- 1Frontier Research Institute for Interdisciplinary Sciences, Tohoku University, Sendai, Japan
- 2Smart-Aging Research Center, Tohoku University, Sendai, Japan
- 3Japan Society for the Promotion of Science, Tokyo, Japan
- 4Department of Medical Sciences, Graduate School of Medicine, Tohoku University, Sendai, Japan
Intergenerational transmission is a crucial aspect of human development. Although prior studies have demonstrated the continuity of psychopathology and maladaptive upbringing environments between parents and offspring, the underlying neurobiological mechanisms remain unclear. We have begun a novel neuroimaging research project, the Transmit Radiant Individuality to Offspring (TRIO) study, which focuses on biological parent-offspring trios. The participants of the TRIO study were Japanese parent-offspring trios consisting of offspring aged 10–40 and their biological mother and father. Structural and functional brain images of all participants were acquired using magnetic resonance imaging (MRI). Saliva samples were collected for DNA analysis. We obtained psychosocial information, such as intelligence, mental health problems, personality traits, and experiences during the developmental period from each parent and offspring in the same manner as much as possible. By April 2023, we completed data acquisition from 174 trios consisting of fathers, mothers, and offspring. The target sample size was 310 trios. However, we plan to conduct genetic and epigenetic analyses, and the sample size is expected to be expanded further while developing this project into a multi-site collaborative study in the future. The TRIO study can challenge the elucidation of the mechanism of intergenerational transmission effects on human development by collecting diverse information from parents and offspring at the molecular, neural, and behavioral levels. Our study provides interdisciplinary insights into how individuals’ lives are involved in the construction of the lives of their descendants in the subsequent generation.
1. Introduction
Interdisciplinary research involving human neuroimaging has contributed to understanding individuality by exploring the associations between genes, the environment, and gene–environment interactions and brain structure or function. Several large cohort studies, such as the Adolescent Brain Cognitive DevelopmentSM (ABCD study®) study1 (1), IMAGEN study2 (2), Philadelphia Neurodevelopmental Cohort (PNC)3 (3), Generation R study4 (4), and Chinese Imaging Genetics (CHIMGEN) study5 (5) have taken the lead of this research area. However, in many cases, these large-scale studies do not cover a crucial aspect of human development—intergenerational transmission.
Intergenerational transmission refers to offspring inheriting behaviors and characteristics from parents through genetic and non-genetic pathways (6). Numerous studies have reported on the intergenerational continuities in psychopathology (7–12). Recent large-sample studies have revealed that children with parents who have a history of psychiatric hospitalization are at 2–3 times higher risk of developing mental illness in adolescence compared with those whose parents do not (13). Children who have experienced a parental suicide attempt during early childhood are likely to exhibit suicide attempts in their adolescence (14). Thus, intergenerational transmission of psychopathology is likely one of the crucial topics in current psychiatry (12). Furthermore, children tend to experience social interactions that are similar to those of their parents. For instance, it has been reported that families with a parent who was abused in childhood are confronted with a higher incidence of maltreatment than those with no abused parent (15, 16). Aside from maltreatment, variations in parenting behavior in a normative range, such as affection and harsh discipline, also seem to be transmitted intergenerationally (17–20). In addition, children whose fathers have experienced peer rejection in childhood are reportedly more likely to be victims of bullying themselves (21).
The experience of psychopathology or social interactions, as well as the effect of the experience could be inherited by offspring. Intergenerational transmission of trauma effect (intergenerational trauma) means that individuals’ adverse experiences, particularly in their childhood, affect their offspring (22). Adverse childhood experiences (ACEs) refer to exposure to threat and/or deprivation during childhood, including physical, psychological, or sexual abuse, and household dysfunction such as mental illness, substance abuse, criminal behavior, and violence between family members (23). Many recent studies have demonstrated the associations between maternal ACEs and offspring’s behavioral and neural phenotypes. For example, maternal ACEs are positively correlated with internalizing or externalizing symptoms and negatively correlated with resilience and self-affirmation in offspring (24, 25). However, it remains unclear what mechanisms are in place for parents and offspring to have the same experiences, or for the effect of parental life experiences to be passed on to offspring.
There are three notable concepts in the exploration of mechanisms of intergenerational transmission: intergenerational neuroimaging, epigenetic inheritance, and genetic nurture. First, intergenerational neuroimaging—a research field that uses brain images as endophenotypes to estimate the mechanisms of intergenerational transmission—is beginning to progress (26). Several pioneering studies have investigated the structural or functional brain similarities between parent–offspring dyads using magnetic resonance imaging (MRI) (27–40). These studies assumed that the stronger the correlation of brain characteristics between parents and offspring, the more similar their brains. It has been confirmed that parent–offspring dyads show greater similarity in brain characteristics than randomly selected adult–child pairs (35, 38, 39). Parent–offspring similarities have been reported in various characteristics, such as in gray matter volume (28, 35, 40), cortical thickness (29), surface area (39, 40), sulcal morphology (38, 39), fractional anisotropy (34), white matter microstructure (31, 32), resting-state functional connectivity (35–37), gumma-amino butyric acid and glutamate ratio (33), and task-evoked neural activation during reward processing (30). It seems that parent-offspring neural similarities do not reflect the offspring’s inheritance of particular brain characteristics from the parent but rather reflect the similarities in the process of brain development. Therefore, while it is difficult to clarify the molecular basis of intergenerational transmission through intergenerational neuroimaging, putative mechanisms can be deepened by understanding the brain characteristics that are similar between parent and offspring, genetic or environmental factors that are associated with the similarity, genetic or environmental factors associated with the development of brain characteristics that parent-offspring similarities are detected, including how the neural susceptibility for those factors are determined. Additionally, the association between parental ACEs and offspring’s brain development has been investigated to show the putative mechanisms of intergenerational trauma. For example, significant associations between maternal experiences of childhood maltreatment and infants’ total brain volume (41), total intracranial volume (42), amygdala volume (43), and functional connectivity in the frontoamygdala circuits (44) have also been reported. In one study, children whose mothers experienced a great earthquake in Turkey demonstrated smaller gray matter volumes in the hippocampus and amygdala (45). Second, epigenetic inheritance has been suggested to underlie the intergenerational transmission of trauma effect. Epigenetic inheritance means that life experiences and environmental exposures cause stable changes in non-DNA molecules in germ cells and alter gene expression patterns after embryonic development, thereby affecting the offspring’s phenotype (46). DNA methylation due to the effects of traumatic experiences has been observed in the same genes in both parents who had experienced it themselves and their children who had not (47–49). However, details of the association between epigenetic inheritance and intergenerational transmission of trauma effects have not been elucidated clearly. Third, the genetic nurture effect refers to the phenomenon that parental nature (i.e., parental genotype) affects offspring’s outcome through shaping the nurture (environments that parents provide to their offspring) (50). A prior study has robustly confirmed that parental polygenic risk scores of educational attainment calculated with non-transmitted alleles affect children’s educational outcomes (51). The mechanisms by which the genetic nurture effect is established include the effects of parental non-transmitted alleles on the prenatal environment, placenta, and postnatal environment, such as breast milk composition and nurturing behavior (46). Some researchers argue that clarification of what environmental factors related to parental non-transmitted alleles affect offspring’s educational outcomes will enable the development of interventions to break the chain of low educational attainment (51).
One of the reasons why the mechanisms of intergenerational transmission are unclear, despite the accumulation of previous studies as described above, is the lack of data on fathers. Although some studies examined brain similarities among all patterns of parent–offspring gender combinations (mother–daughter, mother–son, father–daughter, and father–son) (28, 40), most studies have focused on mother–offspring dyads (29, 30, 37–39), with some focusing on mother–daughter dyads (29, 30). Even in the context of intergenerational trauma, studies that focused on fathers are rare, but some interesting findings have been reported. For instance, paternal early-life stress was significantly correlated with neonates’ white matter microstructure in the corpus callosum (52). Furthermore, paternal ACEs are associated with offspring attention problems at 3 years of age, and this association is affected by the offspring’s blood DNA methylation in neonates (53). On the other hand, genetic studies have emphasized the significance of family-based designs like parent-offspring trios (54, 55). These previous studies have revealed gene–environment correlations (rGE) in the context of child development. Krapohl et al. found that offspring’s polygenic scores confer schizophrenia or educational attainment predicts the exposure to environmental factors such as paternal age at birth, maternal smoking during pregnancy, breastfeeding, parental smacking, household income, watching television, and maternal education level (54). Baldwin et al. have confirmed the genetic confounding effects in the association between ACEs and mental health, by clarifying that children with higher polygenic scores for mental health problems (attention-deficit hyperactivity disorder, depression, and schizophrenia) are more likely to be exposed to ACEs (55). However, both of these rGE studies had only offspring’s genotype data; therefore, parental genotype data is necessary to distinguish the observed rGE as passive (parental genotype associated with some environments are inherited by offspring) or evocative (offspring’s genotype evokes parental behavior). As suggested in previous studies, most offspring’s environments are parental phenotypes (54). Therefore, utilizing paternal, maternal, and offspring genotype and phenotype data is worthwhile for understanding the intergenerational effects of genetics and environments on human development.
In the field of neuroimaging, data from both parents and offspring are rarely available. Although birth cohorts and three-generational cohorts have contributed to a wealth of genomic information and physiological indices for parents and offspring, in many cases, brain images are obtained only from parents or offspring. For example, the Norwegian Mother and Child Cohort Study (MoBa)6 (56) and Avon Longitudinal Study of Parents and Children (ALSPAC)7 (57) have acquired brain MRIs from only a subset of children (58, 59). Additionally, although the Developing Human Connectome Project (dHCP)8 (60), which investigates typical and atypical brain development beginning from the fetal period, has collected maternal medical and obstetric histories, brain MRIs are only available for offspring. Three-generation cohorts such as the Tohoku Medical Megabank Project Birth and Three-Generation Cohort Study (TMM BirThree Cohort Study)9 (61) and LifeLines10 (62) have the possibility of collecting multi-generational brain images in the future. However, brain imaging has been performed only on some adults in the TMM BirThree Cohort Study and only on participants in a subproject (ImaLife) of LifeLines; thus, at least for now, multi-generational neuroimaging remains unavailable. In contrast, the population-neuroscience cohort studies of the Tokyo TEEN Cohort (pn-TTC)11 (63) and Generation R study (64) have obtained brain images of both parents and offspring. Most parental brain images in the pn-TTC were from the mother (35). For the Generation R study, it has not yet been reported whether both mothers and fathers completed MRI acquisition. Nonetheless, it seems that data on fathers are still lacking in studies on intergenerational transmission.
Consequently, for elucidating the mechanisms of intergenerational transmission, it is necessary to collect behavioral indices, brain images, environmental factors, and genetic/epigenetic information on at least two generations of parents and offspring. Hence, we started a novel neuroimaging research project—the Transmit Radiant Individuality to Offspring (TRIO) study—which is subject to biological parent-offspring trios comprising fathers, mothers, and offspring. This study aimed to elucidate the bio-psycho-social mechanism of intergenerational transmission using brain MRI as an endophenotype. We anticipate that the understanding of the role of parental genotype and/or life experiences in the development of personality and risk of mental illness in the subsequent generation would be deepened in this study. The findings of this study will contribute to the growth of effective interventions for individuals’ adaptive development. We hypothesized that: (1) maternal and paternal intergenerational transmissions are established by different mechanisms, and (2) the interaction between the paternal and maternal effects involves intergenerational transmission. The procedure for testing these hypotheses is presented using the effect of the parental experience of childhood maltreatment on offspring development as an example. Hypothesis (1) would be examined by comparing the result of the analyses using father-offspring dyads and mother-offspring dyads. For example, the paternal experience of childhood maltreatment and such experience of mothers may associate with neural and/or behavioral phenotype of offspring in a different way. Hypothesis (2) would be tested by analyzing whether the effect of the maternal experience of childhood maltreatment on offspring’s brain structure is moderated by the paternal childhood experience of being reared, and vice versa.
In this paper, we describe the acquiring data content, procedures of data collection, current progress, and future directions of our project.
2. Methods
2.1. Participants
The participants of the TRIO study were parent-offspring trios consisting of three members: a male or female offspring aged 10 (a 5th-grade elementary school student in Japan) to 40 and their biological mother and father. Most previous studies aiming to elucidate the mechanisms of intergenerational transmission have focused on offspring from infancy to adolescence. Therefore, it is unclear whether the effects of intergenerational transmission are observed at any age, or whether they are limited to certain ages (6). To the best of our knowledge, the maximum age of offspring is late 20s to 30 years in previous studies of parent-offspring brain similarity and the association of parental ACEs and offspring’s brain (40, 65). Thus, we set the upper age limit at 40 years, which is not included in previous studies and is classified as young adulthood in the developmental stage (66). In studies exploring early childhood, children’s personality and mental health problems are often assessed by parents. However, age-related differences in certain traits may prevent the detection of intergenerational transmission effects, for example, adult and child aggression appear as different behaviors. To mitigate this limitation, it is considered necessary to unify methods of data collection between parents and offspring. Thus, we set the lower age limit at 10 years, the age at which a person is considered to be able to answer the self-administered questionnaires. No maximum or minimum age limit was set for parents. To align the genetic backgrounds of the participants, it was required for all relatives within the third degree of kinship to be Japanese. Participants were required to have no history of cerebrovascular disease, brain tumor, intracranial disease, degenerative brain disease, epilepsy, serious heart disease, serious brain injury with impaired consciousness, no tendency of claustrophobia and nyctophobia, no metals in the body such as a cardiac pacemaker, and no possibility of current pregnancy. These conditions were verified at the time of participation, and if any member of the trio met the exclusion criteria, their participation was declined. If a participant was found to meet the exclusion criteria during participation in the study, only assessments that guaranteed safety were performed.
2.2. Incentives for participants
Each participant received a gift voucher worth 5,000 JPY. The parents were also given a report on brain health of themselves based on hippocampal volume using BrainSuite, developed by CogSmart, Inc. Offspring were given a printed copy of the T1-weighted brain image and a score report from the Wechsler Adult Intelligence Scale Fourth Edition (WAIS-IV) (67) or Wechsler Intelligence Scale for Children Fourth Edition (WISC-IV) (68).
2.3. Recruitment of participants
Advertisements for recruiting participants were published in a local magazine on town information. Additionally, flyers and posters were displayed at universities, high schools, vocational schools, government offices, public facilities, and stores in Sendai. All advertisements included information on the eligibility criteria, an overview of the inspections to be conducted, and the time required along with dates and rewards. A QR code on the advertisement could be scanned to access a website with details of the study. This website allowed applicants to access an application form created using Google Forms, in which applicants and their family members input their name, age, gender, role in the family (father, mother, or child), phone number, e-mail address, preferred date of participation, and confirmation that they do not meet the exclusion criteria. After reviewing the applicants’ input data, the staff coordinated the date and time of participation by e-mail or phone. If the number of applicants exceeded the targeted number of trios, a waiting list was created. Applicants in this list were requested to participate as soon as additional survey dates were added.
2.4. Experimental procedure
2.4.1. Overview
Data were collected at the Institute of Development, Aging, and Cancer of Tohoku University. On the day of the visit, the participants underwent brain MRI, global intelligence test, face morphological scanning, and 2D palm scanning. On the same day, they provided saliva samples and answered the questionnaires. Parents brought the mother and child health handbook and the score reports of their offspring’s physical fitness test. Several questionnaires were administered in this study. To reduce the psychological burden on participants as much as possible, most questionnaires were taken home so that they could be self-administered at the participants’ own pace. In addition, a time schedule for participation was documented on the website to help participants keep a track of the progression of tests in the study. Participants were asked to check the time schedule in advance. Questionnaires taken home were requested to be submitted by post within approximately 2 weeks of the date of participation.
The duration of all experiments on the day of participation is approximately 4 h, and the time required to complete the questionnaire test to be taken home is approximately 1 h.
2.4.2. Sociological information
Participants were asked to provide the following information through originally formatted questionnaires: educational background (69), personal annual income, employment status, type of business, job role, changes in income due to the COVID-19 pandemic (70), family members, sibling composition, availability, and type of pets. In the case of the participating offspring as students, questions about occupation and income were omitted.
2.4.3. Health and physical information
Height and weight were measured and recorded before the MRI.
All participants answered the Japanese version of the Flinders handedness questionnaire (FLANDERS) (71, 72) to evaluate handedness.
Participants were asked about their current illness under treatment, medications they are currently taking, medical history, history of COVID-19 infection, and history of psychiatric diseases and developmental disorders in relatives, using the original survey form. For participants aged under 15, questions other than those regarding the history of COVID-19 infection were answered by the mother. Female participants also answered questions on the menstrual cycle and the last menses start date.
Pubertal status was also assessed for underage participants using the Pubertal Development Scale (73, 74), a self-administered questionnaire comprising five questions about the progression of secondary sexual characteristics.
Only parents of children aged under 18 were asked to bring their offspring’s score reports from physical fitness tests conducted recently at school. The Physical Fitness Test is an official physical ability test mandated by the Japan Ministry of Education, Culture, Sports, Science, and Technology to investigate the nation’s physical strength and athletic ability, and is administered annually at schools. This test score captures a child’s flexibility and agility. The score reports were scanned by the research staff.
2.4.4. Global intelligence
To measure global intelligence, participants aged 16 or older completed the WAIS-IV, and those aged 15 or younger completed the WISC-IV. Ten core subtests were conducted to calculate the full-scale intelligence quotient along with four factors (verbal comprehension, perceptual reasoning, working memory, and processing speed indices). The tests were conducted in a quiet, non-stimulating room, one-on-one with staff familiar with the procedure and participants. The time required to complete the test ranged approximately 60–90 min.
2.4.5. Brain imaging
Prior to scanning, participants were asked to confirm that they had no metal in their bodies, tattoos, permanent makeup, claustrophobia, nyctophobia, pregnancy, and were not wearing thermal undershirts and contact lenses. Participants’ signatures were obtained for this confirmation.
Brain images were acquired using a 3-Tesla dStream Achieva scanner (Philips Medical Systems, Best, Netherlands) with a 20-channel headneck coil. Participants wore earplugs and headphones to protect their ears from MRI noise. An emergency buzzer was given to the participants to alert the research staff if any problems occurred during the imaging. The total scan time was approximately 24 min.
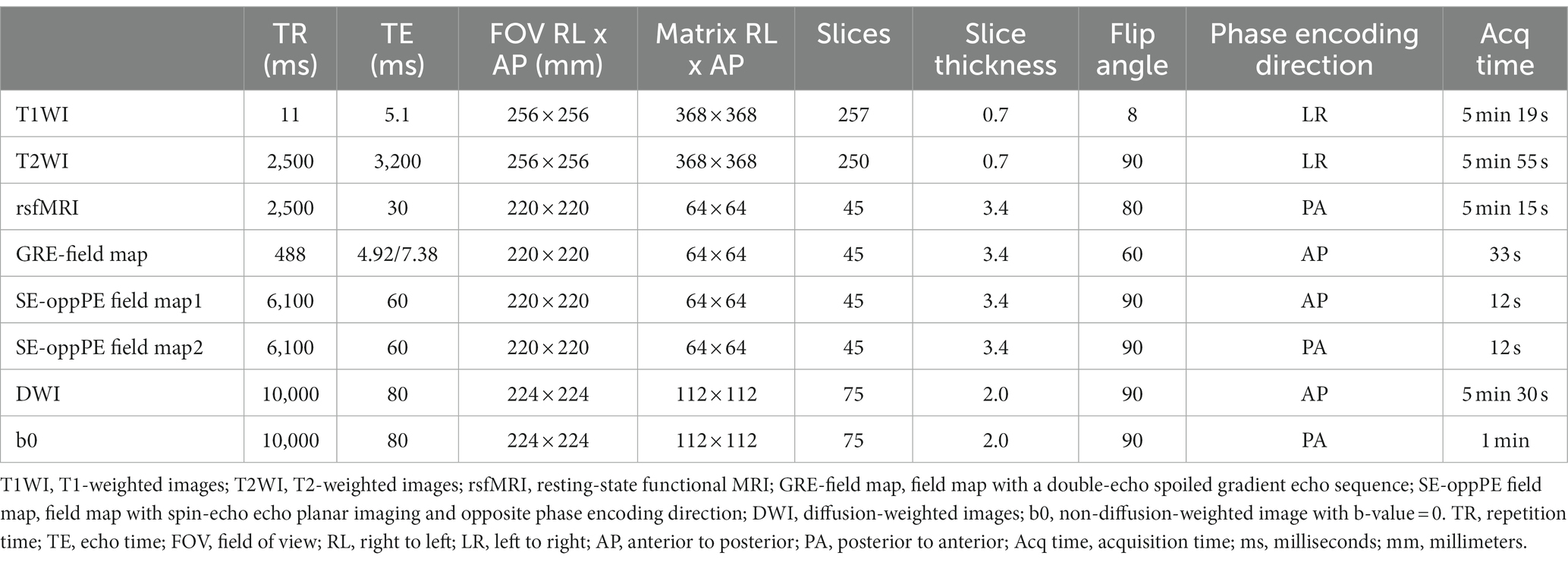
The following images were acquired for each participant. First, sagittal T1-weighted images (T1WIs) were obtained using a magnetization-prepared rapid gradient-echo (MPRAGE) sequence. Second, sagittal T2-weighted images (T2WIs) were acquired using a spin-echo sequence. Third, resting-state functional MRI (rsfMRI) was performed using gradient-echo echo-planar imaging (EPI). Each run contained 120 image volumes preceded by 7 dummy volumes, with each volume comprising 45 slices. Participants were asked to stay awake, not think about anything as much as possible, and gaze at the center of the black fixation point on the gantry. Fourth, field maps were acquired for distortion correction using rsfMRI. As the usefulness of each type of distortion correction remains controversial, we obtained two types of field maps: images with a double-echo spoiled gradient echo sequence (GRE-field map) and a pair of spin-echo EPI images with opposite anterior–posterior (AP) and posterior–anterior (PA) phase encoding direction (SE-oppPE-field map) (75–77). Fifth, diffusion-weighted images (DWI) was obtained in 30 different directions using a spin-echo sequence. The b-value was set to 1,000s/mm2 for 30 volumes. Sixth, two non-diffusion weighted (b=0) images were acquired with reversed-phase encoding directions (AP and PA) for distortion correction (78). Details of the acquisition parameters are presented in Table 1.
2.4.6. Genome and epigenome
Saliva samples were collected for genetic/epigenetic analysis using Oragene® Discover (DNA Genotek, Inc., Ottawa, ON, Canada) following the manufacturer’s protocol. The collected samples were stored at room temperature until DNA extraction was performed. Genomic DNA was extracted from the samples using an Oragene® purifier. Thereafter, the extracted DNA samples were measured for concentration using a Nanodrop 2000 (Thermo Fisher, Applied Biosystems, Foster City, CA, United States) and then stored in a freezer at −30°C. In future research, we plan to use microarrays for single-nucleotide polymorphism (SNP) typing and epigenomic analyses.
2.4.7. Psychological measures
We used psychological questionnaires to collect information about the participants’ behavioral phenotypes, including mental health problems, well-being, personality traits, and socioemotional competencies. We selected scales for which reliability and validity were confirmed and are widely used internationally.
Our first priority was to assess behavioral phenotypes using the same scale for both parents and offspring. However, because it is difficult for children aged under 15 to answer the same questionnaires as adults, we adopted appropriately developed scales for children whenever possible. Moreover, self-administered questionnaires were used unless only parent-administered questionnaires for children were available.
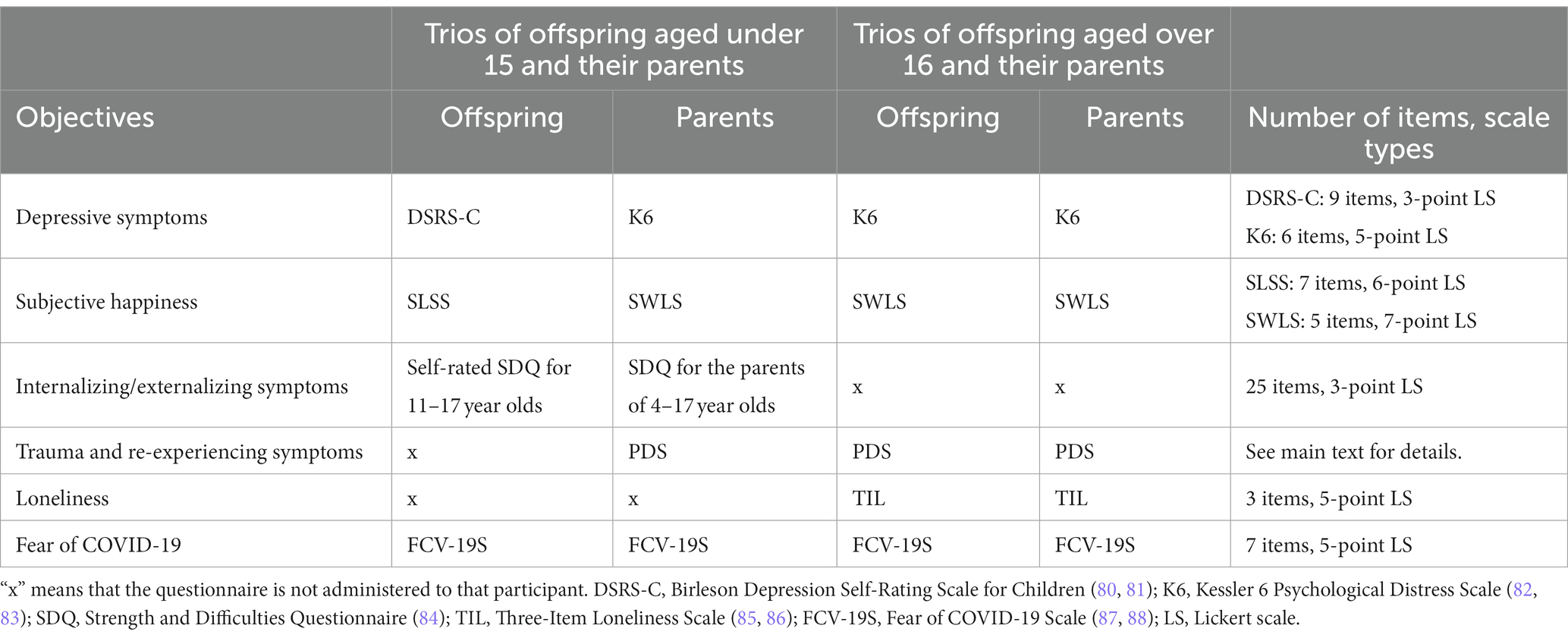
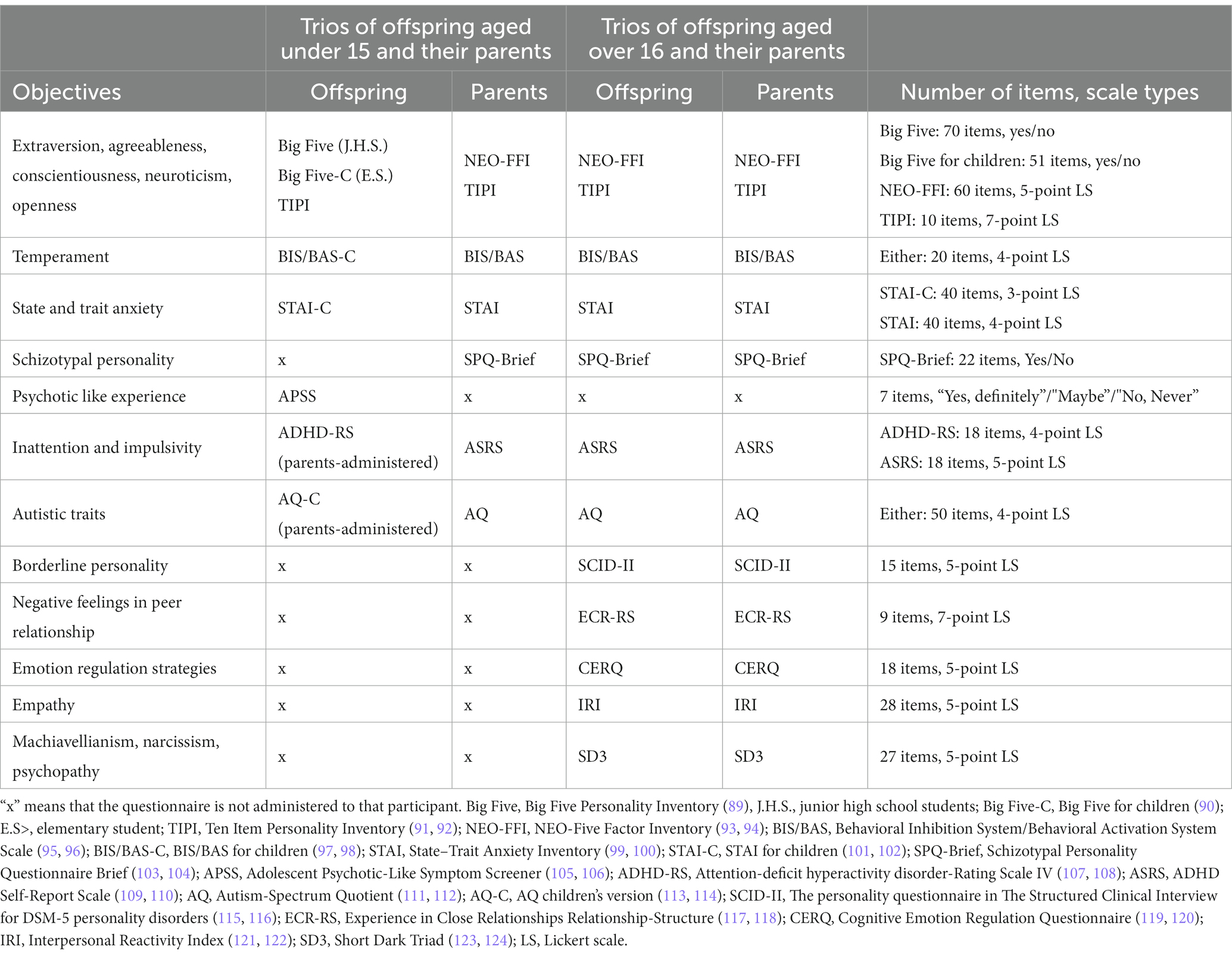
The response burden for questionnaires varies by respondents’ age. Children are more likely to be burdened (79). Therefore, to roughly equalize the response burden, fewer questionnaires were administered to offspring aged 10–15 compared with those aged 16 and older. Which participants answered which questionnaires are summarized in Tables 2–4.
2.4.7.1. Mental health problems and well-being
Participants’ depressive symptoms, subjective happiness, eudaimonic well-being, internalizing/externalizing symptoms, serious trauma, re-experiencing symptoms, loneliness, and fear of COVID-19 were assessed. The names of the questionnaires, number of items, and responses are summarized in Table 2. Here, we discuss only the details necessary for special mention.
The Satisfaction with Life Scale (SWLS) (139, 140) is used to assess subjective happiness. Participants were asked to respond not only for themselves but also for the two others (e.g., the mother answers about herself, her offspring, and her husband) to examine how the trio perceives each other’s perspectives on life, and whether their self-evaluation matches or diverges from the two others’ evaluations. Prior studies have evaluated others’ assessment of well-being using this scale (141). On the other hand, for offspring aged 15 and younger, the Student’s Life Satisfaction Scale (SLSS) (142, 143) was used to evaluate subjective happiness. Trios of offspring aged under 15 and their parents did not evaluate each other’s well-being.
The Strengths and Difficulties Questionnaire (SDQ) (84) was used to assess internalizing and externalizing symptoms in offspring aged 15 and lower. We administered the self-report version of the SDQ for offspring. Additionally, parents were administered the parent-report version of the SDQ to assess the offspring’s internalizing and externalizing symptoms from multiple perspectives. The Japanese version of this questionnaire was obtained from YouthinMind.12
The Posttraumatic Diagnostic Scale (PDS) (144–146) was used to assess the presence or absence of serious trauma and re-experiencing symptoms. Respondents selected what they have experienced thus far from a list of several traumatic events, such as natural disasters and assaults; further, if any of the selected events have bothered them in the past month, they were asked to state when the event began and ended, and the extent to which they re-experience the event.
2.4.7.2. Personality traits and socioemotional competencies
Participants were assessed for the following: extraversion, agreeableness, conscientiousness, neuroticism, openness, temperaments of behavioral inhibition/activation, state and trait anxiety, schizotypal personality, psychotic-like experience, inattention and impulsivity, autistic traits, borderline personality, negative feelings in peer relationships (discomfort in close relationships and abandonment anxiety), emotion regulation strategies, empathic traits, Machiavellianism, narcissism, and psychopathy. The names of the questionnaires, number of items, and choices are summarized in Table 3. Here, we discuss only the details necessary for special mention.
The Ten-Item Personality Inventory (TIPI) (91, 92, 147) comprises 10 items to assess five-factor personality traits. Each participant responded about themselves and the two others (e.g., the offspring answered about themself, their mother, and their father) to examine their perception of each other’s personality, and whether their self-evaluations match or diverge from the others.
The Schizotypal Personality Questionnaire Brief (SPQ-Brief) (103, 104) was used to assess traits believed to have a common biological basis with schizophrenia. For offspring aged under 15, the Adolescent Psychotic-Like Symptom Screener (APSS) (105, 106) was used to assess psychotic-like experiences, which are associated with schizophrenia. The APSS consists of seven items—four taken from the schizophrenia section of the Diagnostic Interview Schedule for Children (148, 149) and three items on visual hallucinations, delusions of control, and grandiosity added by Kelleher et al. (105). The Japanese version of the APSS was obtained from the Japanese paper on which Ando et al. were based.
The personality questionnaire in the Structured Clinical Interview for DSM-5 personality disorders (SCID-II) (115) was used to evaluate the tendency toward borderline personality disorder (BPD). We only used 15 items assessing BPD. Although the original format was to answer “yes” or “no,” a 5-point Likert scale is sometimes used to capture individual differences in more detail (150–152), which we adopted in this study.
Experience in Close Relationships Relationship-Structure (ECR-RS) (117) was used to evaluate discomfort in close relationships and abandonment anxiety. Participants were asked to indicate their thoughts on their relationships with people such as their father, mother, friend, and lover/spouse.
2.4.7.3. Environmental factors during development
The definition of environment is broad and includes all “non-genetic” factors (153). According to a previous study that proposed the concept of exposome (all environmental exposures from the prenatal period through the entire life) (153), environmental factors are divided into three domains: an internal domain, comprising metabolism, inflammation, hormones, and other bodily processes that can be quantified using high-throughput molecular omics technology; a specific external domain, comprising the individual-level psychosocial factors that can be assessed by questionnaires, such as educational achievement, social deprivation, and traumatic experience; and a general external domain, comprising community-level physical factors such as temperature, green space, air pollution, transportation, and population density. The findings of large neuroimaging cohort studies with abundant data belonging to the general external domain, such as CHIMGEN (5) and ABCD study® (154), indicate the importance of physical environmental factors in brain development. In contrast, internal domain data are an advantage of birth cohorts (155). As mentioned in the introduction, the interest of this study was to elucidate the mechanisms of intergenerational transmission of experiences and their influences. Therefore, we focused on the environmental factors that corresponded with the specific external domain during the developmental periods of both parents and offspring. The environmental factors that we focused on can be roughly divided into three subdomains: in-home, out-of-home, and pre/postnatal.
2.4.7.3.1. In-home environment
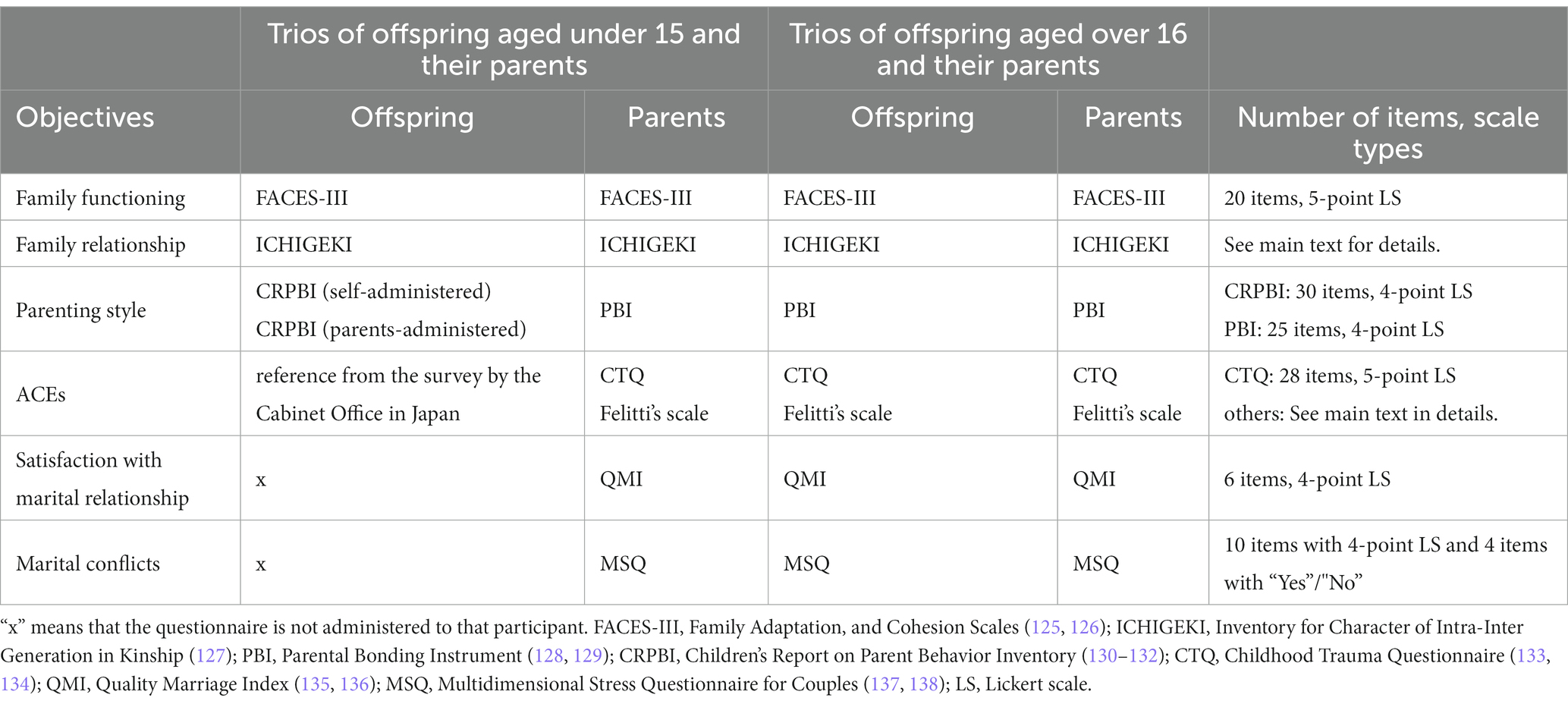
We assessed family functioning, family relationships, perceived parenting style, ACEs, satisfaction with marital relationships, and marital conflicts. The names of the questionnaires, number of items, and responses are summarized in Table 4.
Family Adaptation and Cohesion Scales (FACES-III) (125, 126) were used to assess two dimensions of family functioning: cohesion (emotional bonding among family members) and adaptability (the ability to change power relations and roles within the family depending on the situation).
The Inventory for Character of Intra-Inter Generation in Kinship (ICHIGEKI) (127) was used to identify how respondents perceived their relationships with the two others in the trio. For example, a father will respond with a score of 1–10 for the magnitude of his emotional bonding and conflicts with his wife and offspring.
The Parental Bonding Instrument (PBI) (128, 129) was used to assess emotional warmth and overprotectiveness received from parents before the age of 16. Participants were asked to answer about both their father and mother. For offspring aged under 15, the Children’s Report on Parent Behavior Inventory (CRPBI-30) (130–132) was used to assess parenting style in terms of emotional acceptance and control. Offspring aged under 15 were asked to answer about both their father and mother. The CRPBI-30 scale allows both children and parents to assess parenting style from each perspective, and thus, parents of offspring aged under 15 are also asked to complete this questionnaire.
ACEs were assessed using several questionnaires. First, the Childhood Trauma Questionnaire (CTQ) (133, 134) was used to assess childhood experiences of physical/emotional/sexual abuse and physical/emotional neglect. Second, based on previous studies, we investigated the presence or absence of parental divorce or separation, domestic violence, criminal behavior in the household, mental illness in the household, death of a caregiver, and poverty before age 18 (23, 156). Although mental illness in the household is also included as an ACE in Felitti’s scale, it was omitted in this study because we already obtained this information in the health and physical domains. For offspring aged under 15, the presence or absence of experiences of physical/emotional abuse and neglect, parental divorce or separation, witnessing domestic violence, alcohol or substance abuse addiction in the household, and mental illness in the household were examined based on a survey of child poverty by the Cabinet Office of Japan.
Quality Marriage Index (QMI) (135, 136) was used to evaluate the parents’ satisfaction with marital relationships through questions about bonding. As this scale is also used to assess the offspring’s perception of the relationship between their parents (157), we also asked the offspring participants to answer questions about the bonding between their father and mother. However, owing to the difficulty of the questions, this scale was not administered to offspring aged under 15.
The Multidimensional Stress Questionnaire for Couples (MSQ) (137, 138) was used to assess marital conflict between parents. The questionnaire comprises 10 items rated on a 4-point Likert scale to indicate the presence or absence of daily conflicts occurring in the last 7 days and 1 year; additionally, 4 items on more serious marital problems (e.g., violence and infidelity) were answered with “yes” or “no.”
Using retrospective questions on their experiences up to adolescence, participants were also asked about the frequency of conversations during mealtimes at home, family members who are home when they return from school, the relationship between the spatial composition of their house and their private room, and their experience of pet ownership and emotional unity with their pets.
2.4.7.3.2. Out-of-home environment
We asked participants the following information about their experiences till high school: friendships, relationships with teachers, meeting others who influenced their lives, bullying victimization/perpetration/bystanders, reading, sports, playing a musical instrument, leadership positions, changing schools, living abroad, excitement about nature and the arts, and enthusiasm for a hobby. They were also asked about the elementary, junior high, and high schools that they attended, including the approximate number of students, whether they took entrance exams, and whether they were coeducational schools. Furthermore, we asked about whether they played outdoors or indoors from preschool to elementary school, when they began using cell phones or smartphones, and whether their parents restricted them from using TV, games, or phones. As there are few reliable and validated internationally used scales for the above information, the questions used in the Japanese literature or the survey forms of Japanese public institutions were used as references.
As Japan is prone to natural disasters, we asked respondents about the locations of homes they had lived in by age 20 to capture their experiences with natural disasters. Respondents were asked to indicate in which prefecture and municipality and the ages at which they lived in each home. This question was answered by the mother for offspring aged 15 or younger.
2.4.7.3.3. Pre-and-postnatal environment
To examine the impact of the pre- and postnatal environments on offspring development and intergenerational transmission, we asked mothers about the following information: experience of fertility treatment and artificial abortion prior to conceiving the offspring participating in this study; presence or absence of any perinatal problems such as breech baby; neonatal asphyxia; obstetric complications; perinatal depression; anticipation of childbirth; duration and method of breastfeeding; social support during and after pregnancy; and the frequency of alcohol, caffeine, raw food, and junk food consumption from when the pregnancy was discovered to delivery and from delivery to weaning. Additionally, both mothers and fathers were asked about their smoking habits before, during, and after pregnancy; whether there were smokers around them during their childhood; and whether their working patterns changed due to marriage and childbirth.
This study was conducted in Sendai City, Miyagi Prefecture, Japan, where many residents experienced the Great East Japan Earthquake. In particular, participants born between 2008 and 2013 may have experienced an earthquake either prenatally or as infants. Therefore, we asked their parents about the damage caused by the Great East Japan Earthquake. Parents were asked about the prefecture in which they lived at the time of the earthquake, the extent of damage to their household, and whether their relatives were affected.
Furthermore, perinatal information was also collected from the Mother and Child Health Handbook, which is issued for women in Japan at the time pregnancy is confirmed and is used during pregnancy and postpartum health checkups. This handbook includes information on the mother’s weight, fundal height, blood pressure, fetal growth at each prenatal checkup, method of delivery, birth weight, infant growth, and developmental milestones up to age 6. We scanned all pages after obtaining the mother’s permission.
2.4.7.4. Lifestyle habits
Participants’ lifestyle habits were also evaluated using questionnaires. For participants aged 16 and older (both parents and offspring), physical activity was assessed using the Global Physical Activity Questionnaire (GPAQ) (158, 159); eating and drinking habits were assessed using the Food Frequency Questionnaire (FFQ) short-form (160). Subjective sleep quality was assessed using the Athens Insomnia Scale (AIS) (161, 162), and participants were asked about their awareness of snoring or sleep apnea. Smartphone use and online game habits were assessed using the Smartphone Addiction Scale Short Version (SAS-SV) (163, 164) and Ten-Item Internet Gaming Disorder Test (IGDT-10, the Japanese version was translated by the Kurihama Medical and Addiction Center) (165). We also assessed bathing habits (166, 167). Smoking habits were assessed using the question items used in the survey by the Ministry of Health, Labour and Welfare of Japan was used. Additionally, the Multidimensional Scale of Perceived Social Support (168, 169) was used to ask about help obtained from daily interpersonal relationships.
For offspring aged under 15, physical activity was assessed using the WHO Health Behavior in School-aged Children (HBSC) (170, 171). Eating habits were assessed using the same scale as adults (FFQ short-form); however, as it is difficult for children to assess their own eating habits (172), we asked one of the parents to complete this scale. Additionally, the offspring were asked if they ate breakfast, lunch, and dinner every day. Subjective sleep quality was assessed using five items in the sleep disturbance factor of the General Health Questionnaire (GHQ-30) (173). Children’s sleep quality was assessed using the GHQ-30 in a previous study (174). Additionally, an isolated question asked the offspring whether they went to bed at the same time each day. The Japanese version of the Korean Scale for Internet Addiction for Adolescents (K-scale) was used to assess Internet-use habits. The Japanese version of the K-scale was translated by the Kurihama Medical and Addiction Center, with permission from the National Information Society Agency. Furthermore, we asked the offspring about the average number of study hours per day.
2.4.7.5. Other measurements of phenotypes
To examine the effects of intergenerational transmission on phenotypes other than brain structure and function or behavioral traits assessed by questionnaires, we obtained the following measurements: face morphology, ratio of second to fourth digit length (2D/4D ratio), and characteristics of the drawn tree. The brain and face have been reported to share several genetic loci (175), and facial asymmetry is associated with broader autistic phenotypes (176, 177). Moreover, 2D/4D ratio is considered to reflect sex steroid hormone exposure during embryonic development (178). Several findings have indicated a sex-specific association between the 2D/4D ratio and brain structure or function (179–181). Additionally, the associations between the 2D/4D ratio and personality traits, such as schizotypal personality (182), neuroticism (183), and emotional stability (184), are shown. The tree-drawing test (Baum test) (185) is a projective personality assessment technique. As the characteristics of drawn trees, such as canopy area and trunk width, have been reported to be significantly associated with schizophrenia (186) and depression (187), the usefulness of this tool to quantitatively assess some facets of psychological traits that cannot be captured with questionnaires has been suggested (188). We collected these data from the parents and offspring using the following procedure.
2.4.7.5.1. Face morphology
Digital facial stereophotogrammetry was used to capture 3D facial surfaces; 3D stereophotogrammetric imaging is a well-established approach for generating dense 3D points that represent the surface geometry of a face using multiple 2D images with overlapping fields of view (189). Facial surfaces were obtained using EinScan Pro HD (SHINING 3D Tech. Co., Ltd., Hangzhou, China). According to a standard facial imaging protocol (189), participants were instructed to close their mouths, relax their faces, and maintain a neutral expression. The participants were also asked to keep their eyes closed. 2D face images were also acquired using iPhone 6 (Apple Inc., Cupertino, CA, United States) for reference.
2.4.7.5.2. 2D/4D ratio
Brother PRIVIO DCP-J962N (Brother Industries, Ltd., Aichi, Japan) was used to acquire 2D palm scanned images. According to a prior study’s protocol (190), both hands are placed flat on the scanner glass, ensuring that they are clearly separated from each other. Next, the scanner cover is closed, following which the scanner generates a PDF file of the image (210 mm × 297 mm, 200 dpi resolution).
2.4.7.5.3. Tree-drawing test
A sheet of A4 paper and a 4B pencil were provided to each participant, and they were instructed to “draw a tree on the drawing paper. The purpose is not to see how good or bad you are at drawing, so please draw freely, with little care.” When the participants finished drawing, they were asked to freely describe on the back of the paper what kind of tree they drew and how they felt after drawing it.
2.5. Analysis
Brain images will be preprocessed using the analysis software widely used in this field like FreeSurfer13 (191), FMRIB Software Library (FSL)14 (192), MRtrix315 (193), and Advanced Normalization Tools (194) (ANTs),16 and Analysis of Functional NeuroImages (AFNI)17 (195). Structural and functional indices such as gray matter volume, cortical thickness, surface area, local gyrification index, white matter fractional anisotropy, and resting-state functional connectivity will be extracted. Quality controls of preprocessed images will be performed by appropriate scripts and manual editing. All raw and preprocessed images are stored in Brain Imaging Data Structure (BIDS) format for data sharing in the future.
One of the major goals of our study is to understand the putative mechanisms of intergenerational transmission, using brain images as endophenotype. To achieve this objective, we consider the following analyses. First, we plan to determine which characteristics in which brain areas are similar between father and offspring and/or mother and offspring, and whether the similarities can be recognized as the neural basis for the intergenerational transmission of behavioral phenotypes. This theme includes a replication of previous studies of intergenerational neuroimaging. In previous studies, parent–offspring brain similarity has often been described using the correlation of the characteristics of brain region of interest (ROI) between parents and offspring (28, 32, 35, 39, 40). Therefore, we will also examine parent–offspring brain similarity based on correlation analysis. The power analysis using G*Power (196, 197) calculated the required sample size of 134, with a moderate effect size (r = 0.30, α = 0.05, two-tailed, power = 0.95). Second, we also plan to investigate the association between parental ACEs and offspring’s brain structure or function to present additional knowledge of intergenerational transmission of trauma effect. Linear regression analysis is one of the options for examining these associations (41–44, 198–201). The power analysis using G*Power calculated the required sample size is 138, with a moderate effect size [f2 = 0.15, α = 0.05, power = 0.95, number of predictors = 5 (i.e., predictor variables = maternal ACEs, paternal ACEs, interactive term of maternal and paternal ACEs, offspring’s age, and offspring’s sex, dependent variable = ROI value of cortical thickness in offspring)]. Third, we will examine whether the effect of parenting style on offspring’s brain development differs depending on whether there is intergenerational transmission of parenting style. Specifically, we will examine the differences in offspring’s (Generation 3; G3) brain development depending on whether parents’ (Generation 2; G2) parenting style toward their offspring is similar to the parenting style they received from their own caregivers (Generation 1; G1) during their childhood. The parenting style of G2 and G1 are assessed using PBI or CRPBI as mentioned above. According to our preliminary analysis performed using different data from the present study, whether positive parenting style (assessed by the care factor in PBI) transmitted between G1 and G2 was related to G3’s cortical thickness with a moderate effect size [analysis of covariance (ANCOVA) was conducted to compare the four groups: high-care G1 and high-care G2, high-care G1 and low-care G2, low-care G1 and low-care G2, and low-care G1 and high-care G2]. In line with this, the sample size required to obtain a moderate effect size by ANCOVA with four groups was calculated to be 279 [f = 0.25, α = 0.05, power = 0.95, number of covariates = 2 (i.e., offspring’s age and gender)]. From the above, approximately 310 trios would be required to meet the largest samples for the currently planned analysis, assuming that 10% of the samples are excluded due to incomplete or missing data. In addition to the above, other analyses deemed necessary will be performed. For each analysis, if there are missing values, the data from that participant will be excluded from the analysis.
In the future, the sample size can be increased further to enable analysis using genetic information. In genetic studies, a family-based design has the advantage that it can eliminate the problems of population stratification (202). The parent–offspring trio is the simplest family-based design. Some previous studies using polygenic transmission disequilibrium tests (pTDT), a recently developed family-based design analysis method (203), have been conducted with <100–200 trios (204–206), while others have been conducted with nearly 3,000 trios (203, 207, 208). In contrast, previous studies on the genetic nurture effect often deal with 1,000–2,000 trios (51). Although the optimal sample size of trio-based genetic analysis is unclear, we hope to expand this study by including more than 1,000 trios in the future.
Although this study is a venturesome project with a different budget and staffing compared to existing large cohorts, we intend to eventually develop this project into a multi-site collaborative study and lead the neuroimaging research of parent–offspring trios.
3. Discussion
3.1. Current status and future directions
By April 2023, we completed data acquisition from 174 trios. The mean ages of the offspring, mothers, and fathers were 17.55 ± 6.76, 50.18 ± 6.21, and 51.58 ± 7.03, respectively. The numbers of male and female offspring were 85 and 89, respectively. Most participants completed all the surveys, but brain images were partially omitted for some due to unexpected claustrophobia or technical errors of the MRI scanner.
3.2. Strengths and limitations
Our study has several strengths. First, we were able to accumulate data on fathers, whereas most previous studies have focused only on mothers and offspring (209). We devised to make it easier for fathers to participate in this study by allowing families to separately schedule their participation in the study and by conducting the survey on weekends. Second, data collection was performed in the same manner for both parents and offspring, especially for trios in which the offspring were aged 16 or older. For trios with offspring aged 15 or younger, we attempted to quantify common phenotypes by using age-appropriate self-administered scales for each parent and offspring. This approach allowed us to examine the parent–offspring similarities and their relationships for each phenotype and intermediate phenotype. Third, behavioral phenotypes and biological markers were collected using various methods, including not only brain images and questionnaires but also peer assessment of personality and well-being, projective techniques, face morphology scans, and palm scans. These diverse data of trios provide a novel perspective on the relationship between the formation of individuality and intergenerational transmission.
This study has some limitations. First, because children aged under 10 were not included in this study, it was not possible to determine when the effects of intergenerational transmission on traits become apparent. This limitation should be addressed through future studies targeting younger children and their parents. Second, as our study used a cross-sectional design, we could not investigate the longitudinal changes in intergenerational transmission effects on the offspring’s lifespan development. Ideally, intergenerational transmission should be investigated when two generations are of the same age, because unapparent traits in youth may appear in middle adulthood (6). However, such an ideal study would take a long time to be realized. We believe that our study adopted the most feasible and optimal design that is currently available. Third, most of the environmental factors were assessed retrospectively; therefore, recall bias may have affected the results. Thus, we tried to address this limitation as much as possible by devising questions that can be answered with a simple choice of answer.
3.3. Conclusion
The TRIO study is a novel neuroimaging research project that investigated the association between intergenerational transmission and personality development. Our study provides interdisciplinary insights into how individuals’ lives are involved in the construction of the lives of their descendants in the subsequent generation.
Ethics statement
This study was conducted in accordance with the principles of the Declaration of Helsinki (210). Approval was obtained from the Institutional Review Board of Tohoku University (Approved No. 2022-1-534). Written informed consent and ascent was obtained from all participants before the study. If a participant was aged under 18, written consent was obtained from their parents.
Author contributions
YT supervised the project. IM, RY, and YT designed the study. IM and RY collected the data. IM wrote the manuscript, which was reviewed by RY and YT. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by a Grant-in-Aid for Young Scientists (Grant no. 22K13809) and Grant-in-Aid for Transformative Research Areas (A) (Grant no. 22H05209) from the Ministry of Education, Culture, Sports, and Technology (MEXT) and the Japan Society for the Promotion of Science (JSPS) to IM. This work was also funded by the Program for the Creation of Interdisciplinary Research from the Frontier Research Institute for Interdisciplinary Sciences, Tohoku University to IM. This work was also supported by JST SPRING (Grant No. JPMJSP2114) awarded to RY. This work was also supported by a Grant-in-Aid for Scientific Research (B) (Grant no. 19H04211) from the MEXT to YT. This work was supported by JSPS KAKENHI Grant Number JP22H04926 and Grant-in-Aid for Transformative Research Areas — Platforms for Advanced Technologies and Research Resources “Advanced Bioimaging Support” for the curation and analysis of neuroimaging data.
Acknowledgments
We wish to express our deepest thanks to the participants for their willingness to participate in this study. We thank Yukiko Suginome, Saeko Hoshi, Chieko Miura, Maiko Chiba, Misaki Abe, Junko Kato, and Shuzo Yamamoto for their support. We thank Ryosuke Kimura, Tadashi Imanishi, Hiroaki Tomita, Ayaka Uchiyama, Kanna Oyama, Mihiro Koizumi, Takumi Uchiyama, Yuka Aoki, Fumiaki Nitta, Nanae Moriya, Fumi Seki, Narumi Fujiwara, Kazuki Ozawa, Yuji Yanagiya, Mako Toyota, Sawako Watanabe, Yuka Hatayama, Megumi Kato, Maiko Suenaga, Megumi Shirahama, Rinka Yoshihara, and Ruriko Igarashi for their support with data collection. We also appreciate all the institutions that willingly cooperated with us in recruiting study participants. We would like to thank Editage (www.editage.jp) for English language editing.
Conflict of interest
We used BrainSuite, developed by CogSmart, Inc., as an incentive for the participants. YT was the chief scientific officer of CogSmart, Inc. and has obtained approval from the Conflict of Interest (COI) Management Committee of Tohoku University for his involvement in this study. This study was also supported by the joint research fund of CogSmart, Inc., but these funds have not been used to pay for using BrainSuite.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
2. ^https://imagen-project.org/cgi-sys/suspendedpage.cgi
3. ^https://www.med.upenn.edu/bbl/philadelphianeurodevelopmentalcohort.html
5. ^http://chimgen.tmu.edu.cn/en
6. ^https://www.fhi.no/en/studies/moba/
7. ^http://www.bris.ac.uk/alspac/
8. ^https://www.developingconnectome.org/
9. ^https://www.megabank.tohoku.ac.jp/english/research/cohortbiobank/birthree/
10. ^https://www.lifelines.nl/
11. ^http://ttcp.umin.jp/index.html
12. ^https://sdqinfo.org/py/sdqinfo/b3.py?language=Japanese
13. ^https://surfer.nmr.mgh.harvard.edu/
14. ^https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSL
References
1. Karcher, NR, and Barch, DM. The ABCD study: understanding the development of risk for mental and physical health outcomes. Neuropsychopharmacology. (2020) 46:131–42. doi: 10.1038/s41386-020-0736-6
2. Mascarell Maričić, L, Walter, H, Rosenthal, A, Ripke, S, Quinlan, EB, Banaschewski, T, et al. The IMAGEN study: a decade of imaging genetics in adolescents. Mol Psychiatry. (2020) 25:2648–71. doi: 10.1038/s41380-020-0822-5
3. Satterthwaite, TD, Elliott, MA, Ruparel, K, Loughead, J, Prabhakaran, K, Calkins, ME, et al. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. (2014) 86:544–53. doi: 10.1016/j.neuroimage.2013.07.064
4. Hofman, A, Jaddoe, VWV, Mackenbach, JP, Moll, HA, Snijders, RFM, Steegers, EAP, et al. Growth, development and health from early fetal life until young adulthood: the generation R study. Paediatr Perinat Epidemiol. (2004) 18:61–72. doi: 10.1111/j.1365-3016.2003.00521.x
5. Xu, Q, Guo, L, Cheng, J, Wang, M, Geng, Z, Zhu, W, et al. CHIMGEN: a Chinese imaging genetics cohort to enhance cross-ethnic and cross-geographic brain research. Mol Psychiatry. (2020) 25:517–29. doi: 10.1038/s41380-019-0627-6
6. Branje, S, Geeraerts, S, de Zeeuw, EL, Oerlemans, AM, Koopman-Verhoeff, ME, Schulz, S, et al. Intergenerational transmission: theoretical and methodological issues and an introduction to four Dutch cohorts. Dev Cogn Neurosci. (2020) 45:100835. doi: 10.1016/j.dcn.2020.100835
7. Constantino, JN, and Todd, RD. Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry. (2005) 57:655–60. doi: 10.1016/j.biopsych.2004.12.014
8. Sawyer, KM, Zunszain, PA, Dazzan, P, and Pariante, CM. Intergenerational transmission of depression: clinical observations and molecular mechanisms. Mol Psychiatry. (2019) 24:1157–77. doi: 10.1038/s41380-018-0265-4
9. Aktar, E, Van Bockstaele, B, Pérez-Edgar, K, Wiers, RW, and Bögels, SM. Intergenerational transmission of attentional bias and anxiety. Dev Sci. (2019) 22:e12772. doi: 10.1111/desc.12772
10. Hammen, C, Shih, JH, and Brennan, PA. Intergenerational transmission of depression: test of an interpersonal stress model in a community sample. J Consult Clin Psychol. (2004) 72:511–22. doi: 10.1037/0022-006X.72.3.511
11. Garber, J, and Cole, DA. Intergenerational transmission of depression: a launch and grow model of change across adolescence. Dev Psychopathol. (2010) 22:819–30. doi: 10.1017/S0954579410000489
12. Duarte, CS, Monk, C, Weissman, MM, and Posner, J. Intergenerational psychiatry: a new look at a powerful perspective. World Psychiatry. (2020) 19:175–6. doi: 10.1002/wps.20733
13. Paananen, R, Tuulio-Henriksson, A, Merikukka, M, and Gissler, M. Intergenerational transmission of psychiatric disorders: the 1987 Finnish birth cohort study. Eur Child Adolesc Psychiatry. (2021) 30:381–9. doi: 10.1007/s00787-020-01524-5
14. Ranning, A, Uddin, MJ, Sørensen, HJ, Laursen, TM, Thorup, AAE, Madsen, T, et al. Intergenerational transmission of suicide attempt in a cohort of 4.4 million children. Psychol Med. (2021) 52:3202–9. doi: 10.1017/S0033291720005310
15. Assink, M, Spruit, A, Schuts, M, Lindauer, R, van der Put, CE, and Stams, G-JJM. The intergenerational transmission of child maltreatment: a three-level meta-analysis. Child Abuse Negl. (2018) 84:131–45. doi: 10.1016/j.chiabu.2018.07.037
16. Madigan, S, Cyr, C, Eirich, R, Fearon, RMP, Ly, A, Rash, C, et al. Testing the cycle of maltreatment hypothesis: Meta-analytic evidence of the intergenerational transmission of child maltreatment. Dev Psychopathol. (2019) 31:23–51. doi: 10.1017/S0954579418001700
17. Madden, V, Domoney, J, Aumayer, K, Sethna, V, Iles, J, Hubbard, I, et al. Intergenerational transmission of parenting: findings from a UK longitudinal study. Eur J Pub Health. (2015) 25:1030–5. doi: 10.1093/eurpub/ckv093
18. Kerr, DCR, Capaldi, DM, Pears, KC, and Owen, LD. A prospective three generational study of fathers’ constructive parenting: influences from family of origin, adolescent adjustment, and offspring temperament. Dev Psychol. (2009) 45:1257–75. doi: 10.1037/a0015863
19. Niu, H, Liu, L, and Wang, M. Intergenerational transmission of harsh discipline: the moderating role of parenting stress and parent gender. Child Abuse Negl. (2018) 79:1–10. doi: 10.1016/j.chiabu.2018.01.017
20. Neppl, TK, Diggs, ON, and Cleveland, MJ. The intergenerational transmission of harsh parenting, substance use, and emotional distress: impact on the third-generation child. Psychol Addict Behav. (2020) 34:852–63. doi: 10.1037/adb0000551
21. Kerr, DCR, Gini, G, Owen, LD, and Capaldi, DM. Peer teasing experiences of fathers and their children: intergenerational associations and transmission mechanisms. Child Abuse Negl. (2018) 86:33–44. doi: 10.1016/j.chiabu.2018.09.003
22. Yehuda, R, and Lehrner, A. Intergenerational transmission of trauma effects: putative role of epigenetic mechanisms. World Psychiatry. (2018) 17:243–57. doi: 10.1002/wps.20568
23. Felitti, VJ, Anda, RF, Nordenberg, D, Williamson, DF, Spitz, AM, Edwards, V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med. (1998) 14:245–58. doi: 10.1016/s0749-3797(98)00017-8
24. Doi, S, Fujiwara, T, and Isumi, A. Association between maternal adverse childhood experiences and mental health problems in offspring: an intergenerational study. Dev Psychopathol. (2021) 33:1041–58. doi: 10.1017/S0954579420000334
25. Doi, S, Isumi, A, and Fujiwara, T. Association between maternal adverse childhood experiences and child resilience and self-esteem: results from the K-CHILD study. Child Abuse Negl. (2022) 127:105590. doi: 10.1016/j.chiabu.2022.105590
26. Ho, TC, Sanders, SJ, Gotlib, IH, and Hoeft, F. Intergenerational neuroimaging of human brain circuitry. Trends Neurosci. (2016) 39:644–8. doi: 10.1016/j.tins.2016.08.003
27. Poissant, H, Rapin, L, and Mendrek, A. Intergenerational transmission of fronto-parietal dysfunction during forethought in attention deficit/hyperactivity disorder: a pilot study. Psychiatry Res. (2014) 224:242–5. doi: 10.1016/j.pscychresns.2014.08.011
28. Yamagata, B, Murayama, K, Black, JM, Hancock, R, Mimura, M, Yang, TT, et al. Female-specific intergenerational transmission patterns of the human Corticolimbic circuitry. J Neurosci. (2016) 36:1254–60. doi: 10.1523/JNEUROSCI.4974-14.2016
29. Foland-Ross, LC, Behzadian, N, LeMoult, J, and Gotlib, IH. Concordant patterns of brain structure in mothers with recurrent depression and their never-depressed daughters. Dev Neurosci. (2016) 38:115–23. doi: 10.1159/000444448
30. Colich, NL, Ho, TC, Ellwood-Lowe, ME, Foland-Ross, LC, Sacchet, MD, LeMoult, JL, et al. Like mother like daughter: putamen activation as a mechanism underlying intergenerational risk for depression. Soc Cogn Affect Neurosci. (2017) 12:1480–9. doi: 10.1093/scan/nsx073
31. Billeci, L, Calderoni, S, Conti, E, Lagomarsini, A, Narzisi, A, Gesi, C, et al. Brain network organization correlates with autistic features in preschoolers with autism spectrum disorders and in their fathers: preliminary data from a DWI analysis. J Clin Med Res. (2019) 8:487. doi: 10.3390/jcm8040487
32. Abraham, E, Posner, J, Wickramaratne, PJ, Aw, N, van Dijk, MT, Cha, J, et al. Concordance in parent and offspring cortico-basal ganglia white matter connectivity varies by parental history of major depressive disorder and early parental care. Soc Cogn Affect Neurosci. (2020) 15:889–903. doi: 10.1093/scan/nsaa118
33. Okada, N, Yahata, N, Koshiyama, D, Morita, K, Sawada, K, Kanata, S, et al. Neurometabolic underpinning of the intergenerational transmission of prosociality. Neuroimage. (2020) 218:116965. doi: 10.1016/j.neuroimage.2020.116965
34. Vandermosten, M, Schevenels, K, Economou, M, and Hoeft, F. The influence of intergenerational transfer of white matter tracts on early reading development. bioRxiv. (2020). doi: 10.1101/2020.10.09.333096
35. Takagi, Y, Okada, N, Ando, S, Yahata, N, Morita, K, Koshiyama, D, et al. Intergenerational transmission of the patterns of functional and structural brain networks. iScience. (2021) 24:102708. doi: 10.1016/j.isci.2021.102708
36. Kim-Spoon, J, Lee, T-H, Clinchard, C, Lindenmuth, M, Brieant, A, Steinberg, L, et al. Brain similarity as a protective factor in the longitudinal pathway linking household Chaos, parenting, and substance use. Biol Psychiatry Cogn Neurosci Neuroimaging. (2023). doi: 10.1016/j.bpsc.2023.04.008. [Epub ahead of print].
37. Kim, P, Chen, H, Dufford, AJ, Tribble, R, Gilmore, J, and Gao, W. Intergenerational neuroimaging study: mother-infant functional connectivity similarity and the role of infant and maternal factors. Cereb Cortex. (2021) 32:3175–86. doi: 10.1093/cercor/bhab408
38. Ahtam, B, Turesky, TK, Zöllei, L, Standish, J, Grant, PE, Gaab, N, et al. Intergenerational transmission of cortical Sulcal patterns from mothers to their children. Cereb Cortex. (2021) 31:1888–97. doi: 10.1093/cercor/bhaa328
39. Fehlbaum, LV, Peters, L, Dimanova, P, Roell, M, Borbás, R, Ansari, D, et al. Mother-child similarity in brain morphology: a comparison of structural characteristics of the brain’s reading network. Dev Cogn Neurosci. (2022) 53:101058. doi: 10.1016/j.dcn.2022.101058
40. Minami, F, Hirano, J, Ueda, R, Takamiya, A, Yamagishi, M, Kamiya, K, et al. Intergenerational concordance of brain structure between depressed mothers and their never-depressed daughters. Psychiatry Clin Neurosci. (2022) 76:579–86. doi: 10.1111/pcn.13461
41. Khoury, JE, Ahtam, B, Sisitsky, M, Ou, Y, Gagoski, B, Enlow, MB, et al. Maternal childhood maltreatment is associated with lower infant gray matter volume and amygdala volume during the first two years of life. Biol Psychiatry Glob Open Sci. (2022) 2:440–9. doi: 10.1016/j.bpsgos.2021.09.005
42. Moog, NK, Entringer, S, Rasmussen, JM, Styner, M, Gilmore, JH, Kathmann, N, et al. Intergenerational effect of maternal exposure to childhood maltreatment on newborn brain anatomy. Biol Psychiatry. (2018) 83:120–7. doi: 10.1016/j.biopsych.2017.07.009
43. Demers, CH, Hankin, BL, Hennessey, E-MP, Haase, MH, Bagonis, MM, Kim, SH, et al. Maternal adverse childhood experiences and infant subcortical brain volume. Neurobiol. Stress. (2022) 21:100487. doi: 10.1016/j.ynstr.2022.100487
44. Hendrix, CL, Dilks, DD, McKenna, BG, Dunlop, AL, Corwin, EJ, and Brennan, PA. Maternal childhood adversity associates with Frontoamygdala connectivity in neonates. Biol Psychiatry Cogn Neurosci Neuroimaging. (2021) 6:470–8. doi: 10.1016/j.bpsc.2020.11.003
45. Sarigedik, E, Naldemir, IF, Karaman, AK, and Altinsoy, HB. Intergenerational transmission of psychological trauma: a structural neuroimaging study. Psychiatry Res Neuroimaging. (2022) 326:111538. doi: 10.1016/j.pscychresns.2022.111538
46. Cullen, SM, Hassan, N, and Smith-Raska, M. Effects of noninherited ancestral genotypes on offspring phenotypes†. Biol Reprod. (2021) 105:747–60. doi: 10.1093/biolre/ioab120
47. Yehuda, R, Daskalakis, NP, Bierer, LM, Bader, HN, Klengel, T, Holsboer, F, et al. Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biol Psychiatry. (2016) 80:372–80. doi: 10.1016/j.biopsych.2015.08.005
48. Bierer, LM, Bader, HN, Daskalakis, NP, Lehrner, A, Provençal, N, Wiechmann, T, et al. Intergenerational effects of maternal holocaust exposure on FKBP5 methylation. Am J Psychiatry. (2020) 177:744–53. doi: 10.1176/appi.ajp.2019.19060618
49. Perroud, N, Rutembesa, E, Paoloni-Giacobino, A, Mutabaruka, J, Mutesa, L, Stenz, L, et al. The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. World J Biol Psychiatry. (2014) 15:334–45. doi: 10.3109/15622975.2013.866693
50. Kong, A, Thorleifsson, G, Frigge, ML, Vilhjalmsson, BJ, Young, AI, Thorgeirsson, TE, et al. The nature of nurture: effects of parental genotypes. Science. (2018) 359:424–8. doi: 10.1126/science.aan6877
51. Wang, B, Baldwin, JR, Schoeler, T, Cheesman, R, Barkhuizen, W, Dudbridge, F, et al. Robust genetic nurture effects on education: a systematic review and meta-analysis based on 38,654 families across 8 cohorts. Am J Hum Genet. (2021) 108:1780–91. doi: 10.1016/j.ajhg.2021.07.010
52. Karlsson, H, Merisaari, H, Karlsson, L, Scheinin, NM, Parkkola, R, Saunavaara, J, et al. Association of Cumulative paternal early life stress with white matter maturation in newborns. JAMA Netw Open. (2020) 3:e2024832. doi: 10.1001/jamanetworkopen.2020.24832
53. Merrill, SM, Moore, SR, Gladish, N, Giesbrecht, GF, Dewey, D, Konwar, C, et al. Paternal adverse childhood experiences: associations with infant DNA methylation. Dev Psychobiol. (2021) 63:e22174. doi: 10.1002/dev.22174
54. Krapohl, E, Hannigan, LJ, Pingault, J-B, Patel, H, Kadeva, N, Curtis, C, et al. Widespread covariation of early environmental exposures and trait-associated polygenic variation. Proc Natl Acad Sci U S A. (2017) 114:11727–32. doi: 10.1073/pnas.1707178114
55. Baldwin, JR, Sallis, HM, Schoeler, T, Taylor, MJ, Kwong, ASF, Tielbeek, JJ, et al. A genetically informed registered report on adverse childhood experiences and mental health. Nat Hum Behav. (2023) 7:269–90. doi: 10.1038/s41562-022-01482-9
56. Magnus, P, Birke, C, Vejrup, K, Haugan, A, Alsaker, E, Daltveit, AK, et al. Cohort profile update: the Norwegian mother and child cohort study (MoBa). Int J Epidemiol. (2016) 45:382–8. doi: 10.1093/ije/dyw029
57. Golding, J. Children of the nineties. A longitudinal study of pregnancy and childhood based on the population of Avon (ALSPAC). West Engl Med J. (1990) 105:80–2.
58. Schreuder, P, and Alsaker, E. The Norwegian mother and child cohort study (MoBa) – MoBa recruitment and logistics. Nor Epidemiol. (2014) 24. doi: 10.5324/nje.v24i1-2.1754
59. Sharp, TH, McBride, NS, Howell, AE, Evans, CJ, Jones, DK, Perry, G, et al. Population neuroimaging: generation of a comprehensive data resource within the ALSPAC pregnancy and birth cohort. Wellcome Open Res. (2020) 5:203. doi: 10.12688/wellcomeopenres.16060.1
60. Edwards, AD, Rueckert, D, Smith, SM, Abo Seada, S, Alansary, A, Almalbis, J, et al. The developing human connectome project neonatal data release. Front Neurosci. (2022) 16:886772. doi: 10.3389/fnins.2022.886772
61. Kuriyama, S, Metoki, H, Kikuya, M, Obara, T, Ishikuro, M, Yamanaka, C, et al. Cohort profile: Tohoku medical megabank project birth and three-generation cohort study (TMM BirThree cohort study): rationale, progress and perspective. Int J Epidemiol. (2020) 49:18–19m. doi: 10.1093/ije/dyz169
62. Sijtsma, A, Rienks, J, van der Harst, P, Navis, G, Rosmalen, JGM, and Dotinga, A. Cohort profile update: Lifelines, a three-generation cohort study and biobank. Int J Epidemiol. (2022) 51:e295–302. doi: 10.1093/ije/dyab257
63. Okada, N, Ando, S, Sanada, M, Hirata-Mogi, S, Iijima, Y, Sugiyama, H, et al. Population-neuroscience study of the Tokyo TEEN cohort (pn-TTC): cohort longitudinal study to explore the neurobiological substrates of adolescent psychological and behavioral development. Psychiatry Clin Neurosci. (2019) 73:231–42. doi: 10.1111/pcn.12814
64. Kooijman, MN, Kruithof, CJ, van Duijn, CM, Duijts, L, Franco, OH, MH, VIJ, et al. The generation R study: design and cohort update 2017. Eur J Epidemiol. (2016) 31:1243–64. doi: 10.1007/s10654-016-0224-9
65. Mareckova, K, Marecek, R, Jani, M, Zackova, L, Andryskova, L, Brazdil, M, et al. Association of Maternal Depression during Pregnancy and Recent Stress with Brain age among Adult Offspring. JAMA Netw Open. (2023) 6:e2254581. doi: 10.1001/jamanetworkopen.2022.54581
66. Bethlehem, RAI, Seidlitz, J, White, SR, Vogel, JW, Anderson, KM, Adamson, C, et al. Brain charts for the human lifespan. Nature. (2022) 604:525–33. doi: 10.1038/s41586-022-04554-y
67. Wechsler, D. Wechsler adult intelligence scale-fourth edition (WAIS-IV) administration and scoring manual. San Antonio, TX: The Psychological Corporation (2008).
68. Wechsler, D. Wechsler intelligence scale for children-fourth edition (WISC-IV) administration and scoring manual. San Antonio, TX: The Psychological Corporation (2003).
69. Okada, N, Kasai, K, Takahashi, T, Suzuki, M, Hashimoto, R, and Kawakami, N. Brief rating scale of socioeconomic status for biological psychiatry research among Japanese people:a scaling based on an educational history. Jpn J Biol Psychiatry. (2014) 25:115–7. doi: 10.11249/jsbpjjpp.25.2_115
70. Matsudaira, I, Takano, Y, Yamaguchi, R, and Taki, Y. Core belief disruption amid the COVID-19 pandemic in Japanese adults. Hum Soc Sci Commun. (2021) 8:1–7. doi: 10.1057/s41599-021-00976-7
71. Nicholls, MER, Thomas, NA, Loetscher, T, and Grimshaw, GM. The Flinders handedness survey (FLANDERS): a brief measure of skilled hand preference. Cortex. (2013) 49:2914–26. doi: 10.1016/j.cortex.2013.02.002
72. Okubo, M, Suzuki, H, and Nicholls, MER. A Japanese version of the FLANDERS handedness questionnaire. Jpn Psychol Res. (2014) advpub:85.13235. doi: 10.4992/jjpsy.85.13235
73. Petersen, AC, Crockett, L, Richards, M, and Boxer, A. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc. (1988) 17:117–33. doi: 10.1007/BF01537962
74. Tanaka, M. Personality characteristics, negative life events, and depression in early adolescence. Jpn J Pers. (2006) 14:149–60. doi: 10.2132/personality.14.149
75. Glasser, MF, Smith, SM, Marcus, DS, Andersson, JLR, Auerbach, EJ, Behrens, TEJ, et al. The human connectome project’s neuroimaging approach. Nat Neurosci. (2016) 19:1175–87. doi: 10.1038/nn.4361
76. Abreu, R, and Duarte, JV. Quantitative assessment of the impact of geometric distortions and their correction on fMRI data analyses. Front Neurosci. (2021) 15:642808. doi: 10.3389/fnins.2021.642808
77. Schallmo, M-P, Weldon, KB, Burton, PC, Sponheim, SR, and Olman, CA. Assessing methods for geometric distortion compensation in 7 T gradient echo functional MRI data. Hum Brain Mapp. (2021) 42:4205–23. doi: 10.1002/hbm.25540
78. Andersson, JLR, Skare, S, and Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. (2003) 20:870–88. doi: 10.1016/S1053-8119(03)00336-7
79. Stone, A, Shiffman, S, Atienza, A, and Nebeling, L. The science of real-time data capture: self-reports in health research Oxford University Press (2007) Available at: https://play.google.com/store/books/details?id=LsjLb4KYrJQC.
80. Birleson, P. The validity of depressive disorder in childhood and the development of a self-rating scale: a research report. J Child Psychol Psychiatry. (1981) 22:73–88. doi: 10.1111/j.1469-7610.1981.tb00533.x
81. Namikawa, T, Tani, I, Wakita, T, Kumagai, R, Nakane, A, Noguchi, H, et al. Development of a short-form Birleson depression self-rating scale for children. Seishin Igaku. (2011) 53:489–96. doi: 10.11477/mf.1405101871
82. Kessler, RC, Andrews, G, Colpe, LJ, Hiripi, E, Mroczek, DK, Normand, S-LT, et al. Short screening scales to monitor population prevalences and trends in non-specific psychological distress. Psychol Med. (2002) 32:959–76. doi: 10.1017/S0033291702006074
83. Furukawa, TA, Kawakami, N, Saitoh, M, Ono, Y, Nakane, Y, Nakamura, Y, et al. The performance of the Japanese version of the K6 and K10 in the world mental health survey Japan. Int J Methods Psychiatr Res. (2008) 17:152–8. doi: 10.1002/mpr.257
84. Goodman, R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry. (1997) 38:581–6. doi: 10.1111/j.1469-7610.1997.tb01545.x
85. Hughes, ME, Waite, LJ, Hawkley, LC, and Cacioppo, JT. A short scale for measuring loneliness in large surveys: results from two population-based studies. Res Aging. (2004) 26:655–72. doi: 10.1177/0164027504268574
86. Igarashi, T. Development of the Japanese version of the three-item loneliness scale. BMC Psychol. (2019) 7:20. doi: 10.1186/s40359-019-0285-0
87. Ahorsu, DK, Lin, C-Y, Imani, V, Saffari, M, Griffiths, MD, and Pakpour, AH. The fear of COVID-19 scale: development and initial validation. Int J Ment Health Addict. (2020) 20:1537–45. doi: 10.1007/s11469-020-00270-8
88. Wakashima, K, Asai, K, Kobayashi, D, Koiwa, K, Kamoshida, S, and Sakuraba, M. The Japanese version of the fear of COVID-19 scale: reliability, validity, and relation to coping behavior. PLoS One. (2020) 15:e0241958. doi: 10.1371/journal.pone.0241958
89. Murakami, Y, and Murakami, C. Scale construction of a “big five” personality inventory. Jpn J Pers. (1997) 6:29–39.
90. Murakami, Y, and Hatayama, N. The construction of Big Five personality inventory for children. Jpn J Behav. (2010). 93–104. doi: 10.2333/jbhmk.37.93
91. Gosling, SD, Rentfrow, PJ, and Swann, WB. A very brief measure of the big-five personality domains. J Res Pers. (2003) 37:504–28. doi: 10.1016/S0092-6566(03)00046-1
92. Oshio, A, Abe, S, and Cutrone, P. Development, reliability, and validity of the Japanese version of ten item personality inventory (TIPI-J). Jpn J Pers. (2012) 21:40–52. doi: 10.2132/personality.21.40
93. Costa, PT, and McCrae, RR. Personality inventory (NEO-PI-R) and NEO five-factor inventory (NEO-FFI): professional manual. Odessa, FL: Psychological Assessment Resources (1992).
94. Shimonaka, Y, Nakazato, K, Gondo, Y, and Takayama, M. Revised NEO-personality inventory (NEO-PI-R) and NEO five-factor inventory (NEO-FFI) manual for the Japanese version. Tokyo: Tokyo Shinri (1999).
95. Carver, CS, and White, TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. (1994) 67:319–33. doi: 10.1037/0022-3514.67.2.319
96. Takahashi, Y, Yamagata, S, Kijima, N, Shigemasu, K, Ono, Y, and Ando, J. Gray’s Temperament Model: Development of Japanese Version of BIS/BAS Scales and A Behavior Genetic Investigation Using the Twin Method. Jpn J Personal. (2007) 15:276–89. doi: 10.2132/personality.15.276
97. Muris, P, Meesters, C, de Kanter, E, and Timmerman, PE. Behavioural inhibition and behavioural activation system scales for children: relationships with Eysenck’s personality traits and psychopathological symptoms. Pers Individ Dif. (2005) 38:831–41. doi: 10.1016/j.paid.2004.06.007
98. Koseki, S, Koseki, M, Nakamura, M, Ohtani, T, and Kunisato, Y. Japanese version of the behavioral inhibition system and behavioral activation system scale for children: scale development and examination of reliability and validity. Jpn J Behav Cogn Ther. (2018) 44:29–39. doi: 10.24468/jjbct.16-168
99. Spielberger, CD. Manual for the state-trait anxiety inventory, STAI-form Y. Palo Alto, CA: Consulting Psychologists Press (1983).
100. Hidano, N, Fukuhara, M, Iwawaki, M, Soga, S, and Spielberger, CD. State-trait anxiety inventory-form JYZ. Tokyo: Jitsumu Kyoiku-Shuppan (2000).
101. Spielberger, CD, Edwards, CD, Montuori, J, and Lushene, R. STAIC preliminary manual for the state-trait anxiety inventory for children (“how I feel questionnaire”). Palo Alto, CA: Consulting Psychologists Press (1973) Available at: https://www.worldcat.org/title/staic-preliminary-manual-for-the-state-trait-anxiety-inventory-for-children-how-i-feel-questionnaire/oclc/5841111.
102. Soga, S. A study on standardization of Japanese version of the STAIC. Jpn J Psychol. (1983) 54:215–21. doi: 10.4992/jjpsy.54.215
103. Raine, A, and Benishay, D. The SPQ-B: a brief screening instrument for schizotypal personality disorder. J Personal Disord. (1995) 9:346–55. doi: 10.1521/pedi.1995.9.4.346
104. Ito, S, Obu, S, Ota, M, Takao, T, and Sakamoto, S. Reliability and validity of the Japanese version of SPQ-B (schizotypal personality questionnaire brief). Jpn Bull Soc Psychiatry. (2008) 17:168–76.
105. Kelleher, I, Harley, M, Murtagh, A, and Cannon, M. Are screening instruments valid for psychotic-like experiences? A validation study of screening questions for psychotic-like experiences using in-depth clinical interview. Schizophr Bull. (2011) 37:362–9. doi: 10.1093/schbul/sbp057
106. Ando, S, Nishida, A, Yamasaki, S, Koike, S, Morimoto, Y, Hoshino, A, et al. Cohort profile: the Tokyo Teen cohort study (TTC). Int J Epidemiol. (2019) 48:1414–1414g. doi: 10.1093/ije/dyz033
107. DuPaul, GJ, Power, TJ, Anastopoulos, AD, and Reid, R. ADHD rating scale—IV: checklists, norms, and clinical interpretation. New York, NY: The Guilford Press (1998) Available at: https://psycnet.apa.org/fulltext/1998-06605-000.pdf.
108. Ichikawa, H, and Tanaka, Y. ADHD-rating scale-IV: checklists, norms, and clinical interpretation. AkashiShoten: Tokyo (2008).
109. Kessler, RC, Adler, L, Ames, M, Demler, O, Faraone, S, Hiripi, E, et al. The World Health Organization adult ADHD self-report scale (ASRS): a short screening scale for use in the general population. Psychol Med. (2005) 35:245–56. doi: 10.1017/s0033291704002892
110. Takeda, T, Tsuji, Y, and Kurita, H. Psychometric properties of the Japanese version of the adult attention-deficit hyperactivity disorder (ADHD) self-report scale (ASRS-J) and its short scale in accordance with DSM-5 diagnostic criteria. Res Dev Disabil. (2017) 63:59–66. doi: 10.1016/j.ridd.2017.02.011
111. Baron-Cohen, S, Wheelwright, S, Skinner, R, Martin, J, and Clubley, E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. (2001) 31:5–17. doi: 10.1023/a:1005653411471
112. Wakabayashi, A, Tojo, Y, Baron-Cohen, S, and Wheelwright, S. The autism-spectrum quotient (AQ) Japanese version. Shinrigaku Kenkyu. (2004) 75:78–84. doi: 10.4992/jjpsy.75.78
113. Baron-Cohen, S, Hoekstra, RA, Knickmeyer, R, and Wheelwright, S. The autism-spectrum quotient (AQ)--adolescent version. J Autism Dev Disord. (2006) 36:343–50. doi: 10.1007/s10803-006-0073-6
114. Wakabayashi, A, Baron-Cohen, S, Uchiyama, T, Yoshida, Y, Tojo, Y, Kuroda, M, et al. The autism-spectrum quotient (AQ) children’s version in Japan: a cross-cultural comparison. J Autism Dev Disord. (2007) 37:491–500. doi: 10.1007/s10803-006-0181-3
115. First, MB, Williams, JBW, Benjamin, LS, and Spitzer, RL. SCID-5-PD: structured clinical interview for DSM-5® personality disorders. Arlington, VA: American Psychiatric Association Publishing (2016) Available at: https://www.worldcat.org/title/scid-5-pd-structured-clinical-interview-for-dsm-5-personality-disorders/oclc/956521011.
116. Osone, A, and Takahashi, S. The Japanese version of the structured clinical interview for DSM-5 personality disorders (SCID-5-PD). Tokyo: Igaku-Shoin (2017).
117. Fraley, RC, Heffernan, ME, Vicary, AM, and Brumbaugh, CC. The experiences in close relationships-relationship structures questionnaire: a method for assessing attachment orientations across relationships. Psychol Assess. (2011) 23:615–25. doi: 10.1037/a0022898
118. Komura, K, Murakami, T, and Toda, K. Validation of a Japanese version of the experience in close relationship- relationship structure. Shinrigaku Kenkyu. (2016) 87:303–13. doi: 10.4992/jjpsy.87.15208
119. Garnefski, N, Kraaij, V, and Spinhoven, P. Negative life events, cognitive emotion regulation and emotional problems. Pers Individ Dif. (2001) 30:1311–27. doi: 10.1016/S0191-8869(00)00113-6
120. Sakakibara, R. How does cognitive appraisal moderate the relationship between cognitive emotion regulation strategies and psychological health? Jpn J Soc Psychol. (2017) 32:163–73. doi: 10.14966/jssp.0949
121. Davis, MH. A multidimensional approach to individual differences in empathy. JSAS Catalog Select Doc Psychol. (1980) 10:85.
122. Himichi, T, Osanai, H, Goto, T, Fujita, H, Kawamura, Y, Davis, MH, et al. Development of a Japanese version of the interpersonal reactivity index. Shinrigaku Kenkyu. (2018) 88:61–71. doi: 10.4992/jjpsy.88.15218
123. Jones, DN, and Paulhus, DL. Introducing the short dark triad (SD3): a brief measure of dark personality traits. Assessment. (2014) 21:28–41. doi: 10.1177/1073191113514105
124. Shimotsukasa, T, and Oshio, A. Development and validation of the Japanese version of the short dark triad(SD3-J). Jpn J Pers. (2017) 26:12–22. doi: 10.2132/personality.26.1.2
125. Olson, DH, McCubbin, HI, Larsen, A, Muxen, M, and Wilson, M. Family inventories. St. Paul, MN: Family Social Science, University of Minnesota (1985).
126. Kusada, H. The reliability and validity of FACES III for Japanese. Jpn J Counsel Sci. (1995) 28:154–62.
127. Noguchi, S, Kozuka, T, Usami, T, and Wakashima, K. Development and examination of validity for a new family structure assessment scale “ICHIGEKI.”. Annu Rep Grad Sch Educ. (2009) 58:247–65.
128. Parker, G, Tupling, H, and Brown, LB. A parental bonding instrument. Br J Med Psychol. (1979) 52:1–10. doi: 10.1111/j.2044-8341.1979.tb02487.x
129. Ogawa, M. Reliability and validity of the Japanese version of PBI. Jpn J Psychiatr Treat. (1991) 6:1193–201.
130. Schaefer, ES. Children’s reports of parental behavior: an inventory. Child Dev. (1965) 36:413–24.
131. Kojima, H. Inter-battery factor analysis of parental behavior inventories at the item level. Bull Facul Educ. (1969) 18:55–70.
132. Utsumi, S. Shared and unshared views between parent and child on parental behaviors during adolescence. Proceedings: Science of Human Development for Restructuring the “Gap Widening Society” (2012), 103–112.
133. Bernstein, DP, Stein, JA, Newcomb, MD, Walker, E, Pogge, D, Ahluvalia, T, et al. Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse Negl. (2003) 27:169–90. doi: 10.1016/s0145-2134(02)00541-0
134. Nakajima, M, Hori, H, Itoh, M, Lin, M, Kawanishi, H, Narita, M, et al. Validation of childhood trauma questionnaire-short form in Japanese clinical and nonclinical adults. Psychiatry Res Commun. (2022) 2:100065. doi: 10.1016/j.psycom.2022.100065
135. Norton, R. Measuring marital quality: a critical look at the dependent variable. J Marriage Fam Couns. (1983) 45:141–51. doi: 10.2307/351302
136. Moroi, K. Perceptions of equity in the division of household labor. Jpn J Fam Psyhol. (1996) 10:15–30.
137. Bodenmann, G, Schär, M, and Gmelch, S. Multidimensionaler Stressfragebogen für Paare (MDSP). Unpublished scale, University of Zurich. (2007).
138. Kurosawa, T, and Yokotani, K. Validation of the Japanese version of the multidimensional stress questionnaire for couples: factor structure, validity and reliability. J Relat Res. (2018) 9:e16. doi: 10.1017/jrr.2018.15
139. Diener, E, Emmons, RA, Larsen, RJ, and Griffin, S. The satisfaction with life scale. J Pers Assess. (1985) 49:71–5. doi: 10.1207/s15327752jpa4901_13
141. Saeki, M, Oishi, S, Maeno, T, and Gilbert, E. Self–informant agreement for subjective well-being among Japanese. Pers Individ Dif. (2014) 69:124–8. doi: 10.1016/j.paid.2014.05.018
142. Huebner, ES. Initial development of the Student’s life satisfaction scale. Sch Psychol Int. (1991) 12:231–40. doi: 10.1177/0143034391123010
143. Yoshitake, N. Role of daily positive experiences in life satisfaction in junior high school students: events collection and examination of a relationship. Jpn J Educ Psychol. (2010) 58:140–50. doi: 10.5926/jjep.58.140
144. Foa, EB. The posttraumatic diagnostic scale (PDS) manual. Minneapolis, MN: National Computer Systems (1995).
145. Foa, EB, Cashman, L, Jaycox, L, and Perry, K. The validation of a self-report measure of posttraumatic stress disorder: the posttraumatic diagnostic scale. Psychol Assess. (1997) 9:445–51. doi: 10.1037/1040-3590.9.4.445
146. Itoh, M, Ujiie, Y, Nagae, N, Niwa, M, Kamo, T, Lin, M, et al. A new short version of the Posttraumatic Diagnostic Scale: validity among Japanese adults with and without PTSD. Eur J Psychotraumatol (2017) 8:1364119. doi: 10.1080/20008198.2017.1364119
147. Oshio, A, Abe, S, Cutrone, P, and Gosling, SD. Big Five content representation of the Japanese version of the ten-item personality inventory. Psychology. (2013) 4:924. doi: 10.4236/psych.2013.412133
148. Costello, A, Edelbrock, C, Kalas, R, Kessler, M, and Klaric, SA. Diagnostic interview schedule for children (DISC). Bethesda, MD: National Institute of Mental Health (1982).
149. Yoshida, K. Development of Japanese version diagnostic schedule interview for children. (2001). Available at: https://www.nissan-zaidan.or.jp/wp-content/uploads/101007.pdf
150. Bowles, DP, Armitage, CJ, Drabble, J, and Meyer, B. Self-esteem and other-esteem in college students with borderline and avoidant personality disorder features: an experimental vignette study. Personal Ment Health. (2013) 7:307–19. doi: 10.1002/pmh.1230
151. Ichikawa, R, and Mochizuki, S. Relationships among borderline, narcissistic, and avoidant personality disorders and the discrepancy between explicit and implicit self-esteem. Shinrigaku Kenkyu. (2015) 86:434–44. doi: 10.4992/jjpsy.86.14036
152. Kushibiki, N, and Mochizuki, S. The effect of self-monitoring on the instability of self-image in personality disorder attributes. Jpn J Pers. (2021) 29:172–82. doi: 10.2132/personality.29.3.9
153. Wild, CP. The exposome: from concept to utility. Int J Epidemiol. (2012) 41:24–32. doi: 10.1093/ije/dyr236
154. Fan, CC, Marshall, A, Smolker, H, Gonzalez, MR, Tapert, SF, Barch, DM, et al. Adolescent brain cognitive development (ABCD) study linked external data (LED): protocol and practices for geocoding and assignment of environmental data. Dev Cogn Neurosci. (2021) 52:101030. doi: 10.1016/j.dcn.2021.101030
155. Santos, S, Maitre, L, Warembourg, C, Agier, L, Richiardi, L, Basagaña, X, et al. Applying the exposome concept in birth cohort research: a review of statistical approaches. Eur J Epidemiol. (2020) 35:193–204. doi: 10.1007/s10654-020-00625-4
156. Green, JG, McLaughlin, KA, Berglund, PA, Gruber, MJ, Sampson, NA, Zaslavsky, AM, et al. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. (2010) 67:113–23. doi: 10.1001/archgenpsychiatry.2009.186
157. Moroi, K. How do adolescent females perceive their parents’ equity in the division of household labor? Jpn J Fam Psychol. (1997) 11:69–81.
158. Bull, FC, Maslin, TS, and Armstrong, T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health. (2009) 6:790–804. doi: 10.1123/jpah.6.6.790
159. Inoue, S, Nakata, Y, Ohkawara, K, Oka, K, Oguma, Y, Takada, K, et al. Outline of the project research and the development of Japanese version of GPAQ. Jap J Phys Fit Sports Med. (2016) 65:155. doi: 10.7600/jspfsm.65.155
160. Yokoyama, Y, Takachi, R, Ishihara, J, Ishii, Y, Sasazuki, S, Sawada, N, et al. Validity of short and long self-administered food frequency questionnaires in ranking dietary intake in middle-aged and elderly Japanese in the Japan public health center-based prospective study for the next generation (JPHC-NEXT) protocol area. J Epidemiol. (2016) 26:420–32. doi: 10.2188/jea.JE20150064
161. Soldatos, CR, Dikeos, DG, and Paparrigopoulos, TJ. Athens insomnia scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. (2000) 48:555–60. doi: 10.1016/s0022-3999(00)00095-7
162. Okajima, I, Nakajima, S, Kobayashi, M, and Inoue, Y. Development and validation of the Japanese version of the Athens insomnia scale. Psychiatry Clin Neurosci. (2013) 67:420–5. doi: 10.1111/pcn.12073
163. Kwon, M, Kim, D-J, Cho, H, and Yang, S. The smartphone addiction scale: development and validation of a short version for adolescents. PLoS One. (2013) 8:e83558. doi: 10.1371/journal.pone.0083558
164. Tateno, M. A novel form of internet addiction: smartphone addiction. Seishin Shinkeigaku Zasshi. (2019) 121:549–55.
165. Király, O, Sleczka, P, Pontes, HM, Urbán, R, Griffiths, MD, and Demetrovics, Z. Validation of the ten-item internet gaming disorder test (IGDT-10) and evaluation of the nine DSM-5 internet gaming disorder criteria. Addict Behav. (2017) 64:253–60. doi: 10.1016/j.addbeh.2015.11.005
166. Ishizawa, T. The effects of bathing methods and bathing habits for the physical and psychological state Kanazawa University Repository for Academic Resources (2014).
167. Kameda, S, Hayasaka, S, Saito, M, Sato, E, Juhuku, R, Fujimoto, K, et al. Large-scale web investigation of hot spring and home bathing in Oita prefecture. Jpn J Health Res. (2019) 40:1–13. doi: 10.32279/jjhr.40.0_1
168. Zimet, GD, Dahlem, NW, Zimet, SG, and Farley, GK. The multidimensional scale of perceived social support. J Pers Assess. (1988) 52:30–41. doi: 10.1207/s15327752jpa5201_2
169. Iwasa, H, Gondo, Y, Masui, Y, Inagaki, H, Kawaai, C, Rika, O, et al. Reliability and validity of the Japanese version of the multidimensional scale of perceived social support: a study of middle-aged and older adults. J Health Welf Stat. (2007) 54:26–33.
170. Booth, ML, Okely, AD, Chey, T, and Bauman, A. The reliability and validity of the physical activity questions in the WHO health behaviour in schoolchildren (HBSC) survey: a population study. Br J Sports Med. (2001) 35:263–7. doi: 10.1136/bjsm.35.4.263
171. Tanaka, C, Kyan, A, Takakura, M, Olds, T, Schranz, N, Tanaka, M, et al. The validity of the Japanese version of physical activity questions in the WHO health behaviour in school-aged children (HBSC) survey. Res Exer Epidemiol. (2017) 19:93–101. doi: 10.24804/ree.19.93
172. Livingstone, MBE, Robson, PJ, and Wallace, JMW. Issues in dietary intake assessment of children and adolescents. Br J Nutr. (2004) 92:S213–22. doi: 10.1079/bjn20041169
174. Yamamoto, M, Sato, Y, and Shiwaku, H. Relationship between parents’ role-acceptance, parental role behavior and the mental health of adolescents. J Child Health. (2008) 67:349–56.
175. Naqvi, S, Sleyp, Y, Hoskens, H, Indencleef, K, Spence, JP, Bruffaerts, R, et al. Shared heritability of human face and brain shape. Nat Genet. (2021) 53:830–9. doi: 10.1038/s41588-021-00827-w
176. Tan, DW, Gilani, SZ, Boutrus, M, Alvares, GA, Whitehouse, AJO, Mian, A, et al. Facial asymmetry in parents of children on the autism spectrum. Autism Res. (2021) 14:2260–9. doi: 10.1002/aur.2612
177. Boutrus, M, Gilani, Z, Maybery, MT, Alvares, GA, Tan, DW, Eastwood, PR, et al. Brief report: facial asymmetry and autistic-like traits in the general population. J Autism Dev Disord. (2021) 51:2115–23. doi: 10.1007/s10803-020-04661-7
178. Manning, JT, Scutt, D, Wilson, J, and Lewis-Jones, DI. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod. (1998) 13:3000–4. doi: 10.1093/humrep/13.11.3000
179. Gorka, AX, Norman, RE, Radtke, SR, Carré, JM, and Hariri, AR. Anterior cingulate cortex gray matter volume mediates an association between 2D:4D ratio and trait aggression in women but not men. Psychoneuroendocrinology. (2015) 56:148–56. doi: 10.1016/j.psyneuen.2015.03.004
180. Darnai, G, Plózer, E, Perlaki, G, Orsi, G, Nagy, SA, Horváth, R, et al. 2D:4D finger ratio positively correlates with total cerebral cortex in males. Neurosci Lett. (2016) 615:33–6. doi: 10.1016/j.neulet.2015.12.056
181. Donishi, T, Terada, M, and Kaneoke, Y. Effects of gender, digit ratio, and menstrual cycle on intrinsic brain functional connectivity: a whole-brain, voxel-wise exploratory study using simultaneous local and global functional connectivity mapping. Brain Behav. (2018) 8:e00890. doi: 10.1002/brb3.890
182. Zhu, Y-K, Li, C-B, Jin, J, Wang, J-J, Lachmann, B, Sariyska, R, et al. The 2D:4D ratio of the hand and schizotypal personality traits in schizophrenia patients and healthy control persons. Asian J Psychiatr. (2014) 9:67–72. doi: 10.1016/j.ajp.2014.01.005
183. Lautenbacher, LM, and Neyse, L. Depression, neuroticism and 2D:4D ratio: evidence from a large, representative sample. Sci Rep. (2020) 10:11136. doi: 10.1038/s41598-020-67882-x
184. Rodríguez-Ramos, Á, Moriana, JA, García-Torres, F, and Ruiz-Rubio, M. Emotional stability is related to 2D:4D and social desirability in women: possible implications on subjective well-being and psychopathology. PLoS One. (2021) 16:e0248368. doi: 10.1371/journal.pone.0248368
185. Der, KK. Baumtest: Der Beumzeichen-versuch als psychodiagnostisches Hilfmittel. Bern: Hans Huber (1949).
186. Kaneda, A, Yasui-Furukori, N, Saito, M, Sugawara, N, Nakagami, T, Furukori, H, et al. Characteristics of the tree-drawing test in chronic schizophrenia. Psychiatry Clin Neurosci. (2010) 64:141–8. doi: 10.1111/j.1440-1819.2010.02071.x
187. Gu, S, Liu, Y, Liang, F, Feng, R, Li, Y, Liu, G, et al. Screening depressive disorders with tree-drawing test. Front Psychol. (2020) 11:1446. doi: 10.3389/fpsyg.2020.01446
188. Inadomi, H, Tanaka, G, and Ohta, Y. Characteristics of trees drawn by patients with paranoid schizophrenia. Psychiatry Clin Neurosci. (2003) 57:347–51. doi: 10.1046/j.1440-1819.2003.01130.x
189. Heike, CL, Upson, K, Stuhaug, E, and Weinberg, SM. 3D digital stereophotogrammetry: a practical guide to facial image acquisition. Head Face Med. (2010) 6:18. doi: 10.1186/1746-160X-6-18
190. Twrdik, A, Braumann, U-D, Abicht, F, Kiess, W, and Kirsten, T. Measuring finger lengths from 2D palm scans In: A Maier, T Deserno, H Handels, K Maier-Hein, C Palm, and T Tolxdorff, editors. Bildverarbeitung für die Medizin 2018. Berlin, Heidelberg: Springer (2018). 151–6.
192. Jenkinson, M, Beckmann, CF, Behrens, TEJ, Woolrich, MW, and Smith, SM. FSL. Neuroimage. (2012) 62:782–90. doi: 10.1016/j.neuroimage.2011.09.015
193. Tournier, J-D, Smith, R, Raffelt, D, Tabbara, R, Dhollander, T, Pietsch, M, et al. MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. Neuroimage. (2019) 202:116137. doi: 10.1016/j.neuroimage.2019.116137
194. Avants, BB, Tustison, N, and Johnson, H. Advanced normalization tools (ANTS). Insight J. (2009) 2:1. doi: 10.54294/uvnhin
195. Cox, RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. (1996) 29:162–73. doi: 10.1006/cbmr.1996.0014
196. Faul, F, Erdfelder, E, Lang, A-G, and Buchner, A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. (2007) 39:175–91. doi: 10.3758/bf03193146
197. Faul, F, Erdfelder, E, Buchner, A, and Lang, A-G. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. (2009) 41:1149–60. doi: 10.3758/BRM.41.4.1149
198. Hiscox, LV, Fairchild, G, Donald, KA, Groenewold, NA, Koen, N, Roos, A, et al. Antenatal maternal intimate partner violence exposure is associated with sex-specific alterations in brain structure among young infants: evidence from a south African birth cohort. Dev Cogn Neurosci. (2023) 60:101210. doi: 10.1016/j.dcn.2023.101210
199. Lautarescu, A, Bonthrone, AF, Pietsch, M, Batalle, D, Cordero-Grande, L, Tournier, J-D, et al. Maternal depressive symptoms, neonatal white matter, and toddler social-emotional development. Transl Psychiatry. (2022) 12:323. doi: 10.1038/s41398-022-02073-y
200. Mareckova, K, Marecek, R, Andryskova, L, Brazdil, M, and Nikolova, YS. Impact of prenatal stress on amygdala anatomy in young adulthood: timing and location matter. Biol Psychiatry Cogn Neurosci Neuroimaging. (2022) 7:231–8. doi: 10.1016/j.bpsc.2021.07.009
201. Mareckova, K, Miles, A, Andryskova, L, Brazdil, M, and Nikolova, YS. Temporally and sex-specific effects of maternal perinatal stress on offspring cortical gyrification and mood in young adulthood. Hum Brain Mapp. (2020) 41:4866–75. doi: 10.1002/hbm.25163
202. Brumpton, B, Sanderson, E, Heilbron, K, Hartwig, FP, Harrison, S, Vie, GÅ, et al. Avoiding dynastic, assortative mating, and population stratification biases in Mendelian randomization through within-family analyses. Nat Commun. (2020) 11:3519. doi: 10.1038/s41467-020-17117-4
203. Weiner, DJ, Wigdor, EM, Ripke, S, Walters, RK, Kosmicki, JA, Grove, J, et al. Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders. Nat Genet. (2017) 49:978–85. doi: 10.1038/ng.3863
204. Stella, C, Díaz-Caneja, CM, Penzol, MJ, García-Alcón, A, Solís, A, Andreu-Bernabeu, Á, et al. Analysis of common genetic variation across targets of microRNAs dysregulated both in ASD and epilepsy reveals negative correlation. Front Genet. (2023) 14:1072563. doi: 10.3389/fgene.2023.1072563
205. Ahangari, M, Kirkpatrick, R, Nguyen, T-H, Gillespie, N, Kendler, KS, Bacanu, S-A, et al. Examining the source of increased bipolar disorder and major depressive disorder common risk variation burden in multiplex schizophrenia families. Schizophrenia. (2022) 8:106. doi: 10.1038/s41537-022-00317-w
206. García-Alcón, A, González-Peñas, J, Weckx, E, Penzol, MJ, Gurriarán, X, Costas, J, et al. Oxytocin exposure in labor and its relationship with cognitive impairment and the genetic architecture of autism. J Autism Dev Disord. (2023) 53:66–79. doi: 10.1007/s10803-021-05409-7
207. Warrier, V, and Baron-Cohen, S. Childhood trauma, life-time self-harm, and suicidal behaviour and ideation are associated with polygenic scores for autism. Mol Psychiatry. (2019) 26:1670–84. doi: 10.1038/s41380-019-0550-x
208. Lewis, KJS, Martin, J, Gregory, AM, Anney, R, Thapar, A, and Langley, K. Sleep disturbances in ADHD: investigating the contribution of polygenic liability for ADHD and sleep-related phenotypes. Eur Child Adolesc Psychiatry. (2022) 32:1253–61. doi: 10.1007/s00787-021-01931-2
209. Soubry, A. Epigenetics as a driver of developmental origins of health and disease: did we forget the fathers? Bioessays. (2018) 40. doi: 10.1002/bies.201700113
Keywords: intergenerational transmission, parent-offspring trios, neuroimaging, development, personality
Citation: Matsudaira I, Yamaguchi R and Taki Y (2023) Transmit Radiant Individuality to Offspring (TRIO) study: investigating intergenerational transmission effects on brain development. Front. Psychiatry. 14:1150973. doi: 10.3389/fpsyt.2023.1150973
Edited by:
David Popovic, LMU Munich University Hospital, GermanyReviewed by:
Naoyuki Katagiri, Toho University, JapanEuclides José de Mendonça Filho, McGill University, Canada
Kazutaka Ohi, Gifu University, Japan
Copyright © 2023 Matsudaira, Yamaguchi and Taki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Izumi Matsudaira, aXp1bWkubWF0c3VkYWlyYS5lNEB0b2hva3UuYWMuanA=
†These authors have contributed equally to this work and share first authorship
 Izumi Matsudaira
Izumi Matsudaira Ryo Yamaguchi
Ryo Yamaguchi Yasuyuki Taki
Yasuyuki Taki