
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 15 June 2023
Sec. Perinatal Psychiatry
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1193490
This article is part of the Research TopicWomen in Psychiatry 2023: Perinatal PsychiatryView all 12 articles
Background: Postpartum depression (PPD) is the most common complication associated with childbirth and can lead to adverse outcomes for both mothers and their children. A previous meta-analysis found that PPD prevalence varies widely across countries. One potential underexplored contributor to this cross-national variation in PPD is diet, which contributes to mental health and varies significantly around the world. Here, we sought to update the global and national estimates of PPD prevalence using systematic review and meta-analysis. Further, we examined whether cross-national variation in PPD prevalence is associated with cross-national variation in diet using meta-regression.
Methods: To estimate national rates of PPD prevalence, we conducted an updated systematic review of all papers reporting PPD prevalence using the Edinburgh Postnatal Depression Scale between 2016–2021 and combined our findings with a previous meta-analysis of articles published between 1985–2015. PPD prevalence and methods were extracted from each study. Random effects meta-analysis was used to estimate global and national PPD prevalence. To examine dietary predictors, we extracted data on sugar-sweetened beverage, fruit, vegetable, total fiber, yogurt, and seafood consumption from the Global Dietary Database. Random effects meta-regression was used to test whether between-country and within-country variation in dietary factors predicted variation in PPD prevalence, controlling for economic and methodological variables.
Results: 412 studies of 792,055 women from 46 countries were identified. The global pooled prevalence of PPD was 19.18% (95% confidence interval: 18.02 to 20.34%), ranging from 3% in Singapore to 44% in South Africa. Countries that consumed more sugar-sweetened beverages (SSBs) had higher rates of PPD (Coef. = 0.325, p = 0.044, CI:0.010–0.680); Moreover, in years when higher rates of sugar-sweetened beverages were consumed in a country, there were correspondingly higher rates of PPD in that country (Coef. = 0.129, p = 0.026, CI: 0.016–0.242).
Conclusion: The global prevalence of PPD is greater than previous calculations, and drastically varies by country. Sugar-sweetened beverage consumption explained some of the national variation in PPD prevalence.
Mental illness is a leading cause of death and a major public health concern for countries around the world (1). Mental illness in mothers is particularly concerning, as it is not only a leading cause of maternal death in several countries around the world, but it can interfere with parenting behavior and lead to a multitude of adverse physical and emotional child developmental outcomes (2–4). Children with depressed mothers, for example, are more likely to experience psychopathology, behavior issues, and have lower academic achievement (5–7). The adverse impact of maternal depression on offspring may persist into adulthood, raising lifelong risk for mental health, behavioral, and relational issues throughout the lifespan (8–10). In this way, mental health issues can pass down through generations, highlighting the need to understand and prevent maternal mental illness.
Although awareness and research into maternal mental health has increased dramatically in recent decades, rates of mental illness in mothers is still high (11, 12). The most common mental health disorder in mothers is postpartum depression (PPD), defined by the DSM-5 as depressive symptoms experienced in the first 6 months postpartum (13). A recent meta-analysis of 291 studies from 56 countries estimated the global postpartum depression rate to be 17.7%, although prevalence rates varied dramatically by county (11). The lowest rates of PPD were found in Singapore (3%), Nepal (7%), and the Netherlands (8%); while the highest rates were found in Hong Kong (30%), South Africa (37%), and Chile (38%) (11). Examining country-level factors that might explain why rates of PPD vary so dramatically between nations could provide insight into the etiology of PPD and help inform government-led prevention programs. In an initial attempt to do this, Hahn-Holbrook and colleagues (11) conducted a meta-regression that revealed that cross-national variation in PPD prevalence was explained, in part, by economic disparities, fertility rates, and women's access to quality health care resources. Differences in research methodology used across countries explained very little of the cross-national variation in PPD prevalence. Notably, significant cross-national variation in PPD prevalence remained unexplained, suggesting the need to explore other cultural variables (11, 14).
One potential underexplored contributor to cross-national variation in PPD is diet, as diets vary significantly around the world and certain foods such as vegetables, fruit, legumes, nuts, dairy products, fish, and olive oil, may be protective against depression (15). Indeed, scholars have posited that the high levels of PPD seen in the modern day may be due, in part, to evolutionary “mismatches” between our diets today and the diets that humans ate throughout most of human evolution (16). Some of these changes include a decrease in fiber, omega-3 fatty acids, and micronutrients, and an increase in added sugar (16). Thus, we sought to examine whether national variation in dietary patterns predict cross-national variation in postpartum depression. Several dietary factors that have been previously linked to depression risk were considered including yogurt, fiber, fruit, vegetables, omega-3 fatty acids, and sugar-sweetened beverages (SSBs).
High added sugar consumption can adversely affect physical health and lead to obesity, type II diabetes, cardiovascular disease, and mortality (17–19). Several studies have reported links between added sugar consumption and depression (20, 21). However, very few studies have sought to examine the link between sugar consumption and PPD specifically, although one study from Taiwan found that SSB consumption was associated with higher scores on the Edinburgh postpartum depression scale (EPDS) (22).
Several mechanisms have been proposed as to how sugar consumption may increase depression risk. One proposed mechanism is that sugar consumption causes an increase in certain gut bacteria that disrupt healthy brain function, specifically memory (23). A previous study found that issues in working memory may underlie problems with regulating emotions that lead to mood disorders such as depression (23). Another possible mechanism discussed in previous research is the hypothalamic-pituitary-adrenal (HPA) axis. For example, Harrell and colleagues (24) found that diets high in fructose have the potential to alter HPA function in rats, which was associated with increased risk for depressive-like symptoms (24). As fructose is the most common sweetener in processed foods, especially in SSBs, it is plausible that similar effects could be seen in humans (25). Given that SSBs are the main source of added sugar in diets around the globe (26–29), we sought to test whether countries that consume more SSBs have high higher rates of PPD.
Several studies have also linked higher seafood consumption to reduced risk of depression, and postpartum depression specifically. For example, in a systematic review of six studies, Opie and colleagues (30) concluded that seafood consumption was protective against PPD (30). Another study by Hibbeln (31) examined this relationship at the national level and found that countries that consumed more seafood had lower rates of PPD (31), although national rates of PPD in this study were not derived by meta-analysis, calling into question their relatability. Authors of these studies have suggested that seafood may reduce PPD risk because it is high in omega-3 fatty acids, which can be depleted in mothers during pregnancy.
There are several mechanisms that have been theorized to explain how seafood consumption may reduce depressive symptoms and most focus on the role of omega-3 fatty acids. One important type of such acids is DHA, which is abundant in the brain and alters functions of neural systems that utilize dopamine and serotonin, which play a role in depression (32). Another explanation is that omega-3 fatty acids have anti-inflammatory properties and can reduce the production of pro-inflammatory cytokines that contribute to depressive symptoms (33). In addition, emerging research suggest that omega-3 fatty acids benefit the gut microbiome (34), which is also critical for mental health. Given the previously established connection between seafood consumption and depression, we aimed to explore if national variation in seafood consumption predicted national variation in PPD prevalence using meta-analytically derived national PPD prevalence estimates.
Another dietary factor that varies by country is probiotic consumption, which introduces live microorganisms to the gut, altering the microbiome, which may have benefits for the prevention and treatment of depression (35). One of the pathways through which probiotic consumption is thought to reduce the risk of depression is through the gut-brain axis, the bidirectional system that directly and indirectly allows for microbes in the gut to communicate with the brain (36). Through this axis, microbes can affect the brain by regulating HPA functions, and reducing associated pro-inflammatory cytokines, both of which are heavily implicated in the etiology of depression (36). A recent systematic review concluded that probiotic consumption is a promising avenue for the prevention and treatment of depression, although the review did not focus on PPD specifically (37).
Given that there is significant overlap between major depression and PPD, several studies have examined the effectiveness of probiotic consumption for PPD prevention. For example, a randomized controlled trial found that women who received probiotic supplements during pregnancy (N = 193) reported significantly fewer symptoms of postpartum depression than women in the placebo group (N = 187) (38). These results are consistent with rodent models, in which administering probiotics improves depression-like symptoms in highly stressed rat mothers by altering gut microbiota composition, brain monoamines, oxidative stress, and reversing stress-induced changes in the HPA-axis and brain-derived neurotrophic factor (BDNF) (39). Probiotic supplementation is an effective way of distributing and controlling probiotics exposure in experimental research, however, to examine the effects of probiotics on PPD from a global perspective, focusing on traditional foods containing probiotics may be more fitting.
Although there are many sources of probiotics in diets across the world, yogurt is one of the most ancient and most popular (40, 41). References to yogurt consumption for its health benefits date back to 6000 BC in Indian Ayurveda scripts, although it was not until the 1900s that these benefits were attributed to probiotics (40). Patterns of yogurt consumption vary greatly by country. For example, the majority of people in France consume yogurt every day, which is the case for only 6% of Americans. Research conducted in 15 countries showed that the largest amounts of yogurt were consumed in the Netherlands, France, Turkey, Spain, and Germany. The smallest amounts were consumed in Egypt, Colombia, Russia, Romania, and South Africa (42). In a cohort of 14,539 participants, high consumption of whole-fat yogurt was associated with lower rates of depression in women compared to those who did not consume as much yogurt (43). An additional study of 9,965 participants found that yogurt consumption had a protective effect on depression (44). Thus, it seems plausible that cross-national variation in yogurt consumption might explain some of the national variation in PPD prevalence.
Fiber consumption also varies significantly by country, for example, Brazil's average fiber consumption (12.3 grams/day) is approximately half of Sweden's (23–25 grams/day) (45). Moreover, high fiber consumption has been found to reduce the risk of depressive symptoms (46, 47). One theory to explain this relationship is through the effect of fiber on the gut microbiota (46). Gut microbes can break down foods the human digestive system alone is not easily able to digest (e.g., insoluble fiber found in whole grains, nuts, and certain fruits and vegetables), creating a mutually beneficial relationship (48). Non-human animal studies suggest that fiber consumption may lead to microbiota-driven modification of gene expression and increased production of neurotransmitters that may protect against depressive symptomatology (47). In addition to its benefits for the microbiome, fiber also has anti-inflammatory properties thought to help reduce depression risk (47). Specifically, consuming a diet high in fiber may lower inflammation by modifying the pH and permeability of the gut, and reducing inflammatory compounds that can alter neurotransmitter concentrations and influence depressive symptoms (47).
To the authors' knowledge, no human studies have examined the relationship between insoluble (or any) fiber consumption and postpartum depression risk. However, one rodent study found that high intake of dietary fiber supplements alleviates depression-like symptoms postpartum in female mice (49). Therefore, it seems possible that dietary fiber consumption could play a role in PPD such that countries that consume less fiber have higher rates of PPD.
Several studies have found that mothers who consume diets that include plenty of fruits and vegetables have a lower risk of developing PPD (15, 30, 50). Fruits and vegetables contain high levels of fiber, and so it is perhaps not surprising that diets rich in fruits and vegetables are both associated with decreased inflammation, which is a known risk factor for PPD (51, 52). Additionally, as fiber is the key food source for may beneficial gut-bacteria, adequate fruit and vegetable intake is associated with higher bacterial diversity in the gut microbiome (53), which may be protective against PPD (53). In line with the view that fruit and vegetable consumption may be protective against PPD, a cross-sectional survey of 939 women found that higher consumption of fruits and vegetables was associated with lower likelihood of reporting PPD symptoms (54). Thus, it seems feasible that countries that consume more fruits and vegetables may have lower rates of PPD than countries that consume fewer fruits and vegetables.
The aims of the current study were four-fold. First, we sought to update the global and national estimates of PPD prevalence provided by Hahn-Holbrook et al. (11) by conducting a systematic review and meta-analysis of studies published between 2016 and 2021. The second aim was to conduct a meta-regression to examine whether the dramatic differences in PPD prevalence by country can be explained by cultural variation in dietary factors like sugar, yogurt, fiber, fruit and vegetable, and seafood consumption. We hypothesized that countries with higher yogurt, fiber, fruit, vegetable, and seafood consumption, on average, would report lower levels of PPD. Moreover, we predicted that countries with higher SSB consumption would report higher levels of PPD. Given that diets in a country can and do change overtime, the third aim of this study was to explore if changes in a country's diet over time corresponded to changes in prevalence rates of PPD over time (hereafter termed within-country variation in PPD). We predicted that, in years when countries consumed more sugar and less yogurt, fiber, fruits, vegetables, and seafood, studies within those countries would report higher levels of PPD. Finally, a fourth study aim was to explore the extent to which methodological differences across studies contributed to variation in PPD prevalence across studies and across countries.
This study was carried out in a six-step process. First, we conducted a systematic review of studies reporting PPD prevalence published since the meta-analysis published in 2018 by Hahn-Holbrook and colleagues. Following PRISMA guidelines (55), we extracted information on PPD prevalence and methodological variables from each article. Second, this new dataset was merged with Hahn-Holbrook and colleagues (11) dataset, giving us a database of studies reporting PPD prevalence using the EPDS scale published between 1985 and 2021. Third, we used meta-analysis to estimate an updated global rate of PPD prevalence, as well as updated PPD prevalence estimates by country. Fourth, we conducted a meta-regression to explore the extent to which methodological variation predicted variation in PPD prevalence reported across studies. Fifth, using our meta-analytically derived PPD prevalence estimates for each country, we used meta-regression to test the extent to which national variation in rates of PPD could be explained by cultural variation in dietary factors. In these analyses, we statistically controlled for methodological conventions used across countries and national GDP, as the previous meta-analysis by Hahn-Holbrook et al. (11) identified national poverty as the strongest predictor of cross-national variation PPD. Finally, we conducted a meta-regression to explore whether within-country (mean-centered) variation in dietary consumption patterns across time predicted within-country (mean-centered) variation in rates of PPD across time.
In order to update the global and national estimates of PPD prevalence provided by Hahn-Holbrook et al. (11), who included articles published between January 1, 1985 to December 31st 2015, we followed the same search and methodological strategies. To identify new potentially eligible articles published between January 1, 2016 and July 26, 2021, we searched PubMed, PsychINFO, and CINAHL using a combination of the following MeSH terms in the abstract: (“postpartum depression” or “postnatal depression”) and (“incidence” or “prevalence”). Additionally, we used the measures and instruments qualifier “Edinburgh Postnatal Depression Scale”. The Edinburgh Postpartum Depression Scale (EPDS) is a 10-item self-report, widely-used tool specially designed to measure PPD. We chose to focus on studies that used the EPDS specifically, as this is overwhelmingly the most commonly used tool to measure postpartum depression; 70% of studies that measure PPD use the EPDS (11). Moreover, this measure has been specially designed and validated to detect depressive symptoms in the postpartum period. Other self-report depression scales can be problematic to use in the postpartum period as they contain items about weight gain/loss and sleep changes, which are normal in the postpartum period. Clinical interviews for depressive status typically produce lower prevalence rates than self-report measures. However, we chose to focus on studies reporting prevalence using the gold-standard self-report measure (rather than clinical interviews) given that clinical interviews are rarely conducted in low-income countries given the increased cost and participant burden. We further narrowed our search by only including studies of human females published in English. The exact Boolean searches used for each database are provided in Supplementary material.
To be included in this meta-analysis, studies were required to report PPD prevalence using the EPDS on samples of mothers ≤ 1 year postpartum with a sample size over 20 (56). To address the important issue of the timing of depression postpartum, we coded studies in terms of when in the postpartum period that depression was assessed, allowing us to examine this variable using meta-regression. We also excluded studies reporting PPD prevalence in samples unlikely to be representative of the general population (e.g., studies that exclusively recruited special populations like women with a history of depression, adolescent mothers, mothers seeking treatment for depression, mothers of high-risk infants, etc.).
See Figure 1 for a PRISMA flow diagram reporting identification and selection of studies for this updated meta-analysis. Of the 601 studies that our updated search produced, 407 abstracts were reviewed, and 125 full text articles were assessed for eligibility. Of these, 104 studies published between January 2016 and July 2021 met our inclusion criteria. These studies were coded for methodological and PPD prevalence, and then this dataset was merged with the from Hahn-Holbrook et al. (11), resulting in a full dataset of 412 studies that could be included in this updated meta-analysis.

Figure 1. PRISMA flow diagram reporting identification and selection of studies for the meta-analysis.
The following methodological variables were coded from each study: PPD prevalence, total sample size, EPDS cutoff score employed, and the timeframe postpartum in which PPD was assessed. To get one estimate of PPD prevalence per study, data from longitudinal studies reporting PPD in the same women at multiple time points were merged by averaging the PPD prevalence over the time points weighted by the sample size at each time point. Also, if multiple prevalence rates were reported in the same study using different EPDS cutoffs, the prevalence rate from the lowest EPDS cutoff was chosen by default. This decision could cause a bias toward higher estimates of PPD incidence; therefore, we also used meta- regression to estimate PPD prevalence at the standard recommended EPDS cutoffs for possible (9/10) and probable (12/13) PPD (56). To investigate whether studies including women earlier or later in the postpartum period report higher PPD prevalence, we coded the range of the timeframes postpartum during which PPD was assessed and used this score to predict PPD prevalence in meta-regression.
Previous studies indicated that some of the differences in PPD prevalence found across studies and between countries can be explained, in part, by methodological differences (11, 57). For example, it is possible that scientists in some countries use lower PPD cutoffs scores more often than scientists in other countries, leading to the appearance of cross-national variation in PPD, when really it is just that some countries set lower symptom thresholds than others. To investigate the role of methodological differences in cross-national PPD prevalence, national averages for each study N, EPDS cutoff score, age of baby at start of data collection, length of assessment window, and year published were included in meta-regression models.
This study examined several dietary factors that have been associated with depression in previous research, namely: fiber, yogurt, seafood, SSBs, vegetables, and fruit. To assess national rates of dietary consumption, we used estimates collected by the Global Dietary Database (GDD) (58). The database is part of the Global Nutrition and Policy Consortium, an initiative based at the Tufts Friedman School of Nutrition Science and Policy. GDD data was collected through nationally representative surveys when available, and, when national surveys were not available, multiple smaller surveys that encompassed people from different parts of the country were aggregated. For countries with no surveys available, sources such as the WHO Infobase were used (58). We used the national consumption of foods measured in terms of grams per day, excluding dietary supplements (e.g., pills). Dietary fiber includes intake from all food sources, including fruits, vegetables, grains, legumes, and pulses. Yogurt data includes total intake of all types of yogurts and fermented milk. Seafood includes the total daily intake of fish and shellfish. SSB data includes intake of any beverage with added sugars having ≥ 50 kcal per 8 oz or 236.5 g of added sugar per serving, including commercial or homemade beverages, soft drinks, energy drinks, fruit drinks, punch, lemonade, and frescas. The SSB variable excludes fruit and vegetable juices with no added sugar, and non-caloric artificially sweetened beverages. Fruit includes total intake of fresh, frozen, cooked, canned, or dried fruit, and excludes fruit juices and salted or pickled fruits. Vegetables include total intake of fresh, frozen, cooked, canned, or dried vegetables, and excludes vegetables juices, salted or pickled vegetables, starchy vegetables such as potatoes, and legumes such as beans. Given that dietary patterns in a country can and do change over time, food consumption in the GDD is reported in yearly intervals. This within-country variation in food and beverage consumption was taken into account in our analysis by mean-centering food consumption within each country, giving us a variable that captures whether consumption of each food was higher or lower in a given year than the country's national average.
Previous research has identified Global Domestic Product (GDP) per capita as a significant predictor of cross-national PPD prevalence (11). As GDP may relate to national dietary habits, we wanted to statistically adjust for this factor in models testing the association between PPD and dietary factors. National GDP data (in adjusted US dollars) were obtained from the World Bank (59).
Following recommendations for meta-analysis of prevalence (60), we used a double-arcsine transformation of the PPD prevalence data before calculating the study weights and 95% confidence intervals (CIs) to avoid undue influence of weights obtained for studies with low or high prevalence (prevalence close to 0 or 1). To test for heterogeneity in the data, both the Cochran Q test statistic and the I2 statistic were conducted (61). The same procedure was followed to create meta-analytically derived national estimates of PPD prevalence based solely on the studies available from each country. Meta-analytic estimates of PPD prevalence could not be calculated in countries with fewer than two studies (N = 21) (62). All meta-analyses were conducted using the program MetaXL and the “prev” command (60).
Three sets of meta-regressions were performed, the first addressing which methodological factors predicted variation in PPD across all studies, regardless of the nation in which the study was conducted. The second addressed methodological and dietary predictors of PPD variation across nations. The third addressed methodological and dietary predictors of PPD variation within-nations across time. All meta-regression analyses were performed in STATA 14 (63) using the “metareg” command with random-effects models (because all tests indicated significant heterogeneity). To obtain the standard errors needed to weight studies (or nations) for meta-regression in STATA, we transformed the 95%-CIs provided by MetaXL using the following formula (upper 95% CI—lower 95% CI)/3.92.
Funnel plots were used to test whether papers were more or less likely to be published if they had higher or lower PPD prevalence. Results were considered statistically significant if p-values were under 0.05. Effect sizes are reported as unstandardized coefficients and R2 values obtained in meta-regression models.
792,055 women from 412 studies were included in this meta-analysis. Table 1 presents the data extracted from the 103 new studies published since the Hahn-Holbrook and colleagues (11) meta-analysis. The updated global pooled prevalence of PPD across all 412 studies was 19.18% (95% CI: 18.02 to 20.34%). There was a significant degree of heterogeneity between studies (Q = 40,688.45, p = 0.00, I2 = 99%). Adjusting for the recommended EPDS cut-offs yielded a global PPD prevalence of 19.9% (CI: 18.31 to 21.49%) for possible PPD (EPDS cutoff of 10) and 18.28% (CI: 16.56 to 19.9%) for probable PPD (EPDS cutoff of 13). There was no evidence of publication bias as a function of PPD prevalence rate reported in studies (see Figure 2).
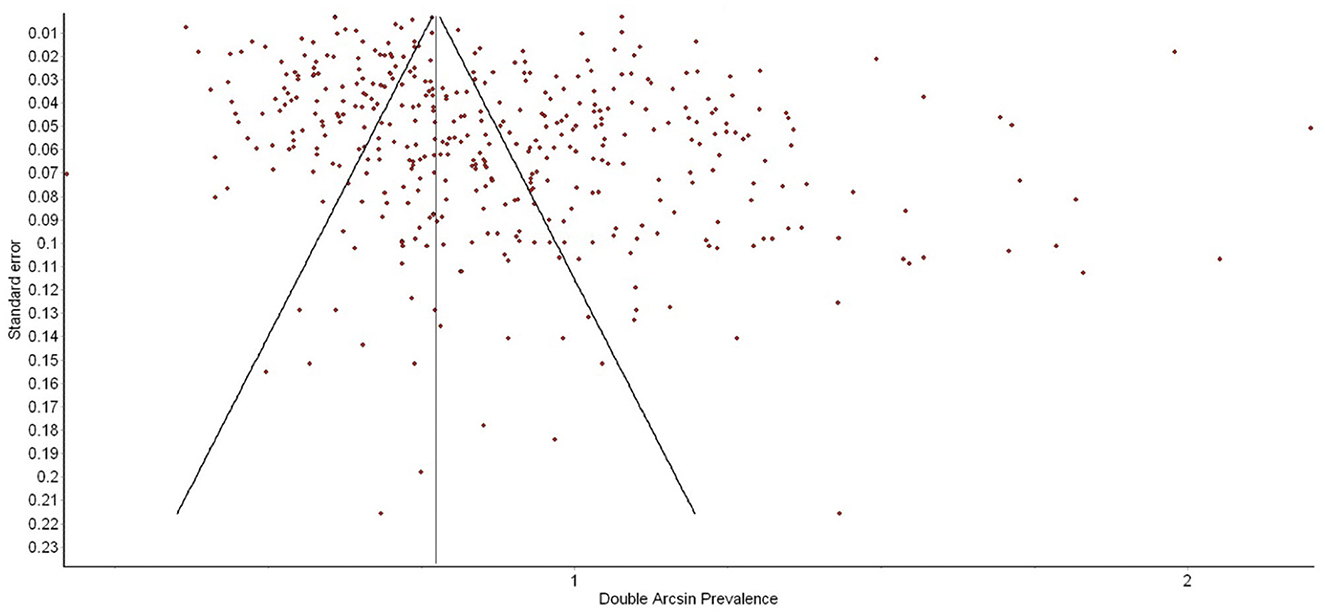
Figure 2. Funnel plot of postpartum depression (PPD) prevalence as a function of prevalence estimate standard error. There was no evidence of publication bias.
Table 2 presents the results of the meta-regression testing the extent to which methodological variables predict variation in PPD prevalence across studies. We found that studies with smaller sample sizes tend to report higher levels of PPD prevalence (Coef. = −0.117, p = 0.019; CI: −0.215 to −0.019). Studies that used lower EPDS cutoff scores reported significantly higher PPD prevalence (Coef. = −0.105, p = 0.44; CI: −0.207 to −0.003). Studies with a longer window of PPD assessment tended to report higher levels of PPD (Coef. = 0.161, p = 0.003, 95% CI: 0.056 to 0.266). No other methodological variables predicted between-study variation in PPD. Together, methodological variables accounted for 4.63% of the variance in PPD prevalence between studies [F[5, 374] = 4.68, p < 0.05].
See Figure 3 for meta-analytically derived estimates of PPD prevalence in 46 countries. National sample sizes ranged from 332 to 353,444 women. National estimates of PPD ranged from 3% in Singapore, 8% in Netherlands, and 11% in Switzerland, to 32% in Vietnam, 38% in Chile, and 44% in South Africa. Meta-analysis suggested that there was significant heterogeneity in PPD prevalence between nations (Q = 8,130.37 p < 0.00, I2 = 99%), suggesting the need for meta-regression to explain cross-national variation in PPD prevalence.
See Table 3 for the results of a meta-regression in which national averages of methodological variables, along with economic and dietary factors, were entered together to predict cross-national variation in PPD prevalence. Of the methodological variables, only the timing of the start of the assessment window predicted cross-national variation in PPD prevalence, with nations that tended to measure PPD earlier in the postpartum reporting higher PPD levels (Coef. = −0.413 p = 0.037; CI: −0.800 to −0.027). Methodological variables alone (when dietary and economic factors were removed from the model) accounted for 10.88% of the total between-country variation in PPD prevalence. GDP did not predict cross national PPD prevalence when methodological and dietary factors were included in the model. In terms of dietary factors, SSB consumption emerged as the only significant dietary factor that predicted cross-national PPD prevalence. This effect remained statistically significant when methodological, economic, and other dietary factors were included in the model. Specifically, countries that consumed more SSBs had higher levels of PPD (Coef. = 0.345, p = 0.044; CI:0.010 to 0.680). SSB consumption alone accounted for 11.42% of between-study variation in PPD prevalence (see Figure 4).
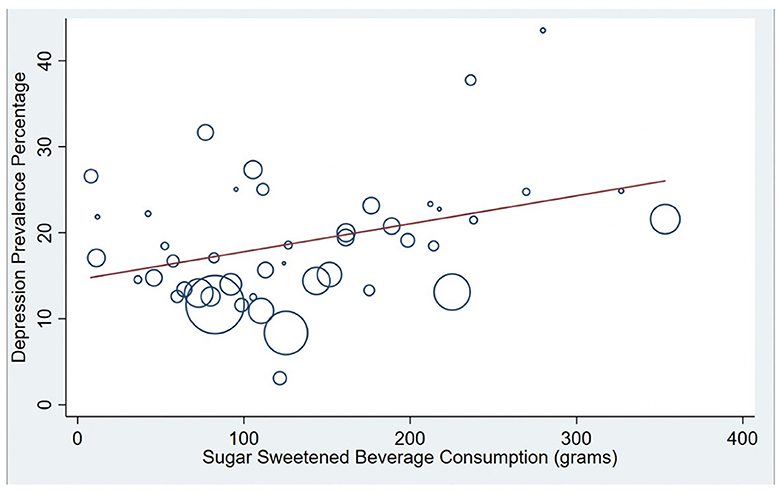
Figure 4. Correlation between National PPD prevalence and National Sugar Sweetened Beverage (SSB) consumption. Bubble plots are presented showing the significant positive association between SSB consumption and national postpartum depression (PPD) prevalence (R-squared = 11.4%). Countries with larger bubbles had larger sample sizes and were weighted accordingly in meta-regression models.
Given that this study includes research on PPD conducted over 40 years, it is important to consider the fact that dietary patterns within a country can change dramatically over time. For example, daily SSB consumption in Japan was approximately 109 grams per person, per day in 1997 but has decreased to about 86 grams in 2018, representing a 21.1% decrease over time. Therefore, we examined whether postpartum depression rates tended to be higher in a country in years when they ate more or less of certain foods. To do this, we matched the year that the PPD study was published to the dietary behavior in that country for that given year. Then, to isolate within-country variation, we created country specific z-scores that mean-center the dietary intake for each country over time, with higher scores representing more consumption of a dietary factor relative to that country's average consumption over-time. We then used these country mean-centered scores to predict country mean-centered variation in PPD (see Table 4 for results). We found that, in years when countries consumed more SSBs, studies within that country tended to report higher levels of PPD (Coef. = 0.129, p = 0.026; CI:0.016 to 0.245. In terms of methodological variables, when studies within a country measured PPD earlier and using a lower EPDS cutoff, compared to the county's average, the studies tended to report higher PPD.
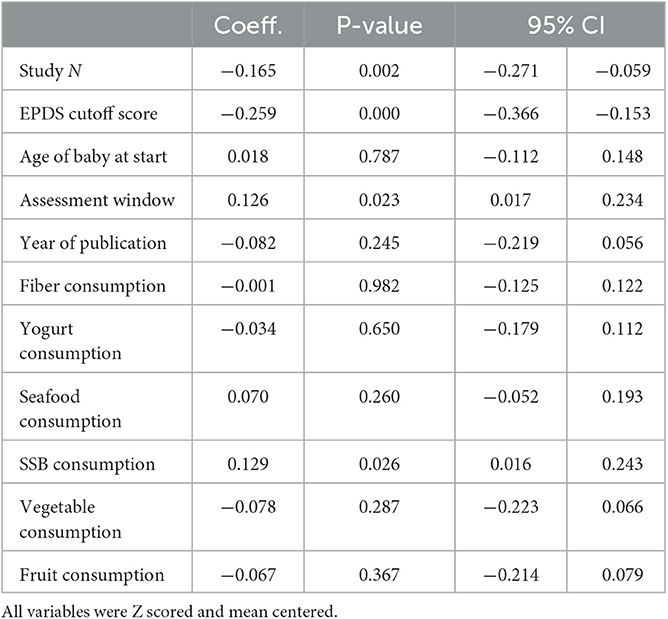
Table 4. Within-country variation in dietary and methodological factors predicting within-country variation of postpartum depression prevalence across time.
This study represents the largest meta-analysis and meta-regression on postpartum depression prevalence in the literature to date. We found that the unadjusted global prevalence rate of PPD in studies utilizing the EPDS scale is 19.18% (95% CI: 18.02 to 20.34%). Adjusting for the recommended EPDS cutoffs yielded a prevalence of 19.9% for possible PPD (>10) and 18.28% for probable PPD (>13). These estimates are slightly higher than the previous calculation by Hahn-Holbrook et al. (11), which found a global PPD prevalence of 17.7% (95% CI: 16.6–18.8%) (11). One explanation for this discrepancy is that the current study adds new data with a higher proportion of studies from relatively low- and middle-income countries, that tend to report higher rates of PPD (57). Between South Africa, which has the highest PPD prevalence rate (44%), and Singapore, which has the lowest PPD prevalence rate, there is a 41% difference. Considering there are many low-income countries missing from this analysis, it is plausible that global PPD is even more prevalent than our findings suggest. While several methodological variables predicted cross-national variation in PPD, specifically the timing of assessment and EPDS cutoff used, the strongest individual predictor in this study was SSB consumption.
Our meta-regression revealed that SSB consumption predicted PPD prevalence both between-countries and within-countries. Specifically, countries that consumed more SSBs, on average, had higher rates of PPD prevalence. This finding alone could be attributed to other cultural differences that co-vary with SSB consumption, although this effect was still significant when methodological factors and GDP were included in the model. However, we also found that, within the same country, in years with higher SSB consumption, studies tended to report higher rates of PPD in that country. This within-country finding lends credence to the hypothesis that it is SSB consumption, and not some other cultural factor, that contributes to higher rates of PPD.
The current study did not find a statistically significant association between seafood consumption and cross-national PPD prevalence, contradicting a previous report by Hibbeln and colleagues (31). Specifically, the previous study correlated DHA in the milk of breastfeeding mothers to PPD rates by country (N = 23), and found that countries that consumed more seafood tended to have lower rates of PPD prevalence (31). There are several possible reasons for the discrepancy between our current findings and this previous report. For example, the current study examines twice the number of countries (N = 46) and includes countries with more diverse economic conditions. Additionally, the current study used meta-regression which weighted countries and studies, and controlled for methodological variables and GDP, while Hibbeln (31) did not control for these confounding variables. Although our findings were not significant, the effect we found was in the same direction as Hibbeln (31). Additionally, Hibbeln (31) was published over two decades ago, and it is possible that breastfeeding women were not discouraged from consuming certain seafood in the same way they are now due to concerns surrounding mercury poisoning (165). Future directions focusing on PPD and seafood consumption, particularly seafood high in omega-3 fatty acids, is warranted. In addition to seafood consumption, we found no relationships between fiber, yogurt, fruit, and vegetable consumption and national PPD prevalence. Despite these null results at the cross-national level, we encourage researchers to continue to examine the relationship between these dietary factors and PPD.
Our results have several important implications for research and policy to prevent PPD. For example, our research suggests that policies that help reduce SSB consumption may help reduce PPD prevalence. There are several existing policies shown to reduce SSB consumption at the national and regional level. For example, implementing a “sugar tax” at the national level disincentivizes the consumer from purchasing SSB by increasing the overall price. Therefore, causing a loss in SSB revenues and encouraging SSB companies to reduce the sugar in their product so that it does not qualify as a high-sugar product. In addition, the extra tax revenue from this strategy can then be used to offset the health care costs associated with sugar-related disease (166). Currently, 45 countries in the world have some type of sugar tax, however, in the US, sugar taxes are in place in only a few cities. Due to the availability of cross-national and within-country sugar consumption data, future research could explore whether implementing sugar taxes changes national rates of depression. While a sugar tax is a good start, more aggressive policies and behavioral health campaigns focusing on limiting SSB consumption may also be necessary. For example, interventions could focus on limiting the consumption of common ingredients in most SSBs. In the last 50 years, the use of high fructose corn syrup has increased dramatically, largely due to the fact that it is inexpensive and manufacturing friendly (167). National and local policies to reduce the use of high fructose corn syrup may be a viable step to improve maternal mental health. However, all types of added sugar are low in nutrient density, and high fructose corn syrup is just one factor in a massive global issue. Therefore, while focusing on types of added sugars in policymaking may lead to some health improvement, reducing consumption of all sugar types would be most impactful.
In addition to gaining a deeper understanding of the impact caused by different types of sweeteners, future work on this topic should focus on identifying additional cultural factors that may further explain variance in national PPD prevalence. For example, environmental and lifestyle factors such as exercise, obesity, partner support, and mindfulness practices, which have been linked to PPD, in individual studies might also be linked to PPD on the national level (168–171). Future research could also focus on micronutrients, such as vitamin D, that previous research have shown play an important role in mental health (172).
Although this study had several strengths, including the large number of studies representing populations with varied lifestyles, economic backgrounds, and diets, our results should be considered alongside several important limitations. First, our study focused on a widely used self-report PPD measure instead of clinical interviews. Self-report PPD measures tend to yield higher estimates than clinical interviews, and therefore, the estimates in this study are likely higher than if we had used studies that conducted interview-based methods. However, given that interview methods are less common than self-report measures, especially in low-income countries, we felt that it was important to utilize a more representative measure. We also felt that potentially overestimating PPD would be safer than underestimating PPD, given its detrimental consequences. We use is nationally representative, and some countries estimates are much more reliable than others. We urge readers to consider the 95% confidence intervals when considering our national PPD prevalence estimates. More research reporting PPD prevalence is essential for providing more accurate prevalence estimates that. Secondly, collecting accurate nutrition data is notoriously challenging. This is why we used the Global Dietary Database, which has the most comprehensive empirical data on dietary intake across and within nations (173). Even so, it is difficult to identify inaccuracies within it. Thirdly, when using aggregate data, it is important to remember the ecological fallacy- that analyses based on area-level averages can yield very different conclusions than those that would be obtained from an analysis at the individual level (174).
PPD is common globally, however, there is significant cross-national and within-country variation. While certain methodological variables may contribute to this variation, SSB consumption may also be a risk factor. Future research is warranted to test whether policy that reduces SSB consumption at the national level may help to reduce PPD prevalence.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
AF-W conducted the review, meta analysis, and meta regression, as well as wrote the manuscript. JH-H oversaw all analysis, edited the manuscript, and provided guidance. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1193490/full#supplementary-material
Figure S1. Forest plot of between-study variation in PPD prevalence.
1. World Health Organization. Public Health Action for the Prevention of Suicide: A Framework. Geneva: World Heal Organization (2012).
2. Burke L. The impact of maternal depression on familial relationships. Int Rev Psychiatry. (2003) 15:243–55. doi: 10.1080/0954026031000136866
3. Miranda JJ, Patel V. Achieving the millennium development goals: does mental health play a role? PLoS Med. (2005) 2:e291. doi: 10.1371/journal.pmed.0020291
4. Oates M. Perinatal psychiatric disorders: a leading cause of maternal morbidity and mortality. Br Med Bull. (2003) 67:219–29. doi: 10.1093/bmb/ldg011
5. Flynn EP, Chung EO, Ozer EJ, Fernald LCH. Maternal depressive symptoms and child behavior among Mexican women and their children. Int J Environ Res Public Health. (2017) 14. doi: 10.3390/ijerph14121566
6. Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: a meta-analytic review. Clin Child Fam Psychol Rev. (2011) 14:1–27. doi: 10.1007/s10567-010-0080-1
7. Pearson RM, Bornstein MH, Cordero M, Scerif G, Mahedy L, Evans J, et al. Maternal perinatal mental health and offspring academic achievement at age 16: The mediating role of childhood executive function. J Child Psychol Psychiatry. (2016) 57:491–501. doi: 10.1111/jcpp.12483
8. Gilliam M, Forbes EE, Gianaros PJ, Erickson KI, Brennan LM, Shaw DS. Maternal depression in childhood and aggression in young adulthood: Evidence for mediation by offspring amygdala–hippocampal volume ratio. J Child Psychol Psychiatry. (2015) 56:1083–91. doi: 10.1111/jcpp.12364
9. Nonnenmacher N, Noe D, Ehrenthal JC, Reck C. Postpartum bonding: the impact of maternal depression and adult attachment style. Arch Women's Mental Health. (2016) 19:927–35. doi: 10.1007/s00737-016-0648-y
10. Pearson RM, Evans J, Kounali D, Lewis G, Heron J, Ramchandani PG, et al. Maternal depression during pregnancy and the postnatal period: risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry. (2013) 70:1312–9. doi: 10.1001/jamapsychiatry.2013.2163
11. Hahn-Holbrook J, Cornwell-Hinrichs T, Anaya I. Economic and health predictors of national postpartum depression prevalence: A systematic review, meta-analysis, and meta-regression of 291 studies from 56 countries. Front Psychiatry. (2018) 8:248. doi: 10.3389/fpsyt.2017.00248
12. Leach LS, Poyser C, Fairweather-Schmidt K. Maternal perinatal anxiety: a review of prevalence and correlates. Clinical Psychologist. (2017) 21:4–19. doi: 10.1111/cp.12058
13. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Virginia: American Psychiatric Association (2013).
14. Halbreich U, Karkun S. Cross-cultural and social diversity of prevalence of postpartum depression and depressive symptoms. J Affect Disord. (2006) 91:97–111. doi: 10.1016/j.jad.2005.12.051
15. Chatzi L, Melaki V, Sarri K, Apostolaki I, Roumeliotaki T, Georgiou V, et al. Dietary patterns during pregnancy and the risk of postpartum depression: the mother–child ‘Rhea' cohort in Crete, Greece. Public Health Nutr. (2011) 14:1663–70. doi: 10.1017/S1368980010003629
16. Hahn-Holbrook J, Haselton M. Is postpartum depression a disease of modern civilization? Curr Dir Psychol Sci. (2014) 23:395–400. doi: 10.1177/0963721414547736
17. Alexander Bentley R, Ruck D J, Fouts HN. U.S. obesity as delayed effect of excess sugar. Econ Hum Biol. (2020) 36:100818. doi: 10.1016/j.ehb.2019.100818
18. Collin LJ, Judd S, Safford M, Vaccarino V, Welsh JA. Association of sugary beverage consumption with mortality risk in us adults: a secondary analysis of data from the REGARDS study. JAMA Network Open. (2019) 2:e193121. doi: 10.1001/jamanetworkopen.2019.3121
19. Taskinen M-R, Packard CJ, Borén J. Dietary fructose and the metabolic syndrome. Nutrients. (2019) 11:1987. doi: 10.3390/nu11091987
20. Fish A, George G. Added sugar consumption and the relationship with mood disorders in women. J Fam Cons Sci. (2020) 112:40–8. doi: 10.14307/JFCS112.2.40
21. Sanchez-Villegas A, Zazpe I, Santiago S, Perez-Cornago A, Martinez-Gonzalez MA, Lahortiga-Ramos F. (2018). Added sugars and sugar-sweetened beverage consumption, dietary carbohydrate index and depression risk in the Seguimiento Universidad de Navarra (SUN) Project. British Journal of Nutrition, 119, 211–221.
22. Ker C-R, Wu C-H, Lee C-H, Wang S-H, Chan T-F. Increased sugar-sweetened beverage use tendency in pregnancy positively associates with peripartum Edinburgh postpartum depression scores. Sci Rep. (2021) 11:1. doi: 10.1038/s41598-021-94790-5
23. Levens SM, Gotlib IH. Updating positive and negative stimuli in working memory in depression. J Exp Psychol Gen. (2010) 139:654–64. doi: 10.1037/a0020283
24. Harrell CS, Burgado J, Kelly SD, Johnson ZP, Neigh GN. High-fructose diet during periadolescent development increases depressive-like behavior and remodels the hypothalamic transcriptome in male rats. Psychoneuroendocrinology. (2015) 62:252–64. doi: 10.1016/j.psyneuen.2015.08.025
25. Hu FB. Resolved: There is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev. (2013) 14:606–19. doi: 10.1111/obr.12040
26. Sanchez-Pimienta T, Batis C, Lutter C, Rivera J. Sugar-sweetened beverages are the main sources of added sugar intake in the mexican population. J Nutr. (2016) 146:1888S–96S. doi: 10.3945/jn.115.220301
27. Lei L, Rangan A, Flood VM, Louie JCY. Dietary intake and food sources of added sugar in the Australian population. Br J Nutr. (2016) 115:868–77. doi: 10.1017/S0007114515005255
28. Popkin BM, Hawkes C. The sweetening of the global diet, particularly beverages: Patterns, trends and policy responses for diabetes prevention. Lancet Diabetes Endocrinol. (2016) 4:174–86. doi: 10.1016/S2213-8587(15)00419-2
29. Welsh JA, Sharma AJ, Grellinger L, Vos MB. Consumption of added sugars is decreasing in the United States1–4. Am J Clin Nutr. (2011) 94:726–34. doi: 10.3945/ajcn.111.018366
30. Opie RS, Uldrich AC, Ball K. Maternal postpartum diet and postpartum depression: a systematic review. Matern Child Health J. (2020) 24:966–78. doi: 10.1007/s10995-020-02949-9
31. Hibbeln JR. Seafood consumption, the DHA content of mothers' milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. J Affect Disord. (2002) 69:15–29. doi: 10.1016/S0165-0327(01)00374-3
32. Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids Health Dis. (2007) 6:21. doi: 10.1186/1476-511X-6-21
33. Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. (2011) 25:1725–34. doi: 10.1016/j.bbi.2011.07.229
34. Vijay A, Astbury S, Le Roy C, Spector TD, Valdes AM. The prebiotic effects of omega-3 fatty acid supplementation: a six-week randomised intervention trial. Gut Microbes. (2021) 13:1863133. doi: 10.1080/19490976.2020.1863133
35. Westfall S, Lomis N, Kahouli I, Dia SY, Singh SP, Prakash S. Microbiome, probiotics and neurodegenerative diseases: deciphering the gut brain axis. Cell Mol Life Sci. (2017) 74:3769–87. doi: 10.1007/s00018-017-2550-9
36. Dinan TG, Cryan JF. Melancholic microbes: A link between gut microbiota and depression? Neurogastroenterol Motility. (2013) 25:713–9. doi: 10.1111/nmo.12198
37. Wallace CJK, Milev R. The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry. (2017) 16:14. doi: 10.1186/s12991-017-0138-2
38. Slykerman RF, Hood F, Wickens K, Thompson JMD, Barthow C, Murphy R, et al. Effect of Lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: a randomised double-blind placebo-controlled trial. EBioMedicine. (2017) 24:159–65. doi: 10.1016/j.ebiom.2017.09.013
39. Yang Y, Zhao S, Yang X, Li W, Si J, Yang X. The antidepressant potential of lactobacillus casei in the postpartum depression rat model mediated by the microbiota-gut-brain axis. Neurosci Lett. (2022) 774:136474. doi: 10.1016/j.neulet.2022.136474
40. Fisberg M, Machado R. History of yogurt and current patterns of consumption. Nutr Rev. (2015) 73:4–7. doi: 10.1093/nutrit/nuv020
41. Sarkar S. Potentiality of probiotic yoghurt as a functional food – a review. Nutr Food Sci. (2018) 49:2. doi: 10.1108/NFS-05-2018-0139
42. Danone. Economic and Social Report. Annual Report. (2013). Available online at: https://www.danone.com/content/dam/danone-corp/danone-com/investors/en-all559publications/2013/integratedreports/Danone-RA2013-EN_01.pdf (accessed September 20, 2021).
43. Perez-Cornago A, Sanchez-Villegas A, Bes-Rastrollo M, Gea A, Molero P, Lahortiga-Ramos F, et al. Intake of high-fat yogurt, but not of low-fat yogurt or prebiotics, is related to lower risk of depression in women of the SUN cohort study. J Nutr. (2016) 146:1731–9. doi: 10.3945/jn.116.233858
44. Sangsefidi ZS, Mirzaei M, Hosseinzadeh M. The relation between dietary intakes and psychological disorders in Iranian adults: a population-based study. BMC Psychiatry. (2020) 20:257. doi: 10.1186/s12888-020-02678-x
45. Miller KB. Review of whole grain and dietary fiber recommendations and intake levels in different countries. Nutr Rev. (2020) 78:29–36. doi: 10.1093/nutrit/nuz052
46. Fatahi S, Matin SS, Sohouli MH, Găman M-A, Raee P, Olang B, et al. Association of dietary fiber and depression symptom: a systematic review and meta-analysis of observational studies. Complement Ther Med. (2021) 56:102621. doi: 10.1016/j.ctim.2020.102621
47. Swann OG, Kilpatrick M, Breslin M, Oddy WH. Dietary fiber and its associations with depression and inflammation. Nutr Rev. (2020) 78:394–411. doi: 10.1093/nutrit/nuz072
48. Berding K, Carbia C, Cryan JF. Going with the grain: fiber, cognition, and the microbiota-gut-brain-axis. Exp Biol Med. (2021) 246:796–811. doi: 10.1177/1535370221995785
49. Liu Z, Li L, Ma S, Ye J, Zhang H, Li Y, et al. High-dietary fiber intake alleviates antenatal obesity-induced postpartum depression: roles of gut microbiota and microbial metabolite short-chain fatty acid involved. J Agric Food Chem. (2020) 68:13697–710. doi: 10.1021/acs.jafc.0c04290
50. Okubo H, Miyake Y, Sasaki S, Tanaka K, Murakami K, Hirota Y, et al. Dietary patterns during pregnancy and the risk of postpartum depression in Japan: the osaka maternal and child health study. Br J Nutr. (2011) 105:1251–7. doi: 10.1017/S0007114510004782
51. Galland L. Diet and Inflammation. Nutr Clin Pract. (2010) 25:634–40. doi: 10.1177/0884533610385703
52. Liu X, Yan Y, Li F, Zhang D. Fruit and vegetable consumption and the risk of depression: a meta-analysis. Nutrition. (2016) 32:296–302. doi: 10.1016/j.nut.2015.09.009
53. Frankenfeld CL, Hullar MAJ, Maskarinec G, Monroe KR, Shepherd JA, Franke AA, et al. The gut microbiome is associated with circulating dietary biomarkers of fruit and vegetable intake in a multiethnic cohort. J Acad Nutr Diet. (2022) 122:78–98. doi: 10.1016/j.jand.2021.05.023
54. Yang C, Zhao A, Lan H, Ren Z, Zhang J, Szeto IM-Y, et al. Association between dietary quality and postpartum depression in lactating women: a cross-sectional survey in urban china. Front Nutr. (2021) 8:705353. doi: 10.3389/fnut.2021.705353
55. Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
56. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry J Ment Sci. (1987) 150:782–6. doi: 10.1192/bjp.150.6.782
57. Fisher J, Cabral de Mello M, Patel V, Rahman A, Tran T, Holton S, et al. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Org. (2012) 90:139–149H. doi: 10.2471/BLT.11.091850
58. Global, Dietary Database,. Summary of Methods and Data Collection. Available online at: https://www.globaldietarydatabase.org/methods/summary-methods-and-data-collection (accessed February 18, 2022).
59. World Bank. GDP (current US$) Data. (2022). Available online at: https://data.worldbank.org/indicator/NY.GDP.MKTP.CD (accessed February 23, 2022).
60. Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Commun Health. (2013) 67:974–8. doi: 10.1136/jech-2013-203104
61. Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol Methods. (2006) 11:193–206. doi: 10.1037/1082-989X.11.2.193
62. Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta-analysis. J Educ Behav Stat. (2010) 35:215–47. doi: 10.3102/1076998609346961
64. Ogbo FA, Eastwood J, Hendry A, Jalaludin B, Agho KE, Barnett B, et al. Determinants of antenatal depression and postnatal depression in Australia. BMC Psychiatry. (2018) 18:49. doi: 10.1186/s12888-018-1598-x
65. Azad R, Fahmi R, Shrestha S, Joshi H, Hasan M, Khan ANS, et al. Prevalence and risk factors of postpartum depression within one year after birth in urban slums of Dhaka, Bangladesh. PLoS ONE. (2019) 14:5. doi: 10.1371/journal.pone.0215735
66. Abuchaim E, de SV, Caldeira NT, Lucca MMD, Varela M, Silva IA. Postpartum depression and maternal self-efficacy for breastfeeding: prevalence and association. Acta Paulista de Enfermagem. (2016) 29:664–70. doi: 10.1590/1982-0194201600093
67. Avilla JC, de Giugliani C, Bizon AMBL, Martins ACM, Senna AFK, de Giugliani ERJ. Association between maternal satisfaction with breastfeeding and postpartum depression symptoms. PLoS ONE. (2020) 15:e0242333. doi: 10.1371/journal.pone.0242333
68. Corrêa H, Castro e Couto T, Santos W, Romano-Silva MA, Santos LMP. Postpartum depression symptoms among Amazonian and Northeast Brazilian women. J Affect Disord. (2016) 204:214–8. doi: 10.1016/j.jad.2016.06.026
69. Farías-Antúnez S, Santos IS, Matijasevich A, de Barros AJD. Maternal mood symptoms in pregnancy and postpartum depression: association with exclusive breastfeeding in a population-based birth cohort. Soc Psychiatry Psychiatr Epidemiol. (2020) 55:5. doi: 10.1007/s00127-019-01827-2
70. Halal CS, Bassani DG, Santos IS, Tovo-Rodrigues L, Del-Ponte B, Silveira MF, et al. Maternal perinatal depression and infant sleep problems at 1 year of age: Subjective and actigraphy data from a population-based birth cohort study. J Sleep Res. (2021) 30:e13047. doi: 10.1111/jsr.13047
71. Lorentz MS, Chagas LB, Perez AV, da Silva Cassol PA, Vettorazzi J, Lubianca JN. Correlation between depressive symptoms and sexual dysfunction in postpartum women during the COVID-19 pandemic. Eur J Obstet Gynecol Reprod Biol. (2021) 258:162–7. doi: 10.1016/j.ejogrb.2020.12.039
72. Dennis CL, Brown HK, Wanigaratne S, Fung K, Vigod SN, Grigoriadis S, et al. Prevalence, incidence, and persistence of postpartum depression, anxiety, and comorbidity among chinese immigrant and nonimmigrant women: a longitudinal cohort study. Can J Psychiatry. (2018) 63:44–53. doi: 10.1177/0706743717720689
73. Emerson MR, Mathews TL, Struwe L. Postpartum depression screening for new mothers at well child visits. MCN Am J Maternal/Child Nurs. (2018) 43:139–45. doi: 10.1097/NMC.0000000000000426
74. Falah-Hassani K, Shiri R, Dennis C-L. Prevalence and risk factors for comorbid postpartum depressive symptomatology and anxiety. J Affect Disord. (2016) 198:142–7. doi: 10.1016/j.jad.2016.03.010
75. Gan Y, Xiong R, Song J, Xiong X, Yu F, Gao W, et al. The effect of perceived social support during early pregnancy on depressive symptoms at 6 weeks postpartum: a prospective study. BMC Psychiatry. (2019) 19:232. doi: 10.1186/s12888-019-2188-2
76. Guo X-J, Chen J, Ren J-H, Deng X, Xu L-Z. Comparisons on perinatal depression between the first-child women and the second-child women in West China under the universal 2-child policy. Medicine. (2020) 99:e20641. doi: 10.1097/MD.0000000000020641
77. Huang Y, Liu Y, Wang Y, Liu D. Family function fully mediates the relationship between social support and perinatal depression in rural Southwest China. BMC Psychiatry. (2021) 21:151. doi: 10.1186/s12888-021-03155-9
78. Li Y, Long Z, Cao D, Cao F. Social support and depression across the perinatal period: a longitudinal study. J Clin Nurs. (2017) 26:17–8. doi: 10.1111/jocn.13817
79. Liang P, Wang Y, Shi S, Liu Y, Xiong R. Prevalence and factors associated with postpartum depression during the COVID-19 pandemic among women in Guangzhou, China: a cross-sectional study. BMC Psychiatry. (2020) 20:557. doi: 10.1186/s12888-020-02969-3
80. Liu S, Yan Y, Gao X, Xiang S, Sha T, Zeng G, et al. Risk factors for postpartum depression among Chinese women: path model analysis. BMC Preg Childbirth. (2017) 17. doi: 10.1186/s12884-017-1320-x
81. Liu Y, Guo N, Li T, Zhuang W, Jiang H. Prevalence and associated factors of postpartum anxiety and depression symptoms among women in Shanghai, China. J Affect Disord. (2020) 274:848–56. doi: 10.1016/j.jad.2020.05.028
82. Long G, Yao ZY, Na Y, Ping Y, Wei S, Mingsheng T. Different types of low back pain in relation to pre- and post-natal maternal depressive symptoms. BMC Preg Childbirth. (2020) 20:551. doi: 10.1186/s12884-020-03139-9
83. Peng K, Zhou L, Liu X, Ouyang M, Gong J, Wang Y, et al. Who is the main caregiver of the mother during the doing-the-month: is there an association with postpartum depression?. BMC Psychiatry. (2021) 21:270. doi: 10.1186/s12888-021-03203-4
84. Peng S, Lai X, Du Y, Meng L, Gan Y, Zhang X. Prevalence and risk factors of postpartum depression in China: a hospital-based cross-sectional study. J Affect Disord. (2021) 282:1096–100. doi: 10.1016/j.jad.2021.01.012
85. Shi P, Ren H, Li H, Dai Q. Maternal depression and suicide at immediate prenatal and early postpartum periods and psychosocial risk factors. Psychiatry Res. (2018) 261:298–306. doi: 10.1016/j.psychres.2017.12.085
86. Wang Y-Y, Li H, Wang Y-J, Wang H, Zhang Y-R, Gong L, et al. Living with parents or with parents-in-law and postpartum depression: a preliminary investigation in China. J Affect Disord. (2017) 218:335–8. doi: 10.1016/j.jad.2017.04.052
87. Xiong R, Deng A. Prevalence and associated factors of postpartum depression among immigrant women in Guangzhou, China. BMC Preg Childbirth. (2020) 20:247. doi: 10.1186/s12884-020-02946-4
88. Zhou C, Zheng W, Yuan Q, Zhang B, Chen H, Wang W, et al. Associations between social capital and maternal depression: results from a follow-up study in China. BMC Preg Childbirth. (2018) 18:45. doi: 10.1186/s12884-018-1673-9
89. Zhou H, Li W, Ren Y. Poor sleep quality of third trimester exacerbates the risk of experiencing postnatal depression. Psychol Health Med. (2020) 25:229–38. doi: 10.1080/13548506.2018.1549738
90. Ding G, Niu L, Vinturache A, Zhang J, Lu M, Gao Y, et al. “Doing the month” and postpartum depression among Chinese women: a Shanghai prospective cohort study. Women and Birth. (2020) 33:e151–8. doi: 10.1016/j.wombi.2019.04.004
91. Stylianides C, Middleton N, Kouta C, Raftopoulos V. The Role of Emotional Intelligence Postpartum Depression in Predicting Mothers' Satisfaction with Quality of Co-Operation with Obstetricians Midwives. (2016). Available online at: https://ktisis.cut.ac.cy/handle/10488/8662 (accessed September 20, 2021).
92. Fiala A, Švancara J, Klánová J, Kašpárek T. Sociodemographic and delivery risk factors for developing postpartum depression in a sample of 3233 mothers from the Czech ELSPAC study. BMC Psychiatry. (2017) 17:104. doi: 10.1186/s12888-017-1261-y
93. Ahmed GK, Elbeh K, Shams RM, Malek MAA, Ibrahim AK. Prevalence and predictors of postpartum depression in Upper Egypt: a multicenter primary health care study. J Affect Disord. (2021) 290:211–8. doi: 10.1016/j.jad.2021.04.046
94. Meky HK, Shaaban MM, Ahmed MR, Mohammed TY. Prevalence of postpartum depression regarding mode of delivery: a cross-sectional study. J Maternal-Fetal Neonatal Med. (2020) 33:3300–7. doi: 10.1080/14767058.2019.1571572
95. Adamu AF, Adinew YM. Domestic violence as a risk factor for postpartum depression among Ethiopian women: facility based study. Clin Pract Epidemiol Mental Health. (2018) 14:109–19. doi: 10.2174/1745017901814010109
96. Dadi AF, Mwanri L, Woodman RJ, Azale T, Miller ER. Causal mechanisms of postnatal depression among women in Gondar town, Ethiopia: application of a stress-process model with generalized structural equation modeling. Reprod Health. (2020) 17:1–15. doi: 10.1186/s12978-020-00912-z
97. Dadi AF, Miller ER, Woodman RJ, Azale T, Mwanri L. Effect of perinatal depression on risk of adverse infant health outcomes in mother-infant dyads in Gondar town: A causal analysis. BMC Preg Childbirth. (2021) 21:255. doi: 10.1186/s12884-021-03733-5
98. Wubetu AD, Engidaw NA, Gizachew KD. Prevalence of postpartum depression and associated factors among postnatal care attendees in Debre Berhan, Ethiopia, 2018. BMC Preg Childbirth. (2020) 20. doi: 10.1186/s12884-020-02873-4
99. Fritel X, Tsegan YE, Pierre F, Saurel-Cubizolles M-J. Association of postpartum depressive symptoms and urinary incontinence. A cohort study. Eur J Obstetr Gynecol Reprod Biol. (2016) 198:62–7. doi: 10.1016/j.ejogrb.2015.12.028
100. Koutra K, Vassilaki M, Georgiou V, Koutis A, Bitsios P, Kogevinas M, et al. Pregnancy, perinatal and postpartum complications as determinants of postpartum depression: the Rhea mother–child cohort in Crete, Greece. Epidemiol Psychiatr Sci. (2016) 27:244–55. doi: 10.1017/S2045796016001062
101. Ana Y, Lewis MG, van Schayck OCP, Babu GR. Is physical activity in pregnancy associated with prenatal and postnatal depressive symptoms? Results from MAASTHI cohort study in South India. J Psychosomatic Res. (2021) 144:110390. doi: 10.1016/j.jpsychores.2021.110390
102. Badiya PK, Siddabattuni S, Dey D, Javvaji SK, Nayak SP, Hiremath AC, et al. Identification of clinical and psychosocial characteristics associated with perinatal depression in the south Indian population. Gen Hosp Psychiatry. (2020) 66:161–70. doi: 10.1016/j.genhosppsych.2020.08.002
103. Jha P, Larsson M, Christensson K, Svanberg AS. Fear of childbirth and depressive symptoms among postnatal women: a cross-sectional survey from Chhattisgarh, India. Women Birth. (2018) 31:e122–33. doi: 10.1016/j.wombi.2017.07.003
104. Joshi M, Raut A. Maternal depression and its association with responsive feeding and nutritional status of infants: a cross-sectional study from a rural medical college in central India. J Postgrad Med. (2019) 65:212–8. doi: 10.4103/jpgm.JPGM_479_18
105. Murry LL, Surbala Devi Y, Joshi P, Dabas S, Kumari V, Jitenkumar Singh K. Postpartum depression and its risk factors among Indian women. Nurs J India. (2020) CXI:04. doi: 10.48029/NJI.2020.CXI408
106. Nurbaeti I, Deoisres W, Hengudomsub P. Postpartum depression in Indonesian mothers: Its changes and predicting factors. Pacific Rim Int J Nurs Res. (2018) 22:93–105.
107. Nurbaeti I, Deoisres W, Hengudomsub P. Association between psychosocial factors and postpartum depression in South Jakarta, Indonesia. Sex Reproduct Healthcare. (2019) 20:72–6. doi: 10.1016/j.srhc.2019.02.004
108. Abdollahi F, Etemadinezhad S, Lye M-S. Postpartum mental health in relation to sociocultural practices. Taiwan J Obst Gynecol. (2016) 55:1. doi: 10.1016/j.tjog.2015.12.008
109. Ezzeddin N, Kalantari N, Zavoshy R, Noroozi M, Miri N. Association of infant exclusive breast feeding with household food security and maternal mental health. Arch Iran Med. (2019) 22:9.
110. Afshari P, Tadayon M, Abedi P, Yazdizadeh S. Prevalence and related factors of postpartum depression among reproductive aged women in Ahvaz, Iran. Health Care Women Int. (2020) 41:255–65. doi: 10.1080/07399332.2019.1578779
111. Iranpour S, Kheirabadi GR, Heidari-Beni M, Maracy MR. Association between caffeine consumption during pregnancy and postpartum depression: a population-based study. J Caffeine Res. (2017) 7:1–6. doi: 10.1089/jcr.2016.0008
112. Daoud N, O'Brien K, O'Campo P, Harney S, Harney E, Bebee K, et al. Postpartum depression prevalence and risk factors among Indigenous, non-Indigenous and immigrant women in Canada. Can J Public Health. (2019) 110:440–52. doi: 10.17269/s41997-019-00182-8
113. Mazor E, Sheiner E, Wainstock T, Attias M, Walfisch A. The association between depressive state and maternal cognitive function in postpartum women. Am J Perinatol. (2019) 36:285–90. doi: 10.1055/s-0038-1667376
114. Bruno A, Laganà AS, Leonardi V, Greco D, Merlino M, Vitale SG, et al. Inside–out: The role of anger experience and expression in the development of postpartum mood disorders. J Mat Fetal Neonatal Med. (2018). 31:3033–3038. doi: 10.1080/14767058.2017.1362554
115. Clavenna A, Seletti E, Cartabia M, Didoni A, Fortinguerra F, Sciascia T, et al. Postnatal depression screening in a paediatric primary care setting in Italy. BMC Psychiatry. (2017) 17:1. doi: 10.1186/s12888-017-1205-6
116. Cozzolino M, Troiano G, Coccia ME. Spontaneous pregnancy versus assisted reproductive technologies: Implications on maternal mental health. Women Health. (2021) 61:303–12. doi: 10.1080/03630242.2021.1881025
117. Ostacoli L, Cosma S, Bevilacqua F, Berchialla P, Bovetti M, Carosso AR, et al. Psychosocial factors associated with postpartum psychological distress during the Covid-19 pandemic: a cross-sectional study. BMC Preg Childbirth. (2020) 20:703. doi: 10.1186/s12884-020-03399-5
118. Cui M, Kimura T, Ikehara S, Dong J-Y, Ueda K, Kawanishi Y, et al. Prenatal tobacco smoking is associated with postpartum depression in Japanese pregnant women: the japan environment and children's study. J Affect Disord. (2020) 264:76–81. doi: 10.1016/j.jad.2019.11.145
119. Honjo K, Kimura T, Baba S, Ikehara S, Kitano N, Sato T, et al. Association between family members and risk of postpartum depression in Japan: Does “who they live with” matter? -The Japan environment and Children's study. Soc Sci Med. (2018) 217:65–72. doi: 10.1016/j.socscimed.2018.09.043
120. Iwata H, Mori E, Sakajo A, Aoki K, Maehara K, Tamakoshi K. Prevalence of postpartum depressive symptoms during the first 6 months postpartum: association with maternal age and parity. J Affect Disord. (2016) 203:227–32. doi: 10.1016/j.jad.2016.06.002
121. Matsumura K, Hamazaki K, Tsuchida A, Kasamatsu H, Inadera H, Kamijima M, et al. Education level and risk of postpartum depression: results from the Japan environment and children's study (JECS). BMC Psychiatry. (2019) 19:419. doi: 10.1186/s12888-019-2401-3
122. Muchanga SMJ, Yasumitsu-Lovell K, Eitoku M, Mbelambela EP, Ninomiya H, Komori K, et al. Preconception gynecological risk factors of postpartum depression among Japanese women: the Japan environment and children's study (JECS). J Affect Disord. (2017) 217:34–41. doi: 10.1016/j.jad.2017.03.049
123. Shibata Y, Suzuki S. Comparison of the Edinburgh postnatal depression scale and the whooley questions in screening for postpartum depression in Japan. J Maternal-Fetal Neonatal Med. (2020) 33:2785–8. doi: 10.1080/14767058.2018.1560413
124. Suzuki S. Relationship between postpartum depression and lactation status at a Japanese perinatal center: a cross-sectional study. F1000Research. (2020) 8:1845. doi: 10.12688/f1000research.20704.2
125. Takehara K, Tachibana Y, Yoshida K, Mori R, Kakee N, Kubo T. Prevalence trends of pre- and postnatal depression in Japanese women: a population-based longitudinal study. J Affect Disord. (2018) 225:389–94. doi: 10.1016/j.jad.2017.08.008
126. Zejnullahu VA, Ukella-Lleshi D, Zejnullahu VA, Miftari E, Govori V. Prevalence of postpartum depression at the clinic for obstetrics and gynecology in Kosovo teaching hospital: demographic, obstetric and psychosocial risk factors. Eur J Obstet Gynecol Reprod Biol. (2021) 256:215–20. doi: 10.1016/j.ejogrb.2020.11.025
127. Inthaphatha S, Yamamoto E, Louangpradith V, Takahashi Y, Phengsavanh A, Kariya T, et al. Factors associated with postpartum depression among women in Vientiane Capital, Lao People's Democratic Republic: a cross-sectional study. PLoS ONE. (2020) 15:e0243463. doi: 10.1371/journal.pone.0243463
128. Badr LK, Ayvazian N, Lameh S, Charafeddine L. Is the effect of postpartum depression on mother-infant bonding universal? Infant Behav Dev. (2018) 51:15–23. doi: 10.1016/j.infbeh.2018.02.003
129. Radzi CWJBWM, Jenatabadi HS, Samsudin N. Postpartum depression symptoms in survey-based research: a structural equation analysis. BMC Public Health. (2021) 21:1–12. doi: 10.1186/s12889-020-09999-2
130. Abdul Raheem R, Chih HJ, Binns CW. Factors associated with maternal depression in the Maldives: a prospective cohort study. Asia Pacific J Public Health. (2018) 30:244–51. doi: 10.1177/1010539518756380
131. Suárez-Rico BV, Estrada-Gutierrez G, Sánchez-Martínez M, Perichart-Perera O, Rodríguez-Hernández C, González-Leyva C, et al. Prevalence of depression, anxiety, and perceived stress in postpartum mexican women during the COVID-19 lockdown. Int J Environ Res Public Health. (2021) 18:4627. doi: 10.3390/ijerph18094627
132. Bhusal BR, Bhandari N, Chapagai M, Gavidia T. Validating the Edinburgh Postnatal Depression Scale as a screening tool for postpartum depression in Kathmandu, Nepal. Int J Ment Health Syst. (2016) 10:71. doi: 10.1186/s13033-016-0102-6
133. Chalise M, Karmacharya I, Kaphle M, Wagle A, Chand N, Adhikari L. Factors associated with postnatal depression among mothers attending at Bharatpur hospital, Chitwan. Depress Res Treat. (2020) 2020:e9127672. doi: 10.1155/2020/9127672
134. Maharjan PL, Lamichhane S, Shrestha PD, Mathias J, Gautam KR, Shah SK. Prevalence and factors associated with depressive symptoms among post-partum mothers in dhanusha District of Nepal. Sleep Hypnosis Int J. (2018) 21:60–68. doi: 10.5350/Sleep.Hypn.2019.21.0173
135. Sulyman D, Ayanda K, Dattijo L, Aminu B. Postnatal depression and its associated factors among Northeastern Nigerian women. Annals Trop Med Public Health. (2016) 9:9565678. doi: 10.4103/1755-6783.179099
136. Shakeel N, Sletner L, Falk RS, Slinning K, Martinsen EW, Jenum AK, et al. Prevalence of postpartum depressive symptoms in a multiethnic population and the role of ethnicity and integration. J Affect Disord. (2018) 241:49–58. doi: 10.1016/j.jad.2018.07.056
137. Qandil S, Jabr S, Wagler S, Collin SM. Postpartum depression in the Occupied Palestinian Territory: a longitudinal study in Bethlehem. BMC Preg Childbirth. (2016) 16:375. doi: 10.1186/s12884-016-1155-x
138. Labrague LJ, McEnroe-Petitte D, Tsaras K, Yboa BC, Rosales RA, Tizon MM, et al. Predictors of postpartum depression and the utilization of postpartum depression services in rural areas in the Philippines. Perspect Psychiatr Care. (2020) 56:2. doi: 10.1111/ppc.12428
139. Drozdowicz-Jastrzebska E, Skalski M, Gdańska P, Mach A, Januszko P, Nowak R, et al. Insomnia, postpartum depression and estradiol in women after delivery. Metab Brain Dis. (2017) 32. doi: 10.1007/s11011-017-0079-0
140. Jaeschke RR, Dudek D, Topór-Madry R, Drozdowicz K, Datka W, Siwek M, et al. Postpartum depression: bipolar or unipolar? Analysis of 434 polish postpartum women. Braz J Psychiatry. (2016) 39:154–9. doi: 10.1590/1516-4446-2016-1983
141. Maliszewska K, Bidzan M, Swiatkowska-Freund M, Preis K. Personality type, social support and other correlates of risk for affective disorders in early puerperium. Ginekol Pol. (2016) 87:6. doi: 10.5603/GP.2016.0023
142. Nasr RSA, Altharwi K, Derbah MS, Gharibo SO, Fallatah SA, Alotaibi SG, et al. Prevalence and predictors of postpartum depression in Riyadh, Saudi Arabia: A cross sectional study. PLoS ONE. (2020) 15:e0228666. doi: 10.1371/journal.pone.0228666
143. Almutairi AF, Salam M, Alanazi S, Alweldawi M, Alsomali N, Alotaibi N. Impact of help-seeking behavior and partner support on postpartum depression among Saudi women. Neuropsychiatr Dis Treat. (2017) 13:1929–36. doi: 10.2147/NDT.S135680
144. Alzahrani A. Risk factors for postnatal depression among primipara mothers. Span J Psychol. (2019) 22. doi: 10.1017/sjp.2019.33
145. Mokwena K, Masike I. The need for universal screening for postnatal depression in South Africa: confirmation from a sub-district in Pretoria, South Africa. Int J Environ Res Public Health. (2020) 17. doi: 10.3390/ijerph17196980
146. Fan Q, Long Q, De Silva V, Gunarathna N, Jayathilaka U, Dabrera T, et al. Prevalence and risk factors for postpartum depression in Sri Lanka: a population-based study. Asian J Psychiatr. (2019) 47:101855. doi: 10.1016/j.ajp.2019.101855
147. Khalifa DS, Glavin K, Bjertness E, Lien L. Course of depression symptoms between 3 and 8 months after delivery using two screening tools (EPDS and HSCL-10) on a sample of Sudanese women in Khartoum state. BMC Preg Childbirth. (2018) 18. doi: 10.1186/s12884-018-1948-1
148. Eckerdal P, Georgakis MK, Kollia N, Wikström A-K, Högberg U, Skalkidou A. Delineating the association between mode of delivery and postpartum depression symptoms: a longitudinal study. Acta Obstet Gynecol Scand. (2018) 97:301–11. doi: 10.1111/aogs.13275
149. Roumieh M, Bashour H, Kharouf M, Chaikha S. Prevalence and risk factors for postpartum depression among women seen at Primary Health Care Centres in Damascus. BMC Preg Childbirth. (2019) 19:519. doi: 10.1186/s12884-019-2685-9
150. Lin YH, Chen CM, Su HM, Mu SC, Chang ML, Chu PY, et al. Association between Postpartum Nutritional Status and Postpartum Depression Symptoms. Nutrients. (2019) 11:1204. doi: 10.3390/nu11061204
151. Lin WC, Chang SY, Chen YT, Lee HC, Chen YH. Postnatal paternal involvement and maternal emotional disturbances: the effect of maternal employment status. J Affect Disord. (2017) 219:9–16. doi: 10.1016/j.jad.2017.05.010
152. Lin P-Y, Chiu T-H, Ho M, Pei-Chen Chang J, Hui-Chih Chang C, Su K-P. Major depressive episodes during pregnancy and after childbirth: A prospective longitudinal study in Taiwan. J Formosan Med Assoc. (2019) 118:1551–9. doi: 10.1016/j.jfma.2019.03.003
153. Bay F, Sayiner FD. Perception of traumatic childbirth of women and its relationship with postpartum depression. Women and Health. (2021) 61:479–89. doi: 10.1080/03630242.2021.1927287
154. Bolak Boratav H, Toker Ö, Küey L. Postpartum depression and its psychosocial correlates: a longitudinal study among a group of women in Turkey. Women and Health. (2016) 56:502–21. doi: 10.1080/03630242.2015.1101737
155. Çelik SB, Bucaktepe GE, Uludag A, Bulut IU, Erdem Ö, Altinbaş K. Screening mixed depression and bipolarity in the postpartum period at a primary health care center. Comprehens Psychiatry. (2016) 71:57–62. doi: 10.1016/j.comppsych.2016.07.013
156. Demirel G, Egri G, Yesildag B, Doganer A. Effects of traditional practices in the postpartum period on postpartum depression. Health Care Women Int. (2018) 39:65–78. doi: 10.1080/07399332.2017.1370469
157. Dikmen-Yildiz P, Ayers S, Phillips L. Depression, anxiety, PTSD and comorbidity in perinatal women in Turkey: a longitudinal population-based study. Midwifery. (2017) 55:29–37. doi: 10.1016/j.midw.2017.09.001
158. Sahin E, Seven M. Depressive symptoms during pregnancy and postpartum: a prospective cohort study. Perspect Psychiatr Care. (2019) 55:430–7. doi: 10.1111/ppc.12334
159. Yilmaz FA, Avci D, Aba YA, Ozdilek R, Dutucu N. Sexual dysfunction in postpartum turkish women: it's relationship with depression and some risk factors. Afr J Reprod Health. (2018) 22:54–63. doi: 10.29063/ajrh2018/v22i4.6
160. Alhammadi SM, Hashem LA, Abusbeih ZR, Alzaabi FS, Alnuaimi SN, Jalabi AF, et al. Predictors of postpartum depression in Dubai, a rapidly growing multicultural society in the United Arab Emirates. Psychiatr Danub. (2017) 29:313–22.
161. Kothari C, Wiley J, Moe A, Liepman MR, Tareen RS, Curtis A. Maternal depression is not just a problem early on. Public Health. (2016) 137:154–61. doi: 10.1016/j.puhe.2016.01.003
162. Magliarditi AT, Lua LL, Kelley MA, Jackson DN. Maternal depression scale: do “Drop-In” laborist patients have increased postpartum screening risks compared to patients with adequate prenatal care? Matern Child Health J. (2019) 23:54–60. doi: 10.1007/s10995-018-2593-z
163. Soffer MD, Adams ZM, Chen YS, Fox NS. Risk factors for positive postpartum depression screen in women with private health insurance and access to care. J Maternal-Fetal Neonatal Med. (2019) 32:4154–8. doi: 10.1080/14767058.2018.1484096
164. Do T, Nguyen T, Thi Thu Huong P. Postpartum depression and risk factors among Vietnamese women. Biomed Res Int. (2018) 2018:1–5. doi: 10.1155/2018/4028913
165. Lin YC, Chang WH, Li TC, Iwata O, Chen HL. Health risk of infants exposed to lead and mercury through breastfeeding. Exp Health. (2022). doi: 10.1007/s12403-022-00485-1
166. Forberger S, Reisch L, Meshkovska B, Lobczowska K, Scheller DA, Wendt J, et al. Sugar-sweetened beverage tax implementation processes: results of a scoping review. Health Res Pol Syst. (2022) 20:33. doi: 10.1186/s12961-022-00832-3
168. LaCoursiere D, Barrett-Connor E, O'Hara M, Hutton A, Varner M. The association between prepregnancy obesity and screening positive for postpartum depression. BJOG Int J Obstet Gynaecol. (2010) 117:1011–8. doi: 10.1111/j.1471-0528.2010.02569.x
169. Marconcin P, Peralta M, Gouveia ÉR, Ferrari G, Carraça E, Ihle A, et al. Effects of exercise during pregnancy on postpartum depression: a systematic review of meta-analyses. Biology. (2021) 10. doi: 10.3390/biology10121331
170. Yamada A, Isumi A, Fujiwara T. Association between lack of social support from partner or others and postpartum depression among Japanese mothers: a population-based cross-sectional study. Int J Environ Res Public Health. (2020) 17:E4270. doi: 10.3390/ijerph17124270
171. Bershadsky S, Trumpfheller L, Kimble HB, Pipaloff D, Yim IS. The effect of prenatal Hatha yoga on affect, cortisol and depressive symptoms. Complement Ther Clin Pract. (2014) 20:106–13. doi: 10.1016/j.ctcp.2014.01.002
172. Menon V, Kar SK, Suthar N, Nebhinani N. Vitamin D and depression: a critical appraisal of the evidence and future directions. Indian J Psychol Med. (2020) 42:11–21. doi: 10.4103/IJPSYM.IJPSYM_160_19
173. Miller V, Singh GM, Onopa J, Reedy J, Shi P, Zhang J, et al. Global Dietary Database 2017: Data availability and gaps on 54 major foods, beverages and nutrients among 5.6 million children and adults from 1220 surveys worldwide. BMJ Glob Health. (2021) 6:e003585. doi: 10.1136/bmjgh-2020-003585
174. Piantadosi S, Byar DP, Green SB. The ecological fallacy. Am J Epidemiol. (1988) 127:893–904. doi: 10.1093/oxfordjournals.aje.a114892
Search Syntax
CINAHL 7/26/21 Here is the Boolean search that yields 195
records: Boolean search
(IN edinburgh postnatal depression scale) AND (AB (postpartum depression OR postnatal depression)) AND (AB (incidence OR prevalence))
With the additional limiters:
(1) Narrow to start 2016
(2) Narrow it to English articles only
(3) Narrow it to Humans only
(4) Narrow it to females only
PSYCHINFO 7/26/21 Here is the Boolean search that yields 162
records: AB (incidence or prevalence) AND AB (postnatal depression or postpartum depression) AND
TM edinburgh postnatal depression scale Here are the limiters: Date: start 2016
* Language: English
* Population: Female
PUBMED 7/26/21
Here is the Boolean search that yields 245 records: ((“postpartum depression”[All Fields] OR “postnatal depression”[All Fields]) AND “prevalence”[All Fields]) AND “edinburgh postnatal depression scale”[All Fields] AND ((“2015/01/01”[PDAT]) AND “humans”[MeSH Terms])
Keywords: postpartum depression (PPD), diet, sugar sweetened beverages (SSBs), added sugar, global, national, review—systematic, meta analyses
Citation: Fish-Williamson A and Hahn-Holbrook J (2023) Nutritional factors and cross-national postpartum depression prevalence: an updated meta-analysis and meta-regression of 412 studies from 46 countries. Front. Psychiatry 14:1193490. doi: 10.3389/fpsyt.2023.1193490
Received: 24 March 2023; Accepted: 02 May 2023;
Published: 15 June 2023.
Edited by:
Soudabeh Givrad, NewYork-Presbyterian, United StatesReviewed by:
Rasmieh Al-amer, Western Sydney University, AustraliaCopyright © 2023 Fish-Williamson and Hahn-Holbrook. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adi Fish-Williamson, YWZpc2hAdWNtZXJjZWQuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.