
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry, 29 March 2023
Sec. Sleep Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1160001
This article is part of the Research TopicNew Insights into Sleep Abnormalities Associated with Alcohol, Cannabis, Cocaine, and Opiate useView all 6 articles
 Melissa E. Milanak1†
Melissa E. Milanak1† Sara M. Witcraft1†
Sara M. Witcraft1† Jie Young Park2
Jie Young Park2 Katharine Hassell3
Katharine Hassell3 Tierney McMahon4
Tierney McMahon4 Allison K. Wilkerson1*
Allison K. Wilkerson1*Introduction: Treatment of substance use disorders (SUDs) is challenging with high rates of treatment dropout and relapse, particularly among individuals with comorbid psychiatric conditions. Anxiety and insomnia are prevalent among those with SUD and exacerbate poor treatment outcomes. Interventions that concurrently target anxiety and insomnia during the early stages of SUD treatment are lacking. To this end, we investigated the feasibility and preliminary effectiveness in a single-arm pilot trial of an empirically informed group transdiagnostic intervention, Transdiagnostic SUD Therapy, to concurrently reduce anxiety and improve sleep among adults receiving treatment for SUD. Specifically, we hypothesized that participants would evidence declines in anxiety and insomnia and improvements in sleep health, a holistic, multidimensional pattern of sleep-wakefulness that promotes wellbeing. A secondary aim was to describe the protocol for Transdiagnostic SUD Therapy and how it may be implemented into a real-world addiction treatment setting.
Method: Participants were 163 adults (Mage = 43.23; 95.1% White; 39.93% female) participating in an intensive outpatient program for SUD who attended at least three of four Transdiagnostic SUD Therapy sessions. Participants had diverse SUDs (58.3% alcohol use disorder, 19.0% opioid use disorder) and nearly a third of the sample met criteria for two SUDs and comorbid mental health diagnoses (28.9% anxiety disorder, 24.6% major depressive disorder).
Results: As anticipated, anxiety and insomnia reduced significantly across the 4-week intervention period from clinical to subclinical severity, and sleep health significantly improved (ps < 0.001). These statistically significant improvements following Transdiagnostic SUD Therapy demonstrated medium to large effects (ds > 0.5).
Conclusion: Transdiagnostic SUD Therapy is designed to be flexibly administered in “real-world” clinical settings and, preliminarily, appears to be effective in improving emotional and behavioral factors that increase risk for return to substance use and poor SUD treatment outcomes. Additional work is needed to replicate these findings, determine the feasibility of widespread uptake of Transdiagnostic SUD Therapy, and examine whether the treatment effects translate to improvement in substance use outcomes.
Substance use disorders (SUDs) are chronic, relapsing conditions with effects that persist beyond detoxification, and in some cases even years into abstinence (1). Over 20 million individuals aged 12 or older in the U.S. experience SUD in a given year (2) with a total annual cost exceeding $13 billion in hospitals alone (3). Mental and behavioral health conditions co-occur with SUDs at elevated rates (4–6), and those with dual diagnoses experience worse functional impairment and more severe substance use and psychiatric symptoms relative to those with single disorders (2). Unfortunately, only 7% of dual diagnosis patients receive concurrent treatment for their substance use and mental health disorder and of those who do seek and receive treatment for SUD, up to 75% drop out (2, 7). It is of critical importance to identify comorbid psychiatric conditions that may be concurrently targeted in SUD treatment to reduce rates of treatment dropout and relapse and to maximize desired outcomes including reduction of substance use and improvement in functional outcomes.
Anxiety (e.g., worry, nervousness, and physiological sensations) and insomnia (i.e., difficulty initiating sleep, maintaining sleep, or waking too early) represent some of the most prevalent problems among individuals with SUD. The lifetime prevalence of SUD is substantially higher among those with anxiety-related disorders [11–25% vs. 7.4%; (2, 8)], and presence of a SUD increases risk for anxiety symptoms two- to four-fold (9). Similarly, insomnia occurs in nearly all individuals with SUD through the course of use, withdrawal, and short- and long-term remission (30–91% vs. 6.4–15.0% in the general population), and lifetime history of insomnia increases risk for substance use relative to those with good sleep (4, 10–15). Substance-induced symptoms of anxiety and insomnia are also common, such as withdrawal symptoms mimicking anxiety or drug use perpetuating wakefulness (4, 16).
Anxiety and insomnia each attenuate the impact and reach of evidence-based SUD interventions. In addition to increasing probability of relapse (17–19), anxiety may also negatively impact SUD treatment seeking (20) and predict SUD treatment dropout (21, 22). Comorbid SUD-anxiety patients are most likely to receive treatment for only their substance use or anxiety disorder (23) and treating only SUD does not adequately reduce anxiety (19), leaving individuals vulnerable to anxiety- or stress-precipitated relapse. Further, higher levels of pre-treatment insomnia are associated with lower likelihood of SUD treatment completion (24), whereas improvement of insomnia early in SUD treatment is associated with broad improvement in overall mental health and functioning (25). For those who achieve abstinence, however, insomnia increases risk of relapse (11, 26, 27) even years into recovery (4, 28). Considering the impact of co-occurring anxiety and insomnia in SUD treatment, anxiety accounted for the relationship between alcohol use severity and insomnia (29), and 70% of patients with comorbid SUD and posttraumatic stress disorder (PTSD) experienced insomnia that did not decrease throughout course of treatment (30, 31). The available literature indicates that anxiety and insomnia both play a critical role in the efficacy (or lack thereof) of SUD treatment, and concurrently addressing both anxiety and insomnia would likely improve SUD treatment outcomes. Yet, this opportunity has not been thoroughly investigated.
Given the frequency of comorbid psychiatric symptoms (32) and high rates of relapse and dropout among those with SUDs (7, 33), transdiagnostic interventions, or principle- or process-based treatments, may effectively and efficiently address SUD and co-occurring emotional distress. Transdiagnostic approaches operate by targeting shared processes underlying comorbid presentations and allow for the treatment of heterogeneous clinical presentations and idiographic adaption of evidence-based intervention (34, 35). Transdiagnostic interventions have gained popularity in the last decade (36–38), yet none concurrently address anxiety and insomnia in the context of substance use or SUD treatment.
Many cognitive-behavioral and acceptance-based strategies have demonstrated efficacy in managing cravings, withdrawal, psychological distress, and sleep disturbances. For instance, interpersonal and emotion regulation, problem-solving, assertiveness training, behavioral activation, grounding, and relaxation strategies (e.g., deep breathing and progressive muscle relaxation) are effective in reducing anxiety, cravings, withdrawal, impulsive substance use, and improving sleep quality (39–44). Cognitive behavioral therapy for insomnia (CBT-I) is the first-line treatment for insomnia (45), however, very little empirical work has investigated CBT-I among those with SUDs. Small randomized clinical trials find that CBT-I relative to control is effective in improving subjective sleep quality and insomnia severity but not at reducing relapse (46–48). Likewise, mindfulness-based interventions reduce frequency and quantity of substance use, cravings, substance-related problems, anxiety, and experiential avoidance, and increase rates of abstinence (49–51). Despite the empirical support for each of these treatment components, research to determine the ideal selection of cognitive-behavioral and acceptance-based skills for addressing co-occurring psychiatric distress and sleep disturbances in SUD patients is needed.
In consideration of the high rates of relapse and treatment dropout among those with SUD, it is essential to target malleable factors that may otherwise increase risk for poor SUD treatment outcomes, and when treated, act as a buffer for such negative outcomes. Albeit limited, the extant literature cumulatively implicates anxiety, insomnia, and especially their co-occurrence in worse clinical and treatment outcomes among those with SUD. Additionally, group-based integrative CBT for anxiety and SUD is found to be feasible and effective, over SUD-only treatment, within a SUD-specialty IOP (52). To this end, we developed and tested a transdiagnostic, modular group intervention for reducing anxiety and insomnia in adults with SUD (Transdiagnostic SUD Therapy) based on evidence-based interventions for non-SUD populations that demonstrate effect for substance use and related outcomes. Where possible, we modified these treatment elements to be more relevant for SUD to maximize impact. We first aimed to outline the protocol for Transdiagnostic SUD Therapy, with the full protocol provided as a Supplementary File. The second aim was to pilot Transdiagnostic SUD Therapy in a single-arm trial within a real-world intensive outpatient program (IOP) for adults with SUD, most of whom were in the early stages of their recovery. We hypothesized that Transdiagnostic SUD Therapy would be associated with reductions in anxiety and insomnia severity and improvements in sleep health, which is a holistic and multidimensional pattern of sleep-wakefulness associated with promotions in wellbeing.
Participants (N = 274) were referred to an IOP in the Center for Drug and Alcohol Problems (CDAP) at the Medical University of South Carolina (MUSC) after an initial triage walk-in evaluation and having completed detoxification, if necessary. MUSC is a major academic medical center in the southeastern U.S. The CDAP IOP is a 20-day program consisting of 3-h of daily group therapy and weekly individual and family therapy. See Wilkerson et al. (24) for more information about the structure of the IOP. Transdiagnostic SUD Therapy was rolled into broader IOP programming; therefore, all IOP patients had access to the Transdiagnostic SUD Therapy pilot trial if they were present in IOP treatment the day it was provided. Specifically, each Tuesday during the study period, a 90-min sleep-focused module was presented followed by a 90-min anxiety-focused module. There were no exclusion criteria for participation in the trial as all patients enrolled in the broader IOP participated in this group treatment if they were present on Tuesdays. The open trial occurred between January 2017 and April 2019 and ended due to a change in organizational structure of the IOP clinic.
A retrospective chart review was conducted to gather pertinent information. Prior to initiating IOP, participants provided demographic information and participated in interviews to assess substance use history, medical and psychiatric health history, and social history. At the beginning of each sleep module, participants were asked to complete self-report measures of insomnia and sleep health, and at the beginning of each anxiety module, participants were asked to complete a self-report measure of anxiety severity (see “Measures” below). All procedures were approved by the institution’s IRB (Pro00081480).
Transdiagnostic SUD Therapy is comprised eight, 90-min modules including four sleep and four anxiety modules that are delivered weekly over the course of four weeks (i.e., one sleep and one anxiety module comprise a session for a total of four sessions). Corresponding sleep and anxiety modules were designed to be administered on the same day (e.g., sleep module 1 at 9 am, anxiety module 1 at 10:30 am) unless a different order is clinically indicated based on group members’ needs. The general structure of each module is consistent with that of other CBT protocols, including starting by setting the agenda. Although the content of each session differs, the agenda structure includes: (1) instructing group members to complete self-report assessments, (2) reviewing and setting sleep schedules, (3) briefly assessing use of strategies since last session, (4) presenting new material, and (5) assigning homework. Outlined below is the overarching content of each sleep and anxiety module. See Table 1 for an overview of each module and the Supplementary File for the full Transdiagnostic SUD Therapy protocol including adaptations to better address the needs of individuals with SUD.
The main intervention components of the sleep modules included sleep education, sleep restriction, stimulus control, values clarification and values-consistent behavioral activation, cognitive restructuring, and sleep hygiene. The first sleep module provided education about how substances influence sleep physiology; sleep restriction therapy and stimulus control were introduced and sleep schedules for each participant were created. In sleep module 2, a value clarification activity was used to generate ideas for behavioral activation to promote healthy use of free time that is created by the sleep restriction schedule (i.e., more time spent awake). Module 3 introduced automatic thoughts about sleep and how to challenge them using cognitive restructuring. Module 4 provided education about the role of sleep and insomnia in recovery from substances and sleep hygiene was illustrated as method to maintain the quality and quantity of sleep.
Primary interventions within the anxiety modules included psychoeducation, worry time, interpersonal effectiveness skills, mindfulness and acceptance, and relaxation strategies. Anxiety module 1 used the cognitive triangle as a model for elucidating the role of anxiety and worry in substance use and risk of relapse, and worry time was introduced as a strategy to prevent worry from negatively influencing behavior and interfering with sleep initiation. In module 2, participants were provided education about how unmet needs can result in anger and subsequent relapse, and emotion regulation skills and conflict resolution strategies were introduced. Module 3 introduced mindfulness and acceptance strategies to promote present-moment awareness and to create space between emotions and impulsive substance use. The fourth anxiety module emphasized relaxation strategies, including diaphragmatic breathing, progressive muscle relaxation (PMR), and grounding techniques to cope with anxiety. Although other intervention targets (e.g., depression) and transdiagnostic processes (e.g., interoceptive exposure for anxiety sensitivity) may be appropriate to include in Transdiagnostic SUD Therapy, the needs and limitations of the IOP necessitated that we choose only two foci. We selected anxiety and sleep in part because depression was addressed in other groups and individual therapy within the IOP programming, and addressing anxiety and sleep disturbances can not only improve SUD outcomes (25, 52), but may lead to reductions in related outcomes including depression, suicidal ideation, and general emotional distress (53, 54).
Relatively little research has been conducted in the implementation of evidence-based psychotherapies (EBP) in real-world clinical settings (55); therefore, little is known about whether adapting EBP in such settings maintains treatment efficacy. In some treatment contexts it may not be possible to maintain fidelity to a treatment manual. Emerging research provides support for flexible administration of manualized treatment [(e.g., 56)], and the proliferation of process-based approaches calls on clinicians to deemphasize protocols and syndromes and instead utilize transdiagnostic procedures (e.g., contingency management, emotion regulation, and exposure; (34)). Rolling admission and other real-world factors inherent to an IOP necessitate that Transdiagnostic SUD Therapy be flexible and “meet patients where they are” as they present to each session. In any given week, group members may have just entered IOP and/or completed detoxification, while other members may be in their second, third, or even final week of IOP. As such, group members may or may not receive sessions in the order presented above.
The Generalized anxiety disorder-7 (GAD-7) (57) is a 7-item self-report instrument that assesses anxiety severity over the last two weeks. Items are rated on a Likert-type scale from 0 to 3, with total scores ranging from 0 (minimal) to 21 (severe). A score of 10 indicates probable generalized anxiety disorder. The GAD-7 is positively correlated with other measures of anxiety and demonstrates excellent internal consistency among anxious (α = 0.92) and SUD patients [α = 0.93; (57, 58)] as well as in the current sample (αs = 0.91–0.95).
The Insomnia severity index (ISI) (59) is a 7-item self-report measure assessing perceived severity of insomnia symptoms over the last 2 weeks. Items are rated on a 5-point Likert-type scale from 0 to 4, with higher scores indicating more severe insomnia. Scores of 0–7 indicate no insomnia, 8–14 indicate subthreshold/mild insomnia, 15–21 indicate moderate insomnia, and scores above 21 indicate severe insomnia, while a score of 10 indicates clinically significant insomnia (60). The ISI demonstrates excellent internal consistency (α = 0.91) and a decrease in 8.4 points is indicative of moderate improvement following intervention (60). Internal consistency in the current study was good (αs = 0.86–0.89).
The Satisfaction, alertness, timing, efficiency, and duration (SATED) (61) is a 5-item self-report measure designed to assess sleep health across five dimensions—satisfaction with sleep, alertness during waking hours, appropriate timing, sleep efficiency, and duration. Each dimension is rated on a 3-point Likert-type scale from 0 (never) to 2 (usually/always) and total score ranges from 0 (“poor sleep health”) to 10 (“good sleep health”). There is no specified timeframe for rating each dimension. The SATED demonstrates acceptable internal consistency (α = 0.77) and is negatively correlated with measures of sleep disturbance and daytime sleepiness (62). Across the four timepoints, the SATED internal consistency was on average questionable, ranging from poor to acceptable (α = 0.59–0.70).
In total, 274 patients attended at least one Transdiagnostic SUD Therapy session, of which 111 were excluded for attending fewer than three sessions. Of note, these participants dropped out of the larger IOP at these timepoints, not specifically the Transdiagnostic SUD Therapy group. The number of patients who dropped out is large but not surprising given the high dropout rates in SUD treatment (7, 63) and outpatient treatment in particular [relative to inpatient; (64)]. The final sample consisted of 163 participants who attended three or four sessions.
As this intervention was implemented in a real-world setting as a clinical program rather than a research study, there are inconsistencies in how the data were collected on any given week. Although participants completed three or even all four sessions, they did not necessarily complete the measures for each of the attended sessions (e.g., arrived after measures were given, opted out of measures one week). As an example, a participant may have attended all four anxiety and sleep sessions but only have completed measures at two sessions or have completed the GAD-7 in all four anxiety modules but only completed the ISI and SATED in three sleep modules. We used a pre-post design to account for missing instruments, wherein the first successful administration (i.e., the first time a participant completed the measure) of the GAD-7, ISI, and SATED was designated as pre-intervention data, and the last successful administration of the three measures was designated as the post-intervention data.
Data were screened for accuracy errors and assumptions. Only individuals with complete data on all outcome measures were included in the analyses. The most common item missing on both the ISI and the SATED was the first question, which appeared on the paper-and-pencil survey as similar in font and appearance to the survey title and thus was missed/skipped over more often than the GAD-7, which more clearly delineated between the title and the survey questions. After removing participants with missing data, sample sizes in each of the final analyses were 152 for GAD-7 analyses (11 participants excluded), 132 for ISI (31 participants excluded), and 148 for SATED (15 participants excluded). Independent samples t-tests and Chi-square analyses examined differences in demographic variables (i.e., age, sex, race), primary substance use diagnosis, and pre-treatment anxiety, insomnia, and sleep health across patients who did (n = 163; 59.5%) and did not (n = 111; 40.5%) complete three sessions. Given low frequencies of racially/ethnically minoritized participants, race/ethnicity was dichotomized (0 = racially/ethnically minoritized, 1 = White). Descriptive statistics were used to characterize the sample, and treatment effects on anxiety, insomnia, and sleep health across the Transdiagnostic SUD sessions (i.e., pre-post) were analyzed with a series of paired samples t-tests. Effect sizes to determine magnitude of change on significant variables were computed using Cohen’s d.
There were significant differences across those who completed ≥3 sessions relative to those who completed ≤2 sessions in race (Χ2 [1] = 14.15, p < 0.001) and age (t[140,95] = 2.49, p = 0.007), such that individuals who completed ≥3 sessions were typically White and older than those who dropped out of IOP after 1 or 2 sessions. There were no differences across sex or pre-intervention anxiety, insomnia, or sleep health (ps > 0.05).
See Table 2 for sample demographic, substance use, and mental health information. The sample was diverse in their substance use, with alcohol use disorder (58.3%) being the primary use disorder followed by opioid use disorder (19.0%). Nearly 30% of the sample met criteria for a second use disorder, and one-in-five participants were in medication assisted treatment for substance use disorder (e.g., buprenorphine). Regarding mental health, anxiety-related disorders (including PTSD) occurred in nearly one-third of participants, while major depressive disorder was prevalent across one-fourth of the sample. At baseline, participants endorsed moderately severe anxiety (per GAD-7), clinically significant insomnia (per ISI), and moderate sleep health (per SATED), on average (Table 3).
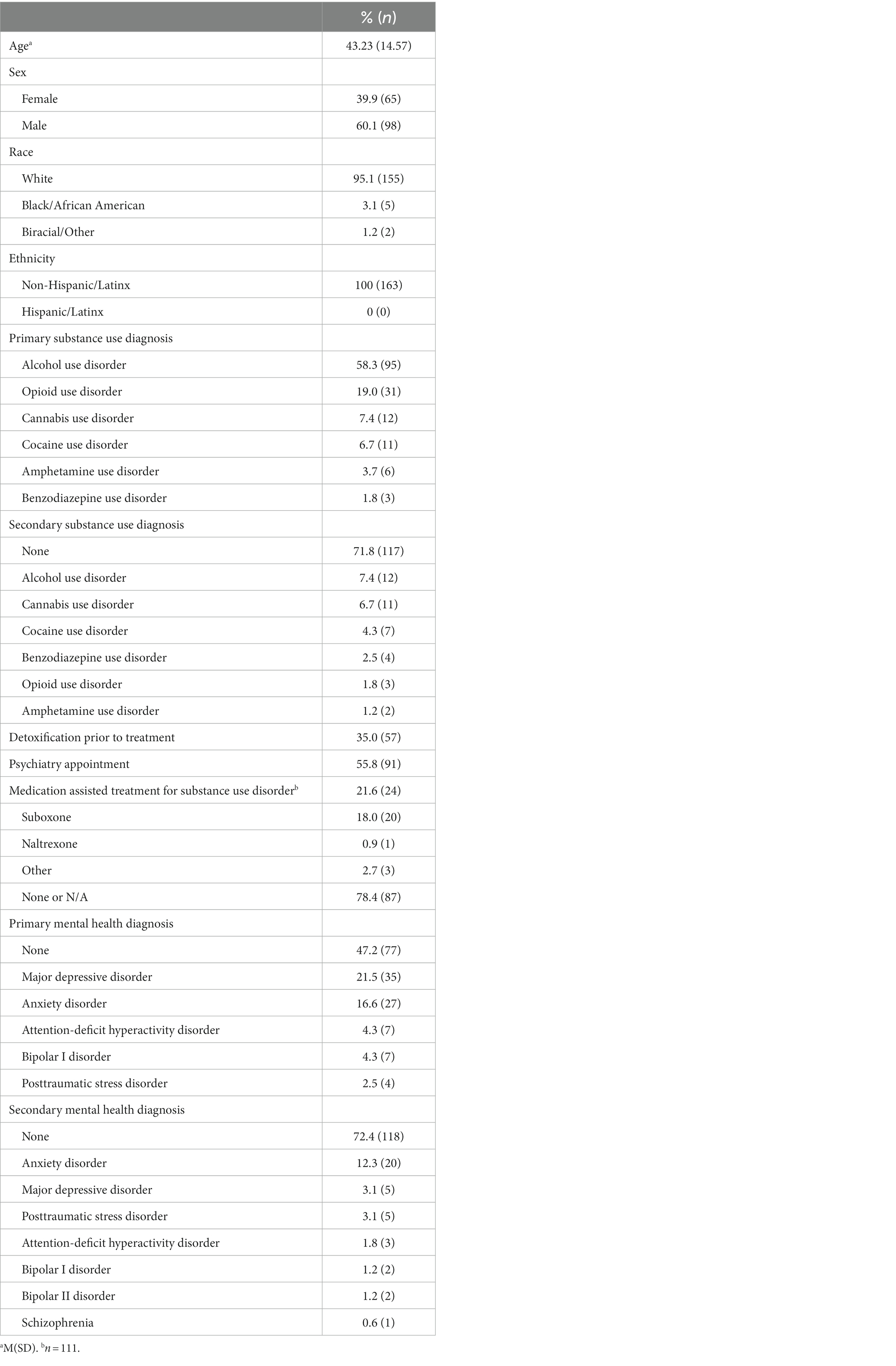
Table 2. Participant pre-intervention demographics, substance use, and mental health characteristics (N = 163).
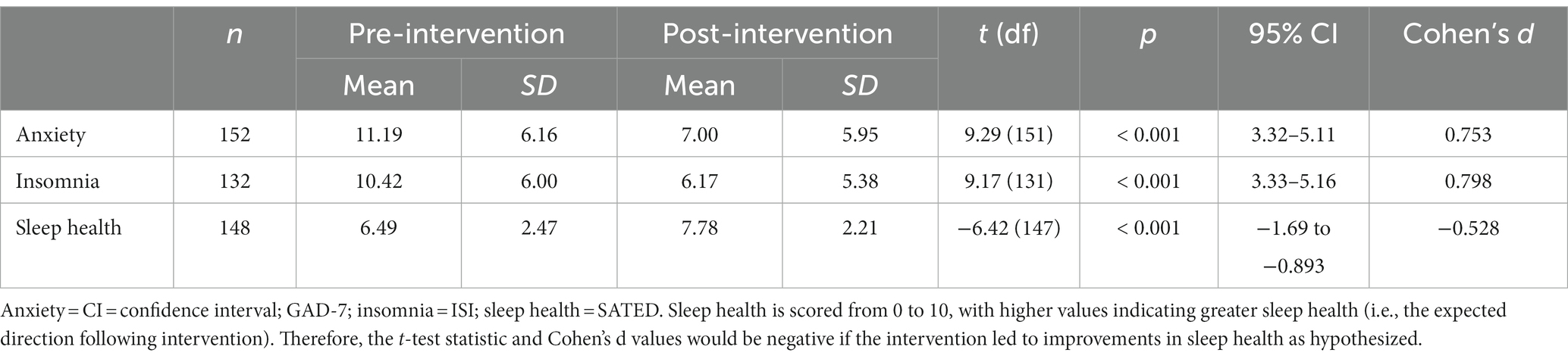
Table 3. Descriptive statistics and paired samples t-tests regarding intervention effects on anxiety, insomnia, and sleep health from pre- to post-intervention.
See Table 3 for paired samples t-test statistics. Anxiety, insomnia, and sleep health all significantly improved from pre- to post-intervention (Figure 1).1 Specifically, anxiety and insomnia both reduced to below clinical significance and sleep health improved throughout the course of Transdiagnostic SUD Therapy. Notably, these findings demonstrated medium to large effects (ds > 0.5). To gather a more fine-grained picture of sleep health, we examined change in individual SATED dimension means across each of the four timepoints. All sleep health dimensions increased over each of the four sessions except Timing, which increased from session 1 to session 2 and then slightly decreased and plateaued at sessions 3 and 4. All dimensions of sleep health, on average, increased from the first administration to the last administration, with Satisfaction increasing the most (0.58 points) followed by Efficiency (0.53), Duration (0.47), Alertness (0.16), and Timing (0.14).
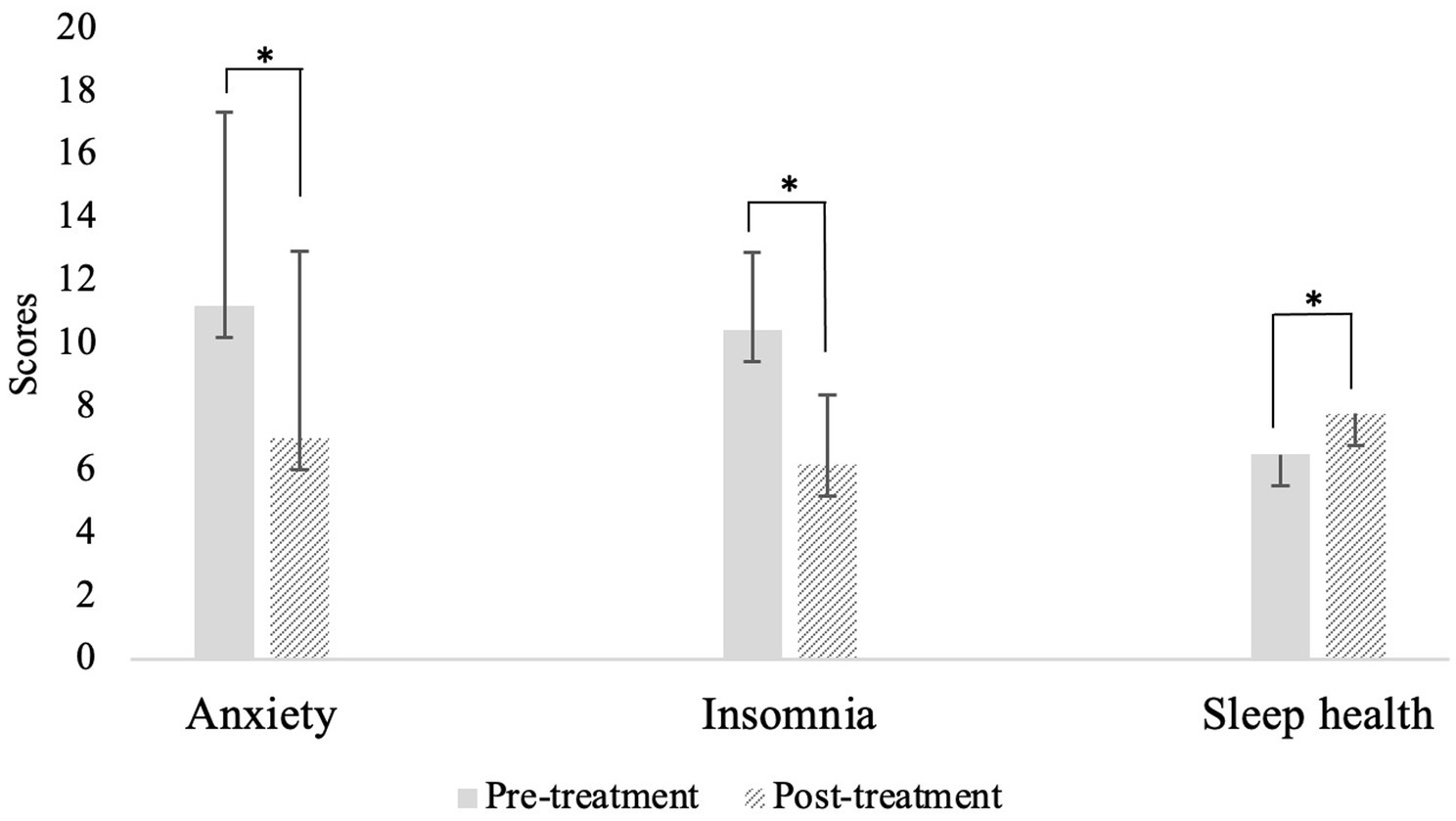
Figure 1. Means and standard error of anxiety, insomnia, and sleep health from pre-to post-intervention. *p < 0.001.
The aims of this manuscript were to describe the protocol and evaluate preliminary support for a transdiagnostic group intervention for anxiety and insomnia among intensive outpatients receiving treatment for SUD. Patients experienced significant reductions in anxiety and insomnia severity and improvement in sleep health throughout the 4-week group intervention. Notably, these findings remained across sex and primary SUD, indicating Transdiagnostic SUD Therapy is effective for both biological males and females and across different types of SUD. Prior to implementation of Transdiagnostic SUD Therapy in this IOP, standard IOP patients experienced clinically significant insomnia symptoms at week 4 of IOP, and reductions in anxiety were 70% less than what was observed in the current study (25). Though the present study did not have a control group, our findings suggest that the implementation of Transdiagnostic SUD Therapy in this IOP may in part contribute to clinical improvement of IOP patients. Importantly, as our sample had various alcohol/substance use and mental health diagnoses, Transdiagnostic SUD Therapy may have the potential to be effective regardless of type of substance(s) used or mental health condition, highlighting its impact and utility as a transdiagnostic intervention. In other words, individuals with SUDs do not necessarily need to have anxiety or sleep disorders to benefit from this intervention, likely given the high prevalence of anxiety and insomnia symptoms throughout the cycle of substance use and recovery. Given anxiety and insomnia occur in withdrawal through early recovery from substances (2, 11), this intervention can supplement traditional SUD treatment by concurrently addressing modifiable factors associated with treatment dropout and relapse (27, 52).
In addition to their prevalence in SUDs, insomnia and anxiety co-occur at heightened rates. Insomnia is the most common sleep disturbance among those with anxiety disorders (up to 50% comorbidity), and individuals with insomnia are at increased risk for anxiety up to 17-fold (65, 66). Insomnia and anxiety reciprocally influence one another, such that lack of sleep can exacerbate distress and diminish one’s ability to cope, while anxiety prolongs sleep onset through negative repetitive thinking and/or physiological arousal. Despite this frequent co-occurrence and bidirectional relationship, insomnia requires targeted intervention, such as CBT-I, to evidence improvements. Indeed, CBT-I is superior to anxiety-focused treatment in improving insomnia symptoms among those with comorbid anxiety disorders and shows moderate to large effects in reducing comorbid anxiety symptoms (67, 68). However, up to 30% of individuals with comorbid anxiety do not experience insomnia-related benefit from CBT-I (69), suggesting that additional intervention components may be needed to mitigate co-occurring anxiety. Cognitive behavioral and acceptance-based interventions are equally effective in the treatment of anxiety disorders (50, 70, 71) and in combination with CBT-I, may be the ideal synergy of strategies to simultaneously improve insomnia and anxiety symptoms. Our findings reflect this sentiment, with integrated elements of CBT-I, CBT for anxiety, and acceptance- and mindfulness-based strategies associated with robust reductions in anxiety and insomnia among SUD patients. However, additional work is needed to examine within-individual differences in anxiety and insomnia outcomes of Transdiagnostic SUD Therapy as different treatment trajectories across individuals may uncover the need for additional, optional Transdiagnostic SUD Therapy modules and/or adaptations to diverse presentations or different clinical settings. Our results speak to this need, in that that racially/ethnically minoritized and younger individuals were less likely to complete ≥3 weeks of IOP than their White and older counterparts. Additionally, whether these treatment components influence substance use outcomes remains to be seen and is the focus of ongoing data collection and analyses.
A major benefit of transdiagnostic interventions is simultaneously addressing overlapping symptoms of comorbid presentations with a single approach (72, 73). Concurrently addressing comorbidities results in better outcomes than single-disorder protocols or treating comorbidities in a sequential manner. For example, Concurrent Treatment of PTSD and Substance Use Disorders Using Prolonged Exposure (COPE) contributes to reductions in PTSD symptoms and SUD severity (74, 75) and yields better SUD outcomes than SUD-only treatment (76). Yet, the relations of sleep disturbance and anxiety among individuals with SUD are complex and little research to date has investigated all three conditions simultaneously, and none have in an intervention context. Experimental studies indicate that anxiety-related factors (e.g., rumination, anxiety sensitivity) may be potential mechanisms influencing the relationship between sleep disturbances and substance use (29, 77, 78), but this work is limited and does not examine the reciprocal role of insomnia on anxiety and substance use. Taken together, this scant literature suggests that transdiagnostic treatment approaches for addressing SUD can be effective and are sorely needed, and that Transdiagnostic SUD Therapy may be a promising approach. Randomized controlled trials are needed to examine the impact of Transdiagnostic SUD Therapy on sleep, anxiety, and substance use outcomes relative to treatment as usual or control.
In addition to improving insomnia symptoms, Transdiagnostic SUD Therapy also appears to contribute to improvements in sleep health. On average, satisfaction, efficiency, and duration of sleep evidenced the greatest improvements across the intervention period. While insomnia consists of difficulty with initiating and maintaining sleep (1), sleep health represents a multidimensional perspective of sleep-wakefulness encompassing physical, mental, and neuro-behavioral wellbeing (61). Rather than focusing on disordered or deficient sleep, sleep health emphasizes positive and holistic sleep characteristics by identifying how well an individual is sleeping (61). As sleep health is negatively correlated with insomnia severity (79), it is not surprising that Transdiagnostic SUD Therapy evidenced improvements in sleep health among our sample. Because sleep health is multifaceted and tied to both sleep-related and overall health, it is possible that the combination of CBT-I and anxiety-focused strategies included in Transdiagnostic SUD Therapy contributed to improvement of sleep health. Empirical work is needed to understand what intervention components influence sleep health. Preliminary evidence implicates increases in sleep onset latency as a salient predictor of declining sleep health (80), suggesting that treatments aimed to enhance sleep health should focus on reducing sleep onset latency.
Preliminary results suggest that Transdiagnostic SUD Therapy may be feasible and effective when administered in a real-world IOP for SUD. Dropout, defined in the current study as attending fewer than three of four total sessions, was approximately 40%, which is similar to or lower than rates found throughout the published literature. A recent meta-analysis indicated that the average dropout rate for in-person psychosocial SUD treatment is 30%, but there is wide variability depending on several demographic and substance use characteristics and can reach as high as 75% (7). Treatment length may be an advantage of 4-session Transdiagnostic SUD Therapy, as fewer sessions are associated with lower rates of dropout (7). Transdiagnostic SUD Therapy is designed to be rolled into existing SUD treatment programming, in this case IOP but other modalities (e.g., individual therapy) and settings (e.g., outpatient, residential) may be appropriate. Integrating Transdiagnostic SUD Therapy along with patients’ attempts to reduce or cease use of substances is recommended given the prevalence of anxiety and insomnia symptoms during withdrawal and early abstinence. Implementing Transdiagnostic SUD Therapy simultaneously with reductions in substance use can provide skills needed to cope with cravings or withdrawal symptoms that may occur at this time, as well as act as a prophylactic method of mitigating triggers for substance use associated with anxiety and/or sleep disturbance that commonly arise during withdrawal and early recovery from substances. Rigorous clinical trials will be needed to examine whether Transdiagnostic SUD Therapy is feasible, acceptable, and effective in alternative settings and in addressing withdrawal and cravings in early SUD treatment. An appropriate next step may be to seek key patient and provider input on the structure (e.g., number of sessions) and intervention elements (e.g., treatment targets) of Transdiagnostic SUD Therapy to determine the optimal composition. Additional trials are needed to determine feasibility of implementation on a larger scale across multiple clinics, as well as designing internally valid, controlled trials to systematically examine additional treatment outcomes and associated transdiagnostic moderators.
Limitations of Transdiagnostic SUD Therapy and the preliminary data presented inform next steps in research and clinical work. Potentially most critical is the homogenous sample, in which nearly all group members identified as White. The effects of this treatment should be replicated within a more racially/ethnically diverse sample to understand the intervention’s efficacy among minoritized individuals, particularly in the context of the finding that ethnically/racially minoritized individuals were less likely to attend at three or more sessions. Cultural adaptations of Transdiagnostic SUD Therapy are likely warranted to account for racial disparities in SUD treatment, and subsequent clinical trials should prioritize recruiting a more diverse sample. In this vein, additional work is needed to understand the impact of other social determinants of health on risk for substance use and the success of Transdiagnostic SUD Therapy, including factors such as socioeconomic status, transportation access, and health literacy. Additionally, a strength of this investigation is the ecologically valid implementation of Transdiagnostic SUD Therapy in a real-world SUD treatment setting. However, clinical trials are needed to investigate whether the cognitive behavioral and acceptance-based strategies selected for Transdiagnostic SUD Therapy are the most effective combination for this population, and the development of additional optional modules for specific populations (e.g., racial/ethnic minorities), motivations for substance use (e.g., coping, provoke/prevent sleep), and other psychiatric symptoms (e.g., depression, posttraumatic stress) may be warranted. Systematic trials may also provide insight into treatment gains provided by Transdiagnostic SUD Therapy above those offered by standard IOP programming. Given the transdiagnostic nature of Transdiagnostic SUD Therapy, inclusion of additional outcome measures to assess global effects (e.g., depression, PTSD symptoms), transdiagnostic mechanisms (e.g., anxiety sensitivity, emotion regulation), and substance use outcomes (e.g., cravings) is warranted to better understand mechanisms of change and scope of intervention effect. Such information can help to refine the intervention to maximize its impact on a wide array of substance use and co-occurring emotional disorders (e.g., need for inclusion of behavioral activation for depression). Further, although over half of our sample were meeting with a psychiatrist, we do not know what psychotropic medications (other than medication assisted treatment) participants were prescribed, if any. Psychotropic medications, such as antidepressants or tranquilizers, could contribute to declines in anxiety and insomnia symptoms; thus, future research should record this information and control for medication effects. In a similar vein, we are underpowered to conclude how medications prescribed for substance use disorders may have influenced the anxiety and sleep findings. Given these medications can reduce anxiety and exacerbate sleep disturbance (81, 82), future research is needed to understand how they may interact with Transdiagnostic SUD Therapy. Finally, 40.5% of the patients who began the Transdiagnostic SUD Therapy (and broader IOP) only completed one or two Transdiagnostic SUD Therapy sessions. These patients were typically racial/ethnic minorities and younger. Future randomized controlled investigations of Transdiagnostic SUD Therapy should seek to examine whether these differences stand and whether other sociodemographic and clinical factors may be contributing to premature dropout. Preliminary results presented herein highlight that Transdiagnostic SUD Therapy is effective across sexes, yet additional work is needed to evaluate whether this intervention is equally effective for sexual and gender diverse individuals. Additionally, researchers should aim to identify how many sessions are needed to experience clinically and statistically significant improvements in anxiety and insomnia symptoms.
Transdiagnostic SUD Therapy may reduce anxiety and insomnia symptoms and improve overall sleep health across sex and various SUDs among adults enrolled in IOP for SUD. These findings meaningfully contribute to the SUD treatment literature by providing preliminary evidence for a brief transdiagnostic intervention that may concurrently address two non-substance-related factors that contribute to relapse and poor SUD treatment outcomes. Further, given that increases in symptoms of anxiety and insomnia are common during active use, withdrawal, early recovery, and even sustained abstinence (12, 83, 84), this protocol may be appropriate for any individual with a history of substance use or SUD, regardless of having an anxiety or insomnia disorder. Our findings suggest that Transdiagnostic SUD Therapy is a promising intervention for integration into real-world SUD treatment settings. Though, replication is needed in community-based settings and randomized controlled trials are warranted to establish its efficacy in not only reducing anxiety and insomnia symptoms but improving substance-related outcomes such as treatment retention, harm reduction/abstinence, and sustained recovery.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Medical University of South Carolina Institutional Review Board. Written informed consent was not provided because data were gathered via a retrospective chart review, for which IRB approval was granted.
SW and AW contributed to writing on the first draft of the manuscript. MM ran intervention groups and was responsible for data collection. JP and KH assisted with running intervention groups. MM and AW contributed to the design of the intervention. SW, TM, and AW acquired data from EMR and ran analyses. All authors contributed to major editing and approved the submission of the manuscript.
This research was supported by the National Institute on Drug Abuse of the National Institutes of Health (2K12 DA031794) awarded to AW.
The authors would like to thank Daniel F. Gros for providing critical feedback on an earlier draft of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1160001/full#supplementary-material
1. ^We used a series of ANCOVAs to examine whether there were differences in post-intervention anxiety, insomnia, and sleep health across sex and primary SUD diagnosis controlling for pre-intervention symptoms. All models were nonsignificant (ps > 0.07).
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). Washington, DC: American Psychiatric Pub (2013).
2. Substance Abuse and Mental Health Services Administration. (2020). Key substance use and mental health indicators in the United States: results from the 2019 National Survey on drug use and health (HHS publication no. PEP20-07-01-001, NSDUH series H-55). Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Available at: https://www.samhsa.gov/data/ (accessed October 6, 2021).
3. Peterson, C, Li, M, Xu, L, Mikosz, CA, and Luo, F. Assessment of annual cost of substance use disorder in US hospitals. JAMA Netw Open. (2021) 4:e210242. doi: 10.1001/jamanetworkopen.2021.0242
4. Dolsen, MR, and Harvey, AG. Life-time history of insomnia and hypersomnia symptoms as correlates of alcohol, cocaine and heroin use and relapse among adults seeking substance use treatment in the United States from 1991 to 1994. Addiction. (2017) 112:1104–11. doi: 10.1111/add.13772
5. Brady, KT, McCauley, JL, and Back, SE. The comorbidity of post-traumatic stress disorder (PTSD) and substance use disorders. In Textbook of Addiction Treatment. eds. N el-Guebaly, G Carrà, M Galanter, and AM Baldacchino. Springer: International Publishing (2021) 1327–39.
6. Marmorstein, NR. Anxiety disorders and substance use disorders: Different associations by anxiety disorder. Journal of Anxiety Disorders. (2012) 26:88–94. doi: 10.1016/j.janxdis.2011.09.005
7. Lappan, SN, Brown, AW, and Hendricks, PS. Dropout rates of in-person psychosocial substance use disorder treatments: a systematic review and meta-analysis. Addiction. (2020) 115:201–17. doi: 10.1111/add.14793
8. Toftdahl, NG, Nordentoft, M, and Hjorthøj, C. Prevalence of substance use disorders in psychiatric patients: a nationwide Danish population-based study. Soc Psychiatry Psychiatr Epidemiol. (2016) 51:129–40. doi: 10.1007/s00127-015-1104-4
9. Lai, HMX, Cleary, M, Sitharthan, T, and Hunt, GE. Prevalence of comorbid substance use, anxiety and mood disorders in epidemiological surveys, 1990–2014: a systematic review and meta-analysis. Drug Alcohol Depend. (2015) 154:1–13. doi: 10.1016/j.drugalcdep.2015.05.031
10. Cao, X-L, Wang, S-B, Zhong, B-L, Zhang, L, Ungvari, GS, Ng, CH, et al. The prevalence of insomnia in the general population in China: a meta-analysis. PLoS One. (2017) 12:e0170772. doi: 10.1371/journal.pone.0170772
11. Chakravorty, S, Chaudhary, NS, and Brower, KJ. Alcohol dependence and its relationship with insomnia and other sleep disorders. Alcohol Clin Exp Res. (2016) 40:2271–82. doi: 10.1111/acer.13217
12. Conroy, DA, and Arnedt, JT. Sleep and substance use disorders: an update. Curr Psychiatry Rep. (2014) 16:487. doi: 10.1007/s11920-014-0487-3
13. Ohayon, MM, and Sagales, T. Prevalence of insomnia and sleep characteristics in the general population of Spain. Sleep Med. (2010) 11:1010–8. doi: 10.1016/j.sleep.2010.02.018
14. Teplin, D, Raz, B, Daiter, J, Varenbut, M, and Tyrrell, M. Screening for substance use patterns among patients referred for a variety of sleep complaints. Am J Drug Alcohol Abuse. (2006) 32:111–20. doi: 10.1080/00952990500328695
15. Grau-López, L, Daigre, C, Grau-López, L, Rodriguez-Cintas, L, Egido, A, Casas, M, et al. Administrative prevalence of insomnia and associated clinical features in patients with addiction during active substance use. Actas Españolas de Psiquiatría. (2016) 44:64–71.
16. Brady, KT, Haynes, LF, Hartwell, KJ, and Killeen, TK. Substance use disorders and anxiety: a treatment challenge for social workers. Soc Work Public Health. (2013) 28:407–23. doi: 10.1080/19371918.2013.774675
17. McHugh, RK. Treatment of co-occurring anxiety disorders and substance use disorders. Harv Rev Psychiatry. (2015) 23:99–111. doi: 10.1097/HRP.0000000000000058
18. Stewart, SH, and Conrod, PJ. Anxiety disorder and substance use disorder co-morbidity: common themes and future directions In: SH Stewart and PJ Conrod, editors. Anxiety and Substance use Disorders. New York: Springer US (2008). 239–57.
19. Wolitzky-Taylor, K, Krull, J, Rawson, R, Roy-Byrne, P, Ries, R, and Craske, MG. Randomized clinical trial evaluating the preliminary effectiveness of an integrated anxiety disorder treatment in substance use disorder specialty clinics. J Consult Clin Psychol. (2018) 86:81–8. doi: 10.1037/ccp0000276
20. Blanco, C, Iza, M, Rodríguez-Fernández, JM, Baca-García, E, Wang, S, and Olfson, M. Probability and predictors of treatment-seeking for substance use disorders in the U.S. Drug Alcohol Depend. (2015) 149:136–44. doi: 10.1016/j.drugalcdep.2015.01.031
21. Elmquist, J, Shorey, RC, Anderson, SE, and Stuart, GL. The relationship between generalized anxiety symptoms and treatment dropout among women in residential treatment for substance use disorders. Subst Use Misuse. (2016) 51:835–9. doi: 10.3109/10826084.2016.1155612
22. Lejuez, CW, Zvolensky, MJ, Daughters, SB, Bornovalova, MA, Paulson, A, Tull, MT, et al. Anxiety sensitivity: a unique predictor of dropout among inner-city heroin and crack/cocaine users in residential substance use treatment. Behav Res Ther. (2008) 46:811–8. doi: 10.1016/j.brat.2008.03.010
23. Mojtabai, R, Olfson, M, and Mechanic, D. Perceived need and help-seeking in adults with mood, anxiety, or substance use disorders. Arch Gen Psychiatry. (2002) 59:77. doi: 10.1001/archpsyc.59.1.77
24. Wilkerson, AK, Sahlem, GL, Bentzley, BS, Lord, J, Smith, JP, Simmons, RO, et al. Insomnia severity during early abstinence is related to substance use treatment completion in adults enrolled in an intensive outpatient program. J Subst Abus Treat. (2019) 104:97–103. doi: 10.1016/j.jsat.2019.06.003
25. Wilkerson, AK, Simmons, RO, Sahlem, GL, Taylor, DJ, Smith, JP, Book, SW, et al. Sleep and substance use disorder treatment: a preliminary study of subjective and objective assessment of sleep during an intensive outpatient program. Am J Addict. (2021) 30:477–84. doi: 10.1111/ajad.13194
26. Brower, KJ, Aldrich, MS, Robinson, EAR, Zucker, RA, and Greden, JF. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatr. (2001) 158:399–404. doi: 10.1176/appi.ajp.158.3.399
27. Geoffroy, PA, Lejoyeux, M, and Rolland, B. Management of insomnia in alcohol use disorder. Expert Opin Pharmacother. (2020) 21:297–306. doi: 10.1080/14656566.2019.1705279
28. Brower, KJ. Insomnia, alcoholism and relapse. Sleep Med Rev. (2003) 7:523–39. doi: 10.1016/S1087-0792(03)90005-0
29. Chaudhary, NS, Wong, MM, Kolla, BP, Kampman, KM, and Chakravorty, S. The relationship between insomnia and the intensity of drinking in treatment-seeking individuals with alcohol dependence. Drug Alcohol Depend. (2020) 215:108189. doi: 10.1016/j.drugalcdep.2020.108189
30. Colvonen, PJ, Ellison, J, Haller, M, and Norman, SB. Examining insomnia and PTSD over time in veterans in residential treatment for substance use disorders and PTSD. Behav Sleep Med. (2019) 17:524–35. doi: 10.1080/15402002.2018.1425869
31. Vandrey, R, Babson, KA, Herrmann, ES, and Bonn-Miller, MO. Interactions between disordered sleep, post-traumatic stress disorder, and substance use disorders. Int Rev Psychiatry. (2014) 26:237–47. doi: 10.3109/09540261.2014.901300
32. Lieb, R. Epidemiological perspectives on comorbidity between substance use disorders and other mental disorders In: G Dom and F Moggi, editors. Co-occurring Addictive and Psychiatric Disorders. Berlin Heidelberg: Springer (2015). 3–12.
33. Bell, J, and Strang, J. Medication treatment of opioid use disorder. Biol Psychiatry. (2020) 87:82–8. doi: 10.1016/j.biopsych.2019.06.020
34. Hofmann, SG, and Hayes, SC. The future of intervention science: process-based therapy. Clin Psychol Sci. (2019) 7:37–50. doi: 10.1177/2167702618772296
35. McHugh, RK, Murray, HW, and Barlow, DH. Balancing fidelity and adaptation in the dissemination of empirically-supported treatments: the promise of transdiagnostic interventions. Behav Res Ther. (2009) 47:946–53. doi: 10.1016/j.brat.2009.07.005
36. Barlow, DH, Farchione, TJ, Sauer-Zavala, S, Murray-Latin, H, Bullis, JR, Ellard, K, et al. Unified protocol for transdiagnostic treatment of emotional disorders: Therapist guide (Second edition). Oxford University Press (2018).
37. Gros, DF. Development and initial evaluation of Transdiagnostic Behavior Therapy (TBT) for veterans with affective disorders. Psychiatry Research. (2014) 220:275–82. doi: 10.1016/j.psychres.2014.08.018
38. Levin, ME, MacLane, C, Daflos, S, Seeley, JR, Hayes, SC, Biglan, A, et al. Examining psychological inflexibility as a transdiagnostic process across psychological disorders. Journal of Contextual Behavioral Science. (2014) 31:155–63. doi: 10.1016/j.jcbs.2014.06.003
39. Alexandru, BV, Róbert, B, Viorel, L, and Basile, B. Treating primary insomnia: a comparative study of self-help methods and progressive muscle relaxation. J Cogn Behav Psychother. (2009) 9:67–82.
40. Barlow, DH ed. Clinical Handbook of Psychological Disorders: A Step-By-Step Treatment Manual. 5th ed. New York: The Guilford Press (2014).
41. Daughters, SB, Magidson, JF, Anand, D, Seitz-Brown, CJ, Chen, Y, and Baker, S. The effect of a behavioral activation treatment for substance use on post-treatment abstinence: a randomized controlled trial. Addiction. (2018) 113:535–44. doi: 10.1111/add.14049
42. Dimeff, LA, and Linehan, MM. Dialectical behavior therapy for substance abusers. Addict Sci Clin Pract. (2008) 4:39–47. doi: 10.1151/ascp084239
43. Limsanon, T, and Kalayasiri, R. Preliminary effects of progressive muscle relaxation on cigarette craving and withdrawal symptoms in experienced smokers in acute cigarette abstinence: a randomized controlled trial. Behav Ther. (2015) 46:166–76. doi: 10.1016/j.beth.2014.10.002
44. McHugh, RK, Hearon, BA, and Otto, MW. Cognitive behavioral therapy for substance use disorders. Psychiatr Clin N Am. (2010) 33:511–25. doi: 10.1016/j.psc.2010.04.012
45. Qaseem, A, Kansagara, D, Forciea, MA, Cooke, M, and Denberg, TD, for the Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American college of physicians. Ann Intern Med. (2016) 165:125–33. doi: 10.7326/M15-2175
46. Arnedt, JT, Conroy, DA, Armitage, R, and Brower, KJ. Cognitive-behavioral therapy for insomnia in alcohol dependent patients: a randomized controlled pilot trial. Behav Res Ther. (2011) 49:227–33. doi: 10.1016/j.brat.2011.02.003
47. Soleimani, R, Modabbernia, MJ, Habibi, S, Roudsary, MH, and Elahi, M. The effect of cognitive behavior therapy in insomnia due to methadone maintenance therapy: a randomized clinical trial. Iranian J Med Sci. (2015) 40:396–403.
48. Speed, TJ, Hanks, L, Turner, G, Gurule, E, Kearson, A, Buenaver, L, et al. A comparison of cognitive behavioral therapy for insomnia to standard of care in an outpatient substance use disorder clinic embedded within a therapeutic community: a RE-AIM framework evaluation. Trials. (2022) 23:965. doi: 10.1186/s13063-022-06885-7
49. Chiesa, A, and Serretti, A. Are mindfulness-based interventions effective for substance use disorders? A systematic review of the evidence. Subst Use Misuse. (2014) 49:492–512. doi: 10.3109/10826084.2013.770027
50. Hofmann, SG, Sawyer, AT, Witt, AA, and Oh, D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. J Consult Clin Psychol. (2010) 78:169–83. doi: 10.1037/a0018555
51. Korecki, JR, Schwebel, FJ, Votaw, VR, and Witkiewitz, K. Mindfulness-based programs for substance use disorders: a systematic review of manualized treatments. Subst Abuse Treat Prev Policy. (2020) 15:51. doi: 10.1186/s13011-020-00293-3
52. Wolitzky-Taylor, K, Niles, AN, Ries, R, Krull, JL, Rawson, R, Roy-Byrne, P, et al. Who needs more than standard care? Treatment moderators in a randomized clinical trial comparing addiction treatment alone to addiction treatment plus anxiety disorder treatment for comorbid anxiety and substance use disorders. Behav Res Ther. (2018) 107:1–9. doi: 10.1016/j.brat.2018.05.005
53. Asarnow, LD, and Manber, R. Cognitive behavioral therapy for insomnia in depression. Sleep Med Clin. (2019) 14:177–84. doi: 10.1016/j.jsmc.2019.01.009
54. García-Escalera, J, Chorot, P, Valiente, RM, Reales, JM, and Sandín, B. Efficacy of transdiagnostic cognitive-behavioral therapy for anxiety and depression in adults, children and adolescents: a meta-analysis. Revista de Psicopatología y Psicología Clínica. (2016) 21:147–75. doi: 10.5944/rppc.vol.21.num.3.2016.17811
55. Leffler, JM, and D’Angelo, EJ. Implementing evidence-based treatments for youth in acute and intensive treatment settings. J Cogn Psychother. (2020) 34:185–99. doi: 10.1891/JCPSY-D-20-00018
56. Schnurr, PP, Chard, KM, Ruzek, JI, Chow, BK, Resick, PA, Foa, EB, et al. Comparison of prolonged exposure vs cognitive processing therapy for treatment of posttraumatic stress disorder among us veterans: a randomized clinical trial. JAMA Netw Open. (2022) 5:e2136921. doi: 10.1001/jamanetworkopen.2021.36921
57. Spitzer, RL, Kroenke, K, Williams, JBW, and Löwe, B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. (2006) 166:1092. doi: 10.1001/archinte.166.10.1092
58. Bentley, KH, Sakurai, H, Lowman, KL, Rines-Toth, L, McKowen, J, Pedrelli, P, et al. Validation of brief screening measures for depression and anxiety in young people with substance use disorders. J Affect Disord. (2021) 282:1021–9. doi: 10.1016/j.jad.2021.01.005
60. Morin, CM, Belleville, G, Bélanger, L, and Ivers, H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. (2011) 34:601–8. doi: 10.1093/sleep/34.5.601
61. Buysse, DJ. Sleep health: can we define it? Does it matter? Sleep. (2014) 37:9–17. doi: 10.5665/sleep.3298
62. Benítez, I, Roure, N, Pinilla, L, Sapiña-Beltran, E, Buysse, DJ, Barbé, F, et al. Validation of the satisfaction, alertness, timing, efficiency and duration (SATED) questionnaire for sleep health measurement. Ann Am Thorac Soc. (2020) 17:338–43. doi: 10.1513/AnnalsATS.201908-628OC
63. Loveland, D, and Driscoll, H. Examining attrition rates at one specialty addiction treatment provider in the United States: a case study using a retrospective chart review. Subst Abuse Treat Prev Policy. (2014) 9:41. doi: 10.1186/1747-597X-9-41
64. Stahler, GJ, Mennis, J, and DuCette, JP. Residential and outpatient treatment completion for substance use disorders in the U.S.: moderation analysis by demographics and drug of choice. Addict Behav. (2016) 58:129–35. doi: 10.1016/j.addbeh.2016.02.030
65. Khurshid, KA. Comorbid insomnia and psychiatric disorders: an update. Innovat Clin Neurosci. (2018) 15:28–32.
66. Taylor, DJ, Lichstein, KL, Durrence, HH, Reidel, BW, and Bush, AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. (2005) 28:1457–64. doi: 10.1093/sleep/28.11.1457
67. Jansson-Fröjmark, M, and Jacobson, K. Cognitive behavioural therapy for insomnia for patients with co-morbid generalized anxiety disorder: an open trial on clinical outcomes and putative mechanisms. Behav Cogn Psychother. (2021) 49:540–55. doi: 10.1017/S1352465821000023
68. Mirchandaney, R, Barete, R, and Asarnow, LD. Moderators of cognitive behavioral treatment for insomnia on depression and anxiety outcomes. Curr Psychiatry Rep. (2022) 24:121–8. doi: 10.1007/s11920-022-01326-3
69. Chellappa, SL, and Aeschbach, D. Sleep and anxiety: from mechanisms to interventions. Sleep Med Rev. (2022) 61:101583. doi: 10.1016/j.smrv.2021.101583
70. Arch, JJ, Eifert, GH, Davies, C, Vilardaga, JCP, Rose, RD, and Craske, MG. Randomized clinical trial of cognitive behavioral therapy (CBT) versus acceptance and commitment therapy (ACT) for mixed anxiety disorders. J Consult Clin Psychol. (2012) 80:750–65. doi: 10.1037/a0028310
71. Swain, J, Hancock, K, Hainsworth, C, and Bowman, J. Acceptance and commitment therapy in the treatment of anxiety: a systematic review. Clin Psychol Rev. (2013) 33:965–78. doi: 10.1016/j.cpr.2013.07.002
72. Barlow, DH, Allen, LB, and Choate, ML. Toward a unified treatment for emotional disorders – republished article. Behav Ther. (2016) 47:838–53. doi: 10.1016/j.beth.2016.11.005
73. Gros, DF, Allan, NP, and Szafranski, DD. Movement towards transdiagnostic psychotherapeutic practices for the affective disorders. Evid Based Mental Health. (2016) 19:e10–2. doi: 10.1136/eb-2015-102286
74. Back, SE, Killeen, T, Badour, CL, Flanagan, JC, Allan, NP, Ana, ES, et al. Concurrent treatment of substance use disorders and PTSD using prolonged exposure: a randomized clinical trial in military veterans. Addict Behav. (2019) 90:369–77. doi: 10.1016/j.addbeh.2018.11.032
75. Tripp, JC, Jones, JL, Back, SE, and Norman, SB. Dealing with complexity and comorbidity: comorbid PTSD and substance use disorders. Curr Treat Opt Psychiatry. (2019) 6:188–97. doi: 10.1007/s40501-019-00176-w
76. Ruglass, LM, Lopez-Castro, T, Papini, S, Killeen, T, Back, SE, and Hien, DA. Concurrent treatment with prolonged exposure for co-occurring full or subthreshold posttraumatic stress disorder and substance use disorders: a randomized clinical trial. Psychother Psychosom. (2017) 86:150–61. doi: 10.1159/000462977
77. Cao, Q, Zhao, H, Yao, J, and Liang, Y. Anxiety and sleep quality in Chinese persons with a substance use disorder: a moderated mediation model of rumination and mindfulness. Curr Psychol. (2021) 40:2715–24. doi: 10.1007/s12144-019-00200-5
78. Dixon, LJ, Lee, AA, Gratz, KL, and Tull, MT. Anxiety sensitivity and sleep disturbance: investigating associations among patients with co-occurring anxiety and substance use disorders. J Anxiety Disord. (2018) 53:9–15. doi: 10.1016/j.janxdis.2017.10.009
79. Benham, G. The sleep health index: correlations with standardized stress and sleep measures in a predominantly Hispanic college student population. Sleep Health. (2019) 5:587–91. doi: 10.1016/j.sleh.2019.07.007
80. Allen, SF, Elder, GJ, Longstaff, LF, Gotts, ZM, Sharman, R, Akram, U, et al. Exploration of potential objective and subjective daily indicators of sleep health in normal sleepers. Nature Sci Sleep. (2018) 10:303–12. doi: 10.2147/NSS.S168841
81. Tenore, PL. Psychotherapeutic benefits of opioid agonist therapy. J Addict Dis. (2008) 27:49–65. doi: 10.1080/10550880802122646
82. Langstengel, J, and Yaggi, HK. Sleep deficiency and opioid use disorder: trajectory, mechanisms, and interventions. Clin Chest Med. (2022) 43:e1–e14. doi: 10.1016/j.ccm.2022.05.001
83. Brower, KJ, Krentzman, A, and Robinson, EAR. Persistent insomnia, abstinence, and moderate drinking in alcohol-dependent individuals. Am J Addict. (2011) 20:435–40. doi: 10.1111/j.1521-0391.2011.00152.x
Keywords: substance use disorder, anxiety, insomnia, transdiagnostic, treatment development
Citation: Milanak ME, Witcraft SM, Park JY, Hassell K, McMahon T and Wilkerson AK (2023) A Transdiagnostic group therapy for sleep and anxiety among adults with substance use disorders: Protocol and pilot investigation. Front. Psychiatry. 14:1160001. doi: 10.3389/fpsyt.2023.1160001
Received: 06 February 2023; Accepted: 09 March 2023;
Published: 29 March 2023.
Edited by:
Caitlin Eileen Martin, Virginia Commonwealth University, United StatesReviewed by:
Emily E. Hartwell, United States Department of Veterans Affairs, United StatesCopyright © 2023 Milanak, Witcraft, Park, Hassell, McMahon and Wilkerson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Allison K. Wilkerson, d2lsa2Vyc2FAbXVzYy5lZHU=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.