
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 29 June 2023
Sec. Psychological Therapy and Psychosomatics
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1158705
This article is part of the Research Topic Community Series in Psychocardiology: Exploring the Brain-Heart Interface, volume II View all 10 articles
Introduction: Adopting an active lifestyle is an important goal, but can be difficult to achieve for people with depressive disorders. Current guidelines recommend the integration of physical activity in the multimodal treatment of depressive disorders. However, the possibilities to provide individual support for physical activities are frequently limited. The aim of our study was to examine how physical activity can be increased in a real-world setting by combining physical training and psychological interventions.
Materials and methods: In this randomized-controlled interventional study, 31 outpatients diagnosed with moderate to severe depression were recruited from the region of Hannover. The intervention group (n = 16) was offered six weekly individual sessions lasting between 60 and 90 min with a sports scientist, including Motivational Interviewing and accompanied exercise activities. The control group (n = 15) received a written booklet with information on steps toward becoming more active. Moderate-to-vigorous physical activity (MVPA) as the primary outcome was analyzed using activity sensors before and after the 6-week intervention, and 3 months subsequently. Secondary outcomes included the Six-Minute Walk Test (6MWT), Sit-to-Stand test (STS), and mental health assessed with self-rating questionnaires.
Results: In the intervention group, MVPA increased significantly between baseline and the first follow-up and remained at an increased level at the second follow-up in comparison to decreased levels in the control group (difference of 15.5 min/day between groups over time, SE = 6.2 min/day, 95%-CI[2.7, 28.3], p = 0.020). The increased activity level was associated with markers of increased fitness (6MWT and STS) in the intervention group. Both groups showed comparable improvements in depressive symptoms, while the number of patients receiving antidepressants increased in the control group and decreased in the intervention group. Two patients dropped out of the intervention group during the trial.
Conclusion: The intervention proved to be a feasible and effective aid to promote a physically active lifestyle for patients diagnosed with depression. Furthermore, the higher level of physical activity was maintained for the follow-up period. Given the success of the approach evaluated in this project, individual support for physical activity should be investigated in larger sample sizes and potentially be considered in the multimodal treatment of depression.
Clinical trial registration: [https://clinicaltrials.gov/], identifier [DRKS00023257].
Achieving a change in exercise habits is an important goal in patient care for depression, but is frequently difficult to implement in practice. Loss of interest, energy, self-confidence and decisiveness are important determinants of major depressive disorder and impede patients in following an active lifestyle. Indeed, studies and meta-analyses demonstrate that people with depression tend toward sedentary lifestyles with decreased physical activity compared with healthy controls (1–4). There is also evidence for an inverse relationship between physical activity and depression [(e.g., 5–8)] and an increased risk for inactivity-induced physical comorbidities [(e.g., 9)]. However, the promotion of physical activity for patients suffering from depression harbors significant therapeutic potential (10). Starting an active lifestyle can lead to a multitude of positive effects through various biological or psychosocial pathways [(e.g., 11)], but there is currently no established or accepted method to increase activity in already depressed and inactive people. In previous studies, we have analyzed the effects of exercise interventions on adipose tissue, muscle mass, cardiorespiratory fitness, metabolic syndrome and brain-derived neurotrophic factor in inpatients with major depressive disorder (12–15) and the effects of physical activity on the severity of depression and anxiety in company employees (16). Building on these findings, the present study evaluates how to increase physical activity in “real-world” settings in outpatients with depressive disorders.
Depressed mood and stress represent the main barriers to physical activity, as do poor levels of social support (17). Lack of time, physical illness, and poor health are also stated as limiting factors (18). Furthermore, a higher body mass index and a lower self-efficacy are associated with lower participation in physical activity (19). Professional assistance in setting goals, overcoming barriers and maintaining motivation (17), and the inclusion of health care professionals qualified in exercise prescription (20) are recommended strategies to assist patients with depressive disorder. The importance of increasing autonomous motivation (21) and self-efficacy (22) is recognized and Motivational Interviewing techniques can support behavioral change on the basis of patients’ own decisions (23, 24). In previous studies, personal or telephone contact provided an opportunity for patients to speak about their goals and any potential reservations regarding exercise (25), and to explain the effects of physical activity (26). Further promising approaches identified in previous projects include personal coaching with individualized training schedules (27), a combination of supervised exercise with recommendations for home-based exercise (28), and a hybrid approach with telehealth options, web-based modules and smart technology [(e.g., 29–31)]. In contrast to many previous approaches, our study neither focuses on group exercise [(e.g., 32)] nor measures efficiency in reducing weight or symptom severity [(e.g., 33)], but rather measures change in physical activity as the primary outcome. On the basis of previous work, we designed an intervention based on a combination of the supporting factors outlined above and techniques such as Motivational Interviewing, flanked by a new component of “accompanied activity.” This involved the discussion of barriers such as those mentioned above, and limiting factors in personal conversations with the study participants.
The aim of this study was to examine how to promote a long-term increase in self-selected physical activity in the patients’ daily life using this combination of physical training and psychological methods. We hypothesized a significantly greater increase in moderate-to-vigorous physical activity (MVPA) as measured with activity trackers when using this combined approach in the intervention group in comparison with the control group. The main focus was on the long-term effects of our intervention as assessed at the second follow-up visit, 3 months after the end of the intervention.
We advertised for potential participants in Hannover (Lower Saxony, Germany) between November 2020 and February 2022. Initial details on the trial were distributed via posts on selected internet platforms (homepage of the “Patienten Universität an der Medizinischen Hochschule Hannover” and Hannover Medical School intranet) and on Hannover Medical School’s social media channel. Handouts were made available by health care professionals in outpatient clinics (Wahrendorff clinical center and Hannover Medical School) and medical practices (in a radius of approximately 5 km of the study site). This resulted in 60 potential participants who were subsequently informed about the study in detail (see Figure 1). The inclusion criteria for patients were: a diagnosis of depression (F32, F33) according to ICD-10 (34), age between 18 and 60 years, and place of residence in the region of Hannover. Exclusion criteria were substance abuse, suicidal tendencies, lack of ability to understand information or give informed consent, pregnancy, lactation, or contraindications for unsupervised physical activity or tests. Written informed consent was obtained from all participants. The study was approved by the local ethics committee at the Hannover Medical School (NR8924_BO_S_2020), registered at “Deutsches Register Klinischer Studien”/WHO International Clinical Trials Registry Platform (DRKS00023257) and performed in accordance with the Declaration of Helsinki. Recruitment was stopped once the required sample size was reached.
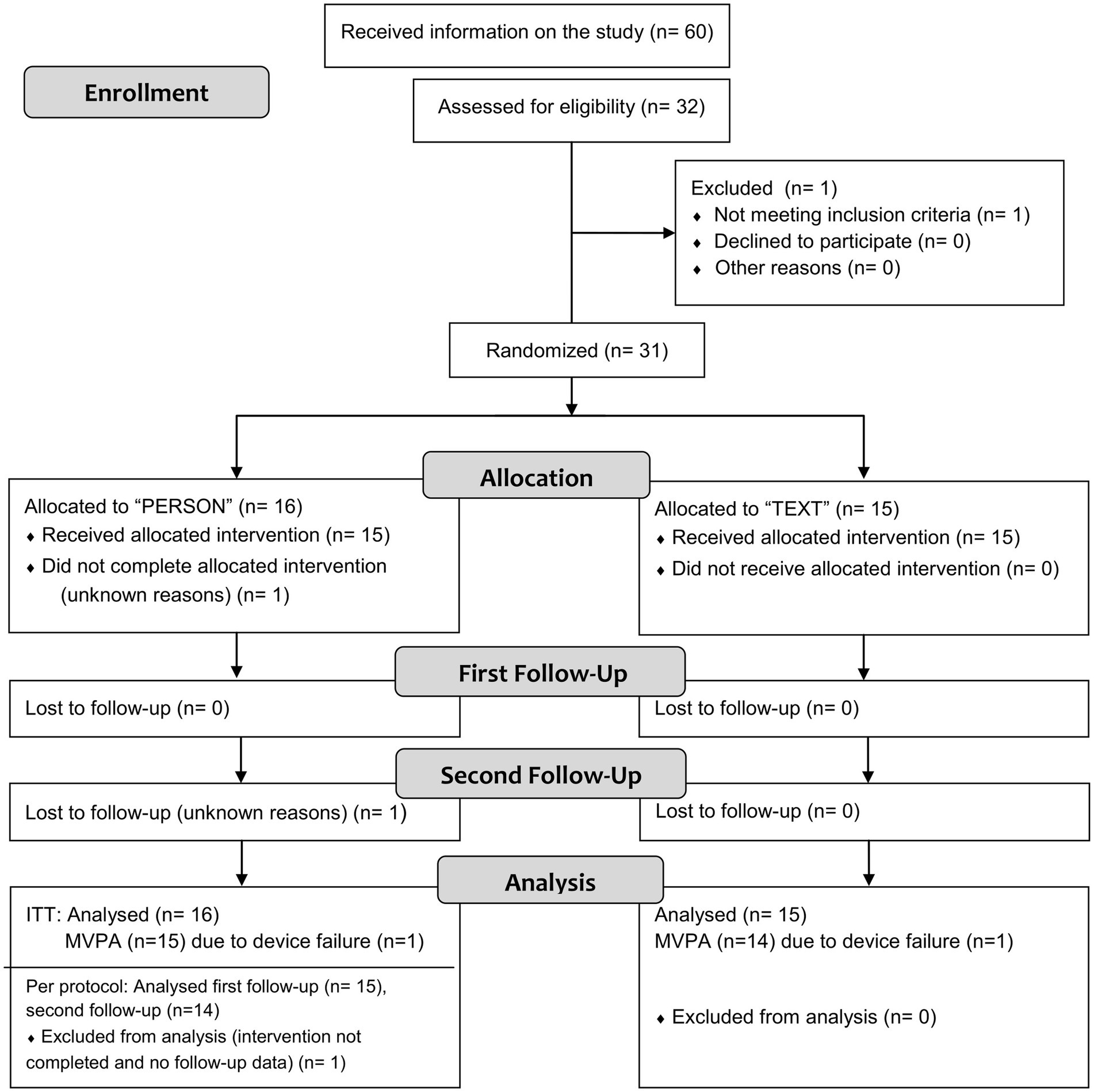
Figure 1. Flowchart of the numbers of participants and dropouts (for details on excluded and missing values, see paragraph “Statistical analysis” and Table 3. MVPA, moderate-to-vigorous physical activity).
Patients were randomly assigned to either the intervention group (n = 16) or the control group (n = 15). All participants continued with their usual medications. Changes in medication were not part of the study procedure, but were monitored at each study visit. Participants changed their medication in consultation with their therapists who were not part of the study team. Twenty-four patients were taking medication; of these 18 were taking antidepressants and six were taking only cardiac or thyroid dysfunction medication. Seven patients were not taking any medication at baseline. The baseline characteristics of the groups are reported in Table 1.
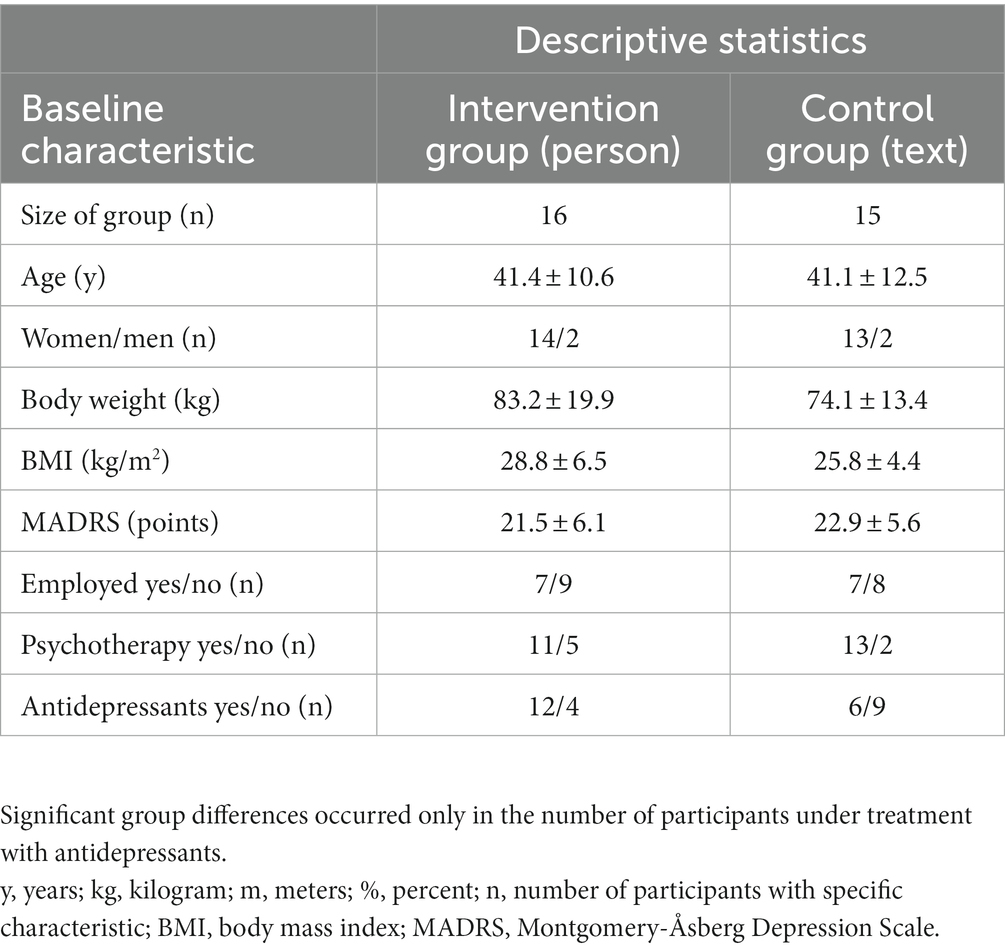
Table 1. Baseline characteristics of participants as mean ± standard deviation or absolute number in the two groups (intervention group, personalized training with Motivational Interviewing and accompanied activity, “person”; control group, written information, “text”).
Patients in the intervention group participated in six individual sessions (one session per week) lasting between 60 and 90 min with a sports scientist, comprising Motivational Interviewing and accompanied physical activity. Sessions included theoretical and practical parts and were standardized with the help of pre-formulated lists of questions and exercises, as well as work sheets and written information. The practical part consisted of a multi-faceted exercise program under the guidance of an experienced sports therapist with training in Motivational Interviewing. The participants had the possibility to try out endurance and strength training, complementary exercises addressing flexibility, coordination, relaxation, and individually requested sports. Participants could individually select a preferred activity based on the guided testing or previous positive experiences. For the theoretical part, the participants’ past exercise experiences and current status were evaluated; goals were defined, analyzed and divided into small tasks. Discrepancies between the current and desired physical activity status were identified. Together with the patient, the sports therapist discussed the advantages and disadvantages of increased sports activity, examined factors which might promote or complicate exercise, and formulated a daily and weekly plan. General information on healthy eating and the effects of sports and exercise on health was provided. The results from the baseline assessments were explained and used to tailor the activities to the patient’s individual health and fitness status. The overall goal was to meet general activity recommendations (35) and to find individual activities that the participants were interested in continuing on their own. The sports therapist was able to act as a sports companion and join the participant’s selected activity, e.g., sports group, fitness center visit or walking tour at home (accompanied activity). The stepwise development of activities has parallels with behavioral activation strategies (36–38), regarding physical activity as a form of healthy behavior with the potential for positive experiences. Following previous studies (39, 40), the intervention is based on principles of self-determination theory (41) and uses Motivational Interviewing techniques (42, 43) (for a detailed description of the contents of the intervention, see Table 2).
Patients in the control group received written information after the results of the baseline assessments had been explained. It contained detailed information on safety, activity recommendations of World Health Organization (WHO) (35) and American College of Sports Medicine (ACSM) (44), training methods, sports disciplines, organization, sports equipment, tips for motivation, information on healthy eating and an exercise log. The control setting did not provide the option of any personal contact in the intervention period, or any individualization of the contents or accompanied activity; all other assessments were identical to those in the intervention group.
As shown in Figure 2, data was collected at baseline (visit 1), directly after the intervention period (visit 2, first follow-up), and after a three-month period following the intervention, during which no training or assistance was offered (visit 3, second follow-up).
Moderate-to-vigorous physical activity (MVPA) in min/day was defined as the primary outcome and was assessed with waist-worn activity sensors (GT9X Link, ActiGraph, Pensacola, Florida, United States). These are medical devices that measure acceleration in the vertical, medio-lateral and antero-posterior axis (45, 46). The activity was measured over 7 days according to a previously defined protocol. Data was processed and analyzed with ActiLife 6 (ActiGraph, Pensacola, Florida, United States) following the recommendations of a systematic review of previous studies (47). In order to detect measurement errors, participants were asked to record non-wear times, activities in water, or activities with high energy expenditure but low acceleration (e.g., stationary bicycle), as well as biking to recognize the use of e-bikes. In the results section we report the measured values without manual correction to minimize evaluator influence or bias.
In addition, the self-rating “Freiburger Questionnaire on Physical Activity” was used (48) to confirm measurements. This also allows the separate analyses of sports activities.
Changes in fitness were assessed using simple tests of everyday activities: Sit-to-Stand Test (STS) (49) and Six-Minute Walk Test (6MWT) (50) with standardized test protocols.
Self-rating questionnaires were used: World Health Organization Quality of Life-Bref (WHOQOL-Bref) (51), Beck Depression Inventory (BDI II) (52), and “Barriers and barrier management in physical exercise” (53). The Montgomery-Åsberg Depression Scale (MADRS) (54, 55) was used as an expert rating in order to measure the severity of depressive symptoms.
The required sample size for the primary outcome (MVPA) was 15 per group according to the a priori power analysis with SAS/STAT Software (SAS Institute Inc., Cary, United States) with the following assumptions: adjusted type I significance level of α = 0.05, power of 1 – β = 0.8, MVPA-differences between groups at visit 3 of 27 min/day, standard deviation 25 min/day. Expected values were based on literature research (10).
Statistical analysis was performed with IBM SPSS Statistics (Version 22, International Business Machines Corporation, Armonk, New York, United States). After testing for normal distribution (Kolmogorov–Smirnov test, Shapiro Wilk test and histogram), the groups were compared either by analysis of variance (ANOVA) or the Mann–Whitney-U-test. The effect of time and time x group interactions were analyzed using the general linear model for repeated measurements with three factor levels and Bonferroni-corrected post-hoc tests or the Friedman test as the appropriate nonparametric alternative (56). We focused on the results of the parametric tests because they are described as robust against violations of the normal distribution (57). As a measure of effect size, partial η2 is reported (η2 ≥ 0.01: small effect; ≥0.06: medium effect; ≥0.14: large effect). Individual changes over time were compared between groups with analysis of covariance (ANCOVA) adjusted for baseline values. The difference between the means of both groups is reported as MD. The level of significance was α = 0.05. In the results section, we report the results of the Intention-To-Treat (ITT) analysis to avoid an overestimation of effects (58). Missing values due to dropout were imputed using the baseline observation carried-forward method. Single missing values, e.g., as a result of device failure, were not imputed. The Spearman-Rho and Pearson correlation coefficients were calculated for the analyses of relationships between parameters. Covariates are stated in the results section.
Figure 1 shows a flowchart of the number of participants and dropouts. Twenty-six women and four men with a mean age of 41 ± 12 years and a mean body mass index (BMI) of 28 ± 6 kg/m2 completed the study. Eighteen participants (58%) were overweight (BMI ≥ 25 kg/m2), 11 Participants (35.5%) were obese (BMI ≥ 30 kg/m2). The baseline comparisons between groups and additional baseline characteristics are given in Table 1. The differences between the groups did not reach statistical significance except for the treatment with antidepressants.
One patient dropped out due to missed appointments (intervention and first follow-up) for unknown reasons. Another patient in the intervention group was lost to follow-up (second follow-up, visit 3) for unknown reasons. The dropout rate over the study period, from baseline to the second follow-up, was 6%.
Attendance, an indicator for compliance, was 96% in the intervention group. Two appointments were canceled because of the participants’ workload, one was forgotten, and one did not take place due to dropout. Appointments were postponed in cases of scheduling difficulties or illness. In general, 73% of the appointments were held face-to-face, 27% were conducted via telephone, e-mail or video conference due to infection containment measures in the pandemic or to promote independent activity (six sessions).
The entire intervention period was impacted by the Covid-19 pandemic, for example lockdown of fitness centers. All activities had to be conducted in accordance with the varying official and individual safety precautions. Participants rated the influence of the pandemic situation on their physical activity with 7 out of 10 points (0: no influence and 10: significant influence) with 57% reporting reduced physical activity. In addition, patients rated the influence of the pandemic on their mental health with 7 out of 10 points (worsening of symptoms reported by 75%). The pandemic situation may also have influenced the choice of sports disciplines. Intervention records show that the main disciplines were walking and jogging, although 88% of the patients combined two activities. No team sports were chosen and a preference for individual disciplines was observed, e.g., swimming, yoga and fitness training. Ninety-four percent of the participants were active on their own; 75% joined a local sports group. Patients rated the intervention with 9 out of 10 points (0: not helpful; 10: very helpful). The items “personal contact” (9.6 points), “free of charge” (9.7 points) and “accompanied activity” (9.5 points) were rated as the most helpful aspects. When asked whether their physical activity was impacted by the intervention, 75% of the intervention group answered with “yes,” while only 13% of the control group reported changes in activity. 81% of interventions resulted in the completion of a sports program and increase in physical activity, and were rated as successful by the sport therapist.
No serious adverse events occurred, but 14 adverse events were reported. Only two were caused by physical activity (twisted knee and heel spur) and occurred in the control group.
Results of the ANOVA with repeated measurements for all three time points revealed a significant interaction between group and time [F(2, 54) = 4.283, p = 0.019, partial η2 = 0.137] as shown in Figure 3. Interactions remain significant after correction with the covariates baseline symptom severity or medication dose (p = 0.029 and p = 0.035, respectively).
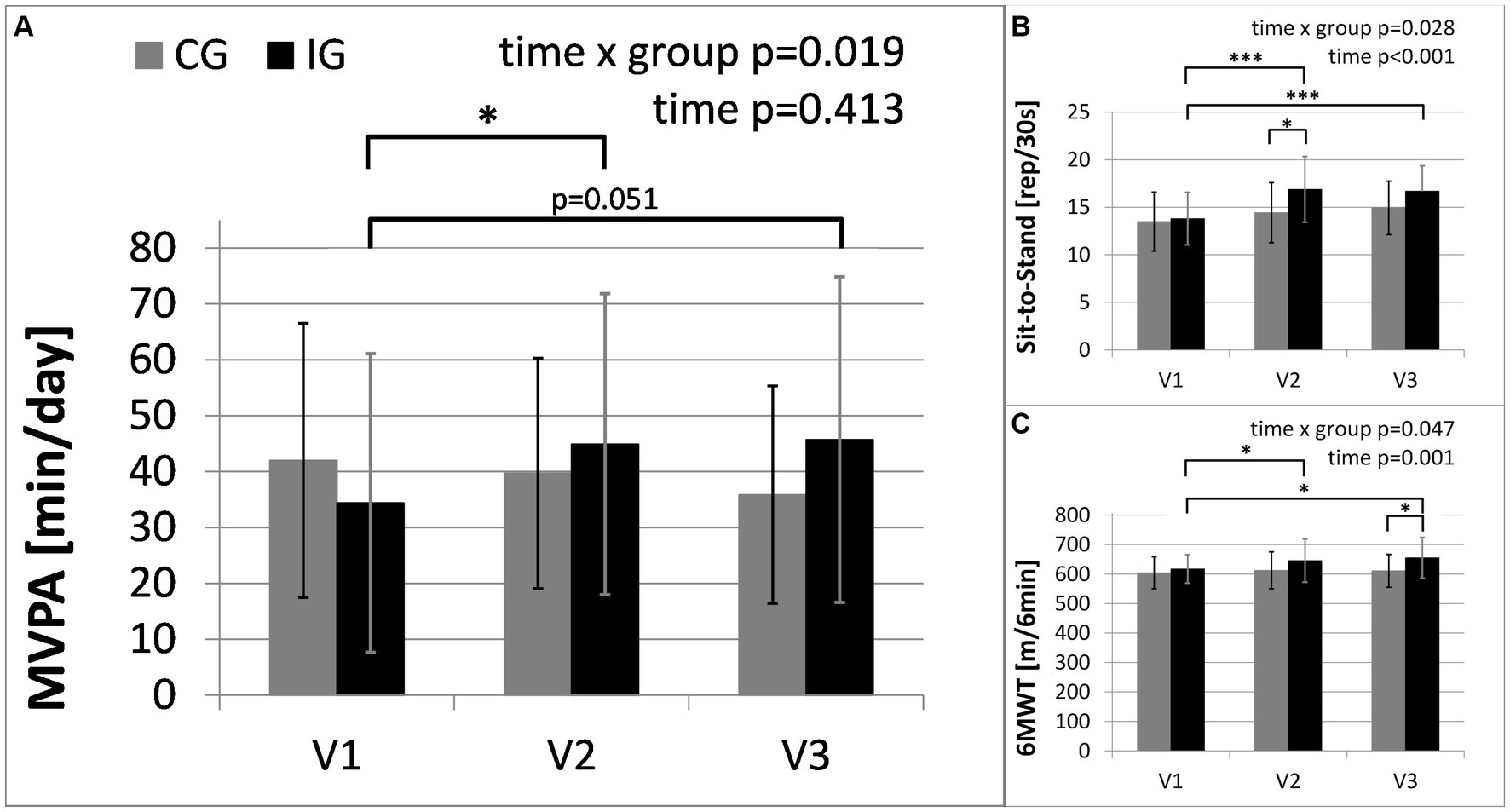
Figure 3. Main outcome of physical activity and secondary outcomes of physical health: (A) moderate-to-vigorous physical activity (MVPA) in minutes per day, assessed with activity sensors (CG, n = 14; IG, n = 15, due to failed measurements); (B) results of Six-Minute Walk Test (6MWT); (C) results of Sit-to-Stand Test. The intervention group (IG) is compared to the control group (CG) over the study period from baseline (V1) to the first follow-up visit (V2) directly after the intervention and the second follow-up (V3), 3 months after the end of the intervention (*: significant time effect/group difference p < 0.05; ***p < 0.001).
Between baseline and the second follow-up, MVPA increased by 10.4 min/day (SE = 4.3 min/day) in the intervention group, but decreased by about 5.1 min/day (SE = 4.5 min/day) in the control group (MD = 15.5 min/day, 95%-CI[2.7, 28.3], p = 0.020). Between baseline and the first follow-up, MVPA increased by 9.8 min/day (SE = 3.4 min/day) in the intervention group, and decreased by 1.5 min/day (SE = 3.5 min/day) in the control group (MD = 11.3 min/day, 95%-CI[1.1, 21.4], p = 0.031). Between the first and second follow-up, MVPA increased by 0.6 min/day (SE = 4.8 min/day) in the intervention group, and decreased further by 3.6 min/day (SE = 4.9 min/day) in the control group (MD = 4.2 min/day, 95%-CI[−10.0, 18.4], p = 0.247).
After adjusting for baseline values, MVPA at the second follow-up was significantly greater in the intervention group compared with the control group [F(1, 26) = 6.157, p = 0.020, partial η2 = 0.191].
The statistical results were confirmed when applying nonparametric tests (for variables with missing normal distribution) and analyzing the according-to-protocol population.
The results of the “Freiburger Questionnaire on Physical Activity” showed that sports activity significantly increased by 2.7 kcal/kg/week (SE = 1.2 kcal/kg/week) in the intervention group, but decreased by 1.6 kcal/kg/week (SE = 1.2 kcal/kg/week) in the control group between baseline and the second follow-up (MD = 4.3 kcal/kg/week, 95%-CI[0.8, 7.8], p = 0.019).
Table 3 provides the results of the exploratory analysis for selected secondary outcomes. Significant time x group interactions occurred in the results of the Sit-to-Stand Test (STS) and Six-Minute Walk Test (6MWT), representing the changes in physical fitness. In the intervention group STS and 6MWT increased by 21 and 6%, respectively. Both groups show significant improvements in the severity of depressive symptoms. Furthermore, Table 3 shows changes in the use of antidepressants. The changes in medication reflected a reduced number of patients taking antidepressants in the intervention group and an increased number of patients taking antidepressants in the control group (see Figure 4). No significant interactions were found between changes in depressive symptoms, physical activity and medication. In the “Barriers and barrier management in physical exercise” questionnaire, significant improvements were seen in barrier management in the intervention group (16% increase) with a significant time x group interaction for barrier management, but not for barrier severity. The management of barriers is significantly correlated with sports activity at visit 2 (r = 0.597, p = 0.001) and visit 3 (r = 0.489, p = 0.007). The perception of barriers to physical activity at baseline (r = −0.412, p = 0.024) and the results of 6MWT at baseline (r = −0.364, p = 0.048) correlated significantly with age, while all other parameters did not show significant correlations with age. No significant correlations with BMI or symptom severity were found.
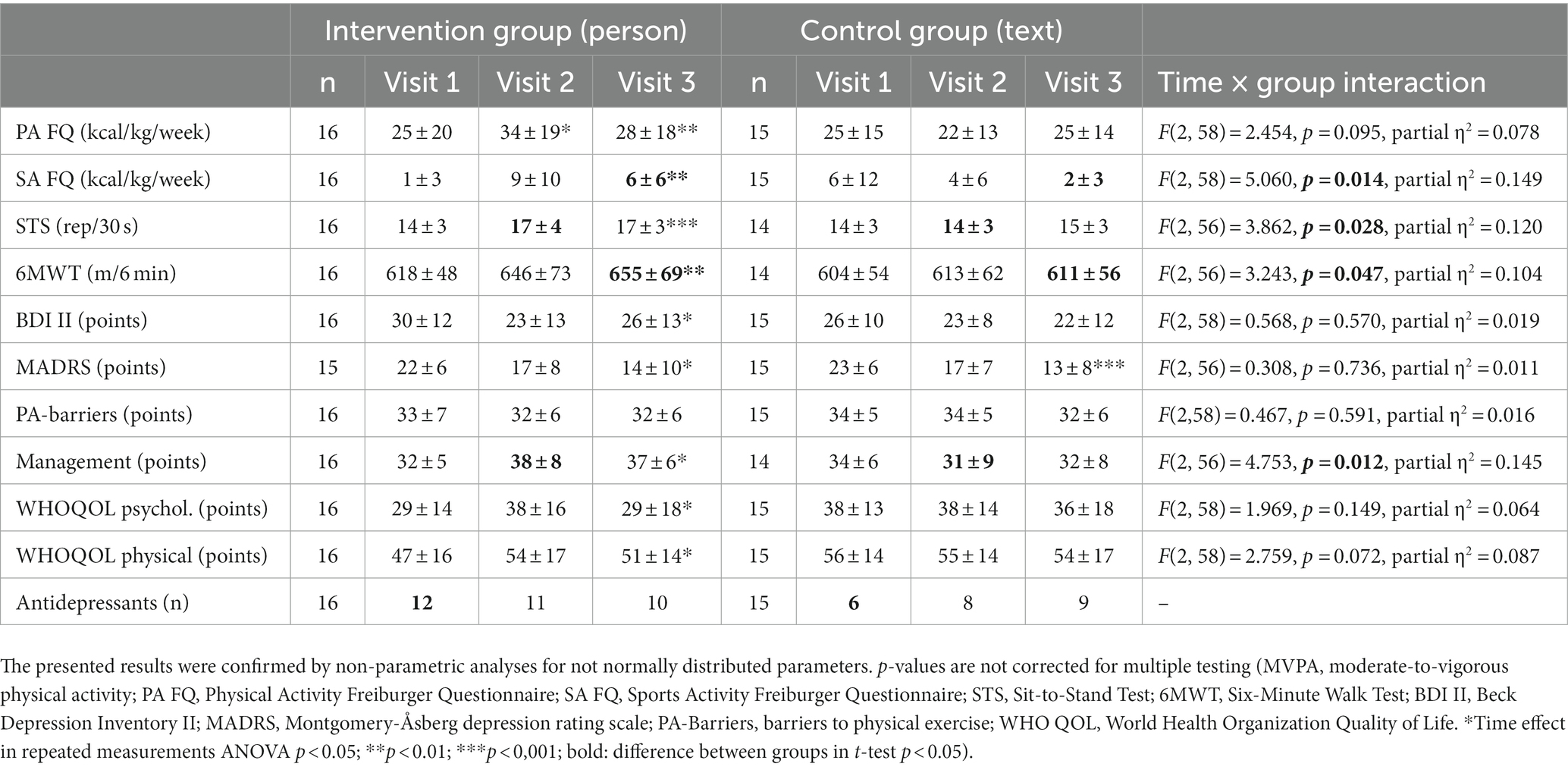
Table 3. Secondary outcomes as mean ± standard deviation in the intervention group and control group before (visit 1), directly after the intervention (visit 2) and at the second follow-up 3 months after the end of the intervention (visit 3).
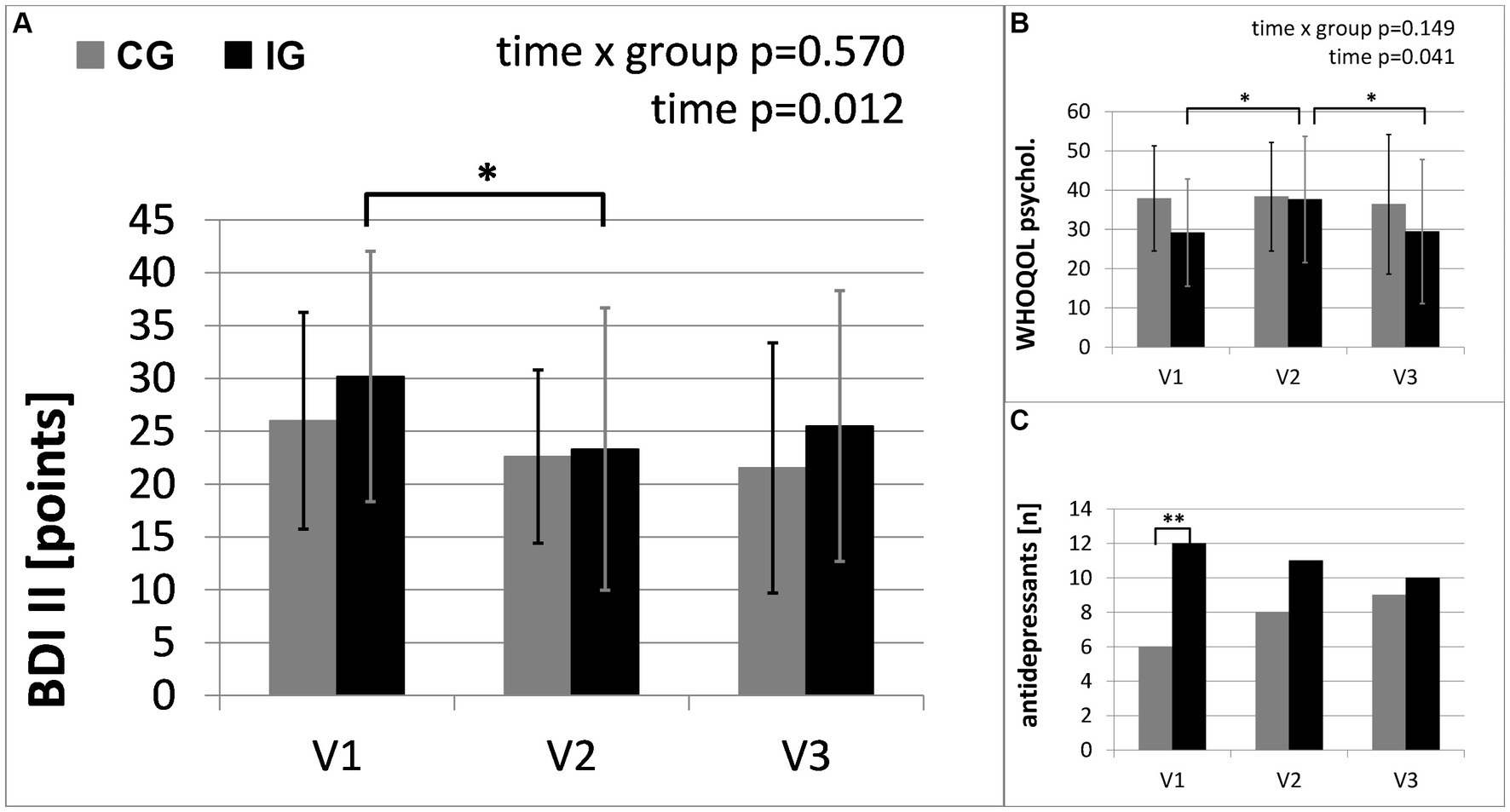
Figure 4. Secondary outcomes of mental health: (A) results of self-rating Beck Depression Inventory (BDI II); (B) results of World health Organization Quality of Life Questionnaire (WHOQOL-Bref); (C) number of participants taking antidepressants. The intervention group (IG) is compared to the control group (CG) over the study period from baseline (V1) to the first follow-up visit (V2) directly after the intervention and the second follow-up (V3), 3 months after the end of the intervention (*: significant time effect/group difference p < 0.05; **p < 0.01).
This study demonstrates a feasible and effective way to promote a long-term increase in self-selected physical activity in a real life setting for patients with depression, combining both physical training and psychological methods.
The intervention group showed a greater increase in moderate-to-vigorous physical activity than the control group. Since the control group was offered the same information via a written booklet and the same assessment visits, this difference can be attributed to the personal contact in combination with accompanied physical activity and an individualized, flexible approach to determine the best way to start activity in the Motivational Interviewing conversations. In addition to measured physical activity increases and the supporting results from the questionnaires, nearly 75% of the participants of the intervention group reported an increase in physical activity due to the intervention. Furthermore, we were able to show that parameters of physical health improved and medication with antidepressants was reduced in the intervention group in the course of the study period. Both groups showed decreased symptoms of depression. This was accompanied by a reduced use of antidepressants in the intervention group and an increased use in the control group. The periodic appointments for study assessments, together with the corresponding social contact and improved connection with the healthcare institution may have contributed to the improved mood in the control group.
In a study by Chalder et al., “physical activity facilitators” offered interviews including Motivational Interviewing techniques primarily via telephone (39, 40). This did not result in any significant reductions in depressive symptoms or medication dosage, but participants stated that they felt well and benefited from the intervention, although they would have wished for more active help. In our study, the increase in activity was smaller than expected, but baseline values were comparable to other measurements with similar activity sensors (10). Possible causes could be linked to the pandemic containment measures and fear of infection. In line with other studies (59), more than 50% of our participants reported reduced physical activity during the COVID-19 pandemic. The chosen activities, which showed a preference for activities which could be done alone and outside, and the rating of the influence of COVID-19 are both relevant in this context. However, despite the limited increase, current research has shown that even small gains in physical activity are effective in improving mental health (60).
In contrast with the INSHAPE program which involved weekly contact with “health mentors” and a free sports program for 6 months leading to increased self-reported activity (61), participants in our project were not supported after the initial six weekly sessions. Nevertheless, the intervention group still showed an increased level of physical and sports activity at the second follow-up 3 months after the end of the intervention (see Figure 3). The parameters of physical health (STS and 6MWT) show similar improvements over time. In contrast, the secondary outcomes of mental health show different changes. Following an initial improvement in mental health during the intervention, they show a slight decline in the follow-up period when there was no contact with the study personnel. Thus, regular appointments and personal contact and support may be critical factors in improving patients’ mental health. Continuous follow-up appointments at a higher frequency than 3-month intervals or alternative methods to increase social support may lead to improved outcomes.
Adverse events caused by physical activity were reported only in the control group and may be explained by too little knowledge of training methods and too much training load without an appropriate habituation process. On the basis of these data, the intervention was deemed safe for the participants. The intervention was also deemed feasible: Symptoms of depression, the COVID-19 lockdown, family difficulties, professional activities, orthopedic problems and pain were reported as the main barriers, but the small dropout rate of 6%, the high attendance rate of 96% and the positive ratings of the components in the intervention group suggest a good feasibility and the effectiveness of motivational strategies for the participants. The dropout rate was smaller than expected and significantly below that reported in a meta-analysis (20) for people with depression (18%). The study was feasible even under pandemic conditions in which there were strict restrictions for sports activity due to the individual training mode chosen for the intervention, in contrast with other study concepts involving group exercise [(e.g., 25)].
Feedback from the patients in the intervention group showed that it was helpful that the intervention was free of charge, presented personally, and included accompanied activity. The analysis of training logs showed that structure, social connections and proximity to their place of residence were important factors. The planning and provision of short workout sequences which could be integrated in daily life and a planned structure for a 7-day period were seen as effective aids. Exploring possibilities for sport near the patient’s place of residence, contacting existing groups or finding partners for sports activity in the family or among friends (to increase social support) were also reported as helpful strategies. Beyond that, feasibility could be further improved by allowing more flexibility in making appointments, e.g., individualizing the time intervals between the appointments. Additional factors for consideration are varying requirements for individual support and the need for extra support during challenging life situations, e.g., change of residence or occupation.
Not all barriers to an active lifestyle are related to the patients. Garvey et al. (24) identified barriers to the prescription of exercise on the part of healthcare providers: lack of knowledge on how to prescribe exercise and establish contact with trained physical activity specialists, concerns about the patient’s ability to exercise, risk factors, such as comorbidities, or placing more stressors on their clients (24). Improvement in cooperation between the different professions is needed in addition to the establishment of routine referral methods with the possibility of individual support for physical activity. Physical exercise is recommended in the guidelines for the treatment of depression (62). Consequently, the translation of the treatment guidelines into clinical practice is the next important step. The German “Rezept für Bewegung” (translation: “prescription of activity”) by the German Olympic Sports Confederation and German Medical Association or global health initiatives such as “Exercise is Medicine®” by the American College of Sports Medicine (ACSM) already pursue this goal, but physical activity is still underused in the therapy of patients with depression (18). A more widespread application is desirable, but would need to take account of differences in country-specific health care systems and lifestyles.
The present study has some limitations: Both groups experienced changes in therapy and medication during the study participation. A critical point for the statistical analysis is the small sample size. While larger effects can be detected with sufficient power, it is possible that small to medium effects would only reach statistical significance in bigger samples. Also, fewer men than women participated. Although the primary outcome was measured with activity sensors, data for secondary outcomes were collected with self-rating questionnaires and therefore may have been influenced by social desirability. The activity sensors used in the study measured acceleration, but not heart rate or effort. Therefore, the classification of activity intensity may not be sufficiently accurate. However, to address this potential limitation, we used wear time and activity logs as well as activity questionnaires and checked each automatic analysis. It should be taken into consideration that absolute values measured with varying activity sensors lack comparability between studies [(e.g., 63)]. Our study procedure was furthermore impacted by the pandemic situation and its effects on physical activity (59) and the follow-up results were influenced by COVID-19 infections in two cases.
Future research should target motivational aspects of being physically active for people with mental illness and seek greater synergies between the fields of psychology and sports and training medicine. On a larger scale, projects are needed which analyze the cost–benefit ratio and test the implementation of programs supporting physical activities in the healthcare system (64). Additionally, larger samples would allow the comparison of single features of the interventions and the analysis of the influence of moderating factors and predictors of response.
Personalized training with Motivational Interviewing and accompanied physical activity was a feasible and effective aid to increase moderate-to-vigorous physical activity and improve physical performance as measured with Sit-to-Stand Test and Six-Minute Walk Test in people with depression. The combination of physical training and psychological methods focusing on individual activity preferences, needs, and possibilities in the everyday life of the participants resulted in a low dropout rate and improved management of barriers preventing physical activity. Participants in the intervention group showed improvements in depressive symptoms in spite of reductions in their pharmacological therapy. The integration of individual support regarding physical activity, including the successful methods evaluated in this project, should be considered for the multimodal treatment of depression.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Hannover Medical School. The patients/participants provided their written informed consent to participate in this study.
KK-V, SH, AK, UT, and KK planned and designed the study. ES and AK recruited the participants. KK-V, ES, and KK collected the data. KK-V and SH were responsible for the analysis and interpretation of the data. KK-V wrote the first draft of the manuscript. SH, ES, AK, UT, and KK contributed to the discussion, reviewed, and edited the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Robert-Enke-Stiftung (Schillerstraße 4, 30890 Barsinghausen, Germany). This publication is funded by the Deutsche Forschungsgemeinschaft (DFG) as part of the “Open Access Publikationskosten” program.
We thank all participants. We acknowledge the support of our colleagues (especially John Adel, Klaus Wiechmann, Gudrun Protte, Lukas Radziwolek, Elke Gützlaff, Kira Wolters, Marie Wojtke, Jule Menge, Julia Dietze, Thorsten Folsche, Farina Linhardt, Sebastian Häckl).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Schuch, F , Vancampfort, D , Firth, J , Rosenbaum, S , Ward, P , Reichert, T, et al. Physical activity and sedentary behavior in people with major depressive disorder: a systematic review and meta-analysis. J Affect Disord. (2017) 210:139–50. doi: 10.1016/j.jad.2016.10.050
2. Difrancesco, S , Lamers, F , Riese, H , Merikangas, KR , Beekman, AT , van Hemert, AM, et al. Sleep, circadian rhythm, and physical activity patterns in depressive and anxiety disorders: a 2-week ambulatory assessment study. Depress Anxiety. (2019) 36:975–86. doi: 10.1002/da.22949
3. Denche-Zamorano, Á , Ajenjo-Gomez, D , Pereira-Payo, D , Galán-Arroyo, C , Vega-Muñoz, A , Contreras-Barraza, N, et al. Physical activity frequency and depression in the Spanish population. Int J Environ Res Public Health. (2022) 19:19. doi: 10.3390/ijerph192214704
4. Ma, R , Romano, E , Vancampfort, D , Firth, J , Stubbs, B , and Koyanagi, A . Association between physical activity and comorbid anxiety/depression in 46 low- and middle-income countries. J Affect Disord. (2023) 320:544–51. doi: 10.1016/j.jad.2022.10.002
5. Chang, Y , Park, K-Y , Hwang, H-S , and Park, H-K . Association between type and intensity of physical activity and depression. Korean J Fam Med. (2022) 43:254–60. doi: 10.4082/kjfm.21.0146
6. Lee, J-M , and Ryan, EJ . The relationship between the frequency and duration of physical activity and depression in older adults with multiple chronic diseases. J Clin Med. (2022) 11:11. doi: 10.3390/jcm11216355
7. Rutherford, ER , Vandelanotte, C , Chapman, J , and To, QG . Associations between depression, domain-specific physical activity, and BMI among US adults: NHANES 2011-2014 cross-sectional data. BMC Public Health. (2022) 22:1618. doi: 10.1186/s12889-022-14037-4
8. Yang, D , Yang, M , Bai, J , Ma, Y , and Yu, C . Association between physical activity intensity and the risk for depression among adults from the National Health and nutrition examination survey 2007-2018. Front Aging Neurosci. (2022) 14:844414. doi: 10.3389/fnagi.2022.844414
9. De Hert, M , Correll, CU , Bobes, J , Cetkovich-Bakmas, M , Cohen, D , Asai, I, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. (2011) 10:52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x
10. Helgadóttir, B , Forsell, Y , and Ekblom, Ö . Physical activity patterns of people affected by depressive and anxiety disorders as measured by accelerometers: a cross-sectional study. PLoS One. (2015) 10:e0115894. doi: 10.1371/journal.pone.0115894
11. Kandola, A , Ashdown-Franks, G , Hendrikse, J , Sabiston, CM , and Stubbs, B . Physical activity and depression: towards understanding the antidepressant mechanisms of physical activity. Neurosci Biobehav Rev. (2019) 107:525–39. doi: 10.1016/j.neubiorev.2019.09.040
12. Kahl, KG , Kerling, A , Tegtbur, U , Gützlaff, E , Herrmann, J , Borchert, L, et al. Effects of additional exercise training on epicardial, intra-abdominal and subcutaneous adipose tissue in major depressive disorder: a randomized pilot study. J Affect Disord. (2016) 192:91–7. doi: 10.1016/j.jad.2015.12.015
13. Kerling, A , Tegtbur, U , Gützlaff, E , Kück, M , Borchert, L , Ates, Z, et al. Effects of adjunctive exercise on physiological and psychological parameters in depression: a randomized pilot trial. J Affect Disord. (2015) 177:1–6. doi: 10.1016/j.jad.2015.01.006
14. Kerling, A , Von, BA , Kück, M , Tegtbur, U , Grams, L , Haufe, S, et al. Exercise therapy improves aerobic capacity of inpatients with major depressive disorder. Brain Behav. (2016) 6:e00469. doi: 10.1002/brb3.469
15. Kerling, A , Kück, M , Tegtbur, U , Grams, L , Weber-Spickschen, S , Hanke, A, et al. Exercise increases serum brain-derived neurotrophic factor in patients with major depressive disorder. J Affect Disord. (2017) 215:152–5. doi: 10.1016/j.jad.2017.03.034
16. Haufe, S , Kahl, KG , Kerling, A , Protte, G , Bayerle, P , Stenner, HT, et al. Employers with metabolic syndrome and increased depression/anxiety severity profit most from structured exercise intervention for work ability and quality of life. Front Psych. (2020) 11:562. doi: 10.3389/fpsyt.2020.00562
17. Firth, J , Rosenbaum, S , Stubbs, B , Gorczynski, P , Yung, AR , and Vancampfort, D . Motivating factors and barriers towards exercise in severe mental illness: a systematic review and meta-analysis. Psychol Med. (2016) 46:2869–81. doi: 10.1017/S0033291716001732
18. Chen, C , Beaunoyer, E , Guitton, MJ , and Wang, J . Physical activity as a clinical tool against depression: opportunities and challenges. J Integr Neurosci. (2022) 21:132. doi: 10.31083/j.jin2105132
19. Vancampfort, D , Stubbs, B , Sienaert, P , Wyckaert, S , De, HM , Rosenbaum, S, et al. What are the factors that influence physical activity participation in individuals with depression? A review of physical activity correlates from 59 studies. Psychiatr Danub. (2015) 27:210–24.
20. Stubbs, B , Vancampfort, D , Rosenbaum, S , Ward, PB , Richards, J , Soundy, A, et al. Dropout from exercise randomized controlled trials among people with depression: a meta-analysis and meta regression. J Affect Disord. (2016) 190:457–66. doi: 10.1016/j.jad.2015.10.019
21. Vancampfort, D , Madou, T , Moens, H , De Backer, T , Vanhalst, P , Helon, C, et al. Could autonomous motivation hold the key to successfully implementing lifestyle changes in affective disorders? A multicentre cross sectional study. Psychiatry Res. (2015) 228:100–6. doi: 10.1016/j.psychres.2015.04.021
22. Milanovic, M , Ayukawa, E , Usyatynsky, A , Holshausen, K , and Bowie, CR . Self efficacy in depression: bridging the gap between competence and real world functioning. J Nerv Ment Dis. (2018) 206:350–5. doi: 10.1097/NMD.0000000000000804
23. Wong-Anuchit, C , Chantamit-O-Pas, C , Schneider, JK , and Mills, AC . Motivational interviewing-based compliance/adherence therapy interventions to improve psychiatric symptoms of people with severe mental illness: meta-analysis. J Am Psychiatr Nurses Assoc. (2019) 25:122–33. doi: 10.1177/1078390318761790
24. Garvey, L , Benson, AC , Benger, D , Short, T , Banyard, H , and Edward, K-L . The perceptions of mental health clinicians integrating exercise as an adjunct to routine treatment of depression and anxiety. Int J Ment Health Nurs. (2022) 32:502–12. doi: 10.1111/inm.13089
25. McNamara, G , Robertson, C , Hartmann, T , and Rossiter, R . Effectiveness and benefits of exercise on older people living with mental illness’ physical and psychological outcomes in regional Australia: a mixed-methods study. J Aging Phys Act. (2022) 31:417–29. doi: 10.1123/japa.2021-0514
26. Nyström, MB , Stenling, A , Sjöström, E , Neely, G , Lindner, P , Hassmén, P, et al. Behavioral activation versus physical activity via the internet: a randomized controlled trial. J Affect Disord. (2017) 215:85–93. doi: 10.1016/j.jad.2017.03.018
27. Martiny, K , Refsgaard, E , Lund, V , Lunde, M , Sørensen, L , Thougaard, B, et al. A 9-week randomized trial comparing a chronotherapeutic intervention (wake and light therapy) to exercise in major depressive disorder patients treated with duloxetine. J Clin Psychiatry. (2012) 73:1234–42. doi: 10.4088/JCP.11m07625
28. Schmitter, M , Spijker, J , Smit, F , Tendolkar, I , Derksen, A-M , Oostelbos, P, et al. Exercise enhances: study protocol of a randomized controlled trial on aerobic exercise as depression treatment augmentation. BMC Psychiatry. (2020) 20:585. doi: 10.1186/s12888-020-02989-z
29. Moreira-Neto, A , Martins, B , Miliatto, A , Nucci, MP , and Silva-Batista, C . Can remotely supervised exercise positively affect self-reported depressive symptoms and physical activity levels during social distancing? Psychiatry Res. (2021) 301:113969. doi: 10.1016/j.psychres.2021.113969
30. D’Amore, C , Reid, JC , Chan, M , Fan, S , Huang, A , Louie, J, et al. Interventions including smart technology compared with face-to-face physical activity interventions in older adults: systematic review and meta-analysis. J Med Internet Res. (2022) 24:e36134. doi: 10.2196/36134
31. Lippke, S , Ratz, T , Keller, FM , Juljugin, D , Peters, M , Pischke, C, et al. Mitigating feelings of loneliness and depression by means of web-based or print-based physical activity interventions: pooled analysis of 2 community-based intervention trials. JMIR Aging. (2022) 5:e36515. doi: 10.2196/36515
32. Daumit, GL , Dalcin, AT , Jerome, GJ , Young, DR , Charleston, J , Crum, RM, et al. A behavioral weight-loss intervention for persons with serious mental illness in psychiatric rehabilitation centers. Int J Obes. (2011) 35:1114–23. doi: 10.1038/ijo.2010.224
33. Goldberg, RW , Reeves, G , Tapscott, S , Medoff, D , Dickerson, F , Goldberg, AP, et al. “MOVE!” outcomes of a weight loss program modified for veterans with serious mental illness. Psychiatr Serv. (2013) 64:737–44. doi: 10.1176/appi.ps.201200314
34. Dilling, H . Internationale Klassifikation psychischer Störungen: ICD-10 Kapitel V (F) diagnostische Kriterien für Forschung und Praxis. Bern: Hogrefe (2016).
35. World Health Organization . WHO guidelines on physical activity and sedentary behaviour. Geneva: World Health Organization (2020).
36. Hopko, DR , Lejuez, CW , Ruggiero, KJ , and Eifert, GH . Contemporary behavioral activation treatments for depression: procedures, principles, and progress. Clin Psychol Rev. (2003) 23:699–717. doi: 10.1016/S0272-7358(03)00070-9
37. Ekers, D , Webster, L , van Straten, A , Cuijpers, P , Richards, D , and Gilbody, S . Behavioural activation for depression; an update of meta-analysis of effectiveness and sub group analysis. PLoS One. (2014) 9:e100100. doi: 10.1371/journal.pone.0100100
38. Euteneuer, F , Dannehl, K , Del Rey, A , Engler, H , Schedlowski, M , and Rief, W . Immunological effects of behavioral activation with exercise in major depression: an exploratory randomized controlled trial. Transl Psychiatry. (2017) 7:e1132. doi: 10.1038/tp.2017.76
39. Chalder, M , Wiles, NJ , Campbell, J , Hollinghurst, SP , Searle, A , Haase, AM, et al. A pragmatic randomised controlled trial to evaluate the cost-effectiveness of a physical activity intervention as a treatment for depression: the treating depression with physical activity (TREAD) trial. Health Technol Assess. (2012) 16:iii–v. doi: 10.3310/hta16100
40. Chalder, M , Wiles, NJ , Campbell, J , Hollinghurst, SP , Haase, AM , Taylor, AH, et al. Facilitated physical activity as a treatment for depressed adults: randomised controlled trial. BMJ. (2012) 344:e2758. doi: 10.1136/bmj.e2758
41. Teixeira, PJ , Carraça, EV , Markland, D , Silva, MN , and Ryan, RM . Exercise, physical activity, and self-determination theory: a systematic review. Int J Behav Nutr Phys Act. (2012) 9:78. doi: 10.1186/1479-5868-9-78
42. Lundahl, B , Moleni, T , Burke, BL , Butters, R , Tollefson, D , Butler, C, et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns. (2013) 93:157–68. doi: 10.1016/j.pec.2013.07.012
43. Rollnick, S , Butler, CC , Kinnersley, P , Gregory, J , and Mash, B . Motivational interviewing. BMJ. (2010) 340:c1900. doi: 10.1136/bmj.c1900
44. Garber, CE , Blissmer, B , Deschenes, MR , Franklin, BA , Lamonte, MJ , Lee, I-M, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. (2011) 43:1334–59. doi: 10.1249/MSS.0b013e318213fefb
45. Sasaki, JE , John, D , and Freedson, PS . Validation and comparison of ActiGraph activity monitors. J Sci Med Sport. (2011) 14:411–6. doi: 10.1016/j.jsams.2011.04.003
46. Santos-Lozano, A , Santín-Medeiros, F , Cardon, G , Torres-Luque, G , Bailón, R , Bergmeir, C, et al. Actigraph GT3X: validation and determination of physical activity intensity cut points. Int J Sports Med. (2013) 34:975–82. doi: 10.1055/s-0033-1337945
47. Migueles, JH , Cadenas-Sanchez, C , Ekelund, U , Delisle Nyström, C , Mora-Gonzalez, J , Löf, M, et al. Accelerometer data collection and processing criteria to assess physical activity and other outcomes: a systematic review and practical considerations. Sports Med. (2017) 47:1821–45. doi: 10.1007/s40279-017-0716-0
48. Frey, I , and Berg, A . Physical activity counseling: assessment of physical activity by questionnaire. Eur J Sport Sci. (2002) 2:1–6. doi: 10.1080/17461390200072406
49. Jones, CJ , Rikli, RE , and Beam, WC . A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport. (1999) 70:113–9. doi: 10.1080/02701367.1999.10608028
50. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. (American Thoracic Society) (2002) 166:111–7. doi: 10.1164/ajrccm.166.1.at1102
51. The WHOQOL Group . The World Health Organization quality of life assessment (WHOQOL): development and general psychometric properties. Soc Sci Med. (1998) 46:1569–85. doi: 10.1016/S0277-9536(98)00009-4
52. Hautzinger, M , Keller, F , and Kühner, C . BDI-II-Depressionsinventar. Frankfurt: Harcourt Test Service (2006).
53. Krämer, L , and Fuchs, R . Barrieren und Barrierenmanagement im Prozess der Sportteilnahme. Zeitsch Gesundheitspsychol. (2010) 18:170–82. doi: 10.1026/0943-8149/a000026
54. Collegium Internationale Psychiatriae Scalarum . Internationale Skalen für Psychiatrie. Göttingen: Hogrefe (2015).
55. Montgomery, SA , and Asberg, M . A new depression scale designed to be sensitive to change. Br J Psychiatry. (1979) 134:382–9. doi: 10.1192/bjp.134.4.382
57. Nahm, FS . Nonparametric statistical tests for the continuous data: the basic concept and the practical use. Korean J Anesthesiol. (2016) 69:8–14. doi: 10.4097/kjae.2016.69.1.8
58. Tripepi, G , Chesnaye, NC , Dekker, FW , Zoccali, C , and Jager, KJ . Intention to treat and per protocol analysis in clinical trials. Nephrology. (2020) 25:513–7. doi: 10.1111/nep.13709
59. Wunsch, K , Kienberger, K , and Niessner, C . Changes in physical activity patterns due to the Covid-19 pandemic: a systematic review and Meta-analysis. Int J Environ Res Public Health. (2022) 19:19. doi: 10.3390/ijerph19042250
60. Pearce, M , Garcia, L , Abbas, A , Strain, T , Schuch, FB , Golubic, R, et al. Association between physical activity and risk of depression: a systematic review and meta-analysis. JAMA Psychiat. (2022) 79:550–9. doi: 10.1001/jamapsychiatry.2022.0609
61. van Citters, AD , Pratt, SI , Jue, K , Williams, G , Miller, PT , Xie, H, et al. A pilot evaluation of the in SHAPE individualized health promotion intervention for adults with mental illness. Community Ment Health J. (2010) 46:540–52. doi: 10.1007/s10597-009-9272-x
62. Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) . Nationale VersorgungsLeitlinie Unipolare Depression – Langfassung. doi: 10.6101/AZQ/000496
63. Sasayama, K , and Adachi, M . Comparison of ActiGraph GT9X link with two Japanese accelerometers for assessments of free-living physical activity in junior high school students. BMC Res Notes. (2020) 13:390. doi: 10.1186/s13104-020-05231-x
Keywords: depression, exercise, physical activity, motivation, motivational interviewing, sport
Citation: Keller-Varady K, Haufe S, Schieffer E, Kerling A, Tegtbur U and Kahl KG (2023) Personalized training as a promoter for physical activity in people with depressive disorder—a randomized controlled trial in Germany. Front. Psychiatry. 14:1158705. doi: 10.3389/fpsyt.2023.1158705
Received: 04 February 2023; Accepted: 13 June 2023;
Published: 29 June 2023.
Edited by:
Melissa Thong, German Cancer Research Center (DKFZ), GermanyReviewed by:
Kaisa Kaseva, University of Helsinki, FinlandCopyright © 2023 Keller-Varady, Haufe, Schieffer, Kerling, Tegtbur and Kahl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katriona Keller-Varady, a2VsbGVyLXZhcmFkeS5rYXRyaW9uYUBtaC1oYW5ub3Zlci5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.