- 1MAPS Public Benefit Corporation, San Jose, CA, United States
- 2Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, San Diego, San Diego, CA, United States
- 3Tulip Medical Consulting LLC, Port Townsend, WA, United States
3,4-Methylenedioxymethamphetamine (MDMA) is currently being investigated as an adjunctive treatment to therapy for posttraumatic stress and other anxiety related disorders in clinical trials. Within the next few years MDMA-assisted therapy is projected for approval by regulatory authorities. MDMA’s primary mechanism of action includes modulation of monoamine signaling by increasing release and inhibiting reuptake of serotonin, norepinephrine, and, to a lesser extent, dopamine. This pharmacology affects sympathomimetic physiology. In controlled trials, special attention has been given to cardiovascular adverse events (AEs), because transient increases in heart rate and blood pressure have been observed during the MDMA-assisted therapy sessions. Finding and quantifying the potential drivers of cardiac AEs in clinical trials is difficult since only a relatively small number of participants have been included in these studies, and a limited set of allowed concomitant drugs has been studied. In this study a more diverse set of reports from the FDA Adverse Event Reporting System was surveyed. We found 17 cases of cardiovascular AEs, in which the individuals had taken one or more substances in addition to MDMA. Interestingly, all of those concomitant medications and illicit substances, including opioids, stimulants, anticholinergics, and amphetamines, had been previously associated with cardiovascular AEs. Furthermore, in none of the reports MDMA was marked as the primary suspect.
Introduction
3,4-methylenedioxymethamphetamine also known as MDMA or its street name Ecstasy, is currently a schedule I controlled substance in the United States and the European Union, and a Class A substance in the United Kingdom. There is growing interest in MDMA’s utilization in psychiatry based on promising efficacy and safety findings from multiple controlled clinical trials including a Phase 3 study for MDMA assisted therapy for post-traumatic stress disorder (PTSD) (1–5).
MDMA’s psychoactive effects are due to its complex pharmacology, including modulation of release and reuptake of serotonin, norepinephrine, and dopamine (6–9), and an increase in oxytocin levels (10). The efficacy of MDMA in PTSD treatment is attributed to supporting fear-extinction learning an increased ability to confront adverse memories, and improved social and interpersonal interactions (5, 11–13).
However, due to the monoamine neurotransmission modulation, cardiovascular physiology may be affected as well (14–16). Arrhythmia-related adverse events (AEs), in addition to hypertensive and ischemia-related AEs have been reported in literature (17–20). In controlled clinical trials in both healthy volunteers and patients with PTSD, AEs of transient increases in blood pressure and heart rate were observed along with muscle tightness, decreased appetite, nausea, hyperhidrosis, and feeling cold (4, 21).
Considering the polypharmacy often present in PTSD populations due to other comorbid conditions such as substance use (22, 23), anxiety (24), depression (25), sleep (26) and pain disorders (27), and the respective treatment drugs, further MDMA AE evaluation is warranted using the FAERS database. This is particularly important since the clinical trials excluded many of these medications, and there is potential for drug–drug interactions (28).
In this study, we evaluated arrhythmic, ischemic, and hypertensive AE reports in MDMA users from the FDA Adverse Event Reporting System (FAERS). These AEs were reviewed in submissions where MDMA was reported to be used alone or with additional therapeutics or illicit substances. The contribution of concomitant drugs and substances to the risk of cardiovascular AEs was evaluated.
Methods
FDA adverse event reporting system
FAERS is a repository of AEs submitted to the FDA through MedWatch (Forms 3,500/3500A) (29) by consumers, legal representatives, healthcare professionals, sponsors and manufacturers.
FAERS was initially intended for post-marketing drug and biologic safety surveillance to detect and re-evaluate drug safety signals that may have been missed in smaller scale controlled trials. However, the database includes reports of drugs still under investigation and Schedule I substances, making it a useful resource to evaluate safety of substances not yet approved by the FDA and other regulatory authorities. Reporting use of unapproved or illegal substances is important, since those agents may be the culprits of the adverse events wrongly attributed to concomitant therapeutics.
Combining and normalizing data sets
FAERS/AERS quarterly data sets, each including a data subset (demographics, drug, indication, outcome, reaction, report source), were downloaded individually from the FDA public repository in dollar sign-separated text format (30–32). At the time of the study FAERS/AERS contained 18,274,795 reports from January 2004 to September 2022. It was convenient to standardize the multiple data tables into a unified single table structure. A set of Unix shell scripts was used for data restructuring and filtering (33). The partially missing fields, relevant to the current analysis, in the MDMA reports were only in the demographic section (age, weight, sex), and were comparable to the rest of the database.
Case selection
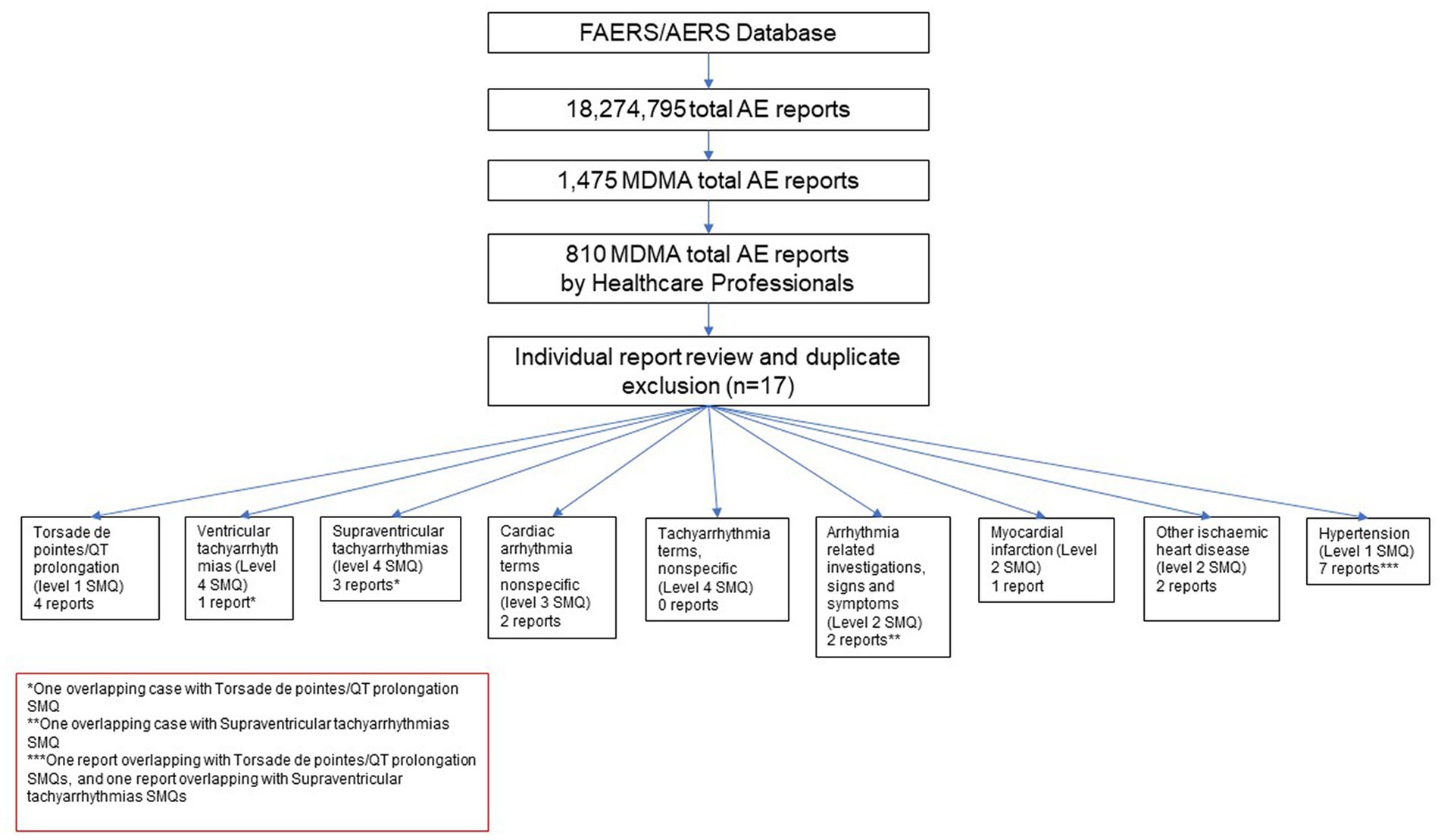
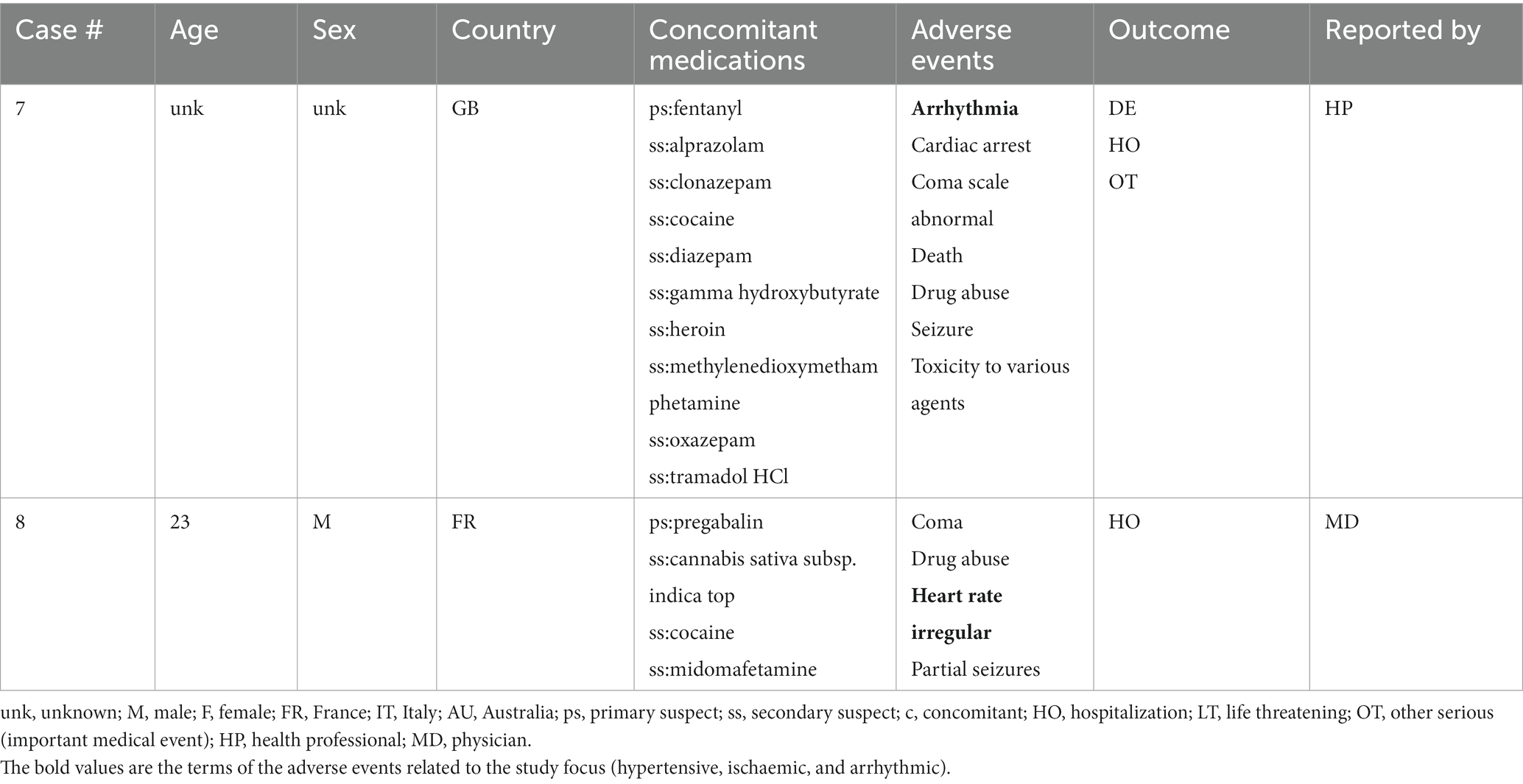
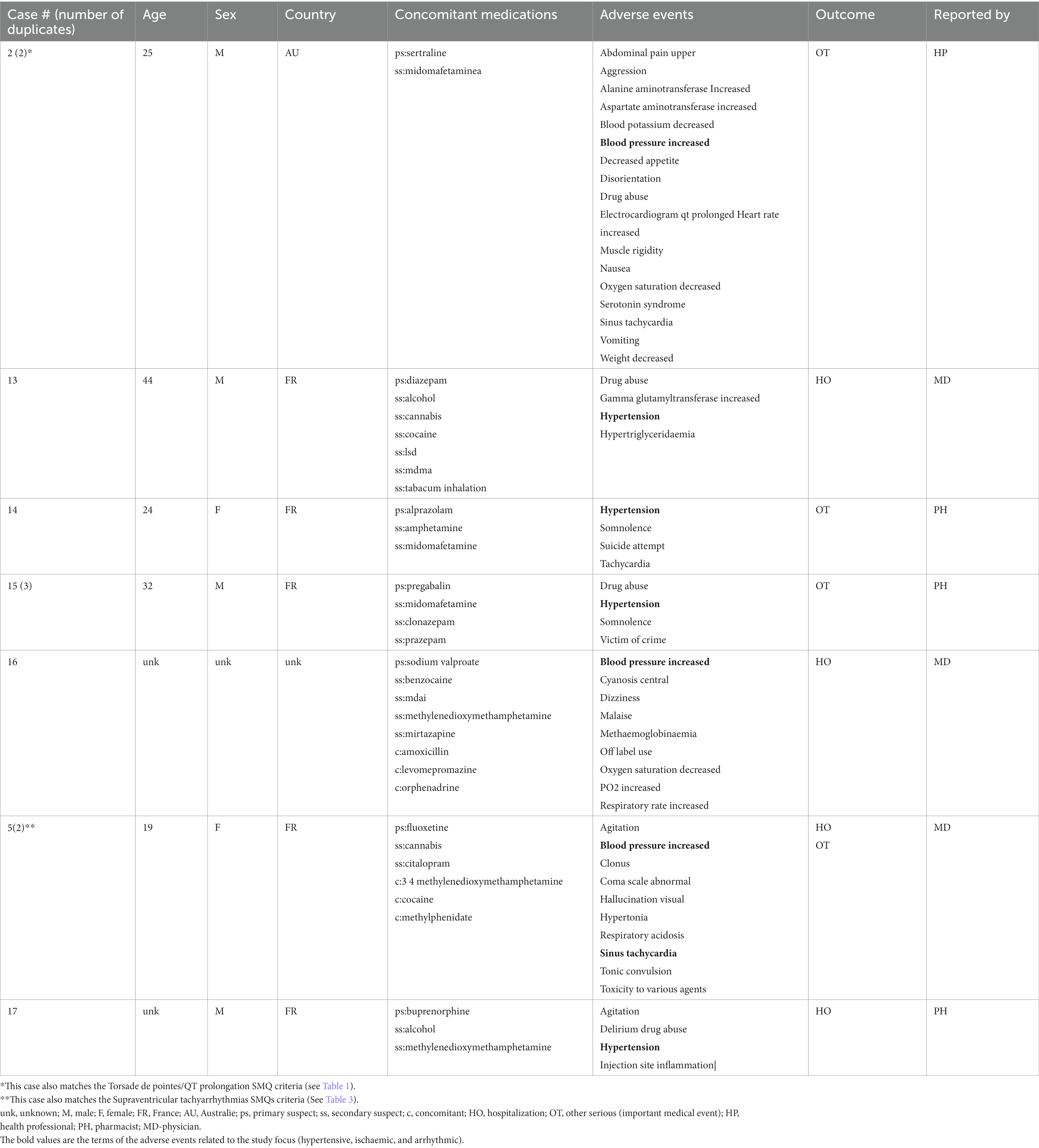
Cases where one of the reported drugs included the terms methylenedioxymethamphetamine, 3,4-methylenedioxymethamphetamine, midomaphetamine, midomafetamine, MDMA, ecstasy, were selected into the MDMA cohort for review. A total of 1,475 reports were selected. Further, cases where reports were submitted by a healthcare professional were selected to avoid reporting bias and add clinical relevance to the reports. The resulting cohort was queried for AEs with Preferred Terms (PT) based on the following Standardized MedDRA queries (SMQs) (34) consisting of a series of specific terms intended to select key symptoms or diagnoses: Torsade de pointes/QT prolongation (level 1 SMQ), Arrhythmia related investigations, signs and symptoms (Level 2 SMQ), Cardiac arrhythmia terms nonspecific (level 3 SMQ), Supraventricular tachyarrhythmias (level 4 SMQ), Hypertension (Level 1 SMQ), Ventricular tachyarrhythmias (Level 4 SMQ), Tachyarrhythmia terms, nonspecific (Level 4 SMQ). A comprehensive list of both narrow and broad scope SMQ PTs used in the query can be found in Supplementary Table S1. Cases were selected if at least one narrow scope PT or two or more broad scope PTs were reported. Reports were further individually reviewed to exclude duplicate submissions from multiple reporting sources resulting in 17 unique reports with AEs of interest (Figure 1). No other inclusion and exclusion parameters were applied in the case selection. All of the screened cases were included in the study (n = 17).
Results
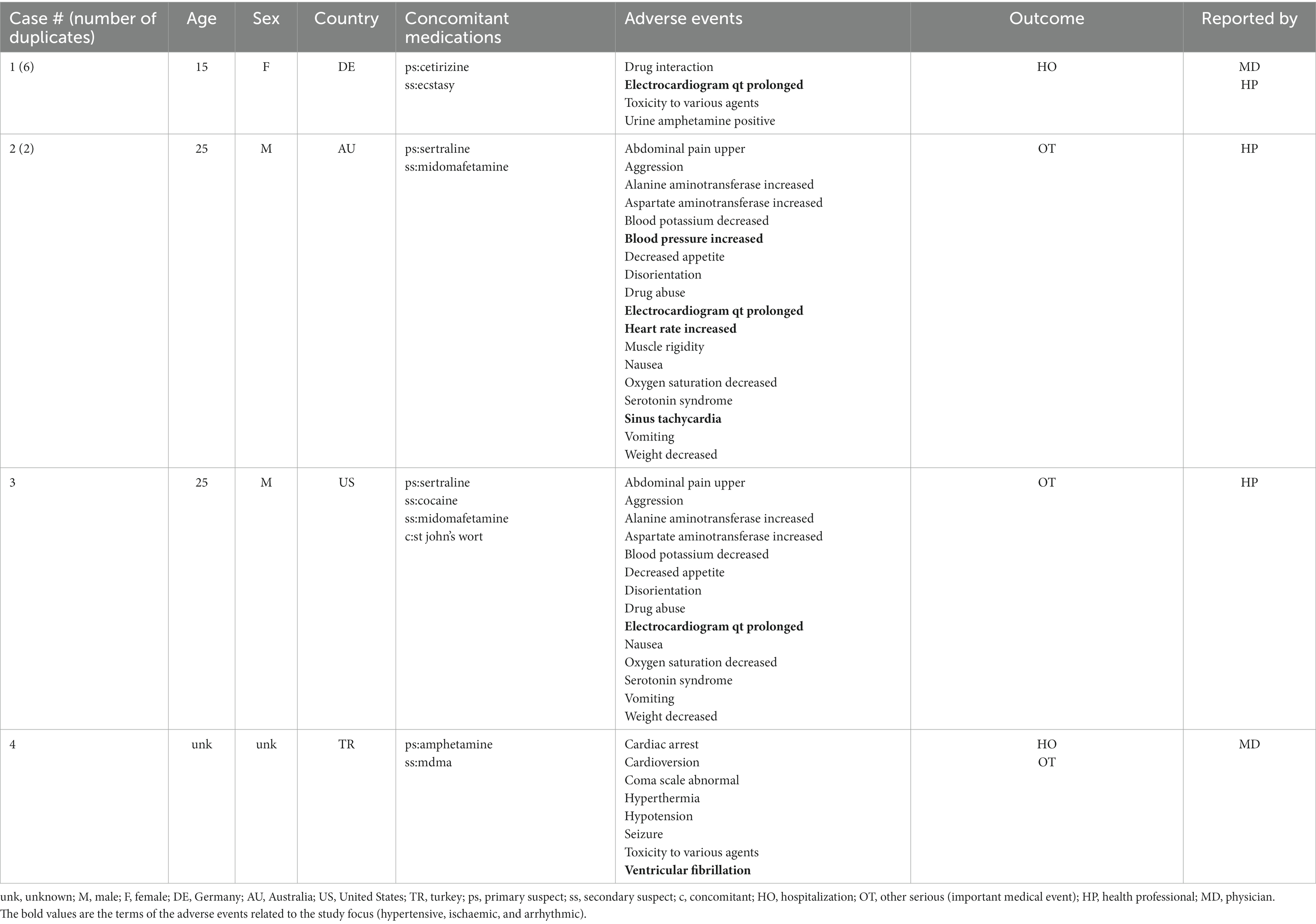
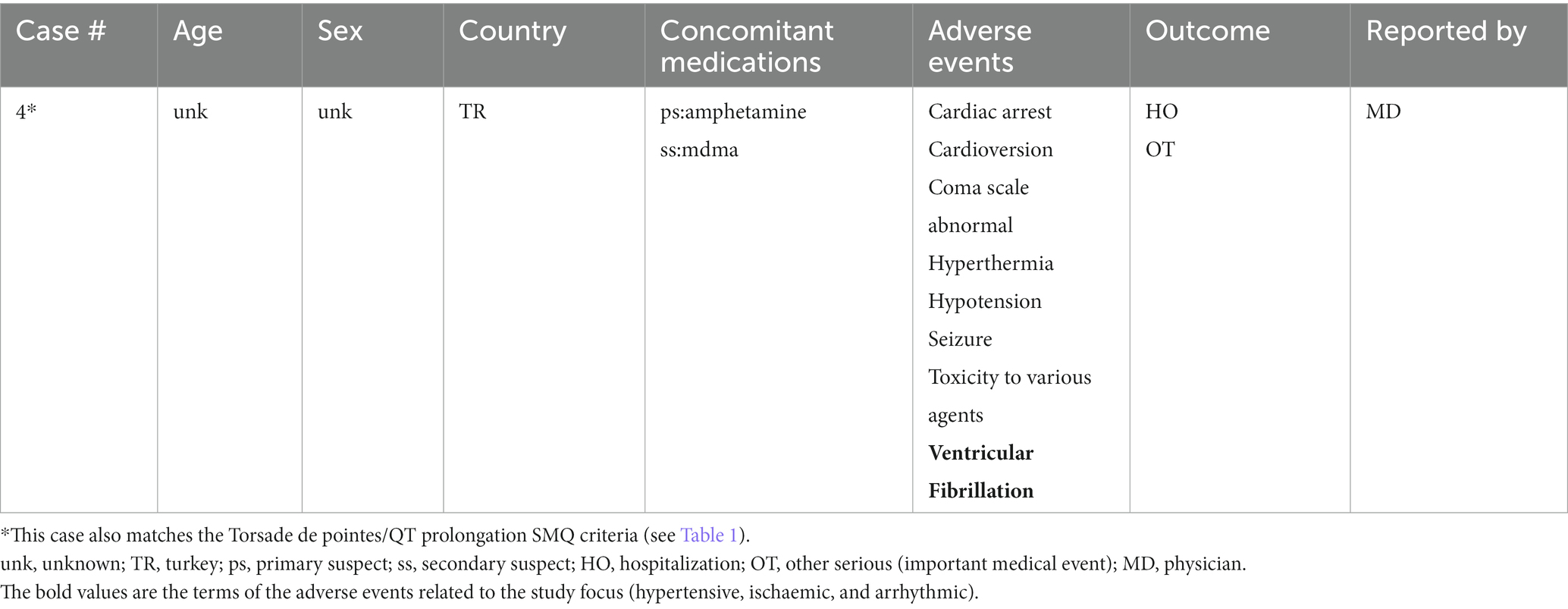
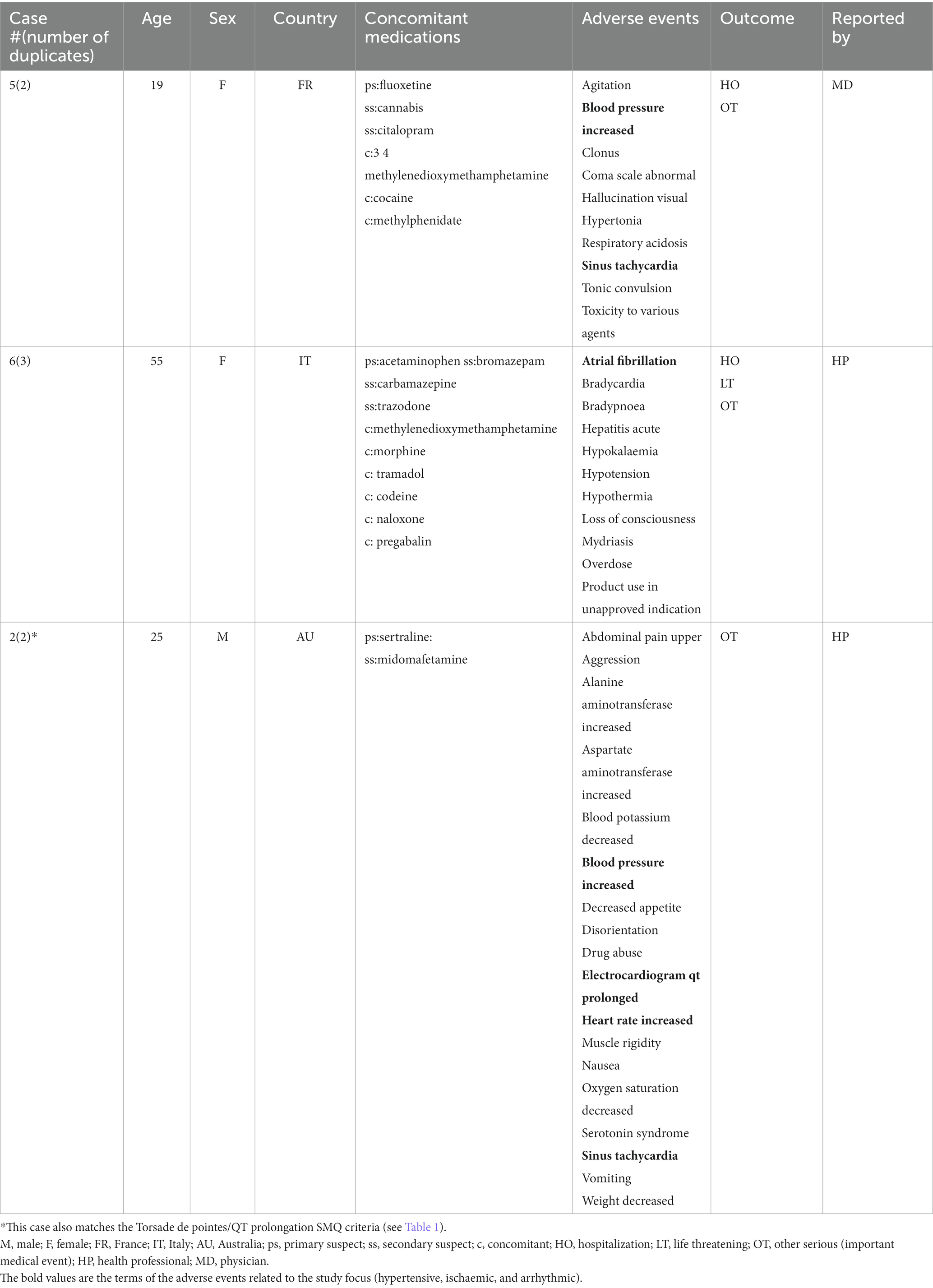
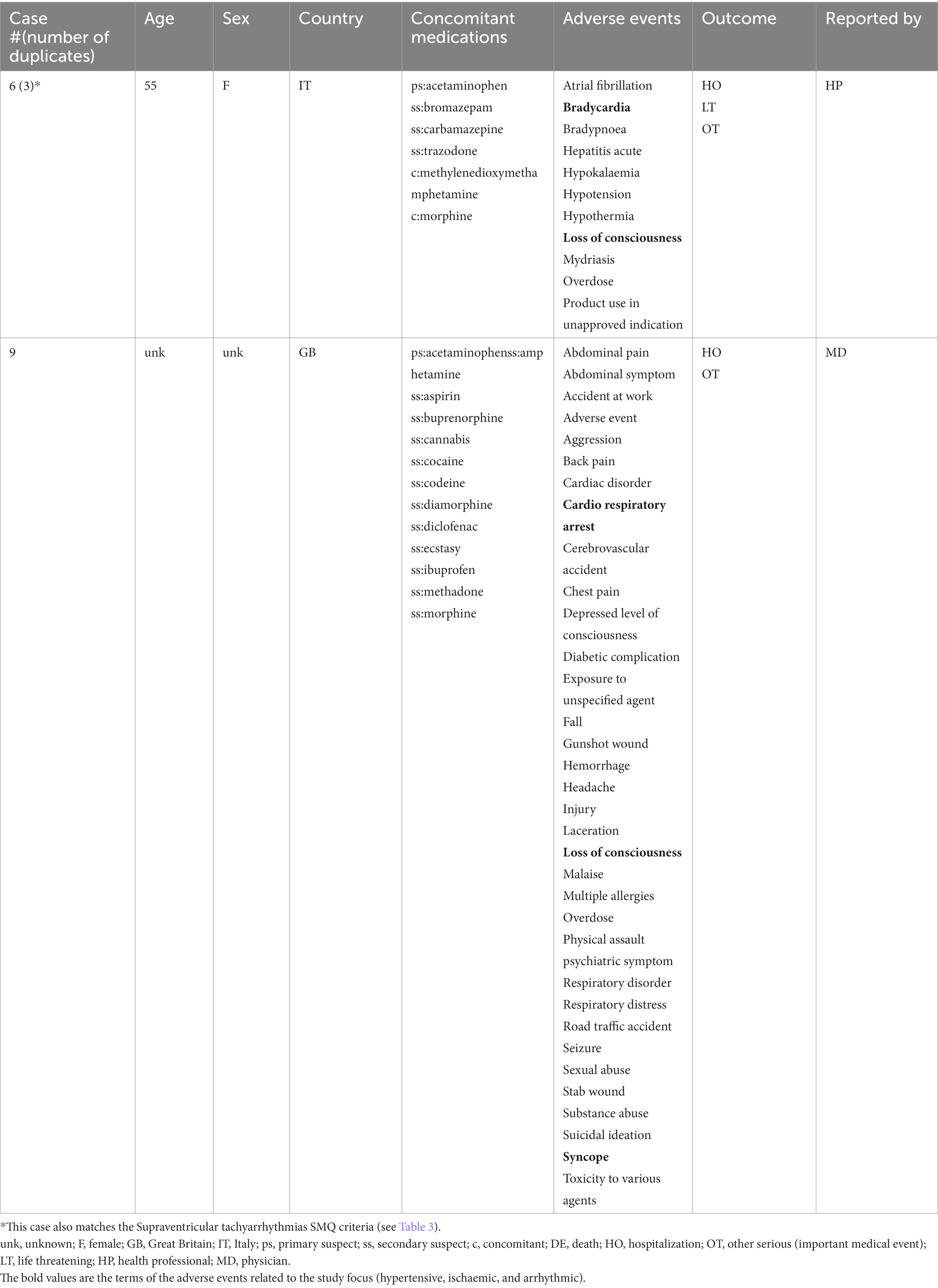
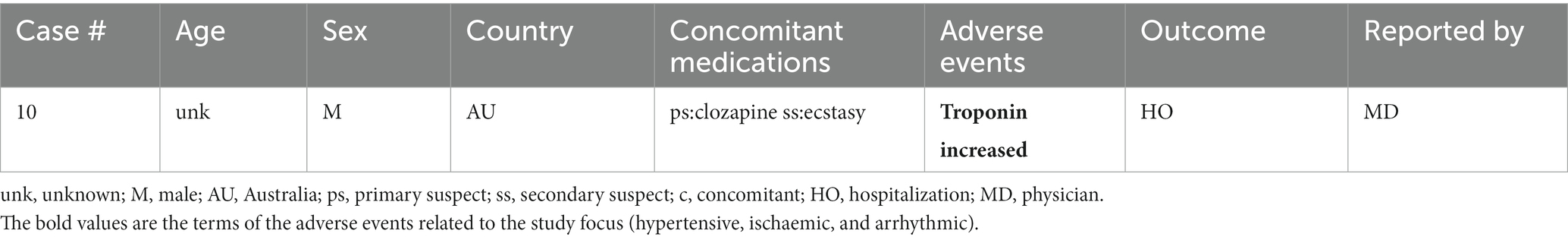
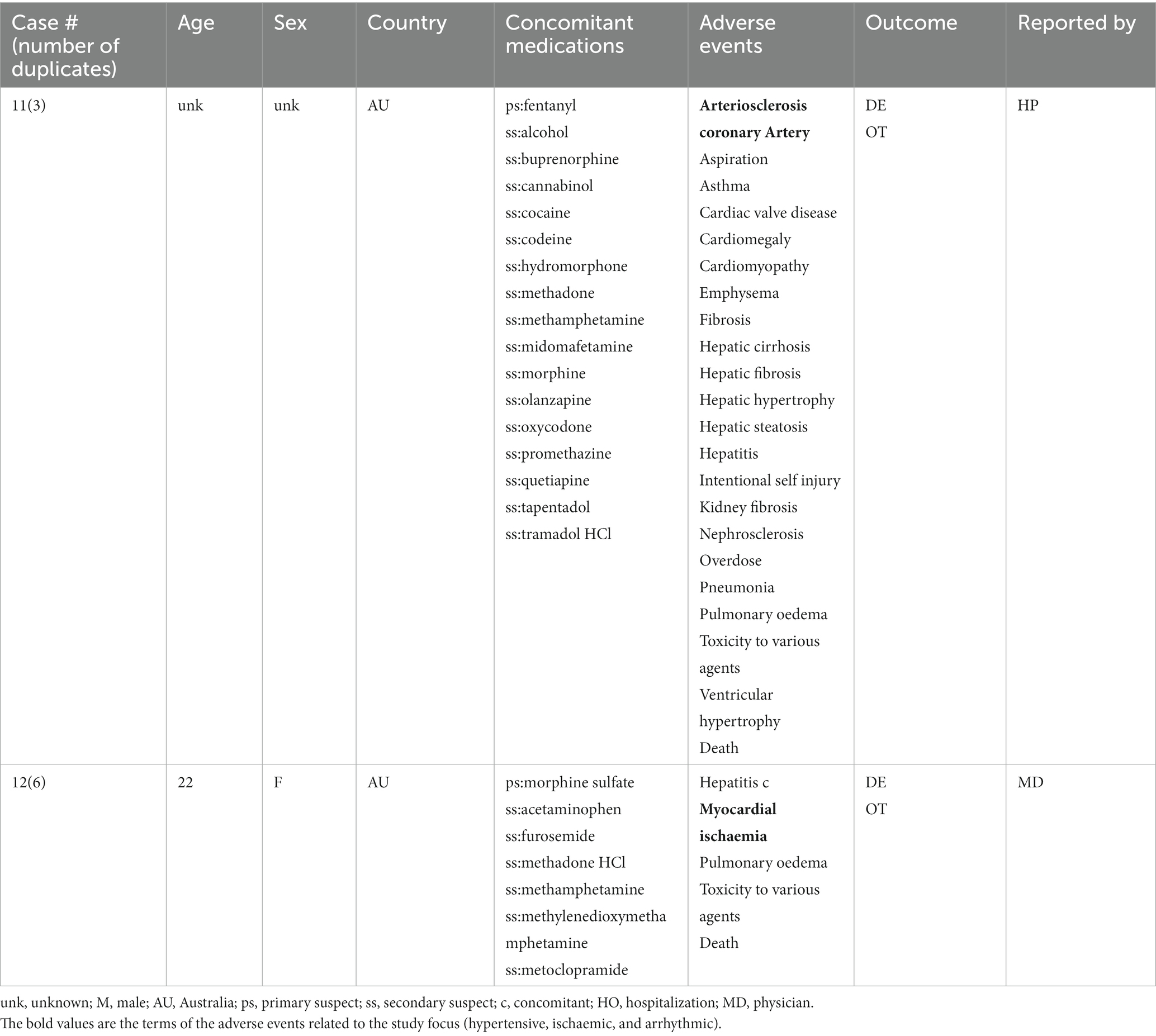
A total of 17 unique cases were reviewed in this study. There were no reports where MDMA was taken as a single agent and ischemic, hypertensive, or arrhythmic AEs were reported. All cases included concomitant medications with known associated cardiac function abnormalities. There were a total of four cases matching the Torsade de pointes/QT prolongation SMQ search criteria (Supplementary Table S1). In all of the cases, MDMA was taken with concomitant medications (SSRIs, antihistamines/anticholinergics, amphetamines) with known effects on cardiac function (Table 1). These cases included one report of ventricular fibrillation which matched the Ventricular tachyarrhythmias SMQ search query (Table 2). The Supraventricular tachyarrhythmia SMQ query produced three reports with one report overlapping with Torsade de pointes/QT prolongation SMQ terms (Table 3). The Cardiac arrhythmia terms nonspecific SMQ search produced two reports with concomitant reported cocaine, opioid, benzodiazepine, gamma hydroxybutyrate, and cannabis use (Table 4). There were two reports in the Arrhythmia related investigations, signs and symptoms query, both based on three broad scope AE PTs (Supplementary Table S1), one of which (case #6) overlapped with the Supraventricular tachyarrhythmia query (Table 5). The Myocardial infarction SMQ based search produced one report of an AE of troponin increased, where MDMA was taken with clozapine (Table 6). The Tachyarrhythmia terms nonspecific SMQ search produced no reports. The Other ischaemic heart disease query found two unique reports and the ‘Hypertension’ query produced seven reports with two overlaps with Torsade de pointes/QT prolongation and Supraventricular tachyarrhythmias SMQs (Table 7). There were seven Hypertension SMQ cases with two reports overlapping with Torsade de pointes/QT prolongation and Supraventricular tachyarrhythmias SMQs (Table 8).
The summary of all of the cases, with the concomitant medications organized by class, is provided in Table 9.
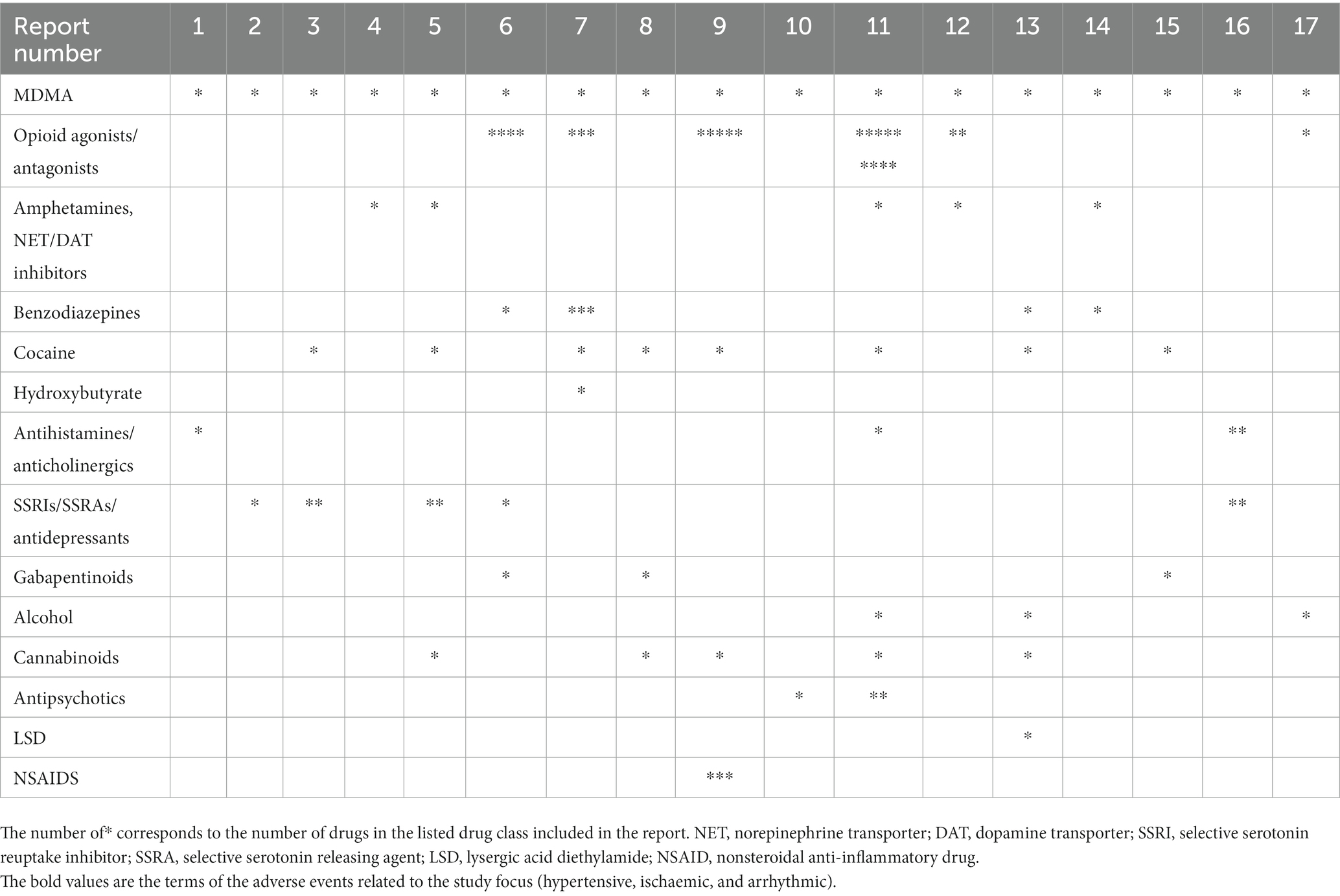
Table 9. Concomitant drugs associated with cardiac function related AEs in MDMA cases summarized by class.
Discussion
In this study, we evaluated AEs related to arrhythmias, hypertension, and ischemia in MDMA reports from the FDA adverse Event Reporting System (See Supplementary Table S1). There were no reports of those AEs in cases where MDMA was the sole reported drug. A limited number of 17 cases associated with MDMA use, reported in the last ~18 years, were evaluated. Interestingly, in every single case, MDMA was not reported as the primary suspect of those AEs. Furthermore, in all the cases, all listed concomitant drugs except one, acetaminophen, had known cardiac function related effects and were marked as primary suspects. There were two unique acetaminophen overdose cases that reported MDMA as a concomitant drug. Additionally, 76% of the reports included two or more drugs or illicit substances associated with arrhythmic, ischemic, or hypertensive AEs. It is interesting to note that in majority of the cases, illicit substances were taken in combination with psychoactive prescription medications supporting previous observations that substance use and abuse are often comorbid with psychiatric disorders. While individuals with mental health are more susceptible to misuse/abuse of illicit substances, this correlation is more complex and multidimensional as misuse/abuse may themselves lead to psychiatric disorders (35, 36).
There is still the possibility that MDMA contributed to the cardiovascular AE due to its sympathomimetic mechanism of action or CYP2D6-mediated drug–drug interaction(s) (37, 38). However, it was neither a sole culprit nor a primary suspect in any of the reported cases. The United Nations Office of Drugs and Crime estimated the number of people who report MDMA/ecstasy use to be nearly 20 million people (39). Surprisingly, considering this number, the number of reported MDMA cases with cardiovascular AE in FAERS/AERS was surprisingly low. Despite the fact that MDMA, as a known sympathomimetic, transiently increases blood pressure which is also observed in clinical trials, the number of FAERS reports on hypertension is relatively low, supporting the transient nature of this observation.
Study limitations. Due to the voluntary nature of FAERS/AERS reporting, with the exception of spontaneous reports from sponsors/manufacturers, the data presented only represents a subset of actual cases and should not be confused with actual population frequencies. Additionally, since the manufacturing and distribution of MDMA is not regulated, it is not clear whether the reported compound was pure MDMA or if it was laced with another compound not caught by the conventional drug tests. Information on the ingested MDMA dose and how the presence of MDMA was evaluated is missing from the reports, since case narratives are kept confidential by the FDA due to privacy concerns. Detailed medical and psychiatric history of the individuals in the reports were also not available. However, all of the 17 cases presented in the study were submitted by healthcare professionals (Form-3,500), thus some level of clinical adjudication prior to reporting is expected.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://fis.fda.gov/extensions/FPD-QDE-FAERS/FPD-QDE-FAERS.html.
Ethics statement
Ethical approval was not provided for this study on human participants because Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Study utilized de-identified postmarketing data available online to the public. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
TM performed the research. TM, DD, LJ, AB, and RA designed the study, drafted the manuscript, and reviewed the final version. RA processed the data sets. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by MAPS Public Benefit Corporation and in part by Skaggs School of Pharmacy and Pharmaceutical Sciences, UC San Diego Health.
Acknowledgments
We thank Da Shi for contributions to the data processing. We also thank Isaac V. Cohen for useful discussions.
Conflict of interest
TM, DD, and LJ were employed by MAPS Public Benefit Corporation. AB was employed by Tulip Medical Consulting LLC.
The remaining author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1149766/full#supplementary-material
References
1. Wolfson, PE, Andries, J, Feduccia, AA, Jerome, L, Wang, JB, Williams, E, et al. MDMA-assisted psychotherapy for treatment of anxiety and other psychological distress related to life-threatening illnesses: a randomized pilot study. Sci Rep. (2020) 10:20442. doi: 10.1038/s41598-020-75706-1
2. Mithoefer, MC, Feduccia, AA, Jerome, L, Mithoefer, A, Wagner, M, Walsh, Z, et al. MDMA-assisted psychotherapy for treatment of PTSD: study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacology. (2019) 236:2735–45. doi: 10.1007/s00213-019-05249-5
3. Jerome, L, Feduccia, AA, Wang, JB, Hamilton, S, Yazar-Klosinski, B, Emerson, A, et al. Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: a longitudinal pooled analysis of six phase 2 trials. Psychopharmacology. (2020) 237:2485–97. doi: 10.1007/s00213-020-05548-2
4. Mitchell, JM, Bogenschutz, M, Lilienstein, A, Harrison, C, Kleiman, S, Parker-Guilbert, K, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. (2021) 27:1025–33. doi: 10.1038/s41591-021-01336-3
5. Yazar-Klosinski, BB, and Mithoefer, MC. Potential psychiatric uses for MDMA. Clin Pharmacol Ther. (2017) 101:194–6. doi: 10.1002/cpt.565
6. Kalant, H. The pharmacology and toxicology of "ecstasy" (MDMA) and related drugs. CMAJ. (2001) 165:917–28.
7. Meyer, JS. 3,4-methylenedioxymethamphetamine (MDMA): current perspectives. Subst Abus Rehabil. (2013) 4:83–99. doi: 10.2147/SAR.S37258
8. Simmler, LD, Buser, TA, Donzelli, M, Schramm, Y, Dieu, LH, Huwyler, J, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. (2013) 168:458–70. doi: 10.1111/j.1476-5381.2012.02145.x
9. Verrico, CD, Miller, GM, and Madras, BK. MDMA (ecstasy) and human dopamine, norepinephrine, and serotonin transporters: implications for MDMA-induced neurotoxicity and treatment. Psychopharmacology. (2007) 189:489–503. doi: 10.1007/s00213-005-0174-5
10. Dumont, GJ, Sweep, FC, van der Steen, R, Hermsen, R, Donders, AR, Touw, DJ, et al. Increased oxytocin concentrations and prosocial feelings in humans after ecstasy (3,4-methylenedioxymethamphetamine) administration. Soc Neurosci. (2009) 4:359–66. doi: 10.1080/17470910802649470
11. Hysek, CM, Schmid, Y, Simmler, LD, Domes, G, Heinrichs, M, Eisenegger, C, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affect Neurosci. (2014) 9:1645–52. doi: 10.1093/scan/nst161
12. Bershad, AK, Miller, MA, Baggott, MJ, and de Wit, H. The effects of MDMA on socio-emotional processing: does MDMA differ from other stimulants? J Psychopharmacol. (2016) 30:1248–58. doi: 10.1177/0269881116663120
13. Maples-Keller, JL, Norrholm, SD, Burton, M, Reiff, C, Coghlan, C, Jovanovic, T, et al. A randomized controlled trial of 3,4-methylenedioxymethamphetamine (MDMA) and fear extinction retention in healthy adults. J Psychopharmacol. (2022) 36:368–77. doi: 10.1177/02698811211069124
14. Foulon, P, and De Backer, D. The hemodynamic effects of norepinephrine: far more than an increase in blood pressure! Ann Transl Med. (2018) 6:S25. doi: 10.21037/atm.2018.09.27
15. Lambert, GW, Kaye, DM, Lefkovits, J, Jennings, GL, Turner, AG, Cox, HS, et al. Increased central nervous system monoamine neurotransmitter turnover and its association with sympathetic nervous activity in treated heart failure patients. Circulation. (1995) 92:1813–8. doi: 10.1161/01.CIR.92.7.1813
16. Côté, F, Fligny, C, Fromes, Y, Mallet, J, and Vodjdani, G. Recent advances in understanding serotonin regulation of cardiovascular function. Trends Mol Med. (2004) 10:232–8. doi: 10.1016/j.molmed.2004.03.007
17. Dowling, GP, McDonough, ET, and Bost, RO. 'Eve' and 'Ecstasy' A report of five deaths associated with the use of MDEA and MDMA. JAMA. (1987) 257:1615–7. doi: 10.1001/jama.1987.03390120077027
18. Sano, R, Hasuike, T, Nakano, M, Kominato, Y, and Itoh, H. A fatal case of myocardial damage due to misuse of the "designer drug" MDMA. Leg Med (Tokyo). (2009) 11:294–7. doi: 10.1016/j.legalmed.2009.09.003
19. Bonsignore, A, Barranco, R, Morando, A, Fraternali Orcioni, G, and Ventura, F. MDMA induced cardio-toxicity and pathological myocardial effects: a systematic review of experimental data and autopsy findings. Cardiovasc Toxicol. (2019) 19:493–9. doi: 10.1007/s12012-019-09526-9
20. Lester, SJ, Baggott, M, Welm, S, Schiller, NB, Jones, RT, Foster, E, et al. Cardiovascular effects of 3,4-methylenedioxymethamphetamine. A double-blind, placebo-controlled trial. Ann Intern Med. (2000) 133:969–73. doi: 10.7326/0003-4819-133-12-200012190-00012
21. Vizeli, P, and Liechti, ME. Safety pharmacology of acute MDMA administration in healthy subjects. J Psychopharmacol. (2017) 31:576–88. doi: 10.1177/0269881117691569
22. Simmons, S, and Suárez, L. Substance abuse and trauma. Child Adolesc Psychiatr Clin N Am. (2016) 25:723–34. doi: 10.1016/j.chc.2016.05.006
23. Najavits, LM, Weiss, RD, and Shaw, SR. The link between substance abuse and posttraumatic stress disorder in women. Res rev Am J Addict. (1997) 6:273–83.
24. Knowles, KA, Sripada, RK, Defever, M, and Rauch, SAM. Comorbid mood and anxiety disorders and severity of posttraumatic stress disorder symptoms in treatment-seeking veterans. Psychol Trauma. (2019) 11:451–8. doi: 10.1037/tra0000383
25. Campbell, DG, Felker, BL, Liu, CF, Yano, EM, Kirchner, JE, Chan, D, et al. Prevalence of depression-PTSD comorbidity: implications for clinical practice guidelines and primary care-based interventions. J Gen Intern Med. (2007) 22:711–8. doi: 10.1007/s11606-006-0101-4
26. Koffel, E, Khawaja, IS, and Germain, A. Sleep disturbances in posttraumatic stress disorder: updated review and implications for treatment. Psychiatr Ann. (2016) 46:173–6. doi: 10.3928/00485713-20160125-01
27. Kind, S, and Otis, JD. The interaction between chronic pain and PTSD. Curr Pain Headache Rep. (2019) 23:91. doi: 10.1007/s11916-019-0828-3
28. Sarparast, A, Thomas, K, Malcolm, B, and Stauffer, CS. Drug-drug interactions between psychiatric medications and MDMA or psilocybin: a systematic review. Psychopharmacology. (2022) 239:1945–76. doi: 10.1007/s00213-022-06083-y
29. Craigle, V. MedWatch: the FDA safety information and adverse event reporting program. J Med Libr Assoc. (2007) 95:224–5. doi: 10.3163/1536-5050.95.2.224
30. Keshishi, D, Makunts, T, and Abagyan, R. Common osteoporosis drug associated with increased rates of depression and anxiety. Sci Rep. (2021) 11:23956. doi: 10.1038/s41598-021-03214-x
31. Wollmer, MA, Makunts, T, Krüger, THC, and Abagyan, R. Postmarketing safety surveillance data reveals protective effects of botulinum toxin injections against incident anxiety. Sci Rep. (2021) 11:24173. doi: 10.1038/s41598-021-03713-x
32. Makunts, T, Jerome, L, Abagyan, R, and de Boer, A. Reported cases of serotonin syndrome in MDMA users in FAERS database. Front Psych. (2021) 12:824288. doi: 10.3389/fpsyt.2021.824288
33. Stein, LD. Unix Survival Guide. Curr Protoc Bioinformatics. (2015) 51:A1.C.1–A1.C.27. doi: 10.1002/0471250953.bia01cs51
34. Mozzicato, P. Standardised MedDRA queries: their role in signal detection. Drug Saf. (2007) 30:617–9. doi: 10.2165/00002018-200730070-00009
35. De Berardis, D, Fornaro, M, Orsolini, L, Ventriglio, A, Vellante, F, and Di Giannantonio, M. Emotional Dysregulation in adolescents: implications for the development of severe psychiatric disorders, substance abuse, and suicidal ideation and behaviors. Brain Sci. (2020) 10:591. doi: 10.3390/brainsci10090591
36. Orsolini, L, Chiappini, S, Papanti, D, De Berardis, D, Corkery, JM, and Schifano, F. The bridge between classical and "synthetic"/chemical psychoses: towards a clinical, psychopathological, and therapeutic perspective. Front Psych. (2019) 10:851. doi: 10.3389/fpsyt.2019.00851
37. de la Torre, R, Yubero-Lahoz, S, Pardo-Lozano, R, and Farré, M. MDMA, methamphetamine, and CYP2D6 pharmacogenetics: what is clinically relevant? Front Genet. (2012) 3:235. doi: 10.3389/fgene.2012.00235
38. Rodgers, JT, Davydova, NY, Paragas, EM, Jones, JP, and Davydov, DR. Kinetic mechanism of time-dependent inhibition of CYP2D6 by 3,4-methylenedioxymethamphetamine (MDMA): functional heterogeneity of the enzyme and the reversibility of its inactivation. Biochem Pharmacol. (2018) 156:86–98. doi: 10.1016/j.bcp.2018.08.010
Keywords: 3,4-methylenedioxymethamphetamine, MDMA, adverse events, cardiovascular, schaemia, hypertension, arrhythmia
Citation: Makunts T, Dahill D, Jerome L, de Boer A and Abagyan R (2023) Concomitant medications associated with ischemic, hypertensive, and arrhythmic events in MDMA users in FDA adverse event reporting system. Front. Psychiatry. 14:1149766. doi: 10.3389/fpsyt.2023.1149766
Edited by:
Roberto Ciccocioppo, University of Camerino, ItalyReviewed by:
Cody Wenthur, University of Wisconsin-Madison, United StatesDomenico De Berardis, Department of Mental Health, ASL 4, Italy
Copyright © 2023 Makunts, Dahill, Jerome, de Boer and Abagyan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tigran Makunts, dGlncmFuLm1ha3VudHNAbWFwc2Jjb3JwLmNvbQ==
 Tigran Makunts
Tigran Makunts Diane Dahill1
Diane Dahill1 Lisa Jerome
Lisa Jerome