
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Psychiatry , 20 October 2023
Sec. ADHD
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1280440
 Yael Richter1*
Yael Richter1* Carlos Gordon1
Carlos Gordon1 Gabriel Vainstein2
Gabriel Vainstein2 Carmel Bublil-Mor1
Carmel Bublil-Mor1 Dario Geisinger1
Dario Geisinger1 Noya Meital-Kfir1
Noya Meital-Kfir1 Zohar Elyoseph3
Zohar Elyoseph3Objective: Stimulation of the peripheral visual field has been previously reported as beneficial for cognitive performance in ADHD. This study assesses the safety and efficacy of a novel intervention involving peripheral visual stimuli in managing attention deficit hyperactivity disorder (ADHD).
Methods: One hundred and eight adults, 18–40 years old, with ADHD, were enrolled in a two-month open-label study. The intervention (i.e., Neuro-glasses) consisted of standard eyeglasses with personalized peripheral visual stimuli embedded on the lenses. Participants were assessed at baseline and at the end of the study with self-report measures of ADHD symptoms (the Adult ADHD Self-Report Scale; ASRS), and executive functions (The Behavior Rating Inventory of Executive Function Adult Version; BRIEF-A). A computerized test of continuous performance (The Conners’ Continuous Performance Test-3; CPT-3) was tested at baseline with standard eyeglasses and at the end of study using Neuro-glasses. The Clinical Global Impression-Improvement scale (CGI-I) was assessed at the intervention endpoint. Safety was monitored by documentation of adverse events.
Results: The efficacy analysis included 97 participants. Significant improvements were demonstrated in self-reported measures of inattentive symptoms (ASRS inattentive index; p = 0.037) and metacognitive functions concerning self-management and performance monitoring (BRIEF-A; p = 0.029). A continuous-performance test (CPT-3) indicated significant improvement in detectability (d’; p = 0.027) and reduced commission errors (p = 0.004), suggesting that the Neuro-glasses have positive effects on response inhibition. Sixty-two percent of the participants met the response criteria assessed by a clinician (CGI-I). No major adverse events were reported.
Conclusion: Neuro-glasses may offer a safe and effective approach to managing adult ADHD. Results encourage future controlled efficacy studies to confirm current findings in adults and possibly children with ADHD.
Clinical trial registration: https://www.clinicaltrials.gov/, Identifier NCT05777785.
Attention deficit hyperactivity disorder (ADHD) poses a significant public health problem estimated to affect up to 11% of children worldwide (1). ADHD is commonly first diagnosed during the early school years due to its substantial role in the emergence of school-related disruptions. Nevertheless, it is a life-long disorder, affecting as many as 7% of the adult population (2). Symptoms of ADHD include inattentive and hyperactive–impulsive behaviors that persistently impact educational, occupational, interpersonal, and emotional functioning (3, 4).
Although ADHD has a wide range of adverse outcomes, it is one of the most treatable neurobehavioral disorders. Stimulants, such as amphetamines and methylphenidates, are a common ADHD treatment with documented safety and efficacy (5, 6). Nevertheless, stimulant treatment is not without limitations. It is frequently associated with bothersome side effects, ambivalence regarding chronic medication usage, and inconvenience or ineffective dosing. More than half of the patients being offered a pharmacotherapy treatment do not follow their medication regimen, even shortly after initiating treatment (7, 8). This low adherence rate and premature treatment termination undermine the benefits of stimulant treatment and support the need for developing new interventions for ADHD.
In this study, we use novel glasses, i.e., Neuro-glasses, as an intervention for treating adults with ADHD. It is worth noting that ADHD has been found to be associated with various visual problems and oculomotor abnormalities (9–12). A recent meta-analysis showed a link between ADHD and atypical corneal curvature (e.g., astigmatism), problems controlling eye muscles (e.g., hyperopia and hypermetropia, and strabismus), reduced contrast sensitivity, and color discriminability (10). Interestingly, ADHD was not directly related to problems in visual acuity, refractive error, or anatomic ocular measures. These findings underscore the necessity for including visual assessments within the scope of ADHD evaluations. Impaired visual perception has a direct impact on attention and cognition. Consequently, if left untreated, vision deficits could potentially worsen existing ADHD symptoms or even manifest as ADHD-like symptoms, leading to a false diagnosis of ADHD and vice versa. It is essential to emphasize, however, that the Neuro-glasses used in this study are not an optometry solution by its very nature. Rather, their impact on attention-related performance is induced using stimuli in the peripheral visual field.
Peripheral vision provides essential information concerning our surroundings. It has a survival function as it guides and relates us to the world around us, showing high sensitivity to movement and brightness changes in the visual scene (13, 14). Although the vast majority of the human retina is devoted to peripheral vision, it is frequently considered uninformative compared to foveal, detailed-oriented vision. Indeed, peripheral vision provides low acuity and poor color vision, however, much of our perception relies on peripheral inputs (13–16). Among other systems, peripheral vision is involved in balance, movement, and stress. A wide range of everyday tasks, such as driving, sports, visual searching, or even walking, do not demand high acuity vision but mainly depend on our ability to see outside the point of fixation (14–16).
Given the key role of peripheral vision in monitoring the environment and facilitating rapid detection of changes in the visual scene, it is expected to affect the allocation of attentional resources. Peripheral vision allows us to react quickly and shift attention to adjust our visually guided behavior to the visual scene. Without sufficient peripheral vision providing the visual context in which objects exist, it is difficult to distinguish relevant from nonrelevant information (14, 17–19). Peripheral vision provides contextual input that helps prioritize the regions in the visual scene relevant to a given task. Based on which the attentional resources are allocated (13–15).
Previous studies have shown that peripheral stimuli external to a task often influence performance (17, 19–21). Facilitation was reported to depend on the features of the stimulus, its relevance to the task, and its novelty (20). Increased sensitivity to peripheral stimuli was reported for nonclinical adults with ADHD-like traits (22). Participants with high levels of ADHD-like traits were more responsive than control to the cueing role of peripheral stimuli, indicating the arrival of a target. This elevated sensitivity allowed the participants with high levels of ADHD-like traits to allocate attentional resources more efficiently to meet task demands. The assumption was that facilitation in ADHD reflects hypersensitivity of the superior colliculus (SC), a structure concerned with peripheral vision (22, 23). However, accumulating evidence indicates that ADHD patients benefit from extra-task stimuli even when they are centrally presented, involve other modalities (i.e., auditory, motor information), or lack informational content (22, 24–27). For example, white noise exposure was highly beneficial for individuals with ADHD (24, 28, 29). A possible explanation is that stimuli external to a task operate via an additive effect, where the addition of noise boosts a naturally too-weak signal above a threshold level, allowing its detectability (28, 29).
The beneficial effect of glasses using peripheral visual stimulation was previously reported in patients with chronic dizziness (30). It was assumed that constant visual cues on the peripheral visual field strengthen the information of real head motion, possibly reducing the mismatch between sensory and locomotor information. Here, we hypothesize that the cueing effect of peripheral vision stimulation is not unisensory and affects a broad spectrum of attention-based performances. This study aims to assess the efficacy and safety of peripheral stimulation glasses, the Neuro-glasses, in adults with ADHD. It is further hypothesized that by stimulating the visual periphery, the Neuro-glasses will attenuate ADHD symptoms and related functions.
Eligible participants included adults aged 18 to 40 with a documented history of ADHD diagnosis. The upper age limit was predetermined to avoid age-related cognitive and visual decline. Exclusion criteria were: 1) any psychiatric or neurological comorbidity (e.g., epilepsy, Autism, depression, traumatic brain injury), 2) concomitant use of ADHD medications (i.e., stimulants or non-stimulants) 4 weeks before entering the study, and 3) undergoing neurofeedback or cognitive training treatment.
This was a two-month, open-label study in which eligible ADHD-diagnosed adults were provided with a pair of Neuro-glasses featuring a personalized visual stimuli pattern for each participant. A baseline assessment of the participants’ ADHD clinical profile pre-intervention included the Adult ADHD Self-Report Scale (ASRS; 31) and the Behavior Rating Inventory of Executive Function Adult Version (BRIEF-A; 32) questionnaires. In addition, participants completed the Conners’ Continuous Performance Test-3 (CPT-3; 33) while wearing standard eyeglasses without stimuli at the peripheral visual field. Optical centration parameters and demographic information were collected for each participant. Participants were then invited to complete a personalization process in which they were fitted with personalized Neuro-glasses (see ‘Neuro-glasses intervention’ section for detailed description). Participants were instructed to wear their Neuro-glasses for at least 2 hours daily for 2 months.
A follow-up assessment at the end of the intervention re-assessed ADHD performance on the ASRS and BRIEF-A questionaries. In addition, participants completed the CPT-3 test while wearing their Neuro-glasses.The Clinical Global Impression-Improvement (CGI-I; 34) rating scale was administered by a clinician (Figure 1).
The Neuro-glasses (Sparkles™, supplied by VIZO Specs Ltd.) consisted of a standard eyeglasses frame and standard optic lenses with semi-transparent marking stimuli, a few millimeters in size, embedded on the lenses (Figure 2). In cases where prescription glasses were needed, the Neuro-glasses were corrected to copy the participant’s existing prescription.

Figure 2. Illustration of a sample Neuro-glasses consisting of a standard eyeglasses frame and standard optic lenses with semi-transparent marking stimuli embedded on the lenses.
The stimuli pattern was adjusted at VIZO’s facility to fit the optimal performance of an individual. This personalization process included three visits. In each visit, participants completed a proprietary battery of neurocognitive tests while wearing eyeglasses with different stimuli patterns. The tests examined performance on tasks of selective attention, endogenous and exogenous attention, shifting of attention, processing speed, visual search, and inhibition of response. The participants were tested in front of an eye tracker (Tobii Pro Spectrum, Tobii, Inc., Sweden) sampling at 150 Hz with a 23.8-inch display mounted on top (EIZO FlexScan EV2451, EIZO Inc., United States) and viewed at 60 cm distance. The tests were carried out using E-Prime 3.0 (Psychology Software Tools Inc., United States) running on a standard personal computer (Intel i7, 64Gb RAM, NVIDIA 1080Ti running Windows 10). Eye-tracking parameters such as oculomotor reaction time and pupil size, as well as behavioral measures of time and accuracy, were collected and processed by VIZO’s proprietary cloud-based system to determine the optimal number and size of the stimuli and their positioning in the peripheral visual field.
The outcome measures provide a broad assessment of ADHD symptoms, executive functions, global functioning, and measures of attention and impulsivity. The assessment involved self-report questionnaires, clinician evaluation, and a task-oriented computerized assessment.
The Adult ADHD Self-Report Scale (ASRS; 31) is a self-report assessment comprising 18 items corresponding to the DSM-V-TR criteria for ADHD. The ASRS is a self-administered instrument whereby participants rate 18 symptom items using a 5-point Likert scale ranging from 0 (‘Never’) to 4 (‘Very Often’). The total score ranges between 0 to 72, with higher scores indicating greater ADHD impairments. The ASRS total score is comprised of two subscales: Inattention and Hyperactivity.
The Behavior Rating Inventory of Executive Function Adult Version (BRIEF-A; 32) is a standardized measure that captures views of adults’ executive functions or self-regulation in their everyday environment. The BRIEF-A comprises 75 items, each rated by the individual, using a 3-point Likert scale ranging from 0 (‘Never’) to 2 (‘Often’). The total score ranges between 0 to 150. BRIEF-A includes a Behavioral Regulation index, a Metacognition index, and a summary score-Global Executive Composite.
The Clinical Global Impression-Improvement (CGI-I; 34) provides an overall clinician-rated summary measure of improvement on a 7-point scale, ranging from 1 (very much improved) to 7 (very much worsened).
The Conners’ Continuous Performance Test-3 (CPT-3; 33) is a computerized task of attention and impulsivity. It includes a serial presentation of target and non-target letters. Participants are instructed to press a button when any letter, except the letter “X” (i.e., target), is presented on the screen. The CPT-3 provides numerous summary measures: 1) d-prime (d’) – measures “signal detectability,” that is, the respondent’s ability to differentiate non-targets (i.e., the letter X) from targets (i.e., all other letters); 2) Omissions – missed targets; 3) Commissions – incorrect responses to non-targets (False alarm); 4) Reaction Time (HRT) – the mean response speed; 5) Hit Reaction Time Standard Deviation (HRT SD) – the consistency of response speed to targets.
To assess adherence, participants were instructed to document their daily usage of the eyeglasses (i.e., total hours per day). Potential side effects and adverse events were assessed by the study investigators during the follow-up assessment.
Paired samples t-tests were used to test the effect of the Neuro-glasses intervention, comparing the baseline and end-of-intervention performances. Normality distribution of data was assessed using the Shapiro–Wilk test. Due to the exploratory nature of the study, no corrections for multiple testing were applied. The safety analysis included all available data on the intent-to-treat (ITT) population.
Mixed model ANOVA was used to explore any potential effect of covariates such as gender, age, eyeglasses, learning disabilities, treatment naïve, and ASRS total score. None of the covariates were found significant, thus were not included in the final model.
Power analyzes determined that a sample size of 90 participants will have 80% power to detect an effect size of 0.30 (Cohen’s D) using a paired t-test with a 0.05 two-sided significance level. Assuming a 20% attrition rate over the course of the study, 108 participants were targeted for inclusion (35). This relatively small effect size was determined given the heterogeneity of the study population, as participation was neither limited by the severity nor occurrence of ADHD symptoms. All analyzes were conducted using SPSS® Version 26.
Of 119 individuals screened, 108 met the inclusion criteria. Two participants failed to comply with the personalization process due to difficulty staying awake or using a cell phone during the test, resulting in 106 participants receiving the intervention. Five participants voluntarily withdrew consent at the end of the intervention, and one was lost to follow-up (Figure 3). One hundred participants completed the study; three were excluded from the efficacy analysis due to a major protocol deviation (concomitant use of ADHD medication). The per-protocol (PP) population included the remaining 97 participants. Participants’ demographics are summarized in Table 1.
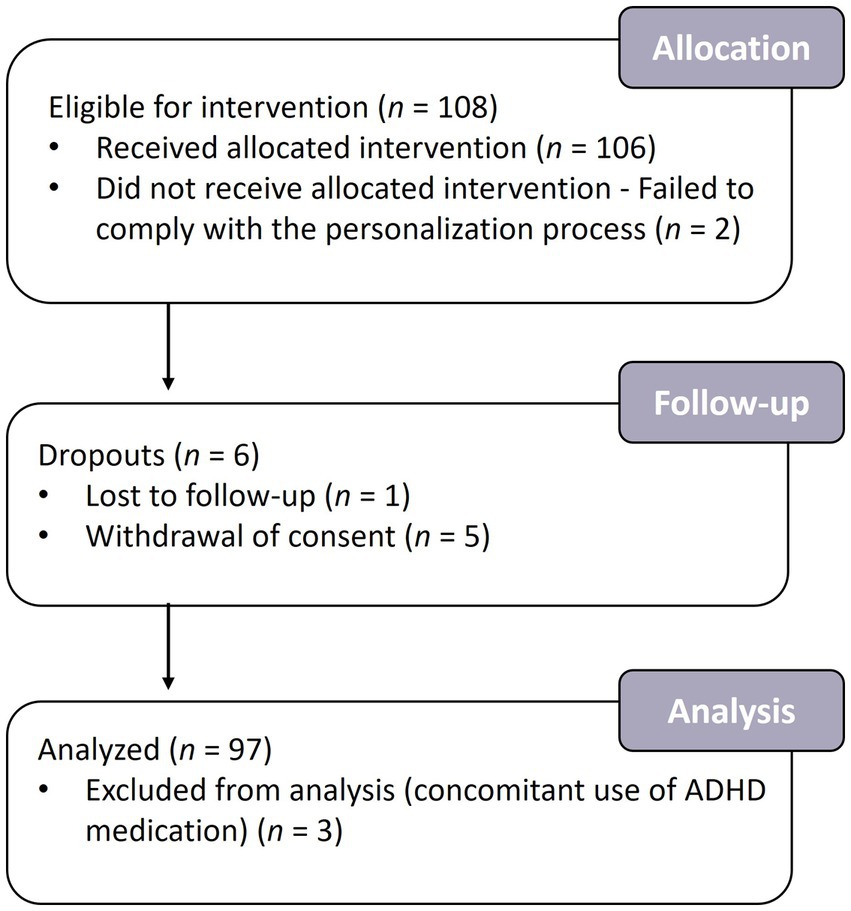
Figure 3. Flowchart of the participants in the study. A total of 108 participants were enrolled in the study, of whom 100 completed the intervention and 97 were analyzed.
The Neuro-glasses were well tolerated. No serious adverse events were reported throughout the two-month intervention, and no events led to intervention discontinuation. All side effects were transient and reported to be mild or moderate. Of the 100 participants, only 65 reported one or more adverse events. The remaining 35 participants had no adverse events (Table 2). The occurrence of adverse events was not significantly different between participants having prescription eyeglasses than those without a history of prescription eyeglasses.
Adherence results indicate high acceptability. Sixty-three percent of the participants reported wearing the glasses for at least 4 hours daily, whereas only 7% wore the glasses for less than 2 hours daily (Table 3).
Table 4 summarizes the outcome measures scores at baseline and end of study for the per-protocol population. Significant improvement was observed on both the ASRS and BRIEF-A questionnaires. Improvement in the ASRS was evident for the inattentive subscale, indicating reduced ADHD inattentive symptoms following intervention [t (94) = 0.12, p = 0.037]. Self-reports on the ASRS total score approached significance [t (94) = 1.87, p = 0.064], showing a 4% reduction in ADHD symptoms post-intervention. No significant effect was found on the ASRS hyperactive subscale [t (94) = 1.08, ns]. Improvement in the BRIEF-A was evident for the Metacognitive Index [t (92) = 2.22, p = 0.029]. There were no significant effects for the BRIEF-A Behavior Regulation index or the Global Executive Composite [t (92) = 0.73, ns., and t (92) = 1.73, ns., respectively].
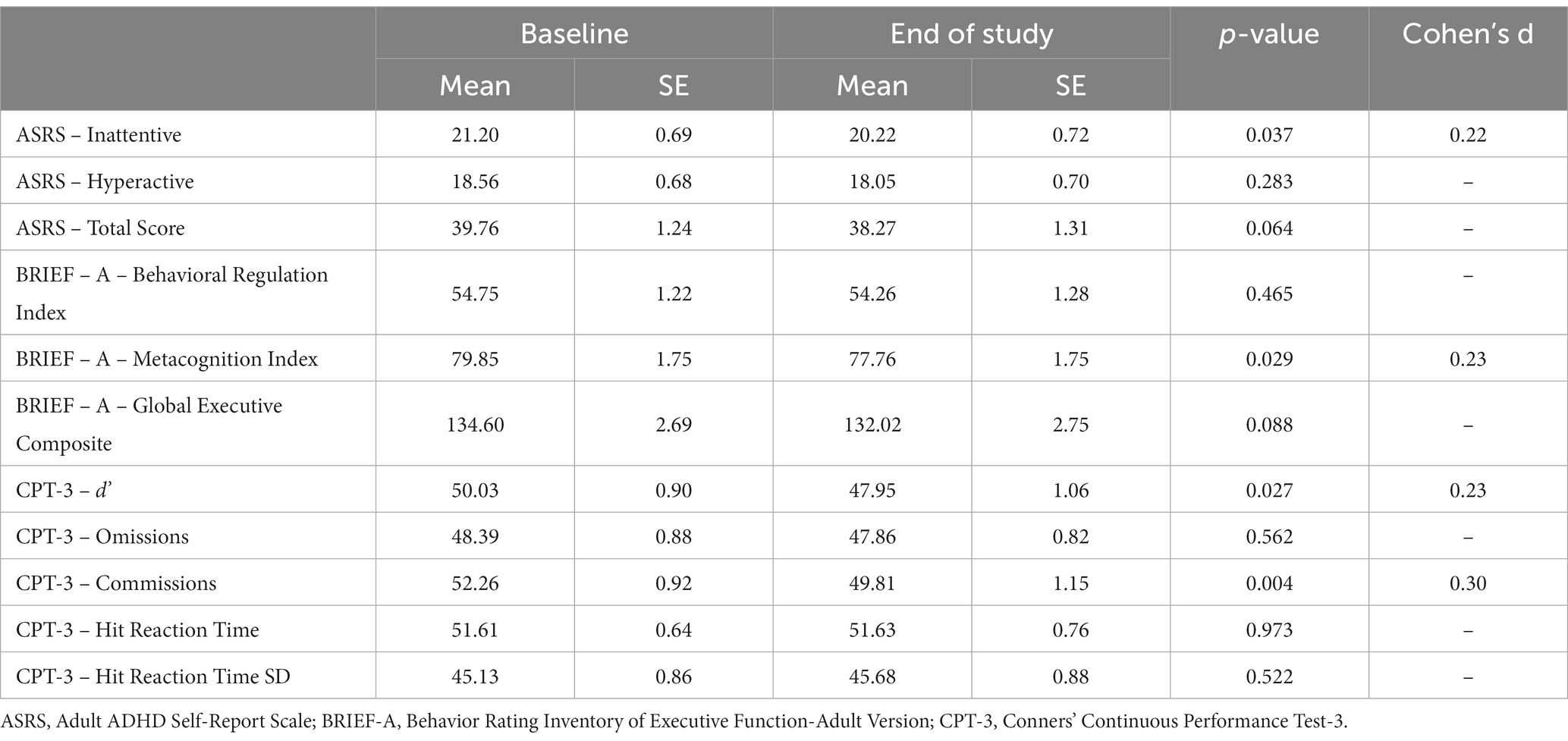
Table 4. Outcome measures scores at baseline and end-of-study for the per-protocol population (n = 97).
The effect of a two-month intervention on attentional control was tested using the CPT-3. Our findings indicate significant improvement in target detectability (i.e., d’; t (92) = 2.25, p = 0.027) and reduced incorrect responses to non-targets (i.e., commission errors; t (92) = 2.92, p = 0.004). All other CPT-3 measures were less sensitive to intervention-based changes in performance (Table 4).
Response to the intervention was assessed using the CGI-I. Participants were considered responders if they had a CGI-I score of “1” or “2” (very much improved or much improved, respectively). Sixty-two percent of the participants met the response criteria, having much or very much symptomatic improvement after using the Neuro-glasses.
No significant covariate effect was observed for demographic or baseline parameters such as gender, age, eyeglasses, learning disabilities, treatment naïve, and ASRS total score.
This study provides preliminary support for the safety and efficacy of a peripheral visual stimulation-based device in managing ADHD in adults. Reduction in ADHD symptoms was demonstrated by the ASRS inattentive subscale, reflecting the rated adult’s difficulty in maintaining attention for long periods, organizing/planning, paying attention to details, and committing reckless mistakes (31). No significant improvement was found on the ASRS hyperactive subscale. The reduced inattentive symptoms occurrence was consistent with an improvement in the metacognition index of the BRIEF-A questionnaire, evaluating the ability to self-manage tasks and monitor performance in different contexts (32). An objective, task-oriented assessment of attention and impulsivity, the CPT-3, revealed reduced difficulty in target detectability and commission errors, suggesting enhanced inhibition of response and attentional control. No significant reduction was found in the number of omission errors. This lack of findings is not unexpected given the low difficulty level of the task and the below-average omission baseline-score that falls outside the clinical range.
Despite the small effect sizes, the results are encouraging, given that the level of ADHD symptom manifestation in the study population was relatively mild, as reflected by the average ASRS baseline score. The clinical meaningfulness of the findings is further supported by the 62% of participants who positively responded to the intervention, as assessed by the CGI-I.
Assessment of the intervention adherence rate revealed that most participants (63%) wore the Neuro-glasses for at least 4 hours daily, whereas only 7% reported wearing the Neuro-glasses occasionally for up to 2 hours daily. These findings, together with the relatively low dropout rate (<10%), are of high importance, as they indicate that the intervention was well tolerated and accepted by ADHD adults. Moreover, the high adherence rate suggests that the effect of the Neuro-glasses is notable and meaningful for the user.
The current pilot study confirms that the Neuro-glasses hold minimal risks, as no clinically significant adverse events were associated with the intervention. Reports involved minor events of headache, dizziness, and eyestrain that were all transient, relatively mild and had not resulted in any discontinuations of the intervention.
The etiology of ADHD is related to various genetic and environmental factors (36). Though ADHD is not preventable or cured, the symptoms can be managed. Central nervous system stimulants are currently the best-known and most widely used ADHD treatment (6). Nevertheless, stimulants have a low adherence rate. The decision to discontinue treatment is primarily associated with medication-related side effects and ambivalence regarding chronic medication usage, especially at young ages (7, 8). In this study, demographic information revealed that 90% of the current study population reported a history of ADHD stimulant usage yet sought alternative treatment options. Alternative non-pharmacological interventions for ADHD include different variations of behavioral and cognitive treatments, Neurofeedback, sports, and diets/supplements. Though most of these treatments hold a more conservative, somewhat natural approach, they either fail to show solid empirical support for their efficacy or involve long-term treatment as they demand the acquisition of new coping skills. As such, some of those treatments are more commonly used as supplementation. Given the limitations of the medicated and non-medicated interventions, there is a pressing need for developing new treatment approaches for ADHD, such as the Neuro-glasses.
The Neuro-glasses presumably attenuate ADHD symptoms by stimulating the visual periphery. However, it is still unclear what is the mechanism underlying their effect. A possible explanation is that the Neuro-glasses might operate via the arousal system. Arousal is crucial for survival, as it regulates consciousness, attention, alertness, motivation, and information processing. Arousal regulation reflects the competence of the brain to deal with ongoing situational demands. Its dysregulation has been linked to psychiatric and physiological disorders and cognitive impairments (37–40). Relevant to this study, arousal dysregulation has been associated with ADHD pathology, reflecting naturally reduced arousal levels in ADHD patients (26, 37, 41). The hypo-arousal state, characterizing ADHD, was suggested to explain the deficit in sustained attention and vigilance as well as the hyperactive and impulsive autoregulatory behaviors, aiming to raise the arousal level (38–40, 42). Although somewhat speculative, we suggest here that the Neuro-glasses may act as a source of arousal. Previous studies have shown that peripheral stimuli external to a task often influence target identification and discrimination (20, 21, 43), decision-making (18), and visual search (19). This facilitative effect was reported to be more pronounced in adults with ADHD-like traits (22). The beneficial effect of extra-task stimulation on cognitive performance of ADHD patients is supported by several models of ADHD, including the optimal stimulation model (27), the cognitive-energetic model (26), and the moderate brain arousal model (MBA) (24). Interestingly, all models postulate that under certain circumstances, rather than being distracted by non-task stimulus, ADHDs benefit from its presence. They link this effect to the regulation of arousal levels and the extent to which variations in these factors can affect performance. It has been proposed that the external task stimuli facilitate performance by enhancing arousal to an optimal level. The resulting elevated arousal levels are assumed to increase the total attention capacity and narrow the focus of attention to the most dominant aspect of a given task, improving performance (26, 37, 38, 40, 42, 44).
While the Neuro-glasses are not an optical device by definition, they may operate via visual correction. A large body of evidence associates ADHD and problems of vision (9–11). ADHD patients were found to be comparable to controls with regard to visual acuity. However, they were more likely to have functional vision problems involving the alignment, focus, and movement of the eyes (10). As this may stress the importance of visual examinations in ADHD, it also implies that, in some cases, visual deficits may confound the diagnosis of ADHD. It is not unlikely to suggest that the Neuro-glasses correct visual deficits that mimic ADHD symptoms. As the incoming sensory inputs are of higher quality, the cognitive performance characterizing ADHD is improved. Future studies should involve a comprehensive visual examination to test the impact of the Neuro-glasses on visual problems.
While the results of this study are encouraging, we acknowledge certain important limitations. First, this is an uncontrolled study. Thus, we cannot rule out the contribution of placebo effects. Second, given the exploratory nature of the study, no primary endpoint was predetermined. Third, the current study population does not represent the high prevalence of comorbidities in the ADHD population and their effect on attention symptoms since ADHD adults having any psychiatric or neurological comorbidity were excluded from the study. Fourth, this study does not control for the possible confounding effects of visual problems and emotional or motivational factors that may affect the conclusive assessment of improvement. Fifth, due to the study design, it is essential to recognize that with regards to the CPT specifically, we cannot rule out a possible acute impact of wearing the Neuro-glasses, as opposed to effects caused by their regular use.
To conclude, Neuro-glasses provide a non-pharmacologically safe approach to managing ADHD in adults. These preliminary encouraging findings merit further research including future controlled studies evaluating the efficacy of a peripheral visual stimulation-based intervention in adults and possibly children with ADHD. Moreover, the current study provides important insights into the utility of visual stimulation as an intervention for treating ADHD and potentially for additional psychiatric and neurological disorders involving dysregulation of arousal levels.
The original data collected in the study is included in the article. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Max Stern Yezreel Valley College Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
YR: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – original draft. CG: Conceptualization, Investigation, Writing – review & editing. GV: Conceptualization, Investigation, Writing – review & editing. CB-M: Data curation, Formal analysis, Methodology, Project administration, Writing – review & editing. DG: Conceptualization, Data curation, Methodology, Software, Writing – review & editing. NM-K: Supervision, Writing – original draft. ZE: Conceptualization, Investigation, Supervision, Writing – review & editing.
We would like to thank all participants for their invaluable contributions to this study.
YR, CG, DG, NM-K, and CB-M are employees of VIZO-Specs Ltd. ZE and GV are consultants for VIZO-Specs Ltd.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received funding from VIZO-Specs Ltd. The funder had the following involvement with the study: study design, data collection, analysis, interpretation of data, the writing of this article, and the decision to submit it for publication.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Visser, SN, Danielson, ML, Bitsko, RH, Holbrook, JR, Kogan, MD, Ghandour, RM, et al. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J Am Acad Child Adolesc Psychiatry. (2014) 53:34–46.e2. doi: 10.1016/j.jaac.2013.09.001
2. Song, P, Zha, M, Yang, Q, Zhang, Y, Li, X, and Rudan, I. The prevalence of adult attention-deficit hyperactivity disorder: a global systematic review and meta-analysis. J Glob Health. (2021) 11:04009. doi: 10.7189/jogh.11.04009
3. Biederman, J, and Faraone, SV. Attention-deficit hyperactivity disorder. Lancet. (2005) 366:237–48. doi: 10.1016/S0140-6736(05)66915-2
4. Bush, G. Attention-deficit/hyperactivity disorder and attention networks. Neuropsychopharmacology. (2010) 35:278–300. doi: 10.1038/npp.2009.120
5. Arnsten, AFT. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. (2006) 31:2376–83. doi: 10.1038/sj.npp.1301164
6. Greenhil, LL, Halperin, JM, and Abikoff, H. Stimulant medications. J Am Acad Child Adolesc Psychiatry. (1999) 38:503–12. doi: 10.1097/00004583-199905000-00011
7. Gajria, K, Lu, M, Sikirica, V, Greven, P, Zhong, Y, Qin, P, et al. Adherence, persistence, and medication discontinuation in patients with attention-deficit/hyperactivity disorder - a systematic literature review. Neuropsychiatr Dis Treat. (2014) 10:1543–69. doi: 10.2147/NDT.S65721
8. Biederman, J, Fried, R, DiSalvo, M, Storch, B, Pulli, A, Yvonne Woodworth, K, et al. Evidence of low adherence to stimulant medication among children and youths with ADHD: an electronic health records study. Psychiatr Serv. (2019) 70:874–80. doi: 10.1176/appi.ps.201800515
9. Martin, L, Aring, E, Landgren, M, Hellström, A, and Andersson, GM. Visual fields in children with attention-deficit/hyperactivity disorder before and after treatment with stimulants. Acta Ophthalmol. (2008) 86:259–64. doi: 10.1111/j.1755-3768.2008.01189.x
10. Bellato, A, Perna, J, Ganapathy, PS, Solmi, M, Zampieri, A, Cortese, S, et al. Association between ADHD and vision problems. A systematic review and meta-analysis. Mol Psychiatry. (2023) 28:410–22. doi: 10.1038/s41380-022-01699-0
11. DeCarlo, DK, Swanson, M, McGwin, G, Visscher, K, and Owsley, C. ADHD and vision problems in the National Survey of Children’s health. Optom Vis Sci. (2016) 93:459–65. doi: 10.1097/OPX.0000000000000823
12. Maron, DN, Bowe, SJ, Spencer-Smith, M, Mellahn, OJ, Perrykkad, K, Bellgrove, MA, et al. Oculomotor deficits in attention deficit hyperactivity disorder (ADHD): a systematic review and comprehensive meta-analysis. Neurosci Biobehav Rev. (2021) 131:1198–213. doi: 10.1016/j.neubiorev.2021.10.012
14. Rosenholtz, R. Capabilities and limitations of peripheral vision. Annu Rev Vis Sci. (2016) 2:437–57. doi: 10.1146/annurev-vision-082114-035733
15. Vater, C, Wolfe, B, and Rosenholtz, R. Peripheral vision in real-world tasks: a systematic review. Psychon Bull Rev. (2022) 29:1531–57. doi: 10.3758/s13423-022-02117-w
16. Day, GS, and Schoemaker, P. Peripheral vision: sensing and acting on weak signals. Long Range Plan. (2004) 37:117–21. doi: 10.1016/j.lrp.2004.01.003
17. Yantis, S, and Jonides, J. Abrupt visual onsets and selective attention: voluntary versus automatic allocation. J Exp Psychol Hum Percept Perform. (1990) 16:121–34. doi: 10.1037//0096-1523.16.1.121
18. Day, RF, Shyi, GCW, and Wang, JC. The effect of flash banners on multiattribute decision making: distractor or source of arousal? Psychol Mark. (2006) 23:369–82. Available at: https://onlinelibrary.wiley.com/doi/full/10.1002/mar.20117
19. Inoue, Y, Tanizawa, T, Utsumi, A, Susami, K, Kondo, T, and Takahashiy, K. Visual attention control using peripheral vision stimulation. 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC).
20. Enns, JT, Austen, EL, Di Lollo, V, Rauschenberger, R, and Yantis, S. New objects dominate luminance transients in setting attentional priority. J Exp Psychol Hum Percept Perform. (2001) 27:1287–302. doi: 10.1037/0096-1523.27.6.1287
21. Lange-Malecki, B, and Treue, S. A flanker effect for moving visual stimuli. Vision Res. (2012) 62:134–8. doi: 10.1016/j.visres.2012.03.016
22. Panagiotidi, M, Overton, PG, and Stafford, T. Attention-deficit hyperactivity disorder-like traits and distractibility in the visual periphery. Perception. (2017) 46:665–78. doi: 10.1177/0301006616681313
23. Overton, PG. Collicular dysfunction in attention deficit hyperactivity disorder. Med Hypotheses. (2008) 70:1121–7. doi: 10.1016/j.mehy.2007.11.016
24. Söderlund, G, Sikström, S, and Smart, A. Listen to the noise: noise is beneficial for cognitive performance in ADHD. J Child Psychol Psychiatry Allied Discip. (2007) 48:840–7. doi: 10.1111/j.1469-7610.2007.01749.x
25. Van Mourik, R, Oosterlaan, J, Heslenfeld, DJ, Konig, CE, and Sergeant, JA. When distraction is not distracting: a behavioral and ERP study on distraction in ADHD. Clin Neurophysiol. (2007) 118:1855–65. doi: 10.1016/j.clinph.2007.05.007
26. Sergeant, J. The cognitive-energetic model: an empirical approach to attention-deficit hyperactivity disorder. Neurosci Biobehav Rev. (2000) 24:7–12. doi: 10.1016/S0149-7634(99)00060-3
27. Zentall, SS, and Zentall, TR. Optimal stimulation: a model of disordered activity and performance in normal and deviant children. Psychol Bull. (1983) 94:446–71. doi: 10.1037/0033-2909.94.3.446
28. Pickens, TA, Khan, SP, and Berlau, DJ. White noise as a possible therapeutic option for children with ADHD. Complement Ther Med. (2019) 42:151–5. doi: 10.1016/j.ctim.2018.11.012
29. Baijot, S, Slama, H, Söderlund, G, Dan, B, Deltenre, P, Colin, C, et al. Neuropsychological and neurophysiological benefits from white noise in children with and without ADHD. Behav Brain Funct. (2016) 12:1–13. doi: 10.1186/s12993-016-0095-y
30. Gordon, CR, Tamir, R, Furas, R, Klein, C, and Roth, R. A pilot study of a novel specs for chronic dizziness. Acta Neurol Scand. (2018) 138:344:351. doi: 10.1111/ane.12968
31. Kessler, RC, Adler, L, Ames, M, Demler, O, Faraone, S, Hiripi, E, et al. The World Health Organization adult ADHD self-report scale (ASRS): a short screening scale for use in the general population. Psychol Med [Internet]. (2005) 35:245–56. doi: 10.1017/S0033291704002892
32. Gioia, GA, Isquith, PK, Guy, SC, and Kenworthy, L. TEST REVIEW behavior rating inventory of executive function. Child Neuropsychol. (2000) 6:235–8. doi: 10.1076/chin.6.3.235.3152
33. Keith Conners, C, Sitarenios, G, and Ayearst, LE. Conners’ continuous performance test third edition In: JS Kreutzer, J DeLuca, and B Caplan, editors. Encyclopedia of clinical neuropsychology. Cham: Springer International Publishing (2018). 929–33.
34. Guy, W. ECDEU assessment manual for psychopharmacology. Rockville, MD: US Department of Health, education, and welfare, public health service, alcohol, drug abuse, and mental health administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs (1976).
35. O’Brien, RG, and Muller, KE. Unified power analysis for t-tests through multivariate hypotheses In: LK Edwards, editor. Applied analysis of variance in the behavioral sciences. New York: Marcel Dekker (1993)
36. Millichap, JG. Etiologic classification of attention-deficit/hyperactivity disorder. Pediatrics. (2008) 121:e358–65. doi: 10.1542/peds.2007-1332
37. Martella, D, Aldunate, N, Fuentes, LJ, and Sánchez-Pérez, N. Arousal and executive alterations in attention deficit hyperactivity disorder (ADHD). Front Psychol. (2020) 11:1–7. doi: 10.3389/fpsyg.2020.01991
38. Strauß, M, Ulke, C, Paucke, M, Huang, J, Mauche, N, Sander, C, et al. Brain arousal regulation in adults with attention-deficit/hyperactivity disorder (ADHD). Psychiatry Res. (2018) 261:102–8. doi: 10.1016/j.psychres.2017.12.043
39. Yoon, SYR, Jain, U, and Shapiro, C. Sleep in attention-deficit/hyperactivity disorder in children and adults: past, present, and future. Sleep Med Rev. (2012) 16:371–88. doi: 10.1016/j.smrv.2011.07.001
40. Bellato, A, Arora, I, Hollis, C, and Groom, MJ. Is autonomic nervous system function atypical in attention deficit hyperactivity disorder (ADHD)? A systematic review of the evidence. Neurosci Biobehav Rev. (2020) 108:182–206. doi: 10.1016/j.neubiorev.2019.11.001
41. Hegerl, U, and Hensch, T. The vigilance regulation model of affective disorders and ADHD. Neurosci Biobehav Rev. (2014) 44:45–57. doi: 10.1016/j.neubiorev.2012.10.008
42. Sergeant, J. Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biol Psychiatry. (2005) 57:1248–55. doi: 10.1016/j.biopsych.2004.09.010
43. Carrasco, M, and McElree, B. Covert attention accelerates the rate of visual information processing. Proc Natl Acad Sci. (2001) 98:5363–7. doi: 10.1073/pnas.081074098
Keywords: ADHD, ADHD intervention, Adult ADHD, peripheral vision, peripheral visual stimulation
Citation: Richter Y, Gordon C, Vainstein G, Bublil-Mor C, Geisinger D, Meital-Kfir N and Elyoseph Z (2023) A novel intervention for treating adults with ADHD using peripheral visual stimulation. Front. Psychiatry. 14:1280440. doi: 10.3389/fpsyt.2023.1280440
Received: 20 August 2023; Accepted: 03 October 2023;
Published: 20 October 2023.
Edited by:
Paul Geoffrey Overton, The University of Sheffield, United KingdomReviewed by:
Eleanor Dommett, King’s College London, United KingdomCopyright © 2023 Richter, Gordon, Vainstein, Bublil-Mor, Geisinger, Meital-Kfir and Elyoseph. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yael Richter, eWFlbHJAdml6by1vLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.