
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 18 October 2023
Sec. Mood Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1278566
This article is part of the Research Topic Community Series in Early Life Stress and Depression: Volume II View all 5 articles
Background: Obesity and overweight are common in young patients with major depressive disorder (MDD). However, the prevalence and associated clinical factors of obesity/overweight in young first-episode and drug-naïve (FEDN) MDD patients are rarely reported in China.
Methods: A cross-sectional study of 917 young patients (aged 18–35 years) with FEDN MDD was performed. Demographic and clinical data were collected. Depression, anxiety, and psychotic symptoms were assessed using the Hamilton Depression Scale (HAMD), the Hamilton Anxiety Scale (HAMA), and the Positive and Negative Syndrome Scale (PANSS) positive subscale, respectively.
Results: Among the young MDD patients, the prevalence of obesity and overweight was 4.14 and 52.89%, respectively. Compared to normal-weight patients, overweight patients were older, had a greater age of onset, and had higher TSH and TG levels. Male MDD patients had a higher risk of obesity than female patients. Compared to obese patients, normal-weight and overweight patients had significantly lower HAMD scores, TC levels, and rates of TSH abnormalities. Logistic regression analysis showed that age, age of onset, and sex were independently associated with obesity, and TSH was independently associated with both obesity and overweight, in young MDD patients.
Conclusion: Our findings suggest a high prevalence of overweight and obesity in young FEDN MDD patients. Several demographic and clinical variables are independently associated with overweight/obesity in these young MDD patients.
The prevalence of major depressive disorder (MDD), especially among young adults, is increasing each year and is of growing concern. One study showed that the prevalence of MDD among young men and young women in the United States was 6.4 and 9.2%, respectively (1). Another survey of youth across the United States showed a 14.3% prevalence of depression in the last month and a 7.8% prevalence of depression for 7 and 14 days or more (2). The youth population tends to have a higher risk of developing depression (3). In addition, vegetative symptoms, such as changes in weight and appetite, low energy, and insomnia, are more common in younger patients with MDD than in adults (4). However, only 30% of young patients achieve remission in current antidepressant treatment (5–7). Therefore, a more in-depth study of the pathogenesis of MDD in young adults is needed. It has been shown that the pathogenesis of depression in youth may involve multiple aspects, including neuroendocrine and glucose/lipid metabolism-related mechanisms (8). Compared with the general population, young MDD patients tend to have higher glucose, total and low-density lipoprotein cholesterol (LDL-C), body mass index (BMI), lower high-density lipoprotein cholesterol (HDL-C), and higher rates of abdominal obesity (9).
The prevalence of overweight and obesity in young populations varies by country. A study of young Australians showed that 20.6% of women were overweight, 20.6% of men were obese, 29.3% of men were overweight, and 13.8% of men were obese (10). Another survey of young Ugandans showed that the overall prevalence of obesity and overweight was 2.3 and 10.4%, respectively (11). A study of young Chinese university students showed an overall prevalence of 9.5% for overweight and obesity, with 13.9% in males and 6.1% in females (12).
In clinical practice, the coexistence of overweight and obesity with depression is common in young adults. A study of young adults in the United States showed a correlation between overweight/obesity and depression in a younger population, but not in an older population (2). In China, fasting glucose, HbA1c, and triglycerides were found to be risk factors for depression in young obese patients (13). There are several studies of depressed patients of all ages, and the results vary across geographic regions. A German survey of depressed patients who changed medication early showed that 31% were overweight and 21% were obese (14). A Portuguese survey of depressed patients showed that 27% of patients were overweight and 46% were obese (15). A survey of outpatients with depression in the United States showed that 51.4% of patients were overweight and 20.0% were classified as obese (16). However, the geographical limitation of patients and the BMI of subjects in these studies may be influenced by antidepressants, which may lead to different results. Our team’s recent survey of depressed patients of all ages in China showed that 3.73% were obese and 56.00% were overweight (17). However, this study was an all-age study of adult depressed patients, whereas there are relatively few studies on the prevalence of obesity and overweight in the young depressed population in China, and associated clinical factors are unclear.
To date, studies on the prevalence of obesity and overweight in young depressed patients are relatively scarce. Therefore, to reduce the effect of medications and other factors on BMI (18, 19), we recruited 917 first-episode and drug naïve MDD patients aged 18–35 years in mainland China as study subjects and collected clinical data and lipid metabolism and neuroendocrine-related biomarkers. The main objectives of this study were (1) to investigate the prevalence of obesity and overweight in young MDD patients, and (2) to examine the associated clinical factors associated with obesity and overweight in young MDD patients.
The study was approved by the Institutional Review Board (IRB) of the First Hospital of Shanxi Medical University. All subjects signed a written consent form prior to participation in this study.
In this cross-sectional study, 917 consecutive outpatients were recruited from the First Hospital of Shanxi Medical University. Participants were recruited in accordance with the Declaration of Helsinki. Each patient was then independently evaluated by two experienced psychiatrists according to the Chinese version of the Structured Clinical Interview for DSM-IV (SCID) to confirm the diagnosis of MDD. Subjects met the following inclusion criteria: (1) 18–35 years old, Han Chinese; (2) no previous antidepressant or antipsychotic treatment; (3) first episode of MDD; (4) duration of illness less than two years; and (5) not pregnant or breastfeeding.
After a detailed medical history review, physical examination, and laboratory testing, we acquired a complete medical history and clinical/anthropometric data for all patients. Patients meeting the following criteria were excluded: (1) axis I disease other than MDD; (2) central nervous system disease, acute, unstable, or life-threatening disease (e.g., infection, cancer, and organ failure); (3) history of drug/alcohol abuse or dependence; and (4) refusal to sign a written consent form.
Sociodemographic information was collected using a standard questionnaire that included gender, age, age of onset, duration of illness, marital status, education level, and suicide attempts. The study questionnaire was administered by trained research staff. BMI was calculated as weight (kg) divided by height (m) squared and patients were classified as normal weight (BMI < 24 Kg/m2), overweight (24 Kg/m2 ≤ BMI < 28 Kg/m2) or obese (BMI ≥ 28 Kg/m2) (20).
The HAMD-17 was used for the comprehensive assessment of depressive symptoms. The scale contains 8 items on a 5-point scale from 0 (no symptoms) to 4 (very severe symptoms) and 9 items on a 3-point scale from 0 (no symptoms) to 2 (severe symptoms). Patients with a score of 24 or more were considered to have severe depressive symptoms (21).
The PANSS positive subscale was used to assess the severity of psychotic symptoms in patients with MDD. Each item was scored from 1–7. Patients with a total score > 15 were considered to have psychotic symptoms (22).
The 14-item Hamilton Anxiety Rating Scale (HAMA) was used to quantify the severity of anxiety symptoms. Patients with a total score > 29 were considered to have severe anxiety symptoms.
The Clinical Global Impression of Severity Scale (CGI-S) was used to measure clinicians’ impression of the current severity of illness, with scores ranging from 1 (normal) to 7 (most severe) (23).
Prior to the investigation, two qualified psychiatrists were trained in the use of these rating scales. After repeated assessments, interobserver correlation coefficients for HAMD and PANSS scores exceeded 0.8, respectively.
Blood samples were collected in the morning after an overnight fast and immediately sent to the hospital’s laboratory center for measurement on the same day. In this study, blood lipids and thyroid function were measured, including HDL-C, LDL-C, triglycerides (TG), total cholesterol (TC), free triiodothyronine (FT3), free thyroxine (FT4), and thyroid stimulating hormone (TSH). Serum TSH, FT3, and FT4 were performed by chemiluminescent immunoassay. The normal range of TSH was 0.27–4.20 mIU/L, FT3 was 3.10–6.8 pmol/L and FT4 was 10–23 pmol/L. The lipid profile was measured by enzyme-labeled colorimetric assay.
First, the normality of all variables was tested by the Shapiro–Wilk test, and the homogeneity of variance was determined by the Levene test. Categorical variables were expressed as frequencies and percentages, and the X2 test was used for comparison of categorical variables. All normally distributed continuous variables were expressed as mean ± SD, and non-normally distributed variables were expressed as median (quartiles). Comparisons of continuous variables conforming to the normal distribution were performed using analysis of variance (ANOVA), and non-normally distributed variables were tested using the Kruskal-Wallis rank sum test.
For post hoc analyses, paired comparisons between the three groups (normal weight, overweight, and obese) were performed using the Nemenyi test. To further explore the effect of each clinical factor on BMI, a multivariate unordered logistic regression model was constructed to clarify the effect of each factor on BMI levels (normal weight, overweight, and obesity). BMI was considered as the dependent variable.
All statistical analyses in this study were performed using the SPSS 20.0 platform, and all p-values were calculated using two tails, with significance set at p < 0.05.
As shown in Table 1, the following variables did not follow a normal distribution: age at onset, age, A-TG, A-TPO, FT3, FT4, HDL-C, LDL-C, TC, TG, HAMA, and HAMD (all p < 0.05). Among 917 young MDD patients (351 men and 566 women), the mean age of onset was 24 years. The mean duration of the disease was 4 months. The mean total scores of HAMD and HAMA were 30 and 21, respectively.
Among these young MDD patients, the prevalence of obesity and overweight was 4.14% (38/917) and 52.89% (485/917), respectively. The chi-square analysis showed that gender was significantly different in the three groups (p = 0.015). The Kruskal-Wallis rank test showed that age (p = 0.010), age of onset (p = 0.012), HAMD (p = 0.015), A-TPO (p = 0.013), and TC (p = 0.010), TG (p = 0.043), TSH (p < 0.001), CGI (p = 0.016), and suicide attempt rate (p = 0.013) were significantly different among the three groups.
As shown in Table 2, post hoc analysis showed that overweight patients were older (p = 0.01), had a greater age of onset (p = 0.01), and had higher rates of TSH abnormalities (p < 0.001) and TG levels (p = 0.04) compared to the normal weight group. HAMD score, TC levels, and the rate of TSH abnormalities were lower in overweight patients (PHAMD = 0.04, PTC = 0.03, PTSH = 0.02) and normal-weight patients (PHAMD = 0.02, PTC = 0.01, PTSH < 0.001) than in the obese group.
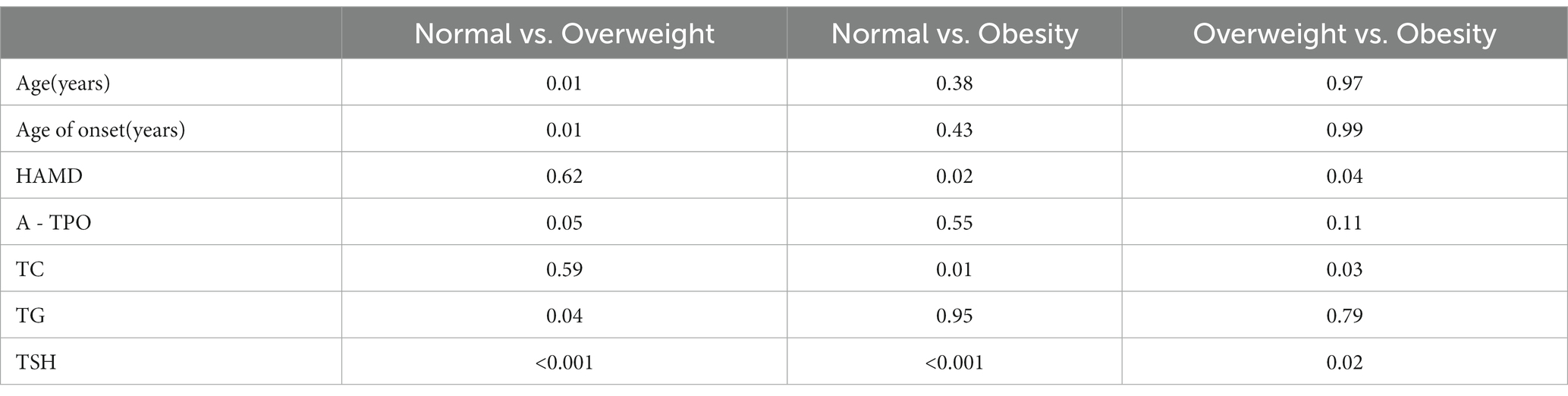
Table 2. All p values for posthoc comparison of selected variables among normal, overweight, and obesity groups.
An ordered logistic regression model was built using three ordered categorical variables (normal weight, overweight, and obesity), and the significance indicators in Table 1 were extracted to further explore their relationship with BMI levels. Using the multivariate ordered logit model, the parallel line test was p = 0.000, which did not meet the requirement. Therefore, an unordered multi-classification logit regression model was used: regression models for normal-weight, obesity, and overweight were established with normal-weight as a reference.
As shown in Table 3, age and age of onset were higher in the obese group, and TSH (two categories, defined by 4.20 mol/L) was higher in both obese and overweight groups than in the normal-weight group. For each year increase in patient age, the risk of obesity was approximately 2.497 times higher than in the normal-weight group (p = 0.002, OR: 2.497, 95% C.I.: 1.391–4.484). For each year increase in age of onset, the risk of obesity was about 2.433 times higher than the normal-weight group (p = 0.003, OR: 2.433, 95% C.I.: 1.344–4.405). In young MDD patients, the risk of obesity was nearly 2.549 times higher in men than in women (p < 0.0001, OR: 2.549, 95% C.I.: 1.628–3.99), but there was no significant sex difference in the risk of being overweight (p = 0.593). Compared with normal-weight patients, the risk of obesity was 4.551 times greater in patients with TSH abnormalities than in patients with normal TSH (p < 0.0001, OR: 4.551, 95% C.I.: 2.419–8.563). The risk of being overweight in patients with TSH abnormalities was 2.286 times higher than in patients with normal TSH (p < 0.0001, OR: 2.286, 95% C.I.: 1.707–3.06).
To our knowledge, this is the first clinical study showing that the prevalence of overweight was 52.89%, and the prevalence of obesity was 4.14% in young Chinese patients with FEDN MDD. Age, age of onset and gender were associated clinical factors for obesity, and TSH was a common risk factor for obesity and overweight in young FEDN MDD patients. In addition, the prevalence of obesity in young MDD patients in this study was higher than in our previous study of MDD patients of all ages (3.73%), while the prevalence of overweight was lower (56.00%) (17), suggesting that younger MDD patients may have a higher risk of obesity than older MDD patients.
Our study found that age was positively correlated with BMI in younger MDD patients, and that the older the patient, the greater the risk of higher BMI. A large US population-based survey on the relationship between risk factors for depression and age showed a stronger association between higher BMI and depression at younger age (24), which is not inconsistent with our present study because the criteria for age grouping were different. Our current study focused on young MDD patients aged 18–35 years, whereas previous US study categorized age into three groups aged 18–39, 40–59, and 60 years, with age being a continuous variable for each subgroup in each 10-year model. It is worthy noting that differences in ethnicity or dietary patterns, as well as the use of antidepressant medication, may influence the relationship between age and BMI to a greater or lesser extent. In the United States, the prevalence of obesity among young adults in 2007–2009 was 29.9% (25), whereas the prevalence of obesity among young Chinese was only 5.6% in 2002 (26). Another survey of young Chinese college students showed that the prevalence of obesity among college students was only 0.4% (27). This study was conducted on young Chinese adults and may differ from the U.S. study in terms of ethnicity and dietary patterns. A previous study also showed that antidepressant use affected patients’ BMI (19).
Further, our study found that age of onset was an associated clinical factor for obesity in young MDD patients. One study found that early-onset depression was associated with a history of depressive episodes and a longer duration of symptoms (28). Another study also showed that patients with early-onset depression had higher levels of neuroticism and a more prevalent abnormal personality (29). Therefore, we hypothesized that a long history of depressive episodes, symptom duration, and higher levels of neuroticism would correlate with patients’ appetite/BMI, making the age of onset an associated clinical factor for obesity in young MDD patients.
In addition, we found significant gender differences in the risk of obesity in young MDD patients. Specifically, young male MDD patients had a higher risk of obesity than young female MDD patients. This interesting finding is inconsistent with previous studies. A study based on US patients showed that female MDD patients had a substantially higher BMI than male patients (30). Another survey of young Americans showed that obese women were more likely to suffer from depression than non-obese women, but there was no significant difference among young male patients (2). This opposite gender difference between young Chinese and young American adults may be due to different criteria for age grouping and dietary patterns, which needs to be explored in future studies.
HAMD score reflects the severity of depression and several surveys have shown that BMI is positively correlated with depression severity in patients with MDD (14, 31), which is consistent with our current study. Possible reasons for this discrepancy may be due to differences in ethnicity, heterogeneity in medication use, disease duration, and inclusion criteria. Moreover, our previous study also showed that HAMD score was a risk factor for weight gain only within two specific score ranges (28 < HAMD score < =29 and > 32) (17). Therefore, more large-sample surveys and animal studies are needed to confirm the relationship between HAMD score and BMI.
TSH is a unique associated clinical factor for overweight and obesity in young MDD patients, which is consistent with our previous findings in all-age FEDN MDD patients (17). This phenomenon can be explained by biological factors, such as a decrease in 5-hydroxytryptamine, an important neurotransmitter in the formation of MDD, which leads to increased concentrations of thyrotropin-releasing hormone and consequently to thyrotropin secretion in brain tissue (32). Another study also found that elevated cortisol concentrations were strongly associated with the development of MDD disease (33). Patients with depression tend to over-activate the HPA axis, the end product of which is cortisol, and elevated cortisol levels decrease the activity of 5-HT1A receptors in the brain, which in turn exacerbates depression (34). It is also reasonable to find no independent association of either FT3 or FT4 with both obesity and overweight in young MDD patients. Although TSH, FT3, and FT4 are critical criteria for thyroid function, changes in TSH do not always correlate positively with changes in FT3 or FT4. For example, subclinical hypothyroidism is defined by elevated TSH (> 4.2 mIU/L) and normal FT4 (35), whereas hyperthyroidism is recognized by low TSH, high FT4, or high FT3 (14). Further animal studies are needed to reveal the relationship between TSH, FT3, FT4, and BMI.
We found that CGI was an associated clinical factor for obesity in young MDD patients. In the group of obese subjects, CGI of 5 appeared to be much less frequent, suggesting that obese MDD patients have more severe disease. Similarly, a previous study also found that higher levels of CGI were a risk factor for obesity in pediatric and adolescent MDD patients (36). All of these findings suggest that close monitoring of weight changes in clinical care is important for young MDD patients.
This study still has several limitations. First, the current international definition of youth is inconsistent. For the age definition of youth, we used an age range that is more appropriate for the Chinese population, which may lead to inconsistent results with other studies. Second, we used BMI rather than waist/hip circumference (WC) to measure obesity/overweight. Although BMI is a widely accepted screening measure for overweight and obesity, other studies have found that waist circumference rather than BMI explains the health risks associated with obesity (37). Therefore, it may be better to calculate both BMI and WC. Third, our participants included only FEDN MDD outpatients in the Chinese Han population, and our findings may not be generalized to MDD patients in other settings. Fourth, previous studies have shown that low-weight MDD patients are of significant research value. However, only two of the 917 young FEDN MDD patients in this study were underweight. In future studies, we will expand the sample size and investigate the prevalence of low weight and associated risk factors in MDD patients.
In summary, our study showed for the first time that the prevalence of overweight and obesity among young FEDN MDD patients was 52.89 and 4.14%, respectively. Our study also found that TSH was a common risk factor for obesity and overweight in young MDD patients. Therefore, TSH testing in MDD patients should be enhanced in clinical practice, which may be predictive of BMI changes in younger patients. In addition, key population information (age, age of onset, and gender) can be screened in clinical practice to guide patients on weight control, which is important to improve the prognosis of MDD.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The studies involving humans were approved by Institutional Review Board (IRB) of the First Hospital of Shanxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
J-JZ: Conceptualization, Formal analysis, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing. X-QW: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. QZ: Writing – review & editing. NG: Writing – review & editing. X-YZ: Conceptualization, Funding acquisition, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Chinese National Programs for Brain Science and Brain-like Intelligence Technology (2021ZD0202102), the National Natural Science Foundation of China (31871111), Youth Innovation Promotion Association CAS (2020088). These sources had no further role in this study design, in the data collection and analysis, in the writing of the report, and in the decision to submit the paper for publication.
We are grateful to all the physicians and nurses who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Beydoun, MA, and Wang, Y. Pathways linking socioeconomic status to obesity through depression and lifestyle factors among Young us adults. J Affect Disord. (2010) 123:52–63. doi: 10.1016/j.jad.2009.09.021
2. Heo, M, Pietrobelli, A, Fontaine, KR, Sirey, JA, and Faith, MS. Depressive mood and obesity in us adults: comparison and moderation by sex, age, and race. Int J Obes. (2006) 30:513–9. doi: 10.1038/sj.ijo.0803122
3. Eaton, WW, Shao, H, Nestadt, G, Lee, HB, Bienvenu, OJ, and Zandi, P. Population-based study of first onset and chronicity in major depressive disorder. Arch Gen Psychiatry. (2008) 65:513–20. doi: 10.1001/archpsyc.65.5.513
4. Rice, F, Riglin, L, Lomax, T, Souter, E, Potter, R, Smith, DJ, et al. Adolescent and adult differences in major depression symptom profiles. J Affect Disord. (2019) 243:175–81. doi: 10.1016/j.jad.2018.09.015
5. Fava, M, and Davidson, KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. (1996) 19:179–200. doi: 10.1016/s0193-953x(05)70283-5
6. Entsuah, AR, Huang, H, and Thase, ME. Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. J Clin Psychiatry. (2001) 62:869–77. doi: 10.4088/jcp.v62n1106
7. Cassano, P, Soares, CN, Cohen, LS, Lyster, AK, and Fava, M. Sex- and age-related differences in major depressive disorder with comorbid anxiety treated with fluoxetine. Arch Womens Ment Health. (2004) 7:167–71. doi: 10.1007/s00737-004-0051-y
8. Chen, YH, Wang, HN, Lang, XE, and Zhang, XY. Prevalence and clinical correlates of abnormal glucose metabolism in Young, first- episode and medication-naive outpatients with major depressive disorder. Psychiatry Res. (2023) 325:115250. doi: 10.1016/j.psychres.2023.115250
9. Moreira, FP, Jansen, K, Cardoso, TA, Mondin, TC, Magalhaes, P, Kapczinski, F, et al. Metabolic syndrome in subjects with bipolar disorder and major depressive disorder in a current depressive episode: population-based study: metabolic syndrome in current depressive episode. J Psychiatr Res. (2017) 92:119–23. doi: 10.1016/j.jpsychires.2017.03.025
10. Grech, A, and Allman-Farinelli, M. Prevalence and period trends of overweight and obesity in Australian Young adults. Eur J Clin Nutr. (2016) 70:1083–5. doi: 10.1038/ejcn.2016.41
11. Baalwa, J, Byarugaba, BB, Kabagambe, EK, Kabagambe, KE, and Otim, AM. Prevalence of overweight and obesity in Young adults in Uganda. Afr Health Sci. (2010) 10:367–73.
12. Jiang, S, Peng, S, Yang, T, Cottrell, RR, and Li, L. Overweight and obesity among Chinese college students: an exploration of gender as related to external environmental influences. Am J Mens Health. (2018) 12:926–34. doi: 10.1177/1557988317750990
13. Li, H, Huang, Y, Zang, X, Zhu, Z, Yang, M, Lang, XE, et al. The relationship between overweight and thyroid function in first-episode, untreated Chinese patients with major depressive disorder with different ages of onset. J Affect Disord. (2021) 294:932–8. doi: 10.1016/j.jad.2021.07.110
14. Dreimuller, N, Lieb, K, Tadic, A, Engelmann, J, Wollschlager, D, and Wagner, S. Body mass index (Bmi) in major depressive disorder and its effects on depressive symptomatology and antidepressant response. J Affect Disord. (2019) 256:524–31. doi: 10.1016/j.jad.2019.06.067
15. Correia, J, and Ravasco, P. Weight changes in Portuguese patients with depression: which factors are involved? Nutr J. (2014) 13:117. doi: 10.1186/1475-2891-13-117
16. Papakostas, GI, Petersen, T, Iosifescu, DV, Burns, AM, Nierenberg, AA, Alpert, JE, et al. Obesity among outpatients with major depressive disorder. Int J Neuropsychopharmacol. (2005) 8:59–63. doi: 10.1017/S1461145704004602
17. Si, T, Yang, K, Lang, X, Dong, X, Wang, N, Zhang, X, et al. Prevalence and risk factors of overweight and obesity in Chinese patients with first-episode drug-naive major depressive disorder. J Affect Disord. (2021) 286:351–9. doi: 10.1016/j.jad.2021.01.037
18. Toups, MS, Myers, AK, Wisniewski, SR, Kurian, B, Morris, DW, Rush, AJ, et al. Relationship between obesity and depression: characteristics and treatment outcomes with antidepressant medication. Psychosom Med. (2013) 75:863–72. doi: 10.1097/PSY.0000000000000000
19. Rihmer, Z, Purebl, G, Faludi, G, and Halmy, L. Association of Obesity and Depression. Neuropsychopharmacol Hung. (2008) 10:183–9.
20. Zhou, BF. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. (2002) 15:83–96.
21. Zimmerman, M, Martinez, JH, Young, D, Chelminski, I, and Dalrymple, K. Severity classification on the Hamilton depression rating scale. J Affect Disord. (2013) 150:384–8. doi: 10.1016/j.jad.2013.04.028
22. Kay, SR, Fiszbein, A, and Opler, LA. The positive and negative syndrome scale (Panss) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
23. Busner, J, and Targum, SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). (2007) 4:28–37.
24. Schaakxs, R, Comijs, HC, van der Mast, RC, Schoevers, RA, Beekman, ATF, and Penninx, B. Risk factors for depression: differential across age? Am J Geriatr Psychiatry. (2017) 25:966–77. doi: 10.1016/j.jagp.2017.04.004
25. Koebnick, C, Smith, N, Huang, K, Martinez, MP, Clancy, HA, and Kushi, LH. The prevalence of obesity and obesity-related health conditions in a large, multiethnic cohort of Young adults in California. Ann Epidemiol. (2012) 22:609–16. doi: 10.1016/j.annepidem.2012.05.006
26. Li, LM, Rao, KQ, Kong, LZ, Yao, CH, Xiang, HD, Zhai, FY, et al. A description on the Chinese National Nutrition and health survey in 2002. Zhonghua Liu Xing Bing Xue Za Zhi. (2005) 26:478–84.
27. Sakamaki, R, Toyama, K, Amamoto, R, Liu, CJ, and Shinfuku, N. Nutritional Knowledge, Food Habits and Health Attitude of Chinese University Students--a Cross Sectional Study. Nutr J. (2005) 4:4. doi: 10.1186/1475-2891-4-4
28. Essau, CA, Lewinsohn, PM, Seeley, JR, and Sasagawa, S. Gender differences in the developmental course of depression. J Affect Disord. (2010) 127:185–90. doi: 10.1016/j.jad.2010.05.016
29. Sneed, JR, Kasen, S, and Cohen, P. Early-life risk factors for late-onset depression. Int J Geriatr Psychiatry. (2007) 22:663–7. doi: 10.1002/gps.1727
30. Sutin, AR, and Zonderman, AB. Depressive symptoms are associated with weight gain among women. Psychol Med. (2012) 42:2351–60. doi: 10.1017/S0033291712000566
31. Agarwal, A, Agarwal, M, Garg, K, Dalal, PK, Trivedi, JK, and Srivastava, JS. Metabolic syndrome and central obesity in depression: a cross-sectional study. Indian J Psychiatry. (2016) 58:281–6. doi: 10.4103/0019-5545.192021
32. Gupta, S, Mukherjee, A, Biswas, S, Bose, S, Nath, S, and Das, HN. Evaluation of endocrine parameters as predictor of major depressive disorder. Indian J Psychol Med. (2017) 39:766–9. doi: 10.4103/IJPSYM.IJPSYM_120_17
33. Kahl, KG, Bens, S, Ziegler, K, Rudolf, S, Dibbelt, L, Kordon, A, et al. Cortisol, the cortisol-Dehydroepiandrosterone ratio, and pro-inflammatory cytokines in patients with current major depressive disorder comorbid with borderline personality disorder. Biol Psychiatry. (2006) 59:667–71. doi: 10.1016/j.biopsych.2005.08.001
34. Pitchot, W, Herrera, C, and Ansseau, M. Hpa Axis dysfunction in major depression: relationship to 5-Ht(1a) receptor activity. Neuropsychobiology. (2001) 44:74–7. doi: 10.1159/000054919
35. Zhao, M, Yang, T, Chen, L, Tang, X, Guan, Q, Zhang, B, et al. Subclinical hypothyroidism might worsen the effects of aging on serum lipid profiles: a population-based case-control study. Thyroid. (2015) 25:485–93. doi: 10.1089/thy.2014.0219
36. Liu, Z, Sun, L, Zhang, Y, Wang, J, Sun, F, Zhang, Z, et al. The prevalence of underweight and obesity in Chinese children and adolescents with major depressive disorder and relationship with suicidal ideation and attempted suicide. Front Psych. (2023) 14:1130437. doi: 10.3389/fpsyt.2023.1130437
Keywords: overweight, obesity, major depressive disorder (MDD), young, first episode and drug naïve (FEDN) patients
Citation: Zhang J-J, Wang X-Q, Zeng Q, Gao N and Zhang X-Y (2023) Prevalence and associated clinical factors for overweight and obesity in young first-episode and drug-naïve Chinese patients with major depressive disorder. Front. Psychiatry. 14:1278566. doi: 10.3389/fpsyt.2023.1278566
Received: 16 August 2023; Accepted: 28 September 2023;
Published: 18 October 2023.
Edited by:
Fang Pan, Shandong University, ChinaReviewed by:
Fengchun Wu, The Affiliated Brain Hospital of Guangzhou Medical University, ChinaCopyright © 2023 Zhang, Wang, Zeng, Gao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang-Yang Zhang, emhhbmd4eUBwc3ljaC5hYy5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.