
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry, 01 June 2023
Sec. Sleep Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1157790
This article is part of the Research TopicThe Relationship between Sleep and Pain in Chronic Pain ConditionsView all 4 articles
Chronic pain (CP) is a prevalent problem, and more than half of patients with CP have sleep disorders. CP comorbidity with sleep disorders imposes immense suffering and seriously affects the patient’s quality of life, which is a challenging issue encountered by clinicians. Although the reciprocal interactions between pain and sleep have been studied to some degree, there is still a lack of awareness and comprehensive description of CP comorbidity with sleep disorders. In this narrative review article, we summarize the current knowledge about the present estimates of the prevalence of comorbid sleep disorders in CP patients, sleep detection methods, sleep characterization in CP, and the effect of sleep disorders on CP and current therapies. We also summarize current knowledge of the neurochemical mechanisms of CP comorbidity with sleep disorders. In conclusion, insufficient attention has been paid to the role of sleep disorders in CP patients, and CP patients should be screened for sleep disorders in the clinic. Special attention should be given to a possible risk of drug–drug interaction when using two types of drugs targeting pain and sleep simultaneously. The current insight into the neurobiological mechanisms underlying CP comorbidity with sleep disorders is still rather limited.
Chronic pain (CP) refers to pain that persists or recurs for more than 3 months, which was listed as an independent disease for the first time in the International Classification of Diseases (ICD-11) revised by the World Health Organization (WHO) in 2018 (1). About 13%–25% of the general population is affected by CP, which is a critical clinical issue and one of the major causes of global impairment (2, 3). Numerous CP patients suffer from poor quality of life, including sleep issues, anxiety, and depression (4–6). According to a survey report in the United States (US), the annual economic loss caused by CP is approximately 560–635 billion US dollars (7).
Sleep is an important physiologic process to maintain homeostasis and function of the body (8). Over 65% of CP patients report having trouble sleeping, making it one of their main complaints (9). Sleep problems include difficulty falling asleep, sleep insufficiency, and low sleep quality, which can lead to a wide range of physical and mental problems (10–12). There is a direct relationship between the degree of sleep issues and the intensity of pain (13). For instance, fibromyalgia can significantly alter the architecture of sleep (14–16). Similarly, poor sleep has negative impacts on CP. According to the Trøndelag Health (HUNT) study, which monitored participants for up to 22 years, people who reported having insomnia symptoms for more than 10 years in combination with short sleep were at an especially high risk of experiencing recurrent spinal pain, and improvement in insomnia symptoms was associated with a favorable prognosis (17). A systematic review and meta-analysis also found that sleep disturbances and disorders were significantly related to chronic postsurgical pain (18). Additionally, people with sleep issues have higher levels of pain-related anxiety, medicine use, and self-reported diseases compared to pain patients who sleep properly (13). Thus, it is clear that CP and sleep disorders are frequently thought to interact with each other. The relevance of the mutual interaction between the two has been gradually realized as more in-depth studies on the comorbidity of CP and sleep problems have been conducted, which has also become a hot topic in pain research. As a result, therapeutic care becomes more intricate and difficult when a sleep disturbance develops in a patient with CP. Given the importance of sleep disorder in the development and unfavorable prognosis of CP, research has begun to increase in recent years to explore the basic neurochemical mechanisms underlying this reciprocal relationship. We examined and condensed an overview of the development of this problem into key areas in this article: clinical presentation and sleep monitoring, therapy and prognosis, and underlying mechanism.
Sleep disorders are also a major public health problem that plagues human physical and mental health (19). According to research data, 27% of people in the world have sleep disorders (20). In numerous experimental sleep deprivation models, limiting or interrupting sleep for a day or a few days can cause sleep disorders such as insufficient sleep time and poor sleep quality, which can result in hyperalgesia or spontaneous pain, exacerbating chronic pain (21). According to studies, 10%–15% of the general population suffers from insomnia (22). Sleep and pain are two vital physiological functions that interact with each other and have an impact on one another in humans. According to research, at least 40% of people with insomnia also have CP, and 50%–88% of CP patients have sleep difficulties (20). Diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria, insomnia is more common in the CP community than in the general population, with a frequency of 24%–32% (22). Meanwhile, a recent meta-analysis has shown that individuals with CP experience significant sleep disturbances, particularly with respect to sleep initiation and maintenance (23). This study has also found that the pooled prevalence of sleep disorders in CP was 44%, with insomnia (72%), restless legs syndrome (32%), and obstructive sleep apnea (32%) being the most common diagnoses (23).
Various sleep indicators can be used to assess CP patients’ sleep quality or duration, including sleep onset time, wakefulness after sleep onset (WASO), sleep onset latency (SOL), sleep efficiency (SE), and total sleep time (TST). There are both subjective and objective methods to assess sleep quality. In terms of objective methods for monitoring sleep, polysomnography (PSG) and actigraphy have high reliability in obtaining information on sleep parameters. PSG is the gold standard method for analyzing sleep quality, using many sensors and electronics; however, it requires a cumbersome, complex setup of electronic sensors and needs to be performed in a laboratory under the control of trained technicians. These requirements may disrupt natural sleep patterns, and one-night sleep data are often insufficient to represent normal sleep behavior. Therefore, it cannot be used frequently in clinical practice due to the disadvantages of economic cost and time costs (24). Nowadays, actigraphy is the most extensively used device to assess sleep quality at home. Actigraphy is a relatively inexpensive and non-invasive method of assessing sleep–wake rhythms over long periods, from days to months (25). It has certain clinical value for sleep disorders accompanied by sleep rhythm disturbance, emotional disorder and body movement abnormality (26). Compared with PSG, actigraphy has limited accuracy in detecting wakefulness during sleep episodes and does not provide information on sleep architecture (27). In terms of subjective methods, many self-report questionnaires have been developed to assess sleep, such as Pittsburgh Sleep Quality Index (PSQI), Sleep Severity Scale (AIS), Sleep Severity Index (ISI), Mini Sleep Questionnaire (MSQ), Jenkins Sleep Scale (JSS), Leeds Sleep Assessment Questionnaire (LSEQ), and Epworth Sleep Scale (ESS) (24). These questionnaires can record sleep quality by estimating subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleep medications, and daytime dysfunction as indicators. Among these questionnaires, the PSQI is the most commonly used measure of subjective self-report sleep quality, which is considered an accepted reference or gold standard for self-perceived sleep quality (28, 29).
Sleep disturbances include reduced SE and altered sleep architecture, which occur in patients with CP (30). CP patients have a decrease in TST and SE and an increase in SOL, WASO, and a number of awakenings, indicating that patients with CP have less sleep time, take longer to fall asleep, and spend more time awake (23, 31). SE is the ratio of total sleep time to total time in bed. Many studies have found that lower SE has a strong association with next-day pain intensity in CP patients (32, 33). A review has found that there is high heterogeneity in sleep architecture according to individual study reports (31). However, a recent meta-analysis has found that CP patients spent more time in the first stage of sleep during the non-rapid eye movement (NREM) and experienced more sleep fragmentation (23). Sleep fragmentation refers to shortened sleep bouts and frequent transitions between sleep and wake states. Patients with CP had a more frequent transition from sleep to wakefulness (23). Sleep fragmentation could impair endogenous pain-inhibitory function in both healthy adults and CP patients (34, 35). One animal experiment has also confirmed that sleep fragmentation combined with musculoskeletal sensitization could exacerbate mechanical hypersensitivity; increase the number of sleep–wake state transitions during the light and dark periods; change NREM sleep, rapid eye movement sleep, and wakefulness; and alter delta power during NREM sleep (36).
Poor sleep is a key factor in the development and maintenance of CP (9). Sleep deprivation can increase subjective pain intensity and worsen peripheral/central pain sensitization in healthy individuals (37). Sleep disorders significantly increase the risk for reduced pain tolerance, and sleep extension increases pain tolerance in healthy individuals (38, 39). Sleep disorders are also one of the most common triggers of migraine (40). Patients with both CP and sleep disturbances have greater pain severity, longer duration of pain, greater disability, are less physically active than those without sleep disturbances, and are more likely to have concurrent depression, catastrophizing, anxiety, and suicidal ideation (22). For example, restless sleep can increase depressive symptoms and fatigue among individuals with knee osteoarthritis (41). The coexistence of insomnia and chronic musculoskeletal pain results in greater pain intensity and alterations in sleep homeostasis (42). Among patients with neuropathic pain, those with poor sleep quality experience more pain, more severe depressive states, and worse quality of life than patients with good sleep quality, with a positive correlation between sleep quality and emotional state (43). In patients with musculoskeletal disorders, poorer sleep quality is associated with higher pain levels, and a high frequency of poor sleep quality is more prevalent in females (44). Another study has also found that sleep deficiency, particularly insomnia and poor sleep quality, might modify the effectiveness of psychological treatments for CP (45). If participants report better sleep quality, they will have less pain and better self-rated health on the following day (46). Improving sleep quality is an important approach to reduce the CP burden (47). Another systematic scoping review has suggested that sleep disturbances, and sleep disorders were associated with worse pain outcomes and treatment-induced sleep improvements ameliorated pain outcomes among veterans with CP (48). Even short-term improvements in sleep can predict long-term improvements in CP and fatigue in older adults with osteoarthritis (49, 50). On the other hand, a cross-sectional study has found that CP can directly affect sleep quality, and poor sleep quality can further lead to depression (51). The aforementioned results indicate that the accurate assessment and timely treatment of sleep problems are important treatment modalities for pain management.
Due to the significant sleep-pain interactions, it has been suggested that multidisciplinary treatment is required to manage CP. Currently, treatments for sleep disorder of patients with CP include pharmacotherapy and non-pharmacological therapy.
In terms of pharmacotherapy, two aspects are particularly important to consider. The first aspect is that medications used to treat pain or sleep can also have direct effects on sleep or pain, respectively. The second aspect is that multiple drugs are frequently used in clinical practice to target sleep disorders and CP simultaneously, which might increase the risk of drug interactions. This topic is discussed in detail in a review article by Herrero et al. (52). Here, therefore, only a brief summary is provided.
Opioids are widely used for the treatment of chronic pain. Recently, a study has found that current and previous chronic users of opioids had differed significantly from the opioid-naïve regarding sleep quality, sleep duration, sleep disturbances, and daytime dysfunction after controlling for sleep medications in CP patients, and opioid-naïve participants had better sleep quality (53, 54). Another study has also found that opioid use was associated with a 30% increased risk of poor-quality sleep and an approximately 15% increased risk for short sleep duration in older adults with CP (53, 54). In addition, chronic opioid use increases the risk of sleep apnea and sleep-disordered breathing (55–57) and may lead to an increased risk of death in patients with chronic non-cancer pain (55). A prospective cohort observational study has suggested that chronic non-cancer pain patients with OPRM1 118-GG genotype were more susceptible to an increase in sleep problems and worsening sleep patterns while taking opioids (58).
In response to the opioid epidemic, medical cannabinoids are increasingly used to manage chronic pain (59, 60). Multiple systematic reviews and meta-analyses have shown that medical cannabis and cannabinoids may improve impaired sleep in patients with CP, including sleep quality, insomnia, obstructive sleep apnea, REM sleep behavior disorder, and excessive daytime sleepiness (61–63).
Gabapentin and pregabalin are both gamma-aminobutyric acid (GABA) analogs for the treatment of neuropathic pain and have positive effects on sleep disturbances in neuropathic pain (64). A meta-analysis has demonstrated that longer-duration gabapentin treatment could significantly improve sleep health in patients with chronic neuropathic pain (65). Pregabalin has also demonstrated efficacy for sleep improvement by reducing sleep interference scores and improving the mean sleep scores in patients with neuropathic pain (66, 67).
Tricyclic antidepressants (TCAs), as first-line or augmenting drugs, were widely used to treat CP conditions, including headache, migraine, neuropathic pain, chronic low back pain, fibromyalgia, chronic widespread pain, and abdominal and gastrointestinal pain (68). Amitriptyline is the most useful TCA for various pain syndromes (68). Meanwhile, TCAs can also improve sleep quality (69). Recently, a systematic review and network meta-analysis showed that amitriptyline has higher efficacy for improving sleep, fatigue, and overall quality of life in patients with fibromyalgia (70).
BZRAs are the most well-known extensively prescribed medication to treat sleeping disorders used as adjuvant therapy for pain management. Recently, a narrative review has suggested that BZRAs have analgesic benefits for burning mouth syndrome and stiff person syndrome and for treating co-occurring insomnia and anxiety disorders for short periods of time (2–4 weeks) in CP management (71). Special attention should be required because co-prescribing of BZRAs with opioids for CP and insomnia is common in clinical practice (72). In 2016, the U.S. Food and Drug Administration (FDA) issued a strong official strong warning for the co-usage of opioids and benzodiazepines due to an increased risk of overdose deaths.1 Meanwhile, the US Centers for Disease Control and Prevention (CDC) also released the Guideline for prescribing opioids for chronic pain in March 2016, and explicitly stated that “clinicians should avoid prescribing opioid pain medication and benzodiazepines concurrently whenever possible” (73). For example, BZRAs combined with buprenorphine can produce serious respiratory depression (74).
Melatonin has a significant role in regulating the sleep–wake cycle and inhibits arousal signals. The existing evidence shows that melatonin can reduce the CP (75). A randomized, double-blinded, controlled trial has demonstrated that melatonin as an adjunct therapy to pregabalin reduced the pain score and pain-related sleep interference scores in patients with painful diabetic neuropathy (PDN) (76).
Suvorexant is a selective, dual orexin receptor antagonist approved in the USA and Japan for the treatment of insomnia (77). A double-blind, crossover study has shown that suvorexant improved sleep time and reduced next-day pain sensitivity in patients with fibromyalgia (78).
Cognitive behavioural therapy (CBT) is also the most commonly used psychological approach to treat CP (79). Meanwhile, CBT is recommended as a non-pharmacologic multimodal combination of treatments for coping with sleep problems and as a first-line therapy for insomnia (80, 81). A pilot trial has suggested that hybrid CBT is effective in reducing headache days and insomnia symptoms, which are maintained for 3 months, in youth with co-occurring chronic migraine and insomnia (82). A randomized controlled trial (RCT) study has found that CBT for insomnia (CBT-I) was superior to CBT for pain (CBT-P), which could improve self-reported WASO, SE, SQ, and dysfunctional beliefs and attitudes about sleep (83). Likewise, a systematic review and network meta-analysis has also confirmed that CBT-I might be the most effective treatment option for individuals with comorbid insomnia and CP (84).
Althogh not recommended by current guidelines, many alternative and complementary therapies are popular worldwide and have been increasingly studied to treat CP and sleep disorders, including music therapy, aromatherapy, massage, and acupuncture (85, 86). Among these therapies, acupuncture is widely known as one of the common forms to alleviate pain and improve sleep (87, 88). For example, acupuncture treatment can reduce pain pressure thresholds and improve sleep disturbances in patients with fibromyalgia (89). A systematic review and meta-analysis has also shown that acupuncture can relieve pain and improve sleep quality in patients with CP-related insomnia (90).
Although CP accompanied by sleep disorders is commonly encountered clinically, our knowledge about the basic neurochemical mechanisms remains rudimentary. Here, we review the majority of recent findings on the perspective of neurochemical mechanisms.
Serotonin, dopamine, and norepinephrine are neurotransmitters of the monoaminergic system, which are involved in the regulation of the endogenous pain system and the sleep–wake system (91, 92). Hyperactivity of monoamine neurons is one of the mechanisms of sleep disorders secondary to CP (93, 94).
Serotonin (5-hydroxytryptamine, 5-HT), the main effector of the serotonergic system, exhibits its effect by activating different receptor subtypes. Neuropathic pain can accelerate the activity of 5-HTergic neurons of the dorsal raphe nucleus (DRN), and activated 5-HTergic neurons produce a significant increase in wakefulness and a significant decrease in NREM sleep in a sciatic nerve ligation (CCI) mouse model (93). Mirtazapine, a noradrenergic and specific serotonergic antidepressant, normalizes the reduction in sleep time and fragmented sleep, regaining the sleep depth at sleep onset in the CP state, and increases the percentage of REM sleep in nerve-ligated mice (95). The selective 5-hydroxy-tryptamine 2A (5-HT2A) antagonist (MDL 100907), administered by intraperitoneal injection, significantly reduces the wake time and improves non-REM sleep time in a CCI mouse model but is not associated with pain relief (96).
Dopamine is a neurotransmitter and neuromodulator that can target dopamine neurons to cause inhibitory or excitatory effects (97). A cross-sectional study has shown that 32.6% of migraine patients have dopaminergic symptoms, including yawning, somnolence, and nausea, and suggested that dopaminergic system modulation should be carefully considered (98). The mesolimbic DA system plays an important role in CP, insomnia, and depression, and the three frequently co-occur (99). Intraperitoneal injection of Levo-tetrahydropalmatine (l-THP), a partial agonist for dopamine D1 receptors (D1R) and an antagonist of D2R, exerts analgesic effects by agonism of D1R and antagonism of D2R, and the antagonism of D2R mediates the hypnotic effect of l-THP in a partial sciatic nerve ligation (PSNL) mouse model (100).
Norepinephrine (NE) is an important neurotransmitter in the central nervous system. The locus coeruleus (LC) is the primary source of NE in the brain. The LC-spinal cord noradrenergic pathway is one of the most important pain inhibitory pathways that release norepinephrine (NE) to inhibit the ascending of pain signals (101). NE level increase in the brain is responsible for many of the sleep loss-associated symptoms (102). The activity of LC-NE determines the likelihood of sensory-evoked awakenings (103). However, there is no direct evidence showing that NE is involved in CP-induced sleep disorders.
Adenosine is a purine nucleoside that exerts a broad range of biological effects by binding to adenosine receptors (ARs). Adenosine exerts the analgesic effect primarily via the activation of A1AR located at peripheral, spinal, and supraspinal sites (104). On the other hand, adenosine plays an important role in integrating light and sleep signaling by the activation of A1/A2AR for the regulation of circadian timing (105). Animal experiments have confirmed that adenosinergic signaling regulates sleep-pain interactions. Systemic administration of the nonselective adenosine receptor antagonist caffeine prevents the sleep deprivation-induced increase in postoperative hypersensitivity, while microinjection of the adenosine A2A receptor antagonist into the median preoptic nucleus blocks the increase in surgical pain levels and duration caused by prior sleep deprivation and eliminats the thermal hyperalgesia induced by sleep deprivation in naive rats (106).
Melatonin is a neuroendocrine hormone, mainly synthesized and secreted by the pineal gland, which has a wide range of physiological functions, including regulation of circadian rhythms, enhancement of immune function, improvement of sleep, and pain (107). Sleep deprivation enhances microglial activation and aggravates neuropathic pain by suppressing melatonin secretion in a CCI rat model (108). Melatonin receptor agonist (piromelatine) significantly prolongs thermal and mechanical latencies and increases NREM sleep in a partial sciatic nerve ligation (PSL) mouse model (109). Furthermore, The antinociceptive effect of piromelatine is mediated by melatonin, opioid, and 5HT1A receptors; while the hypnotic effect of piromelatine is mediated by melatonin receptors (109).
Gamma-aminobutyric acid (GABA) and glutamate (Glu) are major inhibitory and excitatory neurotransmitters, respectively. A proton magnetic resonance spectroscopy study has found that patients with chronic migraine had significantly lower levels of GABA in the dentate nucleus (DN) and higher levels of Glu in the periaqueductal gray (PAG), and higher GABA levels in the PAG were significantly associated with poorer sleep quality in all patients with migraine (110). The extracellular GABA concentration deceases in the cingulate cortex, which is associated with sleep disturbance in neuropathic pain mice (111).
Sleep disorders secondary to chronic pain are a very common phenomenon. There is considerable evidence showing a reciprocal association between pain and sleep, especially in CP. CP results in insufficient sleep time and quality, which in turn increases pain sensitivity and severely compromises the pain management and treatment outcomes of patients. CP can affect sleep in terms of sleep time, sleep structure, sleep depth, etc., resulting in reduced sleep time, sleep structure disorder, sleep fragmentation, and reduced sleep depth. Sleep fragmentation in synergy with CP may lead to prolonged and exacerbated allodynia. Moreover, sleep quality can be a predictor of next-day pain, and short-term improved sleep can contribute to long-term clinical benefits in CP patients. However, sleep assessment is not performed in patients with CP in routine clinical practice (112). Thus, evaluation of sleep is recommended for investigating patients with CP. On the one hand, this may assist in the selection of rational therapeutic strategies for clinicians in clinical practice. On the other hand, this can also help further understand sleep characteristics and sleep disorders mechanisms, which is beneficial for the development of new potential therapeutic agents and treatment strategies in CP patients with comorbid sleep disorders. Of course, we should note that the sleep disorders and CP are “equal” in some cases. Painful and nonpainful somatic symptoms, including sleep disturbance, appetite disturbance, and fatigue or loss of energy, essentially characterize clinical states of depressive mood (113). It is worth to mention, as in the case even “adequate pain management” will not improve insomnia being not just a secondary to CP.
At present, the first choice for CP control is still drug therapy; however, there is still a lack of ideal drugs that are proven to be effective in both aspects. In CP, many drugs can only relieve pain but cannot improve sleep disorders or have hypnotic effects but cannot solve the pain problem. Therefore, it is of great significance to elucidate the underlying mechanisms of the interaction between CP and sleep disorders and seek new treatments. Special attention should be given to the possibility of a risk of drug–drug interaction when using two types of drugs targeting pain and sleep simultaneously. Therefore, more clinical data and basic research are required. At the same time, the available clinical evidence has suggested that nonpharmacologic therapy (CBT and complementary and alternative therapies) has certain therapeutic effects on pain and sleep and should receive more attention.
There is a considerable amount of research on underlying mechanisms for the development of CP and sleep disorders. Neurotransmitters, such as melatonin, cortisol, norepinephrine, and dopamine, are involved in the control of the circadian clock, as well as the regulation of pain perception and pain. CP-induced sleep disorders are closely related to the monoaminergic, adenosine, histamine, melatonin, GABAergic, and orexinergic systems. The initiation and maintenance of sleep, as well as sleep homeostasis, are regulated by complex pathways in these systems (Figure 1). Such results clearly demonstrate that control over the neurophysiological mechanisms medicating pain may provide an important route for treating pain and sleep disorder behavior.
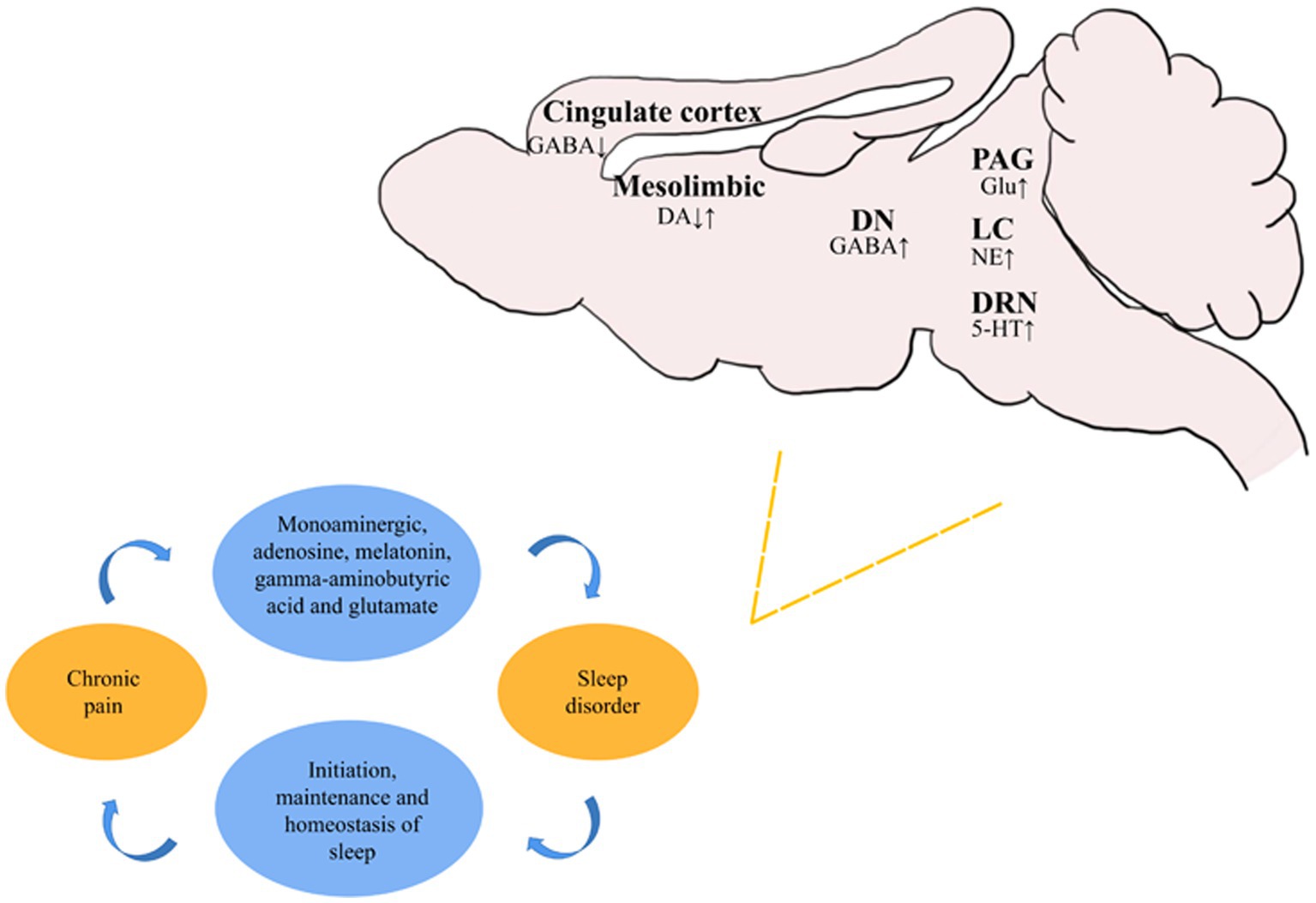
Figure 1. Recent studies on the changes of different neurons in various brain regions during sleep disorders secondary to chronic pain. ↑ represents the activation or activity enhancement of neurons, and ↓ represents the inhibition or activity reduction of neurons.
This study has several limitations. As a narrative review, some relevant articles may have not been included. We only focused on neurochemical mechanisms. Other pathologic mechanisms in neuroplasticity and neural circuitry on sleep disorders secondary to CP are also very important, which were not involved here. Furthermore, sleep problems in persons with CP were a complicated phenomenon involving not only physiological but also psychological and social factors.
Our review aimed to raise awareness for both psychiatric and non-psychiatric practitioners about the importance of sleep disorders secondary to chronic pain. The presence of sleep disorders in CP can aggravate the pain, as well as seriously affect the quality of life of patients. In terms of treatment, CBT is the best non-pharmacological intervention, while pharmacological treatments require further in-depth research. The research on sleep disorders secondary to CP is still in its infancy, and further elucidation of the underlying mechanisms of the interaction between CP and sleep disorders is crucial for developing more effective therapeutic strategies to improve pain and sleep.
KW and JZ directed the project and revised the manuscript. KW, LD, and XY designed research. LD, XY, RH, and XD were involved in bibliographic research and data collection. LD and XY wrote the manuscript. LD, XY, RH, XD, JZ, and KW discussed the results and commented on the manuscript. All authors contributed to the article and approved the submitted version.
This work was financially supported by the National Natural Science Foundation of China (81973940), Shanghai Clinical Research Center for Acupuncture and Moxibustion (20MC1920500), and Shanghai Municipal Commission of Health and Family Planning (ZY(2021-2023)-0208).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^ https://www.fda.gov/news-events/press-announcements/fda-requires-strong-warnings-opioid-analgesics-prescription-opioid-cough-products-and-benzodiazepine
1. Treede, RD, Rief, W, Barke, A, Aziz, Q, Bennett, MI, Benoliel, R, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. (2019) 160:19–27. doi: 10.1097/j.pain.0000000000001384
2. Cohen, SP, Vase, L, and Hooten, WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. (2021) 397:2082–97. doi: 10.1016/S0140-6736(21)00393-7
3. Mills, S, Nicolson, KP, and Smith, BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. (2019) 123:e273–83. doi: 10.1016/j.bja.2019.03.023
4. Dahlhamer, J, Lucas, J, Zelaya, C, Nahin, R, Mackey, S, DeBar, L, et al. Prevalence of chronic pain and high-impact chronic pain among adults-United States, 2016. MMWR Morb Mortal Wkly Rep. (2018) 67:1001–6. doi: 10.15585/mmwr.mm6736a2
5. Lindholm, P, Lamusuo, S, Taiminen, T, Virtanen, A, Pertovaara, A, Forssell, H, et al. The analgesic effect of therapeutic rTMS is not mediated or predicted by comorbid psychiatric or sleep disorders. Medicine. (2016) 95:e5231. doi: 10.1097/MD.0000000000005231
6. Serafini, RA, Pryce, KD, and Zachariou, V. The mesolimbic dopamine system in chronic pain and associated affective comorbidities. Biol Psychiatry. (2020) 87:64–73. doi: 10.1016/j.biopsych.2019.10.018
7. Larson, RA, and Carter, JR. Total sleep deprivation and pain perception during cold noxious stimuli in humans. Scand J Pain. (2016) 13:12–6. doi: 10.1016/j.sjpain.2016.05.037
8. Yamazaki, R, Toda, H, Libourel, PA, Hayashi, Y, Vogt, KE, and Sakurai, T. Evolutionary origin of distinct NREM and REM sleep. Front Psychol. (2020) 11:567618. doi: 10.3389/fpsyg.2020.567618
9. Finan, PH, Goodin, BR, and Smith, MT. The association of sleep and pain: an update and a path forward. J Pain. (2013) 14:1539–52. doi: 10.1016/j.jpain.2013.08.007
10. Lin, Y, Hu, Y, Guo, J, Chen, M, Xu, X, Wen, Y, et al. Association between sleep and multimorbidity in Chinese elderly: results from the Chinese longitudinal healthy longevity survey (CLHLS). Sleep Med. (2022) 98:1–8. doi: 10.1016/j.sleep.2022.06.007
11. Nguyen, VV, Zainal, NH, and Newman, MG. Why sleep is key: poor sleep quality is a mechanism for the bidirectional relationship between major depressive disorder and generalized anxiety disorder across 18 years. J Anxiety Disord. (2022) 90:102601. doi: 10.1016/j.janxdis.2022.102601
12. Shi, W, Chen, C, Cui, Q, Deng, F, Yang, B, Cao, Y, et al. Sleep disturbance exacerbates the cardiac conduction abnormalities induced by persistent heavy ambient fine particulate matter pollution: a multi-center cross-sectional study. Sci Total Environ. (2022) 838:156472. doi: 10.1016/j.scitotenv.2022.156472
13. Miettinen, T, Sverloff, J, Lappalainen, OP, Linton, SJ, Sipila, K, and Kalso, E. Sleep problems in pain patients entering tertiary pain care: the role of pain-related anxiety, medication use, self-reported diseases, and sleep disorders. Pain. (2022) 163:e812–20. doi: 10.1097/j.pain.0000000000002497
14. Emery, PC, Wilson, KG, and Kowal, J. Major depressive disorder and sleep disturbance in patients with chronic pain. Pain Res Manag. (2014) 19:35–41. doi: 10.1155/2014/480859
15. Hernandez-Leon, A, Fernandez-Guasti, A, Martinez, A, Pellicer, F, and Gonzalez-Trujano, ME. Sleep architecture is altered in the reserpine-induced fibromyalgia model in ovariectomized rats. Behav Brain Res. (2019) 364:383–92. doi: 10.1016/j.bbr.2018.01.005
16. Lerman, SF, Campbell, CM, Buenaver, LF, Medak, M, Phillips, J, Polley, M, et al. Exploring the role of negative cognitions in the relationship between ethnicity, sleep, and pain in women with temporomandibular joint disorder. J Pain. (2018) 19:1342–51. doi: 10.1016/j.jpain.2018.05.009
17. Nordstoga, AL, Mork, PJ, Meisingset, I, Nilsen, T, and Skarpsno, ES. The joint effect of sleep duration and insomnia symptoms on the risk of recurrent spinal pain: the HUNT study. Sleep Med. (2022) 99:11–7. doi: 10.1016/j.sleep.2022.07.003
18. Varallo, G., Giusti, EM., Manna, C., Castelnuovo, G., Pizza, F., Franceschini, C., et al. (2022). Sleep disturbances and sleep disorders as risk factors for chronic postsurgical pain: a systematic review and meta-analysis. Sleep Med Rev, 63,:101630. doi: 10.1016/j.smrv.2022.101630
19. Riemann, D, Krone, LB, Wulff, K, and Nissen, C. Sleep, insomnia, and depression. Neuropsychopharmacology. (2020) 45:74–89. doi: 10.1038/s41386-019-0411-y
20. Ostovar-Kermani, T, Arnaud, D, Almaguer, A, Garcia, I, Gonzalez, S, Mendez, MY, et al. Painful sleep: insomnia in patients with chronic pain syndrome and its consequences. Folia Med. (2020) 62:645–54. doi: 10.3897/folmed.62.e50705
21. Al-Khudhairy, MW, AlOtaibi, A, Abdul Rahman, L, Al-Garni, M, Yaslam, R, and Fatani, R. The association of self-reported iron and vitamin D levels on sleep quality and pain perception in a subset of Saudi population. Risk Manag Healthc Policy. (2021) 14:4853–65. doi: 10.2147/RMHP.S318698
22. Husak, AJ, and Bair, MJ. Chronic pain and sleep disturbances: a pragmatic review of their relationships, comorbidities, and treatments. Pain Med. (2020) 21:1142–52. doi: 10.1093/pm/pnz343
23. Mathias, JL, Cant, ML, and Burke, A. Sleep disturbances and sleep disorders in adults living with chronic pain: a meta-analysis. Sleep Med. (2018) 52:198–210. doi: 10.1016/j.sleep.2018.05.023
24. Fabbri, M, Beracci, A, Martoni, M, Meneo, D, Tonetti, L, and Natale, V. Measuring subjective sleep quality: a review. Int J Environ Res Public Health. (2021) 18:1082. doi: 10.3390/ijerph18031082
25. Fekedulegn, D, Andrew, ME, Shi, M, Violanti, JM, Knox, S, and Innes, KE. Actigraphy-based assessment of sleep parameters. Ann Work Expo Health. (2020) 64:350–67. doi: 10.1093/annweh/wxaa007
26. Smith, MT, McCrae, CS, Cheung, J, Martin, JL, Harrod, CG, Heald, JL, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. (2018) 14:1231–7. doi: 10.5664/jcsm.7230
27. Smith, MGÖMTP. A laboratory study on the effects of wind turbine noise on sleep: results of the polysomnographic WiTNES study. Sleep. (2020) 43:46. doi: 10.1093/sleep/zsaa046
28. Buysse, DJ, Reynolds, CR, Monk, TH, Berman, SR, and Kupfer, DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
29. Van Looveren, E, Bilterys, T, Munneke, W, Cagnie, B, Ickmans, K, Mairesse, O, et al. The association between sleep and chronic spinal pain: a systematic review from the last decade. J Clin Med. (2021) 10:3836. doi: 10.3390/jcm10173836
30. Onen, SH, Onen, F, Courpron, P, and Dubray, C. How pain and analgesics disturb sleep. Clin J Pain. (2005) 21:422–31. doi: 10.1097/01.ajp.0000129757.31856.f7
31. Bjurstrom, MF, and Irwin, MR. Polysomnographic characteristics in nonmalignant chronic pain populations: a review of controlled studies. Sleep Med Rev. (2016) 26:74–86. doi: 10.1016/j.smrv.2015.03.004
32. Lee, HJ, and Kim, ST. A questionnaire-based study of sleep-wake patterns and sleep quality in a TMJ and orofacial pain clinic. Cranio. (2020) 38:213–20. doi: 10.1080/08869634.2018.1550134
33. Lewandowski, AS, Palermo, TM, De la Motte, S, and Fu, R. Temporal daily associations between pain and sleep in adolescents with chronic pain versus healthy adolescents. Pain. (2010) 151:220–5. doi: 10.1016/j.pain.2010.07.016
34. Edwards, RR, Grace, E, Peterson, S, Klick, B, Haythornthwaite, JA, and Smith, MT. Sleep continuity and architecture: associations with pain-inhibitory processes in patients with temporomandibular joint disorder. Eur J Pain. (2009) 13:1043–7. doi: 10.1016/j.ejpain.2008.12.007
35. Smith, MT, Edwards, RR, McCann, UD, and Haythornthwaite, JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. (2007) 30:494–505. doi: 10.1093/sleep/30.4.494
36. Sutton, BC, and Opp, MR. Sleep fragmentation exacerbates mechanical hypersensitivity and alters subsequent sleep-wake behavior in a mouse model of musculoskeletal sensitization. Sleep. (2014) 37:515–24. doi: 10.5665/sleep.3488
37. Chang, JR, Fu, SN, Li, X, Li, SX, Wang, X, Zhou, Z, et al. The differential effects of sleep deprivation on pain perception in individuals with or without chronic pain: a systematic review and meta-analysis. Sleep Med Rev. (2022) 66:101695. doi: 10.1016/j.smrv.2022.101695
38. Simonelli, G, Mantua, J, Gad, M, St, PM, Moore, L, Yarnell, AM, et al. Sleep extension reduces pain sensitivity. Sleep Med. (2019) 54:172–6. doi: 10.1016/j.sleep.2018.10.023
39. Sivertsen, B, Lallukka, T, Petrie, KJ, Steingrimsdottir, OA, Stubhaug, A, and Nielsen, CS. Sleep and pain sensitivity in adults. Pain. (2015) 156:1433–9. doi: 10.1097/j.pain.0000000000000131
40. Almoznino, G, Benoliel, R, Sharav, Y, and Haviv, Y. Sleep disorders and chronic craniofacial pain: characteristics and management possibilities. Sleep Med Rev. (2017) 33:39–50. doi: 10.1016/j.smrv.2016.04.005
41. Gilbert, AL, Lee, J, Song, J, Semanik, PA, Ehrlich-Jones, LS, Kwoh, CK, et al. Relationship between self-reported restless sleep and objectively measured physical activity in adults with knee osteoarthritis. Arthritis Care Res. (2021) 73:687–92. doi: 10.1002/acr.23581
42. Frange, C, Hachul, H, Hirotsu, C, Tufik, S, and Andersen, ML. Temporal analysis of chronic musculoskeletal pain and sleep in postmenopausal women. J Clin Sleep Med. (2019) 15:223–34. doi: 10.5664/jcsm.7622
43. Guntel, M, Huzmeli, ED, and Melek, I. Patients with neuropathic pain have poor sleep quality. J Nerv Ment Dis. (2021) 209:505–9. doi: 10.1097/NMD.0000000000001325
44. Bascour-Sandoval, C, Belmar-Arriagada, H, Albayay, J, Lacoste-Abarzua, C, Bielefeldt-Astudillo, D, Gajardo-Burgos, R, et al. The effect of sleep quality on pain in Chilean individuals with musculoskeletal disorders. Int J Environ Res Public Health. (2021) 18:11370. doi: 10.3390/ijerph182111370
45. Palermo, TM, Law, EF, Kim, A, de la Vega, R, and Zhou, C. Baseline sleep disturbances modify outcome trajectories in adolescents with chronic pain receiving internet-delivered psychological treatment. J Pain. (2022) 23:1245–55. doi: 10.1016/j.jpain.2022.03.003
46. Lucke, AJ, Wrzus, C, Gerstorf, D, Kunzmann, U, Katzorreck, M, Hoppmann, C, et al. Bidirectional links of daily sleep quality and duration with pain and self-rated health in older Adults’ daily lives. J Gerontol A Biol Sci Med Sci. (2022) c192:glac192. doi: 10.1093/gerona/glac192
47. Li, R, Dworkin, RH, Chapman, BP, Becerra, AZ, Yang, L, Mooney, CJ, et al. Moderate to severe chronic pain in later life: risk and resilience factors for recovery. J Pain. (2021) 22:1657–71. doi: 10.1016/j.jpain.2021.05.007
48. Saconi, B, Polomano, RC, Compton, PC, McPhillips, MV, Kuna, ST, and Sawyer, AM. The influence of sleep disturbances and sleep disorders on pain outcomes among veterans: a systematic scoping review. Sleep Med Rev. (2021) 56:101411. doi: 10.1016/j.smrv.2020.101411
49. Koffel, E, Kroenke, K, Bair, MJ, Leverty, D, Polusny, MA, and Krebs, EE. The bidirectional relationship between sleep complaints and pain: analysis of data from a randomized trial. Health Psychol. (2016) 35:41–9. doi: 10.1037/hea0000245
50. Vitiello, MV, McCurry, SM, Shortreed, SM, Baker, LD, Rybarczyk, BD, Keefe, FJ, et al. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain. (2014) 155:1547–54. doi: 10.1016/j.pain.2014.04.032
51. Alhalal, EA, Alhalal, IA, Alaida, AM, Alhweity, SM, Alshojaa, AY, and Alfaori, AT. Effects of chronic pain on sleep quality and depression: a cross-sectional study. Saudi Med J. (2021) 42:315–23. doi: 10.15537/smj.42.3.20200768
52. Herrero, BA, Beetz, G, Bruneau, A, Martel, MO, Cistulli, PA, Nixdorf, DR, et al. Multitargeting the sleep-pain interaction with pharmacological approaches: a narrative review with suggestions on new avenues of investigation. Sleep Med Rev. (2021) 59:101459. doi: 10.1016/j.smrv.2021.101459
53. Frers, A, Shaffer, J, Edinger, J, and Wachholtz, A. The relationship between sleep and opioids in chronic pain patients. J Behav Med. (2021) 44:412–20. doi: 10.1007/s10865-021-00205-1
54. Zaidel, C, Musich, S, Karl, J, Kraemer, S, and Yeh, CS. Psychosocial factors associated with sleep quality and duration among older adults with chronic pain. Popul Health Manag. (2021) 24:101–9. doi: 10.1089/pop.2019.0165
55. Chung, F, Wong, J, Bellingham, G, Lebovic, G, Singh, M, Waseem, R, et al. Predictive factors for sleep apnoea in patients on opioids for chronic pain. BMJ Open Respir Res. (2019) 6:e523:e000523. doi: 10.1136/bmjresp-2019-000523
56. Correa, D, Farney, RJ, Chung, F, Prasad, A, Lam, D, and Wong, J. Chronic opioid use and central sleep apnea: a review of the prevalence, mechanisms, and perioperative considerations. Anesth Analg. (2015) 120:1273–85. doi: 10.1213/ANE.0000000000000672
57. Garcia, AN, and Salloum, IM. Polysomnographic sleep disturbances in nicotine, caffeine, alcohol, cocaine, opioid, and cannabis use: a focused review. Am J Addict. (2015) 24:590–8. doi: 10.1111/ajad.12291
58. Margarit, C, Ballester, P, Inda, MD, Roca, R, Gomez, L, Planelles, B, et al. OPRM1 gene interaction with sleep in chronic pain patients treated with opioids. Pain Physician. (2019) 22:97–107.
59. Bicket, MC, Stone, EM, and McGinty, EE. Use of cannabis and other pain treatments among adults with chronic pain in US states with medical cannabis programs. JAMA Netw Open. (2023) 6:e2249797. doi: 10.1001/jamanetworkopen.2022.49797
60. McDonagh, MS, Morasco, BJ, Wagner, J, Ahmed, AY, Fu, R, Kansagara, D, et al. Cannabis-based products for chronic pain: a systematic review. Ann Intern Med. (2022) 175:1143–53. doi: 10.7326/M21-4520
61. Babson, KA, Sottile, J, and Morabito, D. Cannabis, cannabinoids, and sleep: a review of the literature. Curr Psychiatry Rep. (2017) 19:23. doi: 10.1007/s11920-017-0775-9
62. Bialas, P, Fitzcharles, MA, Klose, P, and Hauser, W. Long-term observational studies with cannabis-based medicines for chronic non-cancer pain: a systematic review and meta-analysis of effectiveness and safety. Eur J Pain. (2022) 26:1221–33. doi: 10.1002/ejp.1957
63. McParland, AL, Bhatia, A, Matelski, J, Tian, C, Diep, C, Clarke, H, et al. Evaluating the impact of cannabinoids on sleep health and pain in patients with chronic neuropathic pain: a systematic review and meta-analysis of randomized controlled trials. Reg Anesth Pain Med. (2022) 48:180–90. doi: 10.1136/rapm-2021-103431
64. Ferini-Strambi, L. Neuropathic pain and sleep: a review. Pain Ther. (2017) 6:19–23. doi: 10.1007/s40122-017-0089-y
65. Kapustin, D, Bhatia, A, McParland, A, Trivedi, A, Davidson, A, Brull, R, et al. Evaluating the impact of gabapentinoids on sleep health in patients with chronic neuropathic pain: a systematic review and meta-analysis. Pain. (2020) 161:476–90. doi: 10.1097/j.pain.0000000000001743
66. Onakpoya, IJ, Thomas, ET, Lee, JJ, Goldacre, B, and Heneghan, CJ. Benefits and harms of pregabalin in the management of neuropathic pain: a rapid review and meta-analysis of randomised clinical trials. BMJ Open. (2019) 9:e23600:e023600. doi: 10.1136/bmjopen-2018-023600
67. Parsons, B, Fujii, K, Nozawa, K, Yoshiyama, T, Ortiz, M, and Whalen, E. The efficacy of pregabalin for the treatment of neuropathic pain in Japanese subjects with moderate or severe baseline pain. J Pain Res. (2019) 12:1061–8. doi: 10.2147/JPR.S181729
68. Schneider, J, Patterson, M, and Jimenez, XF. Beyond depression: other uses for tricyclic antidepressants. Cleve Clin J Med. (2019) 86:807–14. doi: 10.3949/ccjm.86a.19005
69. Bakker, MH, Hugtenburg, JG, Smits, MG, van der Horst, HE, and Slottje, P. Off-label low dose amitriptyline for insomnia disorder: patient-reported outcomes. Pharmacoepidemiol Drug Saf. (2023) 32:435–45. doi: 10.1002/pds.5561
70. Farag, HM, Yunusa, I, Goswami, H, Sultan, I, Doucette, JA, and Eguale, T. Comparison of amitriptyline and US Food and Drug Administration-approved treatments for fibromyalgia: a systematic review and network meta-analysis. JAMA Netw Open. (2022) 5:e2212939. doi: 10.1001/jamanetworkopen.2022.12939
71. Wright, SL. Limited utility for benzodiazepines in chronic pain management: a narrative review. Adv Ther. (2020) 37:2604–19. doi: 10.1007/s12325-020-01354-6
72. Koffel, E, DeRonne, B, and Hawkins, EJ. Co-prescribing of opioids with benzodiazepines and other hypnotics for chronic pain and insomnia: trends and health outcomes. Pain Med. (2020) 21:2055–9. doi: 10.1093/pm/pnaa054
73. Dowell, D, Haegerich, TM, and Chou, R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. (2016) 315:1624–45. doi: 10.1001/jama.2016.1464
74. Dahan, A, van Lemmen, M, Jansen, S, Simons, P, and van der Schrier, R. Buprenorphine: a treatment and cause of opioid-induced respiratory depression. Br J Anaesth. (2022) 128:402–4. doi: 10.1016/j.bja.2021.12.001
75. Oh, SN, Myung, SK, and Jho, HJ. Analgesic efficacy of melatonin: a meta-analysis of randomized, double-blind, placebo-controlled trials. J Clin Med. (2020) 9:1553. doi: 10.3390/jcm9051553
76. Shokri, M, Sajedi, F, Mohammadi, Y, and Mehrpooya, M. Adjuvant use of melatonin for relieving symptoms of painful diabetic neuropathy: results of a randomized, double-blinded, controlled trial. Eur J Clin Pharmacol. (2021) 77:1649–63. doi: 10.1007/s00228-021-03170-5
77. Atkin, T, Comai, S, and Gobbi, G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. (2018) 70:197–245. doi: 10.1124/pr.117.014381
78. Roehrs, T, Withrow, D, Koshorek, G, Verkler, J, Bazan, L, and Roth, T. Sleep and pain in humans with fibromyalgia and comorbid insomnia: double-blind, crossover study of suvorexant 20 mg versus placebo. J Clin Sleep Med. (2020) 16:415–21. doi: 10.5664/jcsm.8220
79. Terpstra, JA, van der Vaart, R, van Beugen, S, van Eersel, RA, Gkika, I, Erdos, D, et al. Guided internet-based cognitive-behavioral therapy for patients with chronic pain: a meta-analytic review. Internet Interv. (2022) 30:100587. doi: 10.1016/j.invent.2022.100587
80. Qaseem, A, Kansagara, D, Forciea, MA, Cooke, M, and Denberg, TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. (2016) 165:125–33. doi: 10.7326/M15-2175
81. Zuo, X, Dong, Z, Zhang, P, Zhang, P, Chang, G, Xiang, Q, et al. Effects of cognitive behavioral therapy on sleep disturbances and quality of life among adults with type 2 diabetes mellitus: a randomized controlled trial. Nutr Metab Cardiovasc Dis. (2020) 30:1980–8. doi: 10.1016/j.numecd.2020.06.024
82. Law, EF, Wan, TS, Aaron, RV, Dudeney, J, and Palermo, TM. Hybrid cognitive-behavioral therapy intervention for adolescents with co-occurring migraine and insomnia: a single-arm pilot trial. Headache. (2018) 58:1060–73. doi: 10.1111/head.13355
83. McCrae, CS, Williams, J, Roditi, D, Anderson, R, Mundt, JM, Miller, MB, et al. Cognitive behavioral treatments for insomnia and pain in adults with comorbid chronic insomnia and fibromyalgia: clinical outcomes from the SPIN randomized controlled trial. Sleep. (2019) 42:y234. doi: 10.1093/sleep/zsy234
84. Enomoto, K, Adachi, T, Fujino, H, Kugo, M, Tatsumi, S, and Sasaki, J. Comparison of the effectiveness of cognitive behavioral therapy for insomnia, cognitive behavioral therapy for pain, and hybrid cognitive behavioral therapy for insomnia and pain in individuals with comorbid insomnia and chronic pain: a systematic review and network meta-analysis. Sleep Med Rev. (2022) 66:101693. doi: 10.1016/j.smrv.2022.101693
85. Bauer, BA, Tilburt, JC, Sood, A, Li, GX, and Wang, SH. Complementary and alternative medicine therapies for chronic pain. Chin J Integr Med. (2016) 22:403–11. doi: 10.1007/s11655-016-2258-y
86. Hrehova, L, and Mezian, K. Non-pharmacologic treatment of insomnia in primary care settings. Int J Clin Pract. (2021) 75:e14084. doi: 10.1111/ijcp.14084
87. Patel, M, Urits, I, Kaye, AD, and Viswanath, O. The role of acupuncture in the treatment of chronic pain. Best Pract Res Clin Anaesthesiol. (2020) 34:603–16. doi: 10.1016/j.bpa.2020.08.005
88. Shergis, JL, Ni, X, Jackson, ML, Zhang, AL, Guo, X, Li, Y, et al. A systematic review of acupuncture for sleep quality in people with insomnia. Complement Ther Med. (2016) 26:11–20. doi: 10.1016/j.ctim.2016.02.007
89. Valera-Calero, JA, Fernandez-de-Las-Penas, C, Navarro-Santana, MJ, and Plaza-Manzano, G. Efficacy of dry needling and acupuncture in patients with fibromyalgia: a systematic review and meta-analysis. Int J Environ Res Public Health. (2022) 19:9904. doi: 10.3390/ijerph19169904
90. Liu, F, You, J, Li, Q, Fang, T, Chen, M, Tang, N, et al. Acupuncture for chronic pain-related insomnia: a systematic review and meta-analysis. Evid Based Complement Alternat Med. (2019) 2019:5381028–10. doi: 10.1155/2019/5381028
91. Bannister, K, Bee, LA, and Dickenson, AH. Preclinical and early clinical investigations related to monoaminergic pain modulation. Neurotherapeutics. (2009) 6:703–12. doi: 10.1016/j.nurt.2009.07.009
92. Saper, CB, Fuller, PM, Pedersen, NP, Lu, J, and Scammell, TE. Sleep state switching. Neuron. (2010) 68:1023–42. doi: 10.1016/j.neuron.2010.11.032
93. Ito, H, Yanase, M, Yamashita, A, Kitabatake, C, Hamada, A, Suhara, Y, et al. Analysis of sleep disorders under pain using an optogenetic tool: possible involvement of the activation of dorsal raphe nucleus-serotonergic neurons. Mol Brain. (2013) 6:59. doi: 10.1186/1756-6606-6-59
94. Koh, K, Hamada, A, Hamada, Y, Yanase, M, Sakaki, M, Someya, K, et al. Possible involvement of activated locus coeruleus-noradrenergic neurons in pain-related sleep disorders. Neurosci Lett. (2015) 589:200–6. doi: 10.1016/j.neulet.2014.12.002
95. Ito, H, Tsuneki, H, Sasaoka, T, Toyooka, N, Matsuo, M, and Yamazaki, M. Suvorexant and mirtazapine improve chronic pain-related changes in parameters of sleep and voluntary physical performance in mice with sciatic nerve ligation. PLoS One. (2022) 17:e264386:e0264386. doi: 10.1371/journal.pone.0264386
96. Ito, H, Takemura, Y, Aoki, Y, Hattori, M, Horikawa, H, and Yamazaki, M. Analysis of the effects of a tricyclic antidepressant on secondary sleep disturbance induced by chronic pain in a preclinical model. PLoS One. (2020) 15:e243325:e0243325. doi: 10.1371/journal.pone.0243325
97. Neville, V, Nakagawa, S, Zidar, J, Paul, ES, Lagisz, M, Bateson, M, et al. Pharmacological manipulations of judgement bias: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2020) 108:269–86. doi: 10.1016/j.neubiorev.2019.11.008
98. Barbanti, P, Aurilia, C, Egeo, G, Fofi, L, Guadagni, F, and Ferroni, P. Dopaminergic symptoms in migraine: a cross-sectional study on 1148 consecutive headache center-based patients. Cephalalgia. (2020) 40:1168–76. doi: 10.1177/0333102420929023
99. Finan, PH, and Smith, MT. The comorbidity of insomnia, chronic pain, and depression: dopamine as a putative mechanism. Sleep Med Rev. (2013) 17:173–83. doi: 10.1016/j.smrv.2012.03.003
100. Liu, YY, Wang, TX, Zhou, JC, Qu, WM, and Huang, ZL. Dopamine D1 and D2 receptors mediate analgesic and hypnotic effects of l-tetrahydropalmatine in a mouse neuropathic pain model. Psychopharmacology. (2019) 236:3169–82. doi: 10.1007/s00213-019-05275-3
101. Li, J, Wei, Y, Zhou, J, Zou, H, Ma, L, Liu, C, et al. Activation of locus coeruleus-spinal cord noradrenergic neurons alleviates neuropathic pain in mice via reducing neuroinflammation from astrocytes and microglia in spinal dorsal horn. J Neuroinflammation. (2022) 19:123. doi: 10.1186/s12974-022-02489-9
102. Mehta, R, Bhattacharya, R, and Mallick, BN. Sleep and neuroimmunomodulation for maintenance of optimum brain function: role of noradrenaline. Brain Sci. (2022) 12:1725. doi: 10.3390/brainsci12121725
103. Hayat, H, Regev, N, Matosevich, N, Sales, A, Paredes-Rodriguez, E, Krom, AJ, et al. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci Adv. (2020) 6:eaaz4232. doi: 10.1126/sciadv.aaz4232
104. Vincenzi, F, Pasquini, S, Borea, PA, and Varani, K. Targeting adenosine receptors: a potential pharmacological avenue for acute and chronic pain. Int J Mol Sci. (2020) 21:8710. doi: 10.3390/ijms21228710
105. Jagannath, A, Varga, N, Dallmann, R, Rando, G, Gosselin, P, Ebrahimjee, F, et al. Adenosine integrates light and sleep signalling for the regulation of circadian timing in mice. Nat Commun. (2021) 12:2113. doi: 10.1038/s41467-021-22179-z
106. Hambrecht-Wiedbusch, VS, Gabel, M, Liu, LJ, Imperial, JP, Colmenero, AV, and Vanini, G. Preemptive caffeine administration blocks the increase in postoperative pain caused by previous sleep loss in the rat: a potential role for preoptic adenosine A2A receptors in sleep-pain interactions. Sleep. (2017) 40:zsx116. doi: 10.1093/sleep/zsx116
107. Chaudhry, SR, Stadlbauer, A, Buchfelder, M, and Kinfe, TM. Melatonin moderates the triangle of chronic pain. Sleep architecture and Immunometabolic traffic. Biomedicine. (2021) 9:984. doi: 10.3390/biomedicines9080984
108. Huang, CT, Chiang, RP, Chen, CL, and Tsai, YJ. Sleep deprivation aggravates median nerve injury-induced neuropathic pain and enhances microglial activation by suppressing melatonin secretion. Sleep. (2014) 37:1513–23. doi: 10.5665/sleep.4002
109. Liu, YY, Yin, D, Chen, L, Qu, WM, Chen, CR, Laudon, M, et al. Piromelatine exerts antinociceptive effect via melatonin, opioid, and 5HT1A receptors and hypnotic effect via melatonin receptors in a mouse model of neuropathic pain. Psychopharmacology. (2014) 231:3973–85. doi: 10.1007/s00213-014-3530-5
110. Wang, W, Zhang, X, Bai, X, Zhang, Y, Yuan, Z, Tang, H, et al. Gamma-aminobutyric acid and glutamate/glutamine levels in the dentate nucleus and periaqueductal gray with episodic and chronic migraine: a proton magnetic resonance spectroscopy study. J Headache Pain. (2022) 23:83. doi: 10.1186/s10194-022-01452-6
111. Narita, M, Niikura, K, Nanjo-Niikura, K, Narita, M, Furuya, M, Yamashita, A, et al. Sleep disturbances in a neuropathic pain-like condition in the mouse are associated with altered GABAergic transmission in the cingulate cortex. Pain. (2011) 152:1358–72. doi: 10.1016/j.pain.2011.02.016
112. Woo, A, and Ratnayake, G. Sleep and pain management: a review. Pain Manag. (2020) 10:261–73. doi: 10.2217/pmt-2020-0001
Keywords: chronic pain, sleep disorders, pain and sleep comorbidity, pain treatment, sleep disorder treatment
Citation: Duo L, Yu X, Hu R, Duan X, Zhou J and Wang K (2023) Sleep disorders in chronic pain and its neurochemical mechanisms: a narrative review. Front. Psychiatry. 14:1157790. doi: 10.3389/fpsyt.2023.1157790
Received: 03 February 2023; Accepted: 15 May 2023;
Published: 01 June 2023.
Edited by:
Aleksandar Videnovic, Harvard Medical School, United StatesReviewed by:
Dmitry Romanov, I. M. Sechenov First Moscow State Medical University, RussiaCopyright © 2023 Duo, Yu, Hu, Duan, Zhou and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Zhou, cGR6aG91amlhQDE2My5jb20=; Ke Wang, d2FuZ2tlODQzMEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.