- 1Department of Psychiatry, The University of Hong Kong, Pok fu Lam, Hong Kong SAR, China
- 2State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, Pok fu Lam, Hong Kong SAR, China
- 3Division of Physiotherapy, Faculty of Medicine and Health Science, Stellenbosch University, Cape Town, South Africa
Introduction: Pharmacological treatment may be effective for treating positive symptoms of schizophrenia; no evidence of clinically significant effects on negative and cognitive symptoms, social and behavioral functioning. This review investigated treatment outcomes of multiple (at least four sessions in 4 weeks) group music therapy sessions adjunct to standard care in inpatients with chronic schizophrenia.
Methods: A systematic review search of five electronic medical and psychological databases conducted using keywords “music therapy” and “schizophrenia” up to December 2021. Screening was performed for published articles on any adjunct multiple group music therapy (four sessions in 4 weeks minimum) adjunct to “treatment as usual” for inpatients with “chronic” schizophrenia. All study outcomes were all included. Risk of bias of all studies was assessed.
Results: 1160 articles were screened, and 13 randomized controlled trials (RCTs) with a total of 1,114 inpatients were included. Ten RCTs reported open group sessions with active structured music making (ASMM) combining passive music listening (PML) and/or active singing, playing instruments, and improvisations while three other studies applied PML only. Four studies reported significant outcomes for both positive and negative symptoms. Ten of the thirteen studies recorded significant improvements in negative symptoms, behavioral and social functioning. Lasting significant effects were found in a longitudinal RCT with 272 samples evaluated unguided pre-recorded PML as a coping method lasting up to six months and similar results found in another two longitudinal RCTs. Secondary outcomes measured cognition, mood, social interest and function, self-care ability, interpersonal relationships, and QoL all showed significant outcomes. The significance level for pre-post intervention and between-group measures ranged from p < 0.001 to p < 0.05. No negative effects were reported in any studies.
Conclusion: Evidence from this review suggests rehabilitation with adjunctive regular PML or combined ASMM in group settings may provide therapeutic engagement, contributing to improvements in social interest and participation. PML is low-cost and non-invasive therapy. Enhancing overall QoL as one type of psychosocial therapy. More rigorous longitudinal studies with larger sample sizes are needed to investigate whether regular long-term individual PML and active group music therapy have the same significant treatment effects as coping and rehabilitation strategies.
1 Introduction
The Global Burden of Disease Study reported that mental disorders affected 125.3 million people in 2019 worldwide, a 56% increase from a previous report in 1990 (1). Although depressive and anxiety disorders have the highest prevalence among mental disorders, schizophrenia is estimated to have doubled from 1 to 2% (2). According to available data, one in seven individuals diagnosed with schizophrenia can experience functional recovery, suggesting that a major treatment objective should not only be symptomatic clinical remission but also improved social and cognitive functions (3). For these reasons, alternative and adjunctive non-pharmacological treatment approaches maybe required to optimize long-term outcomes.
1.1 Description of schizophrenia and standard treatment
Schizophrenia is a pathological and neurodevelopmental mental illness in which a person’s ideas and perceptions are typically detached from reality, significantly affecting their mood and behavior. It is characterized by a unique combination of symptoms and experiences. In clinical practice, positive symptoms include hallucinations, delusions, and disorganized speech and/or behavior, whereas negative symptoms include blunted affect, alogia, avolition, asociality, and anhedonia. The main treatments for patients with schizophrenia have traditionally been pharmacological, including first-generation antipsychotics (FGA), also known as neuroleptics, which were introduced in the 1950s, followed by second-generation antipsychotics (SGA) in the 1980s. FGA and SGA are effective for treating positive symptoms in some patients with schizophrenia. A meta-analysis of 168 randomized placebo-controlled trials investigating existing treatments for the management of negative and cognitive symptoms found that most treatments had non-statistically significant effects and no clinically significant improvement (4). An updated clinical review reported that antipsychotics might worsen negative and cognitive symptoms if taken over time and that side effects range from weight gain, sedation, acute movement disorders, decreased blood pressure with dizziness, and Parkinsonism (5). Long-term neurodevelopmental illness courses that coincide with progressive brain structural changes are well documented. These include enlarged ventricles as a result of loss of gray matter that are related to positive symptoms, whereas loss of the fusiform gyrus and white matter is related to impaired face recognition, negative symptoms, and reduced cortical thickness and neural connectivity. This affects motor control, motor and sensory integration, and spatial attention, which result in gesture deficits, attention impairments, and reduced verbal fluency in addition to a range of cognitive tasks related to short- and long-term memory, decision-making, and emotion processing across phases of the disorder (6), which in turn may affect normal cognitive and behavioral function. Further, discernment of drug-induced side effects of “secondary” negative symptoms from “primary” negative symptoms can be challenging (7).
Patients with chronic schizophrenia are more resistant to drug treatment than those with acute schizophrenia (8), and pharmacological treatment options for negative and cognitive symptoms are limited (4, 9, 10). Long-term antipsychotic treatment-induced structural brain volume reduction, dopamine receptor sensitization, and reduced cognitive function are also associated with relapse and disease progression (11). Clinical study findings have indicated that negative symptoms and cognitive impairment may be important predictors of poor social and occupational performance (12).
Studies have demonstrated both the potential and limitations of FGA and SGA. Antipsychotics have therapeutic effects mainly on positive symptoms, agitation, aggression, and, to some extent, suicidality, as well as relapse prevention treatment (5). The amelioration of negative and cognitive symptoms remains a largely unmet medical need. Owing to strong associations between negative and cognitive symptoms and poor functional outcomes, as demonstrated in a longitudinal first-episode study with a 7-year follow-up (13), a meta-analysis found that negative symptoms were significantly correlated with functional outcome (14) and psychosocial function (15), while another statistical study demonstrated that cognitive function, both positive and negative symptoms, affected over 56% of the variance in quality of life (QoL) of patients with schizophrenia (16). Improvements in QoL and overall functional “recovery” constitute “real-world” therapeutic aims in which both negative and cognitive symptoms are more relevant, as indicated in a clinical review (5). Another recent review informed urgently needed effective interventions for these domains (9, 17).
1.2 Description of illness course
The illness course of schizophrenia is progressive and is usually classified into three phases (prodrome, acute, and chronic) (9). The Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) defined the “acute” phase as the sudden onset of at least one psychotic symptom (s) for a duration of less than 1 month from onset and classified as a “reactive type” with transient psychotic symptoms. This is distinct from the “chronic” phase of schizophrenia with a symptom duration of greater than 2 years since illness onset (18). In clinical practice, the distinction between the “chronic” and “acute” phases of schizophrenia is key in that better prognosis is found for the acute phase compared to chronic schizophrenia (19). In diagnostic manuals for acute schizophrenia, International Classification of Diseases, 10th Revision (ICD-10) are being named and coded brief psychotic disorder (BPD) (code F23) and is same as in the DSM-V (BPD, code 298.8) in which this disorder may or may not be recurrent (20).
1.3 Music as an intervention for schizophrenia
Roederer cited music as a co-product of the development of human language and an essential environmental sensory stimulus for perception, information processing, analysis, storage, and retrieval operations (21). These are essential for voice sound detection, identification, and speech comprehension in brain development. Music has also been recognized as being socially prominent in gatherings of all cultural and religious backgrounds, with activities such as singing, dancing, and generating music extending beyond personal enjoyment to encourage the social good (22). A recent meta-analysis of 18 randomized controlled trials (RCTs) aimed to evaluate the efficacy of adjunct music therapy in patients with schizophrenia demonstrated improved total and negative symptoms, depressive symptoms, and QoL in people with schizophrenia compared with the control group (23).
Music is a complex, polygenic trait. Genome-wide association studies have shown that genes implicated in musicality (musical ability) are associated with psychiatric disorders and neurodegenerative diseases. Music is more than a sociocultural concept, as several genes related to social and cognitive traits have been identified in children with musical abilities (24).
With the advancement of neuroimaging techniques over the past 30 years, researchers have found evidence of how environmental stimuli such as music impact brain activity. The dynamics of brain activity in numerous cortical and subcortical areas have been identified in association with attention, memory, motor functions, semantics, and music syntactic processing, in addition to areas linking emotions, such as the limbic and paralimbic regions, which are still being studied (25–27). Recent discoveries on neural mechanisms specific to music perception and neural population in the human auditory cortex and its pathways suggest that they respond selectively to music, but not to speech or environmental sounds (28). There are further findings in the neural population selective for music with singing (29), including enhanced brain plasticity by selective music listening (30).
Music therapy is a form of psychosocial rehabilitation because of its unique contribution to facilitating self-expression, communication, socialization, social cohesiveness, and psychological and physiological well-being (31). A comprehensive systematic analysis of all RCTs found that music therapy for schizophrenia and schizophrenia-like diseases improves overall health, mental health (particularly negative symptoms), social function, and QoL when compared with conventional care or no treatment (32). Another expert panel study reported a strong consensus (92.3%) that psychosocial interventions are necessary for the functional recovery of people with schizophrenia (33).
A decade-old systematic review of music-based interventions for hospitalized individuals with acute schizophrenia concluded that at least four sessions of structured active musical participation had significant positive effects (34). A more recent systematic review on the influence of music on symptom management and the rehabilitation of patients with schizophrenia concluded that dosage had a greater impact on the effects of music therapy than type and format (35).
Despite encouraging evidence of the positive effects of music therapy for acute schizophrenia, no systematic review has been conducted on the effects of group music therapy with a duration of greater than 4 weeks for individuals with chronic schizophrenia. This systematic review aimed to address the following question:
“What are the treatment effects of regular group music therapy sessions adjunctive to treatment as usual (TAU) in patients hospitalized with chronic schizophrenia?”
2 Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews, 2020 (36). A single researcher (LL) performed all steps.
2.1 Eligibility criteria
2.1.1 Search framework
2.1.1.1 Population
Adult inpatients with chronic schizophrenia; aged ≥18 years; diagnosed with schizophrenia using the DSM-III diagnostic criteria (37), DSM-IV (38), DSM-V (39), ICD-10 (40), or CCMD-2,3 (41) with greater than 2 years of ongoing symptoms’ duration even with medication or therapy. The chronic phase was defined as illness duration of greater than 2 years from initial onset (18).
2.1.1.2 Intervention
Adjunct multiple-guided (minimum four sessions in four weeks) group music therapy.
2.1.1.3 Comparator
TAU.
2.1.1.4 Outcome
Any reported (for instance, both positive and negative symptoms, mood, social interests, function, and QoL).
2.1.2 Inclusion criteria
Any RCT or non-RCT, as appropriate, reporting outcomes of guided music therapy or music-based intervention (active, receptive, or combination) applied to patients with chronic schizophrenia receiving standard care in hospital settings. Music therapy must be delivered in groups guided by professional music therapists; doctors, including psychiatrists, or nurses; psychotherapists; trained research assistants; or researchers. Articles written in English and Chinese were included. Data were obtained from the inception of databases to December 2021.
2.1.3 Exclusion criteria
Articles in non-English or non-Chinese languages or those with music therapy or music intervention mixed with other activities, such as dancing; trials providing individual or single sessions of music therapy; case reports; or series trials were excluded.
2.2 Sources of information and search strategies
PubMed, the Cochrane Library, MEDLINE, EMBASE, and PsychoINFO databases were searched from inception to December 31, 2021. The search terms were “(music therapy)” and “(schizophrenia).”
2.3 Study selection and data extraction
The abstracts and titles of articles were assessed, and potentially relevant studies were screened for the full text. A bespoke MS Excel spreadsheet was constructed to record the extracted information on the study title, authors, study period, study aims, country, study duration, intervention frequency, guided sessions offered and attended, music therapy methodologies and techniques, protocol designs, unique setting characteristics, patient diagnoses, informed consent, sample size, randomization, and allocation procedures.
2.4 Data items
Music therapy characteristics were recorded in terms of frequency, duration, and intervention protocols/formats. Reported outcomes at baseline and after intervention, measurement timepoints, potential confounders, type of analysis, and treatment effects were also recorded.
2.5 Synthesis methods
The method of synthesis was descriptive analysis of reported interventions and outcomes.
3 Results
3.1 Study selection
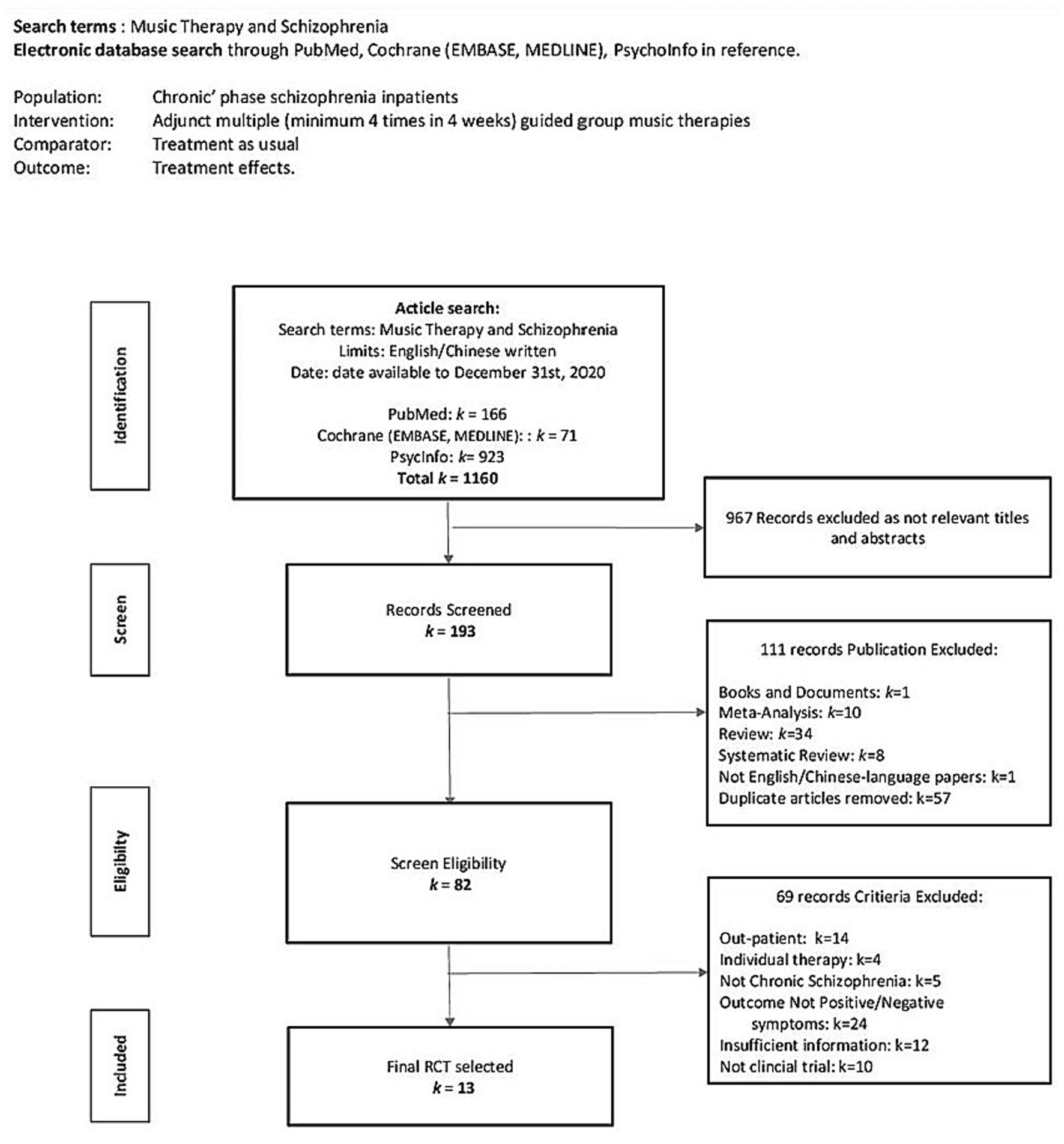
Figure 1 shows the flow diagram of methods used to screen and search the literature. Initially, 1,160 articles were included, of which 967 were excluded because of irrelevant titles and abstracts after the initial screening. On further screening, 111 articles were excluded because they did not meet the inclusion criteria. Diagnosis of acute schizophrenia was excluded because there is no specific definition of the acute phase of schizophrenia spectrum disorder in the ICD-10 diagnostic manual. It was considered whether to include acute schizophrenia as a subgroup. A recent 3-year longitudinal study reported that only 37% of BPD transitioned to schizophrenia; psychotic symptoms were mainly psychosis or positive symptoms and sometimes neurological dysfunction and biological lesions related to substance abuse were reported (42). Furthermore, with the risk of self-harm or suicidal ideation, higher non-adherence, and discontinuation of antipsychotic treatments in patients with acute schizophrenia, the individual might not be sufficiently stabilized in these vulnerable populations and may not be ready for group therapies (43). To reduce the risk of bias, we excluded acute schizophrenia because its treatment strategy is different from that of chronic schizophrenia, which are acute care and usually hospitalization in an emergency psychiatric ward or daycare center for a duration of less than 1 month (44–48); therefore, our primary inclusion criterion of minimum 4 weeks of music intervention period was not met.
One of our main inclusion criteria was the effect of multiple adjunct music therapy of a minimum of four sessions in 4 weeks. We set this minimum dosage (frequency) criterion based on an indication from a recent systematic review on music therapy effects in inpatients with acute schizophrenia, which showed a significant positive effect with more than four sessions of structured active musical participation (34). Another systematic review concluded that dosage had a greater impact on the effect of music therapy compared with music type and format for symptom management and rehabilitation (35). After a clearly defined population, the minimum dosage was determined to be four music therapy sessions in 4 weeks, based on the findings from the above two systematic reviews. Another inclusion criterion was inclusion of RCT and non-RCTs, as appropriate. After exclusion of studies that did not meet our eligibility criteria, the remaining studies were all clinical RCTs.
Four of the included studies had English titles and abstracts, but the main content was in Chinese (49–53) and Korean (54). A free online translation tool for Health Science (55) was used to translate the Korean study. Chinese is the author’s first language. All included studies reported that informed consent was obtained from all participants. Only one trial (52) reported the randomization procedures.
3.2 Risk of bias assessment and reporting
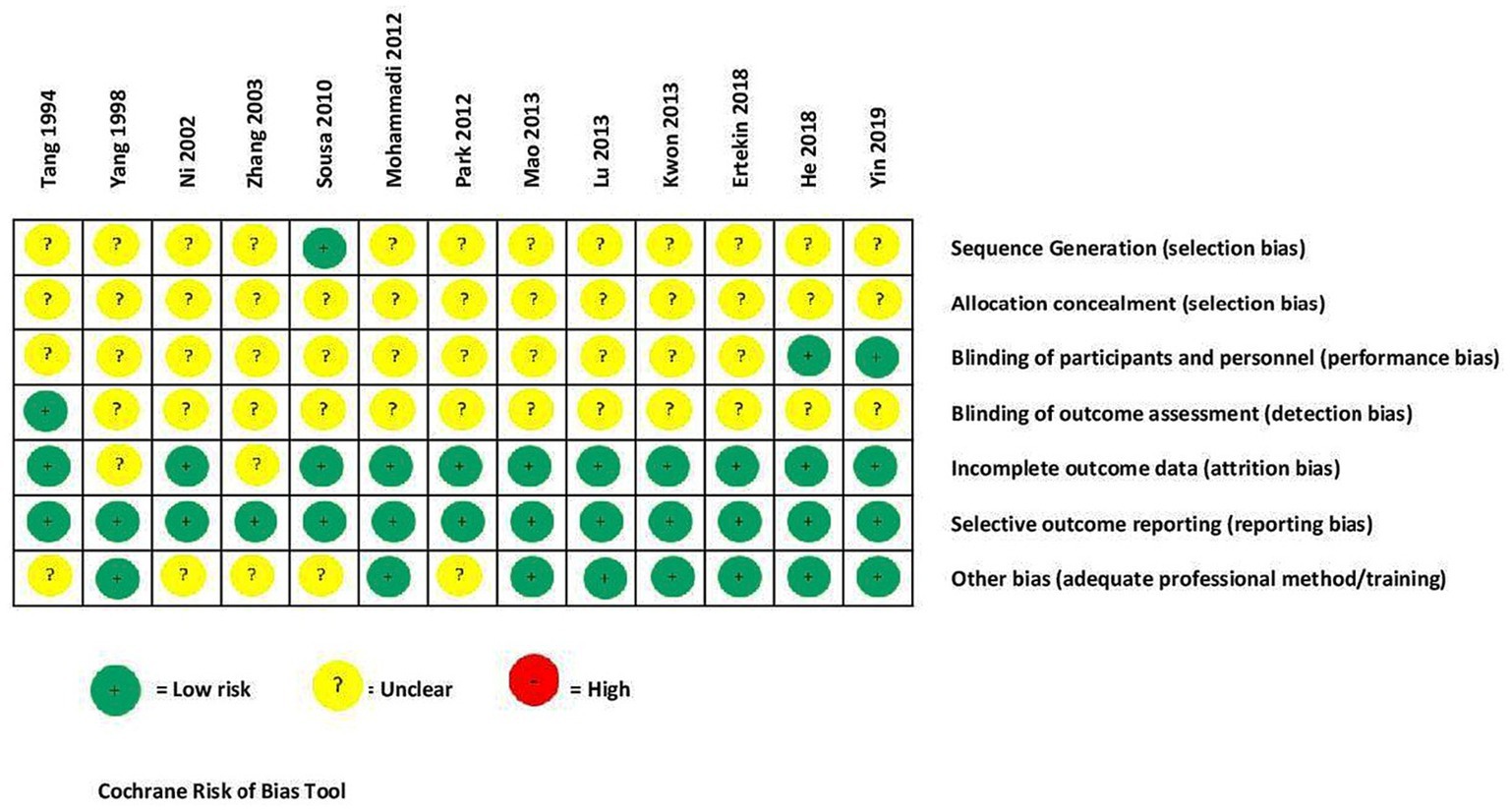
Figure 2 shows the Cochrane risk-of-bias tool (56) was used for bias assessment and reporting. This tool includes seven items: (i) sequence generation, (ii) allocation concealment, (iii) blinding of participants and personnel, (iv) blinding of outcome assessment, (v) blinding of outcome assessment, (vi) incomplete outcome data, (vii) selective outcome reporting, and (vii) other biases.
3.3 Summary of socio-demographic and clinical profiles of study participants
The studies were heterogeneous in sociodemographic profiles, comorbidities, symptom type and severity, illness duration, frequency and length of hospitalization, and medical and family histories.
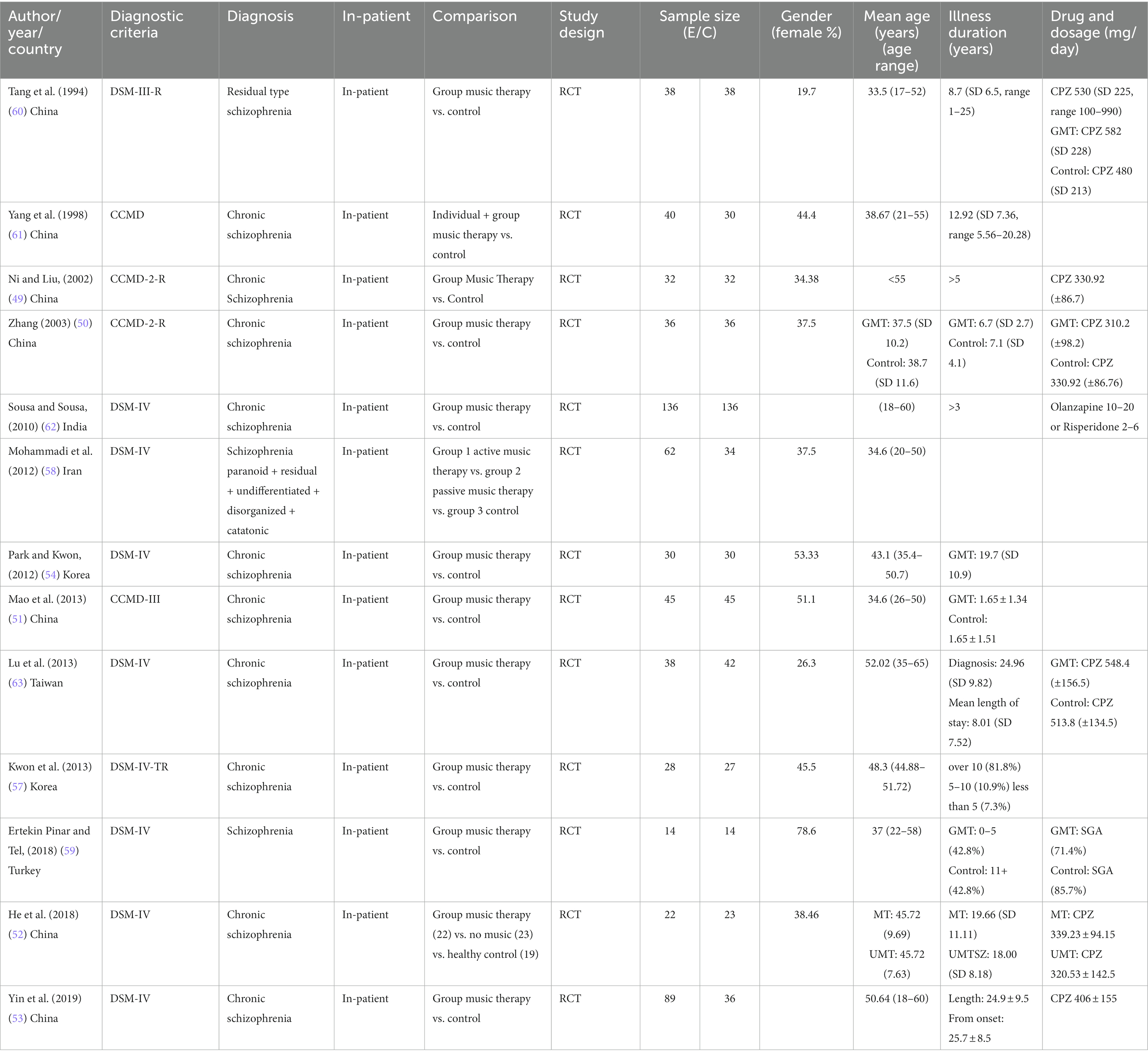
Table 1 is the summary of sociodemographic and clinical characteristics of participants from the included studies. Thirteen trials including 1,114 inpatients were examined. All included studies reported that informed consent was obtained from all participants. The publishing years span from 1990 through 2020. Included studies were conducted in China (49–53, 60, 61), Korea (54, 57), Taiwan (63), Iran (58), India (62), and Turkey (59). The largest sample size was 272 inpatients (62), and the smallest sample size was 28 inpatients (59).
Only eight studies recorded TAU in terms of medication and daily dosage. The mean age in six trials was the mid-30s; three trials, the mid-40s; two trials, the 50s; and two trials, <55 years. The maximum age range was 18–60 years. Two trials (53, 62) did not report the sex ratio, whereas only four trials had 50 ± 10% female participants. The highest sex ratio reported was 78.6% (59) and the lowest was 19.7% (60). Included studies were those with inpatients with chronic schizophrenia with over 2 years since disease diagnosis; however, the disease duration ranged from 1 year to 1–34.78 years, with only seven studies reporting a mean duration of 10–24.84 years (52–54, 57, 59, 61, 63). Six trials (49, 50, 52, 53, 60, 63) reported the medication type, specifically the FGA chloropromazine or equivalent, daily mean dose of >300 mg (SD 80 mg). Two trials (59, 62) reported the use of the SGA olanzapine and risperidone.
3.4 Description of interventions
All trials had specific protocols and reported programs as listed in Table 2; one trial used questions in their music discussion sessions (53) and four trials provided specific activity/content details (53, 54, 57, 58). Three trials (49, 52, 59) used regular passive music listening (PML), whereas one trial (60) added singing to PML. In one trial (58), participants were randomly assigned to one of the three groups: active structured music making (ASMM), PML, or no music therapy as an adjunct to TAU.
The interventions were heterogeneous in structure, session duration and frequency, music type, active improvision methods, and PML. The sessions lasted from 30 to 120 min. The shortest intervention was four sessions in 4 weeks (58), and the longest was 45-min sessions twice daily and 5 days a week for 24 weeks (51). All trials were guided by professional music therapists, psychiatrists, research assistants, or nurses.
In one study (59), music therapy was the only intervention with no specific structure. This allowed participants to engage in PML with their pre-arranged recorded music in MP3 format whenever they had auditory hallucinations as a symptom-coping method. Music types ranged from Western, Chinese, Indian, Turkish, and Korean classical music without lyrics to Taiwanese and Persian pop songs with lyrics for PML. Ten trials included singing in their music therapies (50, 51, 53, 54, 57, 58, 60–63). Four trials provided instruments for participants to play (51, 54, 57, 63), with added improvisation performance (61); two trials added movement (57, 58); and three added songwriting (50, 54, 57).
Four trials added music appreciation through discussions on lyrics, composition, and knowledge (54, 57, 58, 63). Three trials added music games, such as improvised playing concert musical instruments, for inducement of interpersonal relationship; lyrics discussion for positive self-expression (54); singing along with discussion; songwriting; personal and group dancing; and movement improvisations (57). Another form of music appreciation included recitation and adaptation of song lyrics such as “I Believe,” “Invisible Wings,” and, “Starting Again,” and conducting a small chorus to group division and selection of response strategies of different scenarios, etc. (53). Most of these Asian music interventions are structured specifically from song selections, music instruments, rewriting song lyrics, and discussion with specific intentions with varied types, durations, and intents in each session.
3.5 Description of outcome measures
Primary and secondary outcomes were heterogenous; instruments listed in Table 3A.
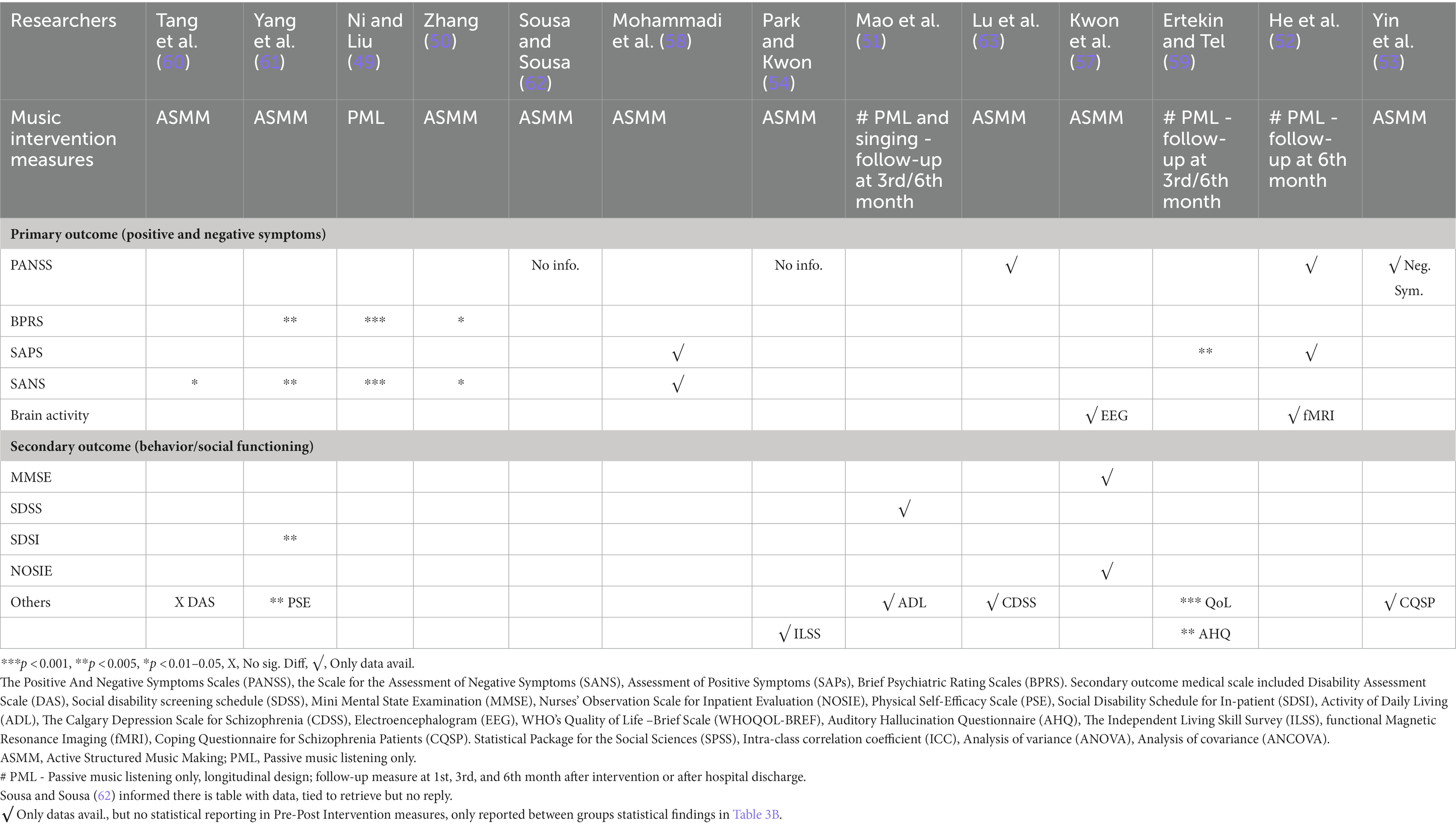
Table 3A. Effect measures of pre-post intervention total scores of both primary and secondary outcomes in experimental group.
Primary outcomes included scores of the Positive and Negative Symptoms Scale (PANSS) (64), Scale for the Assessment of Negative Symptoms (SANS) (65), Scale for the Assessment of Positive Symptoms (SAPS) (66), and Brief Psychiatric Rating Scale (BPRS) (67).
Secondary outcomes measured mental state and social and behavioral changes using the Calgary Depression Scale (68), Nurses’ Observation Scale for Inpatient Evaluation (NOSIE) (69), WHO’s Quality of Life-Brief Scale (WHOQoL-BREF) (70), Depression Anxiety and Stress Scale (DAS) (71), Physical Self-Efficacy Scale (PSE) (72), Social Disability Schedule for Inpatient (SDSI) (61), Social Disability Screening Schedule (SDSS) (73), Activity of Daily Living (74), Mini-Mental State Examination (MMSE) (75), Auditory Hallucination Questionnaire (AHQ) (76), Independent Living Skill Survey (ILSS) (77), and Coping Questionnaire for Schizophrenia Patients (CQSP) (53).
Objective brainwave electroencephalogram (EEG) (78) and functional magnetic resonance imaging (fMRI) (79) measures were also used to record functional brain changes.
3.6 Dropout rate summary
Five studies did not report dropout rates (49–51, 58, 59). In one trial (n = 288), 16 participants (5%) dropped out because of hospital discharge during intervention (62). Yin et al. (53) reported a 12% dropout rate (n = 125) due to hospital discharge, refusal, and other health issues. Lu et al. (63) reported that three participants out of 63 (4.7%) dropped out but provided no reason. One trial (n = 80) reported an 8% dropout rate due to relocation to acute wards and loss to follow-up at post-test and 3 months. Two longitudinal studies with objective measures with EEG and fMRI had higher dropout rates: Kwon et al. (57) reported that 13 participants (19%) dropped out at 7 weeks after intervention, while He et al. (52) reported a 20% dropout rate at 1 month and 31% at 6 months, first due to discharge from hospital, and second due to some patients declining to undergo another fMRI.
3.7 Summary of data analysis results from all studies
Table 3A reports the baseline and post-intervention total scores of both primary and secondary measures in the experimental group. Table 3B reports data analysis of post-intervention outcome total scores between groups after intervention. Trials used heterogenous statistical methods including t-test, chi-square test, analysis of variance, and analysis of covariance to describe pre- and post-intervention outcomes and difference between the groups. Data analysis for sub-domains of measures were reported using SANS (49, 50, 60, 61), SAPS, WHOQoL-BREF (59), PANSS (52, 53), EEG (57), and fMRI (52).
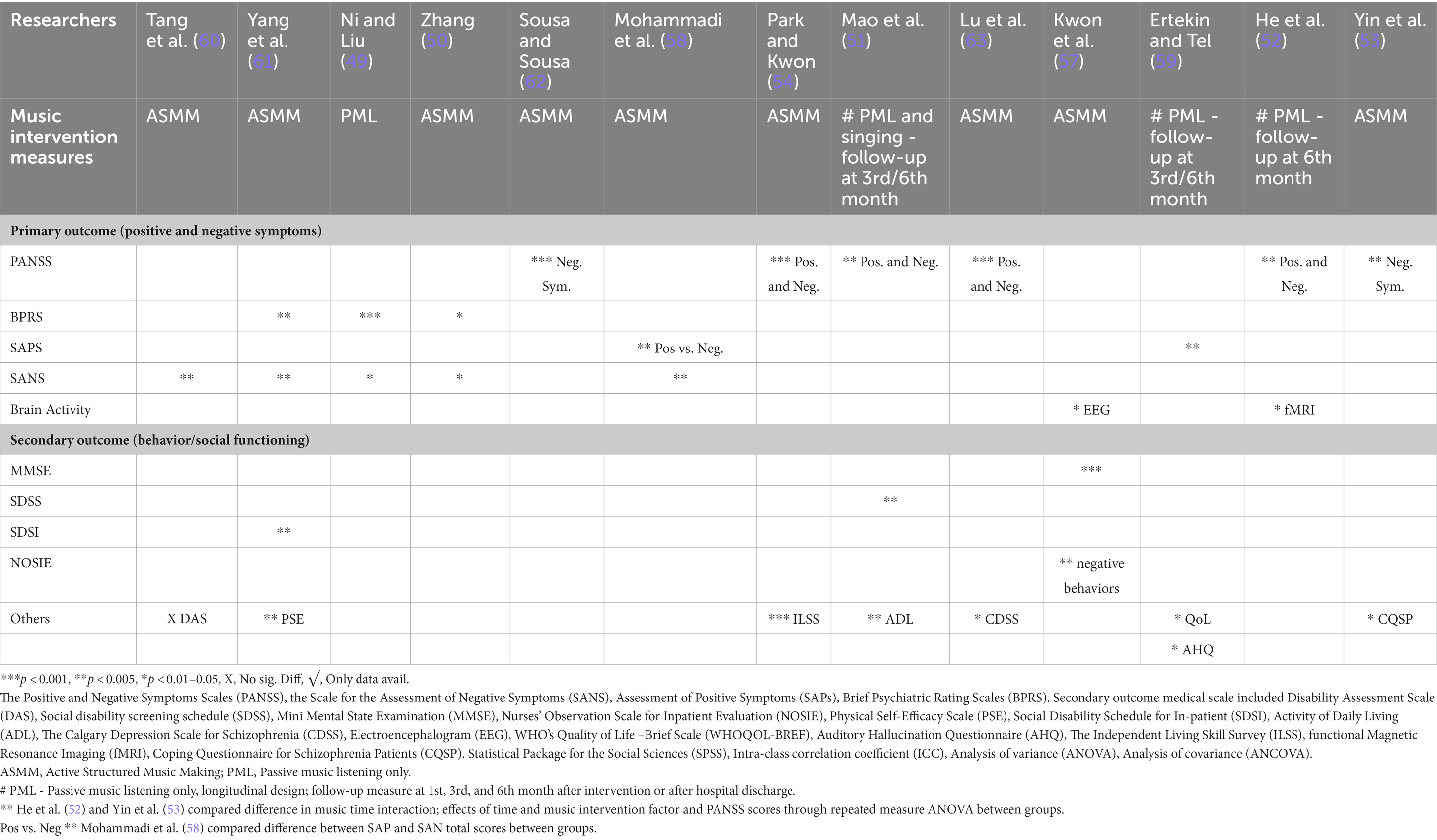
Table 3B. Effect measures of total scores post intervention of both primary and secondary outcomes BETWEEN groups.
3.7.1 Primary outcomes
The most common clinical rating scales used for positive symptoms were the PANSS, SAPS, SANS and BPRS. Only one trial measured EEG as their primary outcome (57, 59) used the AHQ to measure cognitive function and coping with auditory hallucinations. Measurements were taken at baseline, pre-, and post-intervention. All studies reported a significant decrease in total symptom severity (clinical improvement), with p values ranging from <0.001 to 0.05 (see Tables 2, 3A, 3B). One study reported a substantial decrease in verbal and pseudo types of hallucinations in positive symptoms (61), whereas two studies reported a significant decrease in anxiety and lack of energy (49, 61). Two studies (52, 59) applied passive pre-recorded music for PML with little guidance and showed significant results in both primary and sub-domain measures.
For negative symptoms, the common clinical rating scales used were PANSS, SANS, and BPRS in all but three trials (51, 57, 59), while one of these trials did not measure any negative symptoms (59). One study reported a significant improvement in attention deficit after 1 month (60). Significant speech and initiative improvement were recorded in one trial (61). Two studies (49, 50) reported significant improvements in blunted affect, avolition, and interest in external events.
3.7.2 Secondary outcomes
Secondary outcomes measured were behavioral and social function, mental state, and self-care ability. Four studies (51, 57, 60, 61) used DAS, SDSS, SDSI, and NOSIE measures. The SDSS was used in one trial (51). Problem solving and cognitive adjustment domains were reported using the CQSP in one study after 12 weeks, and the ILSS was used by another trial (54).
One study showed alpha brainwave activity in test participants at eight sites more than in controls, where the experimental group had significant increases in cognitive function and decrease in negative behavior (57). It also measured participants’ mental state using the MMSE and observations by nurses on inpatients’ social interest and competence, personal neatness, and mood states using the NOSIE. Another study found that even 6 months after baseline, improvement was observed in neural connectivity function in the dorsal anterior insula and posterior insular networks in the insular cortex, resulting in psychiatric symptom improvement by normalizing the salience and sensorimotor networks. For more details, see Tables 2, 3A, 3B.
3.7.3 Longitudinal effects in patients after multiple group music therapy
Three trials (51, 52, 59) reported follow-up measures at 1, 3, and 6 months after completion of intervention. One of these trials reported significant findings 6 months after hospital discharge in physical, mental, environmental, QoL, and national domains.
Furthermore, the studies primarily measured positive symptoms only and followed up using the AHQ to assess coping effects; the participants only listened to pre-recorded music (PML) whenever they had auditory disturbances within the total experiment duration of 24 weeks. After hospital discharge, nearly 80% of patients in the experimental group still had occasional auditory hallucinations and continued to listen to music; symptoms reduced to almost half from the first month up to the sixth month (59). One trial had music therapy sessions (PML with singing) of 45-min duration for 5 days a week in the morning and evening for 6 months, with a total of 240 sessions. Baseline, 3-month, and 6-month measures were recorded for symptoms, ADL, and social disability screening; significant findings were found in the total scores of all three scales at p < 0.05 (51). Another trial studied neural connectivity and clinical symptoms in schizophrenia and found significant findings in predicting symptom remission in response to daily 30 min (p < 0.01). With PML to Mozart after 1 month; non-significant findings were observed at 1-month after intervention, which vanished after 6 months (52).
3.7.4 Overall outcomes
Overall, there were no significant negative findings in any of the trials, and only one study reported no significant differences in DAS measures (60). Seven trials provided data measuring between pre-post intervention in both groups (Table 3A), but only performed statistical calculations and reported findings between groups (Table 3B). Reports of some subscale results might indicate no difference in the pre-post group music therapy. The implications inform engagement in promoting therapeutic relationships. Active involvement in group music therapy, whether PML or ASMM, fosters motivation and volition, management, and alleviation of negative emotions (anxiety, depressed mood, or arousal) in addition to improving both non-verbal and verbal self-expression. In turn, these non-verbal contact with others might elevate social interests and build and improve teamwork, interpersonal relationships, and socialization.
3.8 Assessment of methodological quality
Figure 2 reports the risk-of-bias assessment results. In most studies, there was unclear reporting in sequence generation, allocation concealment, participant and personnel blinding, and outcome assessment. All trials provided detailed descriptions of the outcome data assessment, reporting of outcomes, and data analysis. One included study by Sousa and Sousa indicated four tables with data (sociodemographic, diagnosis of schizophrenia types, PANSS measure scores), but did not respond to our request for these data. Only one trial (62) reported a sealed-envelope method for allocation.
4 Discussion
Although there are several systematic reviews on music therapies for patients with schizophrenia (32, 80, 81), there are none on multiple sessions of group music therapy for inpatients with chronic schizophrenia. This review found promising evidence for multiple sessions of group music therapy as an effective adjunct treatment to TAU, resulting in greater improvements in both positive and negative symptoms and behavioral and social function, which may contribute to improved QoL and functional recovery.
4.1 Summary of main findings
Music therapy as an adjunct to standard treatment may produce significantly enhanced treatment effects in patients with chronic schizophrenia compared with TAU for both positive and negative symptoms. On the negative symptoms’ subscale, significant improvements have been reported in blunted affect, attention, avolition, asociality, and anhedonia (50, 51, 53, 54, 58, 60, 61, 63). For behavioral and social function, increased social interest, better conversational ability related to motivation to communicate, and social engagement, and increased energy related to better self-care ability translated to improved QoL, even though only one study measured QoL improvement (51, 57, 59, 60, 61). Mental state measures, including mood, such as depression and anxiety, have also shown significant improvements (49, 50, 57, 63). Two trials employed objective measures of brain activities that correlated improved emotional relaxation with increased joyful emotion (57) as well as cognitive function improvement in attention and language with group music therapy (52). There have been reports that these positive effects might last for 1 month after intervention, but these are not conclusive. PML demonstrated positive treatment effects as a coping method to auditory hallucinations. Longitudinal treatment effects and general symptom management and improvements contribute to better social function and enhanced interpersonal relationships (54, 57, 58, 60, 61).
4.1.1 Strengths
This review is comprehensive, having searched relevant library databases for over 30 years of publications, and included all relevant trials. Despite the heterogeneous symptom severity, confounding factors, delivery of interventions, and measurement of outcomes, the consistently reported significant positive effects on the symptom management of mental and social domains are encouraging. Whether active or passive, music therapy stimulates brain activity, producing significant positive adjunctive treatment effects for all symptoms.
4.1.2 Limitations
The data set was screened, extracted, analyzed, and drafted the manuscript by a single author (LL), which may have contributed to the risk of selection and interpretation bias. Included patients and settings were hospital inpatients, which limited the opportunities for independent raters. Individual studies showed significant positive primary outcomes in positive and or negative symptoms. However, heterogeneity of music interventions (active ASSM and or passive PML), intervention duration and variable sample profiles and sizes, measures constrained to generate combined results. The dropout rates reflect high refusal rates in longitudinal studies. Many trials did not provide training details of therapists or practice experience.
No study reported any psychotherapy or counseling intervention provided for subjects which might also help prevent or alleviate symptoms at onset. No study reported the number of relapses. Compared with antipsychotic medication, adherence to music therapy may be important for ongoing symptom management. Poor adherence can be caused by multiple environmental, psychosocial, and economic factors, which result in higher relapse risk, poorer prognosis, longer remission time, higher suicide rates, higher hospitalization rates for individuals (82), and higher costs to the public healthcare system (74). In our systematic review, the participants presumably adhered to the full medication regimen, and the low dropout rates in most studies were potentially due to hospital settings. Further studies are required to test whether participants voluntarily adhere to both pharmacological and music therapies and maintain therapeutic results after hospital discharge.
Ten of the included studies (76.9%) were conducted in developing countries, and all the RCTs were conducted in Asian countries. Therefore, this review may not represent the general population of patients with chronic schizophrenia.
5 Conclusion
This review identified effective objective and subjective measures for symptom reduction and improved psychosocial function. Group music therapy (irrespective of delivery) showed encouraging adjunctive effects compared with TAU in patients with chronic schizophrenia. Music therapy is low-cost, non-invasive, and has no apparent side effects; thus, wider applications for people suffering from schizophrenia are recommended. Rigorous longitudinal study designs with larger sample sizes are suggested to investigate whether regular long-term PML or ASMM and group music therapies have the same significant treatment effects on chronic schizophrenia after hospital discharge.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JL declared a past co-authorship with the author WC to the handling editor.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. IHME. (2014). Global burden of disease (GBD 2019). Institute for Health Metrics and Evaluation. Available at: https://www.healthdata.org/gbd/2019 (Accessed March 17, 2014).
2. GBD 2019 Mental Disorders Collaborators. Global, regional, and National Burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Psychiatry. (2022) 9:137–50. doi: 10.1016/s2215-0366(21)00395-3
3. Silva, MA, and Restrepo, D. Functional recovery in schizophrenia. Revista Colombiana de Psiquiatria (English Ed). (2019) 48:252–60. doi: 10.1016/j.rcp.2017.08.004
4. Fusar-Poli, P, Papanastasiou, E, Stahl, D, Rocchetti, M, Carpenter, W, Shergill, S, et al. Treatments of negative symptoms in schizophrenia: Meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull. (2014) 41:892–9. doi: 10.1093/schbul/sbu170
5. Solmi, M, Murru, A, Pacchiarotti, I, Undurraga, J, Veronese, N, Fornaro, M, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. (2017) 13:757–77. doi: 10.2147/TCRM.S117321
6. DeLisi, LE. The concept of progressive brain change in schizophrenia: implications for understanding schizophrenia. Schizophr Bull. (2007) 34:312–21. doi: 10.1093/schbul/sbm164
7. Kirschner, M, Aleman, A, and Kaiser, S. Secondary negative symptoms - a review of mechanisms, assessment and treatment. Schizophr Res. (2017) 186:29–38. doi: 10.1016/j.schres.2016.05.003
8. Elkis, H, and Buckley, PF. Treatment-Resistant Schizophrenia. Psychiatr Clin N Am. (2016) 39:239–65. doi: 10.1016/j.psc.2016.01.006
9. Kahn, RS, Sommer, IE, Murray, RM, Meyer-Lindenberg, A, Weinberger, DR, Cannon, TD, et al. Schizophrenia. Nat Rev Dis Primers. (2015) 1:15067. doi: 10.1038/nrdp.2015.67
10. Mäkinen, J, Miettunen, J, Isohanni, M, and Koponen, H. Negative symptoms in schizophrenia: a review. Nord J Psychiatry. (2008) 62:334–41. doi: 10.1080/08039480801959307
11. Goff, DC, Peter Falkai,, Fleischhacker, WW, Girgis, RR, Kahn, RM, Uchida, H, et al. The long-term effects of antipsychotic medication on clinical course in schizophrenia. Am J Psychiatr. (2017) 174:840–9. doi: 10.1176/appi.ajp.2017.16091016
12. Grant, PM, and Beck, AT. Defeatist beliefs as a mediator of cognitive impairment, negative symptoms, and functioning in schizophrenia. Schizophr Bull. (2008) 35:798–806. doi: 10.1093/schbul/sbn008
13. Milev, P, Ho, B-C, Arndt, S, and Andreasen, NC. Predictive values of Neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatr. (2005) 162:495–506. doi: 10.1176/appi.ajp.162.3.495
14. Ventura, J, Hellemann, GS, Thames, AD, Koellner, V, and Nuechterlein, KH. Symptoms as mediators of the relationship between Neurocognition and functional outcome in schizophrenia: a Meta-analysis. Schizophr Res. (2009) 113:189–99. doi: 10.1016/j.schres.2009.03.035
15. Hunter, R, and Barry, S. Negative symptoms and psychosocial functioning in schizophrenia: neglected but important targets for treatment. Eur Psychiatry. (2012) 27:432–6. doi: 10.1016/j.eurpsy.2011.02.015
16. Savilla, K, Kettler, L, and Galletly, C. Relationships between cognitive deficits, symptoms and quality of life in schizophrenia. Aust N Z J Psychiatry. (2008) 42:496–504. doi: 10.1080/00048670802050512
17. Veerman, SRT, Schulte, PFJ, and de Hann, L. Treatment for negative symptoms in schizophrenia: a comprehensive review. Drugs. (2017) 77:1423–59. doi: 10.1007/s40265-017-0789-y
18. Appendix: Five Criteria Sets for DSM-IV Field Trials of Schizophrenia and Related Disorders Schizophrenia Bulletin. (1991) 17:143–156. doi: 10.1093/schbul/17.1.143
19. Pillmann, F, Haring, A, Balzuweit, S, Blöink, R, and Marneros, A. The concordance of ICD-10 acute and transient psychosis and DSM-IV brief psychotic disorder. Psychol Med. (2002) 32:525–33. doi: 10.1017/s0033291702005408
20. Castagnini, AC, and Fusar-Poli, P. Diagnostic validity of ICD-10 acute and transient psychotic disorders and DSM-5 brief psychotic disorder. Eur Psychiatry. (2017) 45:104–13. doi: 10.1016/j.eurpsy.2017.05.028
21. Roederer, JG. Introduction to the physics and psychophysics of music. London; New York: Hodder & Stoughton Ltd. (1974).
23. Jia, R, Liang, D, Yu, J, Lu, G, Wang, Z, Zhen, W, et al. The effectiveness of adjunct music therapy for patients with schizophrenia: a Meta-analysis. Psychiatry Res. (2020) 293:113464. doi: 10.1016/j.psychres.2020.113464
24. Mariath, LM, Mauat, A, da Silva, T, Kowalski, W, Gattino, GS, Andrade, G, et al. Music genetics research: association with musicality of a polymorphism in the AVPR1A gene. Genet Mol Biol. (2017) 40:421–9. doi: 10.1590/1678-4685-gmb-2016-0021
25. Popescu, M, Otsuka, A, and Ioannides, AA. Dynamics of brain activity in motor and frontal cortical areas during music listening: a magnetoencephalographic study. NeuroImage. (2004) 21:1622–38. doi: 10.1016/j.neuroimage.2003.11.002
26. Koelsch, S, Kasper, E, Sammler, D, Schulze, K, Gunter, T, and Friederici, AD. Music, language and meaning: brain signatures of semantic processing. Nat Neurosci. (2004) 7:302–7. doi: 10.1038/nn1197
27. Koelsch, S, Fritz, T, Cramon, DY, Müller, K, and Friederici, AD. Investigating emotion with music: an FMRI study. Hum Brain Mapp. (2006) 27:239–50. doi: 10.1002/hbm.20180
28. Norman-Haignere, S, Kanwisher, NG, and McDermott, JH. Distinct cortical pathways for music and speech revealed by hypothesis-free voxel decomposition. Neuron. (2015) 88:1281–96. doi: 10.1016/j.neuron.2015.11.035
29. Norman-Haignere, SV, Feather, J, Boebinger, D, Brunner, P, Ritaccio, A, McDermott, JH, et al. A neural population selective for song in human auditory cortex. Curr Biol. (2022) 32:1454–5. doi: 10.1016/j.cub.2022.03.016
30. Hensch, TK, and Bilmoria, PM. Re-opening windows. Dana Foundation. (2012) Available at: https://www.dana.org/article/re-opening-windows/
31. Oren, R, Orkibi, H, Elefant, C, and Salomon-Gimmon, M. Arts-based psychiatric rehabilitation programs in the community: perceptions of healthcare professionals. Psychiatr Rehabil J. (2019) 42:41–7. doi: 10.1037/prj0000325
32. Geretsegger, M, Mössler, KA, Bieleninik, Ł, Chen, X-J, Heldal, TO, and Gold, C. Music therapy for people with schizophrenia and schizophrenia-like disorders. Cochrane Database Syst Rev. (2017) 2017:CD004025. doi: 10.1002/14651858.cd004025.pub4
33. Lahera, G, Gálvez, JL, Sánchez, P, Miguel Martínez-Roig, JV, Pérez-Fuster, PG-P, Herrera, B, et al. Functional recovery in patients with schizophrenia: recommendations from a panel of experts. BMC Psychiatry. (2018) 18:176. doi: 10.1186/s12888-018-1755-2
34. Carr, C, Odell-Miller, H, and Priebe, S. A systematic review of music therapy practice and outcomes with acute adult psychiatric in-patients. PLoS One. (2013) 8:e70252. doi: 10.1371/journal.pone.0070252
35. Chung, J, and Woods-Giscombe, C. Influence of dosage and type of music therapy in symptom management and rehabilitation for individuals with schizophrenia. Issues Ment Health Nurs. (2016) 37:631–41. doi: 10.1080/01612840.2016.1181125
36. PRISMA. (n.d.). Available at: https://prisma-statement.org/PRISMAStatement/Checklist.aspx.
37. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington DC, USA: American Psychiatric Association (1980).
38. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington DC, USA: American Psychiatric Association (1994).
39. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington DC, USA: American Psychiatric Association (2013).
40. World Health Organization. International statistical classification of disease and related health problems, 10th revision. Geneva: World Health Organization (1992).
41. Wang, SC. Review of the clinical discussion of chronic schizophrenia. Chinese J Neurol Psychiatry. (1987) 14:215.
42. López-Díaz, Á, Ayesa-Arriola, R, Ortíz-García, V, de la Foz, P, Suárez-Pinilla, ML, Ramírez-Bonilla, JV-B, et al. Predictors of diagnostic stability in brief psychotic disorders: findings from a 3-year longitudinal study. Acta Psychiatr Scand. (2021) 144:578–88. doi: 10.1111/acps.13364
43. Boaz, TL, Becker, MA, Andel, R, Van Dorn, RA, Choi, J, and Sikirica, M. Risk factors for early readmission to acute Care for Persons with schizophrenia taking antipsychotic medications. Psychiatr Serv. (2013) 64:1225–9. doi: 10.1176/appi.ps.003382012
44. Ulrich, G, Houtmans, T, and Gold, C. The additional therapeutic effect of group music therapy for schizophrenic patients: a randomized study. Acta Psychiatr Scand. (2007) 116:362–70. doi: 10.1111/j.1600-0447.2007.01073.x
45. Peng, S-M, Koo, M, and Kuo, J-C. Effect of group music activity as an adjunctive therapy on psychotic symptoms in patients with acute schizophrenia. Arch Psychiatr Nurs. (2010) 24:429–34. doi: 10.1016/j.apnu.2010.04.001
46. Volpe, U, Gianoglio, C, Autiero, L, Marino, ML, Facchini, D, Mucci, A, et al. Acute effects of music therapy in subjects with psychosis during inpatient treatment. Psychiatry. (2018) 81:218–27. doi: 10.1080/00332747.2018.1502559
47. Lee, K, Lee, KJ, and Cho, JM. Effect of Korean folk music intervention on schizophrenia inpatients’ emotional behavior and interpersonal relationship functioning. Arch Psychiatr Nurs. (2020) 34:115–21. doi: 10.1016/j.apnu.2020.02.002
48. Jeyan, A, Indu, NV, Sivaprakash, B, and Banipreet, K. Effect of adjunctive music listening in schizophrenia – a randomized controlled trial. Kerala J Psychiatry. (2022) 35:316–6. doi: 10.30834/kjp.35.1.2022.316
49. Ni, J, and Liu, Y. Review of analysis of music therapy effect of patient with chronic schizophrenia [Yin Yue Liao fa Zhi Liao Jing Shen fen lie Zheng Liao Xiao fen xi]. Health Psychol J. (2002) 10:145–6. Available at: http://yyws.alljournals.cn/view_abstract.aspx?pcid=A9DB1C13C87CE289EA38239A9433C9DC&cid=0AAA7D21481497F8&jid=F74CF06C9D955FD84AF6C8A7E932D38E&aid=6D24BE715E823871&yid=C3ACC247184A22C1&vid=F3090AE9B60B7ED1&iid=0B39A22176CE99FB&sid=769BD58726D66E7D&eid=2922B27A3177030F&referenced_num=%E6%80%BB%E8%A2%AB%E5%BC%95%EF%BC%9A6%EF%BC%8C%E8%87%AA%E5%BC%95%EF%BC%9A0%EF%BC%8C%E4%BB%96%E5%BC%95%EF%BC%9A6
50. Zhang, ML. Review of the effect of active and receptive music therapy on patients with chronic schizophrenia [Zhu Bei dong Yin Yue Zhi Lio dui man Xing Jing Shen fen lie Zheng Liao Xiao fen xi]. Health Psychol J. (2003) 11:370–1. Available at: http://yyws.alljournals.cn/view_abstract.aspx?pcid=A9DB1C13C87CE289EA38239A9433C9DC&cid=0AAA7D21481497F8&jid=F74CF06C9D955FD84AF6C8A7E932D38E&aid=634037FE4E852040&yid=D43C4A19B2EE3C0A&vid=708DD6B15D2464E8&iid=94C357A881DFC066&sid=0918129209B14F3E&eid=23410D0BDB501DF5&referenced_num=%E6%80%BB%E8%A2%AB%E5%BC%95%EF%BC%9A3%EF%BC%8C%E8%87%AA%E5%BC%95%EF%BC%9A0%EF%BC%8C%E4%BB%96%E5%BC%95%EF%BC%9A3
51. Mao, ZQ, Li, D, Zhang, GF, Cha, ZQ, and Rong, JK. The effects of music therapy on the rehabilitation of patients with chronic schizophrenia [yin yue zhi liao dui man xing jing shen fen lie zheng huan zhe de kang fu xiao guo guan cha]. Chin J Health Psychol. (2013) 21:56–7.
52. He, H, Yang, M, Duan, M, Chen, X, Lai, Y, Yang, X, et al. Music intervention leads to increased insular connectivity and improved clinical symptoms in schizophrenia. Front Neurosci. (2018) 11:744. doi: 10.3389/fnins.2017.00744
53. Yin, Y, Tong, Y, Li, J, Zhang, Y, Liu, L, and Cui, Y. Review of a randomized controlled trial of song techniques on coping style and clinical effect in patients with schizophrenia. [Ge Qu Zhi Liao Ji Shu dui Jing Shen fen lie Zheng Huan Zhe Ying dui fang Shi Ji Zheng Zhuang Gai Shan Zuo Yong de sui Ji dui Zhao Shi Yan]. Chin Ment Health J. (2019) 33:260–6. Available at: https://cglhub.com/auto/db/detail.aspx?db=950001&rid=16750922&agfi=0&cls=0&uni=True&cid=0&showgp=True&prec=False&md=152&pd=208&msd=152&psd=208&mdd=152&pdd=208&count=10&reds=saker
54. Park, YS, and Kwon, Y. Effects of group music therapy on psychiatric symptoms and interpersonal relationship in patients with schizophrenia. Korean J Rehabil Nurs. (2012) 15:126–32. doi: 10.7587/kjrehn.2012.126
55. Prentice, FM, and Kinden, CE. Paraphrasing tools, language translation tools and plagiarism: an exploratory study. Int J Educ Integr. (2018) 14:11. doi: 10.1007/s40979-018-0036-7
56. Risk of Bias Tools - Archive: RoB 2.0. (2016). Sites.google.com . Available at: https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/archive-rob-2-0-2016
57. Kwon, M, Gang, M, and Kyongok, O. Effect of the group music therapy on brain wave, behavior, and cognitive function among patients with chronic schizophrenia. Asian Nurs Res. (2013) 7:168–74. doi: 10.1016/j.anr.2013.09.005
58. Mohammadi, A, Minhas, LS, Haidari, M, and Panah, FM. Review of a study of the effects of music therapy on negative and positive symptoms in schizophrenic patients. German J Psychiatry. (2012) 15:52–62.
59. Ertekin Pinar, S RN, PhD, and Tel, H RN, PhD. The effect of music on auditory hallucination and quality of life in schizophrenic patients: a randomised controlled trial. Issues Ment Health Nurs. (2018) 40:50–7. doi: 10.1080/01612840.2018.1463324
60. Tang, W, Yao, X, and Zheng, Z. Rehabilitative effect of music therapy for residual schizophrenia. Br J Psychiatry. (1994) 165:38–44. doi: 10.1192/s0007125000292969
61. Yang, WY, Li, Z, Weng, YZ, Zang, HY, and Ma, B. Review of psychosocial rehabilitation effects of music therapy in chronic schizophrenia. Hong Kong J Psychiatry. (1998) 8:38–40. Available at: https://www.easap.asia/index.php/find-issues/past-issue/item/580-9801-p38-40
62. Sousa, A, and Sousa, J. Review of music therapy in chronic schizophrenia. J Pak Psychiatric Soc. (2010) 7:13. Available at: http://www.jpps.com.pk/article/musictherapyinchronicschizophrenia_2394.html
63. Lu, S-F, Lo, C-HK, Sung, H-C, Hsieh, T-C, Shun-Chieh, Y, and Chang, S-C. Effects of group music intervention on psychiatric symptoms and depression in patient with schizophrenia. Complement Ther Med. (2013) 21:682–8. doi: 10.1016/j.ctim.2013.09.002
64. Kay, SR, Fiszbein, A, and Opler, LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. (1987) 13:261–76. doi: 10.1093/schbul/13.2.261
65. Andreasen, NC. The scale for the assessment of negative symptoms (SANS). Lowa City: University of Lowa (1982).
66. Andreasen, NC. Scale for the assessment of positive symptoms (SAPS). Lowa City: University of Lowa (1994).
67. Olivares, JM, Sermon, J, Hemels, M, and Schreiner, A. Definitions and drivers of relapse in patients with schizophrenia: a systematic literature review. Ann General Psychiatry. (2013) 12:32. doi: 10.1186/1744-859x-12-32
68. Addington, D, Addington, J, Maticka-Tyndale, E, and Joyce, J. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. (1992) 6:201–8. doi: 10.1016/0920-9964(92)90003-N
69. Honigfeld, G, and James Klett, C. The nurses’ observation scale for inpatient evaluation. A new scale for measuring improvement in chronic schizophrenia. J Clin Psychol. (1965) 21:65–71. doi: 10.1002/1097-4679(196501)21:1%3C65::aid-jclp2270210122%3E3.0.co;2-i
70. World Health Organization. Measuring quality of life: The development of the World Health Organization quality of life instrument (WHOQOL). Geneva: World Health Organization (1993). 9 p.
71. Lovibond, SH, and Lovibond, PF. Manual for the depression anxiety & stress scales. Sydney, NSW: Psychology Foundation of Australia (1995).
72. Ryckman, RM, Robbins, MA, Thornton, B, and Cantrell, P. Review of development and validation of physical self-efficacy Sale. J Pers Soc Psychol. (1982) 42:891–900. doi: 10.1037/0022-3514.42.5.891
73. Wu, W. Social disability screening schedule-Chinese version In: MY Zhang, editor. Handbook of rating scales in psychiatry. Changsha: Human Science and Technology Press (1998). 163–6.
74. Knapp, M, King, D, Pugner, K, and Lapuerta, P. Review of non-adherence to antipsychotic medication regimens: associations with resource use and costs. Br J Psychiatry. (2004) 184:509–16. doi: 10.1192/bjp.184.6.509
75. Tombaugh, TN, McDowell, I, Kristjansson, B, and Hubley, AM. Mini-mental state examination (MMSE) and the modified MMSE (3MS): a psychometric comparison and normative data. Psychol Assess. (1996) 8:48–59. doi: 10.1037/1040-3590.8.1.48
76. Buffum, MD, Buccheri, R, Trygstad, L, Gerlock, AA, Birmingham, P, Dowling, GA, et al. Behavioral Management of Auditory Hallucinations: implementation and evaluation of a 10-week course. J Psychosoc Nurs Ment Health Serv. (2009) 47:32–40. doi: 10.3928/02793695-20090730-01
77. Wallace, CJ. Functional assessment in rehabilitation. Schizophr Bull. (1986) 12:604–30. doi: 10.1093/schbul/12.4.604
78. NHS. Electroencephalogram (EEG). NHS. (2019) 2019 Available at: https://www.nhs.uk/conditions/electroencephalogram/
79. UC san Diego. What is FMRI? - Center for Functional MRI - UC san Diego. Ucsd.edu. (2019) 2019 Available at: http://fmri.ucsd.edu/Research/whatisfmri.html
80. Mössler, K, Chen, XJ, Heldal, TO, and Gold, C. Music therapy for people with schizophrenia and schizophrenia-like disorders. Cochrane Database Syst Rev. (2011) 5:CD004025. doi: 10.1002/14651858.cd004025.pub3
81. Gold, C, Heldal, TO, Dahle, T, and Wigram, T. Music therapy for schizophrenia or schizophrenia-like illnesses. Cochrane Database Syst Rev. (2005):CD004025. doi: 10.1002/14651858.cd004025.pub2
Keywords: adjunctive therapy, chronic schizophrenia, group music therapy, music-based intervention, psychiatric rehabilitation, psychosocial rehabilitation, coping and rehabilitation
Citation: Lam L, Chang WC and Grimmer K (2023) Treatment effects of adjunct group music therapy in inpatients with chronic schizophrenia: a systematic review. Front. Psychiatry. 14:1215578. doi: 10.3389/fpsyt.2023.1215578
Edited by:
Dennis Kätzel, University of Ulm, GermanyReviewed by:
Jessie Lin, Hong Kong Polytechnic University, Hong Kong SAR, ChinaMassimo Tusconi, University of Cagliari, Italy
Copyright © 2023 Lam, Chang and Grimmer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lissa Lam, bGlzc2FfbGFtQGNvbm5lY3QuaGt1Lmhr
†ORCID: Lissa Lam, http://orcid.org/0009-0000-5004-8983
Wing Chung Chang, http://orcid.org/0000-0002-3581-8895
Karen Grimmer, http://orcid.org/0000-0002-9540-458X
 Lissa Lam
Lissa Lam Wing Chung Chang
Wing Chung Chang Karen Grimmer3†
Karen Grimmer3†