
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 22 March 2023
Sec. Neurostimulation
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1154354
This article is part of the Research Topic Basic and Clinical Research on Neurostimulation Techniques in Major Depressive Disorder View all 5 articles
 Wei Zheng1†
Wei Zheng1† Dong-Bin Cai2†
Dong-Bin Cai2† Sha Nie3†
Sha Nie3† Jian-Hua Chen3
Jian-Hua Chen3 Xing-Bing Huang3
Xing-Bing Huang3 Stephan Goerigk4,5,6
Stephan Goerigk4,5,6 Andre Russowsky Brunoni7,8,9
Andre Russowsky Brunoni7,8,9 Wei Zheng3*
Wei Zheng3*Objective: We performed a meta-analysis of randomized, double-blind, controlled trials (RCTs) to systematically investigate the therapeutic effects and tolerability of transcranial alternating current stimulation (tACS) for the treatment of patients with major depressive disorder (MDD).
Methods: Electronic search of PubMed, PsycINFO, EMBASE, Chinese National Knowledge Infrastructure, Wanfang database, and the Cochrane Library up to 1 April 2022. Double-blind RCTs examining the efficacy and safety of tACS for patients with MDD were included. The primary outcome was the improvement of depressive symptoms following a course of tACS treatment. Data were analyzed using Review Manager Version 5.3 (Cochrane IMS, Oxford, UK). Study quality was assessed using the Cochrane risk of bias and Jadad scale. Publication bias was assessed using a funnel plot and the Egger test.
Results: We identified 883 articles, of which 4 RCTs with 5 active treatment arms covering 224 participants with MDD on active tACS (n = 117) and sham tACS (n = 107) were eligible for inclusion. Meta-analysis of depressive symptoms at post-tACS found an advantage of active tACS over sham tACS (n = 212, standard mean difference (SMD) = −1.14, 95% confidence interval (CI): −2.23, −0.06; I2 = 90%, P = 0.04). The significant superiority of active tACS over sham tACS in improving depressive symptoms remained in a sensitivity analysis. Active tACS was significantly superior to sham tACS regarding depressive symptoms at the 4 week follow-up (SMD = −1.07, 95% CI: −2.05, −0.08; I2 = 88%, P = 0.03) and study-defined remission [risk ratio (RR) = 2.07, 95% CI: 1.36, 3.14, I2 = 9%, P = 0.0006]. The discontinuation rate due to any reason was similar between the two groups (P > 0.05). All included studies were rated as high quality (Jadad score ≥ 3), with funnel plots of primary outcome not suggestive of publication bias.
Conclusion: tACS appeared to be modestly effective and safe for improving depressive symptoms in patients with MDD, although further studies are warranted.
Major depressive disorder (MDD) is the common psychiatric illness, leading to the highest burden of disability among mental and substance use disorders (1). Despite antidepressants (ADs) and psychotherapies being available, many patients suffering from MDD do not adequately respond to ADs (2, 3) or psychotherapies (4). Consequently, augmentation strategies of ADs with non-pharmacological interventions, such as adjunctive non-invasive brain stimulation (NIBS) techniques (5), including transcranial magnetic stimulation (TMS), magnetic seizure therapy (MST), electroconvulsive therapy (ECT), transcranial direct current stimulation (tDCS), and transcranial alternating current stimulation (tACS), have been applied widely for the treatment of MDD in clinical practice.
As a type of NIBS technique, tACS is a newly developed intervention (6). In contrast to tDCS, which applies a constant current, tACS provides brain stimulation by sending alternating electric currents to the scalp (7). A case report found that gamma-tACS contributed to a good response in a patient with MDD during pregnancy, and the patient achieved remission during the 3 month follow-up (6). Although four recent randomized, double-blind, controlled trials (RCTs) (8–11) have examined the feasibility, efficacy and safety of tACS in treating adult patients with MDD, the findings have been inconsistent. Two RCTs consistently reported that tACS could significantly improve depressive symptoms in first-episode drug-naïve patients suffering from MDD (10, 11). However, in Alexander et al.’s (8) study, a negative finding was observed when comparing three different conditions (10 Hz-tACS, 40 Hz-tACS and sham tACS).
To date, no systematic review or meta-analysis on tACS as a therapeutic intervention for adult patients with MDD has been published. We hypothesized that active tACS would show superiority over sham tACS in reducing depressive symptoms of MDD.
The eligibility criteria of this systematic review used the PICOS framework. Participants: adult patients (≥18 years) with MDD. Intervention versus Comparison: active tACS versus sham tACS or active tACS plus ADs versus sham tACS plus ADs. Outcomes: The primary outcome was the improvement of depressive symptoms at post-tACS as measured by standardized rating scales, such as the Montgomery-Åsberg Depression Rating Scale (MADRS) and the Hamilton Depression Rating Scale (HAMD). As recommended previously (12, 13), HAMD was preferred to other scales when multiple rating scales (i.e., HAMD and MADRS) were used in the study. The secondary outcomes were (1) the improvement of depressive symptoms at the 2-week and 4-week follow-ups after tACS; (2) study-defined remission (i.e., HAMD total score ≤ 7) and response (i.e., reduction in HAMD total score ≥ 50%) at post-tACS; (3) adverse effects; and (4) any cause discontinuation. Study: Only published double-blind RCTs examining the efficacy and safety of tACS for the treatment of patients with MDD were included. Studies with a single-session of tACS were excluded.
This meta-analysis was conducted and reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Supplementary Table 1) (14). Two authors (WZ and DBC) independently identified double-blind RCTs published prior to 1 April 2022, which examined the efficacy and safety of tACS for the treatment of MDD by systematically searching PubMed, PsycINFO, EMBASE, Chinese National Knowledge Infrastructure (CNKI), Wanfang databases, and the Cochrane Library. The search terms were as follows: (transcranial alternating current stimulation OR tACS) AND (depression OR depression OR depressive OR depressed OR melancholia). Furthermore, the reference lists of the identified articles (8–11) and relevant reviews (15, 16) were manually searched to avoid missing eligible studies. The more search details were presented in the Supplementary Text.
Two authors (WZ and DB-C) independently extracted the following data from each included RCT using a tailored form: author, year of publication, sample size; number and percentage of male participants; average age of participants; illness duration; primary and secondary outcomes (efficacy, safety and tolerability of tACS for MDD). Any discrepancies were resolved by discussion and adjudication through a senior author. If the study data were unclear, the first/corresponding authors were contacted by email or telephone to obtain further information. As recommended previously (17), we used values from other studies to obtain an average when a study did not report the standard deviation.
Data synthesis was performed using Review Manager Version 5.3 (Cochrane IMS, Oxford, UK). The random-effects model was applied for all meta-analytic outcomes (18). For dichotomous and continuous outcomes, we calculated the risk ratio (RR) and standard mean difference (SMD) with 95% confidence intervals (CIs), respectively. Significant heterogeneity for the meta-analytic outcomes was defined as an I2 of > 50%. In the case of I2 > 50% for the improvement of depressive symptoms at post-tACS, a sensitivity analysis was performed to investigate the source of heterogeneity by excluding one outlying study (SMD ≤ −3.50) (9). One RCT compared three different treatment conditions (10 Hz tACS, 40 Hz tACS and sham). As recommended previously (19, 20), half of the participants were assigned to each active treatment arm (10 Hz-tACS or 40 Hz-tACS) to avoid inflating the number of participants in the sham group. Funnel plots or Egger’s test (21) were used to detect publication bias for the primary outcome. The significance level was set as alpha < 0.05, with two-sided tests.
Study quality was assessed using the Cochrane risk of bias (22) and Jadad scale (23) by the two independent authors (WZ and DBC). A Jadad score of ≥ 3 was defined as “high quality.” The two authors (WZ and DBC) independently assessed the overall quality levels of the primary and secondary outcomes using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system (24).
As depicted in Figure 1, a total of 883 potentially relevant articles were identified and screened in both Chinese (n = 592) and English (n = 291) databases and manual searches (n = 0). In total, 4 double-blind RCTs with 5 active treatment arms (8–11) met the study inclusion criteria.
Across the 4 double-blind RCTs, 3 RCTs (3/4, 75%) were conducted in China, and one (1/4, 25%) was conducted in the USA (Table 1). Four RCTs with 5 active treatment arms (n = 224) compared adjunctive active tACS (n = 117) and sham tACS (n = 107). Almost all patients presented a diagnosis of MDD (n = 216, 96.4%), and only 8 (3.6%) were diagnosed with bipolar disorder. Their mean age was 38.8 (range = 32.1−41.1) years, and 20.1% (range = 13.3%−26.0%) of the patients were male. A 15 mA current intensity (77.5 Hz-tACS) was used in 3 RCTs (9–11), and one RCT with 2 active treatment arms applied a 1–2 mA protocol (10/40 Hz-tACS) (8).
As shown in Supplementary Figure 1, the included 4 RCTs with an adequate double-blinded design mentioned “random” assignment with a specific description. Only one RCT (25%, 1/4) was rated as having an “unclear risk of bias” regarding allocation concealment (11). All included studies (Jadad score = 5) were rated as high quality (Table 1). According to the GRADE approach (Supplementary Table 2), the overall quality of the evidence for all seven meta-analytic outcomes was rated as “high” (5.9%, 1/17) or “moderate” (94.1%, 16/17).
Meta-analysis of depressive symptoms at post-tACS as measured by the HAMD found an advantage of active tACS over sham tACS (4 RCTs with 5 active treatment arms, n = 212, SMD = −1.14, 95% CI: −2.23, −0.06; I2 = 90%, P = 0.04; Figure 2). Significance regarding depressive symptoms at post-tACS (n = 155, SMD = −0.69, 95% CI: −1.19, −0.18; I2 = 42%, P = 0.008) remained robust after removing one study (9) with an outlying effect size.
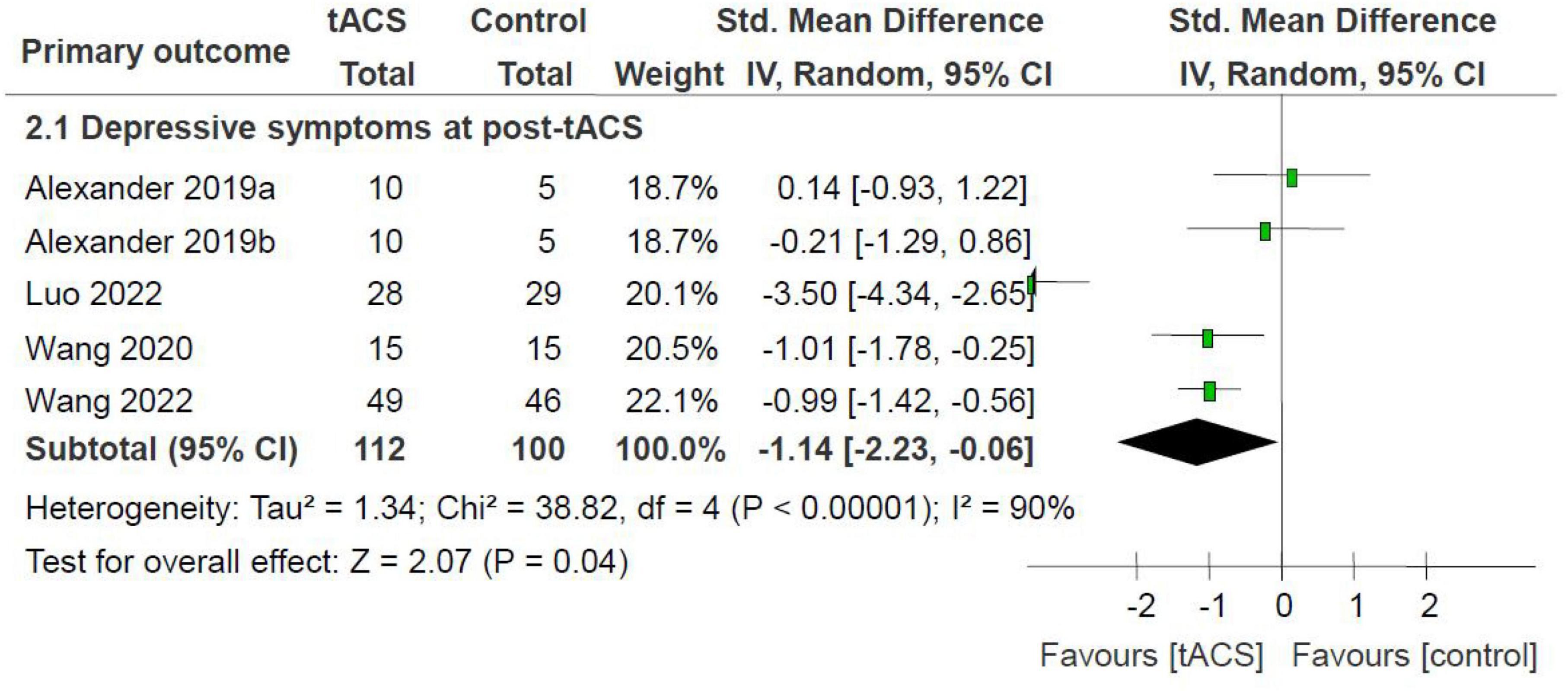
Figure 2. tACS for MDD: depressive symptoms at post-tACS as measured by the HAMD. HAMD, Hamilton Depression Rating scale; MDD, major depressive disorder; tACS, transcranial alternating current stimulation.
Similarly, meta-analysis of the depressive symptoms at the 4-week follow-up found an advantage of active tACS over sham tACS (n = 210, SMD = −1.07, 95% CI: −2.05, −0.08; I2 = 88%, P = 0.03; Table 2). Comparisons between the two groups in depressive symptoms at the 2 week follow-up (n = 29, SMD = −0.07, 95% CI: −0.83, −0.70; I2 = 0%, P = 0.86; Table 2) did not show significant differences.
Active tACS was significantly superior to sham tACS regarding study-defined remission (60.7% versus 29.6%, n = 165, RR = 2.07, 95% CI: 1.36, 3.14, I2 = 9%, P = 0.0006; Table 2), but not regarding study-defined response (70.2% versus 52.1%, n = 165, RR = 1.31, 95% CI: 1.00, 1.73, I2 = 18%, P = 0.05; Table 2).
Compared with sham tACS, active tACS led to significantly increased phosphene perception (n = 32; SMD = 1.19; 95% CI: 0.38, 2.00; I2 = 0%, P = 0.004; Table 2). No significant differences were found with regard to other adverse effects (SMD = −0.51 to 0.55; 95% CI: −1.25, 1.30; I2 = 0%; P = 0.15 to 0.94; Table 2) or discontinuation due to any reason (n = 205; RR = 0.72; 95% CI: 0.23, 2.19; I2 = 0%, P = 0.56; Table 2) between the two groups.
The funnel plot of the primary outcome presented a symmetrical distribution among the included RCTs (Supplementary Figure 2). As a minimum of 10 RCTs are required to run the Egger test, publication bias was not evaluated by conducting the Egger test (25).
In this meta-analysis of 4 double-blind RCTs with 5 active treatment arms (n = 224), active tACS for the treatment of MDD was significantly superior to sham tACS regarding the improvement of depressive symptoms at post-tACS and at the 4-week follow-up, as well as for study-defined remission. At the end of the treatment, 60.7% of patients showed remission and 70.2% showed response to active tACS compared with the corresponding figures of 29.6% and 52.1% for sham tACS. Although the administration of tACS resulted in a significantly higher rate of phosphenes, the rates of other adverse effects and discontinuation due to any reason were similar between the two groups.
The included RCTs, all with relatively small sample sizes (30 to 100), were published within the last 3 years, indicating that tACS for patients with MDD is a new, clinically important topic. The results of this meta-analysis suggest that tACS could provide a non-pharmacological alternative for the treatment of subjects suffering from MDD. For other NIBS techniques such as tDCS (26), the main objective is also to monitor their neurocognitive efficacy. For example, previous meta-analyses found that active tDCS was superior to sham tDCS in treating MDD (27) and improving attention/vigilance (26). Neurocognition is commonly affected in major mental disorders (28). However, in this meta-analysis, data on the neurocognitive efficacy of tACS for MDD were collected in only one RCT (25%, 1/4) (8). The neurocognitive efficacy of tACS for MDD should be further investigated.
The underlying mechanism of tACS in treating MDD maybe attribute to its feasibility in altering disturbed brain oscillations (7). The antidepressant effects of tACS for the treatment of MDD may depend on the different stimulation parameters, especially current and frequency (29). Among the included RCTs, tACS with an alternating current of 15 mA (75 Hz) was used in 3 RCTs (9–11), and tACS with a 2 mA current (10 Hz or 40 Hz) was used in one RCT with 2 active treatment arms (8). The current of 15 mA used in 3 out of 4 RCTs (75%, 3/4) (9–11) in this meta-analysis was higher than that used in previous reports (8, 30–32). Therefore, it remains unclear whether a smaller current or lower frequency can have dramatic antidepressant efficacy (10). Importantly, both alpha-tACS (8, 33) and gamma-tACS (6, 34) can effectively ameliorate depressive symptoms of MDD, suggesting that there are some specific gamma or alpha frequencies or other unknown frequencies that can significantly improve the symptoms of MDD (10).
In this meta-analysis, active tACS led to significantly increased phosphene perception when compared with sham tACS, which was reported in previous studies focusing on healthy subjects (35). A prior study (36) found that phosphene perception was most prominent at frequencies between 14 and 20 Hz-tACS. Numerous studies found that participants did not report phosphene perception at tACS with a higher frequency of ≥ 40 Hz (9–11, 37–39). In this meta-analysis, patients with MDD did not perceive phosphenes in three RCTs (75 Hz-tACS) (9–11) but did in Alexander et al.’s (8) study (10 Hz-tACS or 40 Hz-tACS). Similar to TMS (40) and tDCS (30), tACS used for treating MDD was safe and well tolerated.
This study has several limitations. First, the relatively small number of studies (4 RCTs covering 224 participants with MDD) precluded the evaluation of publication bias. Second, several previous studies reported that a 77.5 Hz pulsed alternating current appeared to produce the best stimulation of the antinociceptive system, eliciting maximal endorphin release and an analgesic effect (10, 41). However, the optimal parameters of tACS as a non-pharmacological alternative for the treatment of MDD should be further examined. Third, the meta-analytic results for the primary outcome were highly heterogeneous (I2 = 90), which was potentially attributed to heterogeneous samples, different study designs and different treatment protocols. However, the findings remained unchanged, and I2 decreased from 90 to 42% in the sensitivity analysis. Finally, only physically healthy patients suffering from MDD were recruited in included RCTs, limiting the generalizability of the findings.
In conclusion, tACS appeared to be modestly effective and safe in improving depressive symptoms for patients with MDD. More high-quality RCTs are warranted to explore the advantages of tACS as a therapeutic intervention for MDD.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
WZ (first author), DB-C, and SN selected the studies, extracted the data, did the quality assessment of the included studies, and wrote the first version of manuscript. WZ (last author) reviewed all the data and helped solve disagreements. All authors have approved and contributed to finalize the final version of manuscript.
This study was funded by the National Natural Science Foundation of China (82101609), Scientific Research Project of Guangzhou Bureau of Education (202032762), Science and Technology Program Project of Guangzhou (202102020658), Guangzhou Health Science and Technology Project (20211A011045), Guangzhou Science and Technology Project of Traditional Chinese Medicine and Integrated Traditional Chinese and Western Medicine (20212A011018), China International Medical Exchange Foundation (Z-2018-35-2002), Guangzhou Clinical Characteristic Technology Project (2019TS67), Science and Technology Program Project of Guangzhou (202102020658), and Guangdong Hospital Association (2019ZD06). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1154354/full#supplementary-material
1. Whiteford H, Degenhardt L, Rehm J, Baxter A, Ferrari A, Erskine H, et al. Global burden of disease attributable to mental and substance use disorders: findings from the global burden of disease study 2010. Lancet. (2013) 382:1575–86. doi: 10.1016/s0140-6736(13)61611-6
2. Voineskos D, Daskalakis Z, Blumberger D. Management of treatment-resistant depression: challenges and strategies. Neuropsychiatr Dis Treat. (2020) 16:221–34. doi: 10.2147/ndt.s198774
3. Cipriani A, Furukawa T, Salanti G, Chaimani A, Atkinson L, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. (2018) 391:1357–66. doi: 10.1016/s0140-6736(17)32802-7
4. Schramm E, Kriston L, Elsaesser M, Fangmeier T, Meister R, Bausch P, et al. Two-year follow-up after treatment with the cognitive behavioral analysis system of psychotherapy versus supportive psychotherapy for early-onset chronic depression. Psychother Psychosom. (2019) 88:154–64. doi: 10.1159/000500189
5. Mutz J, Vipulananthan V, Carter B, Hurlemann R, Fu C, Young A. Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis. BMJ. (2019) 364:l1079. doi: 10.1136/bmj.l1079
6. Wilkening A, Kurzeck A, Dechantsreiter E, Padberg F, Palm U. Transcranial alternating current stimulation for the treatment of major depression during pregnancy. Psychiatry Res. (2019) 279:399–400. doi: 10.1016/j.psychres.2019.06.009
7. Elyamany O, Leicht G, Herrmann C, Mulert C. Transcranial alternating current stimulation (tACS): from basic mechanisms towards first applications in psychiatry. Eur Arch Psychiatry Clin Neurosci. (2021) 271:135–56. doi: 10.1007/s00406-020-01209-9
8. Alexander M, Alagapan S, Lugo C, Mellin J, Lustenberger C, Rubinow D, et al. Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD). Transl Psychiatry. (2019) 9:106. doi: 10.1038/s41398-019-0439-0
9. Luo J, Sun C, Pan W, Wang D, Shi X, Wang Q, et al. Efficacy and safety of transcranial alternating-current stimulation combined with antidepressants in the treatment of depressive episode (in Chinese). J Cap Med Univ. (2022) 43:244–8. doi: 10.3969/j.issn.1006-7795.2022.02.014
10. Wang H, Wang K, Xue Q, Peng M, Yin L, Gu X, et al. Transcranial alternating current stimulation for treating depression: a randomized controlled trial. Brain. (2022) 145:83–91. doi: 10.1093/brain/awab252
11. Wang H, Wang K, Sun Z, Peng M, Xue Q, Li N, et al. A pilot study of transcranial alternating current stimulation in the treatment of drug-naive adult patients with major depressive disorder (in Chinese). Natl Med J China. (2020) 100:197–201. doi: 10.3760/cma.j.issn.0376-2491.2020.03.008
12. Li D, Wang F, Chu C, Chen T, Tang C, Yang W, et al. Significant treatment effect of add-on ketamine anesthesia in electroconvulsive therapy in depressive patients: a meta-analysis. Eur Neuropsychopharmacol. (2017) 27:29–41. doi: 10.1016/j.euroneuro.2016.11.008
13. Zheng W, Li X, Zhu X, Cai D, Yang X, Ungvari G, et al. Adjunctive ketamine and electroconvulsive therapy for major depressive disorder: a meta-analysis of randomized controlled trials. J Affect Disord. (2019) 250:123–31. doi: 10.1016/j.jad.2019.02.044
14. Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:b2535. doi: 10.1136/bmj.b2535
15. Al Qasem W, Abubaker M, Kvašňák E. Working memory and transcranial-alternating current stimulation-state of the art: findings, missing, and challenges. Front Psychol. (2022) 13:822545. doi: 10.3389/fpsyg.2022.822545
16. Grover S, Wen W, Viswanathan V, Gill C, Reinhart R. Long-lasting, dissociable improvements in working memory and long-term memory in older adults with repetitive neuromodulation. Nat Neurosci. (2022) 25:1237–46. doi: 10.1038/s41593-022-01132-3
17. Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, et al. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am J Psychiatry. (2009) 166:152–63. doi: 10.1176/appi.ajp.2008.08030368
18. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
19. Gu X, Chen R, Sun C, Zheng W, Yang X, Wang S, et al. Effect of adjunctive ranitidine for antipsychotic-induced weight gain: a systematic review of randomized placebo-controlled trials. J Int Med Res. (2018) 46:22–32. doi: 10.1177/0300060517716783
20. Zheng W, Cai D, Zheng W, Sim K, Ungvari G, Peng X, et al. Brexanolone for postpartum depression: a meta-analysis of randomized controlled studies. Psychiatry Res. (2019) 279:83–9. doi: 10.1016/j.psychres.2019.07.006
21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
22. Cumpston M, Li T, Page M, Chandler J, Welch V, Higgins J, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.Ed000142
23. Jadad A, Moore R, Carroll D, Jenkinson C, Reynolds D, Gavaghan D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
24. Balshem H, Helfand M, Schünemann H, Oxman A, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
25. Sterne J, Sutton A, Ioannidis J, Terrin N, Jones D, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. (2011) 343:d4002. doi: 10.1136/bmj.d4002
26. Begemann M, Brand B, Ćurčić-Blake B, Aleman A, Sommer I. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. Psychol Med. (2020) 50:2465–86. doi: 10.1017/s0033291720003670
27. Razza L, Palumbo P, Moffa A, Carvalho A, Solmi M, Loo C, et al. A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress Anxiety. (2020) 37:594–608. doi: 10.1002/da.23004
28. Price R, Duman R. Neuroplasticity in cognitive and psychological mechanisms of depression: an integrative model. Mol Psychiatry. (2020) 25:530–43. doi: 10.1038/s41380-019-0615-x
29. Antal A, Paulus W. Transcranial alternating current stimulation (tACS). Front Hum Neurosci. (2013) 7:317. doi: 10.3389/fnhum.2013.00317
30. Matsumoto H, Ugawa Y. Adverse events of tDCS and tACS: a review. Clin Neurophysiol Pract. (2017) 2:19–25. doi: 10.1016/j.cnp.2016.12.003
31. McClure D, Greenman S, Koppolu S, Varvara M, Yaseen Z, Galynker I. A pilot study of safety and efficacy of cranial electrotherapy stimulation in treatment of bipolar II depression. J Nerv Ment Dis. (2015) 203:827–35. doi: 10.1097/nmd.0000000000000378
32. Mischoulon D, De Jong M, Vitolo O, Cusin C, Dording C, Yeung A, et al. Efficacy and safety of a form of cranial electrical stimulation (CES) as an add-on intervention for treatment-resistant major depressive disorder: a three week double blind pilot study. J Psychiatr Res. (2015) 70:98–105. doi: 10.1016/j.jpsychires.2015.08.016
33. Riddle J, Alexander M, Schiller C, Rubinow D, Frohlich F. Reduction in left frontal alpha oscillations by transcranial alternating current stimulation in major depressive disorder is context dependent in a randomized clinical trial. Transl Psychiatry. (2022) 7:302–11. doi: 10.1016/j.bpsc.2021.07.001
34. Haller N, Senner F, Brunoni A, Padberg F, Palm U. Gamma transcranial alternating current stimulation improves mood and cognition in patients with major depression. J Psychiatr Res. (2020) 130:31–4. doi: 10.1016/j.jpsychires.2020.07.009
35. Antal A, Boros K, Poreisz C, Chaieb L, Terney D, Paulus W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. (2008) 1:97–105. doi: 10.1016/j.brs.2007.10.001
36. Kanai R, Chaieb L, Antal A, Walsh V, Paulus W. Frequency-dependent electrical stimulation of the visual cortex. Curr Biol. (2008) 18:1839–43. doi: 10.1016/j.cub.2008.10.027
37. Chaieb L, Antal A, Paulus W. Transcranial alternating current stimulation in the low kHz range increases motor cortex excitability. Restor Neurol Neurosci. (2011) 29:167–75. doi: 10.3233/rnn-2011-0589
38. Moliadze V, Antal A, Paulus W. Boosting brain excitability by transcranial high frequency stimulation in the ripple range. J Physiol. (2010) 588:4891–904. doi: 10.1113/jphysiol.2010.196998
39. Turi Z, Ambrus G, Janacsek K, Emmert K, Hahn L, Paulus W, et al. Both the cutaneous sensation and phosphene perception are modulated in a frequency-specific manner during transcranial alternating current stimulation. Restor Neurol Neurosci. (2013) 31:275–85. doi: 10.3233/rnn-120297
40. Perera T, George M, Grammer G, Janicak P, Pascual-Leone A, Wirecki T. The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. (2016) 9:336–46. doi: 10.1016/j.brs.2016.03.010
41. Lebedev V, Malygin A, Kovalevski A, Rychkova S, Sisoev V, Kropotov S, et al. Devices for noninvasive transcranial electrostimulation of the brain endorphinergic system: application for improvement of human psycho-physiological status. Artif Organs. (2002) 26:248–51. doi: 10.1046/j.1525-1594.2002.06944.x
Keywords: major depressive disorder, transcranial alternating current stimulation (tACS), response, remission, meta-analysis
Citation: Zheng W, Cai D-B, Nie S, Chen J-H, Huang X-B, Goerigk S, Brunoni AR and Zheng W (2023) Adjunctive transcranial alternating current stimulation for patients with major depressive disorder: A systematic review and meta-analysis. Front. Psychiatry 14:1154354. doi: 10.3389/fpsyt.2023.1154354
Received: 30 January 2023; Accepted: 06 March 2023;
Published: 22 March 2023.
Edited by:
Chaomeng Liu, Beijing Anding Hospital, Capital Medical University, ChinaCopyright © 2023 Zheng, Cai, Nie, Chen, Huang, Goerigk, Brunoni and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zheng, emhlbmd3ZWkwNzAyQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.