
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 18 May 2023
Sec. Neuroimaging
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1201256
This article is part of the Research Topic Social-Related Biomarkers and Potential Noninvasive Treatments for Sub-clinical and Clinical Emotional Disorders View all 5 articles
Background: Late-life depression (LLD) is linked to various medical conditions and influenced by aging-related processes. Sleep disturbances and insomnia symptoms may be early indicators or risk factors for depression. Neuroimaging studies have attempted to understand the neural mechanisms underlying LLD, focusing on different brain networks. This study aims to further delineate discriminative brain structural profiles for LLD with insomnia using MRI.
Methods: We analyzed 24 cases in the LLD with insomnia group, 26 cases in the LLD group, and 26 in the healthy control (HC) group. Patients were evaluated using the Hamilton Depression Rating Scale (HAMD-17), Hamilton Anxiety Rating Scale (HAMA), Mini-Mental State Examination (MMSE), and Pittsburgh Sleep Quality Index (PSQI). Structural MRI data were gathered and analyzed using voxel-based morphometry (VBM) to identify differences in gray matter volume (GMV) among the groups. Correlation analyses were conducted to explore the relationships between GMV and clinical characteristics.
Results: Significant difference in sex distribution was observed across the groups (p = 0.029). However, no significant differences were detected in age and MMSE scores among the groups. LLD with insomnia group exhibited significantly higher HAMA (p = 0.041) and PSQI scores (p < 0.05) compared to the LLD group. ANOVA identified significant difference in GMV of anterior lobe of cerebellum (peak MNI coordinate: x = 52, y = −40, z = −30) among HC, LLD, and LLD with insomnia. Post-hoc two-sample t-tests revealed that the significant difference in GMV was only found between the LLD group and the HC group (p < 0.05). The mean GMV in the cerebellum was positively correlated with HAMA scale in LLD patients (r = 0.47, p < 0.05).
Conclusion: There is significant difference in GMV in the LLD group, the association between late-life depression and insomnia may be linked to anxiety. This study provides insights into the discriminative brain structural profiles of LLD and LLD with insomnia, advancing the understanding of the underlying neural mechanisms and potential targets for intervention.
The aging population is a growing global issue, with the United Nations forecasting that by 2050, one in six people worldwide will be over 65 years old (1). Mental disorders, such as depression and anxiety, are a major contributor to the global burden of disease in the elderly (2). Approximately 16% of older adults living in the community may experience clinically significant symptoms of depression, with an incidence of 29% in Europe (3). In Southeast Asia, the overall prevalence of late-life depression (LLD) is estimated to be 42.0%, and in China, the prevalence is approximately 20% (4, 5). LLD is characterized by persistent feelings of sadness, hopelessness, a lack of interest in activities, executive dysfunction, impaired cognitive function, fatigue and insomnia (6–8). Changes in sleep patterns are a normal part of aging, however, older adults may have difficulty falling asleep and staying asleep due to awakenings, and tend to fall asleep earlier and wake up earlier than younger adults (9). Several studies have indicated that insomnia symptoms, excessive daytime sleepiness, and drug use independently increase the risk of subsequent depression in older adults. Sleep disturbance and long-term use of sleeping pills may be early indicators or potentially risk factors for depression (10–13).
LLD is linked to a variety of medical conditions and is thought to be influenced by aging-related processes (14). Potential causes of LLD include vascular damage, chronic inflammation, and dysfunction in brain networks (15–18). Studies using neuroimaging techniques have attempted to understand the neural mechanisms underlying LLD, with a focus on networks such as the executive control network (ECN), default mode network (DMN), cognitive control network (CCN), affective prelimbic network, and corticostriatal circuits (19). Functional connectivity (FC) impairments within or between the frontoparietal, dorsal attention, visual networks (20), as well as subcortical networks of thalamus, basal ganglia, and ventral striatum (21, 22) have been observed in individuals with LLD. Additionally, gray matter reductions have been observed in the anterior cingulate cortex, dorsolateral, and dorsomedial prefrontal cortex in LLD patients (21). Insomnia has been also linked to dysfunction in both intra-hemispheric and inter-hemispheric connectivity (22). Studies have identified increased connectivity in certain brain regions of the DMN and frontoparietal network (23), bilateral hippocampus, and left middle frontal gyrus (24, 25). However, the current existing neuroimaging findings about LLD are lack of consistency since other studies also found reductions in connectivity in areas such as the anterior cingulate cortex, orbital frontal cortex, hippocampus, caudate, and putamen (26). Although several studies have identified structural and functional abnormalities in LLD, the specific brain structural changes associated with LLD with insomnia remain unclear. To further delineate discriminative brain structural profiles for LLD with insomnia, structural MRI were acquired. We employed voxel-based morphometry (VBM) to analyze gray matter volume (GMV) in LLD patients with insomnia. Our hypothesis is that affective brain regions in LLD with insomnia may exhibit different GMV patterns.
In this retrospective study, we scrutinized cases obtained from the Clinical Psychology and Geriatrics departments at the First People’s Hospital of Yunnan Province spanning from January 2021 to October 2022. All included patients were aged over 60 years and right-handed. Additionally, patients underwent Hamilton Depression Rating Scale (HAMD-17), Hamilton Anxiety Rating Scale (HAMA), and Mini-Mental State Examination (MMSE) scoring. Patients with LLD were included based on the DSM-V diagnostic criteria and HAMD-17 scores ≥17. Sleep assessments were conducted using the Pittsburgh Sleep Quality Index (PSQI) criteria, with insomnia defined as PSQI scores ≥5. Scores were based on sleep quality, sleep onset latency, sleep duration, sleep efficiency, sleep disturbances, hypnotic medication use, and daytime dysfunction dimensions (48). Group A comprised patients with LLD and insomnia, Group B consisted of patients with LLD, and Group C served as the healthy control (HC) group with HAMD-17 scores below 17 and PSQI scores below 5.
Exclusion criteria included: severe central nervous system diseases such as ischemic stroke, cerebral hemorrhage, or subarachnoid hemorrhage, accompanied by Parkinson’s disease or multiple sclerosis; abnormal brain white matter caused by carbon monoxide poisoning, hydrocephalus, or hypoxic brain injury; respiratory diseases affecting sleep, like cough variant asthma and obstructive sleep apnea hypopnea syndrome (OSAHS); severe cardiovascular, digestive, and urinary system diseases; long-term alcohol consumption exceeding 14 drinks per week; a history of bipolar disorder, personality disorder, or schizophrenia; and inability to undergo a brain MRI examination.
Initially, 138 patients met these criteria; however, due to factors such as MRI artifacts affecting the magnetic resonance data processing, only 76 cases were ultimately retained. The final sample included 24 cases in the LLD with insomnia group, 26 in the LLD group, and 26 in the healthy control group.
The structural MRI data were acquired using a SIEMENS 1.5-T (Magnetom Skyra, Siemens-Healthcare) scanner with a standard head coil. Image acquisition was performed in the Department of Magnetic Resonance Imaging, The First People’s Hospital of Yunnan Province. For each subject, a high-resolution Fluid Attenuated Inversion Recovery (FLAIR) 3D sequence has been acquired with parameters of Slice Thickness 0.7 mm without gap, repetition time (RT) = 2,200 ms, echo time (ET) = 5.88 ms, flip angle (FA) = 25°, covering 118 axial slices with an in-plane resolution of 0.69 mm × 0.69 mm.
The high-spatial-resolution structural MRI data were processed using the VBM Toolbox (VBM8)1 in the statistical parametric mapping software (SPM 8).2 First, any images with artifacts were removed. Next, the images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) using the default settings in VBM8, and then normalized to the Montreal Neurologic Institute (MNI) space and modulated. After the quality of the segmentation was confirmed, the segmented GM images were smoothed using an 8-mm full-width-at-half-maximum Gaussian kernel for group statistical comparisons. A one-way analysis of variance (ANOVA) with sex, anxiety level, and total brain volume as a covariates was used to identify any significant differences in GMV between LLD with insomnia, LLD, and HC groups. The significant level was determined using Alphasim corrected method with p < 0.05 at the voxel-level threshold of p < 0.005.
A one-way ANOVA was initially employed to detect differences in demographic and clinical features. Subsequently, post-hoc two-sample t-tests were utilized to ascertain between-group differences for all indices. For gender comparisons, a Pearson chi-squared test was conducted. Two-sample t-tests were applied to examine other variables.
In order to establish if there was a relationship between changes in GMV and clinical features, correlation analyses were carried out with a significance level set at p < 0.05, without correction.
The details of demographics and clinical characteristics of all the subjects are shown in Table 1. A significant difference in sex distribution was observed across the groups (p = 0.029), with a higher proportion of females in the LLD with insomnia and LLD groups compared to the HC group. However, no significant differences were detected in age and MMSE scores among the groups. Both the LLD with insomnia (p < 0.05) and LLD (p < 0.05) groups demonstrated significantly higher HAMD and HAMA scores compared to the HC group. Furthermore, the LLD with insomnia group exhibited significantly higher HAMA (p = 0.041) and PSQI scores (p < 0.05) compared to the LLD group.
ANOVA identified significant difference in GMV of anterior lobe of cerebellum (peak MNI coordinate: x = 52, y = −40, z = −30) among HC, LLD, and LLD with insomnia (Figure 1A). Post-hoc two-sample t-tests revealed that the significant difference in GMV was only found between the LLD group and the HC group (p < 0.05) (Figure 1B). The mean GMV in the cerebellum was positively correlated with HAMA scale in LLD patients (r = 0.47, p < 0.05) (Figure 2).
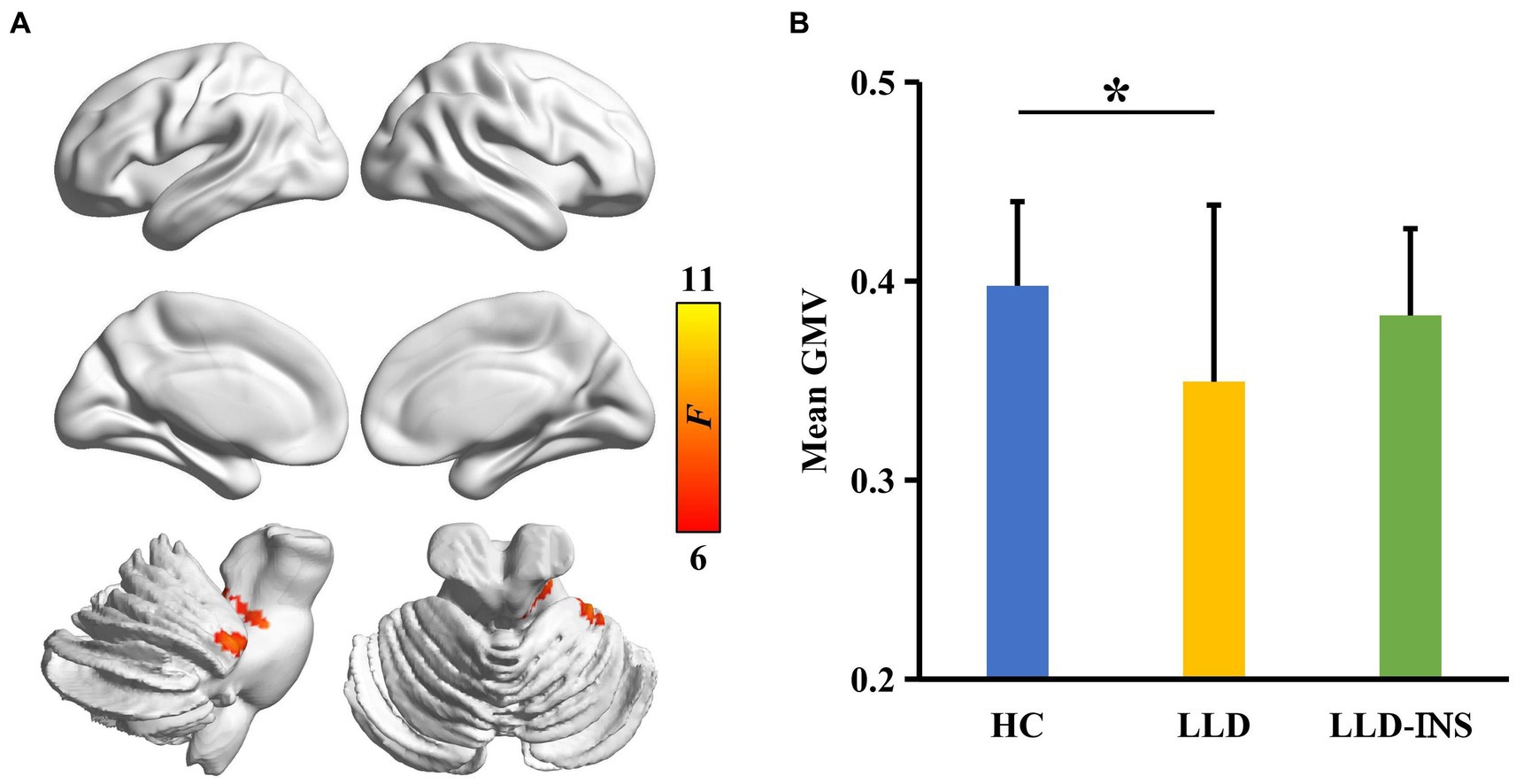
Figure 1. (A) Analysis of variance (ANOVA) with sex as a covariate was performed to identify gray matter volume (GMV) differences across LLD with insomnia, LLD, and HC groups, revealing a significant difference in GMV within the cerebellum. (B) Post-hoc two-sample t-tests were conducted between each pair of groups, identifying a significant difference in GMV between the LLD and HC groups.
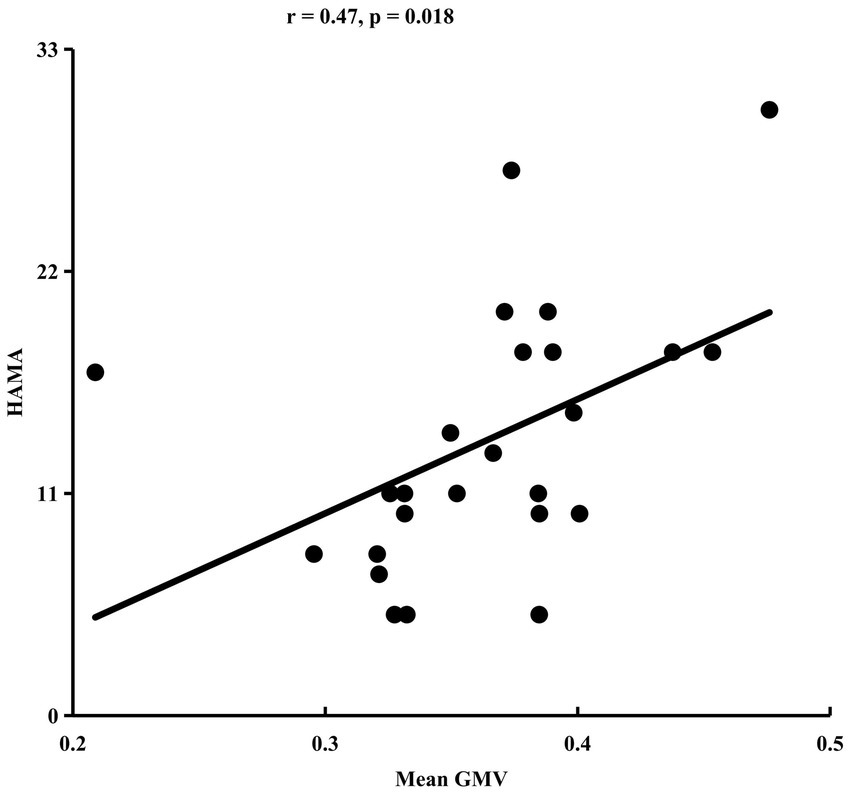
Figure 2. Gray matter volume (GMV) in the cerebellum correlates with HAMA scores in late-life depression (LLD).
Our results have shown that LLD patients with insomnia have higher HAMA scores than the LLD group. This is in line with previous research that has found high rates of comorbid anxiety disorders (83.62%) and insomnia (74.52%) in LLD patients. Vice versa, insomnia serves as a risk factor for the development of both depression (27). Additionally, the large-sample study has revealed that the average cost of the depression-anxiety group was higher than that of the non-anxiety group (28). One of the unique aspects of insomnia in older adults is that it often co-occurs with other chronic medical conditions, such as heart disease, stroke and diabetes (29). In older adults, somatic symptoms associated with these conditions may contribute to anxiety, which in turn may aggravate insomnia or interact with each other (30, 31). The HAMD-17 scale, frequently employed for assessing depression, may lack the sensitivity required to differentiate between somatic symptoms and the impacts of insomnia (30). Therefore, when evaluating insomnia in older adults, it is important to consider both anxiety symptoms and chronic conditions to gain a comprehensive understanding of underlying causes and potential treatment options. It is also important to be aware that when sedative drugs are used in the elderly, they may increase the risk of all-cause dementia and Alzheimer’s disease (AD) (32).
The most significant difference in GMV between the three groups was found in cerebellum. The cerebellum, a vital motor organ, plays a crucial role in maintaining balance and controlling voluntary movement, particularly in coordinating skilled visual movement with the cerebral cortex (33). However, recent researches have uncovered that cerebellum is far more extensive and complex than previously reported (34). The cerebellum is involved in a diverse range of learning and timing processes, and different sub-regions of the cerebellum utilize different forms of plasticity. In addition to the motor functions, it also plays a vital role in regulating cognitive processes, learning and emotions. The cerebellum maintains extensive connections with various brain structures, including the reticular system, hypothalamus, limbic system, and neocortical association areas (35). Moreover, the cerebellum acts as brain connectome and neural circuits, consolidating information originating from nuclei such as the subthalamic nucleus within the basal ganglia and the dentate nucleus situated in the cerebellum itself. This information is then relayed to the cerebral cortex, establishing cohesive networks that orchestrate motor, cognitive, and emotional processes (36, 37). Damage to the cerebellum can cause motor symptoms such as dysmetria, dysarthria, and ataxia, as well as cognitive and affective symptoms, referred as cerebellar cognitive affective syndrome (38). Cerebellar dysfunction also disrupts normal sleep–wake cycles and causes various sleep disorders such as insomnia, excessive daytime sleepiness, rapid eye movement (REM) behavioral disturbances, and sleep apnea (39). Studies have shown a strong correlation between sleep disturbances and cerebellar pathology, possibly due to the close connections between the cerebellum and cerebral cortex. Sleep is crucial for cognitive processes and the interaction between the cerebral cortex and cerebellum during both wakefulness and sleep, contributes to memory consolidation (40).
The brain has complex connectivities, and different brain regions interacting in intricate and dynamic ways are responsible for different functions. The specific pattern of connections plays a crucial role in determining the onset, manifestation, and progression of various brain disorders (41). Understanding the underlying connectivity patterns in the brain may help to better understand the mechanisms that drive these disorders and identify potential targets for treatment. Functional MRI studies have revealed alterations in functional networks within specific brain regions in patients with persistent insomnia, such as changes in activity in the cingulate and right para-hippocampus, cerebellum, and superior frontal gyrus (42). Additionally, anxious patients may have decreased connectivity within hippocampus and amygdala and increased or decreased connectivity with cerebellum (43, 44). A previous study has reported that individuals with poor subjective sleep quality tend to have reduced cognitive function and more severe depressive symptoms (45). Our results indicate that there is a positive correlation between mean cerebellar GMV and scores on the HAMA scale. Previous researches have also demonstrated that anxiety lead to changes in local gray matter volume, which is positively correlated with the volumes of the dorsomedial prefrontal cortex and the anterior cingulate cortex (46, 47). However, this does not establish a causal link between gray matter volume and anxiety. It is important to note that this is a bidirectional relationship, where insomnia may lead to LLD, and vice versa.
Firstly, due to the nature of this observational study, it is incapable of establishing causality, and the findings merely indicate correlations. Secondly, the limited sample size restricts the generalizability of the results and the study’s capacity to discern potential differences. Consequently, cautious interpretation of the results is crucial, and alternative explanations for the findings should be considered. It is also worth noting that VBM is a relatively nascent technique, with ongoing research dedicated to refining methodologies and enhancing accuracy. Moreover, VBM analyses are influenced by the age of the study population, and the understanding of brain structural changes in elderly populations remains limited.
The association between late-life depression and insomnia may be linked to anxiety, which could stem from age-related or somatic diseases in the elderly. When addressing sleep concerns in this population, it is essential to also consider the potential presence of chronic diseases or somatic symptoms. The cerebellum’s role in late-life depression should not be overlooked, as it may offer new perspectives and research directions for understanding and addressing depression in older adults with insomnia.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the institutional review board of the First People’s Hospital of Yunnan Province. The patients/participants provided their written informed consent to participate in this study.
HS, NL, MC, and HC performed the conceptualization, methodology, drafting of the original manuscript, supervision, and validation. JZ collected and processed the MRI data. MZ, JY, and JX handled methodology, statistical analysis, data management, and review. HS and NL reviewed, edited, and proofreaded. All authors contributed to this article and approved the submitted version.
This study was supported by the study of relationship between the expression of vascular growth factor and the prognosis of type 2 diabetes mellitus combined with major depressive disorder (Grant No. 202001AY070001-035); Yunnan Clinical Research Center for Mental Diseases (Grant No. 0105679005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Nations U. Ageing [Internet]. United Nations United Nations. Available at: https://www.un.org/en/global-issues/ageing
2. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Psychiatry. (2022) 9:137–50. doi: 10.1016/S2215-0366(21)00395-3
3. Horackova, K, Kopecek, M, Machů, V, Kagstrom, A, Aarsland, D, Motlova, LB, et al. Prevalence of late-life depression and gap in mental health service use across European regions. Eur Psychiatry. (2019) 57:19–25. doi: 10.1016/j.eurpsy.2018.12.002
4. Tang, T, Jiang, J, and Tang, X. Prevalence of depressive symptoms among older adults in mainland China: a systematic review and meta-analysis. J Affect Disord. (2021) 293:379–90. doi: 10.1016/j.jad.2021.06.050
5. Assariparambil, AR, Noronha, JA, Kamath, A, Adhikari, P, Nayak, BS, Shankar, R, et al. Depression among older adults: a systematic review of south Asian countries. Psychogeriatrics. (2021) 21:201–19. doi: 10.1111/psyg.12644
6. Belvederi Murri, M, Amore, M, Respino, M, and Alexopoulos, GS. The symptom network structure of depressive symptoms in late-life: results from a European population study. Mol Psychiatry. (2020) 25:1447–56. doi: 10.1038/s41380-018-0232-0
7. Kok, RM, and Reynolds, CF III. Management of depression in older adults: a review. JAMA. (2017) 317:2114–22. doi: 10.1001/jama.2017.5706
8. Rodda, J, Walker, Z, and Carter, J. Depression in older adults. BMJ. (2011) 343:d5219. doi: 10.1136/bmj.d5219
9. Gulia, KK, and Kumar, VM. Sleep disorders in the elderly: a growing challenge. Psychogeriatrics. (2018) 18:155–65. doi: 10.1111/psyg.12319
10. Cho, HJ, Lavretsky, H, Olmstead, R, Levin, MJ, Oxman, MN, and Irwin, MR. Sleep disturbance and depression recurrence in community-dwelling older adults: a prospective study. Am J Psychiatry. (2008) 165:1543–50. doi: 10.1176/appi.ajp.2008.07121882
11. Jaussent, I, Bouyer, J, Ancelin, ML, Akbaraly, T, Peres, K, Ritchie, K, et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. (2011) 34:1103–10. doi: 10.5665/SLEEP.1170
12. Oxford. Insomnia, Depression, and Physical Disorders in Late Life: A 2-year Longitudinal Community Study in Koreans | SLEEP | Oxford academic [internet]. doi: 10.1093/sleep/32.9.1221
13. Pigeon, WR, Hegel, M, Unützer, J, Fan, MY, Sateia, MJ, Lyness, JM, et al. Is insomnia a perpetuating factor for late-life depression in the IMPACT cohort? Sleep. (2008) 31:481–8. doi: 10.1093/sleep/31.4.481
14. Alexopoulos, GS. Mechanisms and treatment of late-life depression. Transl Psychiatry. (2019) 9:1–16. doi: 10.1038/s41398-019-0514-6
15. Taylor, WD, Aizenstein, HJ, and Alexopoulos, GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. (2013) 18:963–74. doi: 10.1038/mp.2013.20
16. Alexopoulos, GS, Hoptman, MJ, Kanellopoulos, D, Murphy, CF, Lim, KO, and Gunning, FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. (2012) 139:56–65. doi: 10.1016/j.jad.2011.12.002
17. Leonard, BE, and Myint, A. Changes in the immune system in depression and dementia: causal or coincidental effects? Dialogues Clin Neurosci. (2006) 8:163–74. doi: 10.31887/DCNS.2006.8.2/bleonard
18. Naismith, SL, Norrie, LM, Mowszowski, L, and Hickie, IB. The neurobiology of depression in later-life: clinical, neuropsychological, neuroimaging and pathophysiological features. Prog Neurobiol. (2012) 98:99–143. doi: 10.1016/j.pneurobio.2012.05.009
19. Tadayonnejad, R, and Ajilore, O. Brain network dysfunction in late-life depression: a literature review. J Geriatr Psychiatry Neurol. (2014) 27:5–12. doi: 10.1177/0891988713516539
20. AJP. Coordinate-Based Network Mapping of Brain Structure in Major Depressive Disorder in Younger and Older Adults: A Systematic Review and Meta-Analysis | American Journal of Psychiatry [Internet]. doi: 10.1176/appi.ajp.2021.21010088
21. Bora, E, Fornito, A, Pantelis, C, and Yücel, M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J Affect Disord. (2012) 138:9–18. doi: 10.1016/j.jad.2011.03.049
22. Fasiello, E, Gorgoni, M, Scarpelli, S, Alfonsi, V, Ferini Strambi, L, and De Gennaro, L. Functional connectivity changes in insomnia disorder: a systematic review. Sleep Med Rev. (2022) 61:101569. doi: 10.1016/j.smrv.2021.101569
23. Holub, F, Petri, R, Schiel, J, Feige, B, Rutter, MK, Tamm, S, et al. Associations between insomnia symptoms and functional connectivity in the UK biobank cohort (n = 29,423). J Sleep Res :e13790. doi: 10.1111/jsr.13790
24. Leerssen, J, Wassing, R, Ramautar, JR, Stoffers, D, Lakbila-Kamal, O, Perrier, J, et al. Increased hippocampal-prefrontal functional connectivity in insomnia. Neurobiol Learn Mem. (2019) 160:144–50. doi: 10.1016/j.nlm.2018.02.006
25. Gong, L, Xu, R, Liu, D, Zhang, C, Huang, Q, Zhang, B, et al. Abnormal functional connectivity density in patients with major depressive disorder with comorbid insomnia. J Affect Disord. (2020) 266:417–23. doi: 10.1016/j.jad.2020.01.088
26. Li, M, Wang, R, Zhao, M, Zhai, J, Liu, B, Yu, D, et al. Abnormalities of thalamus volume and resting state functional connectivity in primary insomnia patients. Brain Imaging Behav. (2019) 13:1193–201. doi: 10.1007/s11682-018-9932-y
27. Hertenstein, E, Feige, B, Gmeiner, T, Kienzler, C, Spiegelhalder, K, Johann, A, et al. Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev. (2019) 43:96–105. doi: 10.1016/j.smrv.2018.10.006
28. Wang, X, Rao, W, Chen, X, Zhang, X, Wang, Z, Ma, X, et al. The sociodemographic characteristics and clinical features of the late-life depression patients: results from the Beijing Anding hospital mental health big data platform. BMC Psychiatry. (2022) 22:677.doi: 10.1186/s12888-022-04339-7
29. Foley, DJ, Monjan, A, Simonsick, EM, Wallace, RB, and Blazer, DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep J Sleep Res Sleep Med. (1999) 22:S366–72.
30. Vaccarino, AL, Evans, KR, Sills, TL, and Kalali, AH. Symptoms of anxiety in depression: assessment of item performance of the Hamilton anxiety rating scale in patients with depression. Depress Anxiety. (2008) 25:1006–13. doi: 10.1002/da.20435
31. Cambridge. Comorbid anxiety disorders in late-life depression: results of a cohort study | International Psychogeriatrics | Cambridge Core [Internet]. Available at: https://www.cambridge.org/core/journals/international-psychogeriatrics/article/abs/comorbid-anxiety-disorders-in-latelife-depression-results-of-a-cohort-study/11DE9637A5EBD2955E9DEAB990A72131
32. Cavaillès, C, Berr, C, Helmer, C, Gabelle, A, Jaussent, I, and Dauvilliers, Y. Complaints of daytime sleepiness, insomnia, hypnotic use, and risk of dementia: a prospective cohort study in the elderly. Alzheimers Res Ther. (2022) 14:12. doi: 10.1186/s13195-021-00952-y
33. Glickstein, M, Sultan, F, and Voogd, J. Functional localization in the cerebellum. Cortex. (2011) 47:59–80. doi: 10.1016/j.cortex.2009.09.001
34. de Zeeuw, CI, Lisberger, SG, and Raymond, JL. Diversity and dynamism in the cerebellum. Nat Neurosci. (2021) 24:160–7. doi: 10.1038/s41593-020-00754-9
35. Schmahmann, JD. The role of the cerebellum in affect and psychosis. J Neurolinguistics. (2000) 13:189–214. doi: 10.1016/S0911-6044(00)00011-7
36. Cheng, B, Xu, H, Zhou, H, Guo, Y, Roberts, N, Li, N, et al. Connectomic disturbances in Duchenne muscular dystrophy with mild cognitive impairment. Cereb Cortex. (2023):bhac542. doi: 10.1093/cercor/bhac542
37. Bostan, AC, and Strick, PL. The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci. (2018) 19:338–50. doi: 10.1038/s41583-018-0002-7
38. Stoodley, CJ, and Schmahmann, JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. (2010) 46:831–44. doi: 10.1016/j.cortex.2009.11.008
39. Song, B, and Zhu, JC. A narrative review of cerebellar malfunctions and sleep disturbances. Front Neurosci. (2021) 15. doi: 10.3389/fnins.2021.739138
40. Canto, CB, Onuki, Y, Bruinsma, B, van der Werf, YD, and De Zeeuw, CI. The sleeping cerebellum. Trends Neurosci. (2017) 40:309–23. doi: 10.1016/j.tins.2017.03.001
41. Fornito, A, Zalesky, A, and Breakspear, M. The connectomics of brain disorders. Nat Rev Neurosci. (2015) 16:159–72. doi: 10.1038/nrn3901
42. Jiang, B, He, D, Guo, Z, and Gao, Z. Effect-size seed-based d mapping of resting-state fMRI for persistent insomnia disorder. Sleep Breath. (2020) 24:653–9. doi: 10.1007/s11325-019-02001-3
43. Dai, XJ, Nie, X, Liu, X, Pei, L, Jiang, J, Chang, PD, et al. Gender differences in regional brain activity in patients with chronic primary insomnia: evidence from a resting-state fMRI study. J Clin Sleep Med. 12:363–74. doi: 10.5664/jcsm.5586
44. Kolesar, TA, Bilevicius, E, Wilson, AD, and Kornelsen, J. Systematic review and meta-analyses of neural structural and functional differences in generalized anxiety disorder and healthy controls using magnetic resonance imaging. NeuroImage Clin. (2019) 24:102016. doi: 10.1016/j.nicl.2019.102016
45. Sonni, A, Kurdziel, LBF, Baran, B, and Spencer, RMC. The effects of sleep dysfunction on cognition, affect, and quality of life in individuals with cerebellar Ataxia. J Clin Sleep Med JCSM. (2014) 10:535–43. doi: 10.5664/jcsm.3706
46. Hilbert, K, Lueken, U, and Beesdo-Baum, K. Neural structures, functioning and connectivity in generalized anxiety disorder and interaction with neuroendocrine systems: a systematic review. J Affect Disord. (2014) 158:114–26. doi: 10.1016/j.jad.2014.01.022
47. Schienle, A, Ebner, F, and Schäfer, A. Localized gray matter volume abnormalities in generalized anxiety disorder. Eur Arch Psychiatry Clin Neurosci. (2011) 261:303–7. doi: 10.1007/s00406-010-0147-5
Keywords: voxel-based morphometry, late-life depression, insomnia, anxiety, cerebellum
Citation: Shao H, Li N, Chen M, Zhang J, Chen H, Zhao M, Yang J and Xia J (2023) A voxel-based morphometry investigation of brain structure variations in late-life depression with insomnia. Front. Psychiatry. 14:1201256. doi: 10.3389/fpsyt.2023.1201256
Received: 06 April 2023; Accepted: 28 April 2023;
Published: 18 May 2023.
Edited by:
Xiaolei Xu, Shandong Normal University, ChinaReviewed by:
Bochao Cheng, Sichuan University, ChinaCopyright © 2023 Shao, Li, Chen, Zhang, Chen, Zhao, Yang and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Li, ZzA5NDFhQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.