- 1Department of Medical Psychology and Medical Sociology, Ruhr University Bochum, Bochum, Germany
- 2Department of Cognitive Psychology, Institute of Cognitive Neuroscience, Ruhr University Bochum, Bochum, Germany
Visceral pain and stress are tightly intertwined bodily and emotional phenomena, which enable a flexible adaptation to environmental challenges by activating a response repertoire to restore homeostasis along the gut-brain axis. However, visceral pain and stress can persist widely independent of the initial cause, acquiring independent disease values and posing major health burdens as predominant features in disorders of gut-brain interaction (DGBI). Epidemiological data consistently documents an increased prevalence for women to suffer from chronic visceral pain, possibly shaped by sex hormones and modulated by stress and its biological and psychosocial correlates. Yet, mechanisms underlying the complex interactions between altered visceroception, stress and sex remain widely elusive, especially in clinical populations with DGBI.
We herein selectively review mechanisms of interactions between stress and sex in the complex pathophysiology of DGBI. A particular emphasis is laid on visceral pain, in which stress constitutes a major risk factor as well as mediator, and sex-related differences are particularly pronounced. Building on the neurobiology of stress and mechanisms of gut-brain interactions, we highlight putative target mechanisms via which visceral pain and stress may converge with sex effects into a triad.
Accommodating a global demographic shift, we propose a lifespan perspective in future research, which may enable a more fine-tuned evaluation of this complex interplay exerting distinct challenges during vulnerable developmental phases. This viewpoint may advance our understanding of pathophysiological processes and can ultimately inspire novel tailored prevention strategies and therapeutic approaches in the treatment of chronic visceral pain and DGBI across the lifespan.
1. Introduction
Pain and stress are closely interwoven phenomena with shared conceptual underpinnings, psychological mechanisms and physiological responses (1). Their mutual influences on biological and psychosocial levels enable a flexible adaptation to environmental challenges by activating a response repertoire aimed to restore homeostasis and to regulate health (2, 3). However, both pain and stress can persist widely independent of the initial cause, losing their protective function, and acquiring an independent disease value, which substantially compromises quality of life.
In the context of DGBI, most evidence on the key role of stress and its neurobiological correlates comes from research on irritable bowel syndrome (IBS). With prevalence rates of up to 10% worldwide (4), IBS is not only the most common chronic visceral pain disorder but also exemplary of disturbed gut-brain interactions (5). Constituting key factors in the etiology and pathophysiology of the disease, stress and visceral pain often co-occur in IBS and can exert reciprocal effects. On the one hand, acute stress can increase pain sensitivity in patients (6, 7), affect gut motility and IBS symptomatology [reviewed in (8)] and alter patients’ neural responses to experimental visceral pain (9). On the other hand, pain per se constitutes a meaningful stressor able to trigger systemic changes in neural, neuroendocrine, and immunological systems, which in turn can profoundly impact on gut-brain communication (10). Particularly if stress persists, these systems can become dysregulated, resulting in exaggerated allostatic load (11) and altered stress reactivity (12). As such, stress and its neurobiological correlates demonstrably act as both, relevant risk factors for disease development (13–16) as well as accelerators of symptoms and symptom burden (7, 17, 18), deeming IBS a stress-related condition.
Importantly, women seem to be more prone than men to the development of conditions characterized by pain and stress alike (19, 20). These observations support sex-related factors to play major roles in the transition from adaptive to maladaptive responses and at the same time suggest complex interactions between pain, stress and sex in pathology. Sex differences are most pronounced in the context of visceral pain in disturbances of the gut-brain axis (21, 22) in which, interestingly, stress and its direct and indirect effects on central and peripheral pathways in gut-brain communication constitute key players (23).
While epidemiological data are widely consistent, experimental studies targeting the mechanisms underlying interactions between pain, stress and sex mainly in young healthy participants reveal enormous inter-individual variability and do not allow the clear conclusion that women are per se more sensitive to either stress (24) or pain (25, 26), including visceral pain (27). Especially in the context of complex gut-brain interactions, these inconsistencies underscore the need to systematically identify and target modulating biological and psychosocial factors to gain further insights into the triad of visceral pain, stress and sex and its impact on DGBI.
Based on the neurobiology of stress and its role within the gut-brain axis, we herein selectively review current knowledge regarding sex effects on the interaction between visceral pain and stress and highlight putative underlying mechanisms. Accommodating a demographic shift observed worldwide, we particularly propose a lifespan perspective in future experimental and clinical research to advance our understanding about sexually dimorphic effects in DGBI and stress-related disorders, which may ultimately inspire tailored prevention and resilience strategies particularly during vulnerable developmental phases.
2. The gut-brain axis
The gut-brain axis (Figure 1) conceptualizes the complex and multidirectional communication pathways connecting the brain and gastrointestinal tract, involving modulators within a bio-psychosocial framework (28). Growing evidence supports continuous crosstalk between the enteric (ENS) and central nervous system (CNS) along vagal, spinal (afferent) and humoral (efferent) pathways serving key functions in monitoring and maintaining homeostasis, detecting challenges to the organism and restoring bodily integrity (29). As such, both ENS- and CNS-derived processes and their interactions along the gut-brain axis are capable to shape visceral symptoms including pain but can also modulate stress and stress effects.
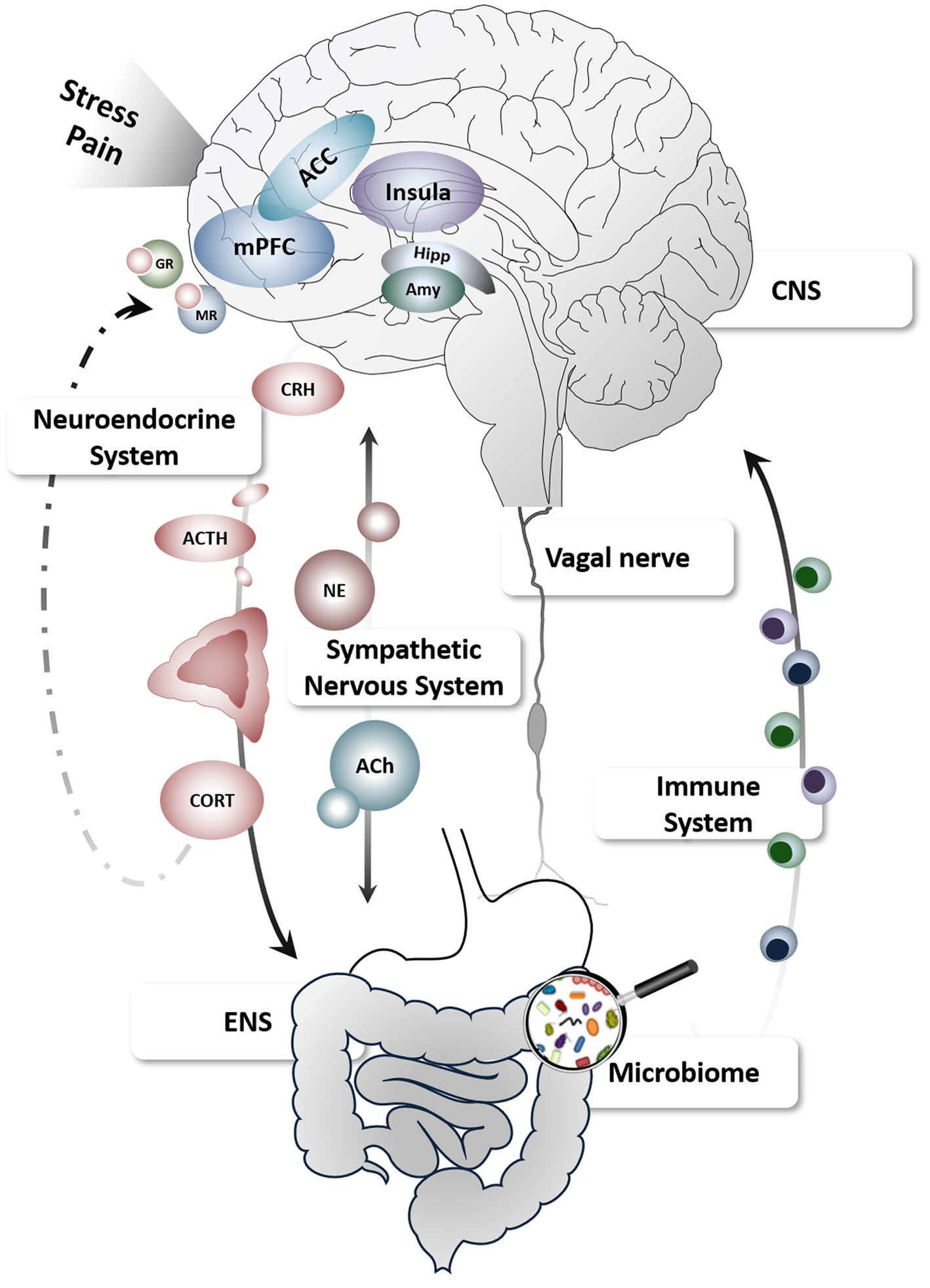
Figure 1. Schematic depiction of the gut-brain axis and key pathways relevant to stress and pain. Figure created using motifolio templates (www.motifolio.com). ACC, anterior cingulate cortex; ACh, acetylcholine; ACTH, adrenocorticotropic hormone; Amy, amygdala; CNS, central nervous system; CORT, cortisol; CRH, corticotropin-releasing hormone; ENS, enteric nervous system; GR, glucocorticoid receptors; Hipp, hippocampus; mPFC, medial prefrontal cortex; MR, mineralocorticoid receptors; NE, norepinephrine.
The ENS is mainly under control of intrinsic enteric neurons and glia, smooth muscles and the lamina propria of the mucosa, but is also extrinsically innervated by primary afferent and autonomic fibers connecting the gastrointestinal tract with the spine and brain (30, 31). The brain together with neuroendocrine, immune, and autonomic nervous systems constitute profound modulators of motility and gut function outside the ENS. In addition, multiple psychological mechanisms including cognitive and emotional factors are integrated with ascending sensory information in distinct brain circuits involving the insula and anterior cingulate cortex as core nodes of the salience network (32), which ultimately shape subjective experiences, as well as autonomic and behavioral responses to visceral sensations (33, 34). Conversely, ENS mechanisms can themselves exert powerful effects on these pathways and brain functions, providing several afferent and efferent target routes for stress-induced modulations of visceral symptoms (35). Finally, increasing evidence supports a key role of gut microbial composition not only in intestinal barrier functioning but also in brain function and behavior. Along this gut-brain-microbiota axis, stress appears to be a prominent modulator via immunological, endocrine, and neural communication channels, deeming stress a key player in this emerging field in the neurosciences (36).
Effects of stress on gastrointestinal function observed in preclinical and clinical studies in patients suffering from chronic visceral pain demonstrably involve alterations along several gut-brain pathways (23, 37). Mediators of the stress response system have been linked to stress-induced changes in intestinal permeability (38), gut motility and visceral hyperalgesia (39), possibly involving inflammatory mechanisms such as mast cell activation and release of pro-inflammatory cytokines (40). Using well-established experimental stress models such as public speech or dichotomous listening, few studies in healthy humans demonstrated acute increases in gut permeability (41, 42). Furthermore, increasing evidence using various experimental stressors supports the notion that stress can induce profound changes in emotion, mood and cognition (14, 16, 43), including effects on brain function (44).
Importantly, applying an experimental visceral pain model with rectal distensions in healthy volunteers, stress and stress mediators were also found to alter visceral perception, particularly aversive visceral symptoms including pain (14, 16) with distinct effects of sex as well as the type of stress model. Specifically, young healthy men and women did not seem to per se differ with respect to visceral sensitivity (27) and sensitivity to aversive visceral sensations was equally lowered in individuals being subject to increased chronic stress load (14). However, pharmacologically increased cortisol concentrations resulted in lowered visceral (but not somatic) pain thresholds particularly in women (16). While warranting further research to substantiate these findings, these first data indicate that sex and sex hormones may directly interact with neurobiological stress modulators in distinctly shaping visceral perception along the gut-brain axis in both health and DGBI (45).
3. The neurobiology of stress
In general, stress sets a fine-tuned orchestration of affective, physiological, immunological and endocrine responses into motion helping the organism to adequately respond to or prepare for a (potential) stressor. The acute stress response is adaptive, while chronic stress represents a potent risk factor for developing mental and somatic disorders (46, 47), including chronic pain (48). Two major systems govern the stress response: first, activation of the sympathetic nervous system leads to a rapid release of (nor)epinephrine from the adrenal medulla and sympathetic nerves (11) increasing heart rate and blood pressure. Second, stress activates the hypothalamus-pituitary-adrenocortical (HPA) axis with an initial release of corticotropin-releasing hormone (CRH) from the hypothalamus. In the anterior pituitary, CRH stimulates the secretion of adrenocorticotropic hormone into the bloodstream, which leads to the release of glucocorticoids, in humans mainly cortisol, from the adrenal cortex. Glucocorticoids pass the blood-brain barrier and occupy mineralocorticoid and glucocorticoid receptors in a plethora of brain regions such as the amygdala, hippocampus or prefrontal cortex (49–52).
Importantly, stress responses differ between men and women (53, 54) and depend on sex hormone availability. For example, reduced cortisol release was observed during the follicular phase of the menstrual cycle, characterized by low female sex hormone concentrations, in comparison to the luteal phase with high concentrations of estradiol and progesterone (55). Intake of hormonal contraceptives leading to low endogenous sex hormone availability also reduces or even blunts cortisol release to acute stress (56), which is, however, restricted to the free, biologically active part of cortisol measured in saliva. Interactions between stress and female sex hormones, including hormonal contraception, have been observed in a variety of processes such as episodic memory, fear conditioning and emotion regulation (57–59), which are also of relevance in the context of pain and its modulation (60–62). In addition to female sex hormones, androgens, particularly testosterone, also impact HPA axis activity, partially mediated by a conversion to estradiol (63). Evidence mainly obtained from rodent models suggests a protective role of testosterone in the context of pain (64–66), including visceral pain (67). However, the dearth of research in humans and partly inconsistent findings (68, 69) hinder clear conclusions regarding a distinct impact of testosterone and its interaction with stress in affecting visceral pain and DGBI. Together, while a putative reciprocity between pain, stress and sex is widely discussed as key in the tremendous interindividual variability in pain- and stress-related disorders (70–73), including DGBI (10, 74), the exact processes of interaction in this triad and underlying mechanisms remain widely elusive.
4. Sex, stress and pain in disorders of gut-brain communication—promoting a lifespan perspective
Particularly in the context of DGBI, both stress and female sex are consistently identified as relevant risk factors (10, 22, 75) and sex differences have been documented for prevalence, incidence, pathophysiologic factors, clinical characteristics, and response to therapy (76). Using a multidimensional approach, a supersystems perspective to describe the connection between pain and stress through reciprocal neural, endocrine, and immune interactions (77) has recently been extended to integrate dysregulations underlying sexually dimorphic effects into a biopsychosocial model (78) (Figure 2). Embracing this concept, distinct central nervous system processing, genetic factors, responsivity of the HPA and sympatho-medullary axes, as well as sex hormones and their influences across the menstrual cycle are undoubtedly involved in visceral pain and DGBI. However, their interplay with psychosocial aspects ranging from proneness to anxiety and depression and sexually dimorphic pain coping strategies to stereotypic gender roles along with cultural and environmental factors is likely what ultimately increases the vulnerability to chronic visceral pain in women (28, 75, 78). In other words, biopsychosocial influences may function as catalyzers to the extent in which stress impacts the development and persistence of pain symptoms along the gut-brain axis in a sexually dimorphic manner. Together, considerable efforts to account for the complexity of relations between visceral pain, stress and sex (hormones) have been made. However, a crucial factor gaining increasing attention in elaborate animal models, yet thus far widely neglected in both conceptual and empirical work in humans, are changes across the lifespan (79). Age-related effects may impact the interaction between visceral pain, stress and sex-related effects in multiple ways: Directly via changes in sex hormone concentrations or HPA axis activity across the lifespan (80, 81), indirectly by inducing secondary changes caused by, e.g., environmental and lifestyle factors or in moderating the extent of biopsychosocial factors affecting the individual, particularly during vulnerable periods of life (79).
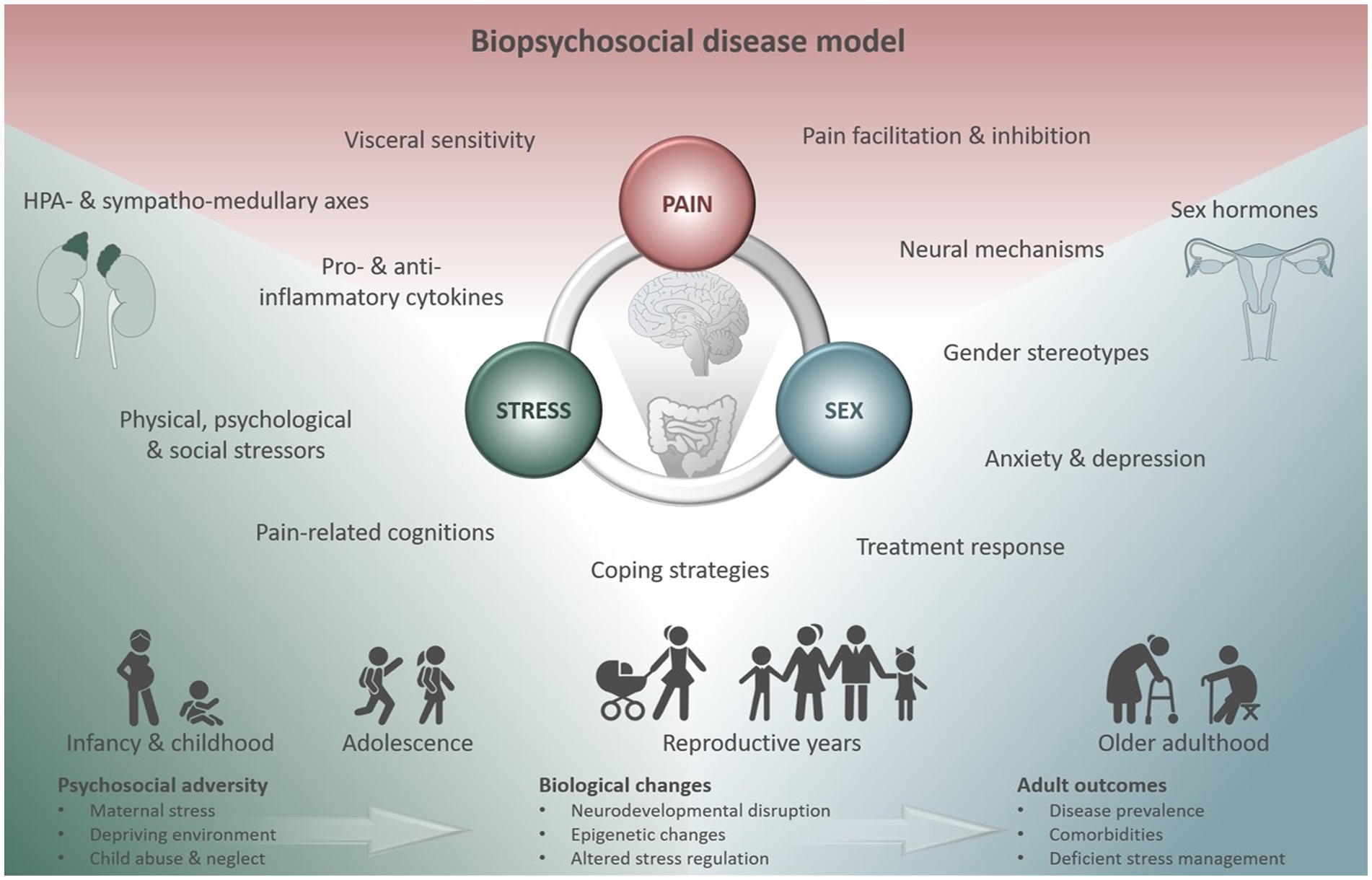
Figure 2. Biopsychosocial disease model summarizing multidimensional factors modulating the triad of visceral pain, stress and sex. Their interactions exert distinct effects in different vulnerable phases across the lifespan affecting prevalence, disease-related biological changes and comorbidities in adulthood. Figure created using motifolio templates (www.motifolio.com).
For example, psychosocial adversity occurring pre- or postnatally, particularly when stress leads to significant or sustained responses of the stress and immune systems (82), can exert massive detrimental effects on the offspring, which may not unfold instantly, but can manifest during sensitive periods later in life. These manifestations appear to be sexually dimorphic with females tending to show increased passive coping, symptoms of anxiety and depression along with aberrant HPA responsivity (79), known to be key risk factors for the development of stress-related disorders including DGBI (83). Consistently, the experience of early adverse life events is able to reliably predict IBS status in women, particularly when associated with intense fear (13) and can have long-term consequences on brain structure and function (84), involving emotion regulation and salience networks in a sex-dependent manner in both health (85) and DGBI (86). Puberty, characterized by physiological changes related to sexual maturation and regulated by several endocrine and genetic factors, appears to be a second critical window when considering interactions between stress, sex and mechanisms along the gut-brain axis. Mainly hormonally driven, puberty is known to interact with the gut’s microbiome and sex-related differences in the microbial composition emerge at the onset of this sensitive developmental phase (87, 88). Given that stress can demonstrably interfere with the gut microbiota (89) and contribute to gut dysbiosis in a sex-specific manner (90), it appears plausible that stressors encountered during puberty may be critical with respect to the vulnerability of dysregulated gut-brain communication. Finally, while rapid changes in estrogen and progesterone levels such as across the menstrual cycle appear to be associated with an exacerbation of bowel symptoms (91), a long-term decline in ovarian hormone concentrations following menopause seems to be related to a decrease in the incidence of DGBI in women (92). This effect, resulting in the elimination of prevalence differences of visceral pain in middle to older aged men and women (22), may, however, be reversed in those women under hormone replacement therapy (93), further supporting the key role of female sex hormones and their fluctuation in the vulnerability to chronic visceral pain conditions.
Together, while challenging, increasingly well-established age-related effects in all dimensions forming the triad of sex (94), stress (95) and visceral pain (96) call for future research particularly considering phenomena related to the lifespan when investigating and evaluating complex sex-stress-pain interactions in healthy humans and in patients suffering from DGBI.
5. Challenges and future directions
Tremendous inter individual variability in experimental and particularly clinical visceral pain in DGBI poses an enormous challenge in health care and effective treatment (36, 97). A biopsychosocial disease model is best suited to conceptualize the complex mosaic of individual and combined influences in moderating and mediating pain experiences and risk for chronification (70). Within this framework, experimental work and translational approaches should ideally be designed to simultaneously assess central, neuroendocrine, immunological and enteric mechanisms. Particularly, effects of acute and chronic stress burden, stress hormones and sex effects in terms of hormonal influences as well as psychosocial factors and the abovementioned interactions need be considered for a holistic appreciation of complex chronic pain conditions. Embedded within these approaches, embracing a lifespan perspective in terms of long-term follow-ups, longitudinal investigations and a particular emphasis on periods in life, which are especially vulnerable to stress-induced insults or associated with dynamic changes in hormonal levels is likely a promising future endeavor.
At first sight, age and changes across the lifespan appear to add yet another level of complexity onto the multifactorial etiology and pathophysiology of disturbed gut-brain interactions, in which several mechanisms are far from understood and others are likely still awaiting their discovery. However, in light of challenges arising from demographic changes, vast interest in mechanisms related to healthy aging is continuously growing (98). Ultimately, joint forces in transdisciplinary research to connect the dots between visceral pain, stress and sex from a lifespan perspective are therefore crucial to inspire transdisciplinary research, identify individualized therapeutic targets, and provide refined approaches for personalized prevention and treatment.
Author contributions
AI and CJM acquired funding. FL, CJM and AI drafted the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG), SFB 1280 Extinction Learning (316803389—projects A09 and A10).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Timmers, I, Quaedflieg, CWEM, Hsu, C, Heathcote, LC, Rovnaghi, CR, and Simons, LE. The interaction between stress and chronic pain through the lens of threat learning. Neurosci Biobehav Rev. (2019) 107:641–55. doi: 10.1016/j.neubiorev.2019.10.007
2. Panerai, AE. Pain emotion and homeostasis. Neurol Sci. (2011) 32:27–9. doi: 10.1007/s10072-011-0540-5
3. Schulz, A, and Vögele, C. Interoception and stress. Front Psychol. (2015) 6:993. doi: 10.3389/fpsyg.2015.00993
4. Ford, AC, Sperber, AD, Corsetti, M, and Camilleri, M. Irritable bowel syndrome. Lancet. (2020) 396:1675–88. doi: 10.1016/S0140-6736(20)31548-8
5. Drossman, DA, and Hasler, WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology. (2016) 150:1257–61. doi: 10.1053/j.gastro.2016.03.035
6. Thoua, NM, Murray, CDR, Winchester, WJ, Roy, AJ, Pitcher, MCL, Kamm, MA, et al. Amitriptyline modifies the visceral hypersensitivity response to acute stress in the irritable bowel syndrome. Aliment Pharmacol Ther. (2009) 29:552–60. doi: 10.1111/j.1365-2036.2008.03918.x
7. Murray, CDR, Flynn, J, Ratcliffe, L, Jacyna, MR, Kamm, MA, and Emmanuel, AV. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome. Gastroenterology. (2004) 127:1695–703. doi: 10.1053/j.gastro.2004.08.057
8. Schaper, SJ, and Stengel, A. Emotional stress responsivity of patients with IBS—a systematic review. J Psychosom Res. (2022) 153:110694. doi: 10.1016/j.jpsychores.2021.110694
9. Elsenbruch, S, Rosenberger, C, Bingel, U, Forsting, M, Schedlowski, M, and Gizewski, ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. (2010) 139:1310–1319.e4. doi: 10.1053/j.gastro.2010.06.054
10. Enck, P, Aziz, Q, Barbara, G, Farmer, AD, Fukudo, S, Mayer, EA, et al. Irritable bowel syndrome. Nat Rev Dis Primers. (2016) 2:16014. doi: 10.1038/nrdp.2016.14
11. McEwen, BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. (1998) 840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x
12. Woda, A, Picard, P, and Dutheil, F. Dysfunctional stress responses in chronic pain. Psychoneuroendocrinology. (2016) 71:127–35. doi: 10.1016/j.psyneuen.2016.05.017
13. Rahal, H, Videlock, EJ, Icenhour, A, Shih, W, Naliboff, B, Gupta, A, et al. Importance of trauma-related fear in patients with irritable bowel syndrome and early adverse life events. Neurogastroenterol Motil. (2020) 32:e13896–10. doi: 10.1111/nmo.13896
14. Icenhour, A, Labrenz, F, Roderigo, T, Benson, S, and Elsenbruch, S. The role of chronic stress in normal visceroception: insights from an experimental visceral pain study in healthy volunteers. Front Psychiatry. (2020) 11:107. doi: 10.3389/fpsyt.2020.00107
15. Koloski, NA, Jones, M, Kalantar, J, Weltman, M, Zaguirre, J, and Talley, NJ. The brain—gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut. (2012) 61:1284–90. doi: 10.1136/gutjnl-2011-300474
16. Benson, S, Siebert, C, Koenen, LR, Engler, H, Kleine-Borgmann, J, Bingel, U, et al. Cortisol affects pain sensitivity and pain-related emotional learning in experimental visceral but not somatic pain: a randomized controlled study in healthy men and women. Pain. (2019) 160:1719–28. doi: 10.1097/j.pain.0000000000001579
17. Dickhaus, B, Mayer, EA, Firooz, N, Stains, J, Conde, F, Olivas, TI, et al. Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. Am J Gastroenterol. (2003) 98:135–43. doi: 10.1111/j.1572-0241.2003.07156.x
18. Posserud, I, Agerforz, P, Ekman, R, Björnsson, ES, Abrahamsson, H, and Simrén, M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. (2004) 53:1102–8. doi: 10.1136/gut.2003.017962
19. Fillingim, RB, King, CD, Ribeiro-Dasilva, MC, Rahim-Williams, B, and Riley, JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. (2009) 10:447–85. doi: 10.1016/j.jpain.2008.12.001
20. Swaab, DF, and Bao, A-M. Sex differences in stress-related disorders: major depressive disorder, bipolar disorder, and posttraumatic stress disorder. Handb Clin Neurol. (2020) 175:335–58. doi: 10.1016/B978-0-444-64123-6.00023-0
21. Mogil, JS, and Bailey, AL. Sex and gender differences in pain and analgesia. Prog Brain Res. (2010) 186:141–57. doi: 10.1016/B978-0-444-53630-3.00009-9
22. Lovell, RM, and Ford, AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. (2012) 10:712–721.e4. doi: 10.1016/j.cgh.2012.02.029
23. Labanski, A, Langhorst, J, Engler, H, and Elsenbruch, S. Stress and the brain-gut axis in functional and chronic-inflammatory gastrointestinal diseases: a transdisciplinary challenge. Psychoneuroendocrinology. (2020) 111:104501. doi: 10.1016/j.psyneuen.2019.104501
24. Dalla, C, Antoniou, K, Drossopoulou, G, Xagoraris, M, Kokras, N, Sfikakis, A, et al. Chronic mild stress impact: are females more vulnerable? Neuroscience. (2005) 135:703–14. doi: 10.1016/j.neuroscience.2005.06.068
25. Racine, M, Tousignant-Laflamme, Y, Kloda, LA, Dion, D, Dupuis, G, and Choinière, M. A systematic literature review of 10 years of research on sex/gender and experimental pain perception—part 1: are there really differences between women and men? Pain. (2012) 153:602–18. doi: 10.1016/j.pain.2011.11.025
26. Greenspan, JD, Craft, RM, LeResche, L, Arendt-Nielsen, L, Berkley, KJ, Fillingim, RB, et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. (2007) 132:S26–45. doi: 10.1016/j.pain.2007.10.014
27. Icenhour, A, Labrenz, F, Roderigo, T, Siebert, C, Elsenbruch, S, and Benson, S. Are there sex differences in visceral sensitivity in young healthy men and women? Neurogastroenterol Motil. (2019) 31:e13664. doi: 10.1111/nmo.13664
28. Black, CJ, Drossman, DA, Talley, NJ, Ruddy, J, and Ford, AC. Functional gastrointestinal disorders: advances in understanding and management. Lancet. (2020) 396:1664–74. doi: 10.1016/S0140-6736(20)32115-2
29. Carabotti, M, Scirocco, A, Maselli, MA, and Severi, C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. (2015) 28:203–9. http://www.ncbi.nlm.nih.gov/pubmed/25830558.
30. Margolis, KG, Gershon, MD, and Bogunovic, M. Cellular organization of neuroimmune interactions in the gastrointestinal tract. Trends Immunol. (2016) 37:487–501. doi: 10.1016/j.it.2016.05.003
31. Rao, M, and Gershon, MD. The bowel and beyond: the enteric nervous system in neurological disorders. Nat Rev Gastroenterol Hepatol. (2016) 13:517–28. doi: 10.1038/nrgastro.2016.107
33. Mayer, EA, Gupta, A, Kilpatrick, LA, and Hong, J-Y. Imaging brain mechanisms in chronic visceral pain. Pain. (2015) 156:S50–63. doi: 10.1097/j.pain.0000000000000106
34. Borsook, D, Edwards, R, Elman, I, Becerra, L, and Levine, J. Pain and analgesia: the value of salience circuits. Prog Neurobiol. (2013) 104:93–105. doi: 10.1016/j.pneurobio.2013.02.003
35. Browning, KN, and Travagli, RA. Central control of gastrointestinal motility. Curr Opin Endocrinol Diabetes Obes. (2019) 26:11–6. doi: 10.1097/MED.0000000000000449
36. Margolis, KG, Cryan, JF, and Mayer, EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. (2021) 160:1486–501. doi: 10.1053/j.gastro.2020.10.066
37. Molina-Torres, G, Rodriguez-Arrastia, M, Roman, P, Sanchez-Labraca, N, and Cardona, D. Stress and the gut microbiota-brain axis. Behav Pharmacol. (2019) 30:187–200. doi: 10.1097/FBP.0000000000000478
38. Rodiño-Janeiro, BK, Alonso-Cotoner, C, Pigrau, M, Lobo, B, Vicario, M, and Santos, J. Role of Corticotropin-releasing factor in gastrointestinal permeability. J Neurogastroenterol Motil. (2015) 21:33–50. doi: 10.5056/jnm14084
39. Taché, Y, and Million, M. Role of corticotropin-releasing factor signaling in stress-related alterations of colonic motility and hyperalgesia. J Neurogastroenterol Motil. (2015) 21:8–24. doi: 10.5056/jnm14162
40. Overman, EL, Rivier, JE, and Moeser, AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-α. PLoS One. (2012) 7:e39935. doi: 10.1371/journal.pone.0039935
41. Vanuytsel, T, van Wanrooy, S, Vanheel, H, Vanormelingen, C, Verschueren, S, Houben, E, et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. (2014) 63:1293–9. doi: 10.1136/gutjnl-2013-305690
42. Gerdin, L, González-Castro, AM, Ericson, A-C, Persborn, M, Santos, J, Walter, SA, et al. Acute psychological stress increases paracellular permeability and modulates immune activity in rectal mucosa of healthy volunteers. United European Gastroenterol J. (2023) 11:31–41. doi: 10.1002/ueg2.12329
43. Roderigo, T, Benson, S, Schöls, M, Hetkamp, M, Schedlowski, M, Enck, P, et al. Effects of acute psychological stress on placebo and nocebo responses in a clinically relevant model of visceroception. Pain. (2017) 158:1489–98. doi: 10.1097/j.pain.0000000000000940
44. Rosenberger, C, Elsenbruch, S, Scholle, A, De Greiff, A, Schedlowski, M, Forsting, M, et al. Effects of psychological stress on the cerebral processing of visceral stimuli in healthy women. Neurogastroenterol Motil. (2009) 21:740–e45. doi: 10.1111/j.1365-2982.2009.01295.x
45. Kano, M, Muratsubaki, T, Van Oudenhove, L, Morishita, J, Yoshizawa, M, Kohno, K, et al. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci Rep. (2017) 7:12425. doi: 10.1038/s41598-017-09635-x
46. McEwen, BS, and Akil, H. Revisiting the stress concept: implications for affective disorders. J Neurosci. (2020) 40:12–21. doi: 10.1523/JNEUROSCI.0733-19.2019
47. Sanacora, G, Yan, Z, and Popoli, M. The stressed synapse 2.0: pathophysiological mechanisms in stress-related neuropsychiatric disorders. Nat Rev Neurosci. (2022) 23:86–103. doi: 10.1038/s41583-021-00540-x
48. Crofford, LJ. Chronic pain: where the body meets the brain. Trans Am Clin Climatol Assoc. (2015) 126:167–83. Available at: https://pubmed.ncbi.nlm.nih.gov/26330672/
49. Joëls, M, and Baram, TZ. The neuro-symphony of stress. Nat Rev Neurosci. (2009) 10:459–66. doi: 10.1038/nrn2632
50. Rodrigues, SM, LeDoux, JE, and Sapolsky, RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. (2009) 32:289–313. doi: 10.1146/annurev.neuro.051508.135620
51. Arnsten, AFT. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. (2009) 10:410–22. doi: 10.1038/nrn2648
52. Roozendaal, B, McEwen, BS, and Chattarji, S. Stress, memory and the amygdala. Nat Rev Neurosci. (2009) 10:423–33. doi: 10.1038/nrn2651
53. Kudielka, BM, and Kirschbaum, C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. (2005) 69:113–32. doi: 10.1016/j.biopsycho.2004.11.009
54. Taylor, SE, Klein, LC, Lewis, BP, Gruenewald, TL, Gurung, RA, and Updegraff, JA. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol Rev. (2000) 107:411–29. doi: 10.1037/0033-295x.107.3.411
55. Kajantie, E, and Phillips, DIW. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. (2006) 31:151–78. doi: 10.1016/j.psyneuen.2005.07.002
56. Gervasio, J, Zheng, S, Skrotzki, C, and Pachete, A. The effect of oral contraceptive use on cortisol reactivity to the Trier social stress test: a meta-analysis. Psychoneuroendocrinology. (2022) 136:105626. doi: 10.1016/j.psyneuen.2021.105626
57. Jentsch, VL, Pötzl, L, Wolf, OT, and Merz, CJ. Hormonal contraceptive usage influences stress hormone effects on cognition and emotion. Front Neuroendocrinol. (2022) 67:101012. doi: 10.1016/j.yfrne.2022.101012
58. Merz, CJ, and Wolf, OT. Sex differences in stress effects on emotional learning. J Neurosci Res. (2017) 95:93–105. doi: 10.1002/jnr.23811
59. Stockhorst, U, and Antov, MI. Modulation of fear extinction by stress, stress hormones and estradiol: a review. Front Behav Neurosci. (2015) 9:359. doi: 10.3389/fnbeh.2015.00359
60. Meulders, A. Fear in the context of pain: lessons learned from 100 years of fear conditioning research. Behav Res Ther. (2020) 131:103635. doi: 10.1016/j.brat.2020.103635
61. Elsenbruch, S, Benson, S, Koenen, LR, Labrenz, F, and Icenhour, A. From gut feelings to memories of visceral pain. Neuroforum. (2020) 26:171–7. doi: 10.1515/nf-2020-0016
62. Koechlin, H, Coakley, R, Schechter, N, Werner, C, and Kossowsky, J. The role of emotion regulation in chronic pain: a systematic literature review. J Psychosom Res. (2018) 107:38–45. doi: 10.1016/j.jpsychores.2018.02.002
63. Zuloaga, DG, Heck, AL, De Guzman, RM, and Handa, RJ. Roles for androgens in mediating the sex differences of neuroendocrine and behavioral stress responses. Biol Sex Differ. (2020) 11:44. doi: 10.1186/s13293-020-00319-2
64. Craft, RM, Mogil, JS, and Aloisi, AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. (2004) 8:397–411. doi: 10.1016/j.ejpain.2004.01.003
65. Aloisi, AM. Gonadal hormones and sex differences in pain reactivity. Clin J Pain. (2003) 19:168–74. doi: 10.1097/00002508-200305000-00004
66. Aloisi, AM, and Bonifazi, M. Sex hormones, central nervous system and pain. Horm Behav. (2006) 50:1–7. doi: 10.1016/j.yhbeh.2005.12.002
67. Ji, Y, Hu, B, Li, J, and Traub, RJ. Opposing roles of estradiol and testosterone on stress-induced visceral hypersensitivity in rats. J Pain. (2018) 19:764–76. doi: 10.1016/j.jpain.2018.02.007
68. Archey, M, Goldey, K, Crockett, E, and Boyette-Davis, J. An investigation of the effects of testosterone and behavioral expressions of pain on sex/gender differences in pain perception. Psychol Rep. (2019) 122:826–40. doi: 10.1177/0033294118781320
69. Racine, M, Tousignant-Laflamme, Y, Kloda, LA, Dion, D, Dupuis, G, and Choinière, M. A systematic literature review of 10 years of research on sex/gender and pain perception—part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain. (2012) 153:619–35. doi: 10.1016/j.pain.2011.11.026
70. Fillingim, RB. Individual differences in pain: understanding the mosaic that makes pain personal. Pain. (2017) 158:S11–8. doi: 10.1097/j.pain.0000000000000775
71. Bartley, EJ, and Fillingim, RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. (2013) 111:52–8. doi: 10.1093/bja/aet127
72. Slavich, GM, and Sacher, J. Stress, sex hormones, inflammation, and major depressive disorder: extending social signal transduction theory of depression to account for sex differences in mood disorders. Psychopharmacology. (2019) 236:3063–79. doi: 10.1007/s00213-019-05326-9
73. Martínez-Lavín, M. Fibromyalgia in women: somatisation or stress-evoked, sex-dimorphic neuropathic pain? Clin Exp Rheumatol. (2021) 39:422–5. doi: 10.55563/clinexprheumatol/0c7d6v
74. Van Oudenhove, L, Crowell, MD, Drossman, DA, Halpert, AD, Keefer, L, Lackner, JM, et al. Biopsychosocial aspects of functional gastrointestinal disorders. Gastroenterology. (2016) S0016–5085(16)00218-3. doi: 10.1053/j.gastro.2016.02.027
75. Black, CJ, and Ford, AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. (2020) 17:473–86. doi: 10.1038/s41575-020-0286-8
76. Narayanan, SP, Anderson, B, and Bharucha, AE. Sex- and gender-related differences in common functional gastroenterologic disorders. Mayo Clin Proc. (2021) 96:1071–89. doi: 10.1016/j.mayocp.2020.10.004
77. Chapman, CR, Tuckett, RP, and Song, CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain. (2008) 9:122–45. doi: 10.1016/j.jpain.2007.09.006
78. Bartley, EJ, and Fillingim, RB. Chapter 4—sex differences in pain and stress In: M Al’absi and MA Flaten, editors. Neuroscience of pain, stress, and emotion. San Diego: Academic Press (2016). 77–95.
79. Hodes, GE, and Epperson, CN. Sex differences in vulnerability and resilience to stress across the life span. Biol Psychiatry. (2019) 86:421–32. doi: 10.1016/j.biopsych.2019.04.028
80. Gupta, D, and Morley, JE. Hypothalamic-pituitary-adrenal (HPA) axis and aging. Compr Physiol. (2014) 4:1495–510. doi: 10.1002/cphy.c130049
81. Chahal, HS, and Drake, WM. The endocrine system and ageing. J Pathol. (2007) 211:173–80. doi: 10.1002/path.2110
82. Murgatroyd, C, Patchev, AV, Wu, Y, Micale, V, Bockmühl, Y, Fischer, D, et al. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. (2009) 12:1559–66. doi: 10.1038/nn.2436
83. Chaloner, A, and Greenwood-Van Meerveld, B. Early life adversity as a risk factor for visceral pain in later life: importance of sex differences. Front Neurosci. (2013) 7:13. doi: 10.3389/fnins.2013.00013
84. Dannlowski, U, Stuhrmann, A, Beutelmann, V, Zwanzger, P, Lenzen, T, Grotegerd, D, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. (2012) 71:286–93. doi: 10.1016/j.biopsych.2011.10.021
85. Gupta, A, Mayer, EA, Acosta, JR, Hamadani, K, Torgerson, C, van Horn, JD, et al. Early adverse life events are associated with altered brain network architecture in a sex—dependent manner. Neurobiol Stress. (2017) 7:16–26. doi: 10.1016/j.ynstr.2017.02.003
86. Gupta, A, Kilpatrick, L, Labus, JS, Tillisch, K, Braun, A, Hong, J-Y, et al. Early adverse life events and resting state neural networks in patients with chronic abdominal pain: evidence for sex differences. Psychosom Med. (2014) 76:404–12. doi: 10.1097/PSY.0000000000000089
87. Korpela, K, Kallio, S, Salonen, A, Hero, M, Kukkonen, AK, Miettinen, PJ, et al. Gut microbiota develop towards an adult profile in a sex-specific manner during puberty. Sci Rep. (2021) 11:23297. doi: 10.1038/s41598-021-02375-z
88. Calcaterra, V, Rossi, V, Massini, G, Regalbuto, C, Hruby, C, Panelli, S, et al. Precocious puberty and microbiota: the role of the sex hormone-gut microbiome axis. Front Endocrinol. (2022) 13:1000919. doi: 10.3389/fendo.2022.1000919
89. Tsilimigras, MCB, Gharaibeh, RZ, Sioda, M, Gray, L, Fodor, AA, and Lyte, M. Interactions between stress and sex in microbial responses within the microbiota-gut-brain axis in a mouse model. Psychosom Med. (2018) 80:361–9. doi: 10.1097/PSY.0000000000000572
90. Esposito, P, and Ismail, N. Linking puberty and the gut microbiome to the pathogenesis of neurodegenerative disorders. Microorganisms. (2022) 10:2163. doi: 10.3390/microorganisms10112163
91. Farage, MA, Neill, S, and MacLean, AB. Physiological changes associated with the menstrual cycle: a review. Obstet Gynecol Surv. (2009) 64:58–72. doi: 10.1097/OGX.0b013e3181932a37
92. Cain, KC, Jarrett, ME, Burr, RL, Rosen, S, Hertig, VL, and Heitkemper, MM. Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Dig Dis Sci. (2009) 54:1542–9. doi: 10.1007/s10620-008-0516-3
93. Ruigómez, A, García Rodríguez, LA, Johansson, S, and Wallander, M-A. Is hormone replacement therapy associated with an increased risk of irritable bowel syndrome? Maturitas. (2003) 44:133–40. doi: 10.1016/s0378-5122(02)00321-3
94. Hägg, S, and Jylhävä, J. Sex differences in biological aging with a focus on human studies. eLife. (2021) 10:e63425. doi: 10.7554/eLife.63425
95. Gaffey, AE, Bergeman, CS, Clark, LA, and Wirth, MM. Aging and the HPA axis: stress and resilience in older adults. Neurosci Biobehav Rev. (2016) 68:928–45. doi: 10.1016/j.neubiorev.2016.05.036
96. Soenen, S, Rayner, CK, Jones, KL, and Horowitz, M. The ageing gastrointestinal tract. Curr Opin Clin Nutr Metab Care. (2016) 19:12–8. doi: 10.1097/MCO.0000000000000238
97. Camilleri, M. Diagnosis and treatment of irritable bowel syndrome: a review. JAMA. (2021) 325:865–77. doi: 10.1001/jama.2020.22532
Keywords: cortisol, gonadal hormones, gut-brain axis, pain, sex differences, visceroception
Citation: Labrenz F, Merz CJ and Icenhour A (2023) Connecting dots in disorders of gut-brain interaction: the interplay of stress and sex hormones in shaping visceral pain. Front. Psychiatry. 14:1204136. doi: 10.3389/fpsyt.2023.1204136
Edited by:
Andreas Stengel, University Hospital Tübingen, GermanyReviewed by:
Anthony C. Johnson, United States Department of Veterans Affairs, United StatesShengliang Chen, Shanghai Jiao Tong University, China
Copyright © 2023 Labrenz, Merz and Icenhour. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adriane Icenhour, YWRyaWFuZS5pY2VuaG91ckBydWIuZGU=
 Franziska Labrenz
Franziska Labrenz Christian J. Merz
Christian J. Merz Adriane Icenhour
Adriane Icenhour