable insights for improving clinical practice, and the identified risk factors could serve as important indicators for clinicians to closely monitor and manage individuals with chronic schizophrenia. Regular screening for DM, hypertension, and heart failure, along with vigilant management of gastrointestinal issues such as GERD and peptic ulcer disease may help to prevent adverse outcomes. In addition, efforts should be directed toward optimizing nutritional status, including addressing underweight and ensuring adequate levels of albumin and hemoglobin. Moreover, in terms of integrative care, a multidisciplinary approach could be beneficial, and collaborative efforts involving psychiatrists, primary care physicians, nutritionists, and other healthcare professionals would enhance the comprehensive care of individuals with schizophrenia. Integrated care models that focus on both mental and physical health, incorporating regular health assessments, lifestyle interventions, and patient education, may contribute to better outcomes. Mental health and medical providers should work together to develop personalized care plans that address the specific needs of individuals with schizophrenia, considering the identified risk factors for mortality. Furthermore, promoting patient engagement and self-management could play an important role in achieving holistic wellbeing in this population. Our findings suggest that a proactive and integrated approach to healthcare delivery, considering the complex interplay of risk factors, is essential for improving the overall health and longevity of individuals with chronic schizophrenia.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by The Human Research Ethics Committee of Kaohsiung E-Da Hospital (EMRP61110N and EMRP66111N). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent from the participants was not required to participate in this study in accordance with the national legislation and the institutional requirements.
T-HY: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. T-LL: Data curation, Investigation, Resources, Writing—original draft, Writing—review & editing. C-FH: Data curation, Investigation, Resources, Writing—original draft, Writing—review & editing. C-CW: Data curation, Investigation, Resources, Writing—original draft, Writing—review & editing. C-PW: Data curation, Investigation, Resources, Writing—original draft, Writing—review & editing. Y-CL: Data curation, Investigation, Resources, Writing—original draft, Writing—review & editing. C-TW: Writing—original draft, Writing—review & editing. F-MC: Formal analysis, Project administration, Writing—original draft, Writing—review & editing. Y-JL: Methodology, Visualization, Writing—original draft, Writing—review & editing. I-TT: Writing—review & editing, Conceptualization, Methodology, Validation, Visualization, Writing—original draft. W-HT: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from Taipei Veterans General Hospital, Yuli Branch and E-Da Hospital of the Republic of China, Taiwan (contract no. VHYL111-002, VLVH-112-03, and EDAHI112001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ventriglio A, Bellomo A, Ricci F, Magnifico G, Rinaldi A, Borraccino L, et al. New pharmacological targets for the treatment of schizophrenia: a literature review. Curr Top Med Chem. (2021) 21:1500–16. doi: 10.2174/1568026621666210701103147
2. Ebisch SJ, Mantini D, Northoff G, Salone A, De Berardis D, Ferri F, et al. Altered brain long-range functional interactions underlying the link between aberrant self-experience and self-other relationship in first-episode schizophrenia. Schizophr Bull. (2014) 40:1072–82. doi: 10.1093/schbul/sbt153
3. De Berardis D, De Filippis S, Masi G, Vicari S, Zuddas A. A Neurodevelopment approach for a transitional model of early onset schizophrenia. Brain Sci. (2021) 11:275. doi: 10.3390/brainsci11020275
4. Jauhar S, Johnstone M, McKenna PJ. Schizophrenia. Lancet. (2022) 399:473–86. doi: 10.1016/S0140-6736(21)01730-X
5. National Institute of Mental Health. Schizophrenia. Available online at: https://www.nimh.nih.gov/health/topics/schizophrenia/index.shtml (accessed February 1, 2016).
6. Sutterland AL, Fond G, Kuin A, Koeter MW, Lutter R, van Gool T, et al. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta Psychiatr Scand. (2015) 132:161–79. doi: 10.1111/acps.12423
7. Henssler J, Brandt L, Müller M, Liu S, Montag C, Sterzer P, et al. Migration and schizophrenia: metaanalysis and explanatory framework. Eur Arch Psychiatry Clin Neurosci. (2020) 270:325–35. doi: 10.1007/s00406-019-01028-7
8. Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, et al. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr Bull. (2018) 44:1195–203. doi: 10.1093/schbul/sby058
10. Institute of health Metrics and Evaluation (IHME). Global Health Data Exchange (GHDx). http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/27a7644e8ad28e739382d31e77589dd7 (accessed September 25, 2021).
11. Simeone JC, Ward AJ, Rotella P, Collins J, Windisch R. An evaluation of variation in published estimates of schizophrenia prevalence from 1990-2013: a systematic literature review. BMC Psychiatry. (2015) 15:193. doi: 10.1186/s12888-015-0578-7
12. Solmi M, Seitidis G, Mavridis D, Correll CU, Dragioti E, Guimond S, et al. Incidence, prevalence, and global burden of schizophrenia-data, with critical appraisal, from the Global Burden of Disease (GBD) 2019. Mol Psychiatry. (2023). doi: 10.1038/s41380-023-02138-4. [Epub ahead of print].
13. Martin A, Bessonova L, Hughes R, Doane MJ, O'Sullivan AK, Snook K, et al. Systematic review of real-world treatment patterns of oral antipsychotics and associated economic burden in patients with schizophrenia in the United States. Adv Ther. (2022) 39:3933–56. doi: 10.1007/s12325-022-02232-z
14. He H, Liu Q, Li N, Guo L, Gao F, Bai L et al. Trends in the incidence and DALYs of schizophrenia at the global, regional and national levels: results from the Global Burden of Disease Study 2017. Epidemiol Psychiatr Sci. (2020) 29:e91. doi: 10.1017/S2045796019000891
15. Cho SJ, Kim J, Kang YJ, Lee SY, Seo HY, Park JE, et al. Annual prevalence and incidence of schizophrenia and similar psychotic disorders in the Republic of Korea: A national health insurance data-based study. Psychiatry Investig. (2020) 17:61–70. doi: 10.30773/pi.2019.0041
16. Chien IC, Chou YJ, Lin CH, Bih SH, Chou P, Chang HJ. Prevalence and incidence of schizophrenia among national health insurance enrollees in Taiwan, 1996-2001. Psychiatry Clin Neurosci. (2004) 58:611–8. doi: 10.1111/j.1440-1819.2004.01311.x
17. Vermeulen J, van Rooijen G, Doedens P, Numminen E, van Tricht M, de Haan L. Antipsychotic medication and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis. Psychol Med. (2017) 47:2217–28. doi: 10.1017/S0033291717000873
18. Joukamaa M. Heliövaara M, Knekt P, Aromaa A, Raitasalo R, Lehtinen V. Schizophrenia, neuroleptic medication, and mortality. Br J Psychiatry. (2006) 188:122–7. doi: 10.1192/bjp.188.2.122
19. Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis JM, et al. Physical health monitoring of patients with schizophrenia. Am J Psychiatry. (2004) 161:1334–49. doi: 10.1176/appi.ajp.161.8.1334
20. Chang CK, Chesney E, Teng WN, Hollandt S, Pritchard M, Shetty H, et al. Life expectancy, mortality risks and cause of death in patients with serious mental illness in South East London: a comparison between 2008-2012 and 2013-2017. Psychol Med. (2023) 53:887–96. doi: 10.1017/S0033291721002257
21. Björk Brämberg E, Torgerson J, Norman Kjellström A, Welin P, Rusner M. Access to primary and specialized somatic health care for persons with severe mental illness: a qualitative study of perceived barriers and facilitators in Swedish health care. BMC Fam Pract. (2018) 19:12. doi: 10.1186/s12875-017-0687-0
22. Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia. Arch Gen Psychiatry. (2007) 64:1123–31. doi: 10.1001/archpsyc.64.10.1123
23. Chan JKN, Wong CSM, Or PCF, Chen EYH, Chang WC. Risk of mortality and complications in patients with schizophrenia and diabetes mellitus: population-based cohort study. Br J Psychiatry. (2021) 219:375–82. doi: 10.1192/bjp.2020.248
24. Sudarshan Y, Cheung BMY. Hypertension and psychosis. Postgrad Med J. (2023) 99:411–5. doi: 10.1136/postgradmedj-2021-141386
25. Westman J, Eriksson SV, Gissler M, Hällgren J, Prieto ML, Bobo WV, et al. Increased cardiovascular mortality in people with schizophrenia: a 24-year national register study. Epidemiol Psychiatr Sci. (2018) 27:519–27. doi: 10.1017/S2045796017000166
26. Suetani S, Honarparvar F, Siskind D, Hindley G, Veronese N, Vancampfort D, et al. Increased rates of respiratory disease in schizophrenia: a systematic review and meta-analysis including 619,214 individuals with schizophrenia and 52,159,551 controls. Schizophr Res. (2021) 237:131–40. doi: 10.1016/j.schres.2021.08.022
27. Ali S, Santomauro D, Ferrari AJ, Charlson F. Schizophrenia as a risk factor for cardiovascular and metabolic health outcomes: a comparative risk assessment. Epidemiol Psychiatr Sci. (2023) 32:e8. doi: 10.1017/S2045796023000045
28. Nordentoft M, Plana-Ripoll O, Laursen TM. Cancer and schizophrenia. Curr Opin Psychiatry. (2021) 34:260–5. doi: 10.1097/YCO.0000000000000697
29. Charlson FJ, Baxter AJ, Dua T, Degenhardt L, Whiteford HA, Vos T. Excess mortality from mental, neurological and substance use disorders in the Global Burden of Disease Study 2010. Epidemiol Psychiatr Sci. (2015) 24:121–40. doi: 10.1017/S2045796014000687
30. Galderisi S, De Hert M, Del Prato S, Fagiolini A, Gorwood P, Leucht S, et al. Identification and management of cardiometabolic risk in subjects with schizophrenia spectrum disorders: a Delphi expert consensus study. Eur Psychiatry. (2021) 64:e7. doi: 10.1192/j.eurpsy.2020.115
31. Peritogiannis V, Ninou A, Samakouri M. Mortality in schizophrenia- spectrum disorders: recent advances in understanding and management. Healthcare (Basel). (2022) 10:2366. doi: 10.3390/healthcare10122366
32. De Hert M, Vancampfort D, Correll CU, Mercken V, Peuskens J, Sweers K, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. (2011) 199:99–105. doi: 10.1192/bjp.bp.110.084665
33. Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). (2007) 4:28–37.
34. Health Promotion Administration. Ministry of Health and Welfare. Taiwan's Obesity Prevention and Management Strategy. 1st edn, 1, 55. Health Promotion Administration, Ministry of Health and Welfare (2018).
35. Byrne BM. Structural Equation Modeling with AMOS: Basic Concepts, Applications, and Programming. New York: Routledge. (2009).
36. Correll CU, Solmi M, Croatto G, Schneider LK, Rohani-Montez SC, Fairley L, et al. Mortality in people with schizophrenia: a systematic review and meta-analysis of relative risk and aggravating or attenuating factors. World Psychiatry. (2022) 21:248–71. doi: 10.1002/wps.20994
37. Tsai KY, Lee CC, Chou YM, Su CY, Chou FH. The incidence and relative risk of stroke in patients with schizophrenia: a five-year follow-up study. Schizophr Res. (2012) 138:41–7. doi: 10.1016/j.schres.2012.02.013
38. Kapral MK, Kurdyak P, Casaubon LK, Fang J, Porter J, Sheehan KA. Stroke care and case fatality in people with and without schizophrenia: a retrospective cohort study. BMJ Open. (2021) 11:e044766. doi: 10.1136/bmjopen-2020-044766
39. Kelly DL, McMahon RP, Wehring HJ, Liu F, Mackowick KM, Boggs DL, et al. Cigarette smoking and mortality risk in people with schizophrenia. Schizophr Bull. (2011) 37:832–8. doi: 10.1093/schbul/sbp152
40. Osborn DP, Nazareth I, King MB. Physical activity, dietary habits and Coronary Heart Disease risk factor knowledge amongst people with severe mental illness: a cross sectional comparative study in primary care. Soc Psychiatry Psychiatr Epidemiol. (2007) 42:787–93. doi: 10.1007/s00127-007-0247-3
41. Chen J, Perera G, Shetty H, Broadbent M, Xu Y, Stewart R. Body mass index and mortality in patients with schizophrenia spectrum disorders: a cohort study in a South London catchment area. Gen Psychiatr. (2022) 35:e100819. doi: 10.1136/gpsych-2022-100819
42. Hsu MC, Ouyang WC. Subsequent dyslipidemia and factors associated with mortality in schizophrenia: a population-based study in Taiwan. Healthcare (Basel). (2021) 9:545. doi: 10.3390/healthcare9050545
43. Cheng CS, Chen WY, Chang HM, Pan CH, Su SS, Tsai SY, et al. Unfavorable cancer mortality-to-incidence ratios in patients with schizophrenia: a nationwide cohort study in Taiwan, 2000-2019. Acta Psychiatr Scand. (2023) 148:347–58. doi: 10.1111/acps.13604
44. Ko YS, Tsai HC, Chi MH, Su CC, Lee IH, Chen PS, et al. Higher mortality and years of potential life lost of suicide in patients with schizophrenia. Psychiatry Res. (2018) 270:531–7. doi: 10.1016/j.psychres.2018.09.038
45. Mitchell AJ, Vancampfort D, De Herdt A, Yu W, De Hert M. Is the prevalence of metabolic syndrome and metabolic abnormalities increased in early schizophrenia? A comparative meta-analysis of first episode, untreated and treated patients. Schizophr Bull. (2013) 39:295–305. doi: 10.1093/schbul/sbs082
46. Stubbs B, Vancampfort D, De Hert M, Mitchell AJ. The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: a systematic review and comparative meta-analysis. Acta Psychiatr Scand. (2015) 132:144–57. doi: 10.1111/acps.12439
47. Mukherjee S, Schnur DB, Reddy R. Family history of type 2 diabetes in schizophrenic patients. Lancet. (1989) 1:495. doi: 10.1016/S0140-6736(89)91392-5
48. Miron IC, Baroană VC, Popescu F, Ionică F. Pharmacological mechanisms underlying the association of antipsychotics with metabolic disorders. Curr Health Sci J. (2014) 40:12–7.
49. Brenner K, Liu A, Laplante DP, Lupien S, Pruessner JC, Ciampi A, et al. Cortisol response to a psychosocial stressor in schizophrenia: blunted, delayed, or normal? Psychoneuroendocrinology. (2009) 34:859–68. doi: 10.1016/j.psyneuen.2009.01.002
50. Onaolapo OJ, Onaolapo AY. Nutrition, nutritional deficiencies, and schizophrenia: an association worthy of constant reassessment. World J Clin Cases. (2021) 9:8295–311. doi: 10.12998/wjcc.v9.i28.8295
51. Andrews RC. Diabetes and schizophrenia: genes or zinc deficiency? Lancet. (1992) 340:1160. doi: 10.1016/0140-6736(92)93186-Q
52. Ratliff JC, Palmese LB, Reutenauer EL, Liskov E, Grilo CM, Tek C. The effect of dietary and physical activity pattern on metabolic profile in individuals with schizophrenia: a cross-sectional study. Compr Psychiatry. (2012) 53:1028–33. doi: 10.1016/j.comppsych.2012.02.003
53. Daumit GL, Goldberg RW, Anthony C, Dickerson F, Brown CH, Kreyenbuhl J, et al. Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis. (2005) 193:641–6. doi: 10.1097/01.nmd.0000180737.85895.60
54. Suzuki Y, Sugai T, Fukui N, Watanabe J, Ono S, Tsuneyama N, et al. High prevalence of underweight and undernutrition in Japanese inpatients with schizophrenia. Psychiatry Clin Neurosci. (2014) 68:78–82. doi: 10.1111/pcn.12082
55. Dipasquale S, Pariante CM, Dazzan P, Aguglia E, McGuire P, Mondelli V. The dietary pattern of patients with schizophrenia: a systematic review. J Psychiatr Res. (2013) 47:197–207. doi: 10.1016/j.jpsychires.2012.10.005
56. Nielsen RE, Banner J, Jensen SE. Cardiovascular disease in patients with severe mental illness. Nat Rev Cardiol. (2021) 18:136–45. doi: 10.1038/s41569-020-00463-7
57. Osimo EF, Brugger SP, de Marvao A, Pillinger T, Whitehurst T, Statton B, et al. Cardiac structure and function in schizophrenia: cardiac magnetic resonance imaging study. Br J Psychiatry. (2020) 217:450–7. doi: 10.1192/bjp.2019.268
58. Korkmaz S, Korkmaz H, Özer Ö, Atmaca M. Assessment of left ventricle systolic and diastolic functions in schizophrenia patients. Psychiatry Res. (2016) 240:348–51. doi: 10.1016/j.psychres.2016.04.025
59. Kilbourne AM, Morden NE, Austin K, Ilgen M, McCarthy JF, Dalack G, et al. Excess heart-disease-related mortality in a national study of patients with mental disorders: identifying modifiable risk factors. Gen Hosp Psychiatry. (2009) 31:555–63. doi: 10.1016/j.genhosppsych.2009.07.008
60. Liao CH, Chang CS, Chang SN, Muo CH, Lane HY, Sung FC, et al. The association of peptic ulcer and schizophrenia: a population-based study. J Psychosom Res. (2014) 77:541–6. doi: 10.1016/j.jpsychores.2014.08.005
61. Kasap E, Ayer A, Bozoglan H, Ozen C, Eslek I, Yüceyar H. Schizophrenia and gastroesophageal reflux symptoms. Indian J Psychiatry. (2015) 57:73–7. doi: 10.4103/0019-5545.148529
62. Nielsen J, Meyer JM. Risk factors for ileus in patients with schizophrenia. Schizophr Bull. (2012) 38:592–8. doi: 10.1093/schbul/sbq137
63. Huang TL. Decreased serum albumin levels in Taiwanese patients with schizophrenia. Psychiatry Clin Neurosci. (2002) 56:627–30. doi: 10.1046/j.1440-1819.2002.01066.x
64. Moramarco S, Morciano L, Morucci L, Messinese M, Gualtieri P, Carestia M, et al. Epidemiology of hypoalbuminemia in hospitalized patients: a clinical matter or an emerging public health problem? Nutrients. (2020) 12:3656. doi: 10.3390/nu12123656
65. Feldman JG, Burns DN, Gange SJ, Bacchetti P, Cohen M, Anastos K, et al. Serum albumin as a predictor of survival in HIV-infected women in the Women's Intergency HIV study. AIDS. (2000) 14:863–70. doi: 10.1097/00002030-200005050-00013
66. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. (2004) 17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x
67. Korkmaz S, Yildiz S, Korucu T, Gundogan B, Sunbul ZE, Korkmaz H, et al. Frequency of anemia in chronic psychiatry patients. Neuropsychiatr Dis Treat. (2015) 11:2737–41. doi: 10.2147/NDT.S91581
68. Polcwiartek C, Loewenstein D, Friedman DJ, Johansson KG, Graff C, Sørensen PL, et al. Clinical heart failure among patients with and without severe mental illness and the association with long-term outcomes. Circ Heart Fail. (2021) 14:e008364. doi: 10.1161/CIRCHEARTFAILURE.121.008364
69. Adams SB. Recognition and management of ileus. Vet Clin North Am Equine Pract. (1988) 4:91–104. doi: 10.1016/S0749-0739(17)30652-1
70. Grant RK, Brindle WM, Donnelly MC, McConville PM, Stroud TG, Bandieri L, et al. Gastrointestinal and liver disease in patients with schizophrenia: a narrative review. World J Gastroenterol. (2022) 28:5515–29. doi: 10.3748/wjg.v28.i38.5515
71. Yang Y, Wan C, Li H, Zhu H, La Y, Xi Z, et al. Altered levels of acute phase proteins in the plasma of patients with schizophrenia. Anal Chem. (2006) 78:3571–6. doi: 10.1021/ac051916x
72. Wan C, La Y, Zhu H, Yang Y, Jiang L, Chen Y, et al. Abnormal changes of plasma acute phase proteins in schizophrenia and the relation between schizophrenia and haptoglobin (Hp) gene. Amino Acids. (2007) 32:101–8. doi: 10.1007/s00726-005-0292-8
73. Lai KY, Wu TH, Liu CS, Lin CH, Lin CC, Lai MM, et al. Body mass index and albumin levels are prognostic factors for long-term survival in elders with limited performance status. Aging (Albany NY). (2020) 12:1104–13. doi: 10.18632/aging.102642
74. Kamruzzaman M. Is BMI associated with anemia and hemoglobin level of women and children in Bangladesh: a study with multiple statistical approaches. PLoS ONE. (2021) 16:e0259116. doi: 10.1371/journal.pone.0259116
75. Qian WL, Xu R, Shi R, Li Y, Guo YK, Fang H. et al. The worsening effect of anemia on left ventricular function and global strain in type 2 diabetes mellitus patients: a 30 T CMR feature tracking study. Cardiovasc Diabetol. (2023) 22:15. doi: 10.1186/s12933-023-01745-3
76. Siddiqui SW, Ashok T, Patni N, Fatima M, Lamis A, Anne KK. Anemia and heart failure: a narrative review. Cureus. (2022) 14:e27167. doi: 10.7759/cureus.27167
Keywords: chronic schizophrenia, all-cause mortality, clinical and biochemical factors, lifestyle, comorbid illnesses, causal pathways, inter-relationship, Structural Equation Modeling
Citation: Yu T-H, Lee T-L, Hsuan C-F, Wu C-C, Wang C-P, Lu Y-C, Wei C-T, Chung F-M, Lee Y-J, Tsai I-T and Tang W-H (2024) Inter-relationships of risk factors and pathways associated with all-cause mortality in patients with chronic schizophrenia. Front. Psychiatry 14:1309822. doi: 10.3389/fpsyt.2023.1309822
Received: 17 October 2023; Accepted: 18 December 2023;
Published: 20 May 2024.
Edited by:
Francesco Monaco, Azienda Sanitaria Locale Salerno, ItalyReviewed by:
Annarita Vignapiano, Department of Mental Health, ItalyCopyright © 2024 Yu, Lee, Hsuan, Wu, Wang, Lu, Wei, Chung, Lee, Tsai and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: I-Ting Tsai, dHNhaS5pdGluZ0BnbWFpbC5jb20=; Wei-Hua Tang, YWZyaWNhcGF1bDEyQHlhaG9vLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.