- 1Faculty of Medicine, University of Lisbon, Lisbon, Portugal
- 2CISP – Centro de Investigação em Saúde Pública, ENSP, Lisbon, Portugal
- 3Centro Hospitalar Universitário de Lisboa Norte (CHULN), Lisbon, Portugal
- 4Portuguese Institute for Sea and Atmosphere (IPMA), Lisbon, Portugal
Numerous studies have described associations between the omega-3 index (defined as the RBC percentage of EPA and DHA) and mental conditions, but no risk stratification or target value has gathered consensus so far. This narrative review aims to summarize the published data on the association between omega-3 index and mental illness and to contribute to the concept of an omega-3 index in the field of mental health. The bibliographic searches have been carried out in PubMed, Scopus and Web of Science databases to find relevant English language original research studies related to that association. The study search and selection process were registered in a PRISMA flow. Thirty-six studies were included in this review examining the links between omega-3 index and postpartum depression (3), major depression (15), major depression and bipolar disorder (1), bipolar disorder (4), schizophrenia and major depression (1), schizophrenia and other psychosis (5) and dementia (7). Thirty of these studies found either significant differences in omega-3 index between patients and controls or inverse relationships between omega-3 index and disease severity. The published evidence is compelling enough to suggest omega-3 index as a risk factor for some psychiatric diseases, specifically, major depression, postpartum depression, psychosis, and dementia. In occidental populations, we propose a risk threshold of (a) 4–5% in major depression and dementia, (b) 5% in postpartum depression, and (c) 4% for psychosis transition.
1. Introduction
The relationship of health with long-chain omega-3 fatty acids – also referred to as long chain polyunsaturated fatty acids or n-3 fatty acids (n-3 FA) - mainly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) – was discovered in 1979 (1) and a plethora of studies have been carried since then, reflecting an increasing interest in the prognostic value of their body levels (2).
The main dietary source of n-3 FA intake is fish (especially fatty species). While EPA and DHA can be synthesized from alpha-linolenic acid (ALA) in humans, most studies suggest that the conversion of ALA to EPA is less than 5% and the conversion to DHA is less than 0.05% (3). N-3 FA blood levels, metabolism and uptake from bloodstream can be affected by individual conditions and genetic determinants (4, 5).
Various means of measuring the expression of n-3 FA status have been used in research and clinical medicine. The omega-3 index (O3I) – defined as the sum of EPA and DHA content of red blood cells (RBC), expressed as a percentage of their total fatty acids - was initially proposed by Harris and von Schacky as a biomarker of the cardiac membrane content in omega-3 fatty acids and as a risk factor for coronary heart disease, especially sudden cardiac death. These authors concluded that the risk decreased by about 90% when O3I increased from 4% to values greater than 8% and defined a risk scale for death from coronary disease (0–4% high risk; 4–8%: intermediate risk; greater than 8%: desirable situation) (6).
O3I reflects other tissues’ content in EPA and DHA (7). It is acknowledged as a reliable long-term marker of n-3 FA intake and has shown to independently deliver predictive information for several illnesses (2). Depression, psychological stress, schizophrenia and dementia have been associated to the elevation of proinflammatory cytokines, including interleukin-1 beta, −2, −6 and − 18, interferon-gamma, and tumor necrosis factor alpha, which results in alterations of neurotransmitter precursors and metabolism as well as in activation of the hypothalamic–pituitary axis (8–10).
The role of n-3 FA in brain structure and functioning is not completely understood. Current knowledge has been largely built on the effects of their deficiency in animal models and on clinical trials that assessed the outcomes of supplementation in various psychiatric disorders. N-3 FA influence central nervous system health through molecular, cellular and neurobiological mechanisms (11) in three main areas – neuronal membranes, cytokine modulation and perfusion (12–15), all involved in the pathophysiology of depression, anxiety, schizophrenia and dementia.
As components of central nervous system membrane phospholipid acyl chains, n-3 FA are critical to their structure and function (16). Proteins embedded in the lipid bilayer of the cell act as transporters and receptors. Their conformation and, consequently, membrane fluidity, ion exchange, signaling and neurotransmission can be changed by n-3 FA (17, 18).
N-3 FA deficiency has been shown to alter the reservoir and release of serotonin and dopamine in animal models (19). The improvement in mood disorders associated with n-3 FA high levels may result from a modulation of membrane-bound receptors and enzymes involved in serotonergic neurotransmission and dopaminergic function (20) and better brain perfusion (21).
DHA has exhibited a protective effect against amyloid beta (Aβ) accumulation and its associated oxidative stress (15), inflammation, synaptic loss, tau protein hyperphosphorylation and the formation of neurofibrillary tangles (22) that occur in dementia.
The effects of n-3 FA supplementation on psychiatric illnesses have been studied in randomized clinical trials that are beyond the scope of this review. They have, nevertheless, brought complementary insights into the actions and mechanisms involved in n-3 FA interactions with the brain structure and function (23–26).
Despite numerous studies describing associations between O3I and mental conditions, no risk stratification or target O3I has so far gathered consensus in the field of mental health.
This narrative review aims to summarize the published data on the relationships between O3I and mental illness as well as to contribute to the concept of an O3I into the field of mental health raised by Milte et al. (27) and endorsed by other authors (28–32), helping physicians in identifying patients at higher risk and monitoring disease progression.
2. Materials and methods
The bibliographic searches were performed in PubMed, Scopus and Web of Science databases to find relevant English language original research studies evaluating associations between O3I and mental conditions. Each database was searched from inception to December 2022. These database searches validate exhaustive exploration and limit publication bias.
The primary search strategy was (“omega-3 index” OR “omega 3 index” OR EPA OR DHA) AND (depression OR anxiety OR bipolar OR schizophrenia OR psychosis OR attention OR hyperactivity OR dementia OR mental disease). One reviewer decided on the eligibility and data to extract, assisted in screening and selection by the web application Ryyan® 2022. Only publications including at least one association of O3I (described as such or as the percentage of EPA and DHA sum in total fatty acids) measured in RBC with a mental illness score calculated through a validated scale were included. Omega-3 supplementation clinical trials were included when baseline data was provided. The PRISMA flow diagram (33) was used to register the search steps and outcomes. The quality of the manuscript was evaluated by all the authors as per the SANRA six-item scale (34). As this review involved data from published studies only, institutional review board approval was not required.
3. Results
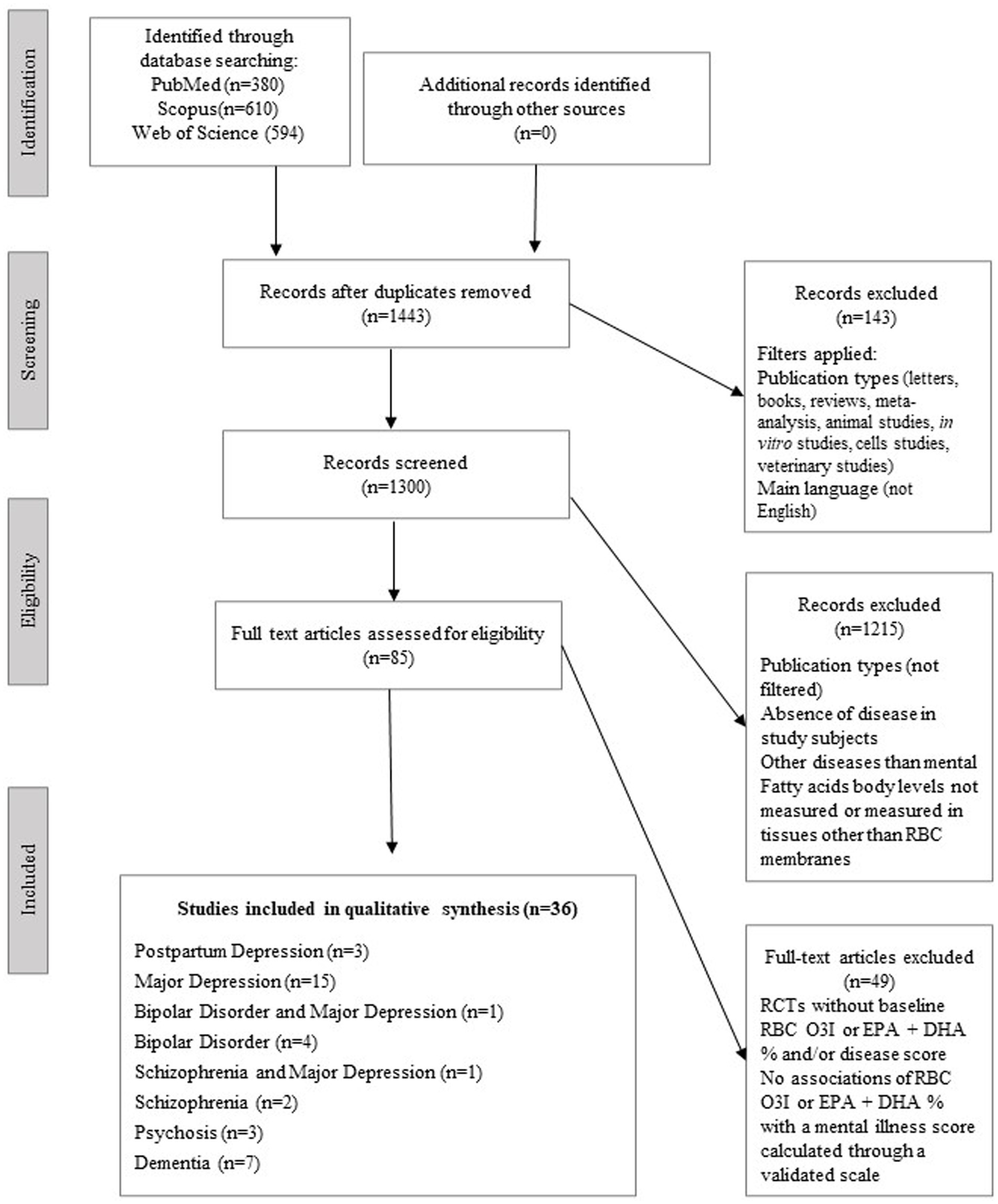
The study selection process is presented in Figure 1. Overall, thirty-six articles were included in the final qualitative analysis, published from 2008 to 2022. The design characteristics and major findings of each publication are presented in Table 1.
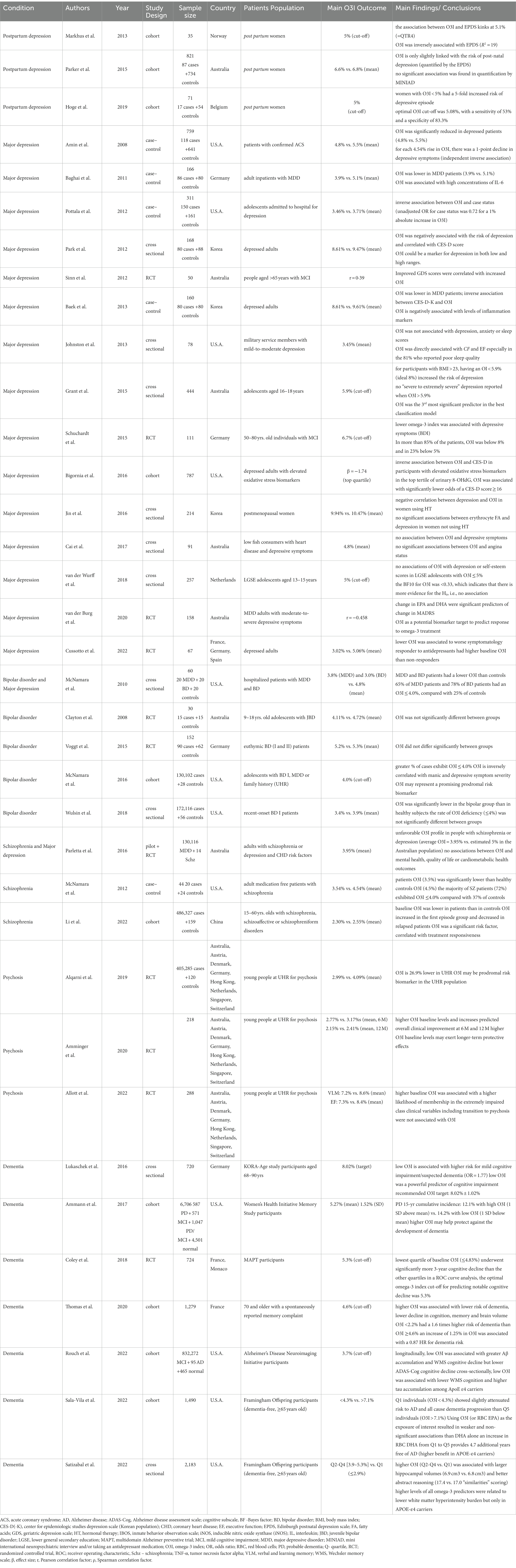
Table 1. Studies exploring relationships between omega-3 index and psychiatric diseases: designs and major findings.
Thirty of these studies found either significant differences in omega-3 index between patients and controls or inverse relationships between omega-3 index and disease severity.
4. Discussion
The literature has yielded heterogeneous results with respect to the links between O3I and psychiatric disorders.
The articles returned by our search show a satisfactory diversity in populations’ age groups, baseline diseases and countries of origin. Most publications included in this review – albeit not all – showed a beneficial effect of higher O3I levels over disease severity in O3I lower ranges. Correlations between O3I and symptoms gravity were found in most studies where O3I was analyzed as a continuous variable. Those that compare O3I values of ill and healthy individuals, show small differences, usually not greater than 1%.
Although the diversity of methods, designs, sample sizes, O3I cut-offs and O3I target values proposed entangles the construction of an O3I as risk marker in mental health, relevant conclusions and suggestions for future research can be drawn.
In postpartum depression both Markhus et al. (29) and Hoge et al. (35) findings aid the concept of an O3I in mental health with a well-supported cut-off of 5% for low vs. high risk. Even though the variable antepartum depression has not been fully controlled by these authors [contrary to Parker et al. (36)], for the purpose of anticipating an increased risk of depression after delivery, an O3I < 5% in blood collected up to the 28th week of gestation should be considered a risk factor.
MDD is the psychiatric disease with the largest body of evidence involving inverse relationships with O3I analyzed both as a continuous and as a discrete variable (28, 37–47). Some of its evidence also add to the knowledge of the disease mechanisms, including the roles that inflammation (38, 41) and oxidative stress (44) play in its pathophysiology.
Authors whose work contradicts this inverse relationship (Cai et al. (48), van der Wurff et al. (49) and Johnston et al. (50)) explain their results on the grounds of methodologic imperfections, low depression severity in homogenous population or O3I values (high overall values and narrow ranges). Cai et al. (48) admit an O3I influence on depression below a threshold of 4–5%. In adolescent populations, van der Wurff et al. (49) propose that the deep changes in brain development, social and emotional behaviors could have offset the protective effect of n-3 FA. Their findings were not replicated by Pottala et al. (28) and Grant et al. (42) who underline their contributions to the expansion of an O3I concept to the field of psychiatry and highlight the role of O3I in depression predicting models.
Studies carried out in Korea (39, 41, 45) generally found significant O3I differences between ill and healthy individuals, with the exception of depressed menopausal women who were not under hormone therapy, explained by Jin et al. (45) as resulting from a previously described synergistic interaction between n-3 FA and estrogen. Despite the potential value of O3I as a marker of depression both in low and high ranges (39) we confine our discussion of an O3I in mental health to occidental populations, suggesting that more Asian populations should be included in future research.
Cussotto et al. (47) realized that O3I significantly predicted response to antidepressants. Considering Bigornia et al. (44), where associations with O3I have only been found within the top quartile of oxidative stress biomarkers concentration, the discussion of whether O3I usefulness as a risk marker is stronger in certain groups of patients ought to be continued.
Two out of five publications on bipolar disorder could not find a significant difference between groups [Clayton et al. (51) and Voggt et al. (52)]. Explanations provided include small sample size and difficulty in matching cases and controls. The authors also point out that both cases and controls had low O3I values, all in the range considered as medium risk for cardiovascular disease. McNamara et al. (53) suggest that an O3I ≤4% has the potential to become a prodromal risk biomarker and help to identify individuals at increased risk for bipolar disorder, underlining the low O3I levels found in these patients more as a risk indicator for sudden cardiac death than a mental disease marker.
The evidence provided by the remaining bipolar disorder studies (54, 55), although valuable and signaling a higher risk in individuals with lower O3I levels, is insufficient to propose O3I ≤4% as a prodromal risk of manic onset. The difference between percentages of patients and healthy controls with an O3I ≤4% has an overall weak clinical significance.
In the mixed schizophrenia and MDD population studied by Parletta et al. (56) (where schizophrenic patients were a minority) no associations between O3I and mental health outcomes were found. The authors compare the 3.95% O3I of their patients with the estimated 5% in the country’s population and underline the higher cardiovascular risk to which schizophrenia and MDD patients are exposed. Although they acknowledge the potential of O3I to estimate the risk of mental illness, their suggestions on future research are confined to the definition of supplementation targets and of critical periods for this intervention.
Despite the significant O3I difference shown by McNamara et al. (57) between schizophrenic patients and controls, the authors only discuss it as evidence of increased risk for cardiovascular morbidity and mortality.
Li et al. (58) findings fuel the debate about predicting antipsychotics responsiveness in schizophrenic patients raised by previously published papers that suggested biomarkers’ research could potentially eliminate resistance to antipsychotics (59).
The NEURAPRO trial (60) is the main available source of knowledge regarding relationships between O3I and the risk of psychosis onset in an ultra-high-risk population. Alqarni et al. (61) propose it as a prodromal risk biomarker. Due to the small effect size the concept of O3I < 4% as a risk factor for transition should be further discussed. Amminger et al. (62) suggest O3I as a prognosis marker, with higher O3I anticipating better clinical outcomes in the medium term but only discuss this finding in the context of n-3 FA supplementation and the effect size found is smaller (O3I approximately 0.5% higher in better responders). Finally, Allott et al. (63) could not find an association between O3I and clinical variables including transition to psychosis.
As to dementia, most of the longitudinal analyses showed a negative association between O3I and the probability of dementia and/or cognitive decline (32, 64–67). O3I effect size varied across studies as did cut-offs and target values proposed. Coley et al. (32) have suggested an O3I of 5% as protective against dementia (above which there would be no benefit), whereas Lukaschek et al. (64) recommended a target O3I of 8.02% ± 1.02%. Rouch et al. (67) have identified an O3I of 3.7% as a cut-off below which some dementia surrogate markers (e.g., higher Aβ and tau accumulation) were significantly increased. In analysis carried out within the Framingham offspring cohort, subjects aged 65 or over whose O3I was ≤2.9% had slightly smaller hippocampal volumes and showed worse abstract reasoning (68). Those whose O3I was <4.3% had a slightly lower risk to develop Alzheimer and all cause dementia, although DHA alone was a better predictor than O3I (69).
Based on the available literature, O3I seems to be a relevant and graded risk factor for the development of dementia with a stratification similar to proposed by Harris and von Shacky (6) for cardiovascular diseases.
This review has some limitations. Most of these studies were cross-sectional or based on cross-sectional analyses within studies with other designs. Causations cannot therefore be inferred.
Dichotomizations and/or risk stratifications could not be derived from several publications where O3I was analyzed as a continuous variable. On the other hand, O3I analyses as a discrete variable raise the questions of criteria for choices and loss of information. Examples of the latest are cut-offs previously adopted in non-psychiatric studies; it is not clear whether those are the most appropriate for the psychiatric arena. The proposal for an O3I in mental diseases should result from analyses carried out on raw data where different cut-offs could be tested.
In the light of most of the selected publications, O3I determination seems to be useful mainly to decide whether supplementation with n-3 FA is recommended. Its interest as an independent marker for diagnosis and independent predictor of response to medication and psychotherapy has not been, so far, exploited in depth.
5. Conclusion
Psychiatric disorders are typically heterogeneous. Several symptoms and phenotypes are shared across diagnoses and biological measurements do not separate completely from those of healthy individuals. This heterogeneity can be observed in the course of disease, in response to treatments and in genetic polymorphisms. Probably for these reasons, no psychiatric biomarker has been found so far (70).
It is still unclear whether O3I can be clinically useful as a marker for diagnosis and treatment response prediction. The published evidence is compelling enough to suggest omega-3 index as a risk factor for some psychiatric diseases, specifically, major depression, postpartum depression, psychosis, and dementia. In occidental populations, we propose a risk threshold of (a) 4–5% in major depression and dementia, (b) 5% in postpartum depression, and (c) 4% for psychosis transition (warranting further research). The concept of a protective O3I in mental health should be studied in more depth in forthcoming studies.
For the purpose of a more solid risk stratification, future research on these and other diseases should privilege analyses of O3I as a discrete variable and test several cut-offs.
Author contributions
HA designed the study, defined the search flow, managed the literature searches, selected the articles, extracted and analysed the data with inputs from ES-L, NB, and MF. HA wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank Teresa Costa, PhD, and Sofia Serra, MD, from NOVA Medical School Library, Faculdade de Ciências Médicas (NMS, FCM), Universidade NOVA de Lisboa, for their assistance in the bibliographic search and study selection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dyerberg, J, and Bang, HO. Haemostatic function and platelet polyunsaturated fatty acids in Eskimos. Lancet. (1979) 314:433–5. doi: 10.1016/S0140-6736(79)91490-9
2. Harris, WS. The Omega-6: Omega-3 ratio: a critical appraisal and possible successor. Prostaglandins Leukot Essent Fatty Acids. (2018) 132:34–40. doi: 10.1016/j.plefa.2018.03.003
3. Cardoso, C, Afonso, C, and Bandarra, NM. Dietary DHA, bioaccessibility, and neurobehavioural development in children. Crit Rev Food Sci Nutr. (2018) 58:2617–31. doi: 10.1080/10408398.2017.1338245
4. Žák, A, Jáchymová, M, Burda, M, Staňková, B, Zeman, M, Slabý, A, et al. FADS polymorphisms affect the clinical and biochemical phenotypes of metabolic syndrome. Meta. (2022) 12:568. doi: 10.3390/metabo12060568
5. Yassine, HN, Croteau, E, Rawat, V, Hibbeln, JR, Rapoport, SI, Cunnane, SC, et al. DHA brain uptake and APOE4 status: a PET study with (1-11C)-DHA. Alzheimers Res Ther. (2017) 9:1–8. doi: 10.1186/s13195-017-0250-1
6. Harris, WS, and Von Schacky, C. The Omega-3 index: a new risk factor for death from coronary heart disease? Prev Med. (2004) 39:212–20. doi: 10.1016/j.ypmed.2004.02.030
7. Fenton, JI, Gurzell, EA, Davidson, EA, and Harris, WS. Red blood cell LC-PUFAs reflect the phospholipid PUFA composition of major organs. Prostaglandins Leukot Essent Fatty Acids. (2016) 112:12–23. doi: 10.1016/j.plefa.2016.06.004
8. Maes, M, and Smith, RS. Fatty acids, cytokines, and major depression. Biol Psychiatry. (1998) 43:313–4. doi: 10.1016/s0006-3223(97)00401-0
9. Tsuboi, H, Sakakibara, H, Tatsumi, A, Yamakawa-Kobayashi, K, Matsunaga, M, Kaneko, H, et al. Serum IL-6 levels and oxidation rate of LDL cholesterol were related to depressive symptoms independent of omega-3 fatty acids among female hospital and nursing home workers in Japan. J Affect Disord. (2019) 249:385–93. doi: 10.1016/j.jad.2019.02.031
10. Fond, G, Lançon, C, Korchia, T, Auquier, P, and Boyer, L. The role of inflammation in the treatment of schizophrenia. Front. Psychiatry. (2020) 11:160. doi: 10.3389/fpsyt.2020.00160
11. Zhou, L, Xiong, JY, Chai, YQ, Huang, L, and Tang, ZY, (, et al.et.al). Possible antidepressant mechanisms of omega-3 polyunsaturated fatty acids acting on the central nervous system. Front Psychiatry (2022);:31. doi: 10.3389/fpsyt.2022.933704, 13
12. Von Schacky, C. Importance of EPA and DHA blood levels in brain structure and function. Nutrients. (2021) 13:1074. doi: 10.1016/s0006-3223(97)00401-0
13. Logan, AC. Omega-3 fatty acids and major depression: a primer for the mental health professional. Lipids Health Dis. (2004) 3:1–8. doi: 10.1186/1476-511X-3-25
14. Das, UN. Polyunsaturated fatty acids and their metabolites in the pathobiology of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. (2013) 42:122–34. doi: 10.1016/j.pnpbp.2012.06.010
15. Cunnane, SC, Plourde, M, Pifferi, F, Bégin, M, Féart, C, and Barberger-Gateau, P. Fish, docosahexaenoic acid and Alzheimer’s disease. Prog Lipid Res. (2009) 48:239–56. doi: 10.1016/j.plipres.2009.04.001
16. Simopoulos, AP, Kifer, RR, Martin, RE, and Barlow, S. Health effects of omega 3 polyunsaturated fatty acids in Seafoods. Proceedings of the 2nd international conference; 1990 mar; Washington, D.C.; [cited 1991]. World Rev Nutr Diet. (1991) 66:103–17. doi: 10.1159/000419283
17. Glaser, C, Lattka, E, Rzehak, P, Steer, C, and Koletzko, B. Genetic variation in polyunsaturated fatty acid metabolism and its potential relevance for human development and health. Matern Child Nutr. (2011) 7:27–40. doi: 10.1111/j.1740-8709.2011.00319.x
18. Gorjão, R, Azevedo-Martins, AK, Rodrigues, HG, Abdulkader, F, Arcisio-Miranda, M, Procopio, J, et al. Comparative effects of DHA and EPA on cell function. Pharmacol Ther. (2009) 122:56–64. doi: 10.1016/j.pharmthera.2009.01.004
19. Chalon, S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. (2006) 75:259–69. doi: 10.1016/j.plefa.2006.07.005
20. Mischoulon, D, and Freeman, MP. Omega-3 fatty acids in psychiatry. Psychiatr Clin North Am. (2013) 36:15–23. doi: 10.1016/j.psc.2012.12.002
21. Amen, DG, Harris, WS, Kidd, PM, Meysami, S, and Raji, CA. Quantitative erythrocyte Omega-3 EPA plus DHA levels are related to higher regional cerebral blood flow on brain SPECT. J Alzheimers Dis. (2017) 58:1189–99. doi: 10.3233/jad-170281
22. Calon, F, and Cole, G. Neuroprotective action of omega-3 polyunsaturated fatty acids against neurodegenerative diseases: evidence from animal studies. Prostaglandins Leukot Essent Fatty Acids. (2007) 77:287–93. doi: 10.1016/j.plefa.2007.10.019
23. Wolters, M, von der Haar, A, Baalmann, AK, Wellbrock, M, Heise, TL, and Rach, S. Effects of N-3 polyunsaturated fatty acid supplementation in the prevention and treatment of depressive disorders—a systematic review and meta-analysis. Nutrients. (2021) 13:1070. doi: 10.3390/nu13041070
24. Suradom, C, Suttajit, S, Oon-arom, A, Maneeton, B, and Srisurapanont, M. Omega-3 polyunsaturated fatty acid (n-3 PUFA) supplementation for prevention and treatment of perinatal depression: a systematic review and meta-analysis of randomized-controlled trials. Nord J Psychiatry. (2021) 75:239–46. doi: 10.1080/08039488.2020.1843710
25. Xu, X, Shao, G, Zhang, X, Hu, Y, Huang, J, Su, Y, et al. The efficacy of nutritional supplements for the adjunctive treatment of schizophrenia in adults: a systematic review and network meta-analysis. Psychiatry Res. (2022) 311:114500. doi: 10.1016/j.psychres.2022.114500
26. Araya-Quintanilla, F, Gutiérrez-Espinoza, H, Sánchez-Montoya, U, Muñoz-Yañez, MJ, Baeza-Vergara, A, Petersen-Yanjarí, M, et al. Effectiveness of omega-3 fatty acid supplementation in patients with Alzheimer disease: a systematic review and meta-analysis. Neurologia. (2020) 35:105–14. doi: 10.1016/j.nrl.2017.07.009
27. Milte, CM, Sinn, N, and Howe, PR. Polyunsaturated fatty acid status in attention deficit hyperactivity disorder, depression, and Alzheimer's disease: towards an omega-3 index for mental health? Nutr Rev. (2009) 67:573–90. doi: 10.1111/j.1753-4887.2009.00229.x
28. Pottala, JV, Talley, JA, Churchill, SW, Lynch, DA, von Schacky, C, and Harris, WS. Red blood cell fatty acids are associated with depression in a case-control study of adolescents. Prostaglandins Leukot Essent Fatty Acids. (2012) 86:161–5. doi: 10.1016/j.plefa.2012.03.002
29. Markhus, MW, Skotheim, S, Graff, IE, Frøyland, L, Braarud, HC, Stormark, KM, et al. Low omega-3 index in pregnancy is a possible biological risk factor for postpartum depression. PLoS One. (2013) 8:e67617. doi: 10.1371/journal.pone.0067617
30. Hibbeln, JR, and Gow, RV. The potential for military diets to reduce depression, suicide, and impulsive aggression: a review of current evidence for omega-3 and omega-6 fatty acids. Mil Med. (2014) 179:117–28. doi: 10.7205/milmed-d-14-00153
31. Harris, WS, and Polreis, J. Measurement of the Omega-3 index in dried blood spots. Ann Clin Lab Res. (2016) 04:4. doi: 10.21767/2386-5180.1000137
32. Coley, N, Raman, R, Donohue, MC, Aisen, PS, Vellas, B, and Andrieu, S. Defining the optimal target population for trials of polyunsaturated fatty acid supplementation using the erythrocyte omega-3 index: a step towards personalized prevention of cognitive decline? J Nutr Health Aging. (2018) 22:982–8. doi: 10.1007/s12603-018-1052-2
33. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
34. Baethge, C, Goldbeck-Wood, S, and Mertens, S. SANRA—a scale for the quality assessment of narrative review articles. Res integr peer rev. (2019) 4:5–7. doi: 10.1186/s41073-019-0064-8
35. Hoge, A, Tabar, V, Donneau, AF, Dardenne, N, Degée, S, Timmermans, M, et al. Imbalance between omega-6 and omega-3 polyunsaturated fatty acids in early pregnancy is predictive of postpartum depression in a Belgian cohort. Nutrients. (2019) 11:876. doi: 10.3390/nu11040876
36. Parker, G, Hegarty, B, Granville-Smith, I, Ho, J, Paterson, A, Gokiert, A, et al. Is essential fatty acid status in late pregnancy predictive of post-natal depression? Acta Psychiatr Scand. (2015) 131:148–56. doi: 10.1111/acps.12321
37. Amin, AA, Menon, RA, Reid, KJ, Harris, WS, and Spertus, JA. Acute coronary syndrome patients with depression have low blood cell membrane omega-3 fatty acid levels. Psychosom Med. (2008) 70:856–62. doi: 10.1097/FPSY.0b013e318188a01e
38. Baghai, TC, Varallo-Bedarida, G, Born, C, Haefner, S, Schüle, C, Eser, D, et al. Major depression, cardiovascular risk factors and the Omega-3 index. Eur Psychiatry. (2011) 26:603–3. doi: 10.4088/jcp.09m05895blu
39. Park, Y, Kim, M, Baek, D, and Kim, SH. Erythrocyte n–3 polyunsaturated fatty acid and seafood intake decrease the risk of depression: case-control study in Korea. Ann Nutr Metab. (2012) 61:25–31. doi: 10.1159/000339264
40. Sinn, N, Milte, CM, Street, SJ, Buckley, JD, Coates, AM, Petkov, J, et al. Effects of n-3 fatty acids, EPA v. DHA, on depressive symptoms, quality of life, memory and executive function in older adults with mild cognitive impairment: a 6-month randomised controlled trial. Br J Nutr. (2012) 107:1682–93. doi: 10.1017/s0007114511004788
41. Baek, D, and Park, Y. Association between erythrocyte n-3 polyunsaturated fatty acids and biomarkers of inflammation and oxidative stress in patients with and without depression. Prostaglandins Leukot Essent Fat Acids. (2013) 89:291–6. doi: 10.1016/Fj.atherosclerosis.2015.03.043
42. Grant, R, Bilgin, A, Guest, J, Morris, MJ, Garg, M, and Pearce, R. The relative value of measures of omega-3 index, perceived stress, cortisol and sleep time in identifying depression among a cohort of Australian adolescents. Int J Child Health Nutr. (2015) 4:40–9. doi: 10.6000/1929-4247.2015.04.01.4
43. Schuchardt, JP, Köbe, T, Witte, V, Willers, J, Gingrich, A, Tesky, V, et al. Genetic variants of the FADS gene cluster are associated with erythrocyte membrane LC PUFA levels in patients with mild cognitive impairment. J Nutr Health Aging. (2016) 20:611–20. doi: 10.1007/s12603-016-0720-3
44. Bigornia, SJ, Harris, WS, Falcón, LM, Ordovás, JM, Lai, CQ, and Tucker, KL. The omega-3 index is inversely associated with depressive symptoms among individuals with elevated oxidative stress biomarkers. J Nutr. (2015) 146:758–66. doi: 10.3945/Fjn.115.222562
45. Jin, Y, Kim, TH, and Park, Y. Association between erythrocyte levels of n-3 polyunsaturated fatty acids and depression in postmenopausal women using or not using hormone therapy. Menopause. (2016) 23:1012–8. doi: 10.1097/gme.0000000000000667
46. van der Burg, KP, Cribb, L, Firth, J, Karmacoska, D, Mischoulon, D, Byrne, GJ, et al. EPA and DHA as markers of nutraceutical treatment response in major depressive disorder. Eur J Nutr. (2020) 59:2439–47. doi: 10.1007/s00394-019-02090-6
47. Cussotto, S, Delgado, I, Oriolo, G, Kemper, J, Begarie, D, Dexpert, S, et al. Low omega-3 polyunsaturated fatty acids predict reduced response to standard antidepressants in patients with major depressive disorder. Depress Anxiety. (2022) 39:407–18. doi: 10.1002/da.23257
48. Cai, S, Coates, AM, Buckley, JD, Berry, NM, Burres, L, Beltrame, J, et al. There is no association between the omega-3 index and depressive symptoms in patients with heart disease who are low fish consumers. Heart Lung Circ. (2017) 26:276–84. doi: 10.1016/j.hlc.2016.07.003
49. van der Wurff, ISM, von Schacky, C, Bergeland, T, Leontjevas, R, Zeegers, MP, Kirschner, PA, et al. Exploring the association between whole blood Omega-3 index, DHA, EPA, DHA, AA and n-6 DPA, and depression and self-esteem in adolescents of lower general secondary education. Eur J Nutr. (2019) 58:1429–39. doi: 10.1007/s00394-018-1667-4
50. Johnston, DT, Deuster, PA, Harris, WS, MacRae, H, and Dretsch, MN. Red blood cell omega-3 fatty acid levels and neurocognitive performance in deployed US Servicemembers. Nutr Neurosci. (2013) 16:30–8. doi: 10.1179/1476830512y.0000000025
51. Clayton, EH, Hanstock, TL, Hirneth, SJ, Kable, CJ, Garg, ML, and Hazell, PL. Long-chain omega-3 polyunsaturated fatty acids in the blood of children and adolescents with juvenile bipolar disorder. Lipids. (2008) 43:1031–8. doi: 10.1007/s11745-008-3224-z
52. Voggt, A, Berger, M, Obermeier, M, Löw, A, Seemueller, F, Riedel, M, et al. Heart rate variability and Omega-3 index in euthymic patients with bipolar disorders. Eur Psychiatry. (2015) 30:228–32. doi: 10.1016/j.eurpsy.2014.11.010
53. McNamara, RK, Jandacek, R, Tso, P, Blom, TJ, Welge, JA, Strawn, JR, et al. Adolescents with or at ultra-high risk for bipolar disorder exhibit erythrocyte docosahexaenoic acid and eicosapentaenoic acid deficits: a candidate prodromal risk biomarker. Early Interv Psychiatry. (2016) 10:203–11. doi: 10.1111/eip.12282
54. Wulsin, LR, Blom, TJ, Durling, M, Welge, JA, DelBello, MP, Adler, CM, et al. Cardiometabolic risks and omega-3 index in recent-onset bipolar I disorder. Bipolar Disord. (2018) 20:658–65. doi: 10.1111/bdi.12633
55. McNamara, RK, Jandacek, R, Rider, T, Tso, P, Dwivedi, Y, and Pandey, GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. (2010) 126:303–11. doi: 10.1016/Fj.jad.2010.03.015
56. Parletta, N, Zarnowiecki, D, Cho, J, Wilson, A, Procter, N, Gordon, A, et al. People with schizophrenia and depression have a low omega-3 index. Prostaglandins Leukot Essent. (2016) 110:42–7. doi: 10.1016/j.plefa.2016.05.007
57. McNamara, RK, Jandacek, R, Rider, T, Tso, P, Dwivedi, Y, and Pandey, GN. Adult medication-free schizophrenic patients exhibit long-chain omega-3 fatty acid deficiency: implications for cardiovascular disease risk. Cardiovasc Psychiatry Neurol. (2013) 2013:1–10. doi: 10.1155/F2013/F796462
58. Li, N, Yang, P, Tang, M, Liu, Y, Guo, W, Lang, B, et al. Reduced erythrocyte membrane polyunsaturated fatty acid levels indicate diminished treatment response in patients with multi-versus first-episode schizophrenia. Schizophrenia. (2022) 8:7–12. doi: 10.1038/s41537-022-00214-2
59. Nasrallah, HA. Treatment resistance is a myth. Curr Psychiatr (2021). (2021) 20:14–6. doi: 10.12788/cp.0105
60. Markulev, C, McGorry, PD, Nelson, B, Yuen, HP, Schaefer, M, Yung, AR, et al. NEURAPRO-E study protocol: a multicentre randomized controlled trial of omega-3 fatty acids and cognitive-behavioural case management for patients at ultra high risk of schizophrenia and other psychotic disorders. Early Interv Psychiatry. (2017) 11:418–28. doi: 10.1111/eip.12260
61. Alqarni, A, Mitchell, TW, McGorry, PD, Nelson, B, Markulev, C, Yuen, HP, et al. Comparison of erythrocyte omega-3 index, fatty acids and molecular phospholipid species in people at ultra-high risk of developing psychosis and healthy people. Schizophr Res. (2020) 226:44–51. doi: 10.1016/j.schres.2019.06.020
62. Amminger, GP, Nelson, B, Markulev, C, Yuen, HP, Schäfer, MR, Berger, M, et al. The NEURAPRO biomarker analysis. NEURAPRO biomarker analysis: long-chain omega-3 fatty acids improve 6-month and 12-month outcomes in youths at ultra-high risk for psychosis. Biol Psychiatry. (2020) 87:243–52. doi: 10.1016/j.biopsych.2019.08.030
63. Allott, K, Schmidt, SJ, Yuen, HP, Wood, SJ, Nelson, B, Markulev, C, et al. Twelve-month cognitive trajectories in individuals at ultra-high risk for psychosis: a latent class analysis. Schizophr Bull. (2022) 3:sgac008. doi: 10.1093/schizbullopen/sgac008
64. Lukaschek, K, von Schacky, C, Kruse, J, and Ladwig, KH. Cognitive impairment is associated with a low omega-3 index in the elderly: results from the KORA-age study. Dement Geriatr Cogn Disord. (2016) 42:236–45. doi: 10.1159/000448805
65. Ammann, EM, Pottala, JV, Robinson, JG, Espeland, MA, and Harris, WS. Erythrocyte omega-3 fatty acids are inversely associated with incident dementia: secondary analyses of longitudinal data from the women's health initiative memory study (WHIMS). Prostaglandins Leukot Essent Fatty Acids. (2017) 121:68–75. doi: 10.1016/j.plefa.2017.06.006
66. Thomas, A, Baillet, M, Proust-Lima, C, Féart, C, Foubert-Samier, A, Helmer, C, et al. Long-chain omega-3 polyunsaturated fatty acids, brain atrophy, cognitive decline and dementia risk: epidemiology/risk and protective factors in MCI and dementia. Alzheimers Dement. (2020) 17:e042968:407–16. doi: 10.1002/alz.12195
67. Rouch, L, Virecoulon Giudici, K, Cantet, C, Guyonnet, S, Delrieu, J, Legrand, P, et al. Associations of erythrocyte omega-3 fatty acids with cognition, brain imaging and biomarkers in the Alzheimer's disease neuroimaging initiative: cross-sectional and longitudinal retrospective analyses. Am J Clin Nutr. (2022) 116:e069256:1492–506. doi: 10.1093/ajcn/nqac236
68. Satizabal, CL, Himali, JJ, Beiser, AS, Ramachandran, V, Melo van Lent, D, Himali, D, et al. Association of red Blood Cell Omega-3 fatty acids with MRI markers and cognitive function in midlife: the Framingham heart study. Neurology. (2022) 99:e2572–82. doi: 10.1212/WNL.0000000000201296
69. Sala-Vila, A, Satizabal, CL, Tintle, N, Melo van Lent, D, Vasan, RS, Beiser, AS, et al. Red blood cell DHA is inversely associated with risk of incident Alzheimer's disease and all-cause dementia: Framingham offspring study. Nutrients. (2022) 14:2408. doi: 10.3390/nu14122408
Keywords: Omega-3 index, biomarker, risk factor, depression, bipolar disorder, psychosis, schizophrenia, dementia
Citation: Antao HS, Sacadura-Leite E, Bandarra NM and Figueira ML (2023) Omega-3 index as risk factor in psychiatric diseases: a narrative review. Front. Psychiatry. 14:1200403. doi: 10.3389/fpsyt.2023.1200403
Edited by:
Francisco Navarrete Rueda, Miguel Hernández University of Elche, SpainReviewed by:
Cristiano Capurso, University of Foggia, ItalyWilliam Harris, Fatty Acid Research Institute, Inc., United States
Copyright © 2023 Antao, Sacadura-Leite, Bandarra and Figueira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Helena Sofia Antao, c29maW50YW9AZ21haWwuY29t
 Helena Sofia Antao
Helena Sofia Antao Ema Sacadura-Leite
Ema Sacadura-Leite Narcisa Maria Bandarra
Narcisa Maria Bandarra Maria Luisa Figueira
Maria Luisa Figueira