
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 07 September 2023
Sec. Autism
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1192669
This article is part of the Research Topic Wellbeing in Parents of Neurodivergent Children View all 8 articles
Objective: Depression scores in caregivers of autistic children often fall in the clinical range. The attention of clinically depressed individuals tends to be biased toward negatively toned information. Whether caring for an autistic child might also be characterized by a negative attentional bias was explored here.
Methods: A sample of N = 98 (57 caregivers and 41 controls) completed questionnaires assessing depressive symptoms. Orienting attention to (i.e., vigilance), and shifting attention away from (i.e., disengagement), negative information was assessed via an online version of the emotional face dot probe task.
Results: Mean depression scores in caregivers, falling in the borderline clinical range, were significantly higher compared with controls. Groups, however, were indistinguishable with respect to vigilance and disengagement, and these attentional indices were unrelated to depression scores.
Conclusion: Caring for an autistic child, while associated with borderline clinical depression scores, was not characterized by a negative attentional bias. Findings are discussed in the context of methodological shortcomings and recommendations for future research.
Psychological distress, of which depression is one common marker, tends to be elevated in the context of caring for an autistic child. Indeed, a plethora of cross-sectional research has linked caring for an autistic child with increased depression scores (1–4). The negative psychological impact of caring for an autistic child appears to be enduring. Indeed, caregivers’ use of emotional coping behaviors increases over time, and emotional coping behaviors are established risk factors for depression in this sample (5, 6). Caregivers have also commented in qualitative studies that concern about who will care for the autistic child in the future, at a time when they might be physically unable, cause their feelings of depression to increase over time (7). Caregivers’ depression scores often satisfy the criteria for a clinical case (8, 9). It was recently estimated that clinically significant psychopathology, in the form of depression, is evident in approximately 33% of caregivers of autistic children (10).
Cognitive models of depression implicate difficulties with cognitive control in the etiology and maintenance of the disorder (11). The cognitive schemas of depressed individuals are typically defined by themes of loss, rejection, and failure, and depressed individuals’ attention, guided by these schemas, can become biased toward the processing of negative information (12). In other words, depressed individuals appear to be hypersensitive to negatively toned information in their environment. For example, the emotional face dot probe task presents two faces, one with a negative (i.e., sad) and one with a neutral expression, side by side on the screen and asks participants to indicate, when cued, which face has been replaced by a large red dot. Response latencies, captured in milliseconds, provide attentional bias indices of vigilance (i.e., orienting attention to negative stimuli) and disengagement (i.e., shifting attention from negative stimuli). Several studies have reported that depressed individuals found it more challenging, after receiving a visual cue, to disengage their attention from negatively toned stimuli. In other words, depressed individuals processed negative stimuli for longer periods following fixation. Some evidence, though less prevalent, shows that depressed individuals are also more vigilant to negative stimuli, detecting them faster following visual cues (13–18). This attentional bias toward the processing of negative content, characterized predominately by problems with disengagement, has been replicated with several other exogenous cueing tasks (19). It was the recent conclusion of several researchers, following comprehensive meta-analysis and systematic review, that increased depressive symptomology provides one robust psychological marker for negative attentional bias (20, 21). Researchers have also found that depressed individuals sometimes lack the positive attentional bias evident in non-clinical samples. Indeed, vigilance for positive stimuli (i.e., faces conveying positive expressions such as happiness) was slower, and disengagement much faster, in depressed individuals compared with controls (22–24). Studies using more sophisticated eye-tracking designs, allowing for continuous monitoring of visual attention, have also found evidence for negative attentional bias in the context of depression. Indeed, when presented with facial expressions varying in emotion (i.e., some positive, some neutral, some negative), depressed individuals glanced more often at the negative, and less often at the positive, stimuli compared with controls (25). Total fixation time on negative stimuli, assessed with eye tracking, has also been shown to be longer in depressed individuals (26). The evidence suggests the attention of depressed individuals, and those with conditions characterized by increased depression scores, is biased in favor of negative information. Depressed individuals seem to pick up on negative content faster and, finding disengaging from it more challenging, process it for longer. The reverse appears to be the case for positive content, with depressed individuals identifying it slower and, disengaging faster, processing it for shorter periods.
Depression scores tend to be high in the context of caring for an autistic child, often falling in the clinical range. Whether caring for an autistic child is characterized by a negative attentional bias, however, has not yet been explored. This study aims to fill this gap. Several lines of evidence converge to suggest this might indeed be the case. For example, caregivers of autistic children self-report more attentional errors compared with their non-caregiving counterparts (27). In the lab, using objective, performance-based measures of attentional control, caregivers were found to make more errors than their non-caregiving counterparts (28–30). Much of the research involving objective tests of attention, however, has involved spousal dementia caregivers, and generalizing these findings to caregivers of autistic children, therefore, should be done cautiously. That said, it was the conclusion of researchers following a comprehensive review that caring for individuals with neurological conditions such as dementia is comparable, in terms of psychological demand and burden, to caring for individuals with developmental conditions such as autism (31). Whether the attention of informal caregivers is biased toward the processing of negatively toned information has rarely been explored. Only four studies have been completed to date as far as we can find, with findings showing caring for a relative experiencing chronic pain to be associated with slower disengaging attention from negative (i.e., faces expressing pain), and faster disengaging attention from positive (i.e., faces expressing happiness), stimuli (32, 33). Most recently, researchers found that spousal dementia caregivers were faster, when their anxiety levels were high, at shifting their attention to faces displaying negative (i.e., sad) expressions compared with controls (34, 35). Familial caregiving has also been linked with increased rumination (i.e., excessive thinking about negative thoughts and feelings), and some researchers have implicated ruminative thinking as one psychological marker for negative attentional bias (36, 37). Ruminative thinking and negative attentional bias, each characterized by the prolonged processing of negative information, are closely related constructs (38).
The negative emotional impact of caring for an autistic child has been well-documented, with caregivers’ depression scores often satisfying clinical standards (10). Clinically depressed individuals preferentially attend to negative information, detecting it faster and processing it for longer (13, 18). Attention, as assessed via self-report and objective lab-based measures, tend to be poorer in familial caregivers (27, 29). Negative attentional biases have been detected in caregiving samples that are comparable, in terms of psychological demand and burden, with parents of autistic children (32–34). Whether caring for an autistic child might be associated with an attentional bias for negative information, and whether this bias takes the form of vigilance (faster orienting to) or disengagement (slower orienting away from), will be explored here. This study will be the first, we believe, to assess attentional control biases in the context of caring for an autistic child. It was hypothesized that caregivers of autistic children, scoring higher than controls in depression symptoms, would be faster to orient their attention to, and slower disengaging their attention from, negatively toned stimuli on an online version of the emotional face dot probe task.
A’priori power analysis configured for medium effect (f = 0.25), alpha of 0.08, and with two groups indicated a sample of N = 128 would be needed. A sample of N = 234 participants, of which 156 were caregivers of autistic children and 77 were controls (i.e., parents of non-autistic children), were recruited via adverts posted in parenting support/information groups on social media. A link to the study survey, hosted by Qualtrics, was included in the recruitment advert. Participation was open to those (a) aged 18 years or older, (b) parenting at least one child aged 3–21 years and living at home full time, and (c) not currently experiencing, or in the last 12 months experienced, any long term stressors (e.g., bereavement, divorce). The child’s autism diagnosis was confirmed by parent report.
Of the N = 234 participants that consented to take part, five failed to complete the depression questionnaire and 14 failed to provide code words for matching questionnaire and dot-probe data. These participants were removed, as were two participants with poor quality data (i.e., <30% accuracy) on the dot-probe task (39). Data for another 115 participants who failed to start the dot probe or complete it to the point where data could be used (i.e., <25% missing trials) were also removed (39). Failure to complete the dot-probe task was equally likely for caregivers and controls (χ2 = 0.74, df = 1, p = 0.39). The high attrition rate (58.1%) was not unexpected in light of changes imposed on the study protocol by COVID-19. Our lab space was indefinitely closed due to COVID-19 which meant the dot-probe task, originally intended to be lab-based, needed to be modified to run online. Research has shown retention for online studies, especially those with complex cognitive tasks, are much poorer compared with lab-based studies (40, 41). The final sample taken forward for analysis, therefore, was N = 98, and this included 57 caregivers and 41 controls. Sample characteristics by group are presented in Table 1.
Data with respect to socio-demographic (e.g., age, gender, annual income, employment status) and lifestyle (e.g., relationship status, number of children, sleep, exercise, alcohol) variables, known to be influential for study outcomes of interest, were collected. Data with respect to characteristics of the autistic child (i.e., current age, diagnosed age) were also collected to safeguard against spurious relationships emerging.
The Hospital Anxiety and Depression Scale (HADS) was used to assess depressive symptoms (42). The HADS is composed of 14 items, seven assessing depression (e.g., “I feel as if I am slowed down”) and seven assessing anxiety (e.g., “I feel tense or wound up”), with each item scored using a four-point Likert type scale (0, never - 3, always). Subscale scores for depression are calculated by summing across all seven items, with total scores ranging between 0 and 21. Higher scores are indicative of greater depressive symptoms. Subscale scores falling in the range of 0–7 are deemed normal. Scores ranging from 8 to 10 are indicative of a borderline clinical, and scores >11 are a clinical, case. Internal consistency for the HADS depression subscale was good (α = 0.86) in recent studies (43), as was the case here (α = 0.85).
A series of 20 face photographs of 10 different adult models (50% female), 10 of them expressing negative emotions (i.e., sadness) and 10 with neutral expressions, were taken from the Karolina Emotional Recognition Facial Database with the consent of authors (44). Hair and neckline, potentially conveying emotional information, were cropped from each photograph, as was any peripheral information. A series of 10 face pairs standardized for shape (i.e., oval), size (500 × 700 pixels), and background (i.e., black) was the result. The emotional face version of the dot-probe task, using expressive faces as opposed to emotive words, was preferred here, and this was because expressive faces, research has found, convey more affective information than words (23). Faces, therefore, might be particularly sensitive for detecting negative attentional biases. Faces might also be particularly suitable stimuli for parents who are accustomed, until language develops, to decoding the emotional needs of their children via facial cues (45). It has been estimated in fact that 25–35% of autistic children are minimally or non-verbal, and caregivers have reported that facial cues are critical for understanding the feelings and intentions of their children (46).
Clicking the link in the recruitment advert directed participants to the survey collection platform, Qualtrics. Questionnaires assessing the socio-demographic and lifestyle factors, and characteristics of the autistic child were completed first. This was followed by the relevant HADS subscale to quantify depressive symptoms. Participants were then redirected, at survey completion, to an online version of the emotional dot probe task. Instructions for task completion were presented on screen. Participants were advised that two faces of the same person, one displaying negative (i.e., sad) and one with a neutral expression, would appear on the screen in left and right positions for 500 ms, with one face then replaced by a large red dot (i.e., dot probe). Participants were asked to indicate, as quickly as possible, which face had been replaced by the probe, pressing the Z key for the face on the left and the M key for the face on the right. The detection latency for the probe was captured in milliseconds. Accuracy feedback was provided following each trial, with “correct,” for correctly identified probe locations, or “incorrect,” for incorrectly identified probe locations, displayed on screen for 500 ms. The time delay between trials was 1,000 ms. Negative-neutral face pairs accounted for half of the experimental trials and neutral-neutral face pairs the other half, with probes replacing either face with equal probability. Face pairs of the same model, of which there was 10 total, were repeated in presentation 9–10 times for a total of 96 experimental trials. The relative position of each face pair (left or right) was counterbalanced. Face pairs were presented in random order to safeguard against order effects were and always presented horizontally. The mean accuracy for correctly identifying probe locations across experimental trials was 99% in the current sample. This is comparable with studies using the emotional face dot probe in controlled lab conditions (13). Researcher instruction in the lab, therefore, appears to be no more advantageous than on-screen instruction in terms of task accuracy, and an online version of the emotional face dot probe appears to be methodologically robust.
Informed consent was gained in all cases. All participants were entered into a prize draw to win an Apple iPad as compensation for their time. The study received all necessary approvals for it to take place in accordance with the Faculty of Health and Life Sciences Ethics Committee. Fully informed consent was provided by all participants.
Mean accuracy across experimental trials (i.e., correctly identifying probe location) was high at 99%, and this did not statistically differentiate groups (t = 0.31, df = 96, p = 0.76). Only reaction times for correct trials were taken forward for statistical analysis. Reaction times <200 ms, indicative of anticipatory responding, were eliminated, as were responses >1,000 ms, likely caused by lapses in concentration (47). Outlying responses on the dot-probe accounted for <1% of all experimental trials, and outlying responses did not differentiate groups (t = 0.33, df = 95, p = 0.74).
Vigilance, characterized by speeding attention to negative stimuli, was calculated by subtracting mean response times for dots replacing neutral faces paired with another neutral face (i.e., neutral: neutral trials) from response times where dots replaced negative (sad) faces paired with neutral faces (i.e., valid sad: neutral trials). Positive scores, indicating faster speeding of attention to dots replacing sad faces compared with neutral faces when paired with another neutral face, indicated greater vigilance to negative stimuli. Disengagement, characterized by problems shifting attention away from negative stimuli, was calculated by subtracting mean reaction times for dots replacing neutral faces paired with sad faces (i.e., invalid sad: neutral trials) from response times where dots replaced neutral faces paired with another neutral face (i.e., neutral: neutral trials). Positive scores, indicating slower shifting of attention away from dots replacing sad faces compared with neutral faces when paired with another neutral face, indicated greater problems disengaging attention from negative stimuli.
A series of bivariate and, for categorical variables, point bi-serial correlations were used to explore whether depression scores, and scores for attentional indices (vigilance and disengagement), might be related to sociodemographic and lifestyle variables, and characteristics of the autistic child. Group differences with respect to depression scores were assessed via one-way univariate ANOVA. MANOVA, supplemented with univariate tests, was used to explore whether groups might be differentiated with respect to vigilance and disengagement. Whether vigilance and disengagement might be related to depression scores was assessed with bivariate correlation.
Depression scores (all ps > 0.08), and scores for vigilance (all ps > 0.12) and disengagement (all ps > 0.11), were unrelated to almost all socio-demographic and lifestyle variables. Children living at home full time were the only exception; depression scores were higher for parents with more children living at home full time (r = 0.23, p = 0.02). Depression scores, and scores for vigilance and disengagement, were unrelated to the current age of the autistic child and age at diagnosis (all ps > 0.18). Number of children living at home full time, therefore, was controlled in relevant subsequent analyses.
One way ANCOVA, adjusting for the number of children living at home full time, revealed depression scores [F(1, 95) = 26.28, p < 0.001, ηp2 = 0.22] were higher in caregivers. Caregivers’ mean depression score was 8.3 (SD = 2.6), placing them in the range for a borderline clinical case. Mean depression scores for controls, by contrast, were 5.1 (SD = 3.1), falling well within the normal range. Caregivers were in fact around 50% more likely, based on their depression scores, to meet the criteria for a borderline or clinical case (χ2 = 7.46, p < 0.01).
Wilks Lambda multivariate test for overall group differences in negative attentional bias was non-significant [F(2, 95) = 0.67, p = 0.52, ηp2 = 0.01]. Follow-up univariate tests revealed no group differences with respect to vigilance [F(1, 96) = 0.64, p = 0.43, ηp2 = 0.00] or disengagement [F(1, 96) = 0.52, p = 0.47, ηp2 = 0.00]. Vigilance and disengagement scores were also unrelated to depression scores (all ps > 0.50). Means and standard deviations for attentional bias indices and depression scores by group are displayed in Table 2.
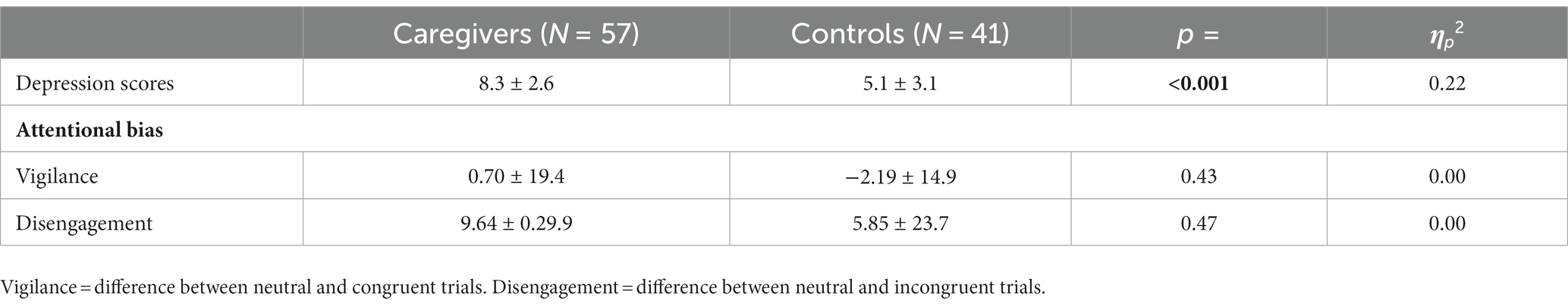
Table 2. Means and standard deviations (M ± SD) for depression scores, and for vigilance and disengagement (milliseconds), by group.
This study explored whether caring for an autistic child might be associated, in the form of vigilance or disengagement, with a negative attentional bias. Results revealed that caregivers’ depression scores were markedly higher compared with controls. This was in keeping with our original hypothesis and commensurate with other recent findings (1–4, 10). Caregivers’ mean depression scores were in fact >8.0, placing them in the range for a borderline clinical case. Mean depression scores for controls, by contrast, were 5.1, falling well inside the normal range. Caregivers were around twice as likely to satisfy the criteria for a borderline or clinical case based on their depression scores. The high depression scores characteristic of caring for an autistic child could have serious implications. Indeed, the relationship between caregivers’ emotional well-being and child autism severity has been shown to be bi-directional, with one affecting the other and vice versa. Autistic children of more depressed parents tend to do less well socially and behaviorally, forming fewer peer relationships and displaying more hyperactive and aggressive behaviors (47, 48). More depressed caregivers have also reported feeling less confident about providing good quality caregiving and being more likely to distance themselves from important caregiving tasks (49, 50), Finding ways to alleviate caregivers’ depressive symptomology, potentially improving the quality of life for the autistic child, should remain a high priority for future researchers.
With respect to attention, findings revealed caregivers were not more vigilant to, and no slower to disengage from, sad faces compared with controls. Caring for an autistic child, while associated with borderline clinical depression scores, does not seem to be characterized by an attentional bias for negative information. Moreover, vigilance and disengagement were unrelated to depression scores. These findings are incongruous with those from recent studies. Indeed, attentional errors, assessed via self-report and more objectively in the lab, were found to be greater in familial caregivers compared with controls (27–30). Several caregiving populations that match parents of autistic children for psychological stress and burden have also displayed attentional processing biases for negative information (32–35). Rumination, providing one psychological marker of, and closely related as a construct with, negative attentional bias, is also typically higher in the context of caring for an autistic child (36). Clinical depression is also one well-known psychological marker for negative attentional bias, and caregivers’ depression scores here averaged >8.0, satisfying a borderline clinical case.
Group disparities with respect to depression scores, but not attentional bias indices, might be explained by methodological choices. The dot-probe task here was configured for faces to appear for 500 ms only. Configuring the task so faces appeared for longer, for 1,000 ms, might have been more sensitive for detecting negative attentional biases. Indeed, whether attentional disparities can be observed between stressed and non-stressed samples has been shown to be moderated by stimuli presentation time. Caregivers of autistic children often report depression scores within the clinical range, and clinically depressed individuals were quicker to shift their attention to negative stimuli presented for 1,000 ms, but not 500 ms, compared with controls (51, 52). Whether attentional disparities between caregivers of autistic children and controls vary by stimuli presentation time, manifesting for stimuli presented for longer periods, could be investigated in future research. The negative attentional bias observed in depressed samples also tends to be a content-specific one. That is, it manifests for sad faces, as those congruent with the depressed person’s mood, but not for mood incongruent faces (e.g., anger). Whether the negative attentional bias characteristic of caring for an autistic child, to the degree one exists, might also be differentially affected by stimuli type, manifesting for certain types of faces and not others, could also be explored in the future. Infant faces, for example, preferentially engage the attentional systems of adults. For example, target detection on a visual search task was significantly slower when the target was paired with an infant, compared with an adult, face. The infant face, more than the adult face, interfered with task performance, slowing the detection of the visual target. This attentional sensitivity for infants’ faces, as evidenced by slower target detection, was also magnified for parents and when infant faces expressed negative emotions (53). The attentional system of adults, therefore, appears to be particularly sensitive to infants’ faces, and parents’ attentional system is particularly sensitive. The preferential attention of parents to infants’ faces has been explained as an evolutionary adaptation, ensuring that infants are attended to and, absent any developed language, receive essential caregiving (54). It has been estimated that language is absent or minimal in 25–35% of autistic children, with their intentions and needs instead communicated to parents instead via facial expressions and other non-verbal cues (46). Parents rely heavily on the nonverbal communication of their autistic child, especially facial expressions, and this seems to be reciprocal. Indeed, autistic children performed poorer than controls on emotion recognition tasks, finding decoding emotions from faces presented in photographs much more challenging (55). More recently, however, researchers demonstrated this effect was moderated by familiarity. That is, autistic children were poorer than controls at recognizing emotions on unfamiliar (i.e., stranger), but not familiar (i.e., parent), faces (56). Caring for an autistic child, therefore, might be associated with an attentional bias for faces displaying negative emotions, but this might be age-specific, observable for infant, but not adult, faces. Future research might explore this.
The findings reported here should be tempered by limitations. The dot-probe task had been intended to run in the lab, in controlled settings where researchers could deliver task instructions orally, but COVID-19 forced a change in study design. Accuracy rates for the online emotional face dot probe task used here were high (99%), matching those of lab-based protocols. Completion rates, by contrast, were poor, with 58.1% of participants failing to start the dot-probe task or complete it to a point where data could be used for analysis. Retention, however, was equally poor across the groups; caregivers were no more likely than controls to drop out at the dot probe stage. The high attrition rate resulted in a final sample of N = 98, and this failed to satisfy the conditions of the apriori power analysis. That said, a high rate of attrition was expected. Indeed, attrition rates are typically high for online studies, particularly where cognitively demanding tasks such as the one used here are incorporated (40, 41). Moreover, it is well known that caregivers of autistic children, making up more than half of the current sample, are a notoriously hard-to-reach population, with most of their time dedicated to the unremitting demands of caretaking. The findings reported here, therefore, should be interpreted cautiously until corroborated with a larger sample. Moreover, whether results from an online version of the emotional dot-probe task such as the one used here are robust, correlating with results from the same task completed in controlled lab settings, also remains to be seen, as does how the reliability of online protocols might vary as a function of the internet service provider. This might be a target for future research. Autism diagnosis in the current study was via parent report only, and a more formal diagnosis would have been better. Parent reports of autism diagnosis, however, do tend to be reliable (57). Much of the variation in caregivers’ depression scores has been found to be accounted for by characteristics of the autistic child, especially problematic behaviors and autism severity (58). These variables were not measured here, and this represents a notable limitation. Indeed, caregivers of children with greater behavioral problems typically report higher depression scores, and it might be this subset of caregivers who are particularly sensitive to negative attentional biases. All caregivers were recruited via online caregiving support groups, and the stress-alleviating effects of social support are well established (59, 60). The baseline level of social support in the current sample, therefore, might be higher than usual, and the current sample might not be representative. It might be that less socially supported caregivers, who also tend to report higher depression scores, are particularly sensitive to negative attentional biases. Finally, reaction time tasks such as dot probe quantify the time taken to shift attention to and from negative stimuli and, therefore, provide only indirect assessments of negative attentional bias. Eye tracking on the other hand captures the continuous flow of visual attention and, as a more rigorous protocol capturing a range of other attentional indices (e.g., fixation time, glance duration), might be more sensitive at detecting attentional biases.
In conclusion, caregivers of autistic children, while more depressed than controls, did not display an attentional bias for negative information. Attentional bias indices of vigilance and disengagement were also unrelated to depression scores. Whether attentional disparities between caregivers and controls might manifest with methodological adjustments, when negative stimuli are presented for longer periods and using more robust protocols involving eye tracking, might be explored in the future.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Faculty of Health and Life Sciences Ethics Committee, Northumbria University. The patients/participants provided their written informed consent to participate in this study.
BL designed the study, collected data, completed analysis, and write the paper. KM designed the dot probe task. PP collected data and helped with data analysis. MW helped design the study and co-wrote the paper. All authors contributed to the article and approved the submitted version.
Thanks to all the caregivers who took the time to contribute to the research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kütük, MÖ, Tufan, AE, Kılıçaslan, F, Güler, G, Çelik, F, Altıntaş, E, et al. High depression symptoms and burnout levels among parents of children with autism spectrum disorders: a multi-center, cross-sectional, case-control study. J Autism Dev Disord. (2021) 51:4100. doi: 10.1007/s10803-021-04914-z
2. Li, F, Tang, Y, Li, F, Fang, S, Liu, X, Tao, M, et al. Psychological distress in parents of children with autism spectrum disorder: a cross-sectional study based on 683 mother-father dyads. J Pediatr Nurs. (2022) 65:e49–55. doi: 10.1016/j.pedn.2022.02.006
3. Marshall, B, Kollia, B, Wagner, V, and Yablonsky, D. Identifying depression in parents of children with autism spectrum disorder: recommendations for professional practice. J Psychosoc Nurs Ment Health Serv. (2018) 56:23–7. doi: 10.3928/02793695-20171128-02
4. Scherer, N, Verhey, I, and Kuper, H. Depression and anxiety in parents of children with intellectual and developmental disabilities: a systematic review and meta-analysis. PLoS One. (2019) 14:e0219888. doi: 10.1371/journal.pone.0219888
5. Gray, DE. Coping over time: the parents of children with autism. J Intellect Disabil Res. (2006) 50:970–6. doi: 10.1111/j.1365-2788.2006.00933.x
6. Vernhet, C, Dellapiazza, F, Blanc, N, Cousson-Gélie, F, Miot, S, Roeyers, H, et al. Coping strategies of parents of children with autism spectrum disorder: a systematic review. Eur Child Adolesc Psychiatry. (2019) 28:747–58. doi: 10.1007/s00787-018-1183-3
7. Samsell, B, Lothman, K, Samsell, EE, and Ideishi, RI. Parents’ experiences of caring for a child with autism spectrum disorder in the United States: a systematic review and metasynthesis of qualitative evidence. Fam Syst Health. (2022) 40:93–104. doi: 10.1037/fsh0000654
8. Gallagher, S, Phillips, AC, Oliver, C, and Carroll, D. Predictors of psychological morbidity in parents of children with intellectual disabilities. J Pediatr Psychol. (2008) 33:1129–36. doi: 10.1093/jpepsy/jsn040
9. Lovell, B, Moss, M, and Wetherell, MA. The psychophysiological and health corollaries of child problem behaviours in caregivers of children with autism and ADHD: problem behaviours in autism and ADHD caregivers. J Intellect Disabil Res. (2015) 59:150–7. doi: 10.1111/jir.12081
10. Schnabel, A, Youssef, GJ, Hallford, DJ, Hartley, EJ, McGillivray, JA, Stewart, M, et al. Psychopathology in parents of children with autism spectrum disorder: a systematic review and meta-analysis of prevalence. Autism. (2020) 24:26–40. doi: 10.1177/1362361319844636
11. Beck, AT. The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatr. (2008) 165:969–77. doi: 10.1176/appi.ajp.2008.08050721
12. Dozois, DJA, and Beck, AT. Cognitive schemas, beliefs and assumptions In: KS Dobson and DJA Dozois, editors. Risk factors in depression. Amsterdam: Elsevier Academic Press (2008). 121–43.
13. Fritzsche, A, Dahme, B, Gotlib, IH, Joormann, J, Magnussen, H, Watz, H, et al. Specificity of cognitive biases in patients with current depression and remitted depression and in patients with asthma. Psychol Med. (2010) 40:815–26. doi: 10.1017/S0033291709990948
14. Gotlib, IH, and Joormann, J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. (2010) 6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305
15. Gotlib, IH, Kasch, KL, Traill, S, Joormann, J, Arnow, BA, and Johnson, SL. Coherence and specificity of information processing biases in depression and social phobia. J Abnorm Psychol. (2004) 113:386–98. doi: 10.1037/0021-843X.113.3.386
16. Gotlib, IH, Krasnoperova, E, Yue, DN, and Joormann, J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. (2004) 113:121–35. doi: 10.1037/0021-843X.113.1.121
17. Peckham, AD, McHugh, RK, and Otto, MW. A meta-analysis of the magnitude of biased attention in depression. Depress Anxiety. (2013) 30:407–7. doi: 10.1002/da.22092
18. Trapp, W, Kalzendorf, C, Baum, C, Hajak, G, and Lautenbacher, S. Attentional biases in patients suffering from unipolar depression: results of a dot probe task investigation. Psychiatry Res. (2018) 261:325–31. doi: 10.1016/j.psychres.2018.01.005
19. Zhou, Z, Cao, S, Li, H, and Li, Y. Treatment with escitalopram improves the attentional bias toward negative facial expressions in patients with major depressive disorders. J Clin Neurosci. (2015) 22:1609–13. doi: 10.1016/j.jocn.2015.03.036
20. Mennen, AC, Norman, KA, and Turk-Browne, NB. Attentional bias in depression: understanding mechanisms to improve training and treatment. Curr Opin Psychol. (2019) 29:266–73. doi: 10.1016/j.copsyc.2019.07.036
21. Suslow, T, Hußlack, A, Kersting, A, and Bodenschatz, CM. Attentional biases to emotional information in clinical depression: a systematic and meta-analytic review of eye tracking findings. J Affect Disord. (2020) 274:632–42. doi: 10.1016/j.jad.2020.05.140
22. Elgersma, HJ, Koster, EHW, van Tuijl, LA, Hoekzema, A, Penninx, BWJH, Bockting, CLH, et al. Attentional bias for negative, positive, and threat words in current and remitted depression. PLoS One. (2018) 13:e0205154. doi: 10.1371/journal.pone.0205154
23. Ji, JL, Grafton, B, and MacLeod, C. Referential focus moderates depression-linked attentional avoidance of positive information. Behav Res Ther. (2017) 93:47–54. doi: 10.1016/j.brat.2017.03.004
24. Joormann, J, and Gotlib, IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. (2007) 116:80–5. doi: 10.1037/0021-843X.116.1.80
25. Duque, A, and Vázquez, C. Double attention bias for positive and negative emotional faces in clinical depression: evidence from an eye-tracking study. J Behav Ther Exp Psychiatry. (2015) 46:107–14. doi: 10.1016/j.jbtep.2014.09.005
26. Lu, S, Xu, J, Li, M, Xue, J, Lu, X, Feng, L, et al. Attentional bias scores in patients with depression and effects of age: a controlled, eye-tracking study. J Int Med Res. (2017) 45:1518–27. doi: 10.1177/0300060517708920
27. Mackenzie, CS, Smith, MC, Hasher, L, Leach, L, and Behl, P. Cognitive functioning under stress: evidence from informal caregivers of palliative patients. J Palliat Med. (2007) 10:749–58. doi: 10.1089/jpm.2006.0171
28. Caswell, LW, Vitaliano, PP, Croyle, KL, Scanlan, JM, Zhang, J, and Daruwala, A. Negative associations of chronic stress and cognitive performance in older adult spouse caregivers. Exp Aging Res. (2003) 29:303–18. doi: 10.1080/03610730303721
29. Corrêa, MS, de Lima, DB, Giacobbo, BL, Vedovelli, K, de Lima Argimon, II, and Bromberg, E. Mental health in familial caregivers of Alzheimer’s disease patients: are the effects of chronic stress on cognition inevitable? Stress. (2019) 22:83–92. doi: 10.1080/10253890.2018.1510485
30. Oken, BS, Fonareva, I, and Wahbeh, H. Stress-related cognitive dysfunction in dementia caregivers. J Geriatr Psychiatry Neurol. (2011) 24:191–8. doi: 10.1177/0891988711422524
31. Lovell, B, and Wetherell, MA. The cost of caregiving: endocrine and immune implications in elderly and non-elderly caregivers. Neurosci Biobehav Rev. (2011) 35:1342–52. doi: 10.1016/j.neubiorev.2011.02.007
32. Mohammadi, S, Dehghani, M, Khatibi, A, Sanderman, R, and Hagedoorn, M. Caregivers’ attentional bias to pain: does it affect caregiver accuracy in detecting patient pain behaviors? Does it affect caregiver accuracy in detecting patient pain behaviors? Pain. (2015) 156:123–30. doi: 10.1016/j.pain.0000000000000015
33. Mohammadi, S, Dehghani, M, Sharpe, L, Heidari, M, Sedaghat, M, and Khatibi, A. Do main caregivers selectively attend to pain-related stimuli in the same way that patients do? Pain. (2012) 153:62–7. doi: 10.1016/j.pain.2011.08.021
34. Cabrera, I, Márquez-González, M, Gallego-Alberto, L, Pedroso-Chaparro, MDS, Barrera-Caballero, S, and Losada, A. To pay attention or not: the associations between attentional bias towards negative emotional information and anxiety, guilt feelings, and experiential avoidance in dementia family caregivers. Aging Ment Health. (2022) 26:328–36. doi: 10.1080/13607863.2021.1871883
35. Márquez-González, M, Cabrera, I, Losada, A, and Knight, BG. Attentional avoidant biases as mediators in the association between experiential avoidance and blood pressure in dementia family caregivers. Aging Ment Health. (2018) 22:669–77. doi: 10.1080/13607863.2017.1293003
36. Galfin, JM, Watkins, ER, and Harlow, T. Psychological distress and rumination in palliative care patients and their caregivers. J Palliat Med. (2010) 13:1345–8. doi: 10.1089/jpm.2010.0139
37. Hur, J, Gaul, K, and Berenbaum, H. Different patterns of attention bias in worry and rumination. Cognit Ther Res. (2019) 43:713–25. doi: 10.1007/s10608-018-09993-4
38. Grafton, B, Southworth, F, Watkins, E, and MacLeod, C. Stuck in a sad place: biased attentional disengagement in rumination. Emotion. (2016) 16:63–72. doi: 10.1037/emo0000103
39. Chen, NTM, Clarke, PJF, Watson, TL, MacLeod, C, and Guastella, AJ. Attentional bias modification facilitates attentional control mechanisms: evidence from eye tracking. Biol Psychol. (2015) 104:139–46. doi: 10.1016/j.biopsycho.2014.12.002
40. Finley, AJ, and Penningroth, SL. Online versus in-lab: pros and cons of an online prospective memory experiment In: AM Columbus, editor. Advances in psychology research. Hauppauge, NY: Nova Science Publishers, Inc (2015). 135–62.
41. Zhou, H, and Fishbach, A. The pitfall of experimenting on the web: how unattended selective attrition leads to surprising (yet false) research conclusions. J Pers Soc Psychol. (2016) 111:493–504. doi: 10.1037/pspa0000056
42. Snaith, RP, and Zigmond, AS. The hospital anxiety and depression scale. BMJ. (1986) 292:344–4. doi: 10.1136/bmj.292.6516.344
43. Unsar, S, Erol, O, and Ozdemir, O. Caregiving burden, depression, and anxiety in family caregivers of patients with cancer. Eur J Oncol Nurs. (2021) 50:101882. doi: 10.1016/j.ejon.2020.101882
44. Lundqvist, D, Flykt, A, and Öhman, A. The Karolinska directed emotional faces (KDEF). CD-ROM. Stockholm: Department of Clinical Neuroscience, Psychology Section. Psychology Section Karolinska Institute (1998);91–630.
45. Boerner, KE, Chambers, CT, Craig, KD, Pillai Riddell, RR, and Parker, JA. Caregiver accuracy in detecting deception in facial expressions of pain in children. Pain. (2013) 154:525–33. doi: 10.1016/j.pain.2012.12.015
46. Rose, V, Trembath, D, Keen, D, and Paynter, J. The proportion of minimally verbal children with autism spectrum disorder in a community-based early intervention programme. J Intellect Disabil Res. (2016) 60:464–77. doi: 10.1111/jir.12284
47. Akram, U, Beattie, L, Ypsilanti, A, Reidy, J, Robson, A, Chapman, AJ, et al. Sleep-related attentional bias for tired faces in insomnia: evidence from a dot-probe paradigm. Behav Res Ther. (2018) 103:18–23. doi: 10.1016/j.brat.2018.01.007
48. Zaidman-Zait, A, Mirenda, P, Duku, E, Szatmari, P, Georgiades, S, Volden, J, et al. Examination of bidirectional relationships between parent stress and two types of problem behavior in children with autism spectrum disorder. J Autism Dev Disord. (2014) 44:1908–17. doi: 10.1007/s10803-014-2064-3
49. Batool, SS, and Khurshid, S. Factors associated with stress among parents of children with autism. J Coll Physicians Surg Pak. (2015) 25:752–6. doi: 10.2015/JCPSP.752756
50. Wang, H, Hu, X, and Han, ZR. Parental stress, involvement, and family quality of life in mothers and fathers of children with autism spectrum disorder in mainland China: a dyadic analysis. Res Dev Disabil. (2020) 107:103791. doi: 10.1016/j.ridd.2020.103791
51. Donaldson, C, Lam, D, and Mathews, A. Rumination and attention in major depression. Behav Res Ther. (2007) 45:2664–78. doi: 10.1016/j.brat.2007.07.002
52. Segal, ZV, Gemar, M, Truchon, C, Guirguis, M, and Horowitz, LM. A priming methodology for studying self-representation in major depressive disorder. J Abnorm Psychol. (1995) 104:205–13. doi: 10.1037//0021-843x.104.1.205
53. Thompson-Booth, C, Viding, E, Mayes, LC, Rutherford, HJV, Hodsoll, S, and McCrory, E. I can’t take my eyes off of you: attentional allocation to infant, child, adolescent and adult faces in mothers and non-mothers. PLoS One. (2014) 9:e109362. doi: 10.1371/journal.pone.0109362
54. Thompson-Booth, C, Viding, E, Mayes, LC, Rutherford, HJV, Hodsoll, S, and McCrory, EJ. Here’s looking at you, kid: attention to infant emotional faces in mothers and non-mothers. Dev Sci. (2014) 17:35–46. doi: 10.1111/desc.12090
55. Dawson, G, Carver, L, Meltzoff, AN, Panagiotides, H, McPartland, J, and Webb, SJ. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev. (2002) 73:700–17. doi: 10.1111/1467-8624.00433
56. Shanok, NA, Jones, NA, and Lucas, NN. The nature of facial emotion recognition impairments in children on the autism spectrum. Child Psychiatry Hum Dev. (2019) 50:661–7. doi: 10.1007/s10578-019-00870-z
57. Rosenberg, RE, Kaufmann, WE, Law, JK, and Law, PA. Parent report of community psychiatric comorbid diagnoses in autism spectrum disorders. Autism Res Treat. (2011) 2011:405849. doi: 10.1155/2011/405849
58. Yorke, I, White, P, Weston, A, Rafla, M, Charman, T, and Simonoff, E. Correction to: the association between emotional and behavioral problems in children with autism spectrum disorder and psychological distress in their parents: a systematic review and meta-analysis. J Autism Dev Disord. (2018) 48:3416. doi: 10.1007/s10803-018-3656-0
59. Zhao, M, Fu, W, and Ai, J. The mediating role of social support in the relationship between parenting stress and resilience among Chinese parents of children with disability. J Autism Dev Disord. (2021) 51:3412–22. doi: 10.1007/s10803-020-04806-8
Keywords: autism, caregivers, depression, negative attentional bias, dot probe
Citation: Lovell B, McCarty K, Penfold P and Wetherell MA (2023) Clinically elevated depression scores do not produce negative attentional biases in caregivers of autistic children. Front. Psychiatry. 14:1192669. doi: 10.3389/fpsyt.2023.1192669
Received: 23 March 2023; Accepted: 22 August 2023;
Published: 07 September 2023.
Edited by:
Fengyu Zhang, Global Clinical and Translational Research Institute, United StatesReviewed by:
Shadi Beshai, University of Regina, CanadaCopyright © 2023 Lovell, McCarty, Penfold and Wetherell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian Lovell, YnJpYW4ubG92ZWxsQG5vcnRodW1icmlhLmFjLnVr
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.