- Department of Psychiatry, Dr. Carol Davila University Emergency Central Military Hospital, Bucharest, Romania
Eating disorders (EDs) represent a contradictory chapter of clinical psychiatry, i.e., although they are associated with significant prevalence and risks in the long term (including vital risk, especially for anorexia nervosa), the therapeutic resources are minimal and based on low-quality data. Another contradiction arose in the last few decades, i.e., a variety of new EDs have been described, either by clinicians or signaled by mass media, but their systematic exploration is progressing very slowly. Entities like “food addiction,” “orthorexia nervosa,” or “emotional eating disorder” still require intensive exploration in order to find the most accurate diagnostic instruments, diagnosis criteria, prevalence data, vulnerability factors, and therapeutic approaches. This article is focused on integrating into a comprehensive model a variety of EDs not specified or loosely defined by the current international classifications of psychiatric disorders. This framework is intended as an instrument for stimulating clinical and epidemiological research, with potential favorable consequences for therapeutic research. The dimensional model suggested here includes four main categories that accommodate the already recognized EDs (i.e., anorexia nervosa, bulimia nervosa, and binge eating disorder) as well as ten EDs that still need intensive research to find their clinical and pathophysiological characteristics. More good-quality studies are urgently required regarding this topic, based on the mental and physical negative impact these EDs may have in the short and long term, especially in vulnerable populations (e.g., pregnant women, athletes, adolescents, etc.).
1. Introduction
Eating disorders (ED) are associated with a tendency toward chronicity and severe functional impairment, high rates of comorbidity and mortality, especially for anorexia nervosa (AN) (1, 2). The worldwide lifetime prevalence of these disorders was between 2.2 and 8.4% (higher for women), while the 12-month prevalence was between 0.7 and 2.2% (3). This indicator increased between 2000 and 2018 from 3.5 to 7.8%, indicating the challenge of this type of disorder for the healthcare systems (3). However, the actual prevalence of EDs may be higher because many EDs have not yet been systematically evaluated by epidemiological research due to insufficiently validated diagnostic criteria. The main EDs currently recognized by the international classifications are AN, bulimia nervosa (BN), and binge-eating disorder (BED) (4–7). Insufficiently defined EDs have begun to attract attention from clinicians and researchers in the last two decades, although they are far from being new in the context of eating pathology. These disorders have been classified by different authors as either a stage between full EDs and recovery or a transition phase between a less severe pathology and a complete ED. (1) It is also worth to be mentioned that the COVID-19 pandemic is considered by several authors as a “wake-up call for making eating disorders a priority,” especially among adolescents and young adults (8). During this pandemic, eating behavior pathology has increased, and this signals the need for developing EDs screening and early treatment policies (8, 9).
2. Well-defined eating disorders-main characteristics
AN is reputedly associated with the highest mortality rate within the category of psychiatric disorders, and it affects mainly teenage girls (10). The core symptoms of AN are lower body mass index (BMI), self-induced weight loss (by dieting/purging or using appetite suppressants, diuretics, or laxatives), and distorted body image (4). Associated features are endocrine dysfunctions reflected in clinical and biological changes (4). The therapeutic resources are very few, and almost 30% of these patients will not recover (10, 11). Individual, group, and family therapy are recommended, with antidepressants and antipsychotics having limited success (10). Different interventions targeting the change of gut microbiota have been attempted, but until now, with very limited success (12, 13).
BN is characterized by the presence of repetitive episodes of binge eating and compensatory behaviors destined to avert BMI increase, consisting of self-provoked vomiting, abuse of diuretics/laxatives/fasting/extreme physical activity, etc. (6, 7). Self-perception is heavily correlated with body shape/weight (7). Antidepressants and cognitive-behavioral therapy (CBT) or interpersonal therapy are considered helpful for these patients (14–16). Fluoxetine is so far the only agent approved for the treatment of BN (17). BED consists of binge eating repetitive episodes, which are accompanied by marked distress, although the patient does not present compensatory behaviors targeting weight loss (7). Program-based self-help interventions, CBT, and pharmacotherapy are available for BED, but the quality of evidence to support these interventions is very low (15). Pharmacological approaches are also explored but insufficiently validated by clinical, good-quality data (16, 18). Lisdexamfetamine dimesylate is the only drug currently approved to treat BED (19).
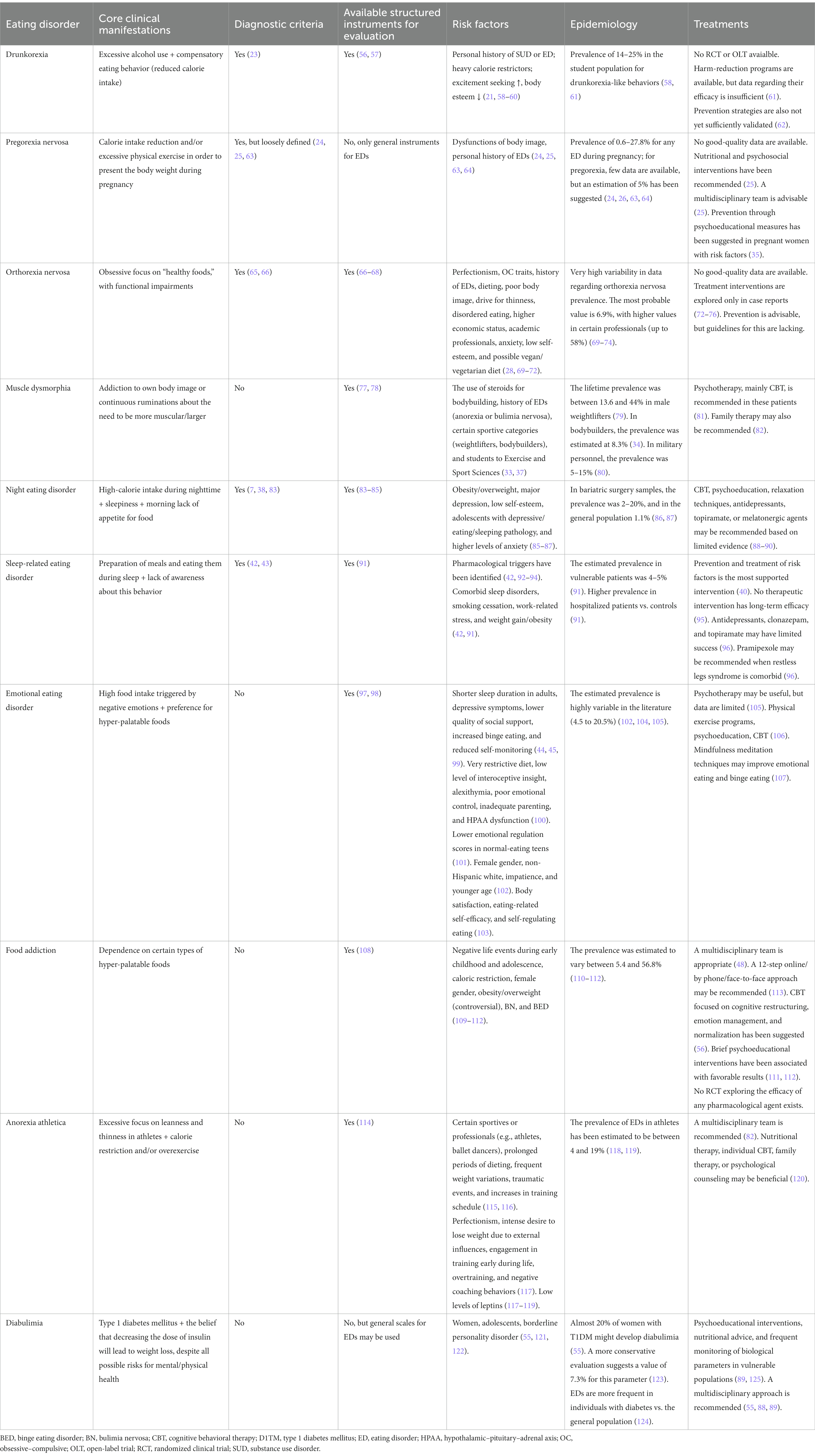
3. Other eating disorders-general characteristics
3.1. Drunkorexia
The core elements are specific compensatory behaviors destined to mitigate the calorie intake due to excessive alcohol consumption (2). Also called “alcoholimia,” or “food and alcohol disturbance,” drunkorexia is observed especially in young drinkers who plan in advance their participation in excessive physical exercise or improper dieting before heavy alcohol ingestion (20). The main objective of these individuals is to be able to engage in heavy alcohol drinking, while dysfunctional eating behavior may be considered only a preemptive measure (21). The consequences of these behavioral dysfunctions for mental and physical health could be severe, i.e., nutritional deficiencies, cognitive impairment, anxious and depressive symptoms, hepatic toxicity, etc. (21, 22). It was suggested by several authors that drunkorexia may be classified as a component of the “Other specified feeding and eating disorders (OSFED)” within the latest edition of the American Psychiatric Association’s Manual of Diagnostic and Statistics (DSM) to provide insurance coverage for this pathology (23).
3.2. Pregorexia nervosa
EDs detected in pregnant women may lead to a significant risk for negative health effects in mothers (e.g., maternal hypertension, anemia, postpartum depression) and children (e.g., low birth weight, spontaneous abortion, microcephaly) (24, 25). For clinicians, it is, however, difficult to diagnose EDs in pregnant women based solely on routine examination, because manifestations of eating behavior pathology and physiological changes associated with pregnancy may overlap (26). Therefore, collecting a detailed anamnesis, with a focus on specific eating habits and body image distortions, especially where symptoms of EDs may be suspected, is needed in this vulnerable population. Also, the clinician is encouraged to detect more discrete ED symptoms, evaluate possible dysfunctions in daily life that may be caused by such symptoms, and determine the level of the patient’s awareness of her health problems based on behavioral cues (26).
The origins of pregorexia nervosa (PN) are found in mass media as far back as 2008 when it was used to describe pregnant women who intentionally reduce calories and increase exercise to control pregnancy weight gain (27). A high level of worry related to the risk of becoming overweight and intense preoccupations with behaviors destined to mitigate this trend toward weight or shape modifications due to a healthy pregnancy is considered central to this diagnosis (27).
3.3. Orthorexia nervosa
This disorder is defined by obsessive thoughts about “healthy” meals and a lack of flexibility regarding the composition of daily meals, which lead to clinically significant medical or psychological impairments (28). Steven Bratman launched the concept of “orthorexia nervosa” (ON) in the late 90s to delineate the pathological preoccupation with “healthy food” or strict dietary rules (29).
The definition of ON has changed in time, and new elements have been added: the patients’ focus is on the avoidance of becoming ill, and the correspondent behavior is excessive dieting; several specific categories of foods are avoided (e.g., those rich in carbohydrates, saturated fats, artificially flavored, etc.); malnutrition or certain nutritional deficits may be detected as a consequence of restricted food ingestion; social isolation, high levels of fears and worries related to eating (30). Unlike “healthy orthorexia,” defined by a constructive interest in eating healthy foods, ON is associated with mental and physical negative consequences.
It is challenging to include ON between the OSFEDs or the obsessive–compulsive spectrum disorders because there are no unanimously accepted diagnostic criteria or sound epidemiological data (31). An online survey (N = 343 Italian healthcare professionals) showed that ON is considered a variant of ED by most responders, while others classified this pathology as a prodromal phase or a stage in the evolution of anorexia nervosa (AN) (32). Most responders (over 80%) favored the inclusion of ON diagnosis in the Diagnostic and Statistical Manual of Mental Disorders (DSM) classification within the category of EDs (32).
3.4. Muscle dysmorphia or bigorexia nervosa
This condition affects male bodybuilders, who continuously ruminate about their body mass and aspect and consider they should be larger or more muscular (33, 34). If obsessions are clearly related to the inadequacy of their body shape, compulsions refer to overexercising in the gym, overbuying sports supplements, dysfunctional eating behavior, or substance use disorders (SUD) (33). Muscle dysmorphia is synonymous with “reverse anorexia,” first described in a study that included 108 bodybuilders (34). Also, the term “Adonis complex” has been vehiculated about an excessive focus on men’s body image, based on the name of the Greek god who represented a standard of masculinity (35).
However, based on the DSM-5 criteria, muscle dysmorphia is a subtype of body dysmorphic disorder, part of the obsessive–compulsive spectrum (7). This classification of bigorexia nervosa can be seriously challenged, based on the shared clinical features with individuals diagnosed with AN and on the fact that almost one of each five patients had a history of AN, while almost one out of three had a past diagnosis of any ED (36, 37). These individuals consider themselves small and weak, although the reality is completely different, and tend to avoid social gatherings due to fears they would be seen as too fragile (34).
3.5. Night eating disorder
Originally described by Stunkard et al. in 1955 and associated with periods of weight gain and life stress (38), night eating syndrome (NES) is still an elusive entity with debated diagnostic criteria. In the original paper, NES was described as consuming large meals during the evening and night (≥25% of the daily caloric intake is distributed in these periods), combined with sleepiness and morning anorexia (38, 39). These manifestations have been considered secondary to a lag in the circadian distribution of food intake (39). Unlike other EDs, there are no compensatory behaviors and the episodes of night eating are not similar to the binge episodes because of the lower quantity of food ingested (38, 39). Although patients may become obese or overweight, this is not a rule (38, 39). NES is characterized by full awareness of nocturnal meals, unlike sleep-related disorders with automatic eating behaviors (38, 39).
The DSM-5 classification mentions NES in a residual category and requires for its diagnosis the existence of repetitive episodes of night eating (during awakenings or after dinner), the complete consciousness during these episodes, and the patient’s ability to remember them. There are no other confounding factors (e.g., sleep–wake cycle changes or socio-cultural norms, organic/toxic/psychiatric pathology), but there are dysfunctions and/or distress due to this problematic behavior (7).
3.6. Sleep-related eating disorder
This clinical entity is included in the category of “parasomnias,” and its onset is during non-REM sleep. The core elements consist in preparing and consuming food during sleep, with no memory of these behaviors when the individual wake up. Sleep-related eating disorder (SRED) is considered a “disorder of arousal,” together with sleepwalking, sexsomnia, sleep terrors, confusional arousals, and sleep-related choking syndrome (40). Unlike individuals with NES, these patients are not aware of what and when they are eating; therefore they do not remember such episodes. Several authors consider this condition represents a non-motor cluster of symptoms belonging to the restless legs syndrome (41).
For the diagnosis of SRED, the following criteria have been formulated: frequent episodes of nighttime eating with onset after sleep initiation; consumption of inedible foods or combinations of comestible and peculiar foods; potentially dangerous behaviors during sleep that are related to obtaining or preparing food; adverse health effects due to the abnormal eating behavior; either partial or complete lack of awareness regarding these episodes; there is no other condition, pharmacological, organic or psychiatric, that could explain this behavior (42, 43).
3.7. Emotional eating disorder
Stress, depression, irritability, and anxiety have been associated with a significant impact on eating behaviors (44). The rise of food intake behaviors as a direct reaction to negative emotional stimuli, with a focus on hyper-palatable foods, was termed “emotional eating” (EE) (44). This type of eating behavior may be considered a coping mechanism for stressful internal or external events, leading to weight increases. However, this behavior is not necessarily associated with higher body weight or obesity (44, 45). Patients with EE may frequently report concerns about their weight and dysfunctions in their body representation/general health perception and may consider EE as an acquired coping strategy they cannot control (45).
There are still doubts if EE stands for a specific phenomenon or if it is just a consequence of other EDs, and several theories have been elaborated in an attempt to explain the pathophysiology of this behavior (46). In healthy individuals, naturalistic studies based on sequential evaluation of negative emotions and eating behaviors led to contradictory results regarding the stability of the EE construct (46). However, in a study that enrolled 127 normal-weight women that explored the relationships between EE, bulimic behaviors, and restrained eating, researchers reported EE might be responsible for excessive food ingestion, and it may also be associated with general psychopathological symptoms met in EDs and chronic dieting (47). Also, individuals with significant overeating behaviors, e.g., BED, presented a consistent correlation between unpleasant emotions and food ingestion, suggesting possible classical conditioning through repeated pairing (46).
3.8. Food addiction
Food addiction is also known as “eating addiction,” and it is focused on the dependence on certain types of hyper-palatable foods (48). Neurobiological changes related to the reward system, preoccupation related to specific substances, impaired control of eating, social dysfunctions, risky use of a substance (e.g., hyper-palatable foods), tolerance/withdrawal, chronic evolution, and high risk of relapse have been found as key diagnostic elements of food addiction (49). Also, genetic vulnerability, substance sensitization, cross-sensitization, and impulsivity are supported by evidence as presenting a pathophysiological role in animal models of food addiction, but also in human studies (49).
Because no unanimously accepted diagnostic criteria exist for this disorder, it is difficult to interpret the results of epidemiological studies exploring food addiction and it is even more challenging to design trials with therapeutic goals. The excessive consumption of specific foods, usually hyper-palatable, rich in saturated fats, hydrocarbonates, and artificial flavors, that follows a pattern resembling the addictive behavior in SUD is considered the common ground of food addiction (48, 50).
3.9. Anorexia Athletica
Body image is essential for many athletes, and in several sports, low body weight may represent an advantage over one’s opponents (51). The obsessive focus on leanness and thinness in athletes may lead to anorexia if they decrease their calorie intake severely and/or tend to resort to excessive physical exercise to achieve or maintain a low body mass (51). Athletes may have lower than expected body weights and fat mass, even without presenting AA; therefore, more accurate instruments for detecting this disorder are needed, except for anthropometric parameters (51).
There are no well-defined and unanimously-accepted diagnostic criteria for AA. Still, several characteristics have been reported: the loss of body weight is due to sport/performance-related concerns, not to the appearance/body shape worries, except for cases where the degree of fatness may be related to lower chances of being successful in the respective sport; the beginning of restricting eating or excessive exercising is self-imposed or recommended by the coaching team, as part of the regular training; frequent weight cycling although some athletes may preserve a very low BMI on long-term (51).
3.10. Diabulimia
The co-existence of type 1 diabetes mellitus (T1DM) and an ED has been termed “diabulimia,” and the core belief of these patients is that decreasing the daily dose of insulin is needed for losing weight or maintaining their current body weight (52). This disorder is also called “type 1 disordered eating” and has been described in children, adolescents, and adults (53). Although diabulimia has only recently been systematically explored, its history is quite long, with the first cases of T1DM and comorbid ED being described more than four decades ago (54).
The risk of severe acute (e.g., diabetic ketoacidosis) or long-term (e.g., retinopathy) complications due to irregular or constantly lower insulin doses are evident in these patients; therefore, psychoeducational and therapeutic interventions should follow the detection of vulnerability factors. Because of the irregular insulin administration and disordered eating behavior, diabulimics have a 3-fold higher risk for death than non-diabetic individuals, based on an 11-year follow-up study in female patients with T1DM and a history of insulin restriction detected during the enrollment visit (55).
4. Discussion
A review of the current data available in the literature for less well-defined EDs was considered necessary due to the need to increase the awareness of the general population about their existence (Table 1). An integrative, four-category dimensional model for EDs has been elaborated based on the limited available data (Figure 1).
This perspective is based on the core elements of each nosographic category: (1) dysfunctions in the self-image, mainly body shape and weight, are shared by patients with anorexia nervosa, bulimia nervosa, anorexia athletica (also called “reverse anorexia”), and pregorexia; (2) dysfunctions of the self-control, or lack of control over their own impulses, are present in the first line of clinical manifestations in patients with BED, NES, and EE; (3) an addictive component is reflected by patients behaviors toward eating: food addiction (where the focus of attention is represented by hyper-palatable foods), orthorexia nervosa (the focus of attention is represented by “healthy foods”), and muscle dysmorphia (the focus of attention is own body); (4) there is an association of abnormal eating behavior and an organic pathology (type I diabetes, in diabulimia), or a psychiatric disorder (alcohol use disorder, in drunkorexia, or a parasomnia, in sleep-related eating disorder).
Each category previously presented shares compensatory behaviors for the main clinical element identified as the core dysfunction. The behaviors are activated to maintain an idealized self-image for the first category, to compensate for the lack of control through excessive eating in the second category, to gather the desired resources and to derive pleasure after consuming them in the third case, and to make up for possible biological or psychological self-perceived threats, in the fourth category.
The efficacy of therapeutic interventions in EDs is still inconclusive. However, during psychotherapy, the core beliefs referring to self-image distortions may be targeted, and techniques like mindfulness, relaxation training, increasing awareness about own impulses, and psychoeducation can lead to favorable effects. Multidisciplinary approaches have been recommended for most EDs (31, 52, 88, 120, 126). While nutritional and psychoeducation interventions are frequently suggested for EDs, very few therapeutic-specific recommendations have been identified: CBT or family therapy for muscle dysmorphia, CBT, relaxation techniques, and pharmacotherapy (antidepressants, topiramate, melatonergic agents) for NES; antidepressants, clonazepam or topiramate for SRED; mindfulness, physical exercise program, or CBT for EE;12-step approach or CBT for food addiction; CBT or family therapy for AA (55, 81, 82, 88–90, 96, 106, 107, 113).
The limitations of this model are (1) lack of pathophysiological evidence for each disorder; (2) large-scale confirmatory epidemiological studies are needed, and this model may be modified by further data; (3) there is a certain degree of overlap between clinical entities because several of their main symptoms are similar (e.g., self-image dysfunctions and addictive features are equally important in muscle dysmorphia).
In conclusion, the current model, although still in its early conceptualization phase, may present pragmatic and theoretical benefits, e.g., conducting epidemiological and clinical trials, finding therapeutic approaches based on common clinical features, and enhancement of public awareness about their consequences on the general health consequences.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Steinhausen, HC. Outcome of eating disorders. Child Adolesc Psychiatr Clin N Am. (2009) 18:225–42. doi: 10.1016/j.chc.2008.07.013
2. Fichter, MM, and Quadflieg, N. Mortality in eating disorders-results of a large prospective clinical longitudinal study. Int J Eat Disord. (2016) 49:391–401. doi: 10.1002/eat.22501
3. Galmiche, M, Déchelotte, P, Lambert, G, and Tavolacci, MP. Prevalence of eating disorders over 2000-2018 period: a systematic literature review. Am J Clin Nutr. (2019) 109:1402–13. doi: 10.1093/ajcn/nqy342
4. WHO. ICD-10 version 2019. Available at: https://icd.who.int/browse10/2019/en. Accessed June 21, 2020
5. WHO. ICD-11 clinical descriptions and diagnostic guidelines for mental and behavioural disorders. Available at: https://icd.who.int/browse11/l-m/en. Accessed June 21, 2020.
6. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Arlington, VA: APA (2000). 546 p.
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: APA (2013).
8. Katzman, DK. The COVID-19 pandemic and eating disorders: a wake-up call for the future of eating disorders among adolescents and young adults. J Adolesc Health. (2021) 69:535–7. doi: 10.1016/j.jadohealth.2021.07.014
9. Vasiliu, O, Vasile, D, Vasiliu, DG, and Ciobanu, OM. Quality of life impairments and stress coping strategies during the COVID-19 pandemic isolation and quarantine-a web-based survey. Rom J Military Med. (2021) CXXIV:10–21.
10. Morris, J, and Twaddle, S. Anorexia nervosa. BMJ. (2007) 334:894–8. doi: 10.1136/bmj.39171.616840.BE
11. Lowe, B, Zipfel, S, Buchholz, C, Dupont, Y, Reas, DL, and Herzog, W. Long-term outcome of anorexia nervosa in a prospective 21-year follow-up study. Psychol Med. (2001) 31:881–90. doi: 10.1017/s003329170100407x
12. Vasiliu, O. The current state of research for psychobiotics use in the management of psychiatric disorders-a systematic literature review. Front Psych. (2023) 14:1074736. doi: 10.3389/fpsyt.2023.1074736
13. Vasiliu, O. Is fecal microbiota transplantation a useful therapeutic intervention for psychiatric disorders? A narrative review of clinical and preclinical evidence. Curr Med Res Opin. (2023) 39:161–77. doi: 10.1080/03007995.2022.2124071
15. Hilbert, A, Petroff, D, Herpertz, S, Pietrowsky, R, Tuschen-Caffier, B, Vocks, S, et al. Meta-analysis of the efficacy of psychological and medical treatments for binge-eating disorder. J Consult Clin Psychol. (2019) 87:91–105. doi: 10.1037/ccp0000358
16. Vasiliu, O, Vasile, D, Mangalagiu, AG, Petrescu, BM, Tudor, C, Ungureanu, D, et al. Efficacy and tolerability of calcium channel alpha-2-delta ligands in psychiatric disorders. Rom J Military Med. (2017) CXX:27–31.
17. Prozac-Fluoxetine Hydrochloride. (1987). Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/018936s091lbl.pdf (Accessed March 12, 2023).
18. Reas, DL, and Grilo, CM. Pharmacological treatment of binge eating disorder: update review and synthesis. Expert Opin Pharmacother. (2015) 16:1463–78. doi: 10.1517/14656566.2015.1053465
19. Vyvanse-Lisdecamfetamine Dimesylate. (2007). Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208510lbl.pdf (Accessed March 12, 2023).
20. Choquette, EM, Rancourt, D, and Thompson, JK. From FAD to FAD: a theoretical formulation and proposed name change for “drunkorexia” to food and alcohol disturbance (FAD). Int J Eat Disord. (2018) 51:831–4. doi: 10.1002/eat.22926
21. Pompili, S, and Laghi, F. Drunkorexia: disordered eating behaviors and risky alcohol consumption among adolescents. J Health Psychol. (2020) 25:2222–32. doi: 10.1177/1359105318791229
22. Roosen, KM, and Mills, JS. Exploring the motives and mental health correlates of intentional food restriction prior to alcohol use in university students. J Health Psychol. (2015) 20:875–86. doi: 10.1177/1359105315573436
23. Thompson-Memmer, C, Glassman, T, and Diehr, A. Drunkorexia: a new term and diagnostic criteria. J Am Coll Heal. (2019) 67:620–6. doi: 10.1080/07448481.2018.1500470
24. Janas-Kozik, M, Zmijowska, A, Zasada, I, Jelonek, I, Cichon, L, Siwiec, A, et al. Systematic review of literature on eating disorders during pregnancy-risk and consequences for mother and child. Front Psych. (2021) 12:777529. doi: 10.3389/fpsyt.2021.777529
25. Tuncer, E, Gumus, AB, and Keser, A. The importance of pregorexia awareness. Clin Exp Health Sci. (2020) 10:186–90. doi: 10.33808/clinexphealthsci.673306
26. Bannatyne, AJ, Hughes, R, Stapleton, P, Watt, B, and Mackenzie-Shalders, K. Signs and symptoms of disordered eating in pregnancy: a Delphi consensus study. BMC Pregnancy Childbirth. (2018) 18:262. doi: 10.1186/s12884-018-1849-3
27. Mathieu, J. What is pregorexia? J Am Diet Assoc. (2009) 109:976–9. doi: 10.1016/j.jada.2009.04.021
29. Bratman, S, and Knight, D. Health Food Junkies: Overcoming the Obsession with Healthful Eating. 1st ed. New York, NY: Broadway Books (2000).
30. Brytek-Mara, A. Restrained eating and vegan, vegetarian and omnivore dietary intakes. Nutrients. (2020) 12:2133. doi: 10.3390/nu12072133
31. Gkiouleka, M, Stavraki, C, Sergentanis, TN, and Vassilakou, T. Orthorexia nervosa in adolescents and young adults: a literature review. Children. (2022) 9:365. doi: 10.3390/children9030365
32. Gramaglia, C, Gattoni, E, Ferrante, D, Abbate-Daga, G, Baldissera, E, Calugi, S, et al. What do Italian healthcare professionals think about orthorexia nervosa? Results from a multicenter survey. Eat Weight Disord. (2022) 27:2037–49. doi: 10.1007/s40519-021-01336-9
33. Mosley, PE. Bigorexia: bodybuilding and muscle dysmorphia. Eur Eat Disord Rev. (2009) 17:191–8. doi: 10.1002/erv.897
34. Pope, HG Jr, Katz, DL, and Hudson, JI. Anorexia nervosa and “reverse anorexia” among 108 male bodybuilders. Compr Psychiatry. (1993) 34:406–9. doi: 10.1016/0010-440x(93)90066-d
35. Pope, HG, Phillips, KA, and Olivardia, R. The Adonis Complex: How to Identify, Treat and Prevent Body Obsession in Men and Boys. New York, NY: Touchstone (2000).
36. Pope, HG Jr, Gruber, AJ, Choi, P, Olivardia, R, and Phillips, KA. Muscle dysmorphia. An underrecognized form of body dysmorphic disorder. Psychosomatics. (1997) 38:548–57. doi: 10.1016/S0033-3182(97)71400-2
37. Olivardia, R, Pope, HG Jr, and Hudson, JI. Muscle dysmorphia in male weightlifters: a case-control study. Am J Psychiatry. (2000) 157:1291–6. doi: 10.1176/appi.ajp.157.8.1291
38. Stunkard, AJ, Grace, WJ, and Wolff, HG. The night-eating syndrome. A pattern of food intake among certain obese patients. Am J Med. (1955) 19:78–86. doi: 10.1016/0002-9343(55)90276-X
39. O’Reardon, JP, Peshek, A, and Allison, KC. Night eating syndrome: diagnosis, epidemiology and management. CNS Drugs. (2005) 19:997–1008. doi: 10.2165/00023210-200519120-00003
40. Idir, Y, Oudiette, D, and Arnulf, I. Sleepwalking sleep terrors, sexsomnia and other disorders of arousal: the old and the new. J Sleep Res. (2022) 31:e13596. doi: 10.1111/jsr.13596
41. Howell, MJ, and Schenck, CH. Restless nocturnal eating: a common feature of Willis-Ekbom syndrome (RLS). J Clin Sleep Med. (2012) 08:413–9. doi: 10.5664/jcsm.2036
42. Irfan, M, Schenck, CH, and Howell, MJ. NonREM disorders of arousal and related parasomnias: an updated review. Neurotherapeutics. (2021) 18:124–39. doi: 10.1007/s13311-021-01011-y
43. The International Classification of Sleep Disorders: Diagnostic and Coding Manual, 2nd Edn Westchester, IL. American Academy of Sleep Medicine. (2005). 174–175.
44. Konttinene, H. Emotional eating and obesity in adults: the role of depression, sleep and genes. Proc Nutr Soc. (2020) 79:283–9. doi: 10.1017/S0029665120000166
45. Frayn, M, Livshits, S, and Knäuper, B. Emotional eating and weight regulation: a qualitative study of compensatory behaviors and concerns. J Eat Disord. (2018) 6:23. doi: 10.1186/s40337-018-0210-6
46. Reichenberger, J, Schnepper, R, Arend, AK, and Blechert, J. Emotional eating in healthy individuals and patients with an eating disorder: evidence from psychometric, experimental and naturalistic studies. Proc Nutr Soc. (2020) 79:290–9. doi: 10.1017/S0029665120007004
47. Lindeman, M, and Stark, K. Emotional eating and eating disorder psychopathology. Eat Disord. (2019) 9:251–9. doi: 10.1080/10640260127552
48. Vasiliu, O. Current status of evidence for a new diagnosis: food addiction-a literature review. Front Psych. (2022) 12:824936. doi: 10.3389/fpsyt.2021.824936
49. Gordon, EL, Ariel-Donges, AH, Bauman, V, and Merlo, LJ. What is the evidence for « food addiction »? A systematic review. Nutrients. (2018) 10:477. doi: 10.3390/nu10040477
50. Zawartailo, L, Attwells, S, de Ruiter, WK, Le, TL, Dawson, D, and Selby, P. Food addiction and tobacco use disorder: common liability and shared mechanisms. Nutrients. (2020) 12:3834. doi: 10.3390/nu12123834
51. Sudi, K, Ottl, K, Payerl, D, Baumagrtl, P, Tauschmann, K, and Müller, W. Anorexia athletica. Nutrition. (2004) 20:657–61. doi: 10.1016/j.nut.2004.04.019
52. Chelvanayagam, S, and James, J. What is diabulimia and what are the implications for practice? Br J Nurs. (2018) 27:980–6. doi: 10.12968/bjon.2018.27.17.980
53. Cainer, A. Recognising and managing type 1 disordered eating in children and young people with diabetes. Nurs Child Young People. (2022) 34:28–32. doi: 10.7748/ncyp.2022.e1396
54. Callum, AM, and Lewis, LM. Diabulimia among adolescents with type 1 diabetes. Clin Nurs Stud. (2014) 2:12. doi: 10.5430/cns.v2n4p12
55. Goebel-Fabbri, AE, Fikkan, J, Franko, DL, Pearson, K, Anderson, BJ, and Weinger, K. Insulin restriction and associated morbidity and mortality in women with type 1 diabetes. Diabetes Care. (2008) 31:415–9. doi: 10.2337/dc07-2026
56. Rahal, CJ, Bryant, JB, Darkes, J, Menzel, JE, and Thompson, JK. Development and validation of the compensatory eating and behaviors in response to alcohol consumption scale (CEBRACS). Eat Behav. (2012) 13:83–7. doi: 10.1016/j.eatbeh.2011.11.001
57. Ward, RM, and Galante, M. Development and initial validation of the drunkorexia motives and behaviors scales. Eat Behav. (2015) 18:66–70. doi: 10.1016/j.eatbeh.2015.04.003
58. Knight, A, Castelnuovo, G, Pietrabissa, G, Manzoni, GM, and Simpson, S. Drunkorexia: an empirical investigation among Australian female university students. Aust Psychol. (2017) 52:414–23. doi: 10.1111/ap.12212
59. Hunt, TK, and Forbush, KT. Is “drunkorexia” an eating disorder, substance use disorder, or both? Eat Behav. (2016) 22:40–5. doi: 10.1016/j.eatbeh.2016.03.034
60. Hill, EM, and Lego, JE. Examining the role of body esteem and sensation seeking in drunkorexia behaviors. Eat Weight Disord. (2020) 25:1507–13. doi: 10.1007/s40519-019-00784-8
61. Burke, SC, Cremeens, J, Vail-Smith, K, and Woolsey, C. Drunkorexia: calorie restriction prior to alcohol consumption among college freshmen. J Alcohol Drug Educ. (2010) 54:17–34.
62. Glassman, T, Paprzycki, P, Castor, T, Wotring, A, Wagner-Greene, V, Ritzman, M, et al. Using the elaboration likelihood model to address drunkorexia among college students. Subst Use Misuse. (2018) 53:1411–8. doi: 10.1080/10826084.2017.1409766
63. Saleem, T, Saleem, S, Shoib, S, Shah, J, and SaeZ, A. A rare phenomenon of pregorexia in Pakistani women: need to understand the related behaviors. J Eat Disord. (2022) 10:74. doi: 10.1186/s40337-022-00589-8
64. Easter, A, Bye, A, Taborelli, E, Corfield, F, Schmidt, U, Treasure, J, et al. Recognising the symptoms: how common are eating disorders in pregnancy? Eur Eat Disord Rev. (2013) 21:340–4. doi: 10.1002/erv.2229
65. Babicz-Zielinska, E, Wadolowska, L, and Tomaszewski, D. Eating disorders: problems of contemporary civilization: a review. Pol J Food Nutr Sci. (2013) 63:133–46. doi: 10.2478/v10222-012-0078-0
66. Cena, H, Barthels, F, Cuzzolaro, M, Bratman, S, Brytek-Matera, A, Dunn, T, et al. Definition and diagnostic criteria for orthorexia nervosa: a narrative review of the literature. Eat Weight Disord. (2019) 24:209–46. doi: 10.1007/s40519-018-0606-y
67. Barthels, F, Meyer, F, and Pietrowsky, R. Die Düsseldorfer Orthorexie Skala-Konstruktion und Evaluation eines Fragebogens zur Erfassung ortho-rektischen Ernährungsverhaltens. Z Klin Psychol Psychother. (2015) 44:97–105. doi: 10.1026/1616-3443/a000310
68. Stunkard, AJ, and Messick, S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. (1985) 29:71–83. doi: 10.1016/0022-3999(85)90010-8
69. Donini, LM, Marsili, D, Graziani, MP, Imbriale, M, and Cannella, C. Orthorexia nervosa: validation of a diagnosis questionnaire. Eat Weight Disord. (2005) 10:e28–32. doi: 10.1007/BF03327537
70. Varga, M, Dukay-Szabó, S, Túry, F, and van Furth, EF. Evidence and gasp in the literature on orthorexia nervosa. Eat Weight Disord. (2013) 18:103–11. doi: 10.1007/s40519-013-0026-y
71. Yilmaz, MN, and Dundar, C. The relationship between orthorexia nervosa, anxiety, and self-esteem: a cross-sectional study in Turkish faculty members. BMC Psychol. (2022) 10:82. doi: 10.1186/s40359-022-00796-7
72. Parra-Fernández, ML, Manzaneque-Cañadillas, M, Onieva-Zafra, MD, Fernández-Martínez, E, Fernández-Muñoz, JJ, Prado-Laguna, MDC, et al. Pathological preoccupation with healthy eating (orthorexia nervosa) in a Spanish sample with vegetarian, vegan, and non-vegetarian dietary patterns. Nutrients. (2020) 12:3907. doi: 10.3390/nu12123907
73. Brytek-Matera, A. Vegetarian diet and orthorexia nervosa: a review of the literature. Eat Weight Disord. (2021) 26:1–11. doi: 10.1007/s40519-019-00816-3
74. Hyrnik, J, Zasada, I, Wilczyński, KW, Jelonek, I, and Janas-Kozik, M. Orthorexia-current approach. Rev Psychiatr Pol. (2021) 55:405–20. doi: 10.12740/PP/115149
75. Lopes, R, Melo, R, and Pereira, BD. Orthorexia nervosa and comorbid depression successfully treated with mirtazapine: a case report. Eat Weight Disord Stud Anorexia Bulimia Obesity. (2020) 25:163–7. doi: 10.1007/s40519-018-0539-5
76. Moroze, RM, Dunn, TM, Holland, C, Yager, J, and Weintraub, P. Micro thinking about micronutrients: a case of transition from obsessions about healthy eating to near-fatal orthorexia nervosa and proposed diagnostic criteria. Psychosomatics. (2015) 56:397–403. doi: 10.1016/j.psym.2014.03.003
77. McCreary, DR, and Sasse, DK. An exploration of the drive for muscularity in adolescent boys and girls. J Am Coll Heal. (2000) 48:297–304. doi: 10.1080/07448480009596271
78. Hildebrandt, T, Walker, DC, Alfano, L, Delinsky, S, and Bannon, K. Development and validation of a male specific body checking questionnaire. Int J Eat Disord. (2010) 43:77–87. doi: 10.1002/eat.20669
79. dos Santo Filho, CA, Tirico, PP, Stefano, SC, Touyz, SW, and Claudino, AM. Systematic review of the diagnostic category muscle dysmorphia. Aust N Z J Psychiatry. (2015) 50:322–33. doi: 10.1177/0004867415614106
80. Phillips, KA. Pharmacotherapy for body dysmorphic disorder. Psychiatr Ann. (2010) 40:325–32. doi: 10.3928/00485713-20100701-05
81. Perelman, H, Schwartz, N, Yeoward-Dodson, J, Quiñones, IC, Murray, MF, Dougherty, EN, et al. Reducing eating disorder risk among male athletes: a randomized controlled trial investigating the male athlete body project. Int J Eat Disord. (2022) 55:193–206. doi: 10.1002/eat.23665
82. Murray, SB, and Griffiths, S. Adolescent muscle dysmorphia and family-based treatment: a case report. Clin Child Psychol Psychiatry. (2015) 20:324–30. doi: 10.1177/1359104514521639
83. Gluck, ME, Geliebter, A, and Satov, T. Night eating syndrome is associated with depression, low self-esteem, reduced daytime hunger, and less weight loss in obese outpatients. Obes Res. (2001) 9:264–7. doi: 10.1038/oby.2001.31
84. Allison, KC, Lundgren, JD, O’Reardon, JP, Martino, NS, Sarwer, DB, Wadden, TA, et al. The night eating questionnaire (NEQ): psychometric properties of a measure of severity of the night eating syndrome. Eat Behav. (2008) 9:62–72. doi: 10.1016/j.eatbeh.2007.03.007
85. Batra, S, Ochani, RK, Memon, ZA, Shaikh, A, Qureshi, NE, Bhimani, S, et al. Relationship between night eating syndrome and self-esteem: a cross-sectional population-based study in Karachi, Pakistan. Cureus. (2019) 11:e5540. doi: 10.7759/cureus.5540
86. de Zwaan, M, Marschollek, M, and Allison, KC. The night eating syndrome (NES) in bariatric surgery patients. Eur Eat Disord Rev. (2015) 23:426–34. doi: 10.1002/erv.2405
87. de Zwaan, M, Müller, A, Allison, KC, Brähler, E, and Hilbert, A. Prevalence and correlates of night eating in German general population. PLoS One. (2014) 9:e97667. doi: 10.1371/journal.pone.0097667
88. Muscatello, MRA, Torre, G, Celebre, L, Dell’Osso, B, Mento, C, Zoccali, RA, et al. “In the night kitchen”: a scoping review on the night eating syndrome. Aust N Z J Psychiatry. (2022) 56:120–36. doi: 10.1177/00048674211025714
89. Wal, JSV. Night eating syndrome: a critical review of the literature. Clin Psychol Rev. (2012) 32:49–59. doi: 10.1016/j.cpr.2011.11.001
90. Pawlow, LA, O’Neil, PM, and Malcolm, RJ. Night eating syndrome effects of brief relaxation training on stress, mood, hunger, and eating patterns. Int J Obes. (2003) 27:970–8. doi: 10.1038/sj.ijo.0802320
91. Winkelman, JW, Herzog, DB, and Fava, M. The prevalence of sleep-related eating disorder in psychiatric and non-psychiatric populations. Psychol Med. (1999) 29:1461–6. doi: 10.1017/s0033291799008272
92. Iranzo, A. Parasomnias and sleep-related movement disorders in older adults. Sleep Med Clin. (2022) 17:295–305. doi: 10.1016/j.jsmc.2022.02.005
93. Mittal, N, Mittal, R, and Gupta, MC. Systematic literature review on zolpidem-induced complex sleep behaviors. Indian J Psychol Med. (2021) 43:373–81. doi: 10.1177/0253717621992372
94. Kanagasundram, S. Quetiapine-induced sleep-related eating disorder: a case report. Clin Case Rep. (2021) 9:e04168. doi: 10.1002/ccr3.4168
95. Shoar, S, Naderan, M, Mahmoodzadeh, H, Shoar, N, and Lotfi, D. Night eating syndrome: a psychiatric disease, a sleep disorder, a delayed circadian rhythm, and/or a metabolic condition? Expert Rev Endocrinol Metab. (2019) 14:351–8. doi: 10.1080/17446651.2019.1657006
96. Chiaro, G, Caletti, MT, and Provini, F. Treatment of sleep-related eating disorder. Curr Treat Options Neurol. (2015) 17:1–11. doi: 10.1007/s11940-015-0361-6
97. Domoff, SE. Dutch eating behavior questionnaire (DEBQ) In: T Wade, editor. Encyclopedia of Feeding and Eating Disorders. Singapore: Springer (2015)
98. Meule, A, Reichenberg, J, and Blechert, J. Development and preliminary validation of the Salzburg emotional eating scale. Front Psychol. (2017) 9:88. doi: 10.3389/fpsyg.2018.00088
99. Ricca, V, Castellini, G, Lo Sauro, C, Ravaldi, C, Lapi, F, Mannucci, E, et al. Correlations between binge eating and emotional eating in a sample of overweight subjects. Appetite. (2009) 53:418–21. doi: 10.1016/j.appet.2009.07.008
100. Van Strien, T. Causes of emotional eating and matched treatment of obesity. Curr Diab Rep. (2018) 18:35. doi: 10.1007/s11892-018-1000-x
101. Shriver, LH, Dollar, JM, Calkins, SD, Keane, SP, Shanahan, L, and Wideman, L. Emotional eating in adolescence: effects of emotion regulation, weight status and negative body image. Nutrients. (2020) 13:79. doi: 10.3390/nu13010079
102. Barak, RE, Shuval, K, Li, Q, Oetjen, R, Drope, J, Yaroch, AL, et al. Emotional eating in adults: the role of sociodemographics, lifestyle behaviors, and self-regulation-findings from a U.S. National Study. Int J Environ Res Public Health. (2021) 18:1744. doi: 10.3390/ijerph18041744
103. Annesi, JJ, Mareno, N, and McEwen, K. Psychosocial predictors of emotional eating and their weight-loss treatment-induced changes in women with obesity. Eat Weight Disord. (2016) 21:289–95. doi: 10.1007/s40519-015-0209-9
104. Sze, KYP, Lee, EKP, Chan, RHW, and Kim, JH. Prevalence of negative emotional eating and its associated psychosocial factors among urban Chinese undergraduates in Hong Kong: a cross-sectional study. BMC Public Health. (2021) 21:583. doi: 10.1186/s12889-021-10531-3
105. Reichenberger, J, Schnepper, R, Arend, AK, Richard, A, Voderholzer, U, Naab, S, et al. Emotional eating across different eating disorders and the role of body mass, restriction, and binge eating. Int J Eat Disord. (2021) 54:773–84. doi: 10.1002/eat.23477
106. Annesi, JJ, and Mareno, N. Indirect effects of exercise on emotional eating through psychological predictors of weight loss in women. Appetite. (2015) 95:219–27. doi: 10.1016/j.appet.2015.07.012
107. Katterman, SN, Kleinman, BM, Hood, MM, Nackers, LM, and Corsica, JA. Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: a systematic review. Eat Behav. (2014) 15:197–204. doi: 10.1016/j.eatbeh.2014.01.005
108. Gearhardt, AN, Corbin, WR, and Brownell, KD. Development of the Yale food addiction scale version 2.0. Psychol Addict Behav. (2006) 30:113–21. doi: 10.1037/adb0000136
109. McKenna, RA, Rollo, ME, Skinner, JA, and Burrows, TL. Food addiction support: website contact analysis. JMIR Cardio. (2018) 2:e10. doi: 10.2196/cardio.8718
110. Wiss, DA, Avena, N, and Gold, M. Food addiction and psychosocial adversity: biological embedding, contextual factors, and public health implications. Nutrients. (2020) 12:3521. doi: 10.3390/nu12113521
111. Pursey, KM, Stanwell, P, Gearhardt, AN, Collins, CE, and Burrows, TL. The prevalence of food addiction as assessed by the Yale food addiction scale: a systematic review. Nutrients. (2014) 6:4552–90. doi: 10.3390/nu6104552
112. Hilker, I, Sanchez, I, Steward, T, Jimenez-Murcia, S, Granero, R, Gearhardt, AN, et al. Food addiction in bulimia nervosa: clinical correlates and association with response to a brief psychoeducational intervention. Eur Eat Disord Rev. (2016) 24:482–8. doi: 10.1002/erv.2473
113. Munguia, L, Gaspar-Perez, A, Jimenez-Murcia, S, Granero, R, Sanchez, I, Vintro-Alcaraz, C, et al. Food addiction in eating disorders: a cluster analysis approach and treatment outcome. Nutrients. (2022) 14:1084. doi: 10.3390/nu14051084
114. Nazem, TG, and Ackerman, KE. The female athlete triad. Sports Health. (2012) 4:302–11. doi: 10.1177/1941738112439685
115. Herbrich, L, Pfeiffer, E, Lehmkuhl, U, and Schneider, N. Anorexia athletica in pre-professional ballet dancers. J Sports Sci. (2011) 29:1115–23. doi: 10.1080/02640414.2011.578147
116. Sundgot-Borgen, J, and Torstveit, MK. Prevalence of eating disorders in elite athletes is higher than in the general population. Clin J Sport Med. (2004) 14:25–32. doi: 10.1097/00042752-200401000-00005
117. Sundgot-Borgen, J, and Torstveit, MK. Aspects of disordered eating continuum in elite high-intensity sports. Scand J Med Sci Sports. (2010) 20:112–21. doi: 10.1111/j.1600-0838.2010.01190.x
118. Matejek, N, Weimann, E, Witzel, C, Mölenkamp, G, Schwidergall, S, and Böhles, H. Hypoleptinemia in patients with anorexia nervosa and in elite gymnasts with anorexia nervosa. Int J Sports Med. (1999) 20:451–6. doi: 10.1055/s-1999-8834
119. Borgen, JS, and Corbin, CB. Eating disorders among female athletes. Phys Sportsmed. (1987) 15:88–95. doi: 10.1080/00913847.1987.11709282
120. Javed, A, Tebben, PJ, Fisher, PR, and Lteif, AN. Female athlete triad and its components: toward improved screening and management. Mayo Clin Proc. (2013) 88:996–1009. doi: 10.1016/j.mayocp.2013.07.001
121. Kinik, MF, Gönüllü, FV, Vatansever, Z, and Karakaya, I. Diabulimia, a type I diabetes mellitus-specific eating disorder. Turk Pediatri Ars. (2017) 52:46–9. doi: 10.5152/TurkPediatriArs.2017.2366
122. Lorettu, L, Pes, GM, Dore, MP, Milia, P, and Nivoli, A. Eating disorders and diabetes: behavioural patterns and psychopathology. Two case reports. Riv Psichiatr. (2020) 55:240–4. doi: 10.1708/3417.34001
123. Falcão, MA, and Francisco, R. Diabetes, eating disorders and body image in young adults: an exploratory study about “diabulimia”. Eat Weight Disord. (2017) 22:675–82. doi: 10.1007/s40519-017-0406-9
124. Jones, JM, Lawson, ML, Daneman, D, Olmsted, MP, and Rodin, G. Eating disorders in adolescent females with and without type 1 diabetes: cross sectional study. BMJ. (2000) 320:1563–6. doi: 10.1136/bmj.320.7249.1563
125. Larrañaga, A, Docet, MF, and García-Mayor, RV. Disordered eating behaviors in type 1 diabetic patients. World J Diabetes. (2011) 2:189–95. doi: 10.4239/wjd.v2.i11.189
126. National Institute for Health and Care Excellence. (2020). Eating disorders: recognition and treatment. NICE Guideline 69. Available at: https://www.nice.org.uk/guidance/ng69. (Accessed June 12, 2022).
Keywords: orthorexia nervosa, eating disorder, food addiction, anorexia athletica, night eating syndrome, emotional eating, diabulimia
Citation: Vasiliu O (2023) An integrative model as a step toward increasing the awareness of eating disorders in the general population. Front. Psychiatry. 14:1184932. doi: 10.3389/fpsyt.2023.1184932
Edited by:
Pasquale Scognamiglio, ASL Napoli 3 Sud, ItalyReviewed by:
Fabio Panariello, University of Bologna, ItalyCopyright © 2023 Vasiliu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Octavian Vasiliu, b2N0YXZ2YXNpbGl1QHlhaG9vLmNvbQ==
 Octavian Vasiliu
Octavian Vasiliu