- 1Department of Psychiatry, The Affiliated Xuzhou Oriental Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
- 2Department of Psychiatry, Xuzhou Medical University, Xuzhou, China
- 3Department of Geriatric Psychiatry, The Affiliated Brain Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
Objective: To explore the pattern of empathy characteristics in male patients with schizophrenia (SCH) and to examine whether empathy deficit is associated with impulsivity and premeditated violence.
Methods: One hundred and fourteen male SCH patients were enrolled in this study. The demographic data of all patients were collected and the subjects were divided into two groups, namely, the violent group, including 60 cases, and the non-violent group, comprising 54 cases, according to the Modified Overt Aggression Scale (MOAS). The Chinese version of the Interpersonal Reactivity Index-C (IRI-C) was used to evaluate empathy and the Impulsive/Predicted Aggression Scales (IPAS) was employed to assess the characteristics of aggression.
Results: Among the 60 patients in the violent group, 44 patients had impulsive aggression (IA) and 16 patients had premeditated aggression (PM) according to the IPAS scale. In the violent group, the scores of the four subfactors of the IRI-C, i.e., perspective taking (PT), fantasy (FS), personal distress (PD), and empathy concern (EC), were significantly lower than in the non-violent group. Stepwise logistic regression showed that PM was independent influencing factor for violent behaviors in SCH patients. Correlation analysis revealed that EC of affective empathy was positively correlated with PM but not with IA.
Conclusion: SCH patients with violent behavior had more extensive empathy deficits compared with non-violent SCH patients. EC, IA and PM are independent risk factors of violence in SCH patients. Empathy concern is an important index to predict PM in male patients with SCH.
1. Introduction
Schizophrenia (SCH) is a spectrum of severe mental disorders which affects 1% of the population globally (1). The majority of patients experience a chronic course of the disease that might result in personality changes, social function impairment and ultimately, complete social function loss that necessitates prolonged hospitalization (2). SCH has a wide range of complex clinical manifestations which can be divided into positive symptoms (hallucinations, delusions, suspicion, anger and so on) and negative symptoms (blunted affect, emotional communication disorders, abstract thinking disorders, etc.) (3). In addition, SCH patients have aberrant mental functioning in cognition, thoughts, emotions, behaviors and other areas. SCH also disrupts social and occupational functioning and adds to the illness burden on families and the society (4, 5).
Public attention has long been drawn to the aggressive conduct of individuals with mental diseases, particularly SCH. Two meta-analyses revealed that one in 10 persons with SCH had aggressive behaviors in public, which is five times more common than in the general population (6, 7). Although instances of violence among those with schizophrenia are infrequent, it is still considered a significant issue. Unfortunately, individuals diagnosed with SCH are often unjustly stigmatized as being prone to violent behavior. It should be noted that while patients with SCH are more likely to engage in violent behaviors (8), only a fraction of violent offenders have SCH (9, 10). As a result of psychotic symptoms, SCH patients frequently exhibit aggressivity (11–13), especially during an acute episode (14, 15). Previous studies have demonstrated that patients with psychopathological conditions were more prone to engage in aggressive actions when suffering from auditory hallucinations, particularly command hallucinations (16, 17). The aggressive behaviors of hospitalized individuals typically manifest in the form of verbal aggression but can also occur as physical aggression in extreme circumstances (7, 18, 19). Violence in SCH complicates clinical treatment and management (20), raises healthcare expenses, lengthens hospital stays and exacerbates the stigma associated with the disease (21, 22).
Different focuses in multiple domains have led to a complicated definition of aggressive behavior. According the most accepted theory, aggressivity can be classified into two distinct subtypes, namely, impulsive and premeditation aggression, depending on the goal, technique and other factors (23, 24). Impulsive aggression is characterized as an emotionally charged and unrestrained type of aggression with high affective arousal and impulsivity (25) whereas premeditated assaults are planned, controlled and non-emotional actions that need foresight and planning (26, 27). While these two forms of violent conduct are independent constructs, they cannot be distinguished from one another in clinical practice and might coexist to varying degrees. Nevertheless, a wealth of research has reported that the neurobiology and neuropsychology of these two categories of aggressivity are clearly distinct (28–33). Therefore, assessing and predicting the likelihood of violent conduct in SCH patients is critical for timely management.
Empathy, the ability to comprehend the emotions of other people, is a social cognitive function that includes affiliative interpersonal communication and is linked to functional outcome in patients (34). It comprises cognitive empathy, which is defined as the identification and comprehension of another person’s emotional state and affective empathy, described as the ability to share another person’s feelings (35). While cognitive impairment is one of the primary symptoms of SCH (36, 37), research on these two forms of empathy has shown conflicting results. A previous study demonstrated that SCH patients had poor cognitive empathy, which led to difficulties in interpreting the feelings of others (38). However, a few studies revealed that emotional empathy was not reduced in SCH, showing that patients could be able to effectively experience the feelings of other people (39–41). Interestingly, violent SCH patients had lower aggressive attitudes after cognitive remediation and social cognitive training (42). Prior research indicated that violent patients exhibited cognitive deficits and higher mentalization, which might be associated with patients committing premeditated violent crimes (43–45).
To date, few researchers have investigated SCH patients who engage in aggressive behaviors. Since empathy deficiencies might be associated with aggression when violence is initiated, it is crucial to forecast the incidence of violent conduct in SCH. In this line, male SCH patients were selected in order to assess the features of violent conduct and empathy capacity as well as to investigate the link between aggressive behaviors and empathy.
2. Methods
2.1. Participants
A total of 114 male patients with SCH, aged between 18 and 55 years old, were recruited from the inpatient department of Xuzhou Oriental Hospital between September 2021 and October 2022. All the patients enrolled in the study were assessed and diagnosed with SCH by two experienced psychiatrists according to the Structured Clinical Interview for DSM-IV (SCID). The participants were classified into 60 violent schizophrenia patients (VSCH) and 54 non-violent controls (NV-SCH) according to The Modified Overt Aggression Scale (MOAS). The Chinese Interpersonal Reactivity Index (C-IRI) and the Impulsive/Premeditated Aggression Scales (IPAS) were also utilized to evaluate the patients. The patients’ general demographic information, such as age, smoking history, family history, height and weight were collected.
The exclusion criteria for all participants comprised of (1) patients with other psychotic disorders, including paranoid psychotic disorders, acute and transient psychotic disorders, schizoaffective disorders, schizotypal personality disorders, affective disorders with psychotic symptoms, mental disorders due to brain damage or physical illness and substance/drug related disorders; (2) patients suffering from psychiatric comorbidity, neurological disorders or unstable physical illnesses; (3) no alcohol consumption in the previous 30 days or drug dependence in the past 6 months.
This research adhered to the ethical principles of the World Medical Association Declaration of Helsinki (46) and was approved by the Medical Ethics Committee of Xuzhou Oriental Hospital. All the patients provided written informed consent before the start of the study.
2.2. Assessment instruments
2.2.1. Modified overt aggression scale
All enrolled patients underwent assessment with Modified overt aggression scale (MOAS) to evaluate the frequency and severity of aggressive episodes and violence within a 1-month period (47, 48). The MOAS consists of four subscales: verbal aggression, physical aggression, self-harm and physical aggression against others (49, 50). The score of each subscale ranges from 0 to 4. A higher score indicates more severe violence, with 0 representing no violence and 4 depicting the most severe level of violence. The subscale scores are multiplied by a factor assigned to that category: 1 point for verbal aggression, 2 points for physical aggression, 3 points for self-aggression and 4 points for aggression toward others. The weighted sums of each subscale are added together to obtain the total weighted score, which ranges from 0 to 40. In this study, the violent group consisted of patients who had a MOAS total score greater or equal to 5 points (51), or an object aggression score greater than 1 point, while the non-violent control group comprised of the remaining patients (52, 53).
2.2.2. Impulsive/premeditated aggression scale
The Impulsive/premeditated aggression scale (IPAS) is a reliable and valid self-report tool for assessing the occurrence of aggressive behaviors occurring over the past 6 months and categorizes aggressive conduct according to impulsive and premeditated aggressive behaviors (54, 55). The IPAS is often employed in the study of violent crimes since it can predict criminal activity (56). The scale has recently gained widespread usage globally and its Chinese version was revised in 2009 (57). The IPAS consists of 30 questions, including 8 items on impulsive aggression (IA), 12 items on premeditated aggression (PM) and 10 items on feeling in control. The items are graded on a scale of 1 to 5, whereby 1 = Strongly Disagree; 2 = Disagree; 3 = Neutral; 4 = Agree; and 5 = Strongly Agree. The proportion of positive items in the IA and PM components were determined separately using qualitative scoring. Items with scores of 5 (strongly agree), or 4 (agree) were rated as positive items, with reversed scores in the 5th and 8th items. After calculating the number of positive items, the percentage of positive items was yielded separately for both IA and PA. Participants with a higher percentage of IA positive items than PM positive items were classified as impulsive aggression (IA) subgroup. Conversely, participants with a higher percentage of PM positive items than IA positive items were classified as premeditated aggression (PM) subgroup. If the percentage of positive items was equal for both IA and PM, participants could not be classified into any subgroups. In the current study, 16 of 60 violent patients were included in the PM subgroup, while 44 of 60 violent patients were included in the IA subgroup.
2.2.3. Chinese interpersonal reactivity index
The Chinese interpersonal reactivity index (IRI-C) is a test that evaluates empathy and has strong reliability and validity. Numerous investigations with Chinese participants have found that the IRI-C results are highly consistent. The IRI-C comprises 22 items which are divided into four subscales, namely, perspective taking (PT), fantasy (FS), personal distress (PD) and empathy concern (EC). While PD and EC are concentrated on the emotional empathy dimension, PT and FS are focused on the cognitive empathy aspect (58). PT measures the capacity of an individual to adopt the viewpoints of other people. FS evaluates the ability to transpose oneself into the feelings and actions of fictional characters. PD scrutinizes aversive emotional reaction that are brought on when witnessing others having negative experiences. EC examines the capacity to sympathize with people who are unfortunate (59). The PT subscale score was calculated as the average of the values for items 6, 9, 15, 19, and 22. The EC subscale score was the total of the scores for items 4, 8, 13, 18, and 21. The score for the FS subscale was the sum of items 3, 5, 10, 12, 17, and 20. The PD subscale score was determined by combining the scores of items 1, 2, 7, 11, 14, and 16. The overall scale score was calculated by adding the scores of all 22 items.
2.3. Statistical analysis
All statistical analyses were conducted in R software. Quantitative data were expressed as mean ± standard deviation (SD) while categorical data were described as the number of cases (n) and percentage (%). Independent sample t-test and chi-square test were utilized for demographic, self-reported violence classification, violence severity and empathy data with Bonferroni corrected comparisons. Stepwise Logistic regression analysis was used to examine the independent factors influencing aggressive behavior. A value of p of <0.05 was considered as statistically significant.
3. Results
3.1. Demographic characteristics
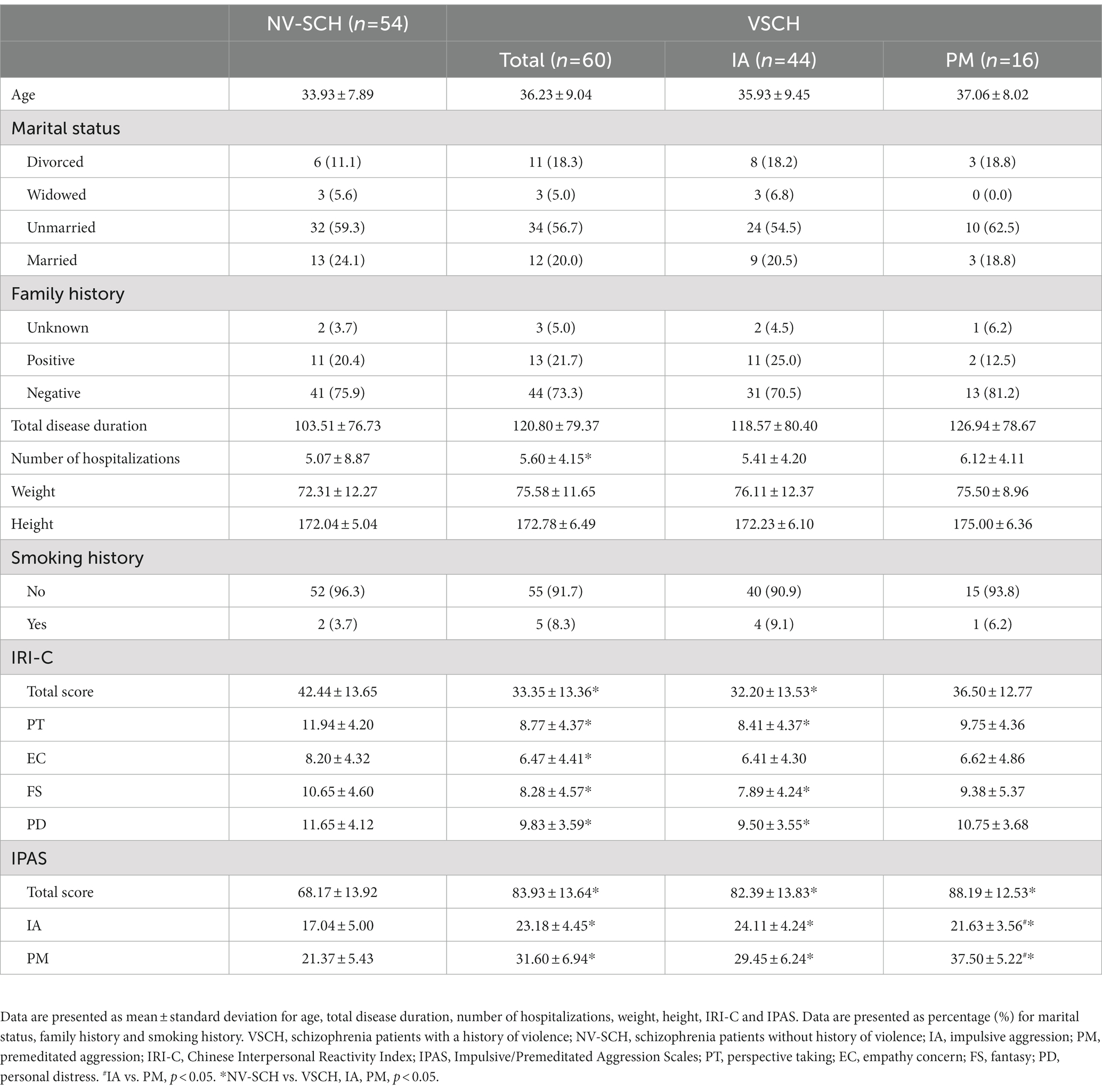
Table 1 shows the demographic data, including age, marital status, family history, total duration of illness, number of hospitalizations, weight, height and smoking history. The VSCH group had a notably higher inpatient frequency than the NV-SCH group (p = 0.035). Further demographic analysis between patients in the IA and PM subgroups of the VSCH group was also not statistically significantly different (p > 0.05).
3.2. Comparison of the impulsive-premeditated aggression scale and interpersonal reactivity index-C
IRI-C intergroup comparisons revealed that the VSCH group had significantly lower IRI-C total score as well as PT, EC, FS, and PD scores compared to the NV-SCH group (p < 0.05). ANOVA which was conducted between the IA subgroup, PM subgroup and the NV-SCH group showed that the total IRI-C score in addition to PT, FS, and PD scores were significantly lower in the IA subgroup in contrast to the NV-SCH group (p < 0.05). No statistical differences were found in the scale ratings between the PM subgroup and NV-SCH group (p > 0.05). However, the IRI-C analysis did not yield statistical significance between the IA and PM groups (see Table 1).
Intergroup comparison of the IPAS demonstrated statistically significant differences between the total IPAS score as well as PM and IA component in the VSCH and NV-SCH groups (p < 0.05). Further analysis of variance between the IA subgroup, PM subgroup and the NV-SCH group revealed that the total IPAS score, PM and IA subscale scores were significantly higher in the IA subgroup and PM subgroup than in the NV-SCH group (p < 0.05). Furthermore, the IA subgroup showed higher IA subscale scores and lower PM subscale scores compared to the PM subgroup (p < 0.05, see Table 1).
3.3. Correlation analyses
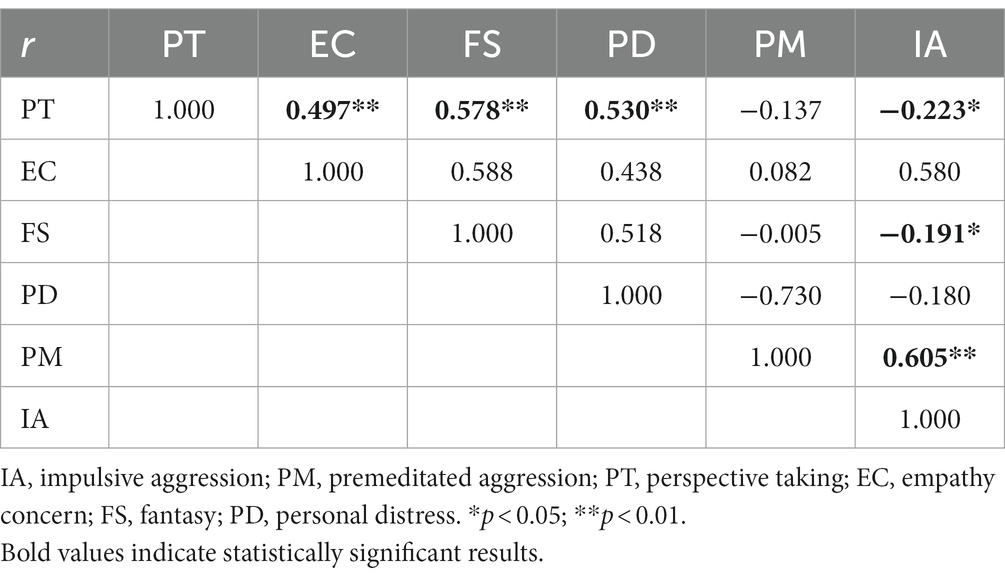
In all patients, correlation analysis between the factors of empathic ability on the IRI-C and aggression characteristics of the IPAS scale revealed that PT was positively correlated with EC, FS and PD (r = 0.497, r = 0.578, r = 0.530, p < 0.01) while PT and FS were negatively correlated with IA (r = −0.223, r = −0.191, p < 0.05). Conversely, PM has a positive correlation with IA (r = 0.605, p < 0.01, see Table 2).
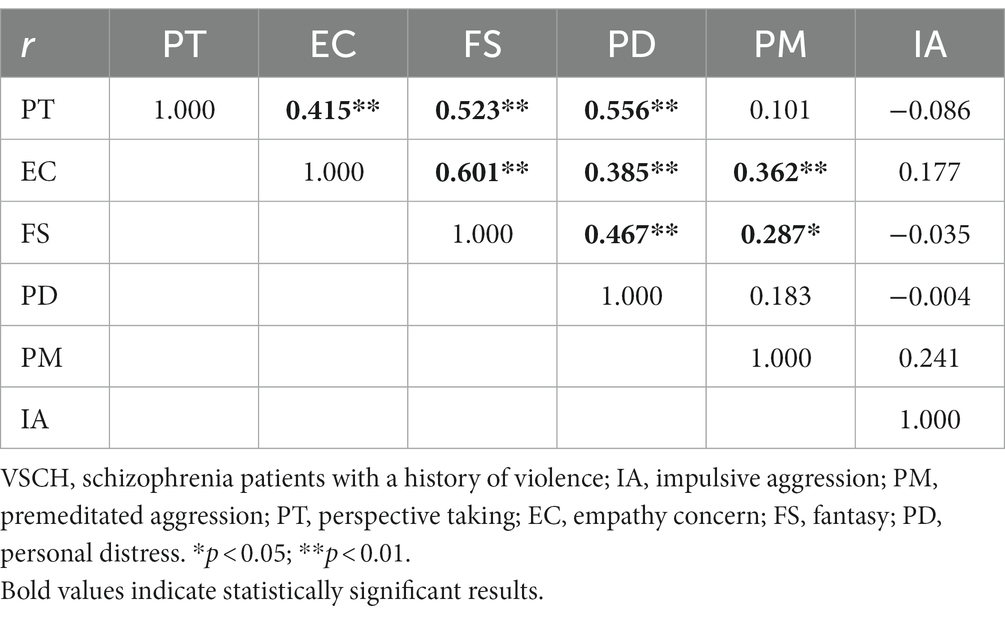
In the VSCH group, correlation analysis between the factors of empathy on the IRI-C and aggression features of the IPAS scale showed that PT was positively correlated with EC, FS, and PD (r = 0.415, r = 0.523, r = 0.556, p < 0.01), while EC and FS were positively correlated to PM (r = 0.362, p < 0.01, r = 0.287, p < 0.05). EC also showed positive correlations with FS and PD (r = 0.601, r = 0.385, p < 0.01), while FS was positively correlated with PD (r = 0.467, p < 0.01, see Table 3).
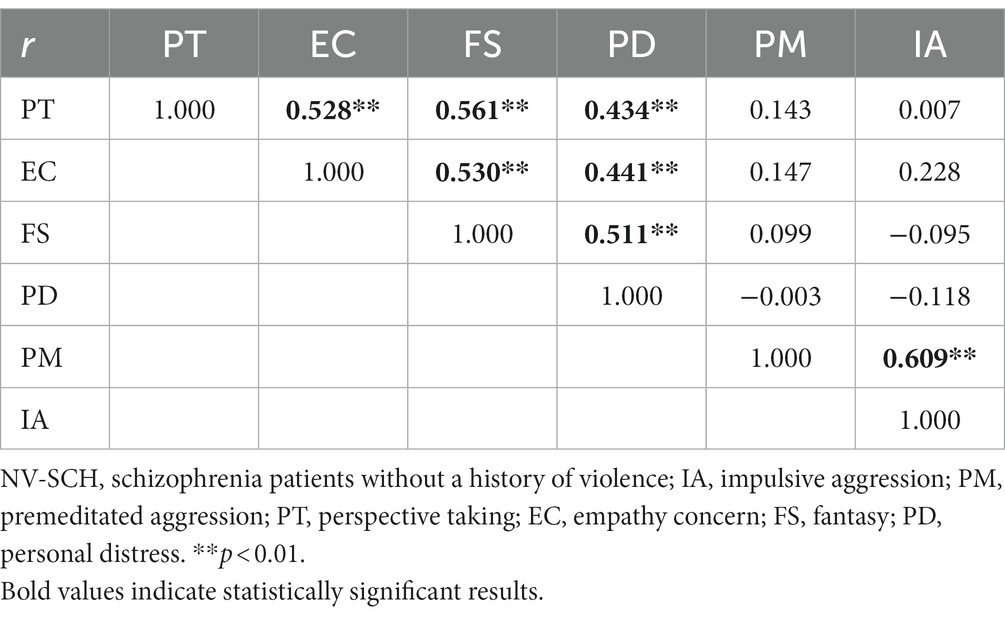
In the NV-SCH group, correlation analysis between the factors of empathy capacity of the IRI-C and aggression traits on the IPAS scale indicated that PT was positively correlated to EC, FS, and PD (r = 0.528, r = 0.561, r = 0.434, p < 0.01). EC also showed positive correlations with FS and PD, while FS was positively correlated with PD (r = 0.530, r = 0.441, r = 0.511, p < 0.01). Additionally, PM showed a positive correlation with IA (r = 0.609, p < 0.01, see Table 4).
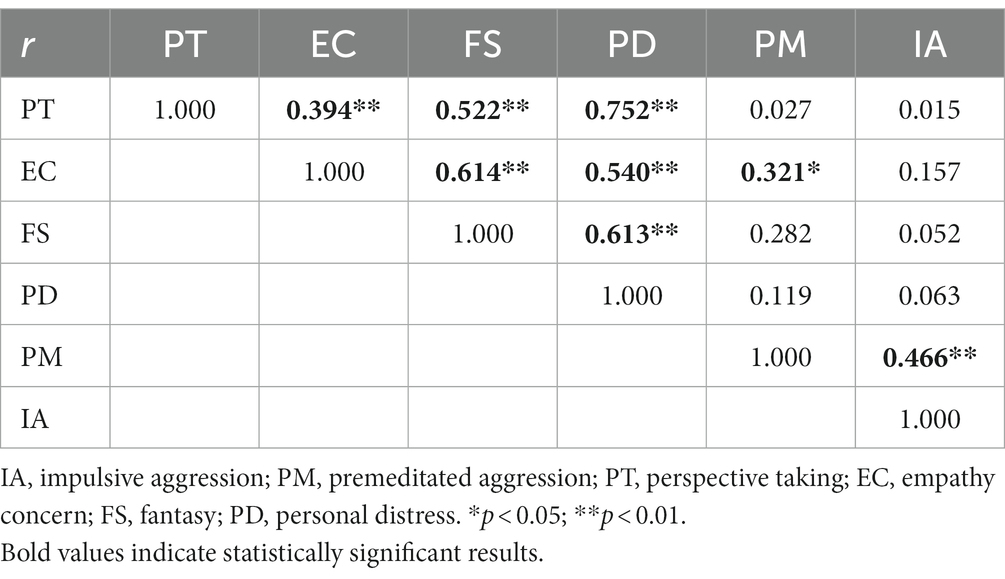
In the IA subgroup, correlation analysis between the factors of empathy components of the IRI-C and aggression elements of the IPAS scale demonstrated that PT was positively correlated to EC, FS and PD (r = 0.394, r = 0.522, r = 0.752, p < 0.01). EC also showed positive correlations with FS and PD (r = 0.614, r = 0.540, p < 0.01), while FS was positively correlated with PD (r = 0.613, p < 0.01). Additionally, EC and IA were positively correlated with PM (r = 0.321, p < 0.05, r = 0.466, p < 0.01, see Table 5).
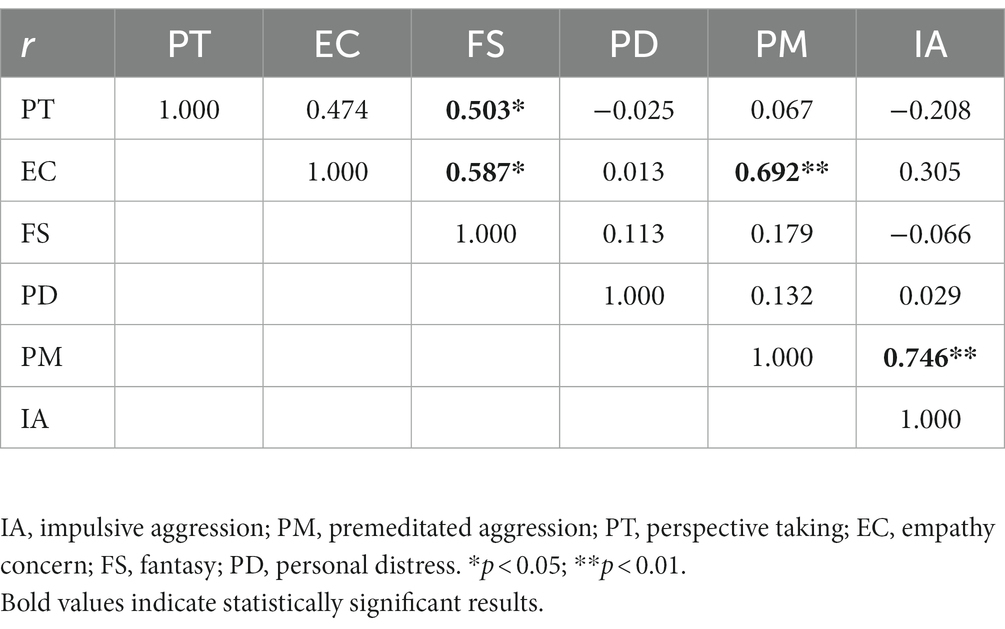
In the PM subgroup, correlation analysis between the factors of empathy aspects of the IRI-C and aggression characteristics on the IPAS scale disclosed that PT and EC were positively correlated to FS (r = 0.503, r = 0.587, p < 0.05) and EC and IA were positively correlated with PM (r = 0.692, r = 0.746, p < 0.01, see Table 6).
3.4. Logistic regression analysis
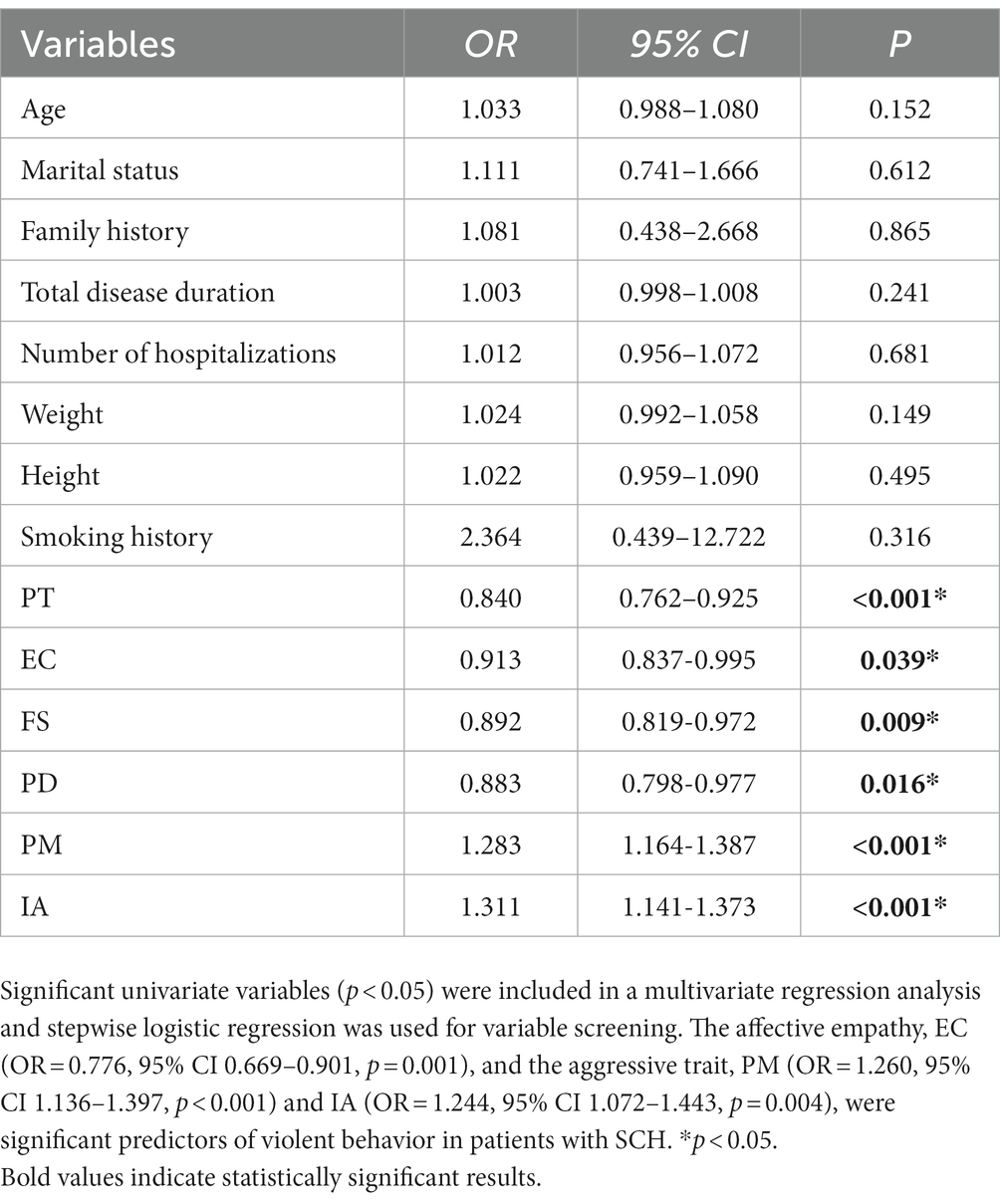
A one-way logistic regression analysis was performed on the demographic data and scale scores of all patients in order to analyze the factors influencing the occurrence of violent behavior in patients with SCH and we found that PT (OR = 0.840, 95% CI 0.762–0.925, p < 0.001), EC (OR = 0.913, 95% CI 0.837–0.995, p = 0.039), FS (OR = 0.892, 95% CI 0.819–0.972, p = 0.009), PD (OR = 0.883, 95% CI 0.798–0.977, p = 0.016), PM (OR = 1.283, 95% CI 1.164–1.387, p < 0.001), and IA (OR = 1.311, 95% CI 1.141–1.373, p < 0.001) were statistically significant (see Table 7). The factors with statistical significance in univariate logistic regression analysis were included in multivariate logistic regression analysis. The analysis revealed that the affective empathy, EC (OR = 0.776, 95% CI 0.669–0.901, p = 0.001), and the aggressive trait, PM (OR = 1.260, 95% CI 1.136–1.397, p < 0.001) and IA (OR = 1.244, 95% CI 1.072–1.443, p = 0.004), were significant predictors of violent behavior in patients with SCH.
4. Discussion
This study examined the empathy deficit in male violent schizophrenic patients and its correlation with impulsive and premeditated violence. Empathy abilities were significantly impaired in VSCH patients compared to NV-SCH patients, but no significant difference was observed in IA and PM subgroups. The results of the correlation analysis examining the relationship between empathy, impulsivity, and premeditated violence indicate a positive correlation between affective empathy and premeditated violence among VSCH, IA, and PM patients. Specifically, the analysis reveals that EC in affective empathy is positively associated with premeditated violence. Regression analysis showed that EC, PM and IA significantly predicted violent behaviors in male patients with schizophrenia. The results align with earlier theories that patients with VSCH exhibit broader empathy impairment in contrast to those with NV-SCH. Additionally, it suggests that premeditated violence is linked with affective empathy.
4.1. Empathy deficits and violent behavior in schizophrenia
In the present study, there were significant differences in empathy among VSCH patients compared to NV-SCH patients. In line with prior studies (44, 60–62), the VSCH patients in the present paper had considerably more impaired empathy, particularly affective empathy and cognitive empathy. Research indicates that violent patients face challenges with empathic reasoning. Additionally, exploratory regression analyses suggest that violent behavior correlates with impaired empathy (43). It was previously shown that the ability to identify others’ emotions and facial expressions were significantly impaired in SCH patients (63, 64), which is thought to play a significant role in social dysfunction. In a study involving patients with chronic SCH, PD in the IRI was identified as a risk factor of suicide which is a serious and violent act against oneself (65). These results suggest that empathy deficits are core characteristics of SCH patients with violent behaviors. Empathy is crucial in comprehending the emotions and sentiments of others. However, impaired empathy can result in a prejudiced view of others’ motives and the belief that they are concealing their true intentions. This can then trigger a sense of victimization and promote aggressive conduct in patients.
In the investigation of the neural basis of empathy, a meta-analysis reported that independent of specific task or stimulus type, there was a sustained activation of the dorsal anterior cingulate cortex-anterior midcingulate cortex-supplementary motor area and bilateral insula, forming a core empathy network (66). One study found that structural alterations and disturbed resting-state functional connectivity in the core empathy network might serve as the neural foundation of social cognitive deficits in individuals with early-onset SCH (67). Another research demonstrated that empathy deficits were associated with lower activation of the amygdala (68). The alterations in these brain regions might underlie the mechanisms for empathy deficits in VSCH patients.
4.2. Empathy deficits and IPAS in VSCH
The study discovered that VSCH patients with the IA subgroup experience more severe empathy deficits. Comparatively, the IA subgroup showed lower scores in the PT, FS, and PD dimensions of the IRI-C scale than other NV-SCH patients. Previous studies suggest that impulsive aggression is correlated with high levels of guilt, hostility, neuroticism, and trait anger (56, 69, 70), but few studies have delved into the empathy of IA subgroups. The frustration-aggression hypothesis (71) suggests that negative emotional states stemming from frustration or social pressure could lead to anger and an increase in impulsive aggression (72). In addition, a lack of empathic ability may exacerbate a patient’s anger and contribute to impulsive aggression.
4.3. Correlations between IRI-C factors and IA of IPAS in all SCH patients
This study reveals a correlation between higher IA scores and lower cognitive empathy scores (PT and FS) in all SCH patients. Cognitive empathy, the ability to comprehend the emotions of others (73, 74), plays a crucial role in social cognition and significantly impacts the social function of patients (75). Earlier research suggests that cognitive empathy can explain changes in social functioning, such as community functioning, in SCH patients (76). This indicates that patients with SCH who exhibit less impairment of cognitive and emotional empathy tend to understand and care for others while showing less impulsive and aggressive behaviors. However, ignoring others’ emotions can lead to impulsive aggression. The study also found a significant association between PT and FS, PD, and EC in all SCH patients, indicating consistent cognitive and affective empathy deficits.
4.4. Correlations between IRI-C factors and PM of IPAS in VSCH
This study yielded results regarding the correlation analysis conducted on IRI-C and IPAS scales in VSCH patients. Specifically, the EC dimension in affective empathy showed a positive correlation with PM scores in a sample population of VSCH patients, as well as IA and PM patients. Prior researches on schizophrenia patients have demonstrated impaired emotional empathy (40, 77). Moreover, literatures have shown a relationship between premeditated aggression and psychopathic personality traits, with premeditated aggression being more associated with the latter than impulsive aggression (78, 79). Individuals demonstrating lack of empathy, remorse, and guilt, alongside manipulative, callous, and grandiose behaviors (80, 81) may exhibit premeditative aggressive behavior. Experiencing affective empathy deficits can result in patients being unable to empathetically respond to the situations and experiences of others, which can increase the likelihood of engaging in premeditated aggression.
4.5. Independent risk factors for violence in SCH
Our study indicates that premeditated aggression scores are linked with aggressive behavior in SCH patients, establishing premeditated aggression trait in these patients as a significant predictor for violent aggression. Premeditated aggression is a learned behavior that is reinforced by receiving rewards. Extensive research evidences suggest that premeditated violence is associated with psychopathic traits (82–84). Antisocial personality disorder (ASPD) is a psychological condition often distinguished by the presence of psychopathy attributes. ASPD is commonly identified in schizophrenic patients with a previous history of violence (85). It is imperative to conduct more research to determine whether the predictive value of premeditated aggression persists after considering the effects of psychopathy on violent behavior among schizophrenia patients. This will aid in identifying suitable measures for early detection, prevention goals and approaches, and reducing the stigma accompanying this disorder.
In addition, patients in the SCH group with higher IA scores were more likely to commit violent acts. This type of aggression is largely linked to negative emotions and psychiatric symptoms, and has been shown to contribute to higher levels of guilt, hostility, and trait anger (79, 86). Negative emotional states resulting from stress and stimulation can lead to anger, which in turn increases the likelihood of impulsive aggression. This behavior is strongly associated with increased volume of the left putamen and decreased volume of the right middle temporal gyrus, superior temporal gyrus, and insula, structures involved in processing environmental stimuli and impacting aggression threshold (87–89). Given these findings, it is important to prioritize attention to male SCH patients displaying impulsive aggression, as their violent behavior is more easily triggered and poses a risk to individuals and property.
Male schizophrenia patients with impaired EC were found to be at higher risk of displaying aggressive behavior. EC refers to empathy for less fortunate others and is associated with improved emotion recognition (90). Empathy interventions have been shown to improve empathy in offenders, with those with more empathy impairment having higher rates of crime (91). These findings suggest the importance of early intervention strategies to improve cognitive empathy and prevent violent incidents.
4.6. Limitations
This study has several limitations that should be acknowledged. The small sample size of the current study makes the results potentially less reliable. The lack of differences in demographic and clinical characteristics in the present study is inconsistent with the results of previous investigations and might be attributed to the small sample size. In the future, the sample size should be further expanded to reduce errors. In this study, there were no healthy controls and thus, we cannot provide further evidence that SCH patients had a deficit in empathy. In addition, the study was designed as cross-sectional research, which makes it impossible to confirm whether there is a direct causal relationship between violent behavior and empathy in SCH patients. Moreover, although IRI-C is widely used to assess empathy, it is a self-report measurement. Multidimensional assessment methods are needed for future studies. Furthermore, we did not take female patients, medication status and education level into account and these factors might affect the findings of this study.
5. Conclusion
In summary, the VSCH patients had a more extensive empathy deficits compared to NV-SCH patients. EC, IA and PM were independent influences of violent behavior in male SCH patients. Further analysis revealed that deficits in the empathic capacity in male SCH patients were closely and positively correlated with PM characteristics, while they were not significantly correlated with IA aggression characteristics. These findings could be used to predict the occurrence of premeditated aggression in male SCH patients via empathy assessments. In addition, targeted interventions for empathic competence would be beneficial in reducing the incidence of premeditated aggression in SCH patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Xuzhou Oriental Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MG, LY, XG, ZL, CZ, YY, NA, CW, and XZ contributed equally to each stage of the study design, data collection, analysis, and write-up. All authors contributed to the article and approved the submitted version.
Funding
This project was funded by National Natural Science Foundation of China (General Program) (81971255), Jiangsu Provincial Department of Science and Technology Social Development Project (BE22019610), Jiangsu Province Frontier Leading Technology Basic Research Project “Robot Emotion Recognition and Interaction Technology Foundation” (BK20192004D), Key Project of Medical Talents in Jiangsu Province (ZDRCA2016075), and Xuzhou Science and Technology Project (KC21181).
Conflict of interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McGrath, J, Saha, S, Chant, D, and Welham, J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol Rev. (2008) 30:67–76. doi: 10.1093/epirev/mxn001
2. Jauhar, S, Johnstone, M, and McKenna, PJ. Schizophrenia. Lancet. (2022) 399:473–86. doi: 10.1016/S0140-6736(21)01730-X
3. Kendler, KS. Phenomenology of schizophrenia and the representativeness of modern diagnostic criteria. JAMA Psychiat. (2016) 73:1082–92. doi: 10.1001/jamapsychiatry.2016.1976
4. Velligan, DI, and Rao, S. The epidemiology and global burden of schizophrenia. J Clin Psychiatry. (2023) 84:MS21078COM5. doi: 10.4088/JCP.MS21078COM5
5. Jin, H, and Mosweu, I. The societal cost of schizophrenia: a systematic review. PharmacoEconomics. (2017) 35:25–42. doi: 10.1007/s40273-016-0444-6
6. Chang, Z, Larsson, H, Lichtenstein, P, and Fazel, S. Psychiatric disorders and violent reoffending: a national cohort study of convicted prisoners in Sweden. Lancet Psychiatry. (2015) 2:891–900. doi: 10.1016/S2215-0366(15)00234-5
7. Fazel, S, Gulati, G, Linsell, L, Geddes, JR, and Grann, M. Schizophrenia and violence: systematic review and meta-analysis. PLoS Med. (2009) 6:e1000120. doi: 10.1371/journal.pmed.1000120
8. Sariaslan, A, Arseneault, L, Larsson, H, Lichtenstein, P, and Fazel, S. Risk of subjection to violence and perpetration of violence in persons with psychiatric disorders in Sweden. JAMA Psychiat. (2020) 77:359–67. doi: 10.1001/jamapsychiatry.2019.4275
9. Varshney, M, Mahapatra, A, Krishnan, V, Gupta, R, and Deb, KS. Violence and mental illness: what is the true story? J Epidemiol Community Health. (2016) 70:223–5. doi: 10.1136/jech-2015-205546
10. O’Reilly, K, Donohoe, G, Coyle, C, O’Sullivan, D, Rowe, A, Losty, M, et al. Prospective cohort study of the relationship between neuro-cognition, social cognition and violence in forensic patients with schizophrenia and schizoaffective disorder. BMC Psychiatry. (2015) 15:155. doi: 10.1186/s12888-015-0548-0
11. Markiewicz, I, Pilszyk, A, and Kudlak, G. Psychological factors of aggressive behaviour in patients of forensic psychiatry wards with the diagnosis of schizophrenia. Int J Law Psychiatry. (2020) 72:101612. doi: 10.1016/j.ijlp.2020.101612
12. Buizza, C, Strozza, C, Sbravati, G, de Girolamo, G, Ferrari, C, Iozzino, L, et al. Positive and negative syndrome scale in forensic patients with schizophrenia spectrum disorders: a systematic review and meta-analysis. Ann General Psychiatry. (2022) 21:36. doi: 10.1186/s12991-022-00413-2
13. Perlini, C, Bellani, M, Besteher, B, Nenadić, I, and Brambilla, P. The neural basis of hostility-related dimensions in schizophrenia. Epidemiol Psychiatr Sci. (2018) 27:546–51. doi: 10.1017/S2045796018000525
14. Van Dorn, RA, Grimm, KJ, Desmarais, SL, Tueller, SJ, Johnson, KL, and Swartz, MS. Leading indicators of community-based violent events among adults with mental illness. Psychol Med. (2017) 47:1179–91. doi: 10.1017/S0033291716003160
15. Ullrich, S, Keers, R, and Coid, JW. Delusions, anger, and serious violence: new findings from the MacArthur violence risk assessment study. Schizophr Bull. (2014) 40:1174–81. doi: 10.1093/schbul/sbt126
16. Fovet, T, Pignon, B, Wathelet, M, Benradia, I, Roelandt, JL, Jardri, R, et al. Admission to jail and psychotic symptoms: a study of the psychotic continuum in a sample of recently incarcerated men. Soc Psychiatry Psychiatr Epidemiol. (2023) 58:25–34. doi: 10.1007/s00127-022-02339-2
17. Li, Q, Zhong, S, Zhou, J, and Wang, X. Delusion, excitement, violence, and suicide history are risk factors for aggressive behavior in general inpatients with serious mental illnesses: a multicenter study in China. Psychiatry Res. (2019) 272:130–4. doi: 10.1016/j.psychres.2018.12.071
18. Weltens, I, Bak, M, Verhagen, S, Vandenberk, E, Domen, P, van Amelsvoort, T, et al. Aggression on the psychiatric ward: prevalence and risk factors. A systematic review of the literature. PLoS One. (2021) 16:e0258346. doi: 10.1371/journal.pone.0258346
19. Zhou, JS, Zhong, BL, Xiang, YT, Chen, Q, Cao, XL, Correll, CU, et al. Prevalence of aggression in hospitalized patients with schizophrenia in China: a meta-analysis. Asia Pac Psychiatry. (2016) 8:60–9. doi: 10.1111/appy.12209
20. Yukhnenko, D, Blackwood, N, Lichtenstein, P, and Fazel, S. Psychiatric disorders and reoffending risk in individuals with community sentences in Sweden: a national cohort study. Lancet Public Health. (2023) 8:e119–29. doi: 10.1016/S2468-2667(22)00312-7
21. Torrey, EF. Stigma and violence: isn't it time to connect the dots? Schizophr Bull. (2011) 37:892–6. doi: 10.1093/schbul/sbr057
22. Pescosolido, BA, Halpern-Manners, A, Luo, L, and Perry, B. Trends in public stigma of mental illness in the US, 1996-2018. JAMA Netw Open. (2021) 4:e2140202. doi: 10.1001/jamanetworkopen.2021.40202
23. Anderson, CA, and Bushman, BJ. Human aggression. Annu Rev Psychol. (2002) 53:27–51. doi: 10.1146/annurev.psych.53.100901.135231
24. Wrangham, RW. Two types of aggression in human evolution. Proc Natl Acad Sci U S A. (2018) 115:245–53. doi: 10.1073/pnas.1713611115
25. Blair, RJ. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. (2004) 55:198–208. doi: 10.1016/S0278-2626(03)00276-8
26. Allen, JJ, Anderson, CA, and Bushman, BJ. The general aggression model. Curr Opin Psychol. (2018) 19:75–80. doi: 10.1016/j.copsyc.2017.03.034
27. Barratt, ES, and Felthous, AR. Impulsive versus premeditated aggression: implications for mens Rea decisions. Behav Sci Law. (2003) 21:619–30. doi: 10.1002/bsl.555
28. Dambacher, F, Schuhmann, T, Lobbestael, J, Arntz, A, Brugman, S, and Sack, AT. Reducing proactive aggression through non-invasive brain stimulation. Soc Cogn Affect Neurosci. (2015) 10:1303–9. doi: 10.1093/scan/nsv018
29. Hubbard, JA, McAuliffe, MD, Morrow, MT, and Romano, LJ. Reactive and proactive aggression in childhood and adolescence: precursors, outcomes, processes, experiences, and measurement. J Pers. (2010) 78:95–118. doi: 10.1111/j.1467-6494.2009.00610.x
30. Nelson, RJ, and Trainor, BC. Neural mechanisms of aggression. Nat Rev Neurosci. (2007) 8:536–46. doi: 10.1038/nrn2174
31. Rosell, DR, and Siever, LJ. The neurobiology of aggression and violence. CNS Spectr. (2015) 20:254–79. doi: 10.1017/S109285291500019X
32. Siever, LJ. Neurobiology of aggression and violence. Am J Psychiatry. (2008) 165:429–42. doi: 10.1176/appi.ajp.2008.07111774
33. Soyka, M. Neurobiology of aggression and violence in schizophrenia. Schizophr Bull. (2011) 37:913–20. doi: 10.1093/schbul/sbr103
34. Mercer, SW, and Reynolds, WJ. Empathy and quality of care. The British journal of general practice. J R Coll Gen Pract. (2002) 52:S9–S12.
35. Hojat, M, Vergare, MJ, Maxwell, K, Brainard, G, Herrine, SK, Isenberg, GA, et al. The devil is in the third year: a longitudinal study of erosion of empathy in medical school. Acad Med. (2009) 84:1182–91. doi: 10.1097/ACM.0b013e3181b17e55
36. Bora, E. Differences in cognitive impairment between schizophrenia and bipolar disorder: considering the role of heterogeneity. Psychiatry Clin Neurosci. (2016) 70:424–33. doi: 10.1111/pcn.12410
37. Sheffield, JM, Karcher, NR, and Barch, DM. Cognitive deficits in psychotic disorders: a lifespan perspective. Neuropsychol Rev. (2018) 28:509–33. doi: 10.1007/s11065-018-9388-2
38. Kuis, DJ, van de Giessen, T, de Jong, S, Sportel, BE, Boonstra, N, van Donkersgoed, R, et al. Empathy and its relationship with social functioning in individuals at ultra-high risk for psychosis. Front Psych. (2021) 12:730092. doi: 10.3389/fpsyt.2021.730092
39. Horan, WP, Reise, SP, Kern, RS, Lee, J, Penn, DL, and Green, MF. Structure and correlates of self-reported empathy in schizophrenia. J Psychiatr Res. (2015) 66-67:60–6. doi: 10.1016/j.jpsychires.2015.04.016
40. Bonfils, KA, Lysaker, PH, Minor, KS, and Salyers, MP. Affective empathy in schizophrenia: a meta-analysis. Schizophr Res. (2016) 175:109–17. doi: 10.1016/j.schres.2016.03.037
41. Koevoets, M, Prikken, M, Hagenaar, DA, Kahn, RS, and van Haren, NEM. The association between emotion recognition, affective empathy, and structural connectivity in schizophrenia patients. Front Psych. (2022) 13:910985. doi: 10.3389/fpsyt.2022.910985
42. Darmedru, C, Demily, C, and Franck, N. Cognitive remediation and social cognitive training for violence in schizophrenia: a systematic review. Psychiatry Res. (2017) 251:266–74. doi: 10.1016/j.psychres.2016.12.062
43. Abu-Akel, A, and Abushua'leh, K. 'Theory of mind' in violent and nonviolent patients with paranoid schizophrenia. Schizophr Res. (2004) 69:45–53. doi: 10.1016/S0920-9964(03)00049-5
44. Kristof, Z, Kresznerits, S, Olah, M, Gyollai, A, Lukacs-Miszler, K, Halmai, T, et al. Mentalization and empathy as predictors of violence in schizophrenic patients: comparison with nonviolent schizophrenic patients, violent controls and nonviolent controls. Psychiatry Res. (2018) 268:198–205. doi: 10.1016/j.psychres.2018.07.021
45. Lysaker, PH, Cheli, S, Dimaggio, G, Buck, B, Bonfils, KA, Huling, K, et al. Metacognition, social cognition, and mentalizing in psychosis: are these distinct constructs when it comes to subjective experience or are we just splitting hairs? BMC Psychiatry. (2021) 21:329. doi: 10.1186/s12888-021-03338-4
46. World Medical A. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. doi: 10.1001/jama.2013.281053
47. He, JF, Hong, W, Shao, Y, Han, HQ, and Xie, B. Application of MOAS for evaluating of violence risk in the inpatients with mental disorders. Fa Yi Xue Za Zhi. (2017) 33:28–31. doi: 10.3969/j.issn.1004-5619.2017.01.007
48. Zhang, XL, and Hu, JM. Application of modified overt aggression scales on risk behavior assessment of patients with mental illness. Fa Yi Xue Za Zhi. (2011) 27:342–5.
49. Yudofsky, SC, Silver, JM, Jackson, W, Endicott, J, and Williams, D. The overt aggression scale for the objective rating of verbal and physical aggression. Am J Psychiatry. (1986) 143:35–9. doi: 10.1176/ajp.143.1.35
50. Silver, JM, and Yudofsky, SC. The overt aggression scale: overview and guiding principles. J Neuropsychiatry Clin Neurosci. (1991) 3:S22–9.
51. Li, W, Yang, Y, Hong, L, An, FR, Ungvari, GS, Ng, CH, et al. Prevalence of aggression in patients with schizophrenia: a systematic review and meta-analysis of observational studies. Asian J Psychiatr. (2020) 47:101846. doi: 10.1016/j.ajp.2019.101846
52. Kay, SR, Wolkenfeld, F, and Murrill, LM. Profiles of aggression among psychiatric patients. I Nature and prevalence. J Nerv Ment Dis. (1988) 176:539–46. doi: 10.1097/00005053-198809000-00007
53. De Benedictis, L, Dumais, A, Stafford, MC, Côté, G, and Lesage, A. Factor analysis of the French version of the shorter 12-item perception of aggression scale (POAS) and of a new modified version of the overt aggression scale (MOAS). J Psychiatr Ment Health Nurs. (2012) 19:875–80. doi: 10.1111/j.1365-2850.2011.01870.x
54. Stanford, MS, Houston, RJ, Mathias, CW, Villemarette-Pittman, NR, Helfritz, LE, and Conklin, SM. Characterizing aggressive behavior. Assessment. (2003) 10:183–90. doi: 10.1177/1073191103010002009
55. Barratt, ES, Stanford, MS, Dowdy, L, Liebman, MJ, and Kent, TA. Impulsive and premeditated aggression: a factor analysis of self-reported acts. Psychiatry Res. (1999) 86:163–73. doi: 10.1016/S0165-1781(99)00024-4
56. Swogger, MT, Walsh, Z, Christie, M, Priddy, BM, and Conner, KR. Impulsive versus premeditated aggression in the prediction of violent criminal recidivism. Aggress Behav. (2015) 41:346–52. doi: 10.1002/ab.21549
57. Yi, Q, Bin, X, and Mingdao, Z. To test the reliability and validity of the Chinese version of the impulse-premeditated aggressive behavior scale. Chin J Behav Med Brain Sci. (2009) 18:366–8.
58. Chrysikou, EG, and Thompson, WJ. Assessing cognitive and affective empathy through the interpersonal reactivity index: An argument against a two-factor model. Assessment. (2016) 23:769–77. doi: 10.1177/1073191115599055
59. Bird, G, and Viding, E. The self to other model of empathy: providing a new framework for understanding empathy impairments in psychopathy, autism, and alexithymia. Neurosci Biobehav Rev. (2014) 47:520–32. doi: 10.1016/j.neubiorev.2014.09.021
60. Corbera, S, Wexler, BE, Bell, MD, Pearlson, G, Mayer, S, Pittman, B, et al. Predictors of social functioning and quality of life in schizophrenia and autism spectrum disorder. Psychiatry Res. (2021) 303:114087. doi: 10.1016/j.psychres.2021.114087
61. Smith, MJ, Horan, WP, Karpouzian, TM, Abram, SV, Cobia, DJ, and Csernansky, JG. Self-reported empathy deficits are uniquely associated with poor functioning in schizophrenia. Schizophr Res. (2012) 137:196–202. doi: 10.1016/j.schres.2012.01.012
62. Michaels, TM, Horan, WP, Ginger, EJ, Martinovich, Z, Pinkham, AE, and Smith, MJ. Cognitive empathy contributes to poor social functioning in schizophrenia: evidence from a new self-report measure of cognitive and affective empathy. Psychiatry Res. (2014) 220:803–10. doi: 10.1016/j.psychres.2014.08.054
63. Silver, H, Goodman, C, Knoll, G, Isakov, V, and Modai, I. Schizophrenia patients with a history of severe violence differ from nonviolent schizophrenia patients in perception of emotions but not cognitive function. J Clin Psychiatry. (2005) 66:300–8. doi: 10.4088/JCP.v66n0305
64. Savla, GN, Vella, L, Armstrong, CC, Penn, DL, and Twamley, EW. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull. (2013) 39:979–92. doi: 10.1093/schbul/sbs080
65. Wang, W, Zhou, Y, Wang, J, Xu, H, Wei, S, Wang, D, et al. Prevalence, clinical correlates of suicide attempt and its relationship with empathy in patients with schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. (2020) 99:109863. doi: 10.1016/j.pnpbp.2020.109863
66. Fan, Y, Duncan, NW, de Greck, M, and Northoff, G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev. (2011) 35:903–11. doi: 10.1016/j.neubiorev.2010.10.009
67. Shi, LJ, Zhou, HY, Wang, Y, Shen, YM, Fang, YM, He, YQ, et al. Altered empathy-related resting-state functional connectivity in adolescents with early-onset schizophrenia and autism spectrum disorders. Asian J Psychiatr. (2020) 53:102167. doi: 10.1016/j.ajp.2020.102167
68. Derntl, B, Finkelmeyer, A, Voss, B, Eickhoff, SB, Kellermann, T, Schneider, F, et al. Neural correlates of the core facets of empathy in schizophrenia. Schizophr Res. (2012) 136:70–81. doi: 10.1016/j.schres.2011.12.018
69. Chase, KA, O'Leary, KD, and Heyman, RE. Categorizing partner-violent men within the reactive-proactive typology model. J Consult Clin Psychol. (2001) 69:567–72. doi: 10.1037/0022-006X.69.3.567
70. Gauthier, KJ, Furr, RM, Mathias, CW, Marsh-Richard, DM, and Dougherty, DM. Differentiating impulsive and premeditated aggression: self and informant perspectives among adolescents with personality pathology. J Personal Disord. (2009) 23:76–84. doi: 10.1521/pedi.2009.23.1.76
71. Berkowitz, L. Frustration-aggression hypothesis: examination and reformulation. Psychol Bull. (1989) 106:59–73. doi: 10.1037/0033-2909.106.1.59
72. Gadea, M, Herrero, N, Picó, A, Espert, R, Salvador, A, and Sanjuán, J. Psychobiological response to an anger induction task in schizophrenia: the key role of anxiety. Psychiatry Res. (2019) 271:541–7. doi: 10.1016/j.psychres.2018.12.044
73. Sparks, A, McDonald, S, Lino, B, O'Donnell, M, and Green, MJ. Social cognition, empathy and functional outcome in schizophrenia. Schizophr Res. (2010) 122:172–8. doi: 10.1016/j.schres.2010.06.011
74. Bora, E, Gökçen, S, and Veznedaroglu, B. Empathic abilities in people with schizophrenia. Psychiatry Res. (2008) 160:23–9. doi: 10.1016/j.psychres.2007.05.017
75. Kee, KS, Green, MF, Mintz, J, and Brekke, JS. Is emotion processing a predictor of functional outcome in schizophrenia? Schizophr Bull. (2003) 29:487–97. doi: 10.1093/oxfordjournals.schbul.a007021
76. Smith, MJ, Horan, WP, Cobia, DJ, Karpouzian, TM, Fox, JM, Reilly, JL, et al. Performance-based empathy mediates the influence of working memory on social competence in schizophrenia. Schizophr Bull. (2014) 40:824–34. doi: 10.1093/schbul/sbt084
77. Achim, AM, Ouellet, R, Roy, MA, and Jackson, PL. Assessment of empathy in first-episode psychosis and meta-analytic comparison with previous studies in schizophrenia. Psychiatry Res. (2011) 190:3–8. doi: 10.1016/j.psychres.2010.10.030
78. Chang, SA, Tillem, S, Benson-Williams, C, and Baskin-Sommers, A. Cognitive empathy in subtypes of antisocial individuals. Front Psych. (2021) 12:677975. doi: 10.3389/fpsyt.2021.677975
79. Azevedo, J, Vieira-Coelho, M, Castelo-Branco, M, Coelho, R, and Figueiredo-Braga, M. Impulsive and premeditated aggression in male offenders with antisocial personality disorder. PLoS One. (2020) 15:e0229876. doi: 10.1371/journal.pone.0229876
80. Hare, RD, and Neumann, CS. Psychopathy: assessment and forensic implications. Can J Psychiatr. (2009) 54:791–802. doi: 10.1177/070674370905401202
81. Hare, RD. Psychopathy: a clinical and forensic overview. Psychiatr Clin North Am. (2006) 29:709–24. doi: 10.1016/j.psc.2006.04.007
82. Bo, S, Sharp, C, Lind, M, Simonsen, S, and Bateman, A. Mentalizing mediates the relationship between psychopathy and premeditated criminal offending in schizophrenia: a 6-year follow-up study. Nord J Psychiatry. (2023) 1-13:1–13. doi: 10.1080/08039488.2023.2186483
83. Bo, S, Abu-Akel, A, Kongerslev, M, and Simonsen, E. Predictors of criminal offending in a clinical sample of patients diagnosed with schizophrenia: a 6-year follow-up study. Pers Disord. (2021) 12:216–27. doi: 10.1037/per0000401
84. Balcioglu, YH, Kirlioglu Balcioglu, SS, Oncu, F, and Turkcan, A. Psychopathy, temperament, and character dimensions of personality as risk determinants of criminal recidivism in schizophrenia patients. J Forensic Sci. (2021) 66:2340–53. doi: 10.1111/1556-4029.14834
85. Hachtel, H, Harries, C, Luebbers, S, and Ogloff, JR. Violent offending in schizophrenia spectrum disorders preceding and following diagnosis. Aust N Z J Psychiatry. (2018) 52:782–92. doi: 10.1177/0004867418763103
86. Peters, JR, Derefinko, KJ, and Lynam, DR. Negative urgency accounts for the association between borderline personality features and intimate partner violence in young men. J Personal Disord. (2017) 31:16–25. doi: 10.1521/pedi_2016_30_234
87. Hofhansel, L, Weidler, C, Votinov, M, Clemens, B, Raine, A, and Habel, U. Morphology of the criminal brain: gray matter reductions are linked to antisocial behavior in offenders. Brain Struct Funct. (2020) 225:2017–28. doi: 10.1007/s00429-020-02106-6
88. Naaijen, J, Mulder, LM, Ilbegi, S, de Bruijn, S, Kleine-Deters, R, Dietrich, A, et al. Specific cortical and subcortical alterations for reactive and proactive aggression in children and adolescents with disruptive behavior. NeuroImage Clin. (2020) 27:102344. doi: 10.1016/j.nicl.2020.102344
89. Yang, Y, Joshi, SH, Jahanshad, N, Thompson, PM, and Baker, LA. Neural correlates of proactive and reactive aggression in adolescent twins. Aggress Behav. (2017) 43:230–40. doi: 10.1002/ab.21683
Keywords: schizophrenic patients, neuropsychology, violence, aggressiveness, empathy
Citation: Gong M, Yao L, Ge X, Liu Z, Zhang C, Yang Y, Amdanee N, Wang C and Zhang X (2023) Empathy deficit in male patients with schizophrenia and its relationships with impulsivity and premeditated violence. Front. Psychiatry. 14:1160357. doi: 10.3389/fpsyt.2023.1160357
Edited by:
Panteleimon Giannakopoulos, University of Geneva, SwitzerlandReviewed by:
Matthew J. Hoptman, Nathan Kline Institute for Psychiatric Research, United StatesXiang Dong Du, Suzhou Psychiatric Hospital, China
Copyright © 2023 Gong, Yao, Ge, Liu, Zhang, Yang, Amdanee, Wang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengdong Wang, NTY0NDU0MzQ2QHFxLmNvbQ==; Xiangrong Zhang, ZHJ4cnpAaG90bWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Muxin Gong
Muxin Gong Lei Yao1†
Lei Yao1† Xiangrong Zhang
Xiangrong Zhang