- Department of Mental Diseases, Shanghai Municipal Hospital of Traditional Chinese Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Study objectives: This meta-analysis analytically reviewed recent studies concerning the potential associations between the brain-derived neurotrophic factor (BDNF) Val66Met polymorphism and susceptibility to major depressive disorder (MDD), with subgroup analyses for race and age.
Methods: Relevant case-control studies were systematically searched for in PubMed, Embase, the Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang, and Sinomed databases. A total of 24 studies were finally identified to have reported outcomes including alleles, dominant genes, recessive genes, homozygosity, and heterozygosity. Subgroup meta-analyses were performed based on participant age and ethnicity. Publication bias was represented by funnel plots. All meta-analyses of the randomized controlled trials included for evaluation were performed using RevMan5.3 software.
Results: The findings revealed no significant association between BDNF Val66Met polymorphism and MDD. However, the Met allele was found to be associated with genetic susceptibility to MDD among white populations on subgroup analysis (OR = 1.25, 95% CI: 1.05–1.48, P = 0.01). In the genetic model, dominant (OR = 1.40, 95% CI: 1.18–1.66, P = 0.0001), recessive (OR = 1.70, 95% CI: 1.05–2.78, P = 0.03), and homozygous (OR = 1.77, 95% CI: 1.08–2.88, P = 0.02) genes were all associated with MDD.
Conclusions: Despite the outcome limitations, this meta-analysis confirmed that the BDNF Val66Met polymorphism is a susceptibility factor for MDD in white populations.
1. Introduction
Major depressive disorder (MDD), also termed clinical depression, manifests with low mood, anhedonia, and fatigue; cognitive decline, autonomic nervous function changes, sleep disturbances, and appetite changes are also frequently reported (1, 2). MDD is an affective regulatory psychiatric disorder characterized by significant and persistent feelings of depression. It is a common and frequently encountered disease (3). It is a prevalent and disabling mood disorder that poses a significant burden to society. The etiology of MDD is complex and multifactorial, involving genetic, neuroendocrine, and sociopsychological factors. It is widely accepted that MDD arises from a complex interplay between these factors, which ultimately contribute to alterations in brain structure and function, leading to the development of depressive symptoms (4, 5).
Recently, brain-derived neurotrophic factor (BDNF) gene polymorphisms have been reported to play important roles in depression and cognitive impairment (6, 7). As the most abundant neurotrophic factor in the central nervous system, BDNF is involved in both developmental and regulatory functions such as plasticity. Abundant in brain areas key to emotional regulation such as the hippocampus, hypothalamus, amygdala, and neocortex, BDNF functions to support cholinergic, dopaminergic, and serotonergic signaling (8, 9). The BDNF genotype was reported to be closely associated with the development of MDD (10). Furthermore, the Val66Met polymorphism was found to be associated with genetic predispositions to anxiety and depression in animals (11).
The BDNF gene is located on human chromosome 11p13. Of several known relevant gene polymorphisms, Val66Met has attracted the greatest interest. This non-synonymous G to A single-nucleotide polymorphism is found at position 196 of exon 2 and results in the substitution of methionine for valine (Val → Met) at codon 66 (12, 13). Although patients suffering depression are considerably more likely to be Met allele carriers, no definitive association has been proven to date (14, 15). To what extent Val66Met associates with susceptibility to MDD thus warrants investigation.
2. Materials and methods
2.1. Database search
On 10 September 2022, the Embase, PubMed, Web of Science, China National Knowledge Internet (CNKI), Wanfang, and Sinomed databases were searched for relevant literature using keywords as follows: (major depressive disorder OR depression disorder OR depression disease) and (brain-derived neurotrophic factor) and (Val66Met OR RS6265) and (polymorphism OR mutation OR variation OR genotype OR gene). Literature in both English and Chinese was searched.
2.2. Selection criteria
All studies included in our meta-analysis conformed to the following inclusion criteria: (a) the study focused on the relationship between MDD and Val66Met; (b) the study was a case-control study published in the form of a treatise; (c) the study included both MDD patients and normal controls; (d) study controls were healthy persons; (e) study sample sizes were adequate and allele and genotype frequency data were available; (f) the study applied the diagnostic criteria of DSM-IV or DSM-V or CCMD-3 or HDRS≥18 or ICD-10 MDD; and (g) the study employed rigorous experimental methods for detecting genetic polymorphisms. For duplicate publications, the study with the greater sample size was included in our analysis.
2.3. Data extraction
All studies selected for analysis were sequentially screened on the basis of title and abstract content; studies which did not meet our selection criteria were excluded while duplicates were removed. The following data were extracted using pre-defined data extraction tables: first author's name; year of publication; country of study; study subject ethnicity; sources of both case and control groups; sample sizes; case and control group genotypes; allele frequencies; allele and genotype identification methods; whether Hardy-Weinberg equilibrium (HWE) conditions were met; and diagnostic criteria. Data evaluation was performed independently by Ou Li and Yuxia Wang; inconsistencies were addressed via research team discussions.
2.4. Statistical analysis
Data were analyzed using Revman 5.3 software. The odds ratio (OR) was used as a measure of BDNF gene polymorphism in MDD and a 95% confidence interval (CI) was considered. The χ2 test was used to evaluate heterogeneity among results of each study. When I2 ≤ 50%, studies were considered to have been homogenous and fixed-effects analysis was conducted. When I2 > 50%, studies were considered to have been heterogeneous and random-effects analysis was conducted. If the heterogeneity between study groups was too great, descriptive analysis was performed. Publication bias was analyzed using funnel plots. The impact of each study on pooled OR was assessed case-by-case.
3. Results
3.1. Literature search and eligible studies
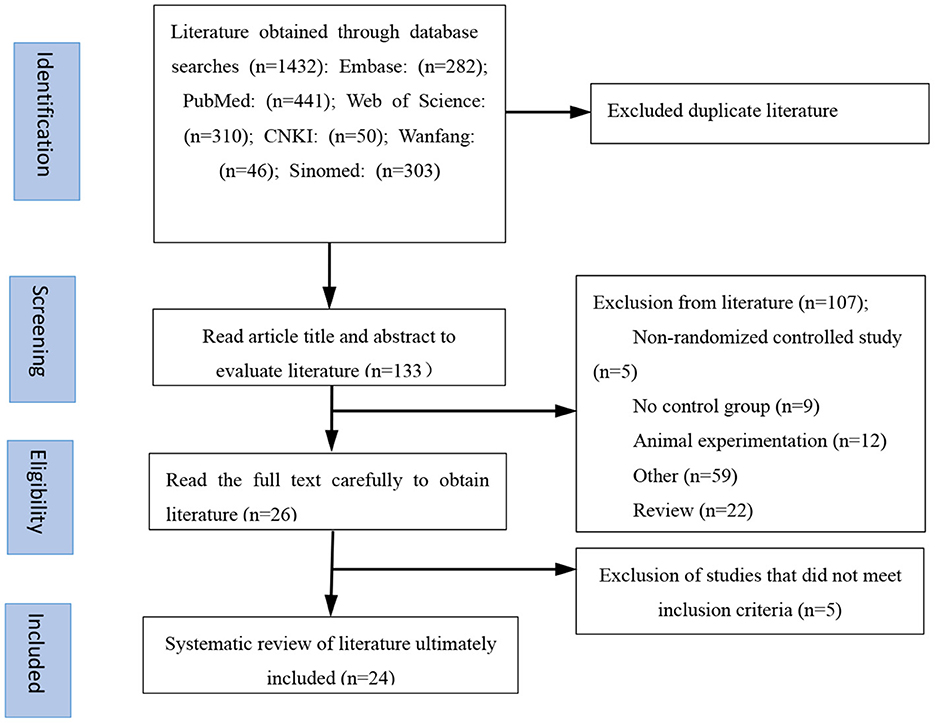
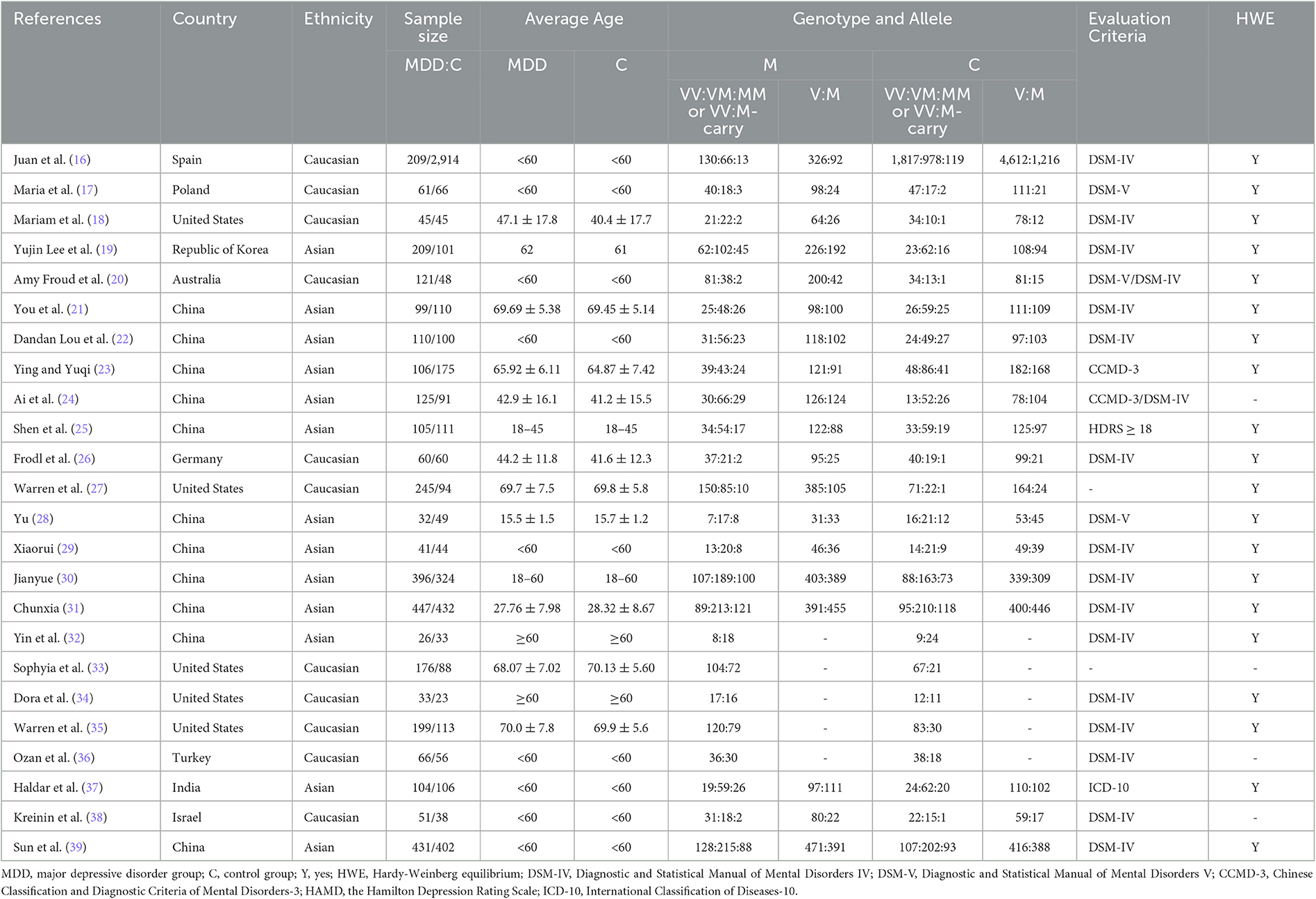
The flow chart in Figure 1 details the process of literature screening used in this study. After elimination of duplicate publications, our initial screening identified a total of 133 potential studies. After reading the study titles and abstracts, 107 studies were excluded either because they lacked a control group, were animal studies, were duplicate publications, or were conference papers. The remaining 26 studies were assessed in more detail and data from five studies were determined to not meet inclusion criteria. The reasons for excluding these five studies from our analysis included a lack of detailed genotype documentation, reports of patients having suffered comorbidities in addition to MDD, or clinical review or meta-analysis publication content. A total of 24 publications were ultimately included in this study. The characteristics of the included literature are detailed in Table 1; eight publications were written in Chinese, covering 4,116 Asian patients, and 16 were written in English, covering 1,662 Caucasian patients. All the literature included in this study was published after 2005 and analyzed minimum and maximum study sample sizes of 58 and 3,123, respectively. Of these 24 studies, 19 reported the Val66Met genotype in detail while the remainder combined the number of allele carriers.
3.2. Quantitative analysis
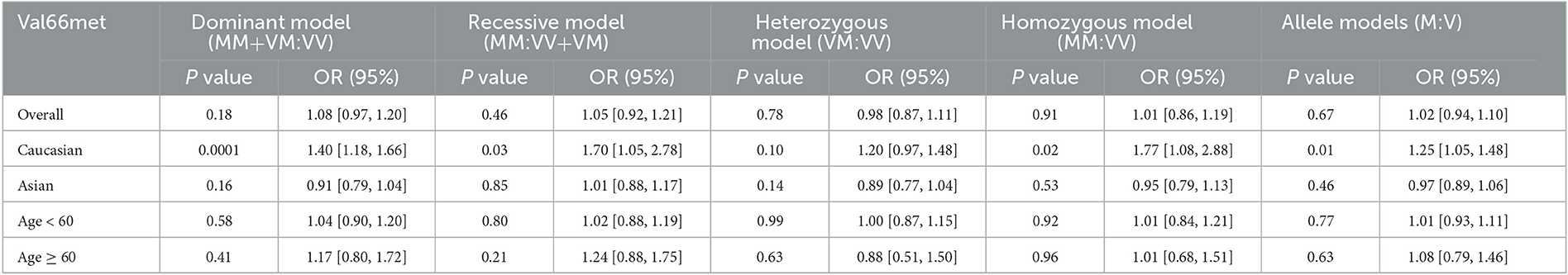
Total effect analysis of the Val66Met genetic model using heterogeneity testing revealed P > 0.05 and I2 ≤ 50% for all genetic models, suggesting that there was little heterogeneity between the studies and all fixed-effects models were adopted for analysis. Analysis of Met allele data revealed a Val OR of 1.02 (95% CI: 0.94, 1.10), confirming that there was no statistically significant difference between Met and Val alleles in MDD patients as compared to the healthy population. Meta-analysis findings based on different genetic models revealed dominant, recessive, heterozygous, and homozygous gene model ORs to be 1.08 (95% CI: 0.97, 1.20), 1.05 (95% CI: 0.92, 1.21), 0.98 (95% CI: 0.87, 1.11), and 1.01 (95% CI: 0.86, 1.19), respectively, confirming that differences between MDD patients and healthy controls were not statistically significant for each gene model. Thus, no significant association of the Val66Met polymorphism with MDD was determined (Table 2, Figure 2).
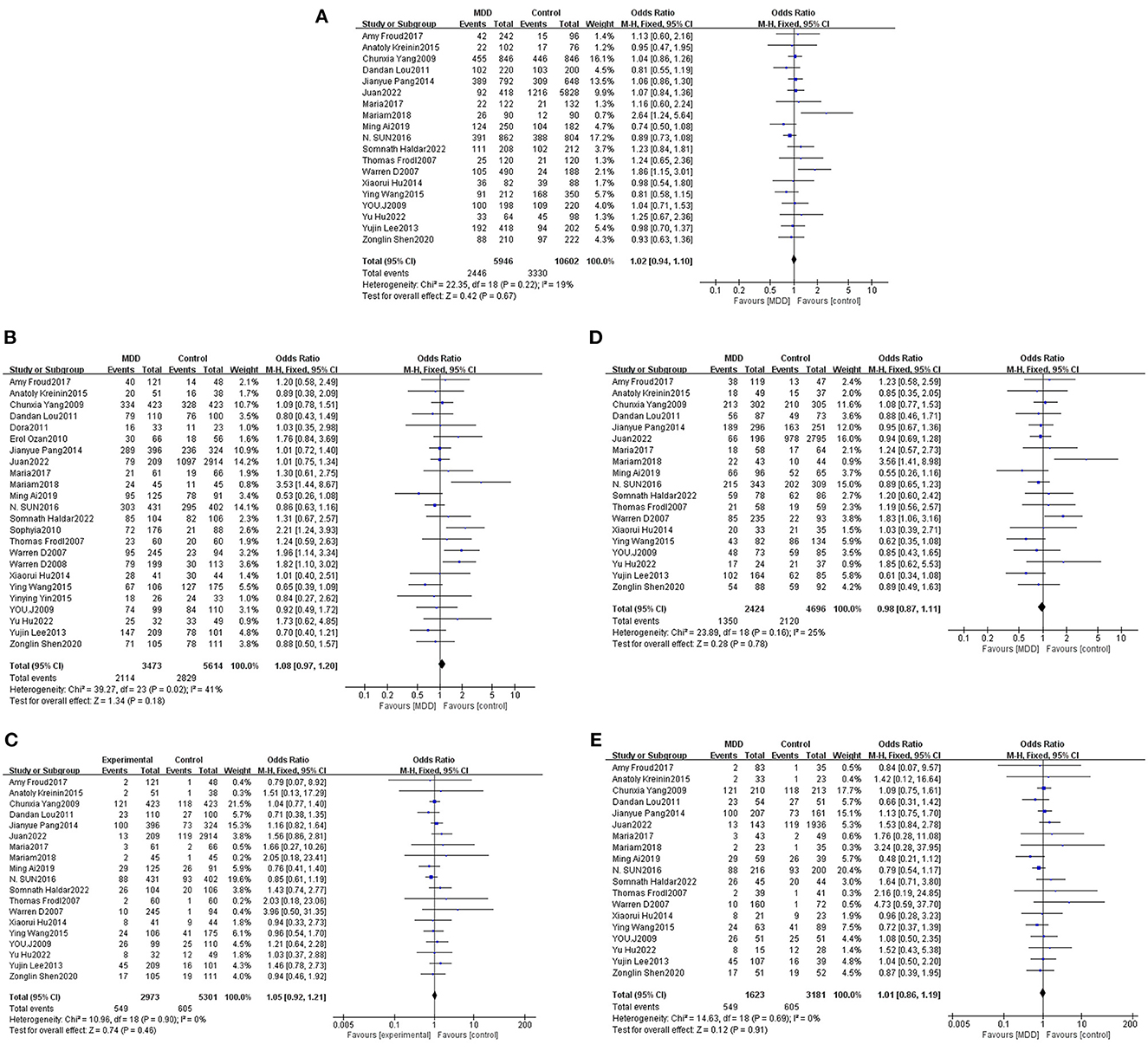
Figure 2. Forest plots of the association of Val66Met with MDD [(A) allele model; (B) dominant model; (C) recessive model; (D) heterozygous model; (E) homozygous model].
Ethnicity-based subgroup analysis of the BDNF Val66Met gene model using heterogeneity testing revealed P > 0.05 and I2 ≤ 50% for all gene models; fixed-effects models were adopted for analysis. The results revealed an OR of 1.25 (95% CI: 1.05, 1.48, P = 0.01) for the Caucasian allele Met:Val, suggesting that Val allele frequency was reduced while Met allele frequency was increased in white MDD patients as compared to controls. Thus, the Met allele was found to be associated with a genetic susceptibility to MDD among white populations. Meta-analysis based on different genetic models revealed dominant, recessive, heterozygous, and homozygous gene model ORs for white populations to be 1.40 (1.18, 1.66, P = 0.0001), 1.70 (1.05, 2.78, P = 0.03), 1.20 (0.97, 1.48, P = 0.10), and 1.77 (1.08, 2.88, P = 0.02), respectively. Statistically significant differences between dominant, recessive, and homozygous gene models were found. These three gene models were found to be associated with a genetic susceptibility to MDD in Caucasian populations. The results of the Asian populations subgroup meta-analysis were not statistically significant and consequently the Val66Met polymorphism was not determined to associate with a genetic susceptibility to MDD in Asian populations (Table 2, Figure 3).

Figure 3. Forest plots of the association of Val66Met with MDD according to ethnicity [(A) allele model; (B) dominant model; (C) recessive model; (D) heterozygous model; (E) homozygous model].
According to the changes in the physiological and psychological structure of modern people, the World Health Organization (WHO) has defined age categories for people, among which elderly people are considered to be those over 60 years old. Age-based subgroup analysis was performed considering 60 years of age as a cut-off for dividing the subjects into two groups. Heterogeneity testing revealed that P < 0.05 and I2 > 50% for alleles, dominant genes, and heterozygous genes in subjects older than 60 years; a random-effects model was thus employed for this population while a fixed-effects model was used for analysis of the remaining gene models with I2 ≤ 50%. Meta-analysis results revealed no statistically significant differences between Met and Val alleles. As such, no statistically significant differences relevant to Met and Val alleles between MDD patients of different ages as compared with healthy persons were found. Thus, age was determined not to be associated with the BDNF Val66Met gene polymorphism or genetic susceptibility to MDD (Table 2, Figure 4).
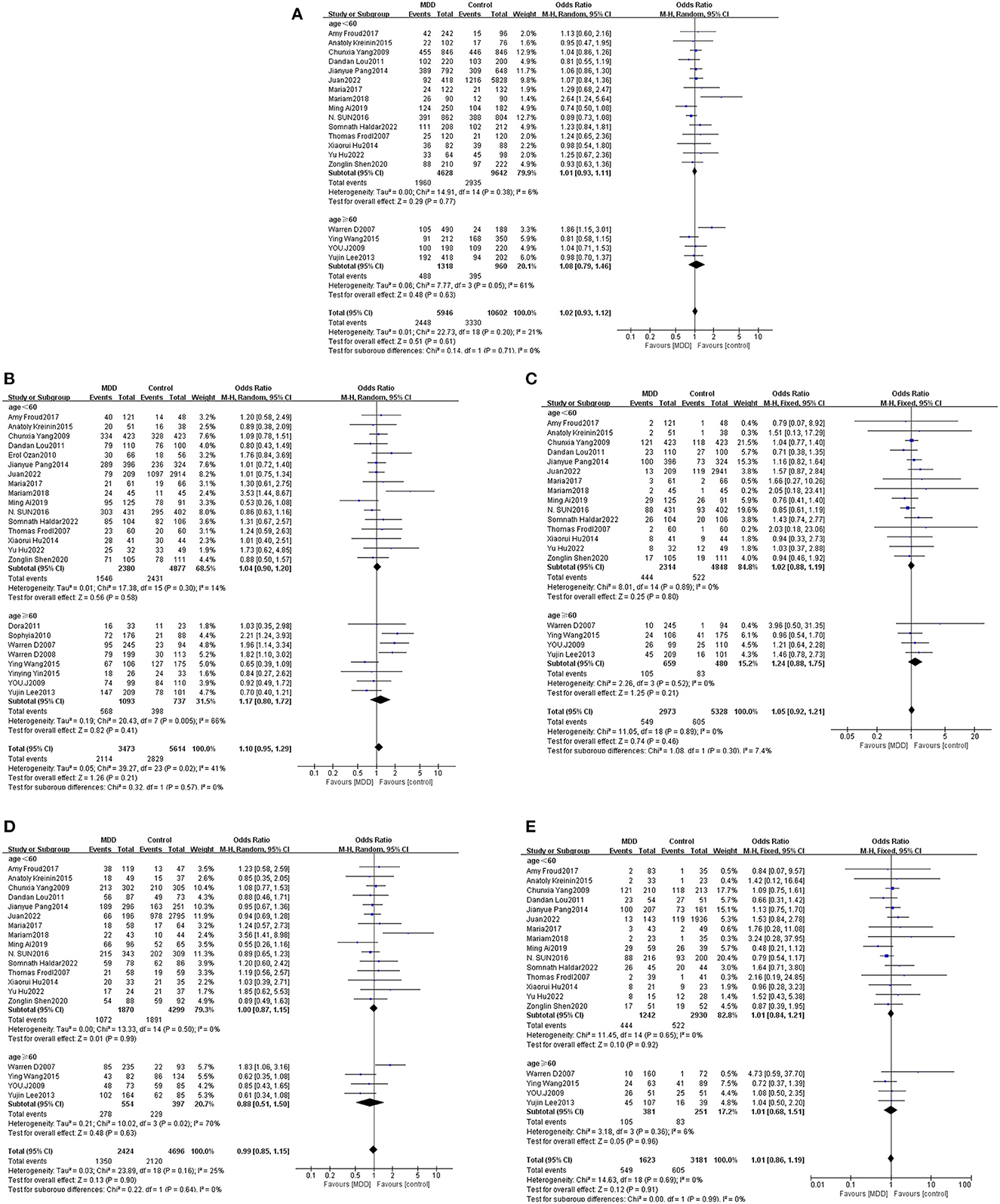
Figure 4. Forest plots of the association of Val66Met with MDD according to age [(A) allele model; (B) dominant model; (C) recessive model; (D) heterozygous model; (E) homozygous model].
3.3. Assessment of sensitivity and publication bias
Sensitivity analysis revealed that risk estimates were not significantly affected upon exclusion of any individual study, confirming that the results of this meta-analysis were reliable. According to genotype indicator, a funnel plot analysis of potential publication bias revealed a roughly symmetrical distribution of values and no significant pattern asymmetry, suggesting that the publication bias in the literature we analyzed was negligible (Figure 5).
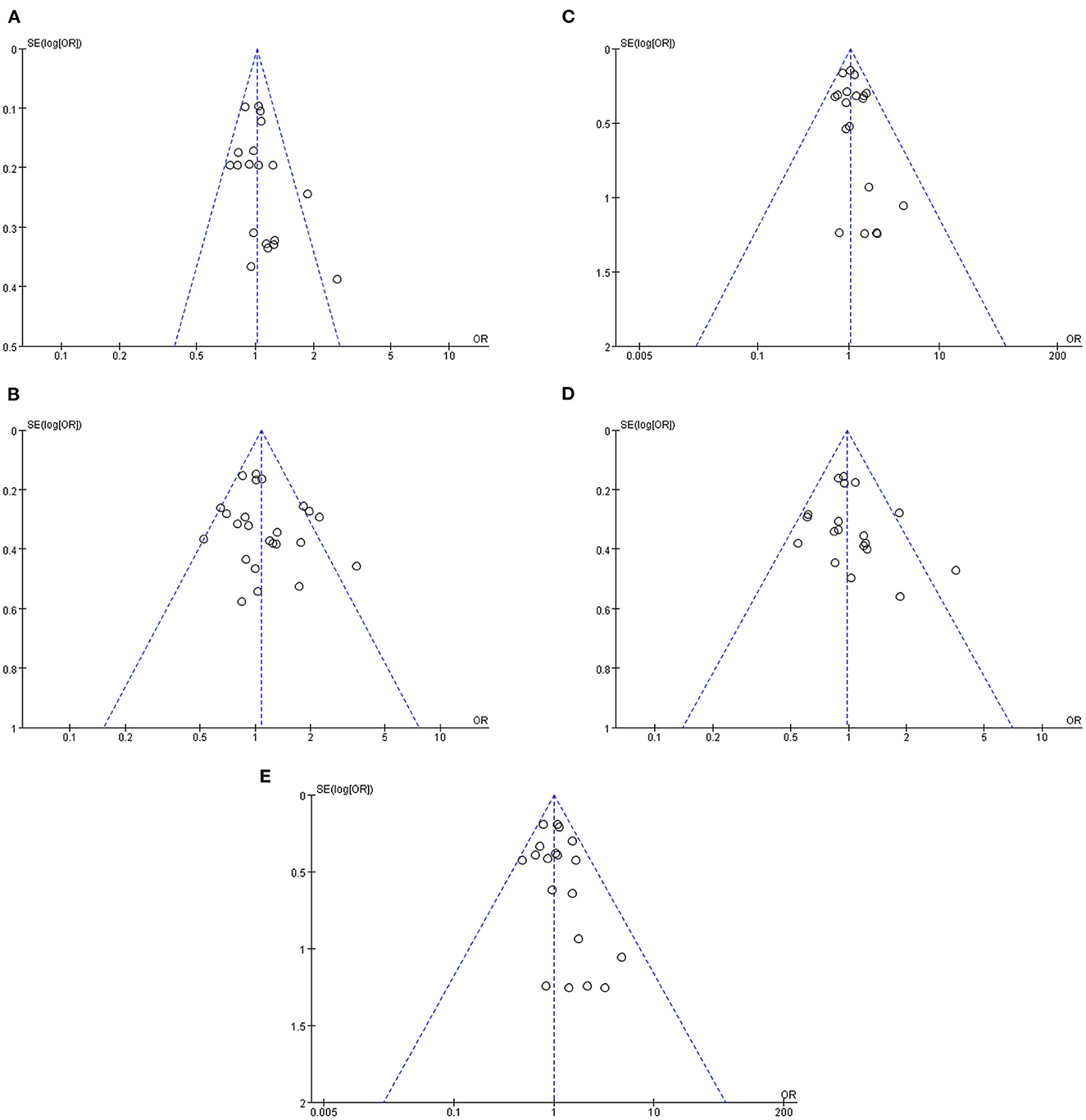
Figure 5. Funnel plots of the association of Val66Met with MDD [(A) allele model; (B) dominant model; (C) recessive model; (D) heterozygous model; (E) homozygous model].
4. Discussion
The pathophysiological mechanisms via which the BDNF Val66Met gene polymorphism acts to induce MDD remain unclear. A dimeric, neurotrophic factor primarily concentrated in the hippocampus and frontal cortex, BDNF is closely related to neuronal plasticity, differentiation, survival, and repair (20). Importantly, BDNF exerts beneficial effects in the setting of depressive and anxiety disorders (40). The role of neurotrophic factor BDNF in psychopathology has attracted more and more attention, and it may be one of the biomarkers of MDD. There are three haplogroups of the BDNF Val66Met polymorphism: Val/Val, Val/Met, and Met/Met. Animal studies have suggested that this polymorphism is associated with a genetic susceptibility to anxiety and depression. Furthermore, this polymorphism was reported to be associated with the prevalence of depression in a population (41, 42). Musazzi (43) exposed adult male BDNF Val/Val and BDNF Val/Met knock-in mice to 30 min of acute restraint stress. Interestingly, BDNF Val/Met mice exhibited higher levels of presynaptic glutamate release and hippocampal cAMP response element binding protein (CREB) phosphorylation as compared to BDNF Val/Val mice, suggesting that Met carriers are more vulnerable to stressful events. The Val66Met polymorphism is a major genetic influence on brain development and neuronal plasticity (44). Cattaneo et al. found significantly lower BDNF protein levels in Met carriers as compared to the Val/Val genotype, suggesting that this polymorphism plays an important role in individual differences in susceptibility to mental illness (45). Bueller reported a significantly increased susceptibility to various neuropsychiatric disorders among M allele carriers. Heterozygous populations carrying the M allele have reduced hippocampal volume and impaired learning memory. Compared to the VV genotype, M carriers were reported to have impaired situational memory, a common symptom in MDD (46). Other studies, however, concluded the opposite. Sen et al. studied a healthy population using the personality factor scale and found that trait anxiety and neuroticism scores were higher in VV genotypes as compared to M allele carriers, suggesting VV populations to be more prone to depression (47). A meta-analysis of the Val66Met gene polymorphisms revealed no statistically significant differences between healthy and depressed persons (48). Although findings reported by Gyekis revealed that the BDNF Val66Met gene polymorphism was not associated with susceptibility to depressive disorders, no further subgroup analysis was performed in that study and its findings thus lacked comprehensiveness. Thus, it is necessary to further investigate whether BDNF Val66Met polymorphism is associated with MDD susceptibility.
The literature used in our study on Caucasian populations included 11 articles, five from the United States and remaining six from Poland, Spain, Turkey, Germany, Israel, and Australia. The literature on Asian populations included 13 articles, 11 of which were from China and one each from the Republic of Korea and India. In this work, we retrieved 24 studies including 3,497 cases and 5,623 controls, thus studying a relatively large sample size that certainly reduced the probability of false negatives. Our findings revealed that the total effect of the BDNF Val66Met gene polymorphism was not significantly associated with MDD. However, a subgroup analysis revealed that the Met allele was associated with a genetic susceptibility to MDD in Caucasian populations, with statistically significant differences between dominant, recessive, and homozygous genotypes. Age was not found to associate with any susceptibility to MDD. No publication bias was noted in our study.
The present study suggests BDNF Val66Met gene polymorphism plays a role in major depressive disorder in Caucasian populations but no such pattern was found in Asian populations. We considered that the possible reasons for the conflicting results include changes in the frequency of the same gene mutation between populations, the impact of the same gene mutation on different races and ages, and the impact of different environments. The frequency of Met alleles in white populations is 25–32% (15), while the frequency of Met alleles in Asian populations is more frequent, about 40–50% (49, 50). There are differences in the lifetime prevalence of major depression between Western and Asian populations, with Asian populations having a lower lifetime prevalence of major depression. A cross-country epidemiological survey showed that the lifetime prevalence of MDD was 1.5% in Taiwan and 2.9% in the Republic of Korea (51), compared with an average of 15–17% for Western populations (52, 53). The high frequency of Met alleles in Asian populations is present in a lower prevalence of major depression, which may indicate that Met alleles protect against major depression. As the M allele occurs more frequently in Asian populations, low frequencies of the V allele and VV genotype likely weakened the association between the polymorphism and susceptibility to depression reported in prior studies (54). Zarza-Rebollo et al. (16) genotyped the BDNF Val66Met gene polymorphism in 3,124 people in the community, 209 of whom suffered from depression, and the incidence of MDD decreased under the interaction between Met allele carriers and the environment. Maria Skibinska (17) and Froud et al. (20) showed that the BDNF Val66Met gene polymorphism was not associated with BDNF serum levels, and this polymorphism did not seem to predict BDNF levels or the incidence of depression. However, Youssef et al. (18) and Taylor et al. (27) noted that Met carriers had a higher risk of depression, and Met allele carriers were almost twice as likely to develop depression in old age compared with pure-allele carriers of Val allele. Frodl et al. (26) suggested that Met allele carriers may be at risk of developing smaller hippocampal volumes and may have implications for vulnerability to MDD. Kanellopoulos et al. (34) believed that Met carriers develop depression at an earlier age than Val/Val homozygotes. Some studies have shown that the relationship between Val66Met polymorphism and MDD may be influenced by environmental factors; for example, negative childhood life events may moderate the relationship between Val66Met gene polymorphism and MDD, with a stronger association observed in individuals who have experienced negative life events (55). In addition, some environmental factors may influence the level and function of BDNF, which is related to the expression and function of Val66Met gene. For example, exercise, antidepressant medication, and dietary fat intake have been shown to influence BDNF level and function (56). Thus, future studies with larger Caucasian sample sizes are required to confirm our findings.
This meta-analysis has some limitations. Since our study only included literature published in Chinese and English, the possibility of publication bias cannot be ruled out. As the number of available case-control studies was relatively small, future meta-analyses evaluating larger samples are required to draw more accurate conclusions. Due to limited data, we were unable to stratify analysis according to factors such as years of education, sex, and exposure to various environmental factors. More precise assessments of the literature should be conducted in future meta-analyses.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
NL, ZZ, and ZS were responsible for the literature collection and collation. YW and OL processed the data and wrote the paper. JX revised the article. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Science and Technology Commission of Shanghai Municipality [grant number 22Y11920800], the National Key R&D Program of China [grant number 2018YFC2002500], the Shanghai Municipal Health Commission [grant number shslczdzk04901], the Shanghai Sailing Program [grant number 20YF1446100], and the Shanghai Municipal Hospital of Traditional Chinese Medicine [grant number WLJH2021ZY-MZY022].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rybak YE, Lai KSP, Ramasubbu R, Vila-Rodriguez F, Blumberger DM, Chan P, et al. Treatment-resistant major depressive disorder: Canadian expert consensus on definition and assessment. Depress Anxiety. (2021) 38:456–67. doi: 10.1002/da.23135
2. Trivedi M H. Major depressive disorder in primary care: strategies for identification. J Clin Psychiatry. (2020) 81:160–6689. doi: 10.4088/JCP.UT17042BR1C
3. Jiang C, Salton S. The role of neurotrophins in major depressive disorder. Transl Neurosci. (2013) 4:46–58. doi: 10.2478/s13380-013-0103-8
4. Pitsillou E, Bresnehan SM, Kagarakis EA, Wijoyo SJ, Liang J, Hung A, et al. The cellular and molecular basis of major depressive disorder: towards a unified model for understanding clinical depression. Mol Biol Rep. (2020) 47:753–70. doi: 10.1007/s11033-019-05129-3
5. Penner-Goeke S, Binder EB. Epigenetics and depression. Dialogues Clin Neurosci. (2019) 21:397–405. doi: 10.31887/DCNS.2019.21.4/ebinder
6. Carniel BP, da Rocha NS. Brain-derived neurotrophic factor (BDNF) and inflammatory markers: Perspectives for the management of depression. Prog Neuropsychopharmacol Biol Psychiatry. (2021) 108:110151. doi: 10.1016/j.pnpbp.2020.110151
7. Lu B, Nagappan G, Lu Y, BDNF. and synaptic plasticity, cognitive function, and dysfunction. Handb Exp Pharmacol. (2014) 220:223–50. doi: 10.1007/978-3-642-45106-5_9
8. Nitta A, Ohmiya M, Sometani A, Itoh M, Nomoto H, Furukawa Y, et al. Brain-derived neurotrophic factor prevents neuronal cell death induced by corticosterone. J Neurosci Res. (1999) 57:227–35.
9. Van Hook M J. Brain-derived neurotrophic factor is a regulator of synaptic transmission in the adult visual thalamus. J Neurophysiol. (2022) 128:1267–77. doi: 10.1152/jn.00540.2021
10. Hwang J-P, Tsai S-J, Hong C-J, Yang C-H, Lirng J-F, Yang Y-M. The Val66Met polymorphism of the brain-derived neurotrophic-factor gene is associated with geriatric depression. Neurobiol Aging. (2006) 27:1834–7. doi: 10.1016/j.neurobiolaging.2005.10.013
11. Chen Z, Jing D, Bath K G, Ieraci A, Khan T, Siao CJ, et al. Genetic Variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. (2006) 314:140–3. doi: 10.1126/science.1129663
12. Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry. 17:306–10. doi: 10.1016/s0924-9338(02)00654-5
13. Nguyen V T, Hill B, Sims N, Heck A, Negron M, Lusk C, et al. Brain-derived neurotrophic factor rs6265 (Val66Met) single nucleotide polymorphism as a master modifier of human pathophysiology. Neural Regen Res. (2022) 18:102. doi: 10.4103/1673-5374.343894
14. Lam P, Cheng C-Y, Hong C-J, Tsai S-J. Association study of a brain-derived neurotrophic factor (val66met) genetic polymorphism and panic disorder. Neuropsychobiology. (2004) 49:178–81. doi: 10.1159/000077362
15. Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am J Med Genet. (2004) 126B:122–3. doi: 10.1002/ajmg.b.20118
16. Zarza-Rebollo JA, Molina E, López-Isac E, Pérez-Gutiérrez AM, Gutiérrez B, Cervilla JA, et al. Interaction effect between physical activity and the bdnf val66met polymorphism on depression in women from the PISMA-ep study. Int J Environ Res Public Health. (2022) 19:2068. doi: 10.3390/ijerph19042068
17. Maria Skibinska AGPK. Val66Met functional polymorphism and serum protein level of brain-derived neurotrophic factor (BDNF) in acute episode of schizophrenia and depression. Pharmacological Rep. (2018) 70:55–9. doi: 10.1016/j.pharep.2017.08.002
18. Youssef MM, Underwood MD, Huang Y-Y, Hsiung S-C, Liu Y, Simpson NR, et al. Association of BDNF Val66Met polymorphism and brain BDNF levels with major depression and suicide. Int J Neuropsychopharmacol. (2018) 21:528–38. doi: 10.1093/ijnp/pyy008
19. Lee Y, Lim SW, Kim SY, Chung JW, Kim J, Myung W, et al. Association between the BDNF Val66Met polymorphism and chronicity of depression. Psychiatry Investig. (2013) 10:56. doi: 10.4306/pi.2013.10.1.56
20. Froud A, Murphy J, Cribb L, Ng CH, Sarris J. The relationship between dietary quality, serum brain-derived neurotrophic factor (BDNF) level, and the Val66met polymorphism in predicting depression. Nutr Neurosci. (2019) 22:513–21. doi: 10.1080/1028415X.2017.1415281
21. You J, Yuan Y, Shi Y, Zhang Z, Zhang X, Li H. Lack of association between BDNF Val66Met gene polymorphism and late-onset depression in a Chinese Han population. Acta Neuropsychiatr. (2009) 21:186–90. doi: 10.1111/j.1601-5215.2009.00401.x
22. Dandan L, Li K, Daqi L, Gan Y, Ai M, Gao X. Efficacy of modified electroconvulsive therapy and brain-derived neurotrophic factor gene polymorphism in patients with major depressive disorder. Chin J Mental Health. (2011) 25:93–7.
23. Ying W, Yuqi Z. Association of BDNF gene polymorphism with geriatric depression and cognitive functions. Mod Med. (2015) 43:1376−9. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=TDYX201511013&DbName=CJFQ2015
24. Ai M, Wang J, Chen J, Wang W, Xu X, Gan Y, et al. Plasma brain-derived neurotrophic factor (BDNF) concentration and the BDNF Val66Met polymorphism in suicide: a prospective study in patients with depressive disorder. Volume. (2019) 12:97–106. doi: 10.2147/PGPM.S201187
25. Shen Z, Lu Y, Jiang H, Ye J, Zhou C, He M, et al. Brain-derived neurotrophic factor Val66Met polymorphism affects cortical thickness of rostral anterior cingulate in patients with major depressive disorder. Neuroreport. (2020) 31:1146–53. doi: 10.1097/WNR.0000000000001528
26. Frodl T, Schüle C, Schmitt G, Born C, Baghai T, Zill P, et al. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry. (2007) 64:410–6. doi: 10.1001/archpsyc.64.4.410
27. Taylor WD, Züchner S, McQuoid DR, Steffens DC, Speer MC, Krishnan KRR. Allelic differences in the brain-derived neurotrophic factor Val66Met polymorphism in late-life depression. Am J Geriatric Psychiat. (2007) 15:850–7. doi: 10.1097/JGP.0b013e318050c9d5
28. Yu H. Association Between Cognitive Impairment and BDNF Gene Polymorphism in Chinese Han Adolescents With Depressive Disorder. Anhui Medical University, Hefei, China. (2022). doi: 10.26921/d.cnki.ganyu.2022.000825.000825
29. Xiaorui H,. The Relationship Between BDNF Gene Polymorphism Brain Structure in First-Episode Untreated Depression. Kunming Medical University, Kunming, China. (2017). Available online at: https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201801&filename=1017274193.nh
30. Jiangyue P. Association between Single Nucleotide Polymorphism Depression. Zhengzhou University, Zhengzhou, China. (2014). Available online at: https://kns.cnki.net/kcms2/article/abstract?v=30M9_8jKqe1lx78p8dN_MSbG7o2saS1WPoLpL9p_H9yEVa3Fzzq8m0L-bo7uNMg0zS-ICJzfPP2BMZE0iXdmnDbHwXChAR4h39_nk9SNq3nsOecltDs4Ucp2yLKhbbwhtzHQ7YDcquQ=&uniplatform=NZKPT&language=CHS
31. Chunxia Y,. BDNF, PKB GSK-3β Association Between Gene Polymorphism Major Depressive Disorder. Shanxi Medical University, Jinzhong, China. (2009). Available online at: https://kns.cnki.net/kcms2/article/abstract?v=30M9_8jKqe2SmVie6Q8YxwL0T8OkoAJ9gHfkN7ntVIidLmmiq8vIZW2jRZqrjaeG1hwq6__NP00uv2z_1KKmVT0_7UomYmq3wT7N3Uwux2EdlL81E6nj_inMXa9CcTTb&uniplatform=NZKPT&language=CHS
32. Yin Y, Hou Z, Wang X, Sui Y, Yuan Y. The BDNF Val66Met polymorphism, resting-state hippocampal functional connectivity and cognitive deficits in acute late-onset depression. J Affect Disord. (2015) 183:22–30. doi: 10.1016/j.jad.2015.04.050
33. Benjamin S, McQuoid DR, Potter GG, Payne ME, MacFall JR, Steffens DC, et al. The brain-derived neurotrophic factor Val66Met polymorphism, hippocampal volume, and cognitive function in geriatric depression. Am J Geriat Psychiat. (2010) 18:323–31. doi: 10.1097/JGP.0b013e3181cabd2b
34. Kanellopoulos D, Gunning FM, Morimoto SS, Hoptman MJ, Murphy CF, Kelly RE, et al. Hippocampal volumes and the brain-derived neurotrophic factor val66met polymorphism in geriatric major depression. Am J Geriat Psychiat. (2011) 19:13–22. doi: 10.1097/JGP.0b013e3181f61d62
35. Taylor WD, Züchner S, McQuoid DR, Payne ME, MacFall JR, Steffens DC, et al. The brain-derived neurotrophic factor VAL66MET polymorphism and cerebral white matter hyperintensities in late-life depression. Am J Geriat Psychiat. (2008) 16:263–71. doi: 10.1097/JGP.0b013e3181591c30
36. Ozan E, Okur H, Eker C, Eker OD, Gönül AS, Akarsu N. The effect of depression, BDNF gene val66met polymorphism and gender on serum BDNF levels. Brain Res Bull. (2010) 81:61–5. doi: 10.1016/j.brainresbull.2009.06.022
37. Haldar S, Roy S, Sen S, Dasgupta A, Ghosh S. Association of the Val66Met polymorphism of the BDNF gene and the deletional mutation of CYP2D6 gene with the prevalence and severity of depressive disorder in an Eastern Indian population. Indian J Psychiatry. (2022) 64:269–76. doi: 10.4103/indianjpsychiatry.indianjpsychiatry
38. Kreinin A, Lisson S, Nesher E, Schneider J, Bergman J, Farhat K, et al. Blood BDNF level is gender specific in severe depression. PLoS ONE. (2015) 10:e0127643. doi: 10.4103/indianjpsychiatry.indianjpsychiatry_541_21
39. Sun N, Yang C-X, Liu Z-F, Li X-R, Xu Y, Zhang K-R. Effects of polymorphisms of serotonin transporter promoter (5-HTTLPR) and brain derived neurotrophic factor gene (G196A rs6265) on the risk of major depressive disorder in the Chinese Han population. Eur Rev Med Pharmacol Sci. (2016) 20:1852−9.
40. Zhang E, Liao P. Brain-derived neurotrophic factor and post-stroke depression. J Neurosci Res. (2020) 98:537–48. doi: 10.1002/jnr.24510
41. Kessing M V J D. Are variations in whole blood BDNF level associated with the BDNF Val66Met polymorphism in patients with first episode depression. Psychiatry Res. (2013) 210:102–8. doi: 10.1016/j.psychres.2013.04.023
42. van Calker D, Serchov T, Normann C, Biber K. Recent insights into antidepressant therapy: Distinct pathways and potential common mechanisms in the treatment of depressive syndromes. Neurosci Biobehav Rev. (2018) 88:63–72. doi: 10.1016/j.neubiorev.2018.03.014
43. Musazzi L, Tornese P, Sala N, Lee FS, Popoli M, Ieraci A. Acute stress induces an aberrant increase of presynaptic release of glutamate and cellular activation in the hippocampus of BDNFVal/Met mice. J Cell Physiol. (2022) 237:3834–44. doi: 10.1002/jcp.30833
44. Pezawas L. The brain-derived neurotrophic factor Val66met polymorphism and variation in human cortical morphology. J Neurosci. (2004) 24:10099–102. doi: 10.1523/JNEUROSCI.2680-04.2004
45. Cattaneo A, Bocchio-Chiavetto L, Zanardini R, Marchina E, Bellotti D, Milanesi E, et al. BDNF Val66Met polymorphism and protein levels in amniotic fluid. BMC Neurosci. (2010) 11:16. doi: 10.1186/1471-2202-11-16
46. Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmeister M, Zubieta J-K. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. (2006) 59:812–5. doi: 10.1016/j.biopsych.2005.09.022
47. Sen S, Nesse R M, Stoltenberg S F, et al. A BDNF coding variant is associated with the NEO personality inventory domain neuroticism, a risk factor for depression. Neuropsychopharmacology. (2003) 28:397–401. doi: 10.1038/sj.npp.1300053
48. Gyekis JP, Yu W, Dong S, Wang H, Qian J, Kota P, et al. No association of genetic variants in BDNF with major depression: A meta- and gene-based analysis. Am J Med Genet B Neuropsychiatr Genet. (2013) 162:61–70. doi: 10.1002/ajmg.b.32122
49. Choi MJ, Kang RH, Lim SW, Oh KS, Lee MS. Brain-derived neurotrophic factor gene polymorphism (Val66Met) and citalopram response in major depressive disorder. Brain Res. (2006) 1118:176–82. doi: 10.1016/j.brainres.2006.08.012
50. Itoh K, Hashimoto K, Kumakiri C, Shimizu E, Iyo M. Association between brain-derived neurotrophic factor 196 G/A polymorphism and personality traits in healthy subjects. Am J Med Genet B Neuropsychiatr Genet. (2004). 124B:61–3. doi: 10.1002/ajmg.b.20078
51. Weissman MM, Bland RC, Canino GJ, et al. Cross-national epidemiology of major depression and bipolar disorder. JAMA. (1996) 276:293–9. doi: 10.1001/jama.276.4.293
52. Bijl RV, Ravelli A, van Zessen G. Prevalence of psychiatric disorder in the general population: results of The Netherlands Mental Health Survey and Incidence Study (NEMESIS). Soc Psychiatry Psychiatr Epidemiol. (1998) 33:587–95. doi: 10.1007/s001270050098
53. Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the national comorbidity survey. Arch Gen Psychiatry. (1994) 51:8–19. doi: 10.1001/archpsyc.1994.03950010008002
54. Xuyi L, Weiwei Q, Pengfei Z, Jiang H, Qian Y, Zong Y, et al. Meta-analysis of BDNF Val66Met gene polymorphism in patients with depression and the efficacy of antidepressants. J Mudanjiang Med Coll. (2018) 39:21–6. doi: 10.13799/j.cnki.mdjyxyxb.2018.03.006
55. Zhao M, Chen L, Yang J, Han D, Fang D, Qiu X, et al. BDNF Val66Met polymorphism, life stress and depression: A meta-analysis of gene-environment interaction. J Affect Disord. (2018) 227:226–235. doi: 10.1016/j.jad.2017.10.024
Keywords: BDNF Val66Met, polymorphism, major depressive disorder, systematic review, meta-analysis
Citation: Wang Y, Li O, Li N, Sha Z, Zhao Z and Xu J (2023) Association between the BDNF Val66Met polymorphism and major depressive disorder: a systematic review and meta-analysis. Front. Psychiatry 14:1143833. doi: 10.3389/fpsyt.2023.1143833
Received: 13 January 2023; Accepted: 01 June 2023;
Published: 21 June 2023.
Edited by:
Sarah Tarbox-Berry, Wayne State University, United StatesReviewed by:
Piyush Pathak, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaQiang Wang, Sichuan University, China
Copyright © 2023 Wang, Li, Li, Sha, Zhao and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Xu, eHVqaWFuMDI5NkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Yuxia Wang
Yuxia Wang Ou Li
Ou Li Nana Li
Nana Li Zhongwei Sha
Zhongwei Sha Zhenghao Zhao
Zhenghao Zhao Jian Xu
Jian Xu