- 1Department of Mental Health, ASL Cuneo 2, Bra, Italy
- 2Department of Mental Health, ASL Napoli 1 Centro, Napoli, Italy
Among individuals receiving an adequate pharmacological treatment for Major Depressive Disorder (MDD), only 30% reach a full symptom recovery; the remaining 70% will experience either a pharmacological response without remission or no response at all thus configuring treatment resistant depression (TRD). After an inadequate response to an antidepressant, possible next step options include optimizing the dose of the current antidepressant, switching to a different antidepressant, combining antidepressants, or augmenting with a non-antidepressant medication. Augmentation strategies with the most evidence-based support include atypical antipsychotics (AAs). Few data are available in literature about switching to another antipsychotic when a first augmentation trial has failed. We present a case-series of patients with unipolar treatment resistant depression who were treated with a combination of antidepressant and low dose of cariprazine after failing to respond to a first augmentation with another AA. We report data about ten patients affected by unipolar depression, visited at the outpatients unit of Mental Health Department of ASL CN2 of Bra and NA1 of Napoli (Italy). All patients failed to respond to conventional antidepressant therapy. A low dose of AA (aripiprazole, risperidone or brexpiprazole) was added for one month to the ongoing antidepressant treatment without clinical improvement. A second augmentation trial was then made with cariprazine. Seven out of ten patients were responders at the end of period, of them 1 patient reached responder status by week 2. HAM-D mean scores decreased from 23.9 ± 3.9 (baseline) to 14.8 ± 5.3 (4 weeks). Cariprazine was well tolerated, no severe side effect was observed during the trial. Our sample of treatment resistant unipolar patients showed good response to augmentation with cariprazine. Failure to a first AA-augmentation trial does not preclude response to a second one. This preliminary result requires confirmation through more rigorous studies conducted over greater samples.
Introduction
Despite the availability of many pharmacological treatment options, nearly about a half of patients affected by Major Depression Disorder (MDD) do not adequately responds to antidepressant (AD) treatment (1, 2). Treatment resistant depression (TRD) is a serious and disabling illness with significant impact on social and occupational outcomes (3). Current strategies to treat patients who do not respond to first-line antidepressant monotherapy include switching AD (either within or between classes) or combining different drugs (4). After failure of 2 AD treatments, current guidelines indeed suggest augmentation strategies (5). Effective agents to add on to ongoing AD, according to literature, could be chosen between mood stabilizers, ADs, thyroid hormones, ketamine or atypical antipsychotic (AA) (5–7). Aripiprazole (8, 9), olanzapine (10, 11), quetiapine (12, 13) and risperidone (14, 15) showed efficacy in augmentation trial for patients affected by TRD. More recently brexpiprazole (16–18) and cariprazine (19–21) also demonstrated their efficacy for TRD. Not withstanding various studies that show efficacy of AAs as dd-on strategy to ameliorate depressive symptoms in TRD, there is a lack of literature, to our knowledge, about efficacy of a second trial with an AA in those patients who failed to respond to a first augmentation trial with antipsychotic. We report a case series of TRD patients who failed to respond to an augmentation with a first AA to their ongoing AD and were subsequently treated with low dose cariprazine (CPZ) as add-on. Cariprazine is a partial agonist of dopamine D2/D3 receptors (preferring D3) and serotonin 5HT1A/5Ht2A receptors (22). This unique receptor profiles may play a role in its efficacy and tolerability and are believed to be involved in the antipsychotic, antidepressant, antianhedonic and pro-cognitive effects (23, 24). FDA has approved cariprazine as an adjunctive treatment for unipolar depression (1.5–3 mg/day) however in Europe it has been approved only for schizophrenia (25).
Materials and methods
Clinical records of inpatients and outpatients with a diagnosis of Major Depressive Disorder according to DSM-5 criteria treated in the Mental Health Department of Alba and Bra (Italy) and Mental Health Department of Napoli 1 (Italy) from July 2022 and March 2023 were analyzed. All patients presented with some form of treatment resistance that was defined according to operational criteria provided by Sourey et al. (26). All patients were treated with a AA (aripiprazole, risperidone or brexpiprazole) added to ongoing AD therapy for 4 weeks without response estimated as reduction of Hamilton Depression Rating Scale (HAM-D) (27) score of at least 50% from the beginning of the augmentation. After a wash-out period from the first AA of 2 weeks maintaining the ongoing AD treatment unchanged, patients underwent a second augmentation trial of 4 weeks with cariprazine. Cariprazine starting dose was 1.5 mg/day for all patients. Dosage changes were established according to clinical judgment (no specific guidelines were followed. Dosage variation was established according to efficacy observed and tolerability). AD dose was maintained unchanged during the weeks of add-on.
All subjects referred to our Service did sign a written informed consent to have their clinical data potentially used for teaching or search purposes, anonymously treated. Written consent was also collected for off-label treatment. Socio-demographic, clinical and safety information were collected for each subject from medical reports. Patients underwent control visits according to clinical practice. All psychiatric diagnoses and clinical assessment were made by psychiatrist with several years of experience. Due to the frequent presence of bipolar spectrum features in TRD patients, careful screening was made by psychiatrist for this diagnosis also by mean of Mood Disorder Questionnaire (MDQ). For the purpose of this report, medical records have been analyzed at the start of treatment with cariprazine, after 2 weeks and after 4 weeks. Clinical symptoms of depression were assessed by means of HAM-D. The effectiveness of cariprazine was assessed evaluating the change of HAM-D scores from baseline to endpoint (4 weeks). Due to exiguity of the sample no statistical analysis was performed.
Results
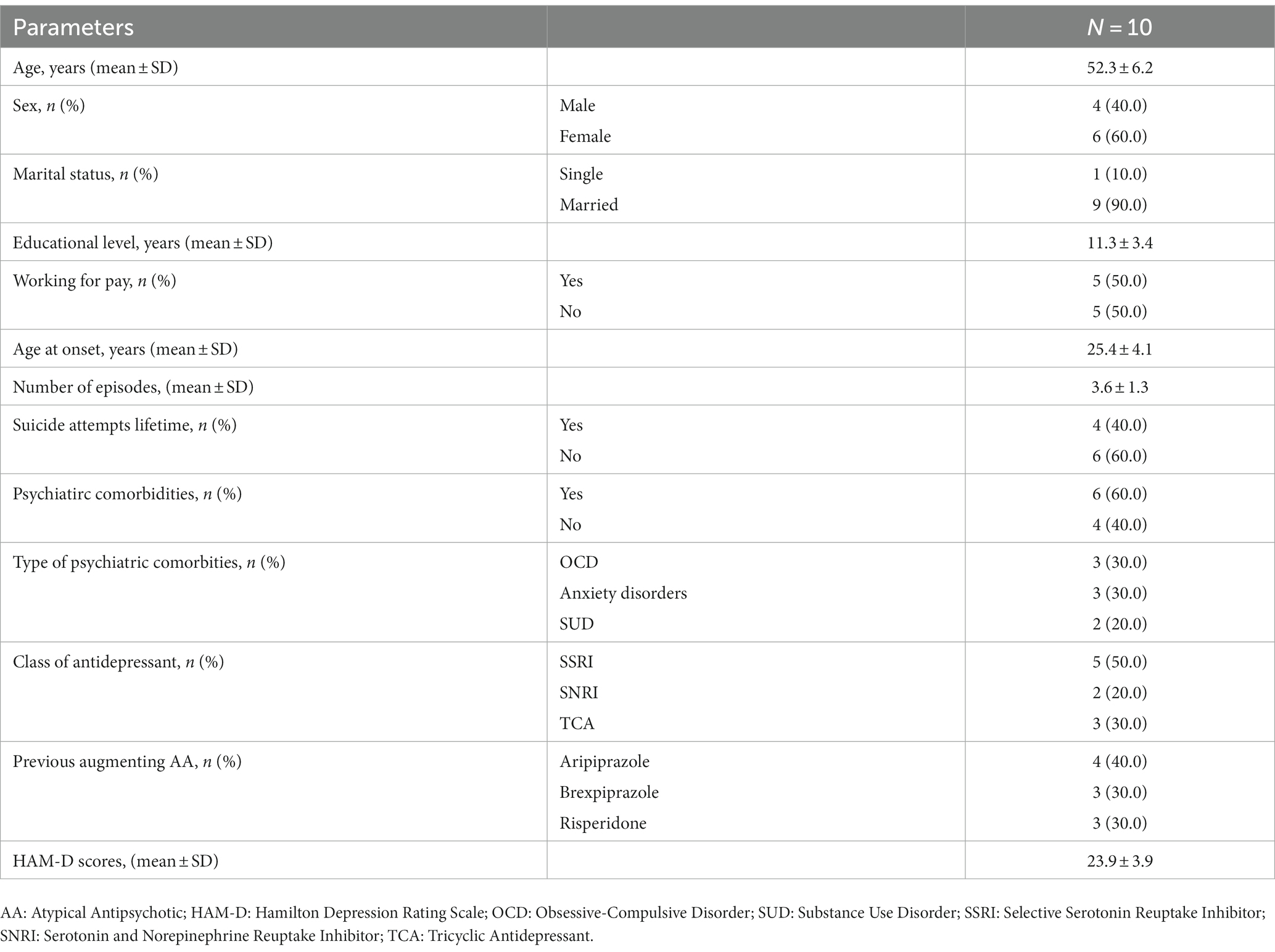
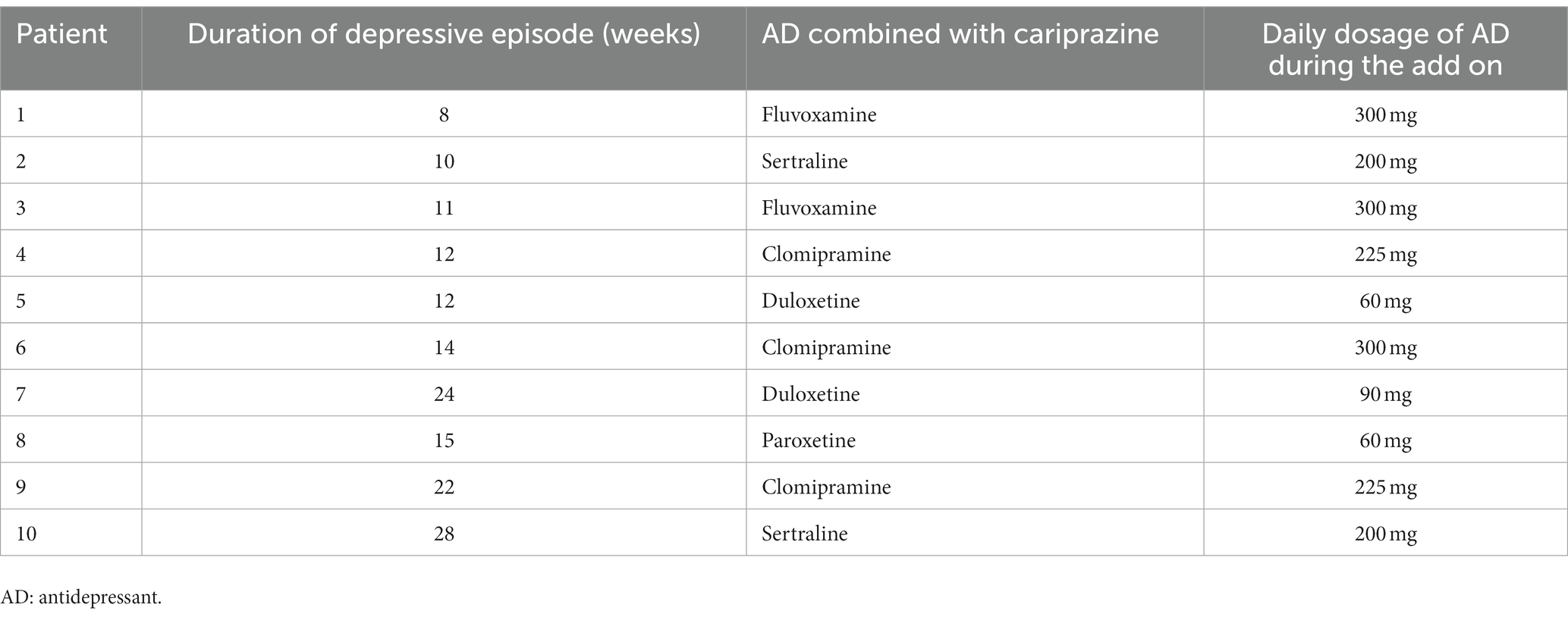
We report on a case series of 10 patients. 6 patients (60.0%) were female. The mean age of the sample was 52.3 ± 6.2 years. The mean age at onset of Major depressive disorder was 25.4 ± 4.1 years. 4 patients (40.0%) had at least one suicidal attempt lifetime. About two-thirds of patients (60%) had other comorbid psychiatric disorders. All socio-demographic and clinical characteristics of the patients are shown in Table 1, including the AA used in the first augmentation trial. Mean doses of antipsychotic in the first trial were, respectively, 4.4 ± 1.2 mg/day for aripiprazole, 1 ± 0 mg/day for brexpiprazole and 0.8 ± 2.3 mg/day for risperidone (risperidone in add on ranged from 0.5 to 1.5 mg/day). Table 2 reports duration of the single episode of treatment resistant depression, the AD combined with cariprazine and its dosage. All patients completed the 4 weeks period of cariprazine add-on, 7 patients (70.0%) experienced at least one adverse event (AE) (see Table 3). HAM-D mean scores decreased from 23.9 ± 3.9 (baseline) to 14.8 ± 5.3 (4 weeks) (Figure 1). 7/10 patients were responders at the end of period, of them 1 patient reached responder status by week 2. No patient met the criteria for remission. Dosage of cariprazine was increased to 3 mg/d in 4 patients. Table 3 summarizes dosage, timing of response and reported AEs in the sample of 10 patients.
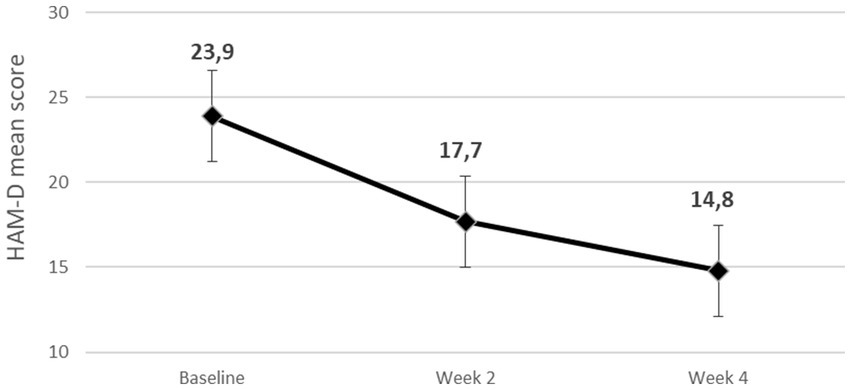
Figure 1. Mean reduction of Hamilton Depression Rating Scale (HAM-D) scores during the 4 weeks observation period.
Discussion
To the best of our knowledge this is the first study focusing on the efficacy and tolerability of cariprazine as add-on agent in TRD real-world patients who failed a previous trial of AA augmentation of their AD therapy. Treating TRD is a clinical challenge due to its cost in terms of continuing disability, consequence for patients’ functioning and quality of life as well as resource utilization (1, 2, 28).
Although not licensed in all countries, cariprazine is one of the so called third generation antipsychotics that showed evidence in treatment of depression. In a phase 2 study flexible –dose cariprazine in adults with MDD and inadequate response to ongoing AD treatment, change from baseline to week 8 in Montgomery-Asberg Depression Rating Scale (MADRS) total score was significantly greater with cariprazine 2–4.5 mg/day compared with placebo (19). In a more recent phase 3 study adjunctive 1.5 mg/day of cariprazine demonstrated efficacy in reducing depressive symptoms in adults with MDD and inadequate response to AD alone (21). Although unipolar and bipolar depression are distinct illnesses, previously published bipolar studies showed positive results with cariprazine add-on (29–31) also when added to mood stabilizers and AD in patients with resistant bipolar depression (32). Collectively these studies support the efficacy of adjunctive cariprazine in reducing depressive symptoms. Our preliminary results show that cariprazine can reduce depressive symptoms in real-world TRD patients in the short-term period also in the sub-population of patients that already failed a first augmentation trial with another AA (in our sample risperidone, aripiprazole or brexpiprazole). At the end of the 4 weeks of observation seven out of ten patients met the criteria for a clinical response, one patient showed response already at week 2, However exiguity of the sample and descriptive nature of our study do not allow a comparison with literature about cariprazine add on. In Durgam et al. (19) rate of responders according to MADRS scores was 48% with cariprazine 1–2 mg/day and 50% with cariprazine 2–4,5 mg/day. In Sachs et al. (21) responders to cariprazine 1.5 mg/day added to ongoing AD therapy were 40.9 and 41% when dosage was 3 mg/day. In our sample most patients responded to a dosage of cariprazine of 1.5 mg/day. These data are congruent with previous observation that lower dose of this antipsychotic seem to be more effective in reducing depressive symptoms (21). In our sample there was no drop-out due to adverse events and there was no severe adverse event reported. In our samples cariprazine was associated with favorable tolerability profiles, low discontinuation rates as previously observed in other study (21). It should be noted that no patients of our study discontinued the previous AA added as augmenting agent, due to side effects but only to lack of efficacy.
In conclusion, our case series suggests that adding low dose cariprazine to AD therapy in TRD patients who failed a previous AA augmentation trial could be an efficacious strategy to ameliorate depressive symptoms and this seems to be true also in real-world patients with other psychiatric comorbidities. To the best of our knowledge this is the first observation in this direction. Our results suffer for several limitations, first the retrospective observational nature of the study and the exiguity of the sample. Further confirmation in larger population and in prospective studies is needed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
EP: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. AM: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. FR: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. VM: Conceptualization, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Fava, M, and Davidson, KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. (1996) 19:179–200. doi: 10.1016/s0193-953x(05)70283-5
2. Trivedi, MH, Rush, AJ, Wisniewski, SR, Nierenberg, AA, Warden, D, Ritz, L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. (2006) 163:28–40. doi: 10.1176/appi.ajp.163.1.28
3. Ivanova, JI, Birnbaum, HG, Kidolezi, Y, Subramanian, G, Khan, SA, and Stensland, MD. Direct and indirect costs of employees with treatment-resistant and non-treatment-resistant major depressive disorder. Curr Med Res Opin. (2010) 26:2475–84. doi: 10.1185/03007995.2010.517716
4. American Psychiatric Association. Practice guidelines for the treatment of patients with major depressive disorder. 3rd ed. Washington, DC: American Psychiatric Association (2010).
5. Kennedy, SH, Lam, RW, McIntyre, RS, Tourjman, SV, Bhat, V, Blier, P, et al. Canadian network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the Management of Adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatr. (2016) 61:540–60. doi: 10.1177/0706743716659417
6. Gelenberg, AJ. A review of the current guidelines for depression treatment. J Clin Psychiatry. (2010) 71:e15. doi: 10.4088/JCP.9078tx1c
7. Nuñez, NA, Joseph, B, Pahwa, M, Kumar, R, Resendez, MG, Prokop, LJ, et al. Augmentation strategies for treatment resistant major depression: a systematic review and network meta-analysis. J Affect Disord. (2022) 302:385–400. doi: 10.1016/j.jad.2021.12.134
8. Berman, RM, Marcus, RN, Swanink, R, McQuade, RD, Carson, WH, Corey-Lisle, PK, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. (2007) 68:843–53. doi: 10.4088/jcp.v68n0604
9. Marcus, RN, McQuade, RD, Carson, WH, Hennicken, D, Fava, M, Simon, JS, et al. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. (2008) 28:156–65. doi: 10.1097/JCP.0b013e31816774f9
10. Corya, SA, Williamson, D, Sanger, TM, Briggs, SD, Case, M, and Tollefson, G. A randomized, double-blind comparison of olanzapine/fluoxetine combination, olanzapine, fluoxetine, and venlafaxine in treatment-resistant depression. Depress Anxiety. (2006) 23:364–72. doi: 10.1002/da.20130
11. Shelton, RC, Osuntokun, O, Heinloth, AN, and Corya, SA. Therapeutic options for treatment-resistant depression. CNS Drugs. (2010) 24:131–61. doi: 10.2165/11530280-000000000-00000
12. McIntyre, A, Gendron, A, and McIntyre, A. Quetiapine adjunct to selective serotonin reuptake inhibitors or venlafaxine in patients with major depression, comorbid anxiety, and residual depressive symptoms: a randomized, placebo-controlled pilot study. Depress Anxiety. (2007) 24:487–94. doi: 10.1002/da.20275
13. El-Khalili, N, Joyce, M, Atkinson, S, Buynak, RJ, Datto, C, Lindgren, P, et al. Extended-release quetiapine fumarate (quetiapine XR) as adjunctive therapy in major depressive disorder (MDD) in patients with an inadequate response to ongoing antidepressant treatment: a multicentre, randomized, double-blind, placebo-controlled study. Int J Neuropsychopharmacol. (2010) 13:917–32. doi: 10.1017/S1461145710000015
14. Nelson, JC, and Papakostas, GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry. (2009) 166:980–91. doi: 10.1176/appi.ajp.2009.09030312
15. Rafeyan, R, Papakostas, GI, Jackson, WC, and Trivedi, MH. Inadequate response to treatment in major depressive disorder: augmentation and adjunctive strategies. J Clin Psychiatry. (2020) 81:OT19037BR3. doi: 10.4088/JCP.OT19037BR3
16. Fava, M, Ménard, F, Davidsen, CK, and Baker, RA. Adjunctive Brexpiprazole in patients with major depressive disorder and irritability: an exploratory study. J Clin Psychiatry. (2016) 77:1695–701. doi: 10.4088/JCP.15m10470
17. Thase, ME, Youakim, JM, Skuban, A, Hobart, M, Augustine, C, Zhang, P, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry. (2015) 76:1224–31. doi: 10.4088/JCP.14m09688
18. Thase, ME, Youakim, JM, Skuban, A, Hobart, M, Zhang, P, McQuade, RD, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. (2015b) 76:1232–40. doi: 10.4088/JCP.14m09689
19. Durgam, S, Earley, W, Guo, H, Li, D, Németh, G, Laszlovszky, I, et al. Efficacy and safety of adjunctive cariprazine in inadequate responders to antidepressants: a randomized, double-blind, placebo-controlled study in adult patients with major depressive disorder. J Clin Psychiatry. (2016) 77:371–8. doi: 10.4088/JCP.15m10070
20. Earley, WR, Guo, H, Németh, G, Harsányi, J, and Thase, ME. Cariprazine augmentation to antidepressant therapy in major depressive disorder: results of a randomized, double-blind, placebo-controlled trial. Psychopharmacol Bull. (2018) 48:62–80.
21. Sachs, GS, Yeung, PP, Rekeda, L, Khan, A, Adams, JL, and Fava, M. Adjunctive Cariprazine for the treatment of patients with major depressive disorder: a randomized, double-blind, placebo-controlled phase 3 study. Am J Psychiatry. (2023) 180:241–51. doi: 10.1176/appi.ajp.20220504
22. Stahl, SM. Mechanism of action of cariprazine. CNS Spectr. (2016) 21:123–7. doi: 10.1017/S1092852916000043
23. Gyertyán, I, Sághy, K, Laszy, J, Elekes, O, Kedves, R, Gémesi, LI, et al. Subnanomolar dopamine D3 receptor antagonism coupled to moderate D2 affinity results in favourable antipsychotic-like activity in rodent models: II. Behavioural characterisation of RG-15. Naunyn Schmiedeberg's Arch Pharmacol. (2008) 378:529–39. doi: 10.1007/s00210-008-0311-x
24. Duric, V, Banasr, M, Franklin, T, Lepack, A, Adham, N, Kiss, B, et al. Cariprazine exhibits anxiolytic and dopamine D3 receptor-dependent antidepressant effects in the chronic stress model. Int J Neuropsychopharmacol. (2017) 20:788–96. doi: 10.1093/ijnp/pyx038
25. European medicines agency reagila assessment report (2017). European medicines agency. Available at: https://www.ema.europa.eu/en/documents/overview/reagila-epar-summary-public_en.pdf
26. Souery, D, Amsterdam, J, de Montigny, C, Lecrubier, Y, Montgomery, S, Lipp, O, et al. Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol. (1999) 9:83–91. doi: 10.1016/s0924-977x(98)00004-2
27. Hamilton, M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
28. Rush, AJ, Trivedi, MH, Wisniewski, SR, Nierenberg, AA, Stewart, JW, Warden, D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. (2006) 163:1905–17. doi: 10.1176/ajp.2006.163.11.1905
29. Durgam, S, Earley, W, Lipschitz, A, Guo, H, Laszlovszky, I, Németh, G, et al. An 8-week randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of Cariprazine in patients with bipolar I depression. Am J Psychiatry. (2016) 173:271–81. doi: 10.1176/appi.ajp.2015.15020164
30. Earley, W, Burgess, MV, Rekeda, L, Dickinson, R, Szatmári, B, Németh, G, et al. Cariprazine treatment of bipolar depression: a randomized double-blind placebo-controlled phase 3 study. Am J Psychiatry. (2019) 176:439–48. doi: 10.1176/appi.ajp.2018.18070824
31. Earley, WR, Burgess, MV, Khan, B, Rekeda, L, Suppes, T, Tohen, M, et al. Efficacy and safety of cariprazine in bipolar I depression: a double-blind, placebo-controlled phase 3 study. Bipolar Disord. (2020) 22:372–84. doi: 10.1111/bdi.12852
Keywords: major depression, treatment resistance, cariprazine, augmentation, atypical antipsychotic
Citation: Pessina E, Martini A, Raffone F and Martiadis V (2023) Cariprazine augmentation in patients with treatment resistant unipolar depression who failed to respond to previous atypical antipsychotic add-on. A case-series. Front. Psychiatry. 14:1299368. doi: 10.3389/fpsyt.2023.1299368
Edited by:
Panagiotis Ferentinos, National and Kapodistrian University of Athens, GreeceReviewed by:
Marcin Siwek, Jagiellonian University, Medical College, PolandAlessandro Cuomo, University of Siena, Italy
Copyright © 2023 Pessina, Martini, Raffone and Martiadis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Enrico Pessina, ZW5yaWNvcGVzc2luYUBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Enrico Pessina
Enrico Pessina Azzurra Martini1†
Azzurra Martini1† Vassilis Martiadis
Vassilis Martiadis