
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry , 24 January 2024
Sec. Neurostimulation
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1273044
This article is part of the Research Topic Women in Psychiatry 2023: Neurostimulation View all 4 articles
Background: Transcranial alternative current stimulation (tACS) refers to a promising non-invasive technique to improve brain functions. However, owing to various stimulation parameters in the literature, optimization of the stimulation is warranted. In this study, the authors aimed to compare the effect of tACS electrode montages on occipital responses.
Methods: In three montage sessions (i.e., Oz-Cz, Oz-cheek, and sham), 10 healthy young adults participated, receiving 20-min 2-mA alpha-tACS. Pattern-reversal visual evoked potentials (VEPs) were measured before tACS (T0), immediately after (T20), and 20 min (T40) after tACS. Normalized changes in time-domain features (i.e., N75, P100 amplitudes, and P100 latency) and frequency-domain features [i.e., power spectral density in alpha (PSDα) and beta (PSDβ) bands] were evaluated.
Results: In contrast to our hypothesis, the occipital response decreased immediately (T20) after receiving the 20-min tACS in all montages in terms of P100 amplitude (p = 0.01). This reduction returned to baseline level (T0) in Oz-cheek and sham conditions but sustained in the Oz-Cz condition (T40, p = 0.03) after 20 min of tACS. The effects on N75 amplitude and P100 latency were statistically insignificant. For spectral analysis, both PSDα and PSDβ were significantly increased after tACS at T20, in which the effect sustained until T40. However, there was no differential effect by montages. There was no significant difference in the occurrence of sensations across the montages. The effectiveness of the blinding is supported by the participants’ rate of guessing correctly.
Conclusion: This study revealed an immediate inhibitory effect of tACS, regardless of the montages. This inhibitory effect sustained in the Oz-Cz montage but faded out in other montages after 20 min.
Transcranial electrical stimulation (tES), encompassing a range of techniques that deliver low-intensity electricity to the brain, has been substantially studied for its effect on brain functions in healthy people and for its therapeutic effects on clinical symptoms in those with psychiatric disorders (1, 2). The therapeutic and remedial effects of tES arise from both acute and long-term neuroplastic alterations in cortical excitability at the macroscopic level, triggered by alterations in brain activity (3). Increasing evidence has demonstrated that the tES targeting on the occipital region (Oz) can enhance, preserve, and even restore visual functions in healthy and visually impaired individuals (4). However, the effects of tES on vision remain inconclusive in the literature [refer to (5) for a recent systematic review]. This variability may stem from diverse stimulation parameters employed across studies, influencing the neuroplastic aftereffects and the subsequent efficacy of tES. Such parameters include, but not limited to, current density, polarity, stimulation duration, and/or geometrical montage of electrodes (6–9). Adjusting these parameters can lead to divergent outcomes in tES effects, including excitation versus inhibition and variations in the persistence of the aftereffect.
The location of the tES electrodes is crucial to determine the cortical area to be modulated and the amount of electrical current to be delivered (6, 10)—active electrode: maximal intracranial effect (11); reference electrode: tissue polarization (12). Regarding the visual system, the effects of transcranial direct current stimulation (tDCS) vary depending on the position of the reference electrode (13). Kraft et al. argued that the post-tES change in the amplitudes of visual evoked potentials (VEPs) reported in Antal et al. were in the opposite direction to that reported in Accornero et al. due to the use of different montages (Oz-Cz vs. Oz-posterior neck base) (7, 10, 13). Reinhart et al. applied Oz-cheek montage and found that tDCS had a larger and longer effect on enhancing visual acuity than that reported in Antal et al. using Oz-Cz montage (14, 15).
In addition to tDCS, transcranial alternating electrical stimulation (tACS) is another mode of tES, which employs specific-frequency electrical stimulation to the brain (16). With tDCS, neuronal firing rates vary with the polarity of direct current due to induced changes in resting membrane potentials (17). Unlike tDCS, tACS has no fixed anode/cathode as the polarity alternates during a half cycle of tACS oscillation; instead, it modulates the wave rate to entrain specific brain rhythm (18). Effects of tACS on visual functions were mixed. On one hand, tACS was found to impair visual detection (19, 20) and temporal resolution (21). On the other hand, it was found to be effective in improving the contrast sensitivity function (22). In terms of visual electrophysiology, tACS was found to increase the amplitude of the steady-state VEP when the frequencies of the tACS and VEP were matched (23).
tACS has been suggested to be superior in modulating brain functions than other modes of tES, given that the stimulating brain areas are the same. According to Manoli et al., 10-Hz tACS was suggested to generate a stronger and more focused effect than tDCS (24). Inukai et al. also found a significant increase in motor evoked potentials with tACS, but not with tDCS, when compared with sham measurements (25). Recent studies demonstrated that tACS directly boosted visual perceptual learning (26). In contrast, tDCS only enhanced visual perceptual learning performance when the subjects had good sleep quality (27).
Several studies also have investigated the effects of tACS by varying its parameters. A recent study by Wang et al. discovered that both the current intensities and the condition of eyes being open or closed have an impact on occipital excitability, with 2 mA in the eyes-open condition inducing notably higher alpha activity (28). Additionally, the selection of the electrode montage, specifically the placement of electrodes, can significantly alter the outcomes of tACS. Hsu et al. reported that facial stimulation montage predominantly produced phosphenes in the upper-left visual field, whereas using the occipital montage led to a more uniform distribution of phosphenes throughout the visual field (29). These studies inspired us to further investigate optimizing these parameters to achieve desired neural outcomes.
Summarizing the above evidence, the stimulation parameters might be a factor in the variation of tES outcomes among studies; hence, optimization is warranted. In this study, we aimed to compare the effects of two tACS montages on occipital excitability. We hypothesized that the Oz-Cz gave a better excitability than the Oz-cheek montage, in terms of amplitudes of VEP, and a better entrainment effect in terms of a longer maintained log difference in power spectral density analyses.
This study adopted a single-blind sham-controlled crossover design and was conducted from September to November 2021. All procedures followed the tenets of the Declaration of Helsinki and were approved by the Institutional Review Board of The Hong Kong Polytechnic University (HSEARS20210713002).
The inclusion criteria included the following: (1) adults of age > 18 years; (2) normal or corrected-to-normal vision (LogMAR 0.00 or equivalent); (3) no contraindications to receive tES (e.g., no wounds on the scalp); (4) no history of any neuropsychiatric disorder; and (5) no prior history of drug abuse or alcoholism. Participants were not allowed to take any medication or stimulating foods/drinks (e.g., alcohol, coffee, tobacco, and chocolate) at least 24 h before the sessions. All participants signed an informed consent form and a modified safety checklist from the Transcranial Magnetic Stimulation Adult Safety Screen (TASS) (Supplementary Appendix 1) (30) before taking part in the study.
Pattern-reversal visual evoked potential (VEP) was applied to measure the occipital responses (Diagnosys Espion, LLC, United States), following a previous version of the ISCEV standard (31). VEP stimuli were displayed on a cathode-ray tube monitor (CM715, Hitachi, Japan) as a 99% contrast black (1 cd/m2) and white (100 cd/m2) checkerboard with 1.5 Hz temporal frequency, i.e., 3.0 reversals per second (rps) at a viewing distance of 1 m. The current standard for pattern reversal is 2.0 rps (32), but a slightly higher 3.0 rps was chosen because of the proximity to the alpha band without triggering a steady-state response (31). The recording position of VEP was located at Fpz (reference) and Oz (active) with grounding on the mastoid bone of the ipsilateral side. The position of Oz was confirmed by measuring the distance D (cm) between the nasion and inion and was located at 10% D above the inion. In each measurement, VEP was tested at least twice to ensure good repeatability for each pattern spatial frequency, i.e., check size with a subtended visual angle of 1.0° and 0.25°, and 75 trials were obtained in each block with the blinked traces removed by a masked observer. The impedance was controlled under 5 kΩ during the whole experiment.
Transcranial alternating current stimulation (tACS) was delivered using a Nurostym (Brainbox, United Kingdom) to modulate the occipital responses. The size of the electrodes was 5 × 5 cm, and the tACS parameters were 2 mA, 10 Hz, for 20 min per session, with a 30-s fade in/out. Based on the 10–20 system, two montages—Oz-Cz (occipital and central midline) and Oz-cheek—were selected and their effects on VEP were compared. For the Oz-cheek montage, the cheek side was contralateral to the randomly selected test eye of VEP. For the sham stimulation, the montage was selected as Oz-Cz or Oz-cheek randomly at a ratio of 1:1. Sham consisted of an active stimulation delivered for 30 s to mimic the initial sensation of active tACS, acting like a placebo to mask the participants. Impedance was controlled below 10 kΩ for tACS electrodes.
All participants took part in all three conditions (Oz-Cz, Oz-cheek, and sham) in a random sequence. For the Oz-Cz and Oz-cheek conditions, the tACS electrode was placed on Oz, while the reference electrode was placed on Cz and cheek, respectively. Sham was one of the two montage positions for each participant using a randomized way. For each condition, VEPs were measured pre-stimulation (T0), immediately after (T20), and 20 min after (T40) tACS in one randomly selected test eye (Figure 1). The duration of each session was approximately 1.5 h, including setting up the equipment and positioning the stimulation electrodes. At the end of the session, participants were asked to subjectively grade their sensations on a scale of 0–4 and speculate whether they had received an active or sham stimulation. The washout period between the two trials was a minimum of 24 h.
Online measurement (i.e., VEPs during tACS) was not adopted because of the difficulty of analyzing the acquired neural responses at the same frequency as the stimulation applied, that is, cortical enhancement in alpha oscillations during the 10-Hz tACS stimulation (33).
To reduce the noises, including eye movements (e.g., blinks and ocular versions), myoelectricity (derived from eyes, head, and forehead), and power frequencies (e.g., powerline hum and radio frequency), a bandpass filter of 0.03–30 Hz was set up as the configuration. VEPs were automatically cut into epochs in the software, and epochs beyond ±800 mV were rejected by a masked observer, in which 60–75 epochs were then averaged to obtain the mean VEP. Time- and frequency-domain features were extracted from the averaged VEP epochs. From the two repeatable waveforms in each check size and stimulation condition, the first trough at approximately 75 ms was marked as N75 (amplitude defined as the difference of trough from zero), and the first peak at approximately 100 ms was marked as P100 (amplitude defined as the difference of peak from N75 trough). The latency was defined as the time at P100. For the frequency-domain features, both the power spectral density values (in mV2/Hz) in alpha (8–12 Hz, PSDα) and beta (12–30 Hz, PSDβ) bands were computed by averaged VEPs using fast Fourier transform with the Welch method (34). The normalized changes of VEP from T0 to T20 and T40 were calculated in percentage, which represented the effect of tACS on the averaged VEP responses:
All statistical procedures were performed using SPSS 26 (IBM, United States). To evaluate the main effects of the stimulus size, time, and montages, three-way repeated-measures ANOVAs on the absolute values of the VEP features were used, while two-way repeated-measures ANOVAs (3 montages * 2 sizes) were used to compare among different montages using the normalized changes. Except P100 amplitude, time-domain VEP features (N75 amplitude and P100 latency) violated the assumption of normal distribution in parametric analyses. Hence, the results were transformed to achieve the normality using percentile ranking followed by an inverse-normal transformation into Z-scores (35). For spectral domain analysis, the PSDα and PSDβ were logarithmically transformed to achieve normal distribution, and the logarithmic difference was analyzed. Holm’s adjustment was applied in post-hoc comparisons (36). Partial eta-squared (η2p) was reported as the effect size, which is a metric reflecting the percentage of the variance in the dependent variable explained by the independent variables. For η2p: small effect = 0.01; medium effect = 0.06; and large effect = 0.14. Bayes factors were also reported to represent the odds of the alternative hypotheses over the null hypotheses (37). Chi-squared tests were used to evaluate the occurrence of subjective sensations and the accuracy of speculating an active stimulation.
All the experimental procedures were completed by 10 normally sighted (corrected LogMAR ≤0.00) adults (6 women, aged 28.2 ± 7.7 years). The impedance caused by tACS in Oz-cheek montage was smaller (8.85 ± 1.22 Ω) than that of Oz-Cz montage (10.6 ± 1.63 Ω). Percentage change in P100 amplitude in all measurements followed a normal distribution (Shapiro–Wilk test, all p > 0.18). After normality transformation, the Shapiro-Wilk test showed an approximately normal distribution for normalized changes in N75 amplitude (all p > 0.13), P100 latency (all p > 0.07), log PSDα (all p > 0.14), and log PSDβ (all p > 0.14).
Supplementary Table S1 shows the time-domain features, including N75 amplitude, P100 amplitude, and latency of the VEP measurements before and after the tACS, while the results of repeated-measures ANOVA on normalized changes of VEP features are summarized in Table 1. For N75 amplitude and P100 latency, the main effects of the montage and size, as well as their interaction, were all insignificant on normally transformed percentage changes immediately and 20 min after the tACS (p > 0.09). For P100 amplitude, the effects were also insignificant immediately after tACS. However, the main effects of both the montage and size were significant at T40 (p = 0.01 and p = 0.03, respectively). In the post-hoc comparison, Oz-Cz montage was significantly smaller than that of sham stimulation after Holm’s adjustment (p < 0.05), but other pairs of montage were similar. Figure 2 shows the percentage change in P100 amplitude immediately and 20 min after tACS, stratified by montages and stimulus sizes, while Figure 3 demonstrates the waveforms obtained from VEP in different conditions.
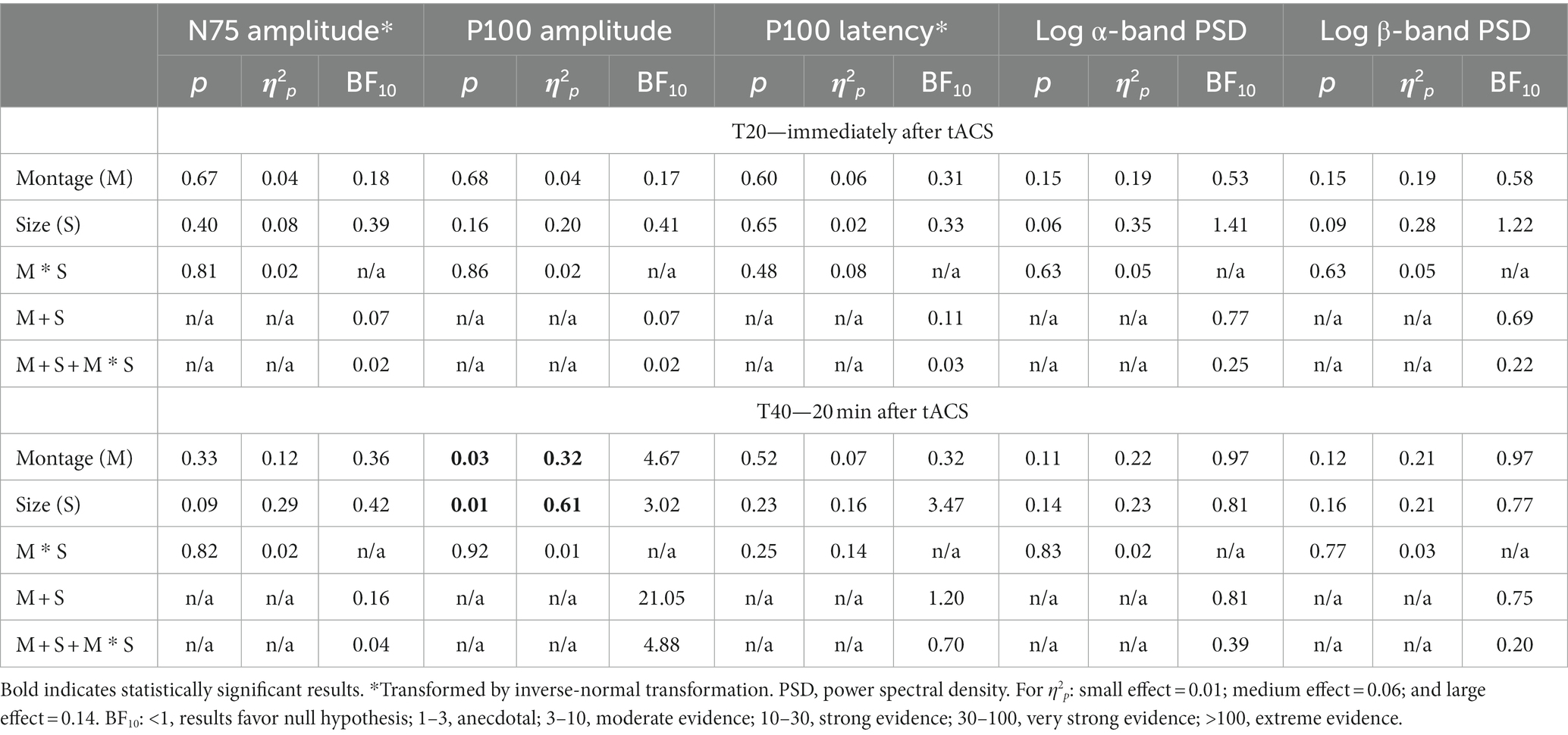
Table 1. Results of two-way repeated-measures ANOVA in normalized percentage changes of VEP time-domain features and logarithmic difference of spectral-domain features.
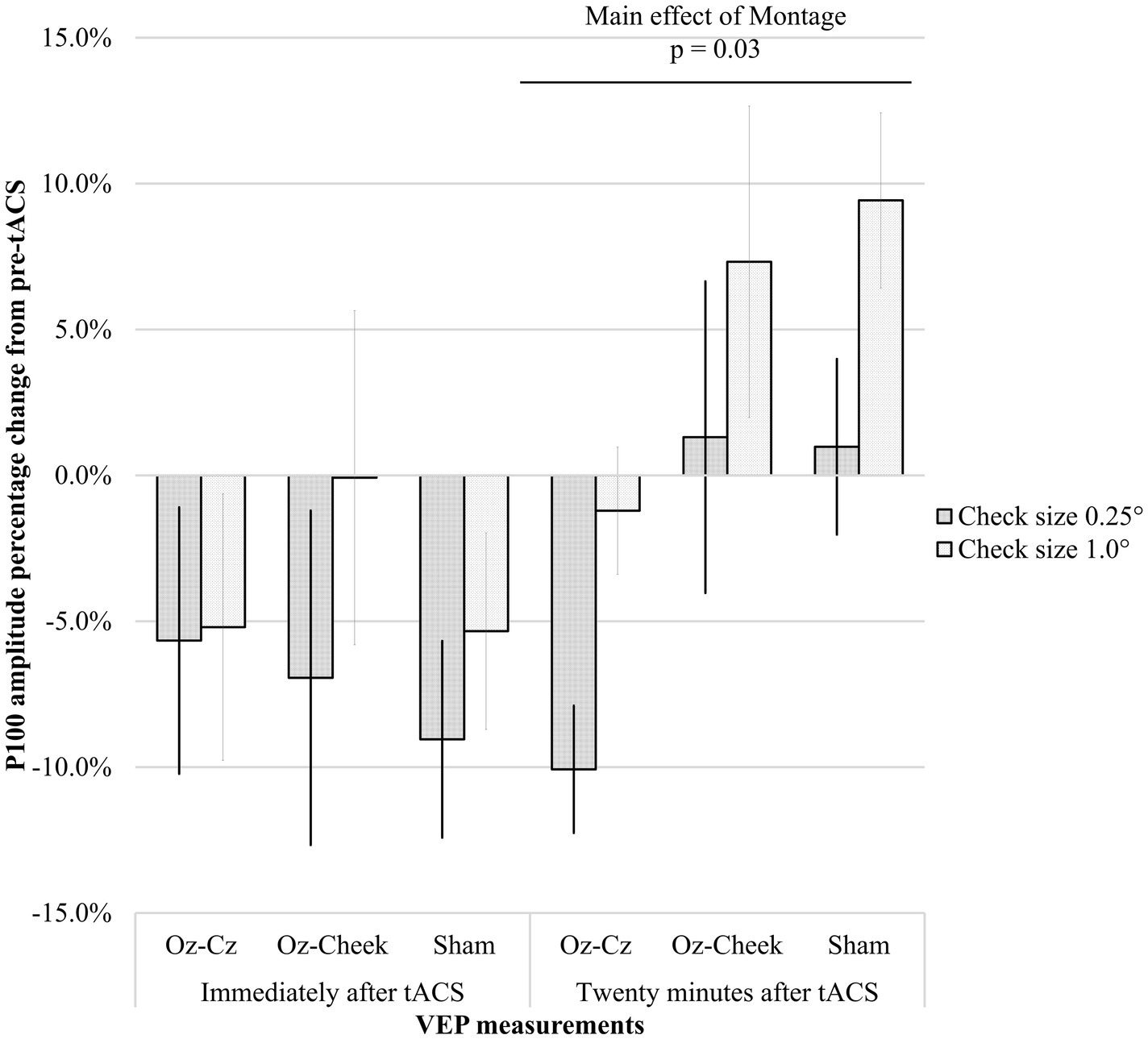
Figure 2. Percentage changes in P100 amplitude immediately after tACS (T20) and 20 min after tACS (T40) for different montages. Error bars represent the standard errors of mean.
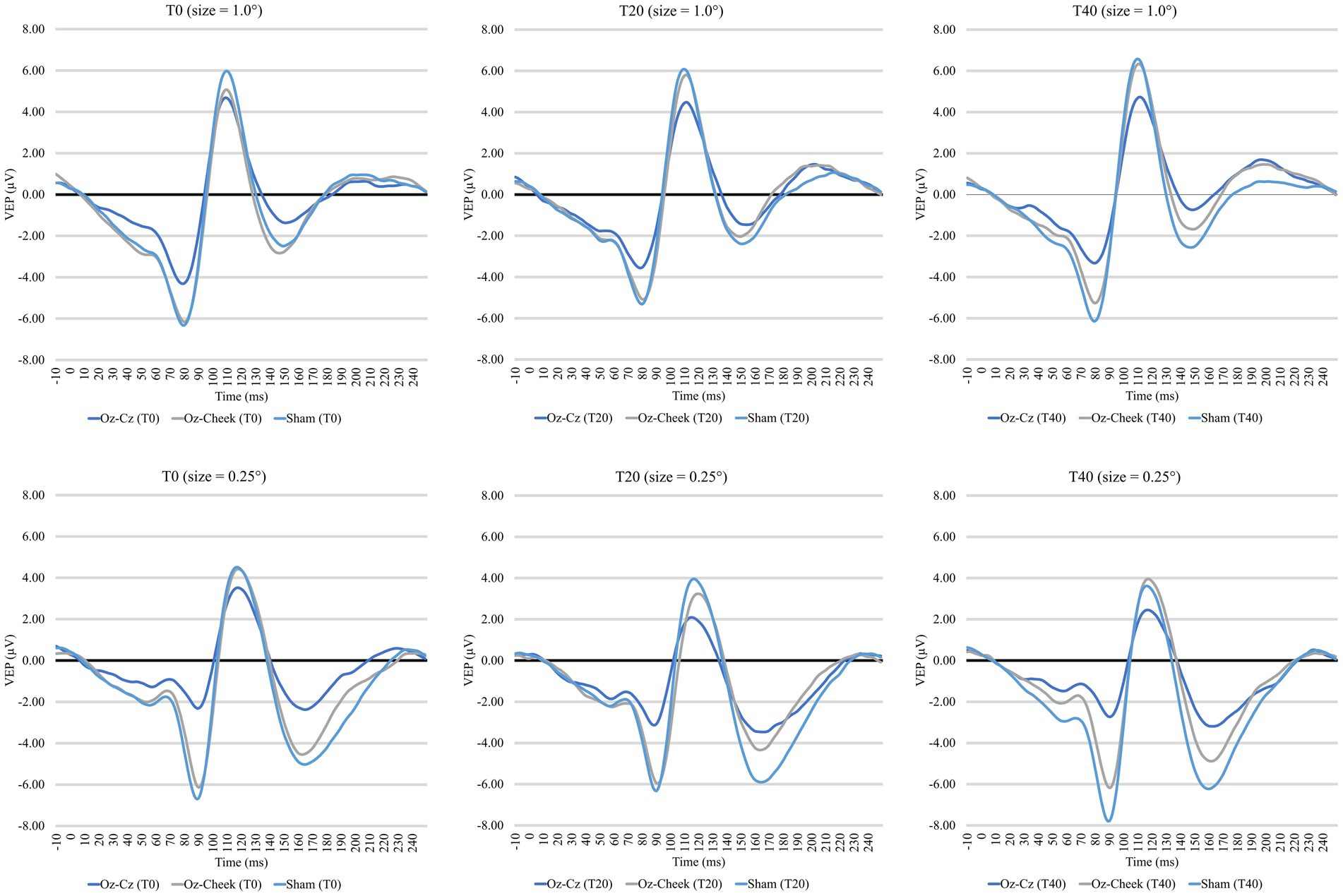
Figure 3. Time-domain visual evoked potentials at three time points for two check sizes. Top panel: 1.0 degree, bottom panel: 0.25 degree.
The logarithmic PSD values, as shown in Supplementary Table S1, of both α- and β-bands all increased after tACS for all montages at T20 and retained until T40. However, neither the main effect of the montage or size nor the interaction was significant in either T20 or T40 (p > 0.06) for both α- and β-bands; the statistical results are summarized in Table 1.
In all tACS montages, 90% of the participants reported at least one type of mild sensation in any of the montages. Stinging, phosphene, and itchiness were the most frequently reported sensations, as shown in Supplementary Figure S1. Results from the chi-square test did not reveal a significant difference in the occurrence of subjective sensation between montages (p > 0.12). As for the grading, almost all reported well tolerated (Score = 1 or 2), except for one grade 3 and another grade 4 itchiness reported during Oz-Cheek stimulation. The chi-square test also demonstrated similar accuracies of speculation for different montages (p = 0.35), supporting the blinding for participants was valid.
The current study compared the effect of tACS at the occipital cortex using different montages on VEP. Our results revealed an immediate inhibitory effect of tACS on P100 amplitude, in which the effect sustained for 20 min after stimulation in the Oz-Cz montage. However, tACS did not have a significant effect on N75 amplitude or P100 latency. In addition, the entrainment effect was inconclusive from the PSD analysis.
Our results demonstrated that P100 amplitude was significantly decreased by either stimulating Oz-Cz or Oz-cheek. However, the difference between active and sham stimulations did not happen as expected. From Figure 2, considering the similar trends of P100 amplitude over time in all the montages, the small sample size in the current study could be a possible reason, which was inadequate to identify the essential difference between the active and sham groups. Another possibility was that the tACS intensity adopted in the current study was too weak to alter the cortical response in humans. Vöröslakos et al. suggested applying at least 4–6 mA currents through the conventional tACS electrodes for modulating the amplitude of alpha waves reliably and instantaneously in human subjects due to the fact that only approximately 25% of the scalp-applied current would be able to enter the brain transcranially (11). However, the efficacy and tolerability of current intensity larger than 4 mA are still in doubt, especially for tACS. De Koninck et al. reported that 1-mA tACS induced stronger aftereffects in alpha power than that stimulated by 4–6 mA (38).
Another interesting finding was that the 10-Hz tACS played an inhibiting role in occipital excitability, which was opposing to our expectation. This inhibitory role was consistently demonstrated in a recent study (39), in which the tDCS significantly reduced the P100 amplitude of the occipital cortex. Other earlier studies also had similar findings that an anodal stimulation decreased phosphene thresholds (40, 41) and tACS diminished the amplitude of motor evoked potentials (42).
The mainstream explanation for the mechanism of tACS was the “entrainment effect,” which indicates that endogenous oscillations during entrainment are synchronized to that of extrinsic and rhythmic stimuli (43). Prior research has provided evidence that tACS can entrain endogenous brain rhythms and increase neural synchronization in the corresponding frequency to enhance cognitive performances (20). Krause et al. found that tACS affected the firing patterns of individual neurons in conscious non-human primates (44). Later, Beliaeva and Polania supported that cortical neurons were entrained via tACS (45). However, our results were inconclusive on the entrainment effect.
The PSD analysis reached a statistical significance in neither of the montages, regardless of the power of alpha and beta bands. However, the PSD values of alpha and beta bands were all increased after tACS in every montage. Although the difference between the montages, sizes, and interaction was not statistically significant, we speculated that the effect size was underestimated by the small sample size, as were the time-domain features. Another possibility was that the changes in the surface electroencephalographic signal were too subtle to be detected. Findings of a recent animal experiment (46) on monkeys, which investigated the entrainment effect caused by low-intensity tACS, were similar to those of the current study. Electroencephalographic signals were recorded from cortical structures near the stimulation electrode to maximize the possibility for the entrainment effect to be detected at the targeted regions. From their results, approximately 10% of all recorded cells showed an entrainment effect with 1.5-mA tACS. In contrast, in the current experimental setup, only the cortical signals at the scalp surface were recorded, making the detection of the entrainment effect more difficult and indirect. Furthermore, Beliaeva and Polania found a large proportion of the recorded cells (approximately 70%) did not respond to tACS (45). Hence, at the population level (i.e., combined data from responsive and non-responsive neurons), the effects of entrainment would be apparently absent. The low prevalence of responsive cortical neurons may explain the noticeable lack of entrainment in low-intensity tACS experiments where neural activity can only be measured based on local field potentials or subcutaneous electroencephalography recordings (11).
Subjective sensations were common during the experiment, but most of them were well-tolerated, supporting 2-mA, 10-Hz tACS being a safe and non-invasive intervention for vision. Compared with the Oz-Cz montage, the Oz-cheek montage had a more frequent (12 vs. 15 times) and obvious (33.3% vs. 46.7% greater than mild) sensation. This outcome was expected since the cheek was directly in contact with the stimulation electrodes, while Cz was protected by the hair. In this study, visual phosphenes were also commonly reported during the active sessions. In the experiment conducted by Kanai et al., tACS was applied at Oz, with the reference electrode applied in different locations remotely from the eye to circumvent the induction of retinal phosphenes (47). Their results showed that the closer the tACS electrodes were to the retina, the more likely retinal stimulation would occur. Another interesting finding was that, when tACS was delivered in darkness, the phosphenes reported during stimulation at alpha frequencies were stronger. With the sham stimulation mimicking the first 30 s of active stimulations, the probability of guessing correctly for active stimulation (2/3) was higher than sham (1/3), indicating that the blinding in this study (especially for sham—50% accuracy) was effectively performed.
The superior–inferior antagonism between the two montages of the current study did not demonstrate any difference in the VEP features. Such similarity could be due to the lack of directional information in standardized VEP to differentiate the localized cortical response from tACS montages (48). Therefore, altitudinal hemifield VEP, i.e., showing only half of the checkerboard above or below the midline, may be a better method to detect the difference between Oz-Cz vs. Oz-cheek montages. It was observed that the Oz-Cz montage generated the highest VEP amplitudes, even at T0. A possible explanation was the shorter distance between Oz-Cz compared to that of Oz-cheek, with the short electrode distance inducing a stronger magnitude of neuromodulation. Another interesting finding was the different aftereffects between the two montages—the onset of tACS action of Oz-Cz was more delayed than that of Oz-cheek. As shown in Figure 2, the P100 amplitude immediately dropped at T20 for Oz-cheek, but not in Oz-Cz. The effect sustained until T40, while the tACS effect from the Oz-cheek montage started to become stronger at T40. We speculated that a quicker conductive speed of Oz-cheek benefited from less interruption from the hair.
Previous studies have illustrated how the montage type, i.e., the positions of the stimulation electrodes, altered the effect of tES on VEP amplitude; however, the evidence was limited to tDCS only (13). In particular, tDCS at Oz-Cz vs. Oz-posterior neck base montages demonstrated opposite effects on the VEP amplitude (7, 10). The difference was attributed to the amount of electricity and the location of the cortical area being stimulated by the tDCS (6, 10). Antal et al. indicated that tACS after-effects were abolished or even reversed, as observed with tDCS (49). In the current study, the tACS montages, i.e., Oz-Cz vs. Oz-cheek, had a stimulation position akin to the ventro-dorsal orientation observed in Oz-Cz vs. Oz-posterior neck base in prior research. This positioning of the stimulation may have an inhibitory effect on the VEP electrophysiological responses.
There are advantages and disadvantages for each montage. From our results, the Oz-Cz montage had fewer and weaker subjective sensations. On the other hand, Oz-cheek had a quicker onset of action and induced smaller impedances, although the efficacies of Oz-Cz and Oz-cheek were similar. It is suggested to balance among the side effects, onset of action, and impedance while choosing a suitable montage for tES interventions.
There were several limitations in this study. First, the sample size was small, which restricted the efficacy of tACS to be detected and limited the generalization of our conclusions. Second, the experimenter was unblinded. This study adopted a single-blind design, in which the impact of the experimenter was not controlled. However, the probability of guessing correctly supported a valid blinding and a minimal effect from the experimenter. Third, there was a lack of comparison of VEP between the left and right hemispheres. A previous study indicated that the unilateral placement of the electrodes restricted the induced electric fields largely to the selected hemisphere, which could be the difference between the Oz-Cz and Oz-cheek (11). Future studies should attempt to utilize a double-blind design, replicate the findings in a larger sample size, and consider comparing differences between the left and right hemispheres.
The current study revealed an immediate inhibitory effect of tACS regardless of the montages. This inhibitory effect sustained in the Oz-Cz montage but faded out in other montages after 20 min. The Oz-Cz montage resulted in fewer and milder side effects, while the Oz-cheek montage produced a faster aftereffect and lower impedances. When selecting an appropriate montage, it is recommended to consider a balance between side effects, onset of action, and impedance.
The original contributions presented in the study are publicly available. This data can be found here: https://doi.org/10.17605/osf.io/khrbt.
The studies involving humans were approved by the Institutional Review Board of The Hong Kong Polytechnic University (ethical approval number: HSEARS20210713002). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
JW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. KC: Data curation, Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. BT: Funding acquisition, Resources, Supervision, Writing – review & editing. HC: Methodology, Supervision, Writing – review & editing. AC: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Research Impact Fund, Research Grants Council, HKSAR (R5047-19), and the InnoHK Initiatives, Hong Kong SAR Government.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1273044/full#supplementary-material
1. Polanía, R, Nitsche, MA, and Ruff, CC. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci. (2018) 21:174–87. doi: 10.1038/s41593-017-0054-4
2. Yavari, F, Jamil, A, Samani, MM, Vidor, LP, and Nitsche, MA. Basic and functional effects of transcranial electrical stimulation (TES)—an introduction. Neurosci Biobehav Rev. (2018) 85:81–92. doi: 10.1016/j.neubiorev.2017.06.015
3. Woods, AJ, and Martin, DM. Clinical research and methodological aspects for Tdcs research In: Transcranial direct current stimulation in neuropsychiatric disorders: clinical principles and management. Springer, Cham. (2021). 265–79. doi: 10.1007/978-3-319-33967-2_26
4. Sehic, A, Guo, S, Cho, K-S, Corraya, RM, Chen, DF, and Utheim, TP. Electrical stimulation as a means for improving vision. Am J Pathol. (2016) 186:2783–97. doi: 10.1016/j.ajpath.2016.07.017
5. Bello, UM, Wang, J, Park, AS, Tan, KW, Cheung, BW, Thompson, B, et al. Can visual cortex non-invasive brain stimulation improve Normal visual function? A systematic review and meta-analysis. Front Neurosci. (2023) 17:1119200. doi: 10.3389/fnins.2023.1119200
6. Nitsche, MA, and Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. (2000) 527:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x
7. Accornero, N, Li Voti, P, La Riccia, M, and Gregori, B. Visual evoked potentials modulation during direct current cortical polarization. Exp Brain Res. (2007) 178:261–6. doi: 10.1007/s00221-006-0733-y
8. Monte-Silva, K, Kuo, M-F, Hessenthaler, S, Fresnoza, S, Liebetanz, D, Paulus, W, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. (2013) 6:424–32. doi: 10.1016/j.brs.2012.04.011
9. Tavakoli, AV, and Yun, K. Transcranial alternating current stimulation (tACS) mechanisms and protocols. Front Cell Neurosci. (2017) 11:214. doi: 10.3389/fncel.2017.00214
10. Antal, A, Kincses, TZ, Nitsche, MA, Bartfai, O, and Paulus, W. Excitability changes induced in the human primary visual cortex by transcranial direct current stimulation: direct electrophysiological evidence. Invest Ophthalmol Vis Sci. (2004) 45:702–7. doi: 10.1167/iovs.03-0688
11. Vöröslakos, M, Takeuchi, Y, Brinyiczki, K, Zombori, T, Oliva, A, Fernández-Ruiz, A, et al. Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nat Commun. (2018) 9:483. doi: 10.1038/s41467-018-02928-3
12. Miranda, PC, Lomarev, M, and Hallett, M. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol. (2006) 117:1623–9. doi: 10.1016/j.clinph.2006.04.009
13. Kraft, A, Roehmel, J, Olma, MC, Schmidt, S, Irlbacher, K, and Brandt, SA. Transcranial direct current stimulation affects visual perception measured by threshold perimetry. Exp Brain Res. (2010) 207:283–90. doi: 10.1007/s00221-010-2453-6
14. Reinhart, RM, Xiao, W, McClenahan, LJ, and Woodman, GF. Electrical stimulation of visual cortex can immediately improve spatial vision. Curr Biol. (2016) 26:1867–72. doi: 10.1016/j.cub.2016.05.019
15. Antal, A, Nitsche, MA, Kruse, W, Kincses, TZ, Hoffmann, K-P, and Paulus, W. Direct current stimulation over V5 enhances visuomotor coordination by improving motion perception in humans. J Cogn Neurosci. (2004) 16:521–7. doi: 10.1162/089892904323057263
16. Paulus, W. Transcranial electrical stimulation (tES–tDCS; tRNS, tACS) methods. Neuropsychol Rehabil. (2011) 21:602–17. doi: 10.1080/09602011.2011.557292
17. Chase, HW, Boudewyn, MA, Carter, CS, and Phillips, ML. Transcranial direct current stimulation: a roadmap for research, from mechanism of action to clinical implementation. Mol Psychiatry. (2020) 25:397–407. doi: 10.1038/s41380-019-0499-9
18. Antal, A, and Herrmann, CS. Transcranial alternating current and random noise stimulation: possible mechanisms. Neural Plast. (2016) 2016:3616807. doi: 10.1155/2016/3616807
19. Brignani, D, Ruzzoli, M, Mauri, P, and Miniussi, C. Is transcranial alternating current stimulation effective in modulating brain oscillations? PLoS One. (2013) 8:e56589. doi: 10.1371/journal.pone.0056589
20. Helfrich, RF, Schneider, TR, Rach, S, Trautmann-Lengsfeld, SA, Engel, AK, and Herrmann, CS. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr Biol. (2014) 24:333–9. doi: 10.1016/j.cub.2013.12.041
21. Battaglini, L, Mena, F, Ghiani, A, Casco, C, Melcher, D, and Ronconi, L. The effect of alpha tACS on the temporal resolution of visual perception. Front Psychol. (2020) 11:1765. doi: 10.3389/fpsyg.2020.01765
22. Nakazono, H, Ogata, K, Takeda, A, Yamada, E, Kimura, T, and Tobimatsu, S. Transcranial alternating current stimulation of Α but not Β frequency sharpens multiple visual functions. Brain Stimul. (2020) 13:343–52. doi: 10.1016/j.brs.2019.10.022
23. Dowsett, J, Herrmann, CS, Dieterich, M, and Taylor, PC. Shift in lateralization during illusory self-motion: EEG responses to visual flicker at 10 Hz and frequency-specific modulation by tACS. Eur J Neurosci. (2020) 51:1657–75. doi: 10.1111/ejn.14543
24. Manoli, Z, Grossman, N, and Samaras, T, editors. Theoretical investigation of transcranial alternating current stimulation using realistic head model. 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society; (2012) San Diego, California: IEEE.
25. Inukai, Y, Saito, K, Sasaki, R, Tsuiki, S, Miyaguchi, S, Kojima, S, et al. Comparison of three non-invasive transcranial electrical stimulation methods for increasing cortical excitability. Front Hum Neurosci. (2016) 10:668. doi: 10.3389/fnhum.2016.00668
26. He, Q, Yang, X-Y, Gong, B, Bi, K, and Fang, F. Boosting visual perceptual learning by transcranial alternating current stimulation over the visual cortex at alpha frequency. Brain Stimul. (2022) 15:546–53. doi: 10.1016/j.brs.2022.02.018
27. Yang, X-Y, He, Q, and Fang, F. Transcranial direct current stimulation over the visual cortex facilitates awake consolidation of visual perceptual learning. Brain Stimul. (2022) 15:380–2. doi: 10.1016/j.brs.2022.01.019
28. Wang, Y, Hou, P, Li, W, Zhang, M, Zheng, H, and Chen, X. The influence of different current-intensity transcranial alternating current stimulation on the eyes-open and eyes-closed resting-state electroencephalography. Front Hum Neurosci. (2022) 16:934382. doi: 10.3389/fnhum.2022.934382
29. Hsu, CY, Liu, TL, Lee, DH, Yeh, DR, Chen, YH, Liang, WK, et al. Amplitude modulating frequency overrides carrier frequency in tACS-induced phosphene percept. Hum Brain Mapp. (2023) 44:914–26. doi: 10.1002/hbm.26111
30. Keel, JC, Smith, MJ, and Wassermann, EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol. (2001) 112:720. doi: 10.1016/S1388-2457(00)00518-6
31. Odom, JV, Bach, M, Barber, C, Brigell, M, Marmor, MF, Tormene, AP, et al. Visual evoked potentials standard (2004). Doc Ophthalmol. (2004) 108:115–23. doi: 10.1023/B:DOOP.0000036790.67234.22
32. Odom, JV, Bach, M, Brigell, M, Holder, GE, McCulloch, DL, Mizota, A, et al. ISCEV standard for clinical visual evoked potentials: (2016 update). Doc Ophthalmol. (2016) 133:1–9. doi: 10.1007/s10633-016-9553-y
33. Liu, A, Vöröslakos, M, Kronberg, G, Henin, S, Krause, MR, Huang, Y, et al. Immediate neurophysiological effects of transcranial electrical stimulation. Nat Commun. (2018) 9:5092. doi: 10.1038/s41467-018-07233-7
34. Welch, P. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified Periodograms. IEEE Trans Audio Electroacoust. (1967) 15:70–3. doi: 10.1109/TAU.1967.1161901
35. Templeton, GF. A two-step approach for transforming continuous variables to Normal: implications and recommendations for is research. Commun Assoc Inf Syst. (2011) 28:4. doi: 10.17705/1cais.02804
37. Lee, MD, and Wagenmakers, E-J. Bayesian cognitive modeling: a practical course. Cambridge, United Kingdom: Cambridge University Press (2014).
38. De Koninck, BP, Guay, S, Blais, H, and De Beaumont, L. Parametric study of transcranial alternating current stimulation for brain alpha power modulation. Brain Commun. (2021) 3:fcab010. doi: 10.1093/braincomms/fcab010
39. Lau, CI, Tseng, L-Y, Walsh, V, and Hsu, T-Y. Revisiting the effects of transcranial direct current stimulation on pattern-reversal visual evoked potentials. Neurosci Lett. (2021) 756:135983. doi: 10.1016/j.neulet.2021.135983
40. Antal, A, Kincses, TZ, Nitsche, MA, and Paulus, W. Manipulation of phosphene thresholds by transcranial direct current stimulation in man. Exp Brain Res. (2003) 150:375–8. doi: 10.1007/s00221-003-1459-8
41. Antal, A, Kincses, TZ, Nitsche, MA, and Paulus, W. Modulation of moving phosphene thresholds by transcranial direct current stimulation of V1 in human. Neuropsychologia. (2003) 41:1802–7. doi: 10.1016/S0028-3932(03)00181-7
42. Zaghi, S, de Freitas, RL, de Oliveira, LM, El-Nazer, R, Menning, S, Tadini, L, et al. Inhibition of motor cortex excitability with 15 Hz transcranial alternating current stimulation (tACS). Neurosci Lett. (2010) 479:211–4. doi: 10.1016/j.neulet.2010.05.060
43. Herrmann, CS, Rach, S, Neuling, T, and Strüber, D. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front Hum Neurosci. (2013) 7:279. doi: 10.3389/fnhum.2013.00279
44. Krause, MR, Vieira, PG, Csorba, BA, Pilly, PK, and Pack, CC. Transcranial alternating current stimulation entrains single-neuron activity in the primate brain. Proc Natl Acad Sci U S A. (2019) 116:5747–55. doi: 10.1073/pnas.1815958116
45. Beliaeva, V, and Polania, R. Can low-intensity tACS genuinely entrain neural activity in vivo? Brain Stimulation (2020). 13:1796–99. doi: 10.1016/j.brs.2020.10.002
46. Johnson, L, Alekseichuk, I, Krieg, J, Doyle, A, Yu, Y, Vitek, J, et al. Dose-dependent effects of transcranial alternating current stimulation on spike timing in awake nonhuman primates. Sci Adv. (2020) 6:eaaz2747. doi: 10.1126/sciadv.aaz2747
47. Kanai, R, Chaieb, L, Antal, A, Walsh, V, and Paulus, W. Frequency-dependent electrical stimulation of the visual cortex. Curr Biol. (2008) 18:1839–43. doi: 10.1016/j.cub.2008.10.027
48. Lennie, P. Single units and visual cortical organization. Perception. (1998) 27:889–935. doi: 10.1068/p270889
Keywords: non-invasive brain stimulation, transcranial alternating current stimulation, transcranial electrical stimulation, montage, occipital excitability, visual evoked potentials
Citation: Wang J, Choi KY, Thompson B, Chan HHL and Cheong AMY (2024) The effect of montages of transcranial alternating current stimulation on occipital responses—a sham-controlled pilot study. Front. Psychiatry. 14:1273044. doi: 10.3389/fpsyt.2023.1273044
Received: 05 August 2023; Accepted: 13 December 2023;
Published: 24 January 2024.
Edited by:
Osama Abulseoud, Mayo Clinic Arizona, United StatesReviewed by:
Jack Jiaqi Zhang, Hong Kong Polytechnic University, Hong Kong SAR, ChinaCopyright © 2024 Wang, Choi, Thompson, Chan and Cheong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Allen Ming Yan Cheong, YWxsZW4ubXkuY2hlb25nQHBvbHl1LmVkdS5oaw==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.