- 1Centre for Healthy Brain Ageing (CHeBA), Discipline of Psychiatry and Mental Health, Faculty of Medicine and Health, University of New South Wales, Sydney, NSW, Australia
- 2The MARCS Institute for Brain, Behaviour and Development, Western Sydney University, Westmead, NSW, Australia
Neurocognitive disorders involves progressive decline in cognition, function, behavior and needs. Recent developments have identified the need to characterize social cognition in individuals with neurocognitive impairments to support uncertainty in clinical decision making, treatment plans and monitoring individual change. Routine social cognition assessments have thus been more recently used and adopted in persons with dementia or mild cognitive impairment. This work serves to summarize current assessments and provide a discourse on the practicality of available social cognition tools, its implication in clinical practice and key future directions. We highlight advantages in establishing validated, multicomponent measures of social cognition for people with neurocognitive disorders.
Introduction
Humans are inherently social beings, and being socially connected with others is a basic human need (1). Yet as we age, we experience a range of physical, cognitive and social changes that can impact our daily functioning and subsequently our wellbeing. In this context, social cognition, which refers to our ability to recognize emotions, social cues, inhibit inappropriate behavior and act appropriately in social situations (2), is a key component of social functioning and can be affected by age-related changes (3). Key social cognitive domains include emotion perception (the ability to identify emotions), theory of mind (the ability to understand others’ mental states) including cognitive theory of mind (understanding others’ thoughts, beliefs or intentions), affective theory of mind (inferring others’ emotional states), empathy (mirroring another’s affective state), and social perception (understanding social cues) (4, 5). Empathy has a cognitive (understanding others’ emotions) and an affective component (feeling what others feel), of which the former overlaps with affective theory of mind, but also includes emotional contagion (unintentional mimicry and synchronizing with others’ emotions) (6, 7).
Social cognitive deficits are a core feature of neurocognitive disorders, which include delirium, mild cognitive impairment (MCI) and dementia, and represent a decline from previous levels of cognitive function (2). Social cognitive deficits in neurocognitive disorders manifest as difficulties with eye contact, turn taking in conversations, social reciprocation, failure to detect social cues in conversations, and making rude or offensive comments (4). Indeed, the DSM-5-TR now states that for a diagnosis of major neurocognitive disorder, a decline must be observed in one of the cognitive domains, such as memory, language, attention, executive function, perceptual-motor function or social cognition (8). For instance, identification of responses requiring interpretation of situational circumstances (i.e., affective theory of mind), but not simple emotions (i.e., emotion perception), is more impaired in people with mild dementia (9).
Early recognition of social cognitive deficits can help identify dementia pathways for individuals, from type [e.g., frontotemporal dementia (FTD) (10)] to progression [e.g., development of behavioral symptoms (11)]. For example, individuals with Alzheimer’s Disease can identify most emotions (e.g., happy, sad, surprise, fear), but have difficulties identifying a range of basic or primary emotions such as disgust or anger in facial expressions, or sarcasm/jokes in conversations (11). There is also evidence for preserved affective empathy in Alzheimer’s Disease and deficits in people with FTD (6) and dementia with Lewy bodies (12). People with Alzheimer’s Disease also show difficulties with identifying some facial emotions (13) and tracking changes in emotions over time (14), There is a lack of research, however, on the differential profiles of social cognitive deficits across the neurocognitive disorders. Routine social cognition assessments can further facilitate appropriate interventions to improve social functioning, with early studies highlighting the potential of psychosocial interventions in strengthening social health for individuals with dementia (14, 15).
Social cognitive skills are needed to maintain social relationships, and vice versa (16). An individual experiencing difficulty reading emotions and/or acting appropriate in social situations may become isolated and lonely. With recent evidence indicating that social isolation is a modifiable risk factor for dementia (17), it is time to consider how we can reliably detect social cognitive deficits in older age and identify changes over time.
Identifying social cognitive deficits continues to be a challenge for multiple reasons. Firstly, current recommendations include a combination of self-report questionnaires, ability based assessments, informant rating, and clinical observation (4), with no standard, or accepted, approach. Secondly, key domains of social cognition (18) typically include broad concepts such as theory of mind, affective empathy, social perception, social behavior (4), and to date, no summary of validated tools has been provided. Furthermore, there are several experimental tasks which are created ad hoc or used primarily in research settings to identify social cognitive deficits. Thus, the majority of extant social cognition measures either have not been rigorously developed or psychometrically validated. To our knowledge, there are few questionnaires or neuropsychological tests which assess social cognitive deficits, as most existing brief and comprehensive cognitive assessments omit the social cognition domain (e.g., Mini Mental State Examination).
Research on the development and validation of social cognition assessments, including measurement of social cognitive deficits, in older adults remains limited. We present some of the existing assessments of social cognition developed for use with older adults experiencing neurocognitive disorders. This information can be used to systematically identify social cognitive deficits, understand the social functioning of older adults with neurocognitive disorders and ensure consistency and standardization in the assessment process.
Overview of social cognition assessments
Thirteen social cognition assessment tools for older adults with neurocognitive disorders (including MCI) are discussed (Table 1). Administration time ranged from 7 min [e.g., Pitfall Intention Explanation task, Pitfall task (12)] to 90 min [e.g., The Awareness of Social Inference Test, TASIT, (24)]. Commonly used assessments were the Interpersonal Reactivity Index (IRI; found in six studies) and TASIT (24) was identified in four studies that assess for social cognition in individuals with neurcognitive disorders. Other scales such as 16-item Toronto Empathy Questionnaire (44) and the 60-item Empathy Quotient (45) have not been used with people with neurocognitive disorders.
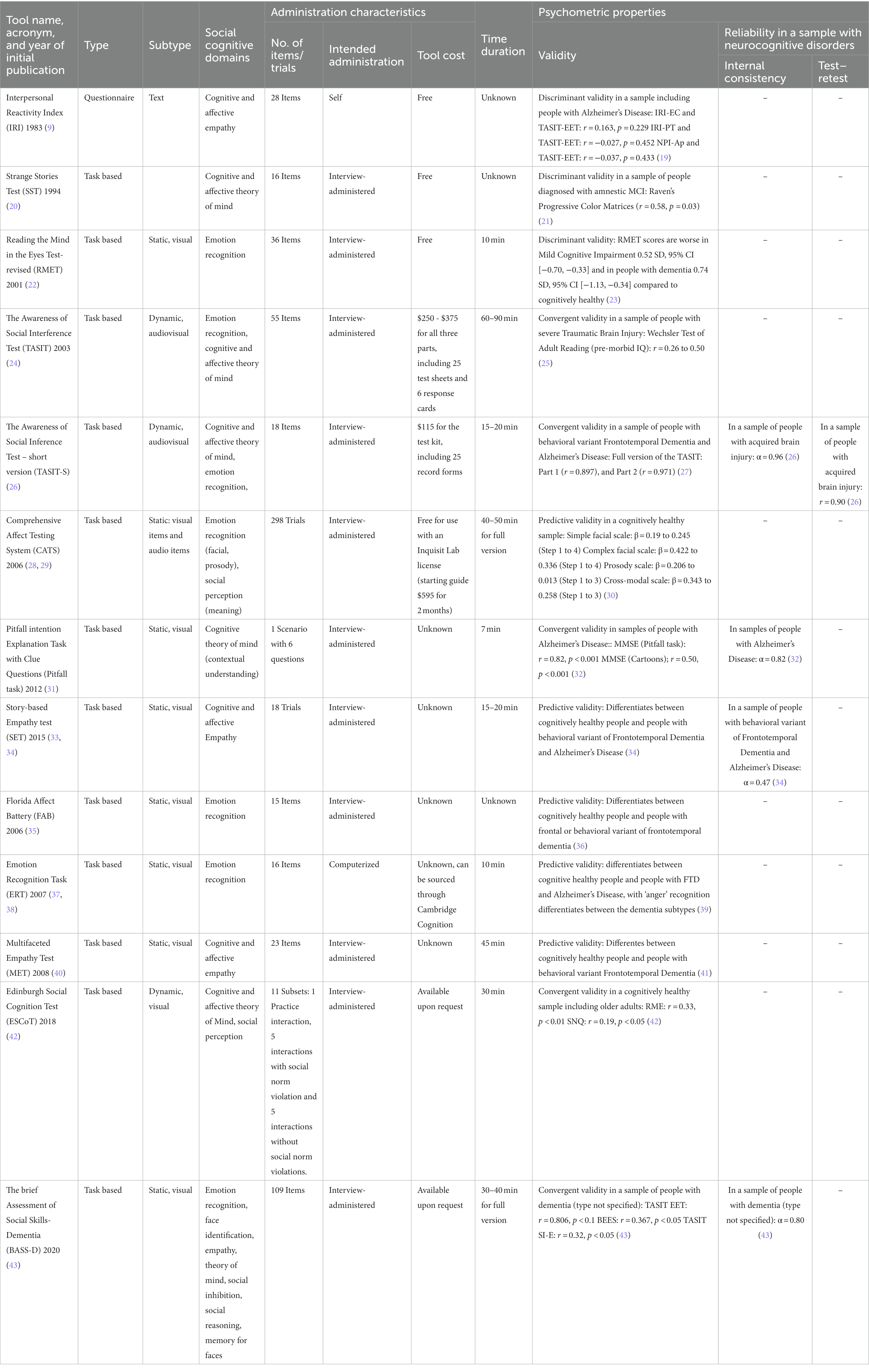
Table 1. Summary of tools used in studies assessing social cognition in adults with neurocognitive disorders.
Social cognition assessments mostly targeted ability-based only approaches [n = 10/13, Strange Stories Task (SST) (20), TASIT (24), TASIT-S (26), Comprehensive Affect Testing System (CATS) (28), Story-based Empathy Test (SET) (33, 34), Florida Affect Battery (FAB) (35), Emotion Recognition Task (ERT) (37, 38), Multifaceted Empathy Test (MET) (40), Edinburgh Social Cognition Test (ESCoT) (42), and Brief Assessment of Social Skills – Dementia (BASS-D) (43)]. Questionnaire-based [Interpersonal Reactivity Index (IRI) (9)] and mixed approaches [PIE (31)] were less frequent.
Assessments over time
Several social cognition assessments were originally developed for general purposes or in other populations, but have since been adapted for or validated with people with dementia. For instance, the IRI is widely used to assess empathy in the general population (46), and was later validated in the context of dementia (9) to better cover empathic concern and personal distress features (47). Similarly, the TASIT was originally developed to assess social cognitive skills following a traumatic brain injury (48), and was later validated for people with dementia (49).
However, other measures lacked specific development with individuals with dementia [e.g., CATS (28, 29), Pitfall Task (31), SET (13, 14)], yet they contain multiple subtests aimed at assessing various aspects of social cognition (e.g., emotion recognition, intention attribution and causal inference).
The majority of instruments focused on one or two domains, predominantly on emotion recognition (identifying a facial expression) or cognitive or affective theory of mind (taking others’ perspectives). Emotion recognition (TASIT, CATS, FAB, ERT) and cognitive or affective theory of mind (TASIT-S, Pitfall Task, ESCoT) were most common, followed by either cognitive and/or affective empathy (IRI, SET, MET).
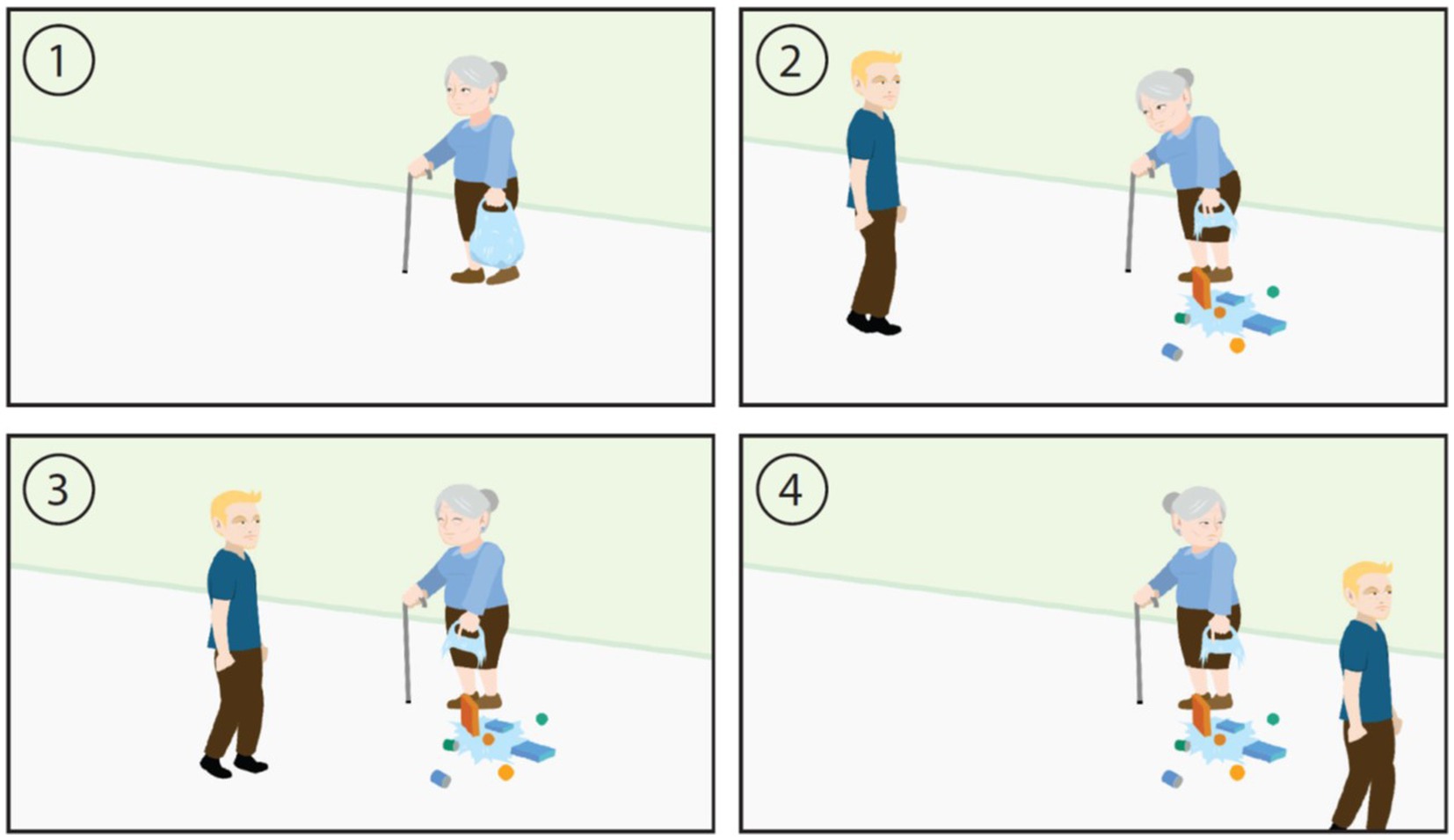
More recently developed assessments such as the ESCoT (42) in 2018 and BASS-D (43) in 2020 cover more detailed social cognition components (e.g., not just a singular domain such as cognitive or affective theory of mind) in a more ecological valid manner (Figure 1). For instance, the ESCoT involves presenting animations of social interactions and the individual is asked about the thoughts and feelings of the characters and the norms that apply in each scenario (Figure 1). One scenario depicts a young man walking past an older woman who’s shopping bag has ripped, causing the contents to fall onto the street. The interviewer asks what the older woman thought and felt at the start of the scenario and at the end (when the young man keeps walking instead of stopping to help her) and what the social norm would be in the scenario (e.g., to help the woman).
Similarly, the BASS-D (43) presents a comprehensive set of photos of faces and videos of interactions to assess the individual’s ability to recognize emotions, identify facial features and expressions, provide appropriate empathic responses, and exhibit social inhibition and reasoning.
Psychometric properties
To the best of our knowledge, all assessments have been tested for validity and some for reliability (6/13 46%) within samples including people with neurocognitive disorders (most commonly reported FTD). Most reported internal consistency rather than test–retest (1/13; 7%; Table 1). Internal consistency ranged from low to moderate (e.g., SET; α = 0.47) to high (TASIT-S; α = 0.96). Validity ranged from low (e.g., CATS) to high (e.g., FAB), with most measures exhibiting moderate to high convergent validity. We were unable to find instruments that were tested for responsiveness to interventions. All of the above mentioned tests have been validated with people with neurocognitive disorders: IRI (50), SST (20), TASIT (19), TASIT-S (27), CATS (51), Pitfall Task (31), SET (34), FAB (52), ERT (37, 38), MET (40), ESCoT (53), and BASS-D (43).
Future directions
Indexing a given social cognitive domain is challenging, due to the current lack of consensus on the definition and theoretical derivation of cognitive domains in neurocognitive disorders. Thus, several assessments provided considerable conceptual overlap between domains. The listed measures assessed multiple domains of social cognition, but tended to focus primarily on the understanding of social situations. This can be problematic as questionnaires (e.g., IRI) often have the limitations of self-report measures, requiring individuals to have insight into their own strengths and weaknesses, which may not be possible during later stages of dementia.
While social reasoning, identifying and remembering faces, and social disinhibition add depth to the assessment of social cognition, a key component remains to be assessed: namely, social behavior. This domain was largely missing and includes skills such as eye contact, turn taking, asking open ended questions, using humor, understanding puns and jokes, and keeping conversations going. Given the ability of emotion recognition and empathy tasks to differentiate between Frontotemporal Dementia and Alzheimer’s Disease (34), it is plausible that individuals who show deficits in these skills may also have difficulties with responding appropriately in social situations. These concepts are understandably difficult to self-report. The main issue is that tools developed to date rely on interpreting the thoughts and feelings of an actor or character, thereby limiting the ecological validity of the tests. The use of actors or characters adds a layer of “pretending” to the tests, which it could be argued require a certain level of theory of mind. The current tests also involve selecting ‘appropriate’ responses based on arbitrary criteria imposed by the researcher, rather than real world dynamic interactions (54). Observational tests might be more suitable to assess social skills (e.g., turn taking and keeping conversations going). However, observational measurements are traditionally time-consuming (e.g., ethnographic studies) and are difficult to standardize, so harnessing the power of technology might be a suitable approach.
The integration of technology in the measurement of social cognition has the potential to transform our understanding of the human brain and behavior. Virtual reality could assess a range of complex social cognitive processes, ranging from emotion recognition, social perception, and cognitive or affective theory of mind, in a more ecologically valid way than traditional approaches (55). Virtual reality can further manipulate social cues, such as facial expressions and tone of voice, capturing the nuance of social interactions and cognitive processes in a controlled, yet naturalistic environment. This method can provide valuable insights into the underlying mechanisms of social cognition, create highly customized social situations tailored to the individual’s specific social cognitive deficits, enabling targeted interventions and treatments.
Technological advances in automatic tracking capabilities (e.g., eye, microfacial expressions) have the potential to further deepen our understanding, and thus definition of, appropriate and inappropriate social behaviors and reactions in individuals with neurocognitive deficits (56). Whilst detection and analysis of common and basic expressions such as happiness, sadness, surprise, anger, disgust has been achieved, recent advancements in machine learning and artificial intelligence have led to the development of more sophisticated algorithms that can detect even more subtle facial movements. These algorithms can now identify patterns in facial expressions that has real-world applications and responses, such as nervousness, disagreement and contempt (57), enabling more accurate detection of microfacial expressions associated with other emotions.
Other common forms of technology such as wearable devices, which can collect physiological data including heart rate variability and skin conductance, can be used to implore the impact of social cognitive processes on the autonomic nervous system, providing a more nuanced understanding of the physiological processes involved in social cognition (58). As technology continues to evolve, we can expect increasingly sophisticated and accurate assessments of social cognition, which will support our understanding and eventual interventions and treatments for individuals with social cognitive deficits.
Recommendations
The use of valid and standardized outcome measures for the assessment of social cognition in older adults with major neurocognitive disorders is necessary to support early intervention and treatment of dementia conditions, as well as strengthening epidemiological studies to further enable our understanding of the trajectory of social cognitive deficits and its association with other factors.
Here we propose four key recommendations to aid the establishment of comprehensive social cognition assessments for older adults with major neurocognitive disorders.
Tool selection and administration
In the absence of other tools, we recommend multicomponent assessments of social cognition, such as the BASS-D and ESCoT, to help increase identification and recognition of social cognitive performance. However, whilst considering which aspects of social cognition should be included in a battery for examining older adults with neurocognitive disorders, a holistic approach should be adopted. While theory of mind is undoubtedly a critical aspect, other components such as emotion recognition, empathy, social perception, and social behavior should also be considered during clinical appraisals. These components collectively provide a more nuanced assessment of social cognitive abilities, allowing for more accuracy of deficits and the development of targeted interventions. However, the selection and delivery of social cognition assessment depends on several factors, including the research objectives, the specific population under study, psychometric properties, cultural appropriateness, the clinical utility of these tools and available resources. Embedding social cognition batteries into standard practice, especially when facing time and funding limitations often present in clinical settings, requires a strategic and pragmatic approach. Practical steps to consider are prioritizing key domains (e.g., focus on a subset of domains most indicative of an individual’s overall social cognitive functioning to streamline the assessment process), using brief and targeted assessment tools that provide meaningful insights within a shorter timeframe, integrating assessments with existing tools, providing flexible administration and leverage technology to automate scoring, analyses and feedback. At times, the implementation of social cognition assessment may need to be selectively based on clinical judgement and determine which individuals are most likely to benefit from a social cognition assessment, considering their presenting symptoms, history, and treatment goals.
Enable better, and more accurate diagnostic measures
We need screening tools for use in research and clinical settings to complement existing cognitive assessments, which often exclude the domain of social cognition. These new measures should be rigorously tested, validated, appropriate, and multidimensional. Social cognitive measures used with other populations (e.g., traumatic brain injury, healthy older adults) could possibly be further validated with people with MCI or dementia [e.g., Emotion Recognition Task (59)] to further identify how affective and cognitive theory of mind are supported by different brain functionalities. There is a lack of knowledge on the timeframe required to detect clinically significant changes and the feasibility of using alternative, targeted versions for assessments carried out within time-limited interventions. Such adaptations may be particularly valuable in clinical settings where efficiency and brevity are necessary considerations. Furthermore, while emerging research suggests the potential utility of social cognition measures in indexing treatment effects more broadly (59), empirical investigations are needed to establish their validity and clinical significance, particularly for individuals with neurocognitive disorders. Currently, there is limited understanding of how quickly treatment interventions may lead to observable improvements in social cognition. Well-designed studies that track individuals over time can provide insights into the temporal dynamics of treatment effects, helping establish realistic expectations for intervention outcomes (60).
Explore the potential additive value of comprehensive social cognitive evaluation
Studies should investigate how social cognition tools can enhance the detection, characterization, and monitoring of cognitive changes in individuals with various forms of dementia. Social cognition measures were predominantly concentrated on individuals with FTD, although there is growing interest in exploring their broader applicability across other neurodegenerative conditions, including MCI and various forms of dementia. Indeed, social cognition tools hold promise beyond FTD and may offer additional insights into the cognitive profiles of individuals with MCI and dementia (60, 61). Future research needs to establish the potential additive value of social cognition measures to traditional cognitive assessments, and the practical implications and clinical utility of incorporating social cognition tools into cognitive assessments on diagnostic accuracy, treatment planning, and prognosis. We expect that specific dimensions will be affected by different (or different rates of progress of) neurocognitive disorders. Multidimensional measures may aid in differential diagnoses and identify potential social cognitive domains requiring interventions or support.
Harness digital technologies to support ecological measures
Developing more sensitive tools using digital technology (such as virtual reality, eye tracking, real-time AI assisted scoring of social behavior) would allow more accurate and sensitive measurement of social cognition, both at home and in the clinic. These tools are likely to be more ecologically valid and engaging assessments, which can enhance the sensitivity of detecting social cognitive impairments across different clinical populations. Technology might combine both performance-based measures and subjective self-report measures to capture subtle, multidimensional aspects of social cognition decline. Adaptive testing approaches, for example, can tailor the assessment difficulty to the individual’s cognitive abilities, capturing the entire spectrum of social cognitive deficits.
Conclusion
Moving forward, more work is needed to elucidate the effective measurement of social cognition and to develop a multidimensional and more ecologically valid diagnostic social cognitive measures that have utility in the clinic. We encourage the development and refinement of existing tools to cover more broadly social behavior to enable more accurate depiction of one’s social functioning. This can be supplemented with technology to enable routine assessments in the clinic.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SS and JS: conceptualization, methodology, supervision, writing – original draft, and writing – review and editing. AM: article search and synthesis and writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding
SS declares payments for lectures from NYU Sydney and University of Sydney, and grant funding from Dementia Australia Research Foundation (not for current manuscript) and grant funding from EU-JPND and NHMRC Australia (not for current manuscript). JS receives a Research Theme Fellowship from Western Sydney University.
Acknowledgments
We would like to thank Victoria Chong for her assistance with the literature search.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Baumeister, RF, and Leary, MR. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol Bull. (1995) 117:497–529. doi: 10.1037/0033-2909.117.3.497
2. Sachdev, PS, Blacker, D, Blazer, DG, Ganguli, M, Jeste, DV, Paulsen, JS, et al. Classifying neurocognitive disorders: the DSM-5 approach. Nat Rev Neurol. (2014) 10:634–42. doi: 10.1038/nrneurol.2014.181
3. Grainger, SA, Crawford, JD, Riches, JC, Kochan, NA, Chander, RJ, Mather, KA, et al. Aging is associated with multidirectional changes in social cognition: findings from an adult life-span sample ranging from 18 to 101 years. J Gerontol Ser B. (2023) 78:62–72. doi: 10.1093/geronb/gbac110
4. Henry, JD, Von Hippel, W, Molenberghs, P, Lee, T, and Sachdev, PS. Clinical assessment of social cognitive function in neurological disorders. Nat Rev Neurol. (2016) 12:28–39. doi: 10.1038/nrneurol.2015.229
5. Happé, F, Cook, JL, and Bird, G. The structure of social cognition: in(ter)dependence of sociocognitive processes. Annu Rev Psychol. (2017) 68:243–67. doi: 10.1146/annurev-psych-010416-044046
6. Bartochowski, Z, Gatla, S, Khoury, R, Al-Dahhak, R, and Grossberg, G. Empathy changes in neurocognitive disorders: a review. Ann Clin Psychiatry. (2018) 30:220–32.
7. Canty, AL, Cao, Y, Neumann, D, and Shum, DHK. The functional significance of cognitive empathy and theory of mind in early and chronic schizophrenia. Psychiatry Res. (2021) 299:113852. doi: 10.1016/j.psychres.2021.113852
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5-TR). 5th Ed., Text Rev ed American Psychiatric Association (2022).
9. Torres, B, Santos, RL, De, SMFB, Simões Neto, JP, Nogueira, MML, Belfort, TT, et al. Facial expression recognition in Alzheimer’s disease: a longitudinal study. Arq Neuropsiquiatr. (2015) 73:383–9. doi: 10.1590/0004-282X20150009
10. Bora, E, Velakoulis, D, and Walterfang, M. Meta-analysis of facial emotion recognition in behavioral variant frontotemporal dementia: comparison with Alzheimer disease and healthy controls. J Geriatr Psychiatry Neurol. (2016) 29:205–11. doi: 10.1177/0891988716640375
11. Bediou, B, Ryff, I, Mercier, B, Milliery, M, Henaff, MA, D’Amato, T, et al. Impaired social cognition in mild Alzheimer disease. J Geriatr Psychiatry Neurol. (2009) 22:130–40. doi: 10.1177/0891988709332939
12. Kemp, J, Philippi, N, Phillipps, C, Demuynck, C, Albasser, T, Martin-Hunyadi, C, et al. Cognitive profile in prodromal dementia with Lewy bodies. Alzheimers Res Ther. (2017) 9:19. doi: 10.1186/s13195-017-0242-1
13. dos Santos, T, de Carvalho, RLS, Nogueira, M, Baptista, MAT, Kimura, N, Lacerda, IB, et al. The relationship between social cognition and executive functions in Alzheimer’s disease: a systematic review. Curr Alzheimer Res. (2020) 17:487–97. doi: 10.2174/1567205017666200626205154
14. Brown, CL, Hua, AY, De Coster, L, Sturm, VE, Kramer, JH, Rosen, HJ, et al. Comparing two facets of emotion perception across multiple neurodegenerative diseases. Soc Cogn Affect Neurosci. (2020) 15:511–22. doi: 10.1093/scan/nsaa060
15. Mabire, JB, Gay, MC, Charras, K, and Vernooij-Dassen, M. Impact of a psychosocial intervention on social interactions between people with dementia: an observational study in a nursing home. Act Adapt Aging. (2022) 46:73–89. doi: 10.1080/01924788.2021.1966574
16. Morese, R, and Palermo, S. Feelings of loneliness and isolation: social brain and social cognition in the elderly and Alzheimer’s disease. Front Aging Neurosci. (2022) 14:896218. doi: 10.3389/fnagi.2022.896218
17. Livingston, G, Huntley, J, Sommerlad, A, Ames, D, Ballard, C, Banerjee, S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
18. Hunt, C, Borgida, E, and Lavine, H. Social cognition In: VS Ramachandran, editor. Encyclopedia of Human Behavior. 2nd ed. San Diego: Academic Press (2012). 456–62.
19. Martinez, M, Multani, N, Anor, CJ, Misquitta, K, Tang-Wai, DF, Keren, R, et al. Emotion detection deficits and decreased empathy in patients with Alzheimer’s disease and Parkinson’s disease affect caregiver mood and burden. Front Aging Neurosci. (2018) 10:120. doi: 10.3389/fnagi.2018.00120
20. Happé, FGE. An advanced test of theory of mind: understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord. (1994) 24:129–54. doi: 10.1007/BF02172093
21. Baglio, F, Castelli, I, Alberoni, M, Blasi, V, Griffanti, L, Falini, A, et al. Theory of mind in amnestic mild cognitive impairment: an fMRI study. J Alzheimers Dis. (2012) 29:25–37. doi: 10.3233/JAD-2011-111256
22. Baron-Cohen, S, Wheelwright, S, Hill, J, Raste, Y, and Plumb, I. The “Reading the mind in the eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. (2001) 42:241–51. doi: 10.1111/1469-7610.00715
23. Eramudugolla, R, Huynh, K, Zhou, S, Amos, JG, and Anstey, KJ. Social cognition and social functioning in MCI and dementia in an epidemiological sample. J Int Neuropsychol Soc. (2022) 28:661–72. doi: 10.1017/S1355617721000898
24. Tsentidou, G, Moraitou, D, and Tsolaki, M. Similar theory of mind deficits in community dwelling older adults with vascular risk profile and patients with mild cognitive impairment: the case of paradoxical sarcasm comprehension. Brain Sci. (2021) 11:627. doi: 10.3390/brainsci11050627
25. McDonald, S, Bornhofen, C, Shum, D, Long, E, Saunders, C, and Neulinger, K. Reliability and validity of the awareness of social inference test (TASIT): a clinical test of social perception. Disabil Rehabil. (2006) 28:1529–42. doi: 10.1080/09638280600646185
26. Honan, CA, McDonald, S, Sufani, C, Hine, DW, and Kumfor, F. The awareness of social inference test: development of a shortened version for use in adults with acquired brain injury. Clin Neuropsychol. (2016) 30:243–64. doi: 10.1080/13854046.2015.1136691
27. Kumfor, F, Honan, C, McDonald, S, Hazelton, JL, Hodges, JR, and Piguet, O. Assessing the “social brain” in dementia: applying TASIT-S. Cortex. (2017) 93:166–77. doi: 10.1016/j.cortex.2017.05.022
28. Weiner, SG. The Comprehensive Affect Testing System: Effects of Age on Performance. California, United States: Pacific Graduate School of Psychology (2005).
29. Froming, KB, Levy, CM, and Ekman, P. Emotion Processing: The Comprehensive Affect Testing System User’s Manual Psychology Software, Inc. (2003).
30. Schaffer, SG, Wisniewski, A, Dahdah, M, and Froming, KB. The comprehensive affect testing system–abbreviated: effects of age on performance. Arch Clin Neuropsychol. (2009) 24:89–104. doi: 10.1093/arclin/acp012
31. Yamaguchi, T, Maki, Y, and Yamaguchi, H. Pitfall intention explanation task with clue questions (pitfall task): assessment of comprehending other people’s behavioral intentions in Alzheimer’s disease. Int Psychogeriatr. (2012) 24:1919–26. doi: 10.1017/S1041610212001147
32. De Lucena, AT, Bhalla, RK, TT, BADS, and MCN, D. The relationship between theory of mind and cognition in Alzheimer’s disease: a systematic review. J Clin Exp Neuropsychol. (2020) 42:223–39. doi: 10.1080/13803395.2019.1710112
33. Dodich, A. A novel task assessing intention and emotion attribution: Italian standardization and normative data of the story-based empathy task | SpringerLink. Neurol Sci. (2015) 36:1907–12. doi: 10.1007/s10072-015-2281-3
34. Dodich, A, Cerami, C, Crespi, C, Canessa, N, Lettieri, G, Iannaccone, S, et al. Differential impairment of cognitive and affective Mentalizing abilities in neurodegenerative dementias: evidence from behavioral variant of frontotemporal dementia, Alzheimer’s disease, and mild cognitive impairment. J Alzheimers Dis. (2016) 50:1011–22. doi: 10.3233/JAD-150605
35. Bowers, D, Blonder, L, and Heilman, K. Florida Affect Battery. USA: Center for Neuropsychological Studies, Department of Neurology Florida (1998).
36. Rosen, HJ, Pace-Savitsky, K, Perry, RJ, Kramer, JH, Miller, BL, and Levenson, RW. Recognition of emotion in the frontal and temporal variants of frontotemporal dementia. Dement Geriatr Cogn Disord. (2004) 17:277–81. doi: 10.1159/000077154
37. Kessels, RPC, Gerritsen, L, Montagne, B, Ackl, N, Diehl, J, and Danek, A. Recognition of facial expressions of different emotional intensities in patients with frontotemporal lobar degeneration. Behav Neurol. (2007) 18:31–6. doi: 10.1155/2007/868431
38. Kessels, RPC, Montagne, B, Hendriks, AW, Perrett, DI, and de Haan, EHF. Assessment of perception of morphed facial expressions using the emotion recognition task: normative data from healthy participants aged 8–75. J Neuropsychol. (2014) 8:75–93. doi: 10.1111/jnp.12009
39. Jiskoot, LC, Poos, JM, Vollebergh, ME, Franzen, S, van Hemmen, J, Papma, JM, et al. Emotion recognition of morphed facial expressions in presymptomatic and symptomatic frontotemporal dementia, and Alzheimer’s dementia. J Neurol. (2021) 268:102–13. doi: 10.1007/s00415-020-10096-y
40. Dziobek, I, Rogers, K, Fleck, S, Bahnemann, M, Heekeren, HR, Wolf, OT, et al. Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the multifaceted empathy test (MET). J Autism Dev Disord. (2008) 38:464–73. doi: 10.1007/s10803-007-0486-x
41. Oliver, LD, Mitchell, DGV, Dziobek, I, MacKinley, J, Coleman, K, Rankin, KP, et al. Parsing cognitive and emotional empathy deficits for negative and positive stimuli in frontotemporal dementia. Neuropsychologia. (2015) 67:14–26. doi: 10.1016/j.neuropsychologia.2014.11.022
42. Baksh, RA, Abrahams, S, Auyeung, B, and MacPherson, SE. The Edinburgh social cognition test (ESCoT): examining the effects of age on a new measure of theory of mind and social norm understanding. PLoS One. (2018) 13:e0195818. doi: 10.1371/journal.pone.0195818
43. Kelly, M, and McDonald, S. Assessing social cognition in people with a diagnosis of dementia: development of a novel screening test, the brief assessment of social skills (BASS-D). J Clin Exp Neuropsychol. (2020) 42:185–98. doi: 10.1080/13803395.2019.1700925
44. Spreng, RN, MC, MK, Mar, RA, and Levine, B. The Toronto empathy questionnaire: scale development and initial validation of a factor-analytic solution to multiple empathy measures. J Pers Assess. (2009) 91:62–71. doi: 10.1080/00223890802484381
45. Baron-Cohen, S, and Wheelwright, S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and Normal sex differences. J Autism Dev Disord. (2004) 34:163–75. doi: 10.1023/B:JADD.0000022607.19833.00
46. Davis, MH. A multidimensional approach to individual differences in empathy. JSAS Cat Sel Doc Psychol. (1980) 10:85.
47. Chrysikou, EG, and Thompson, WJ. Assessing cognitive and affective empathy through the interpersonal reactivity index: an argument against a two-factor model. Assessment. (2016) 23:769–77. doi: 10.1177/1073191115599055
48. McDonald, S, Flanagan, S, Rollins, J, and Kinch, J. TASIT: a new clinical tool for assessing social perception after traumatic brain injury. J Head Trauma Rehabil. (2003) 18:219–38. doi: 10.1097/00001199-200305000-00001
49. Kumfor, F, Irish, M, Leyton, C, Miller, L, Lah, S, Devenney, E, et al. Tracking the progression of social cognition in neurodegenerative disorders. J Neurol Neurosurg Psychiatry. (2014) 85:1076–83. doi: 10.1136/jnnp-2013-307098
50. Hsieh, S, Irish, M, Daveson, N, Hodges, JR, and Piguet, O. When one loses empathy: its effect on Carers of patients with dementia. J Geriatr Psychiatry Neurol. (2013) 26:174–84. doi: 10.1177/0891988713495448
51. Henry, JD, Phillips, LH, and Von Hippel, C. A meta-analytic review of theory of mind difficulties in behavioural-variant frontotemporal dementia. Neuropsychologia. (2014) 56:53–62. doi: 10.1016/j.neuropsychologia.2013.12.024
52. Rosen, HJ, Wilson, MR, Schauer, GF, Allison, S, Gorno-Tempini, ML, Pace-Savitsky, C, et al. Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia. (2006) 44:365–73. doi: 10.1016/j.neuropsychologia.2005.06.012
53. Baksh, RA, Bugeja, T, and MacPherson, SE. Executive functions do not underlie performance on the Edinburgh Social Cognition Test (ESCoT) in healthy younger and older adults. J Int Neuropsychol Soc. (2020) 26:527–38. doi: 10.1017/S1355617719001450
54. Osborne-Crowley, K. Social cognition in the real world: reconnecting the study of social cognition with social reality. Rev Gen Psychol. (2020) 24:144–58. doi: 10.1177/1089268020906483
55. Shen, ZH, Liu, MH, Wu, Y, Lin, QQ, and Wang, YG. Virtual-reality-based social cognition and interaction training for patients with schizophrenia: a preliminary efficacy study. Front Psychiatry. (2022) 13:1022278. doi: 10.3389/fpsyt.2022.1022278
56. Tylen, K, Allen, M, Hunter, B, and Roepstorff, A. Interaction vs. observation: distinctive modes of social cognition in human brain and behavior? A combined fMRI and eye-tracking study. Front Hum Neurosci. (2012) 6:331. doi: 10.3389/fnhum.2012.00331
57. K, R, S, GR, Gautam,, Patil, T, and Shankar, S. Developing an intelligent model to detect micro facial expression. in 2022 International Conference on Advanced Computing Technologies and Applications (ICACTA). (2022), 1–6.
58. Cella, M, Sedgwick, O, Lawrence, M, Grant, N, Tsapekos, D, Harrison, L, et al. Evaluating the mechanisms of social cognition intervention in schizophrenia: a proof-of-concept trial. Psychiatry Res. (2023) 319:114963
59. Montagne, B, Kessels, RPC, De Haan, EHF, and Perrett, DI. The emotion recognition task: a paradigm to measure the perception of facial emotional expressions at different intensities. Percept Mot Skills. (2007) 104:589–98. doi: 10.2466/pms.104.2.589-598
60. Duclos, H, Desgranges, B, Eustache, F, and Laisney, M. Impairment of social cognition in neurological diseases. Rev Neurol (Paris). (2018) 174:190–8. doi: 10.1016/j.neurol.2018.03.003
Keywords: social cognition, assessment, older adults, mild cognitive impairment, dementia
Citation: Samtani S, Meka A and Siette J (2023) Beyond memory: exploring the value of social cognition for older adults with neurocognitive disorders. Front. Psychiatry. 14:1209745. doi: 10.3389/fpsyt.2023.1209745
Edited by:
Sandra Baez, University of Los Andes, ColombiaReviewed by:
Hannah Keage, University of South Australia, AustraliaAmy Jarvis, University of South Australia, Australia, in collaboration with reviewer HK
Despina Moraitou, Aristotle University of Thessaloniki, Greece
Copyright © 2023 Samtani, Meka and Siette. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joyce Siette, am95Y2Uuc2lldHRlQHdlc3Rlcm5zeWRuZXkuZWR1LmF1
 Suraj Samtani
Suraj Samtani Anjani Meka
Anjani Meka Joyce Siette
Joyce Siette