
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 23 November 2023
Sec. Personality Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1243511
This article is part of the Research Topic Women in Psychiatry: Personality Disorders 2023 View all 8 articles
 Carlotta Lunghi1,2,3*
Carlotta Lunghi1,2,3* Lionel Cailhol4,5
Lionel Cailhol4,5 Victoria Massamba3
Victoria Massamba3 Elhadji A. Laouan Sidi3
Elhadji A. Laouan Sidi3 Caroline Sirois3,6
Caroline Sirois3,6 Elham Rahme7
Elham Rahme7 Louis Rochette3
Louis Rochette3 Suzane Renaud5,8
Suzane Renaud5,8 Evens Villeneuve9,10
Evens Villeneuve9,10 Marion Koch11,12
Marion Koch11,12 Robert Biskin11
Robert Biskin11 Cathy Martineau1
Cathy Martineau1 Philippe Vincent13
Philippe Vincent13 Pierre David4,5
Pierre David4,5 Alain Lesage3,4,5
Alain Lesage3,4,5Background: Cluster B personality disorders (PDs) are considered some of the most severe mental health conditions. Scarce evidence exists about the real-world utilization of psychotropics for cluster B PD individuals.
Objective: We aimed to uncover trends and patterns of psychotropic medication use among individuals diagnosed with cluster B PD in the year before and after their diagnosis and to identify factors associated with medication use in a large cohort of individuals newly diagnosed with cluster B PDs.
Methods: We conducted a population-based observational study using Quebec’s health services register. We identified Quebec residents aged ≥14 years and insured with the provincial drug plan with a first diagnosis of cluster B PD recorded between April 1, 2002, and March 31, 2019. Cluster B PD was defined with ICD-9/10 diagnostic codes. We retrieved all claims for the main psychotropic medication classes: antipsychotics, antidepressants, anxiolytics, mood stabilizers, and attention-deficit/hyperactivity disorder (ADHD) medications. We calculated the proportion of individuals exposed to these medication classes and analyzed trends over the years using robust Poisson regression models, adjusting for potential confounders. We used robust Poisson regression to identify factors associated with medication class use.
Results: We identified 87,778 new cases of cluster B PD, with a mean age of 44.5 years; 57.5% were women. Most frequent psychiatric comorbidities in the five years before cluster B PD diagnosis were depression (50.9%), anxiety (49.7%), and psychotic disorders (37.5%). Most individuals (71.0%) received at least one psychotropic during the year before cluster B PD diagnosis, and 78.5% received at least one of these medications in the subsequent year. The proportion of users increased after the diagnosis for antidepressants (51.6–54.7%), antipsychotics (35.9–45.2%), mood stabilizers (14.8–17.0%), and ADHD medications (5.1–5.9%), and remained relatively stable for anxiolytics (41.4–41.7%). Trends over time showed statistically significant increased use of antipsychotics and ADHD medications, decreased use of anxiolytics and mood stabilizers, and a stable use of antidepressants.
Conclusion: Psychotropic medication use is highly prevalent among cluster B PD individuals. We observed an increase in medication use in the months following the diagnosis, particularly for antipsychotics, antidepressants, and mood stabilizers.
Cluster B personality disorders (PDs) are severe and chronic mental health conditions characterized by relational and affective instability, identity disorder, and marked impulsivity (1). People with cluster B PDs present several difficulties in their relational and occupational functioning, decreasing their quality of life (1). A recent systematic review and meta-analysis estimated the lifetime prevalence of cluster B PDs at 5.5%, with differences between subtypes (0.8% for histrionic, 1.2% for narcissistic, 1.9% for borderline, and 3.1% for antisocial) (2). In Quebec, Canada, we estimated cluster B PDs lifetime prevalence at 2.6% (3). Individuals with cluster B PDs are high users of medical services, more than schizophrenia patients (4). They also have high rates of comorbid substance use disorders, particularly alcohol, opioid, and cocaine use disorders (5). Additionally, they exhibit a reduced life expectancy, and 20.5% of premature mortality is attributable to suicide (3, 6, 7). Indeed, borderline personality disorder may specifically indicate a predisposition to suicidal behavior disorder diagnoses (8).
Treatment of cluster B PDs is a clinical challenge because of the high suicidal behaviors and the resistance of clinical symptoms to pharmacological treatments (9, 10). Current clinical guidelines, such as the 2009 United Kingdom’s National Institute for Health Care and Excellence (NICE) recommendations (11) or the European guidelines for PDs (12), and a recent systematic review of clinical recommendations on the treatment of PD patients made by different mental health organizations worldwide (13) do not recommend the use of pharmacotherapy for the treatment of cluster B PD individuals because of the lack of evidence to support their use. Systematic reviews have been conducted in the last decade to identify which medications could benefit patients with cluster B PDs (10, 14–16). A 2010 Cochrane review of randomized clinical trials (RCTs) assessing the effectiveness and safety of different psychotropic medication classes found only fragmentary evidence supporting the use of antipsychotics (especially first generation) in reducing anger (14). More recent reviews (10, 16) and Cochrane updates (17, 18) did not find substantial differences from newer RCTs evaluating second-generation antipsychotics, antidepressants, mood stabilizers, or various medications (e.g., the antiepileptic lamotrigine or the anti-dementia drug memantine). Indeed, no medication has been officially approved for treating patients with cluster B PDs.
Although systematic reviews (10, 18, 19), expert opinions (9), and clinical practice guidelines (11, 12) recognize psychotherapy as the first-line treatment for cluster B PDs, there is evidence of a gap between evidence-based recommendations on pharmacotherapy and current clinical practice, with different psychotropic medications prescribed to treat this condition despite the lack of evidence for their efficacy. Indeed, some studies have reported high consumption of psychotropics in individuals with cluster B PDs (20–31). Nevertheless, most of these studies were cross-sectional (21, 22, 26, 28, 30), focused on borderline individuals (21–23, 25, 26, 28, 31), were conducted in small samples (26, 31), or considered only hospitalized patients (25, 26, 31), those participating in mental health programs (20, 22, 23, 29), or those followed by a psychiatrist (21).
The aim of this study was thus to draw a portrait of the use of psychotropic medications in individuals with cluster B PDs from a publicly managed care system enrolling all 8.5 million inhabitants of the Canadian province of Quebec. Specific objectives were (1) to describe psychotropic medication use in the year before and after cluster B PD diagnosis; (2) to identify trends and patterns in psychotropic use over 16 years; and (3) to identify factors associated with the exposure to psychotropic medication classes.
We conducted a population-based cohort study of Quebec residents covered under the universal provincial health program (32) using medico-administrative data from the Quebec Integrated Chronic Disease Surveillance System (QICDSS) database (33). More than 99% of the Quebec population is covered under this provincial health program and is included in the QICDSS database. QICDSS comprises claims data from physician visits and hospitalizations starting from January 1, 1996. Diagnoses from physician visits and hospitalizations are based on the ninth and tenth revisions of the International Classification of Diseases (ICD-9 and ICD-10). QICDSS also comprises the death registry. Moreover, this database contains information on reimbursed medications for all residents registered under the public drug insurance plan, namely those without a private drug insurance plan, those on a guaranteed income supplement or welfare, and all citizens aged 65 years and older not living in long-term care facilities. In 2021, the public drug plan covered about 3.75 million people (43.5% of the Quebec population) (32).
We identified all Quebec residents ages 14 years old and older with a first cluster B PD diagnosis recorded in the QICDSS database between April 1, 2002, and March 31, 2019. To identify cluster B PD patients in the QICDSS database, we used a case definition that was previously developed by a team of experts (four psychiatrists and one psychologist experienced in treating PDs in Quebec) and described elsewhere (3). Briefly, following two multisite work sessions, the team of experts was able to achieve a consensus on the definitive list of ICD-9 and ICD-10 codes typically employed in the diagnoses of histrionic, narcissistic, borderline, or antisocial personality disorders in Quebec. The cluster B PD case was defined as any patient with at least one ICD-9 (301.1, 301.3, 301.5, 301.7, 301.8, or 301.9) or ICD-10 (F070, F340, F341, F488, F602, F603, F604, F606, F608, F609, F61, F620, F621, F628, F629, F681, F688, F69) diagnostic code. The choice of the ICD codes was based on the practice experience of the expert team to obtain the most clinically relevant data. Indeed, all selected ICD-9 and ICD-10 codes were meant to identify core symptoms of cluster B PDs symptoms based on the DSM. The expert panel also decided on the inclusion of the ICD-9 code 301.9 (unspecified PD) among the codes considered for the case definition, deeming it commonly used for borderline PD in Quebec. To collect information on prescribed medications, we excluded all the cluster B PD individuals who were not fully covered under the public drug plan starting from 1 year before to 1 year after the PD diagnosis. Information on prescribed drugs is unavailable in the QICDSS database for individuals with private medical insurance.
We included all medications listed in the Quebec public drug plan during the study period to identify psychotropic medications claimed during the period going from one year before to one year after cluster B PD diagnosis. We further classified drugs into five main psychotropic medication classes: antipsychotics, antidepressants, anxiolytics, mood stabilizers, and attention-deficit/hyperactivity disorder (ADHD) medications, according to the American Hospital Formulary Service (AHFS) classification (34) and common drug denomination (chemical name of the medication), as reported in Supplementary Table S1. We considered that an individual was exposed to a medication class if they claimed at least one medication from this class in the one year before or after cluster B PD diagnosis.
Baseline socio-demographic variables, assessed at the diagnosis date, included sex, age group, material and social deprivation indexes (in quintiles), and the geographical area based on the Quebec census (Montreal census metropolitan area (CMA), >1,000,000 inhabitants; Other CMA, 100,000-1,000,000 inhabitants; Agglomerations, 10,000–100,000 inhabitants; and Small town/rural area, <10,000 inhabitants). Material and social deprivation indexes are ecological indexes based on the census dissemination area divided into quintiles and represent a proxy of the individual’s socioeconomic status (35). The first quintile includes the least deprived, and the fifth quintile the most disadvantaged areas. For psychiatric conditions, an individual was considered to have a comorbid disorder if they had a claim for a physician visit or a hospitalization with an ICD-9 or ICD-10 code related to that condition recorded in the QICDSS in the five years before cluster B PD diagnosis.
We used descriptive statistics to document the baseline characteristics of the study cohort (age, sex, social and material deprivation, year of diagnosis, and geographical area) and clinical information (psychiatric diagnosis and the number of psychiatric comorbidities) according to age groups (14–24 years; 25–49 years; 50–64 years; 65 years and above). Demographics were reported as mean and standard deviation for age and proportions and 99% confidence intervals (CIs) for categorical variables. For each subject, we assessed the number of different psychotropic medications claimed in the year before and the year after the diagnosis of cluster B PD by using the common drug denominations (identifying the chemical entity). We calculated the proportion of individuals exposed to the main psychotropic medication classes (antipsychotics, antidepressants, anxiolytics, mood stabilizers, and ADHD medications).
We tested trends of change in the prevalence of psychotropic medication class use in the year after cluster B PD diagnosis according to the year of diagnosis using robust Poisson regression models, adjusting for age, sex, material and social deprivation, geographical area, and psychiatric comorbidities. Using robust Poisson regression analyses, we further identified the clinical and socio-demographic factors associated with the use of medication classes in the year after cluster B PD diagnosis, modeling the number of individuals who claimed at least one medication in the psychotropic class under study in that specific model. We calculated unadjusted and adjusted prevalence ratios (PRs) with their 99% CIs. We performed all the analyses using SAS Enterprise Guide 7.1.
We identified 211,974 individuals aged 14 years and above with a first diagnosis of cluster B PD between the fiscal years 2002–2003 and 2018–2019. Among them, we excluded those not fully covered by the public drug plan between 1 year before and one year after the diagnosis. Thus, we gathered a group of 87,778 people, with 6.7% of them diagnosed during a hospital stay, while the remaining 93.3% diagnosed during an outpatient visit (45.7% by a general practitioner and 44.3% by a psychiatrist) and classified according to the fiscal year of their first diagnosis. The general practitioners are commonly available in the Emergency Rooms of hospitals where PD are more likely to visit than patients with other diseases, such as schizophrenia (4). The cohort was predominantly female and socially and materially deprived. Characteristics of the study population are reported in Table 1 according to the individual’s age group at the time of cluster B PD diagnosis.
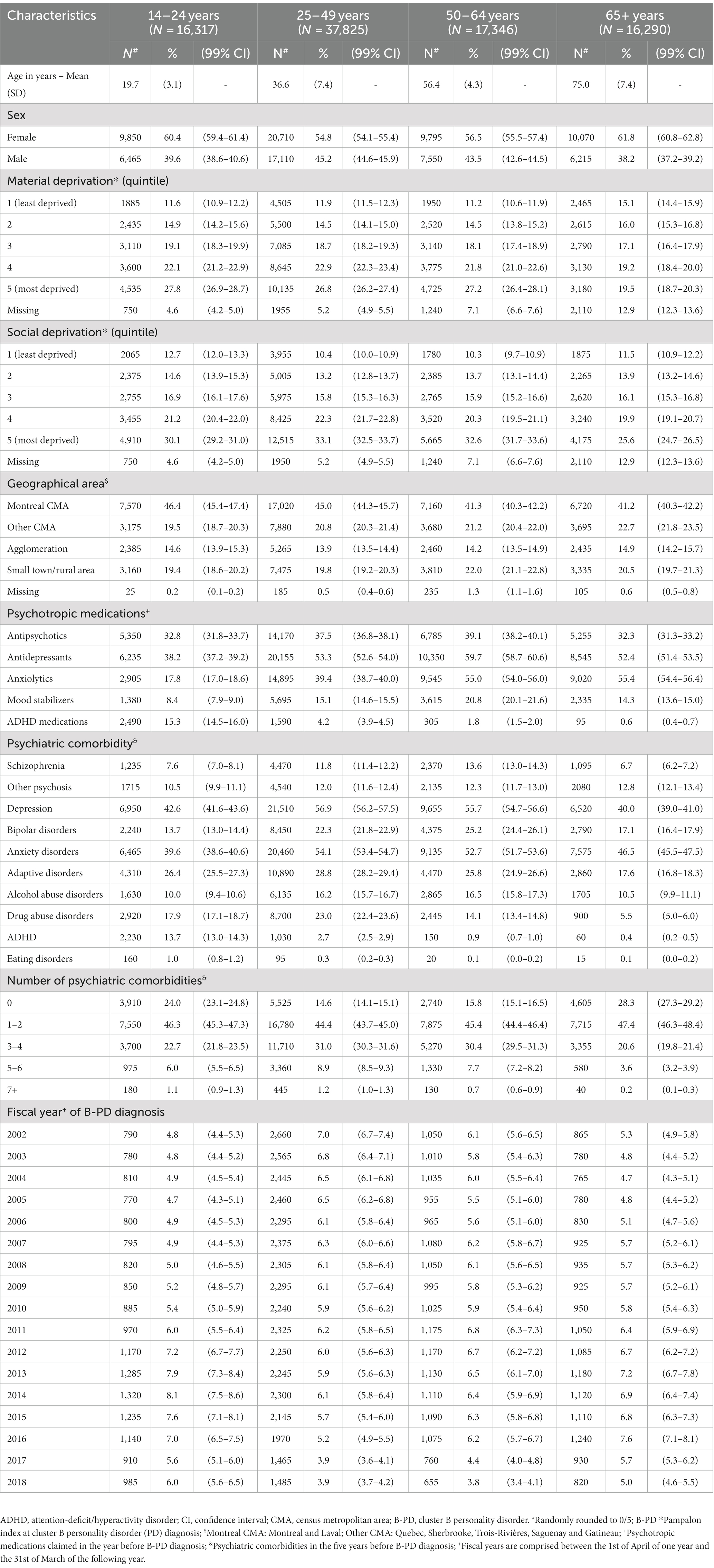
Table 1. Sociodemographic and clinical characteristics of the cohort of individuals at the time of a first diagnosis of cluster B personality disorder.
The proportion of individuals exposed to any psychotropic medication during the year before and after the cluster B PD diagnosis was generally high. It varied only slightly during the study period, between 72.0 and 75.5% before and 77.0 and 80.2% after the diagnosis, depending on the year. These proportions were also constantly higher compared to the proportion of individuals exposed to the same medication classes the year before the diagnosis. The mean number of different psychotropics used has decreased slightly over the study period, from 3.1 in 2002 to 2.8 in 2018 (Supplementary Figure S1). The mean number of antidepressants and mood stabilizers remained relatively stable from 2002 to 2018 (1.53 to 1.51 and 1.22 to 1.18, respectively), while that of antipsychotics and anxiolytics decreased (1.45 to 1.39 and 1.47 to 1.22, respectively), and that of ADHD medications increased (1.05 to 1.19) during the same period.
Figure 1 (panel A) reports the monthly proportion of individuals in the cohort exposed to different psychotropic medication classes during the year before and year after the diagnosis of cluster B PD. We could identify an increase in antidepressant and antipsychotic prescriptions nearby the cluster B PD diagnosis, which was substantially maintained in subsequent months. The use of anxiolytics slightly increased around PD diagnosis, but this increase returned to the previous level within a few months. On the contrary, the other classes remained stable before and after cluster B PD diagnosis. Figure 1 also shows the monthly proportion of individuals exposed to different psychotropic medication classes according to age groups. Antidepressants were the most frequently used medications in all age groups, while ADHD medications were mainly used in the youngest group and anxiolytics in the oldest one. An increase in use nearby the time of cluster B PD diagnosis was evident, especially for antidepressants and antipsychotics in all age groups, but particularly marked in younger individuals.
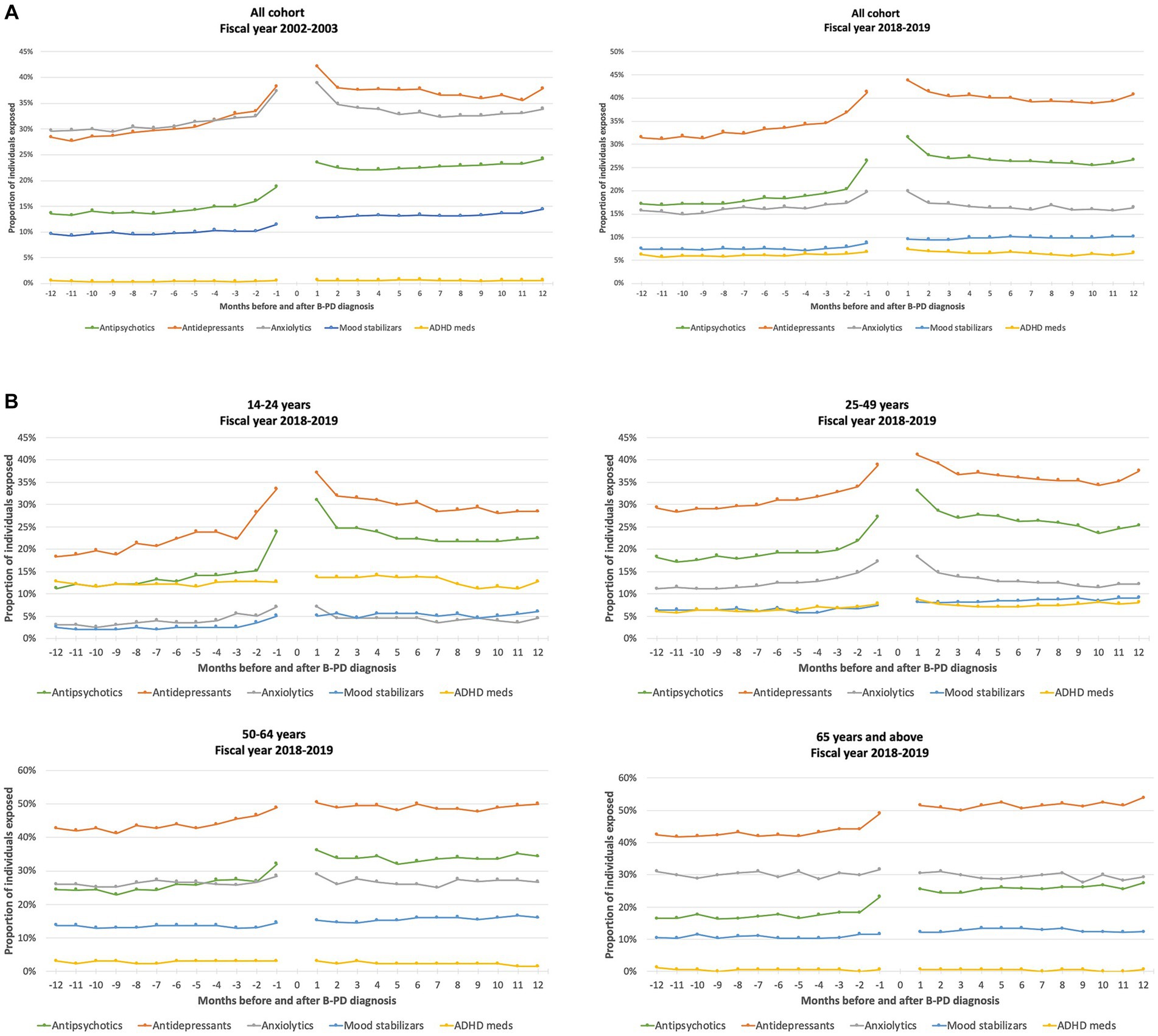
Figure 1. Proportion of individuals exposed to different classes of psychotropic medications in the 12 months before and after a cluster B personality disorder (B-PD) diagnosis, by month (fiscal years 2002–2003 and 2018–2019) (A), and according to the age group, by month (fiscal year 2018–2019) (B).
Figure 2 reports the proportion of individuals exposed to the different psychotropic medication classes according to the cluster B PD diagnosis year. Over the study period, the exposure to antidepressants increased slightly, while the increase in exposure to antipsychotics and ADHD medications was more pronounced. Mood stabilizers and, notably, anxiolytics decreased. As for antipsychotics, the growth was driven by atypical antipsychotics (Supplementary Figure S2).
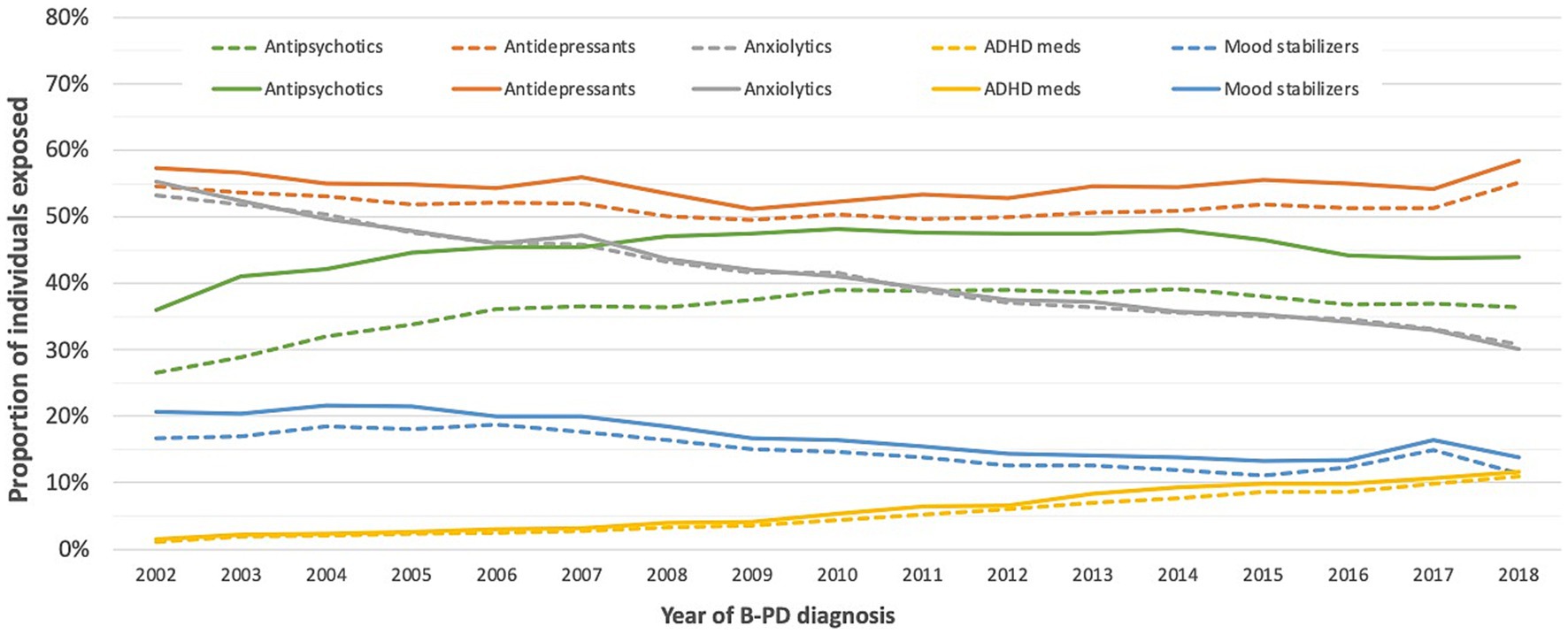
Figure 2. Proportion of individuals exposed to different classes of psychotropic medications in the year before and after a cluster B personality disorder diagnosis.
Sex- and age-adjusted trends over time showed a statistically significant rise in the use of antidepressants (yearly mean change of individuals exposed: +0.51%; 99% CI: +0.36%, +0.67%; p value < 0.0001), antipsychotics (+1.61%; 99% CI: +1.43%, +1.79%; p value < 0.0001), and ADHD medications (+13.1%; 99% CI: +12.5%, +13.8%; p value < 0.0001). On the contrary, anxiolytics showed a sharp decrease (−3.795%; 99% CI: −3.97%, −3.61%; p value < 0.0001), and mood stabilizers a less pronounced one (−2.47%; 99% CI: −2.8%, −2.14%; p value < 0.0001).
Quite a significant proportion of individuals were exposed to combinations of psychotropic medication classes (Figure 3; Supplementary Table S1). The more frequent combination in 2002 (with more than 20% of individuals exposed) was antidepressants and anxiolytics. In 2018, this combination was used by less than 10% of individuals. In recent years, combinations containing ADHD medications have become more frequent. Another more-used combination in 2018 than 2002 was antipsychotics with antidepressants, reaching 15% of usage in 2018.
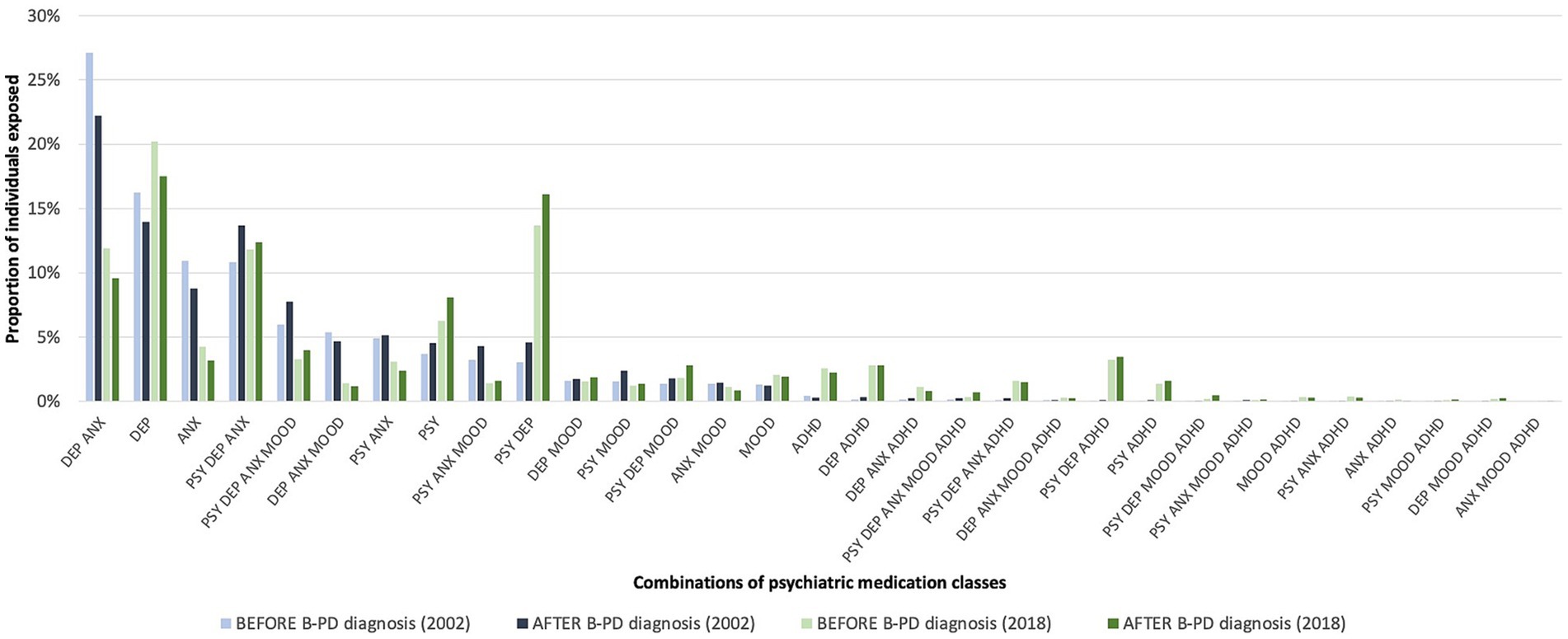
Figure 3. Proportion of individuals exposed to combinations of psychotropic medication classes in the year before and after a cluster B personality disorder (B-PD) diagnosis (2002 vs. 2018).
When analyzing factors associated with antipsychotics, antidepressants, anxiolytics, mood stabilizers, or ADHD medications exposure in the year after cluster B PD diagnosis, we found statistically significant differences according to the medication class for age, sex, social and material deprivation, area of residence and psychiatric comorbidities (Table 2).
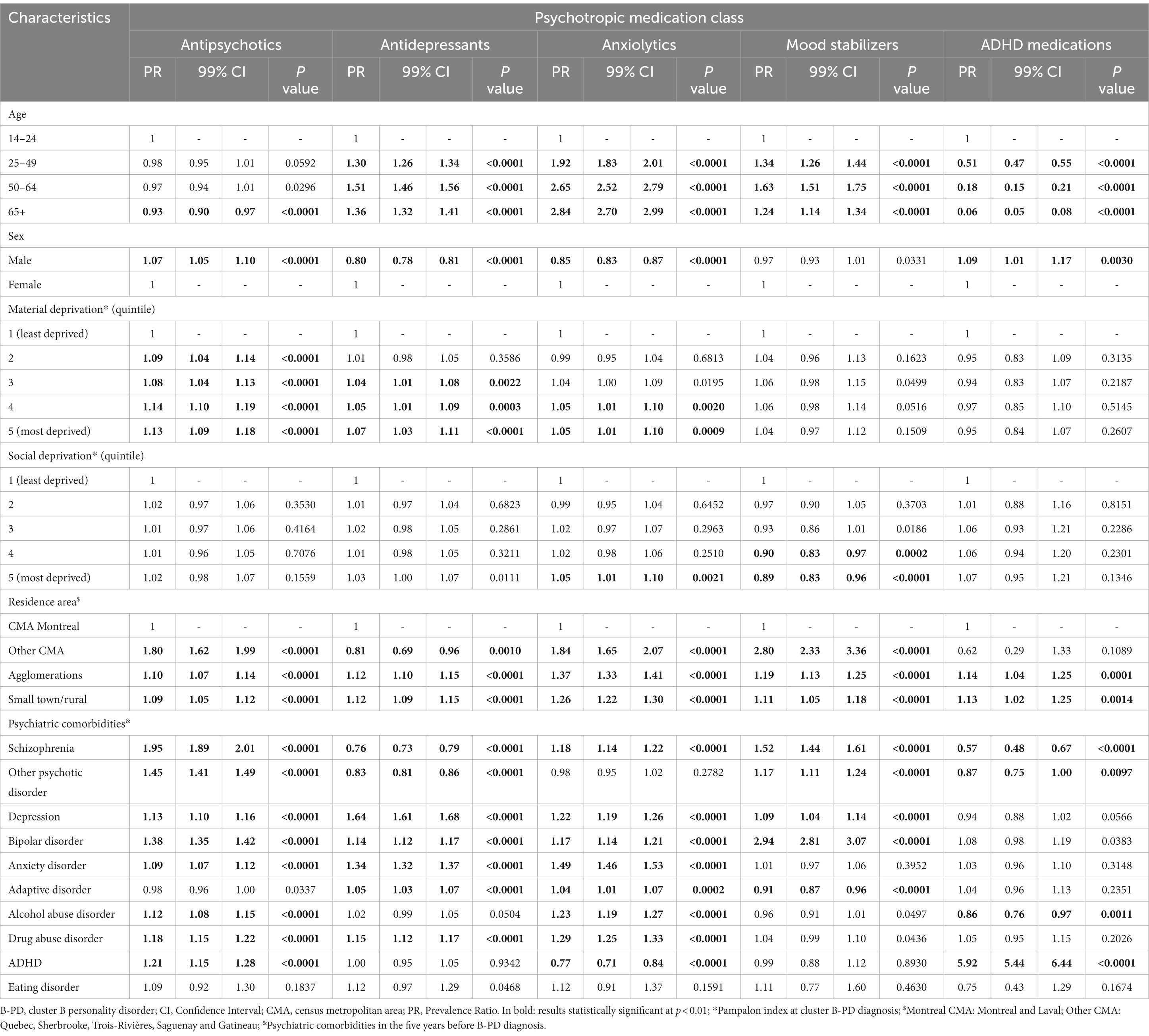
Table 2. Multivariate robust Poisson regression analyses of factors associated with different psychotropic class medication exposure in the year after cluster B personality disorder (PD) diagnosis.
Briefly, we found that age was associated with all the psychotropic classes except antipsychotics, for which only 65 years and older were at lower risk than those 14–24 years old. For anxiolytics, exposure was more likely to occur for older individuals, while for ADHD medications, it happened more frequently for younger individuals. Individuals aged 50 to 64 were more likely to be exposed to antidepressants and mood stabilizers than the youngest. Still, the risk of exposure was generally constantly higher across older age groups.
Being a man was associated with a slightly higher risk of exposure to antipsychotics and ADHD medications (PR: 1.07, 99% CI: 1.05–1.10 and PR: 1.09, 1.01–1.17, respectively) and with a lower risk of exposure to antidepressants and anxiolytics (PR: 0.80, 0.78–0.81 and PR: 0.85, 0.83–0.87, respectively).
Social deprivation was generally not associated with exposure to psychotropics, with few exceptions for anxiolytics and mood stabilizers for the most deprived quintiles. On the contrary, there was a slight increase in the risk of exposure to antidepressants and antipsychotics, and partly to anxiolytics, for increasing material deprivation.
For antipsychotics, the main comorbid conditions associated with a higher risk of exposure were schizophrenia (PR: 1.95), other psychotic disorders (PR: 1.45), bipolar disorders (PR: 1.38), ADHD (PR: 1.21), drug abuse disorders (PR: 1.18), and depression (PR: 1.13). Depression, anxiety disorders, drug abuse disorders, bipolar disorders, and adaptive disorders were associated with an increased risk of exposure to antidepressants, with PRs from 1.64 to 1.05. In contrast, schizophrenia and other psychotic disorders were associated with a lower risk of exposure to this class of medications (PR: 0.76 and PR: 0.83, respectively).
To the best of our knowledge, this is the first study comparing medication use before and after cluster B PD diagnosis in a large cohort of newly diagnosed individuals, with trends and patterns analyzed over a period of 16 years. One of the most striking findings in our study was the extremely high proportion of individuals with cluster B PD receiving psychotropic medications both before and after the first recorded diagnosis, with almost four out of five patients being exposed to at least one psychotropic medication in the year after the diagnosis and a mean 2.8 different psychotropics used. Other studies reported similar high proportions (20–23, 25–31). Moreover, the formal diagnosis of cluster B PD did not seem to reduce the use of psychotropic medications but rather increased the use of some psychotropic medication classes. For those diagnosed with cluster B PD in 2018–2019, the proportion of users remained relatively stable or increased only slightly between 1 year before and after the formal diagnosis for anxiolytics, mood stabilizers, and ADHD medications, with a small peak around the date of the diagnosis for anxiolytics. For antidepressants and antipsychotics, on the contrary, this proportion increased just around the date of the diagnosis and remained higher the entire year afterwards. The peak in the proportion of users around the date of the diagnosis may indicate that the diagnosis of cluster B PD was made after some crisis that required psychotropic medications to control for manifested symptoms, which was a prelude to formal cluster B PD diagnosis.
Nevertheless, while the proportion of users decreased after this crisis period for anxiolytics, it remained stable for antidepressants and, especially, for antipsychotics. Other studies reported extensive antipsychotic utilization, especially atypical, in individuals with PDs (22, 26, 28, 30, 31, 36). While the efficacy of antidepressants seems limited in cluster B PD patients (9), that of antipsychotics, like quetiapine, has been suggested for managing anger, impulsivity and aggressivity (9). This, along with using quetiapine for insomnia, could explain the higher proportion of antipsychotic users after cluster B PD diagnosis (37). The increase in antidepressants and antipsychotic users concurrently with the diagnosis of cluster B PD was especially manifest in younger individuals. Still, it was not neglectable also in older individuals who already had high use. Older individuals were also exposed to anxiolytics in a higher proportion than other age groups, but this was not (or only slightly) affected by cluster B PD diagnosis. It is thus possible that anxiolytics have been used for a long time in older individuals as their use was more prevalent in the past years (38).
Different patterns of psychotropic medication classes use occurred in the last decades. Antidepressants remained the most used medication class, even if their use remained relatively stable over the study period, with only slight differences in the proportion of users depending on the year of diagnosis. Depression and anxiety, the main indications for antidepressant medications (39), were the most frequent comorbid conditions in our sample, with a prevalence of 50.9% for depression and 49.7% for anxiety, often concurrently in the same individual. Nevertheless, no recent clinical trial has been conducted to assess the efficacy of antidepressants in treating cluster B PD (9). Consequently, beyond treating an anxious-depressive comorbid condition, the use of antidepressants for cluster B PDs may have been less frequent or relatively stable.
Antipsychotics showed increased use from 2002 to 2008, remained stable until 2014, and decreased afterwards. In our population, the prevalence of psychotic disorders in the five years before the cluster B PD diagnosis was estimated at 37.5%. For those who were prescribed at least one antipsychotic within a year following their PD diagnosis, the prevalence of schizophrenia, other psychosis, or bipolar disorders was 46.20%. This suggests that more than half of the newly diagnosed PD patients lacked any recorded diagnosis for which an antipsychotic is indicated. Thus, there is a discrepancy between antipsychotic use and recorded diagnoses, which may suggest overuse of medications. However, some differences may result from the lack of diagnoses in the database. Moreover, cluster B PD diagnosis seems to act as a catalyst for antipsychotic prescriptions because of the gap between the proportion of users of these medications before and after the diagnosis. Nonetheless, this gap narrowed slightly in recent years. This pattern could be due to better compliance with clinical guidelines recommending psychotherapy, rather than pharmacotherapy, for cluster B PD treatment and the lack of evidence on antipsychotic treatment efficacy in these individuals (11, 12, 40).
On the other hand, the use of anxiolytics and mood stabilizers has diminished markedly over the study period. The decrease in the utilization of these classes may be related to the increased focus on providing a comprehensive continuum of care for people with PDs. This approach includes psychotherapy, rehabilitation, and all other necessary services across various lines of care (41). Nonetheless, no significant change in the care of patients with mental disorders has been implemented in recent years in Quebec. Moreover, in Quebec, public institutions do not offer coverage for psychotherapy services, leaving residents without personal or work insurance to pay out of pocket for these services. As a result, some individuals cannot access the resources they require, with only a minority of individuals with a mental health problem reporting having sought and received support services (42–44). This significant change could thus be driven by recent clinical guidelines not recommending anxiolytics, such as benzodiazepines, for current mental disorders, with a preference for newer antidepressants (e.g., selective serotonin reuptake inhibitors [SSRIs]) and the consequent change in clinical practice. Indeed, benzodiazepines, the most used anxiolytics, have been associated with injurious falls, fractures, delirium, and long-term cognitive decline, especially in older adults due to their sedative, cognitive-impairing, and motor-impairing effects (45, 46). Benzodiazepines have also been associated in Scandinavian countries’ health administrative databases with the worst health outcomes for schizophrenia, personality disorders, and some substance use disorder (47–50) The decline in the use of anxiolytics has also been reported in older individuals with schizophrenia and in the general aged population in two studies analyzing trends in more than a decade in Quebec (38, 51). Nevertheless, the decline in anxiolytic use we noticed was not compensated for by an equivalent increase in antidepressant use. This suggests that anxiolytics were used more in later years to treat symptomatic aspects of cluster B PDs rather than comorbid anxiety. As for mood stabilizers, their efficacy has not been proven, and their use should be limited to patients with comorbid bipolar disorders (11, 12, 40).
Medications for ADHD showed a constant increase in prescriptions over the study period, from about 1% in 2002–2003 to almost 12% in 2018–2019. This increase aligns with the rise in ADHD diagnosis over the last years in Quebec among children and adults, independently of the diagnosis of cluster B PDs (52, 53). As expected, their use was exceptionally high in younger individuals in recent years, with a proportion of users reaching more than 20% in 2018–2019 (results not shown). Beyond a better recognition of this disorder during the study period, the increased use of ADHD medications could also be due to the favorable risk/benefit profile these medications have shown in clinical practice in reducing ADHD symptoms like hyperactivity and impulsivity (54), with more recent studies also supporting their effectiveness in lowering unintentional injuries (55) and mortality (56), also in PD patients (49).
Trends and patterns in of psychotropic classes combinations first showed that they were more common than single classes. Even if the patterns of combinations changed over time, the proportion of cluster B PD individuals exposed to more than one psychotropic medication only slightly decreased over time (71% in 2002–2003 to 67% in 2018–2019 among users of at least one psychotropic medication). Because of the lack of solid evidence of the effectiveness of psychotropics in cluster B PD individuals, the use of combinations of psychotropic medications raises concerns as they may complexify the therapy of these patients and increase the risk for non-adherence, adverse effects and drug–drug interactions. These concerns are exceptionally elevated in older individuals as they are already at higher risk for multimorbidity and polypharmacy (57–60), which can lead to possible interactions with psychotropic medications, adverse effects and mortality.
Many factors have been associated with exposure to different psychotropic medication classes. Younger age was associated with the use of ADHD medications, which is in line with trends and patterns of diagnosis and treatment of ADHD described in recent studies (61). Age was also associated with exposure to other medication classes, such as antidepressants, antipsychotics, anxiolytics, and mood stabilizers, but in these cases, older individuals were more likely to use them. Generally, those with the higher risk were individuals in the 50–64 years groups, but it was possible to identify a trend for anxiolytics, with an increased risk with increasing age. In a recent study on patients with cluster B PDs, the authors found that older individuals were more likely to use many medications (more elevated than those <65 years) and significantly higher than their peers without cluster B PD diagnosis (62). A cluster B PD diagnosis seems to be a risk factor for receiving many medications, more so than for patients with other psychiatric diagnoses, such as affective disorders (62).
Rather than social deprivation, material deprivation was associated with a higher likelihood of exposure to antipsychotics, antidepressants, and anxiolytics. Our cohort of publicly insured patients was already composed of generally more materially deprived individuals than the general population because, in Quebec, public insurance is offered to those without private employ-related insurance (e.g., unemployed), along with those retired. Nevertheless, the association we found between material deprivation and exposure to psychotropic medications aligns with recent reports for antipsychotic medications in a large retrospective study in the United Kingdom (36). In that study, less deprived individuals with cluster B PDs were less likely to receive antipsychotics than more deprived ones, with adjusted risk ratios between 0.56 and 0.66 (36). More affluent patients may have access to private insurance for psychotherapy since private psychologists are not covered by the public health plan like in the United Kingdom (53).
This study has many strengths. First, it was the first to estimate psychotropic medication use in a large cohort of cluster B PDs individuals throughout the Quebec province. Additionally, the assessment of medication usage prior to and following the initial diagnosis of cluster B personality disorder had not previously been conducted. We thus could find that a not negligible proportion of cluster B PD individuals seemed exposed to psychotropic medications in the period near cluster B PD diagnosis and that this proportion increased after the formal diagnosis. Furthermore, we had access to data spanning nearly two decades, allowing us to analyze trends and patterns in psychotropic medication use. Finally, to identify all the individuals with a registered cluster B personality disorder diagnosis in the QICDSS databases, we used a case definition previously developed through a consensus-leading procedure by a team of experts psychiatrists and psychologists treating PD and using ICD-9 and ICD-10 codes related to DSM symptoms and relevant for clinical practice in Quebec (3).
Nonetheless, the results should be interpreted considering some limitations. Since we used administrative data and claims as a proxy of medication use, we could have overestimated the proportion of people under psychotropic medications. However, our results are consistent with past studies in different countries and settings, suggesting high medication use in cluster B PD individuals. Even in case of an overestimation of medication use, the trends over the 16 years of the study would not have been affected, and the temporal changes observed over time should be accurate. Moreover, the identification of patients with a diagnosis of cluster B PD in the database was based on a case definition using ICD-9 and ICD-10 codes. Since there are no specific ICD codes for cluster B PDs, the codes were chosen through consensus among clinicians. However, the case definition was not formally validated but reflected the consensus reached by a team of experts in the field who treat patients with PDs in Quebec hospitals and clinics. This means that some individuals with cluster A or C may have been misclassified as cluster B PDs due to our definition. Notably, the medico-administrative databases of Quebec, such as QICDSS, are recognized for their high specificity in identifying chronic conditions (33, 63). A form of external validation comes from the important excess mortality we observed in a previous study associated with our definition (3), which is in line with the findings in other countries with comparable linked health administrative databases (64). Moreover, it is worth noting that certain PD patients’ first diagnosis may not have been recorded in the QICDSS because they received it before 1996, the year of developing the QICDSS database, or because they were previously diagnosed in the private sector. To counteract this potential bias, we used a 5-year period to search for diagnosis before the first cohort entry in 2002. Finally, we excluded individuals with private insurance in the two years around cluster B PD diagnosis since the QICDSS database registers only medication use in those under the public drug plan. We cannot thus exclude that medication use would be different in those with private insurance as they are generally less materially deprived and could therefore have better access to psychotherapy in the private sector.
The results of this study indicate that individuals diagnosed with cluster B personality disorders are being prescribed psychotropic medication to a significant extent, especially in the months around the date of diagnosis, which may not be consistent with current best practice recommendations and guidelines. The high use of psychotropic medications may reflect attempts to manage comorbid suicidality and substance use disorders, frequent in cluster B PD patients (3, 5, 6, 65). According to a recent Scandinavian health administrative database study, the long-term benefit of antipsychotics, antidepressants, or benzodiazepines in PD patients was not supported, while the exposure to ADHD medications, clozapine, and bupropion showed a positive effect on these patients (49). A similar and challenging finding was found for patients with methamphetamine use disorder (48), suggesting that the impulsivity dimension present in both PD and substance use disorder (SUD) was regulated by ADHD medication. Medication regulating the craving\sensation seeking dimension of PD and SUD shall also be similarly explored (66, 67). These findings suggest potential challenges to existing guidelines and reflection on the behavioral dimensions of PD and SUD (67), brought by long-term real-life observation made possible by registers of health administrative databases.
We could highlight different trends and patterns in specific psychotropic medication classes over the study period, suggesting essential changes in clinical practice related to certain changes in the management of patients with PDs and more general changes due to better knowledge of psychotropics’ efficacy and safety profiles. Similarly, combinations of psychotropics have been used frequently, with differences in clinical practice during the last sixteen years. In light of these findings, it is vital to conduct further research on the impact of the use of psychotropic medications on health-related outcomes in different healthcare settings, to explore the combination of medications as well as craving\sensation seeking pharmacotherapies.
The datasets presented in this article are not readily available because no permission is granted to use the Quebec Integrated Chronic Diseases Surveillance System (QICDSS) data. This means we will not be able to share data. Requests to access the datasets should be directed to TG91aXMuUm9jaGV0dGVAaW5zcHEucWMuY2E=.
This study is part of the continuous chronic disease surveillance mandate granted to the National Public Health Institute of Quebec (Institut National de Santé Publique du Québec - INSPQ) by the provincial Ministry of Health and Social Services. Ethical approval and written informed consent to participate in this study were not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
AL, CL, and LC conceived the study and developed the protocol. CS, ER, and VM substantially contributed to the development of the methodology. EL and LR performed the statistical analyses. CL and CM wrote the first draft of the manuscript. AL, CS, EL, ER, EV, LC, LR, MK, RB, PD, PV, SR, and VM revised it critically and substantially contributed to the submitted manuscript. All the authors approved the final version of the manuscript.
The authors received no financial support for the research activity related to this study nor any funding for the authorship or the publication of this article.
CS is the recipient of a Junior 2 salary grant from the Fonds de recherche du Québec Santé.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1243511/full#supplementary-material
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Virginia: American Psychiatric Association (2013).
2. Volkert, J, Gablonski, TC, and Rabung, S. Prevalence of personality disorders in the general adult population in Western countries: systematic review and meta-analysis. Br J Psychiatry. (2018) 213:709–15. doi: 10.1192/bjp.2018.202
3. Cailhol, L, Pelletier, É, Rochette, L, Laporte, L, David, P, Villeneuve, É, et al. Prevalence, mortality, and health care use among patients with cluster B personality disorders clinically diagnosed in Quebec: a provincial cohort study, 2001-2012. Can J Psychiatr. (2017) 62:336–42. doi: 10.1177/0706743717700818
4. Cailhol, L, Pelletier, É, Rochette, L, Renaud, S, Koch, M, David, P, et al. Utilization of health care services by patients with cluster B personality disorders or schizophrenia. Psychiatr Serv Wash DC. (2021) 72:1392–9. doi: 10.1176/appi.ps.202000554
5. Trull, TJ, Freeman, LK, Vebares, TJ, Choate, AM, Helle, AC, and Wycoff, AM. Borderline personality disorder and substance use disorders: an updated review. Borderline Pers Disord Emot Dysregulation. (2018) 5:15. doi: 10.1186/s40479-018-0093-9
6. Temes, CM, Frankenburg, FR, Fitzmaurice, GM, and Zanarini, MC. Deaths by suicide and other causes among patients with borderline personality disorder and personality-disordered comparison subjects over 24 years of prospective follow-up. J Clin Psychiatry. (2019) 80:12436. doi: 10.4088/JCP.18m12436
7. Paris, J. Suicidality in borderline personality disorder. Medicina. (2019) 55:223. doi: 10.3390/medicina55060223
8. Ducasse, D, Lopez-Castroman, J, Dassa, D, Brand-Arpon, V, Dupuy-Maurin, K, Lacourt, L, et al. Exploring the boundaries between borderline personality disorder and suicidal behavior disorder. Eur Arch Psychiatry Clin Neurosci. (2020) 270:959–67. doi: 10.1007/s00406-019-00980-8
9. Bozzatello, P, Rocca, P, De Rosa, ML, and Bellino, S. Current and emerging medications for borderline personality disorder: is pharmacotherapy alone enough? Expert Opin Pharmacother. (2020) 21:47–61. doi: 10.1080/14656566.2019.1686482
10. Gartlehner, G, Crotty, K, Kennedy, S, Edlund, MJ, Ali, R, Siddiqui, M, et al. Pharmacological treatments for borderline personality disorder: a systematic review and meta-analysis. CNS Drugs. (2021) 35:1053–67. doi: 10.1007/s40263-021-00855-4
11. National Institute for Health and Clinical Excellence (NICE). (2009). Borderline personality disorder: Recognition and management. Available at: https://www.nice.org.uk/guidance/cg78
12. Simonsen, S, Bateman, A, Bohus, M, Dalewijk, HJ, Doering, S, Kaera, A, et al. European guidelines for personality disorders: past, present and future. Borderline Pers Disord Emot Dysregulat. (2019) 6:9. doi: 10.1186/s40479-019-0106-3
13. Wong, NZY, Barnett, P, Rains, LS, Johnson, S, and Billings, J. Evaluation of international guidance for the community treatment of ‘personality disorders’: a systematic review. PLoS One. (2023) 18:e0264239. doi: 10.1371/journal.pone.0264239
14. Stoffers, J, Völlm, BA, Rücker, G, Timmer, A, Huband, N, Lieb, K, et al. Pharmacological interventions for borderline personality disorder. Cochrane Database Syst Rev. (2010) Jun 16:CD005653. doi: 10.1002/14651858.CD005653.pub2
15. Stoffers, JM, and Lieb, K. Pharmacotherapy for borderline personality disorder--current evidence and recent trends. Curr Psychiatry Rep. (2015) 17:534. doi: 10.1007/s11920-014-0534-0
16. Stoffers-Winterling, J, Storebø, OJ, and Lieb, K. Pharmacotherapy for borderline personality disorder: an update of published, unpublished and ongoing studies. Curr Psychiatry Rep. (2020) 22:37. doi: 10.1007/s11920-020-01164-1
17. Khalifa, NR, Gibbon, S, Völlm, BA, Cheung, NH, and McCarthy, L. Pharmacological interventions for antisocial personality disorder. Cochrane Database Syst Rev. (2020) 9:Cd007667. doi: 10.1002/14651858.CD007667.pub3
18. Stoffers-Winterling, JM, Storebø, OJ, Pereira Ribeiro, J, Kongerslev, MT, Völlm, BA, Mattivi, JT, et al. Pharmacological interventions for people with borderline personality disorder. Cochrane Database Syst Rev. (2022) 2022:12956. doi: 10.1002/14651858.CD012956
19. Cristea, IA, Gentili, C, Cotet, CD, Palomba, D, Barbui, C, and Cuijpers, P. Efficacy of psychotherapies for borderline personality disorder: a systematic review and meta-analysis. JAMA Psychiatry. (2017) 74:319–28. doi: 10.1001/jamapsychiatry.2016.4287
20. Kadra-Scalzo, G, Garland, J, Miller, S, Chang, CK, Fok, M, Hayes, RD, et al. Comparing psychotropic medication prescribing in personality disorder between general mental health and psychological services: retrospective cohort study. BJPsych Open. (2021) 7:e72. doi: 10.1192/bjo.2021.34
21. Knappich, M, Hörz-Sagstetter, S, Schwerthöffer, D, Leucht, S, and Rentrop, M. Pharmacotherapy in the treatment of patients with borderline personality disorder: results of a survey among psychiatrists in private practices. Int Clin Psychopharmacol. (2014) 29:224–8. doi: 10.1097/YIC.0000000000000021
22. Martín-Blanco, A, Ancochea, A, Soler, J, Elices, M, Carmona, C, and Pascual, JC. Changes over the last 15 years in the psychopharmacological management of persons with borderline personality disorder. Acta Psychiatr Scand. (2017) 136:323–31. doi: 10.1111/acps.12767
23. Pascual, JC, Martín-Blanco, A, and Soler, J. Twenty-year trends in the psychopharmacological treatment of outpatients with borderline personality disorder: a cross-sectional naturalistic study in Spain. CNS Drugs. (2021) 35:1023–32. doi: 10.1007/s40263-021-00852-7
24. Alvarez-Tomas, I, Soler, J, Bados, A, Martin-Blanco, A, Elices, M, Carmona, C, et al. Long-term course of borderline personality disorder: a prospective 10-year follow-up study. J Disord. (2017) 31:590–605. doi: 10.1521/pedi_2016_30_269
25. Martinho, E Jr, Fitzmaurice, GM, Frankenburg, FR, and Zanarini, MC. Pro re nata (as needed) psychotropic medication use in patients with borderline personality disorder and subjects with other personality disorders over 14 years of prospective follow-up. J Clin Psychopharmacol. (2014) 34:499–503. doi: 10.1097/JCP.0000000000000132
26. Riffer, F, Farkas, M, Streibl, L, Kaiser, E, and Sprung, M. Psychopharmacological treatment of patients with borderline personality disorder: comparing data from routine clinical care with recommended guidelines. Int J Psychiatry Clin Pract. (2019) 23:178–88. doi: 10.1080/13651501.2019.1576904
27. García-Carmona, JA, Simal-Aguado, J, Campos-Navarro, MP, Valdivia-Muñoz, F, and Galindo-Tovar, A. Off-label use of second-generation antipsychotics in borderline personality disorder: a comparative real-world study among oral and long-acting injectables in Spain. Int Clin Psychopharmacol. (2021) 36:201–7. doi: 10.1097/YIC.0000000000000357
28. Bridler, R, Häberle, A, Müller, ST, Cattapan, K, Grohmann, R, Toto, S, et al. Psychopharmacological treatment of 2195 in-patients with borderline personality disorder: a comparison with other psychiatric disorders. Eur Neuropsychopharmacol. (2015) 25:763–72. doi: 10.1016/j.euroneuro.2015.03.017
29. Crawford, MJ, Kakad, S, Rendel, C, Mansour, NA, Crugel, M, Liu, KW, et al. Medication prescribed to people with personality disorder: the influence of patient factors and treatment setting. Acta Psychiatr Scand. (2011) 124:396–402. doi: 10.1111/j.1600-0447.2011.01728.x
30. Paton, C, Crawford, MJ, Bhatti, SF, Patel, MX, and Barnes, TRE. The use of psychotropic medication in patients with emotionally unstable personality disorder under the care of UK mental health services. J Clin Psychiatry. (2015) 76:e512–8. doi: 10.4088/JCP.14m09228
31. Paolini, E, Mezzetti, FAF, Pierri, F, and Moretti, P. Pharmacological treatment of borderline personality disorder: a retrospective observational study at inpatient unit in Italy. Int J Psychiatry Clin Pract. (2017) 21:75–9. doi: 10.1080/13651501.2016.1235202
32. Régie de l’Assurance Maladie du Québec. Rapport annuel de gestion 2020-2021. Available at: https://www.ramq.gouv.qc.ca/sites/default/files/documents/rapport-annuel-2020-2021.pdf
33. Blais, C, Jean, S, Sirois, C, Rochette, L, Plante, C, Larocque, I, et al. Quebec integrated chronic disease surveillance system (QICDSS), an innovative approach. Chronic Inj Can. (2014) 34:226–35. doi: 10.24095/hpcdp.34.4.06
34. Francke, DE. Uses of AHFS classification system. Am J Hosp Pharm. (1963) 20:119–20. doi: 10.1093/ajhp/20.3.119
35. Pampalon, R, Hamel, D, and Gamache, P. A comparison of individual and area-based socio-economic data for monitoring social inequalities in health. Health Rep. Component of Statistics Canada Catalogue no. 82-003-X. (2009) 20:85–94. Available at: https://www150.statcan.gc.ca/n1/en/pub/82-003-x/2009004/article/11035-eng.pdf?st=wPT3-rYi.
36. Hardoon, S, Hayes, J, Viding, E, McCrory, E, Walters, K, and Osborn, D. Prescribing of antipsychotics among people with recorded personality disorder in primary care: a retrospective nationwide cohort study using the health improvement network primary care database. BMJ Open. (2022) 12:e053943. doi: 10.1136/bmjopen-2021-053943
37. Anderson, SL, and Vande Griend, JP. Quetiapine for insomnia: a review of the literature. Am J Health Syst Pharm. (2014) 71:394–402. doi: 10.2146/ajhp130221
38. Gosselin, E, Simard, M, Lunghi, C, and Sirois, C. Trends in benzodiazepine and alternative hypnotic use in relation with multimorbidity among older adults in Quebec, Canada. Pharmacoepidemiol Drug Saf. (2022) 31:322–33. doi: 10.1002/pds.5383
39. Wong, J, Motulsky, A, Eguale, T, Buckeridge, DL, Abrahamowicz, M, and Tamblyn, R. Treatment indications for antidepressants prescribed in primary Care in Quebec, Canada, 2006-2015. JAMA. (2016) 315:2230–2. doi: 10.1001/jama.2016.3445
40. American Psychiatric Association. Practice guideline for the treatment of patients with borderline personality disorder. Am Psychiatry Assoc Am J Psychiatry. (2001) 158:1–52.
41. Le Corff, C, David, P, Larivière, N, Dahak, J, and Therriault, C. Services et traitements offerts aux personnes ayant un trouble de personnalité limite: état de situation au Québec et perspectives d’avenir. Santé Ment Au Qué. (2022) 47:141–63. doi: 10.7202/1098898ar
42. Statistics Canada. (2003). Canadian community health survey: Mental health and well-being [internet]. Available at: https://www150.statcan.gc.ca/n1/daily-quotidien/030903/dq030903a-eng.htm
43. Roberge, P, Fournier, L, Menear, M, and Duhoux, A. Access to psychotherapy for primary care patients with anxiety disorders. Can Psychol Psychol Can. (2014) 55:60–7. doi: 10.1037/a0036317
44. Cox, DW. Gender differences in professional consultation for a mental health concern: a Canadian population study. Can Psychol Psychol Can. (2014) 55:68–74. doi: 10.1037/a0036296
45. Madhusoodanan, S, and Bogunovic, OJ. Safety of benzodiazepines in the geriatric population. Expert Opin Drug Saf. (2004) 3:485–93. doi: 10.1517/14740338.3.5.485
46. Gress, T, Miller, M, Meadows, C, and Neitch, SM. Benzodiazepine overuse in elders: defining the problem and potential solutions. Cureus. 12:e11042.
47. Heikkinen, M, Taipale, H, Tanskanen, A, Mittendorfer-Rutz, E, Lähteenvuo, M, and Tiihonen, J. Real-world effectiveness of pharmacological treatments of alcohol use disorders in a Swedish nation-wide cohort of 125 556 patients. Addict Abingdon Engl. (2021) 116:1990–8. doi: 10.1111/add.15384
48. Heikkinen, M, Taipale, H, Tanskanen, A, Mittendorfer-Rutz, E, Lähteenvuo, M, and Tiihonen, J. Association of Pharmacological Treatments and Hospitalization and death in individuals with amphetamine use disorders in a Swedish Nationwide cohort of 13 965 patients. JAMA Psychiatry. (2023) 80:31–9. doi: 10.1001/jamapsychiatry.2022.3788
49. Lieslehto, J, Tiihonen, J, Lähteenvuo, M, Mittendorfer-Rutz, E, Tanskanen, A, and Taipale, H. Association of pharmacological treatments and real-world outcomes in borderline personality disorder. Acta Psychiatr Scand. (2023) 147:603–13. doi: 10.1111/acps.13564
50. Tiihonen, J, Mittendorfer-Rutz, E, Torniainen, M, Alexanderson, K, and Tanskanen, A. Mortality and cumulative exposure to antipsychotics, antidepressants, and benzodiazepines in patients with schizophrenia: an observational follow-up study. Am J Psychiatry. (2016) 173:600–6. doi: 10.1176/appi.ajp.2015.15050618
51. Lunghi, C, Rochette, L, Massamba, V, Tardif, I, Ouali, A, and Sirois, C. Psychiatric and non-psychiatric polypharmacy among older adults with schizophrenia: trends from a population-based study between 2000 and 2016. Front Pharmacol. (2023) 14:1080073. doi: 10.3389/fphar.2023.1080073
52. Diallo, FB, Pelletier, É, Vasiliadis, HM, Rochette, L, Vincent, A, Palardy, S, et al. Morbidities and mortality of diagnosed attention deficit hyperactivity disorder (ADHD) over the youth lifespan: a population-based retrospective cohort study. Int J Methods Psychiatr Res. (2022) 31:e1903. doi: 10.1002/mpr.1903
53. Vasiliadis, HM, Diallo, FB, Rochette, L, Smith, M, Langille, D, Lin, E, et al. Temporal trends in the prevalence and incidence of diagnosed ADHD in children and Young adults between 1999 and 2012 in Canada: a data linkage study. Can J Psychiatr. (2017) 62:818–26. doi: 10.1177/0706743717714468
54. Cortese, S, Adamo, N, del Giovane, C, Mohr-Jensen, C, Hayes, AJ, Carucci, S, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. (2018) 5:727–38. doi: 10.1016/S2215-0366(18)30269-4
55. Ruiz-Goikoetxea, M, Cortese, S, Aznarez-Sanado, M, Magallón, S, Alvarez Zallo, N, Luis, EO, et al. Risk of unintentional injuries in children and adolescents with ADHD and the impact of ADHD medications: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2018) 84:63–71. doi: 10.1016/j.neubiorev.2017.11.007
56. Lunghi, C, Vasiliadis, HM, Rahme, E, Rochette, L, Gignac, M, Massamba, V, et al. ADHD medication use and psychiatric comorbidity, trauma and mortality in children and Young adults: a cohort study from Quebec, Canada In: 21st ISoP Annual Meeting “A New Era of Pharmacovigilance: Challenges and Opportunities” 20–23 September 2022 Verona, Italy. Drug Saf. (2022). 45:1277–1278. doi: 10.1007/s40264-022-01219-7
57. Davies, LE, Spiers, G, Kingston, A, Todd, A, Adamson, J, and Hanratty, B. Adverse outcomes of polypharmacy in older people: systematic review of reviews. J Am Med Dir Assoc. (2020) 21:181–7. doi: 10.1016/j.jamda.2019.10.022
58. Franchi, C, Ardoino, I, Ludergnani, M, Cukay, G, Merlino, L, and Nobili, A. Medication adherence in community-dwelling older people exposed to chronic polypharmacy. J Epidemiol Community Health. (2021) 75:854–9. doi: 10.1136/jech-2020-214238
59. Kojima, T, Mizokami, F, and Akishita, M. Geriatric management of older patients with multimorbidity. Geriatr Gerontol Int. (2020) 20:1105–11. doi: 10.1111/ggi.14065
60. Li, Y, Zhang, X, Yang, L, Yang, Y, Qiao, G, Lu, C, et al. Association between polypharmacy and mortality in the older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. (2022) 100:104630. doi: 10.1016/j.archger.2022.104630
61. Raman, SR, Man, KKC, Bahmanyar, S, Berard, A, Bilder, S, Boukhris, T, et al. Trends in attention-deficit hyperactivity disorder medication use: a retrospective observational study using population-based databases. Lancet. Psychiatry. (2018) 5:824–35. doi: 10.1016/S2215-0366(18)30293-1
62. Treagust, N, Sidhom, E, Lewis, J, Denman, C, Knutson, O, and Underwood, BR. The epidemiology and clinical features of personality disorders in later life; a study of secondary care data. Int J Geriatr Psychiatry. (2022) 37. doi: 10.1002/gps.5837
63. Wilchesky, M, Tamblyn, RM, and Huang, A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. (2004) 57:131–41. doi: 10.1016/S0895-4356(03)00246-4
64. Plana-Ripoll, O, Pedersen, CB, Agerbo, E, Holtz, Y, Erlangsen, A, Canudas-Romo, V, et al. A comprehensive analysis of mortality-related health metrics associated with mental disorders: a nationwide, register-based cohort study. Lancet. (2019) 394:1827–35. doi: 10.1016/S0140-6736(19)32316-5
65. Dacosta-Sánchez, D, Díaz-Batanero, C, Fernandez-Calderon, F, and Lozano, ÓM. Impact of cluster B personality disorders in drugs therapeutic community treatment outcomes: a study based on real world data. J Clin Med. (2021) 10:2572. doi: 10.3390/jcm10122572
66. Bahji, A, Bach, P, Danilewitz, M, Crockford, D, Devoe, DJ, el-Guebaly, N, et al. Pharmacotherapies for adults with alcohol use disorders: a systematic review and network meta-analysis. J Addict Med. (2022) 16:630–8. doi: 10.1097/ADM.0000000000000992
Keywords: cluster B personality disorders, borderline personality disorder, histrionic personality disorder, narcissistic personality disorder, antisocial personality disorder, pharmacotherapy, drug utilization, psychiatric medications
Citation: Lunghi C, Cailhol L, Massamba V, Laouan Sidi EA, Sirois C, Rahme E, Rochette L, Renaud S, Villeneuve E, Koch M, Biskin R, Martineau C, Vincent P, David P and Lesage A (2023) Psychotropic medication use pre and post-diagnosis of cluster B personality disorder: a Quebec’s health services register cohort. Front. Psychiatry. 14:1243511. doi: 10.3389/fpsyt.2023.1243511
Received: 20 June 2023; Accepted: 16 October 2023;
Published: 23 November 2023.
Edited by:
Francesca Strappini, University of Bologna, ItalyReviewed by:
Vaios Peritogiannis, Society for the Promotion of Mental Health in Epirus, GreeceCopyright © 2023 Lunghi, Cailhol, Massamba, Laouan Sidi, Sirois, Rahme, Rochette, Renaud, Villeneuve, Koch, Biskin, Martineau, Vincent, David and Lesage. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlotta Lunghi, Y2FybG90dGEubHVuZ2hpQHVuaWJvLml0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.