
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 06 April 2023
Sec. Psychopharmacology
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1130636
This article is part of the Research Topic Safety and Side Effects of Psychotropic Medications, Volume II View all 11 articles
 Giuseppe Cicala1*
Giuseppe Cicala1* Renato de Filippis2†
Renato de Filippis2† Maria Antonietta Barbieri1
Maria Antonietta Barbieri1 Paola Maria Cutroneo3
Paola Maria Cutroneo3 Pasquale De Fazio2†
Pasquale De Fazio2† Georgios Schoretsanitis4,5,6
Georgios Schoretsanitis4,5,6 Edoardo Spina1,3
Edoardo Spina1,3Introduction: Long-acting injectable antipsychotics (LAIs) have proven to be effective in the maintenance treatment of patients suffering from schizophrenia, and their safety and tolerability profiles represent a key factor in their long-term use and choice in clinical practice. Paliperidone palmitate (PP) is the only second-generation LAI (SGA-LAI), available in both one- (PP1M) and 3-month (PP3M) formulations. However, real-world prospective studies on PP1M and PP3M are still few and mostly conducted on small samples. In this context, we aimed to better define the safety and tolerability profile of PP using real world pharmacovigilance data.
Methods: We retrospectively analyzed the publicly available data regarding Individual Case Safety Reports (ICSRs), presenting PP1M and/or PP3M as suspected drugs, reported on EUDRAVigilance between 2011 and June 30th, 2022. ICSRs relative to at least one SGA-LAI other than PP, reported between 2003 and June 30th, 2022, were also examined as reference group. Data were evaluated with a descriptive analysis, and then, as disproportionality measures, crude reporting odds ratio (ROR) and 95% confidence interval (CI) were calculated.
Results: A total of 8,152 ICSRs met the inclusion criteria, of those 77.7% (n = 6,332) presented as suspected drug PP1M, 21.2% (n = 1,731) PP3M, while 89 cases indicated both PP1M and PP3M. Significantly higher probabilities of reporting in PP-related reports were observed for the primary Standardized MedDRA Queries “Sexual Dysfunctions” (ROR = 1.45; 95% CI 1.23-1.70), “Haemodynamic oedema, effusions and fluid overload” (ROR = 1.42; 1.18-1.70), as well as “Fertility disorders” (ROR = 2.69; 1.51-4.80).
Discussion: Our analysis indicates that the tolerability and safety profiles of PP are in line with what is known for the other SGA-LAIs. However, differences regarding endocrine system ADRs have been noticed. The results presented in this work do not discourage the prescription of SGA-LAI formulations but aim to enhance their safety.
Antipsychotic medications represent the mainstay of the pharmacological treatment of schizophrenia (SCZ) (1). They are commonly categorized into three drug classes, first- (FGAs), second- (SGAs) and third-generation antipsychotics (TGAs) (2). Poor adherence to antipsychotic treatment is a critical aspect of the clinical management of patients affected by schizophrenia spectrum disorders (SSDs). In addition, treatment discontinuation represents a relevant risk factor for relapse and rehospitalization (3–6). To improve antipsychotic adherence in patients affected by SCZ in the 1960s the long-acting injectable (LAI) antipsychotic formulations, initially based on FGAs (FGA-LAIs), were introduced (7, 8).
The LAI formulations have proven to reduce the risk of relapse and re-hospitalization due to non-adherence (9). This makes them valuable therapeutic options for the long-term management of patients suffering from SSDs (10–13). Furthermore, robust literature evidence suggests that LAIs may also provide an effective treatment strategy for patients in the early-phase or with a first-episode of psychosis (FEP) (12, 14–16).
As for their oral counterparts, FGA-LAIs have been gradually less prescribed due to the risk of extrapyramidal symptoms and tardive dyskinesia (17, 18). However, over the past 20 years, several SGAs, including olanzapine, risperidone, and paliperidone, and one TGA, aripiprazole, have become available, partially replacing FGA-LAIs thanks to a lower liability for movement disorders (19). There are considerable differences between second-generation LAIs (SGA-LAIs) regarding pharmacodynamic and pharmacokinetic profiles, injection interval, cost, requirements for oral supplementation, and risks of adverse events (20, 21). Safety profiles of SGA-LAIs generally follow the known profiles of the oral molecule, although unexpected safety signals were occasionally observed in clinical practice (22).
Among the currently available SGA-LAIs, paliperidone palmitate (PP) (the esterified form of paliperidone, an active metabolite of risperidone) is the only one already available in both a monthly (PP1M) and a quarterly formulation (PP3M), with a recently approved 6-month PP (PP6M) formulation (23). In particular, the PP3M formulation has shown significant efficacy in delaying the time to relapse in patients suffering from SCZ (24, 25). Candidates for PP3M are patients previously prescribed PP1M (26). In other words, patients introduced to PP3M have been previously exposed PP1M, which they may tolerate well before clinicians switch them to PP3M. This could be related to the low incidence of adverse drug reactions (ADRs) (27).
The aim of the present study was to analyze the ADRs related to PP1M and PP3M and to compare them to those related to the other SGA-LAIs, in the Spontaneous Reporting System (SRS) database (i.e., European Union Drug Regulating Authorities Vigilance database; EUDRAVigilance) of the European Medicines Agency (EMA).
Data on Individual Case Safety Reports (ICSRs) presenting as suspected drugs LAI formulations of PP (i.e., PP1M and/or PP3M) were retrieved using the EUDRAVigilance access platform (publicly available at www.adrreports.eu). EUDRAVigilance functions as a management and analysis platform for information on suspected ADRs regarding drugs that have obtained marketing authorization or are currently under evaluation in clinical trials across the European Economic Area (EEA). More specifically, EUDRAVigilance represents the collection point for all the ICSRs (regarding either drugs or vaccines), reported by healthcare professionals (HCPs) and non-HCP figures to any of the European Union (EU) competent authorities at the national level or the marketing authorization holder. The EU medicines regulatory network, in the form of the EMA, acts as the responsible authority for the maintenance and constant update of EUDRAVigilance. For transparency’s sake, data collected on EUDRAVigilance are publicly available through the previously cited access portal. Data are made available in different tiers of completeness, with the more specific ones requiring access authorization directly licensed from the EMA. The data access level used for the analysis was the one indicated as “Stakeholder Group II: Healthcare professionals, patients and the general public” in the EUDRAVigilance access policy (28).
All ICSRs reported as suspected drugs LAI formulations of PP were retrieved using the line-listing function of the EUDRAVigilance platform. The timeframe used for report collection spanned between January 1st, 2011 (the year of the first market approval for PP1M) and June 30th, 2022. The reference Group (RG) for the analyses was constituted by ICSRs showing at least one SGA-LAI other than PP (i.e., LAI formulations of aripiprazole, olanzapine, and risperidone) as suspected drugs reported to EUDRAVigilance between January 1st, 2003, the year of commercialization of risperidone LAI, and June 30th, 2021. The authors acknowledge that aripiprazole belongs to the class of TGAs (2). However, concerning LAI formulations, a number of literature sources enlist aripiprazole-based LAIs as part of SGA-LAIs (29, 30). Thus, after careful consideration, to improve the applicability of the analysis results, aripiprazole LAI-related ICSRs were considered in the reference group. The retrieved dataset included the following fields: ICSR identification number in EUDRAVigilance; date of receipt; primary source qualification; the presence of an eventual literature reference; patients’ sex and age group; ADRs characteristics (type of ADR, duration, outcome, and seriousness status) and characteristics of suspected and concomitant drugs (Type of drug, use indication, duration of therapy, drug dose and administration route). The level of data completion varied for each ICSR. Once retrieved, ICSRs identified as “non-spontaneous,” ICSRs linked to literature sources, and ICSRs that presented as suspected drugs vaccines have all been excluded.
Data regarding the available demographic characteristics of patients (i.e., sex and age group) were evaluated by means of a descriptive analysis. The descriptive analysis also included adverse event characteristics (i.e., outcome and seriousness), primary source qualification, and the number of suspected drugs other than the LAI formulations of PP. The latter is described cumulatively for all PP-related and for each PP formulation. In addition to that, the annual trend in ICSRs reporting was also evaluated. All the ADRs were classified in accordance with the Medical Dictionary for Regulatory Activities (MedDRA®), which follows a hierarchical structure in which terms are organized into five levels. Observations are codified, at first with more specific lowest-level terms (LLT), to resemble the clinical condition reported closely. Multiple LLTs converge into only one “preferred term” (PT) representing the next structural level. Several PTs can then be grouped using anatomical, pathological, physiological, etiological, or functional criteria in “High-Level Terms” (HLTs). HLTs can then be categorized in “High-Level Group Terms” (HLGTs). Finally, the highest-level terms of this classification are represented by the so-called “System Organ Classes” (SOCs), which provide a broader data overview. As far as seriousness was concerned, a case was defined as ‘serious’ when highlighted at least an ADR resulting in death, hospitalization, persistent or significant disability/incapacity, congenital anomaly/birth defect or conditions deemed as medically important by the reporter, prolonging hospitalization or being life-threatening. For the ADRs outcomes standardized terminology was used with ADRs classified as: ‘recovered/resolved’, ‘recovering/resolving’, ‘recovered/resolved with sequelae’, ‘not recovered/not resolved’, ‘fatal’, and ‘unknown’ on the bases of what was reported in the ICSR. The ADR expectedness was verified based on the Summary of Product Characteristics (SmPCs) available in the EMA database (31). If two or more ADR Symptoms reported in the same ICSR presented different outcomes a global outcome for the case described in the ICSR was computed using the “Lower Level of Resolution” methodology previously described by other authors (32).
For ICSRs characteristics comparisons, we used the Chi-square test and the U Mann–Whitney test for categorical and continuous variables, respectively. The distribution of variables was tested using Shapiro-Wilks and Kolmogorov–Smirnov tests. Continuous variables were reported as median values with associated interquartile ranges (IQRs). Categorical variables were synthesized as frequencies and percentages. ICSRs simultaneously involving a PP-based formulation and another SGA-LAI as suspected drugs were excluded from comparisons between the two groups. The Chi-square test was applied to evaluate differences for ADR characteristics between PP-related ICSRs and the reference group. Values of p < 0.001 were considered statistically significant.
Disproportionalities in the observed ADR frequencies for PP-related ICSRs compared to those of ICSRs presenting as suspected drugs other SGA-LAIs were evaluated by calculating the Reporting Odds Ratios (RORs) and associated 95% confidence intervals (95% CI). The statistical significance threshold was defined as 95% CI lower bound >1 in the presence of ≥3 reports per PP formulation. For ADR regrouping purposes, we used the standardized MedDRA® queries (SMQs), which are groups of MedDRA® terms related to a defined medical condition or area of interest (33). Regrouping terms by SMQs can be done by using either ‘narrow’ or ‘broad’ search strategies. For this analysis, we used the more narrow-scope approach, with terms characterized by a higher likelihood of representing the condition of interest (34). In addition, a sub-analysis using the Chi-square test methodology was performed to compare the ADR reporting frequencies between PP3M and PP1M. All the analyses were carried out using the Statistical Package for Social Science (SPSS, International Business Machines Corporation) Version 28.
Overall, 20,226 ICSRs related to SGA-LAIs were retrieved from the EUDRAVigilance dataset during the observation period. Of those, 8,152 ICSRs indicated PP-based formulations as suspected culprit drugs. Among the PP-related reports, 6,332 (77.7%) presented as suspected drug PP1M and 1,731 (21.2%) PP3M, while 89 ICSRs indicated involvement of both PP formulations. As far as the ICSRs in the reference group were concerned, risperidone LAI was the SGA-LAI most frequently reported (n = 5,317; 43.7%), followed by aripiprazole LAI (n = 4,038; 33.2%) and olanzapine pamoate (n = 2,802; 23%). In the ICSRs for the reference group, 13 cases presenting referred to patients treated with multiple SGA-LAIs. In addition, 96 retrieved cases simultaneously involved a PP-based formulation and another SGA-LAI as suspected drugs. For PP-related ICSRs an initially steady trend was followed by a peak in 2018 (n = 1,569) after the introduction of PP3M in the market and a decreasing trend afterwards (Figure 1).
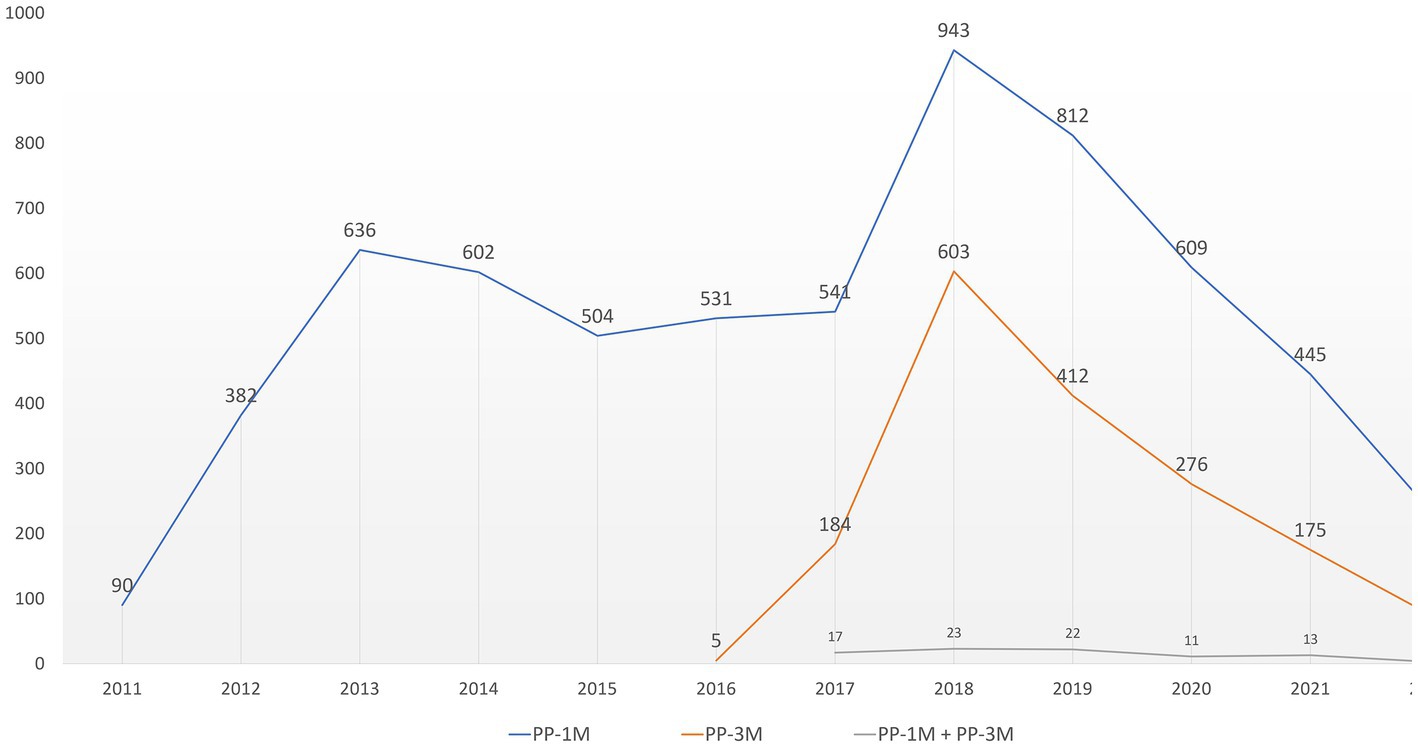
Figure 1. PP-related ICSRs temporal distribution. ICSRs, Individual Case Safety Reports; PP, paliperidone palmitate; PP1M, paliperidone palmitate 1-month; PP3M, paliperidone palmitate 3-month.
PP injection dose data were available in 6,312 (75.2%) ICSRs. The mean observed dose for PP-based formulations was 121,2 mg (±39 SD) for PP1M and 383,9 mg (±132.8 SD) for PP3M. Data for PP treatment duration were available in 430 (5.3%) ICSRs with a median PP treatment duration of 120 days for PP1M (IQR 31–337) and 244 (IQR 91–452) days for PP3.
Treatment indication information for PP based formulations were available in 57.1% (n = 4,655) of ICSRs. Among those, SCZ was the most frequently observed (n = 3,486; 74.9%), followed by psychotic disorders (n = 446; 9.6%), and schizoaffective disorders (n = 253; 5.4%). Table 1 summarizes the main characteristics of PP-related ICSRs compared to those related to the other SGA-LAIs.
Considering the suspected drugs other than PP, 36.8% (n = 3,082) of all PP-related ICSRs presented at least an additional suspected drug. A median value of 1 (IQR 1–2) for the number of co-reported suspected drugs was reported. Stratifying ICSRs by PP formulation, the number of co-reported suspected drugs remained constant for PP1M and PP3M-related ICSRs with the PP3M-related ones exhibited a narrower IQR (1-1). In qualitative terms the most frequently co-reported suspected drugs 65.4% (n = 1,311) belonged to the N05A ATC class (i.e., antipsychotics) namely, risperidone (n = 516; 39.4%), olanzapine (n = 125; 9.5%), and aripiprazole (n = 117; 8.9). Following the N05A was the N03A class (i.e., antiepileptics) (n = 120; 6%), with valproic acid (n = 71; 59.2%), clonazepam (n = 14; 11.7%), and lamotrigine (n = 10; 8.3%). After that, the N06A class drugs (i.e., antidepressants) had the higher frequency (n = 106; 5.3%), namely, escitalopram (n = 15; 14.2%), sertraline (n = 13; 12.3%), and paroxetine (n = 12; 11.3%). More details on the distribution of suspect drugs groups according to the ATC classification, per single PP-derived formulation is available in the Electronic Supplementary Material (ESM) Table 1.
In terms of ADR seriousness, 64.6% (n = 5,264) of PP-related ICSRs indicated at least one ADR classifiable as serious, less frequently than in the reference group (n = 10,091; 64.6%, p < 0.001). Outcome data were available in 52.3% of PP-related ICSRs. In detail 1,543 cases (36.2%) described one ADR deemed as “Recovered/Resolved,” 1,344 (31.5%) cases one labelled as “Not Recovered/Not Resolved,” and 856 (20.1%) one ADR that was still “Recovering/Resolving” at the time of the last available follow-up (Table 1).
ADRs observed in PP-related ICSRs mainly concerned the SOCs “Psychiatric disorders” (n = 2,898; 19.3%), “General disorders and administration site conditions” (n = 2,608; 17.4%), “Nervous system disorders” (n = 1946; 13.0%), “Injury, poisoning and procedural complications” (n = 1,321; 8.8%), and “Investigations” (n = 1,554 179; 7.9%).
ADRs labelled “Endocrine disorders” were more frequently reported in PP-related ICSRs, compared to the reference group (Table 2). The specific ADRs related to this SOC, classified at the MedDRA PT level for PP were hyperprolactinaemia (n = 226; 88.6%), followed by inappropriate antidiuretic hormone secretion (n = 9; 3.5%), hypothyroidism (n = 4; 1.6%), thyroid disorder (n = 3; 1.2%), and diabetes insipidus (n = 2; 0.8%).
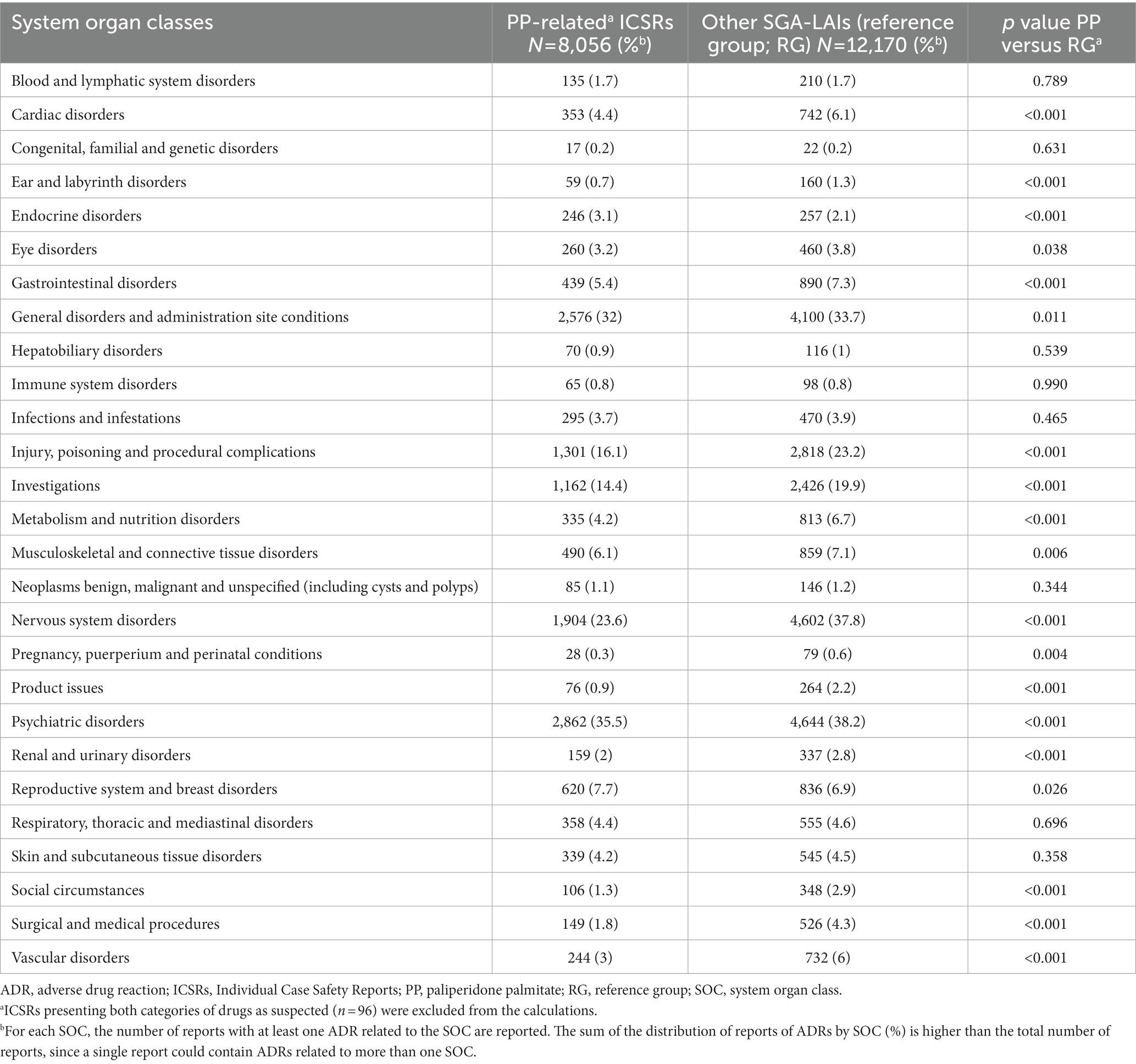
Table 2. Relative ADRs frequencies observed in PP-related ICSRs formulations as compared to reference group ICSRs, stratified by system organ class.
There were 468 ICSRs reporting fatal outcomes. Most of them (n = 303; 64.7%) regarded male patients, and 330 cases (70.5%) were in the 18 to 64 years age group. The number of reported suspected drugs other than PP in this ICSRs was higher when compared to all other PP related ICSR (2.4 ± 2.1 SD vs. 1.6 ± 1.3 SD; p < 0.001) without however, major differences in terms of the type of co-reported suspected drugs. In these ICSRs the most frequently observed ADRs were related to the MedDRA HLTs “Death and sudden death” (n = 181; 17.4%), “Suicidal and self-injurious behavior” (n = 103; 9.9%), “Ischemic coronary artery disorders (n = 26; 2.5%),” “Ventricular arrhythmias and cardiac arrest” and “Product administration errors and issues” (both with n = 24; 2.3%). Among the specific ADRs leading to fatal outcomes those observed with higher frequencies were, aside from death (n = 129; 21.8%) and sudden death (n = 47; 7.9%), completed suicide (n = 98; 16.5%), pulmonary embolism (n = 16; 2.7%), myocardial infarction (n = 14; 2.4%), cardiac failure (n = 13; 2.2%), and cardio-respiratory arrest (n = 12; 2%).
Comparing to PP1M-related ICSRs, the PP3M-related ICSRs more frequently contained the SOCs “psychiatric disorders,” “general disorder and administration site conditions,” and “product issues” (p < 0.001). The relative reporting frequencies for the 10 major SOCs are reported in Figure 2, while full details are available in Table 3. In PP3M-related ICSRs, the specific ADRs more frequently reported as “psychiatric disorders” were SCZ (n = 174; 16.7%), psychotic disorder (n = 97; 9.3%), psychotic symptom (n = 69 6.6%), delusion (n = 54; 5.2%), psychiatric decompensation (n = 53; 5.1%), and anxiety (n = 47; 4.5%). The specific ADRs in PP3M ICSRs, relative to “general disorder and administration site conditions” were mainly “drug ineffective” (n = 158; 20.8%), “condition aggravated” (n = 101; 13.3%), “malaise” (n = 49; 6.5%), “fatigue” (n = 48; 6.3%), and “injection site pain” (n = 36; 4.7%). While for the SOC “product issues” the ADRs observed with the highest frequency in PP3M ICSRs were “device occlusion” (n = 7; 17.9%), “syringe issue” (n = 6; 15.4%), “product complaint” (n = 6; 15.4%), “needle issue” (n = 5; 12.8%), and “product quality issue” (n = 4; 10.3%). More details on specific ADRs related to each SOC at the MedDRA PT level is available in Electronic Supplementary Material (ESM) Table 2.
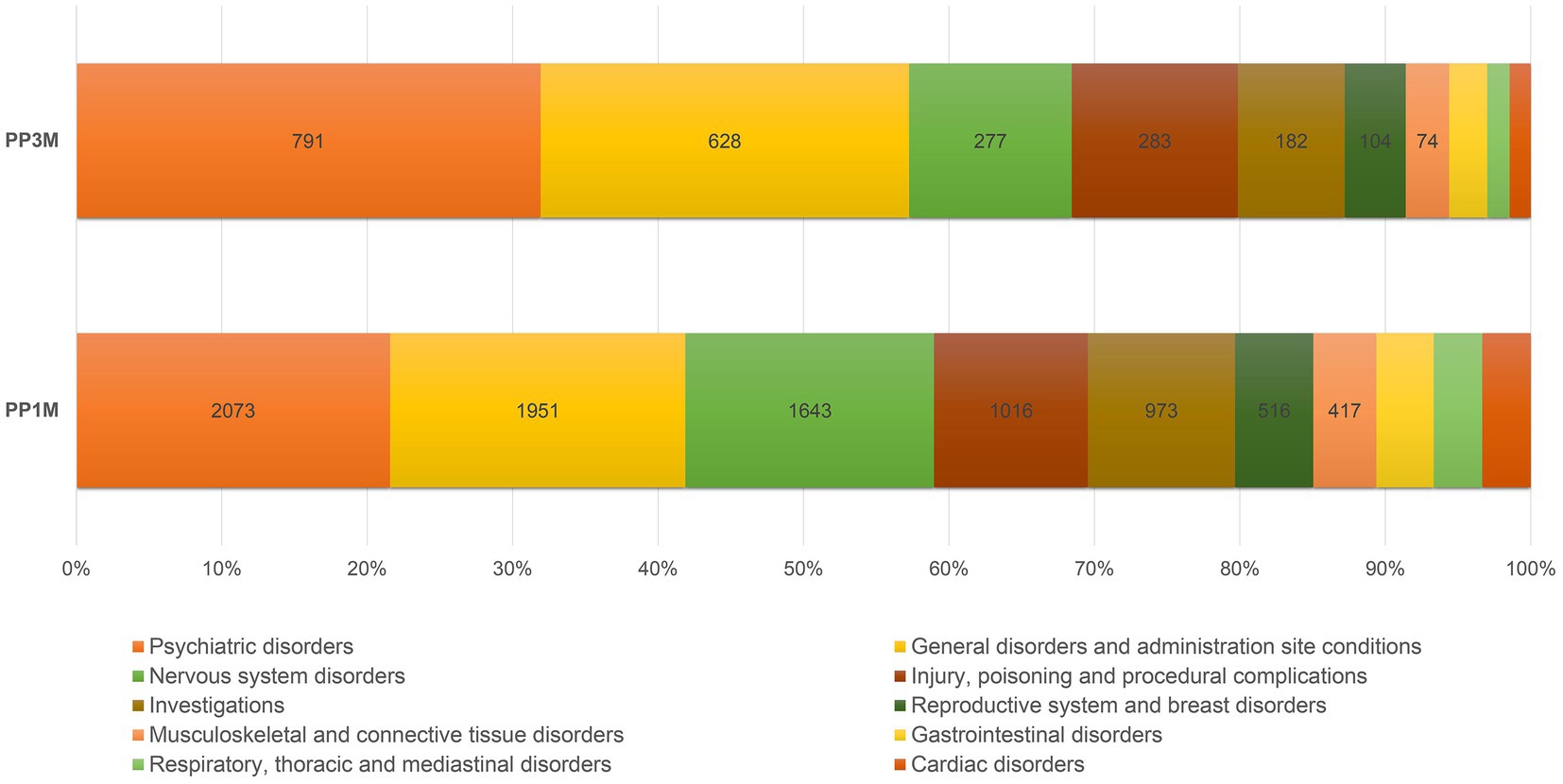
Figure 2. Relative reporting frequencies of ADRs belonging to the 10 most frequently observed SOCs. ADR, adverse drug reaction; PP1M, paliperidone palmitate 1-month; PP3M, paliperidone palmitate 3-month; SOCs, system organ classes.
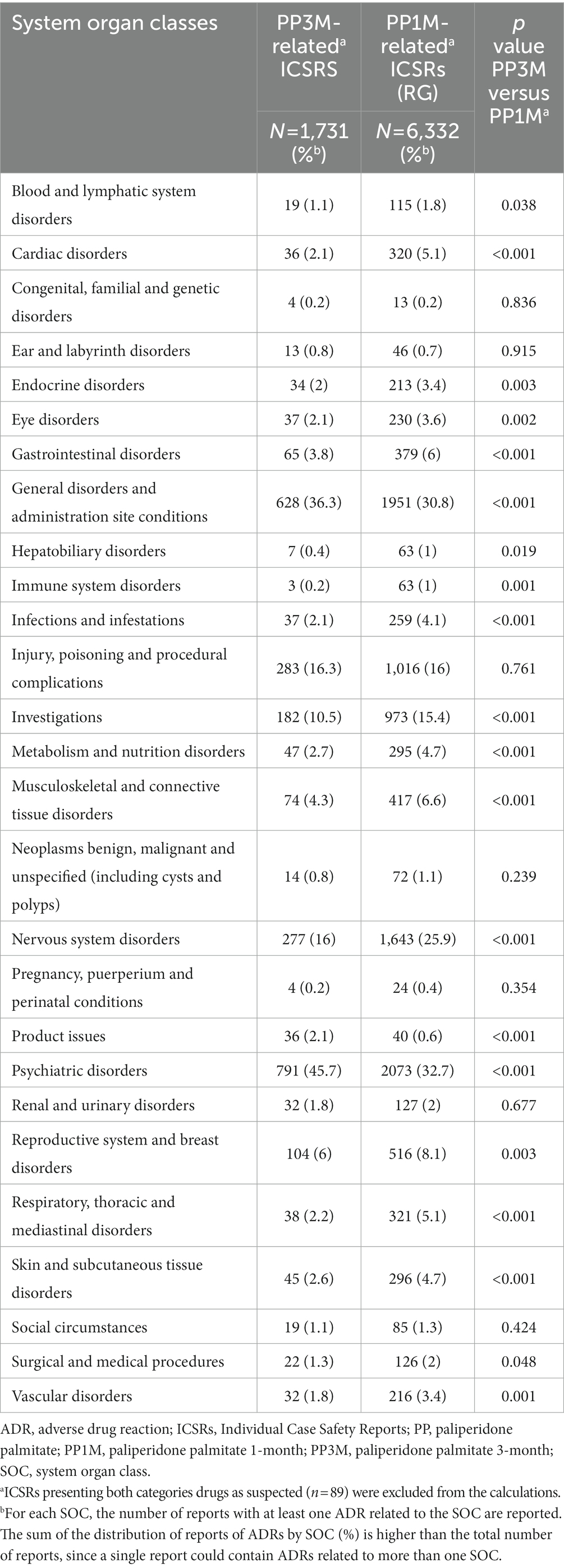
Table 3. Relative ADRs frequencies observed in PP3M-related ICSRs formulations as compared to PP1M-related ICSRs, stratified by system organ class.
Significantly disproportionate reporting, for PP-related reports compared to the reference group, was observed for SMQs “Sexual Dysfunctions” (ROR = 1.45; 95% CI 1.23–1.70), “Haemodynamic oedema, effusions and fluid overload” (ROR = 1.42; 1.18–1.70), as well as “Fertility disorders” (ROR = 2.69; 1.51–4.80) (Table 4). In terms of secondary SMQs only “Parkinson-like events” (ROR = 1.27; 1.06–1.53) were disproportionately reported for PP formulations compared to the reference group Electronic Supplementary Material (ESM) (Table 3).
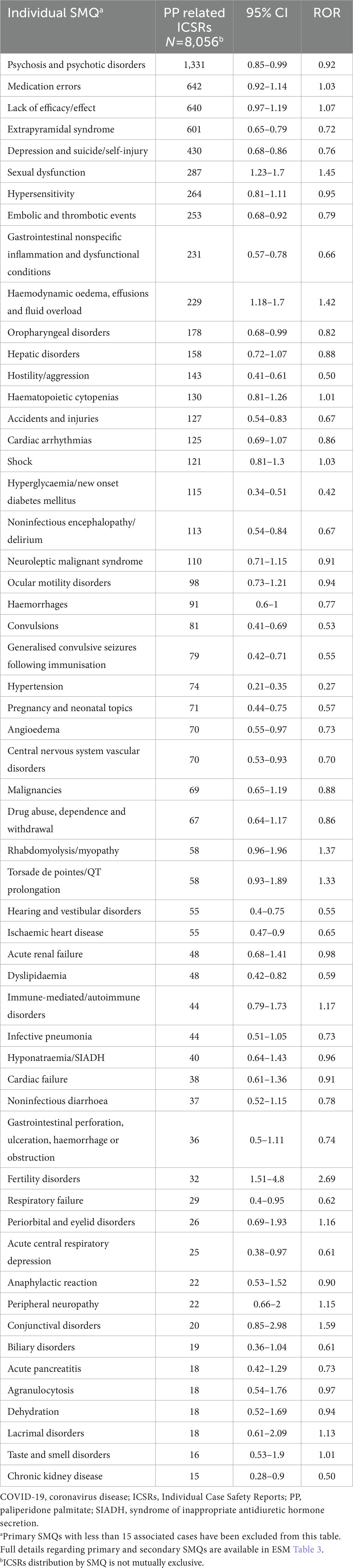
Table 4. Reporting odds ratios for PP-related ICSRs as compared to RG using standardized MedDRA queries.
Among SGA-LAIs PP is the only one currently available not only in a monthly but also a quarterly and more recently a half-yearly administration formulation, thus making it one of the most interesting therapeutic options to maintain treatment adherence in long-term treatment of patients suffering from SCZ (35). Therefore, the constant rising number of ICSRS per year observed in our analysis reflects this continuous level of increasing attention on PP since its market introduction. Furthermore, the ICSRs reporting peak of 2018 following the market introduction of the PP3M formulation shows that this event also had an increasing effect on the yearly reporting frequency of the PP1M-related ICSRs. Thus, we expect an increase for ADRs reports in the coming years following the introduction of the six-monthly formulation as use and clinical experience increase. It must be pointed out however, that the market approval process of SGA-LAIs has not happened simultaneously in all the countries covered by the EUDRAVigilance database. Moreover, differences in the availability of these drugs still persist today.
As far as patient characteristics are concerned, the observed differences in terms of reported patients’ age, between PP and RG-related ICSRs, seem to be in line with routinely clinical practice. PP-based formulations have been introduced more recently than the other LAIs which makes them less likely to be selected by clinicians for treating patients before the age of 18. Also, the lack of EMA-approved indications for their use in pediatric patients limits the use of both PP1M and PP3M in this context (36, 37). The observed differences in terms of reported patient sex may be more attributable to ADRs commonly associated with PP than to effective sex differences in tolerability. In fact, ADRs related to prolactin increases are frequently observed with PP, but they could be considered more in women as they are clinically more impactful (e.g., amenorrhea). This could lead to considering more carefully the administration of PP based in women and by consequence to an observation bias. However, our findings prevent us from formulating any conclusion in this regard.
The data regarding the types of co-reported suspected drugs highlight that almost 40% of ICSRs involved at least one co-medication. Most of the observed co-reported suspected drugs were oral antipsychotics. Adding an oral antipsychotic to LAI-based therapeutic regimens is a common practice in the initial phases to mitigate risks related to the slow release of the LAI formulations (38). Also, antipsychotic polypharmacy (APP) is frequently used in clinical practice. It has been estimated that 10–20% of outpatients and up to 40% of inpatients diagnosed with SCZ are treated with APP mainly as augmentation (39). The frequent combination of PP with mood stabilizers and/or antidepressants and benzodiazepines in the relevant ICSRs highlights the risks that may emerge in the context of such therapeutic regimens (40).
Regarding seriousness of ADRs, PP-related ICSRs were less frequently serious compared to other SGA-LAIs. This is in line with findings from other types of literature that highlighted overall good tolerability for PP when compared to other SGA-LAIs (41). As far as ADR outcomes are concerned, significantly higher (p < 0.001) frequencies of cases describing ADRs deemed as “recovering/resolving” were observed in PP-related ICSRs when compared to the reference group. Significant differences but in a diminutive sense were observed for ADR cases with a complete recovery and with reactions not resolved at the time of the last follow-up between ICSRs PP-related and in the reference group. This data correlates well with the type of observed ADRs in PP-related ICSRs as several of the most frequently observed ADRs such as those relative to “Psychiatric disorders” and “Nervous system disorders” are generally characterized by long resolution periods (e.g., literature sources report a median of 91 days for extrapyramidal symptoms) (42, 43).
In terms of specific ADRs, “Psychiatric disorders” related ones were mainly associated to the onset of psychotic episodes, anxious manifestations, and insomnia (ESM Table 2). While anxiety and insomnia are listed as ADRs frequently associated with PP (36, 37), some considerations must be made regarding symptoms related to SCZ reported as suspected ADRs. Among the ICSRs reporting “schizophrenia” as one of the described specific ADRs, 22.2% presented at least an ADR classifiable within the high-level term “therapeutic and non-therapeutic responses (e.g., Drug ineffective, Treatment noncompliance, Therapeutic product effect decreased) and 12.3% at least one ADR relative to “Product administration errors and issues” (e.g., Inappropriate schedule of product administration; Product dose omission issue; Incorrect dose administered). Furthermore, literature sources indicate that 20 to 30% of patients affected by SCZ are known to not respond to treatment with antipsychotics (44, 45), and data suggest a form of secondary treatment-resistant SCZ (46, 47). Considering this, we could reasonably say that most of these ADRs are more likely to derive from insufficient therapeutic control or relapses of pre-existing diseases rather than from exposure to the drug. This is also confirmed to what is reported for the General disorders and administration site conditions SOC, in which ADRs such as “drug ineffective” and “Condition aggravated” were characterized by the higher frequencies of reporting.
In ICSRs reporting fatal cases, the most frequently reported specific ADRs described suicidal and self-injurious behaviors. These behaviors have been associated with SCZ; a recent study has estimated an increase of 4.5 times of the incidence of these conditions over the general population for patients with SSDs (48). The risk factors for these types of manifestations are highly complex and range from demographic characteristics to psychosocial factors (49). This complexity requires an in-depth case-by-case assessment approach to properly evaluate these reactions, which could require a different study design to investigate. Other common types of ADRs observed in this subgroup of ICSRs included pulmonary embolism and cardiac failure. These ADRs have already been reported in the context of antipsychotic treatment data (50). Data regarding a link between paliperidone and pulmonary thromboembolism, however, are limited to few cases (51, 52). Moreover, the underlying mechanism of this ADR is still largely unknown, although some hypotheses regarding prolactin and its potential role as a platelet aggregation coactivator have been proposed (53). However, the influence of other factors such as obesity, increased levels of antiphospholipid antibodies, and hyperhomocysteinemia remains unclear. On this matter, some authors suggested that using aripiprazole would be preferable in patients presenting possible risk factors (52), but the clinical experience in this sense remains limited. In addition, 8.9% of the total neuroleptic malignant syndrome (NMS) cases observed (n = 113) presented a fatal outcome for the patient. This severe idiosyncratic reaction is linked to the administration of dopamine-blocking agents such as antipsychotics. It presents with symptoms such as fever, muscle rigidity, alterations in mental status, and autonomic functioning (54–56). Since LAI antipsychotics cannot be cleared quickly from the patient’s system, using these formulations can be perceived by the clinicians as limiting in terms of NMS management options, negatively impacting their perceived safety (57). Some recent retrospective studies have, however, estimated a low incidence of NMS cases over the antipsychotic-treated population, equal to 1.99 (95%CI 1.98–2.00) per 10,000 person-years, without any statistically significant differences between oral and LAI antipsychotics formulations (58, 59). This is in line with the results from our analysis, showing no disproportionality in the reporting of NMS between ICSRs PP-related and in the reference group (Table 4).
Our analysis has highlighted an increased probability of reporting for ADRs relative to the SMQs “sexual dysfunction” and “fertility disorder” between PP and the reference group. Sexual dysfunctions are commonly associated with antipsychotics. Literature sources state that up to 75% of treated patients experienced sexual dysfunction (60). However, their incidence could be underestimated due to the reluctancy of patients and physicians to spontaneously discuss and report these kind of reactions (61). These ADRs have multifactorial processes regarding underlying mechanisms. One of the most widely embraced factors is the increase in prolactin levels resulting from the antagonistic action on D2 dopamine receptors that characterizes antipsychotics. Dopaminergic receptors in the hypothalamic tuberoinfundibular tract act as inhibitors for prolactin secretion; thus, inhibition of dopamine D2 receptors in this tract increases prolactin release. This increase results in an inhibition of the release of follicle-stimulating and luteinizing hormones from the pituitary gland. With consequent low gonadal steroids and hypogonadism (62). The impact of these ADRs cannot be underestimated as they can negatively influence patient’s quality of life and potentially reduce treatment compliance (63, 64). The importance of these aspects is particularly central for LAI-treated patients, considering that candidate patients for LAI treatment are usually middle-aged adults, already stabilized in treatment with an AP, for which clinicians seek therapies that could help them improve their quality of life and regain as much social functionality as possible (65). Prolactin-related ADRs could also limit the use of these LAIs in populations of youth with serious mental illness who are at risk for relapse, for which SGA-LAIs could represent an effective treatment strategy (66). A previous prospective study highlighted significant increases in mean prolactin values in risperidone-treated young patients, with long-term consequences of these ADRs still on patients’ development to be clarified (66, 67).
ADRs relative to various forms of peripheral oedema constituted the vast majority of the SMQ “Haemodynamic oedema, effusions and fluid overload” for which a higher probability of reporting in PP-related when compared to RG-related ICSRs emerged from our analysis. These ADRs are already acknowledged as class effects related to the administration of SGA-LAIs. The mechanism underlying this type of ADRs remains unclear; however, several hypotheses have been formulated. Paliperidone being chemically a derivate of risperidone acts with a similar mechanism by blocking the serotonin (5-hydroxytryptamine, 5-HT) 5HT2, Dopaminergic D2, Adrenergic α1, α2 and histaminergic H1 receptors (68). The blockage of α1 receptors results in vasodilation with a consequential increase in hydrostatic pressure in the capillaries that could facilitate the onset of oedema (69). Also, the antagonistic action on 5HT2 receptors could be associated to oedema due to the increase in cyclic adenosine monophosphate concentrations, leading to the relaxation of vascular smooth muscle (70).
Regarding ADRs related to nervous system no disproportionality in primary reporting was reported for the PP-related ICSRs compared to the reference range. However, secondary reports of Extrapyramidal syndrome and Parkinson-like events were more frequent for PP compared to the reference group. However, in these ICSRs there was a higher number of co-reported suspected drugs for extrapyramidal syndrome and Parkinson-like events compared to the rest of PP-related ICSRs (2.2 ± 1.8 SD vs. 1.6 ± 1.3 SD; p = 0.014). When repeating the ROR calculations including on ICSRs with only one suspected drug (either PP or other SGA-LAI) we did not detect disproportionality between PP and the reference group [PP cases = 98; ROR = 1.19 (95%CI: 0.91–1.57)]. Furthermore, the most frequently reported drugs other than PP in these reports were other antipsychotics. Considering these data, we can reasonably assume pharmacodynamic interactions in combination therapies underlying the risk of these ADRs.
The observed disproportionalities in ADR reporting probability while being mostly in line with what is already known about paliperidone-based formulations, might seem puzzling at first since the 44% of ICSRs in the reference group presented LAI formulations of risperidone, which is a chemical precursor of paliperidone (9-hydroxyrisperidone), as a suspected drug. However, risperidone differs substantially from paliperidone from a pharmacokinetic standpoint. In fact, the not negligible fist passage effect, the presence of other metabolites (7-hydroxyrisperidone), and possible influences of cytochrome P450-2D6 and 3A4 individual efficiency status, all represent differentiating factors between the two drugs (71). It has been pointed out by several literature sources that these differences could significantly impact the safety and tolerability profile of these two drugs, as well as provide a different efficacy profile in clinical practice (72, 73). In addition to that, the relative novelty of PP-based formulations compared to risperidone LAI could constitute an attention-increasing factor for ADRs already well-known in previously introduced LAIs for such as those regarding sexual disorders and extrapyramidal manifestations.
Some differences in terms of relative reporting frequencies were noticed between the two PP formulations. Increased reporting frequencies in relation to the SOCs “psychiatric disorders,” “general disorder and administration site conditions,” and “product Issues” were observed for PP3M-related ICSRs when compared to the PP1M-related ones. The reporting of product issues could be linked to the relative novelty of PP3M compared to PP1M and the resulting limited clinical experience with PP3M. A recently marketed drug could be, in fact, more prone to initial product-related issues than a long-time marketed one. In this sense, it must be considered that currently, no meaningful Therapeutic Drug Monitoring (TDM) data are available for PP3M (74). However, from a recently published prospective study, no significant differences were observed for PP3M compared to PP1M in terms of safety (75). In addition, it is well known that in the initial phases of market presence, the attention reserved to the safety and tolerability aspects of a drug is higher. Potentially, the tendency of clinicians to propose newer treatments to patients that have performed well with existing options also needs to be considered (75). This underlines the necessity of further prospective studies involving large patient cohorts and clinicians more directly to properly assess these differences.
To the best of our knowledge, this is one of the first pharmacovigilance studies to evaluate the safety and tolerability profiles of PP-based formulation using data from a European scale pharmacovigilance database.
Considering the relatively recent approval of PP3M and given the general paucity of real-world derived safety data for PP-based LAI formulations, data deriving from large scale SRS databases analyses can contribute to a better characterization of their safety profiles.
Although our findings provide a comprehensive perspective in the evaluation of PP-related ADRs, the results of the present study should be interpreted in the light of some limitations.
First, the granularity of data provided by the EUDRAVigilance platform is limited and frequently managed in a categorical fashion. We acknowledge that we followed a conservative approach in case of lack of sufficient data, frequently leading to case exclusion or downgrade of reported items if information was not consistent. In addition to that, public data access does not allow to use all other drugs reported in the EUDRAVigilance database as a reference group as for other datasets (76). Moreover, we acknowledge that the publicly accessible EUDRAVigilance data level did not allow to access to detailed information about the reporting country, for privacy reasons. We were therefore unable to differentiate the results by reporting country. Likewise, FGA-LAI were not used as a reference group due to the lack of pharmacovigilance data related to the first years of their market presence. Additionally, the provided data limited considerations regarding aspects such as the presence of multiple suspected drugs in ICSRs. It also has to be pointed out that the retrieval of ICSRs regarding formulations with limited geographical availability was not possible due to database limitations.
Second, we performed a retrospective evaluation of cases reported by clinicians without the homogeneous structure of a single research protocol by applying a cluster analysis method not foreseen at the time of original reporting to the EUDRAVigilance platform. This makes secondary analysis of these data speculative, although the use of large pharmacovigilance databases inherently presents this limitation without necessarily limiting the validity of the conclusions.
Third, pharmacovigilance data should be read considering some technical concerns, including under-reporting compared to global clinical population and difficulty in identifying confounders. Indeed, this implies that the ADRs reported may represent only a partial, probably under-representative, percentage of all ADRs which occur in everyday clinical practice. Also, the lack of data related to the number of patients effectively treated with these drugs within the considered period (i.e., the denominator of the incidence fraction) does not allow incidence calculations.
Thus, future prospective clinical studies using a longitudinal design are required to improve the understanding of tolerability and security profile of PP1M, PP3M, and PP6M.
Similarly, further large-scale pharmacovigilance studies of international datasets, and with full access to Level 2A EUDRAVigilance data (77), are required to provide a more reliable estimate of incidence, clinical characteristics, and outcomes of PP-related ADRs compared to other LAIs.
In light of pharmacoepidemiological trends, there is an urgent need to understand SGA-LAI-related ADRs. Compared to other SGA-LAIs increased probabilities of reporting for ADR categorized as referring to the endocrine system impacting patient sexuality and fertility were observed for PP formulations. Also, some clinically irrelevant differences in the ICSRs reporting pattern between PP1M and PP3M emerged requiring further investigation as clinical experience with PP3M increases. The results presented in this work do not discourage the prescription of SGA-LAI formulations but aim to enhance their safety.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
GC and MAB retrieved and analyzed the data. GC and RdF drafted the paper and agreed to be accountable for all aspects of the work, ensuring that questions related to the accuracy or integrity of any part of the work are investigated appropriately. ES, GS, and PMC revised the paper for important intellectual content. ES, GS, and PDF approved the final version of the manuscript to be published. ES, GS, PMC, GC, and RdF developed the concept. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1130636/full#supplementary-material
1. de Bartolomeis, A, Barone, A, Begni, V, and Riva, MA. Present and future antipsychotic drugs: a systematic review of the putative mechanisms of action for efficacy and a critical appraisal under a translational perspective. Pharmacol Res. (2022) 176:106078. doi: 10.1016/j.phrs.2022.106078
2. Mailman, R, and Murthy, V. Third generation antipsychotic drugs: partial Agonism or receptor functional selectivity? Curr Pharm Des. (2010) 16:488–501. doi: 10.2174/138161210790361461
3. Lacro, JP, Dunn, LB, Dolder, CR, Leckband, SG, and Jeste, DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia. J Clin Psychiatry. (2002) 63:892–909. doi: 10.4088/JCP.v63n1007
4. Valenstein, M, Ganoczy, D, McCarthy, JF, Kim, HM, Lee, TA, and Blow, FC. Antipsychotic adherence over time among patients receiving treatment for schizophrenia. J Clin Psychiatry. (2006) 67:1542–50. doi: 10.4088/JCP.v67n1008
5. Velligan, DI, Wang, M, Diamond, P, Glahn, DC, Castillo, D, Bendle, S, et al. Relationships among subjective and objective measures of adherence to Oral antipsychotic medications. Psychiatr Serv. (2007) 58:1187–92. doi: 10.1176/ps.2007.58.9.1187
6. Weiden, PJ. Understanding and addressing adherence issues in schizophrenia: from theory to practice. J Clin Psychiatry. (2007) 68:14–9.
7. Kane, JM, and Correll, CU. Past and present Progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry. (2010) 71:1115–24. doi: 10.4088/JCP.10r06264yel
8. Fleischhacker, WW, and Miyamoto, S. Pharmacological treatment of schizophrenia. Clin Neuropsychopharmacol Ther. (2016) 7:1–8. doi: 10.5234/cnpt.7.1
9. Nasrallah, HA. The case for long-acting antipsychotic agents in the post-CATIE era. Acta Psychiatr Scand. (2007) 115:260–7. doi: 10.1111/j.1600-0447.2006.00982.x
10. Agid, O, Foussias, G, and Remington, G. Long-acting injectable antipsychotics in the treatment of schizophrenia: their role in relapse prevention. Expert Opin Pharmacother. (2010) 11:2301–17. doi: 10.1517/14656566.2010.499125
11. Brissos, S, Veguilla, MR, Taylor, D, and Balanzá-Martinez, V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. (2014) 4:198–219. doi: 10.1177/2045125314540297
12. Correll, CU, Citrome, L, Haddad, PM, Lauriello, J, Olfson, M, Calloway, SM, et al. The use of long-acting injectable antipsychotics in schizophrenia. J Clin Psychiatry. (2016) 77:1–24. doi: 10.4088/JCP.15032su1
13. Miyamoto, S, and Wolfgang, FW. The use of long-acting injectable antipsychotics in schizophrenia. Curr Treat Options Psychiatry. (2017) 4:117–26. doi: 10.1007/s40501-017-0115-z
14. Altamura, AC, Aguglia, E, Bassi, M, Bogetto, F, Cappellari, L, de Giorgi, S, et al. Rethinking the role of long-acting atypical antipsychotics in the community setting. Int Clin Psychopharmacol. (2012) 27:1–349. doi: 10.1097/YIC.0b013e328357727a
15. Heres, S, Lambert, M, and Vauth, R. Treatment of early episode in patients with schizophrenia: the role of long acting antipsychotics. Eur Psychiatry. (2014) 29:1409–13. doi: 10.1016/S0924-9338(14)70001-X
16. Stahl, SM. Long-acting injectable antipsychotics: shall the last be first? CNS Spectr. (2014) 19:3–5. doi: 10.1017/S1092852913001016
17. Johnson, DAW. Historical perspective on antipsychotic long-acting injections. Br J Psychiatry. (2009) 195:s7–s12. doi: 10.1192/bjp.195.52.s7
18. Risio, A, and Lang, A. History and therapeutic rationale of long acting antipsychotics. Curr Clin Pharmacol. (2014) 9:39–52. doi: 10.2174/15748847113089990057
19. de Filippis, R, de Fazio, P, Gaetano, R, Steardo, L, Cedro, C, Bruno, A, et al. Current and emerging long-acting antipsychotics for the treatment of schizophrenia. Expert Opin Drug Saf. (2021) 20:771–90. doi: 10.1080/14740338.2021.1910674
20. Citrome, L. New second-generation long-acting injectable antipsychotics for the treatment of schizophrenia. Expert Rev Neurother. (2013) 13:767–83. doi: 10.1586/14737175.2013.811984
21. Rauch, A-S, and Fleischhacker, WW. Long-acting injectable formulations of new-generation antipsychotics: a review from a clinical perspective. CNS Drugs. (2013) 27:637–52. doi: 10.1007/s40263-013-0083-9
22. Gentile, S. Adverse effects associated with second-generation antipsychotic long-acting injection treatment: a comprehensive systematic review. Pharmacotherapy: the journal of human pharmacology and drug. Therapy. (2013) 33:1087–106. doi: 10.1002/phar.1313
23. Najarian, D, Sanga, P, Wang, S, Lim, P, Singh, A, Robertson, MJ, et al. A randomized, double-blind, multicenter, noninferiority study comparing Paliperidone palmitate 6-month versus the 3-month long-acting injectable in patients with schizophrenia. Int J Neuropsychopharmacol. (2022) 25:238–51. doi: 10.1093/ijnp/pyab071
24. Karslioğlu, EH, Kolcu, Z, Karslioğlu, Nİ, and Çayköylü, A. Prospective analysis of serum prolactin levels, clinical symptomatology and sexual functions in patients with schizophrenia switched to paliperidone palmitate 3-monthly from paliperidone palmitate 1-monthly: preliminary findings of the first 3 months. Hum Psychopharmacol Clin Exp. (2022) 37:e2827. doi: 10.1002/hup.2827
25. Berwaerts, J, Liu, Y, Gopal, S, Nuamah, I, Xu, H, Savitz, A, et al. Efficacy and safety of the 3-month formulation of Paliperidone palmitate vs placebo for relapse prevention of schizophrenia. JAMA Psychiat. (2015) 72:830–9. doi: 10.1001/jamapsychiatry.2015.0241
26. Schoretsanitis, G, Baumann, P, Conca, A, Dietmaier, O, Giupponi, G, Gründer, G, et al. Therapeutic drug monitoring of long-acting injectable antipsychotic drugs. Ther Drug Monit. (2021) 43:79–102. doi: 10.1097/FTD.0000000000000830
27. Ravenstijn, P, Remmerie, B, Savitz, A, Samtani, MN, Nuamah, I, Chang, C-T, et al. Pharmacokinetics, safety, and tolerability of paliperidone palmitate 3-month formulation in patients with schizophrenia: a phase-1, single-dose, randomized, open-label study. J Clin Pharmacol. (2016) 56:330–9. doi: 10.1002/jcph.597
28. European Medicnes Agency. European Medicines Agency Policy on Access to EudraVigilance Data for Medicinal Products for Human Use. EMA/759287/2009 Revision 4. (2019). Available at: https://www.ema.europa.eu/en/documents/other/european-medicines-agency-policy-access-eudravigilance-data-medicinal-products-human-use-revision-4_en.pdf (Accessed September 15, 2022)
29. Jann, MW, and Penzak, SR. Long-acting injectable second-generation antipsychotics: an update and comparison between agents. CNS Drugs. (2018) 32:241–57. doi: 10.1007/s40263-018-0508-6
30. Correll, CU, Kim, E, Sliwa, JK, Hamm, W, Gopal, S, Mathews, M, et al. Pharmacokinetic characteristics of long-acting injectable antipsychotics for schizophrenia: an overview. CNS Drugs. (2021) 35:39–59. doi: 10.1007/s40263-020-00779-5
31. European Medicines Agency. Database of Summary of Product Characteristics (SmPCs). (2022). Available at: https://www.ema.europa.eu/en/medicines/human (Accessed October 03, 2022)
32. Mascolo, A, Scavone, C, Ferrajolo, C, Rafaniello, C, Danesi, R, del Re, M, et al. Immune checkpoint inhibitors and cardiotoxicity: an analysis of spontaneous reports in eudravigilance. Drug Saf. (2021) 44:957–71. doi: 10.1007/s40264-021-01086-8
33. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Introductory Guide for Standardised MedDRA Queries (SMQs) Version 24.0. (2021). Available at: https://www.meddra.org/ (Accessed September 14, 2022)
34. Medical Dictionary for Regulatory Activities. Standardised MedDRA Queries (SMQs). (2022). Available at: https://www.meddra.org/how-to-use/tools/smqs
35. Spoelstra, SK, Bruins, J, Bais, L, Seerden, P, Castelein, S, and Knegtering, H. One-month versus three-month formulation of Paliperidone palmitate treatment in psychotic disorders: patients’, relatives’, and mental health professionals’ perspectives. Patient Prefer Adherence. (2022) 16:615–24. doi: 10.2147/PPA.S349460
36. European Medines Agency. Summary of Product Characteristics: Xeplion; (2015). Available at: https://www.ema.europa.eu/en/documents/product-information/xeplion-epar-product-information_en.pdf (Accessed October 25, 2022)
37. European Mecines Agency. Summary of Product Characteristics: Trevicta; (2014). Available at: https://www.ema.europa.eu/en/documents/product-information/trevicta-epar-product-information_en.pdf (Accessed October 25, 2022)
38. Magliocco, F, de Filippis, R, Aloi, M, Staltari, FA, Gaetano, R, Segura-Garcia, C, et al. Second-generation long-acting injections anti-psychotics improve executive functions in patients with schizophrenia: a 12-month real-world study. Int J Psychiatry Clin Pract. (2020) 24:201–7. doi: 10.1080/13651501.2020.1737134
39. Lähteenvuo, M, and Tiihonen, J. Antipsychotic polypharmacy for the management of schizophrenia: evidence and recommendations. Drugs. (2021) 81:1273–84. doi: 10.1007/s40265-021-01556-4
40. Devrimci Ozguven, H, and Kır, Y. Depot/long acting antipsychotics in the treatment of schizophrenia and bipolar disorder. Arch Neuropsychiatr. (2021) 58:S47–52. doi: 10.29399/npa.27480
41. Jarema, M, Bieńkowski, P, Heitzman, J, Parnowski, T, and Rybakowski, J. Paliperidone palmitate: effectiveness, safety, and the use for treatment of schizophrenia. Psychiatr Pol. (2017) 51:7–21. doi: 10.12740/PP/64581
42. Hatano, M, Kamei, H, Shimato, A, Yamada, S, and Iwata, N. Trend survey on adverse event profiles of antipsychotic long-acting injections and oral agents using the Japanese adverse drug event report database. Psychiatry Res. (2020) 291:113249. doi: 10.1016/j.psychres.2020.113249
43. Mathews, M, Nuamah, I, Savitz, AJ, Hough, DW, Najarian, D, Kim, E, et al. Time to onset and time to resolution of extrapyramidal symptoms in patients with exacerbated schizophrenia treated with 3-monthly vs once-monthly paliperidone palmitate. Neuropsychiatr Dis Treat. (2018) 14:2807–16. doi: 10.2147/NDT.S175364
44. Mørup, MF, Kymes, SM, and Oudin, ÅD. A modelling approach to estimate the prevalence of treatment-resistant schizophrenia in the United States. PLoS One. (2020) 15:e0234121. doi: 10.1371/journal.pone.0234121
45. Siskind, D, Orr, S, Sinha, S, Yu, O, Brijball, B, Warren, N, et al. Rates of treatment-resistant schizophrenia from first-episode cohorts: systematic review and meta-analysis. Br J Psychiatry. (2022) 220:115–20. doi: 10.1192/bjp.2021.61
46. Correll, CU, and Howes, OD. Treatment-resistant schizophrenia. J Clin Psychiatry. (2021) 82:1–2. doi: 10.4088/JCP.MY20096AH1C
47. Lally, J, Ajnakina, O, di Forti, M, Trotta, A, Demjaha, A, Kolliakou, A, et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol Med. (2016) 46:3231–40. doi: 10.1017/S0033291716002014
48. Olfson, M, Stroup, TS, Huang, C, Wall, MM, Crystal, S, and Gerhard, T. Suicide risk in Medicare patients with schizophrenia across the life span. JAMA Psychiat. (2021) 78:876–85. doi: 10.1001/jamapsychiatry.2021.0841
49. Berardelli, I, Rogante, E, Sarubbi, S, Erbuto, D, Lester, D, and Pompili, M. The importance of suicide risk formulation in schizophrenia. Front Psychiatry. (2021) 12:779684. doi: 10.3389/fpsyt.2021.779684
50. Boels, D, Mahé, J, Olry, A, Citterio-Quentin, A, Moragny, J, and Jolliet, P. Fatal and life-threatening ADRs associated with paliperidone palmitate: an observational study in the French pharmacovigilance database. Clin Toxicol. (2021) 59:786–93. doi: 10.1080/15563650.2021.1878206
51. Şengül, MCB, Kaya, K, Yilmaz, A, Şengül, C, and Serinken, M. Pulmonary thromboembolism due to paliperidone: report of 2 cases. Am J Emerg Med. (2014) 32:814.e1–2. doi: 10.1016/j.ajem.2013.12.038
52. Michaud, I, and Landry, P. Case report: Paliperidone palmitate, but not aripiprazole, as a possible risk factor for pulmonary embolism. J Clin Psychopharmacol. (2018) 38:392–4. doi: 10.1097/JCP.0000000000000888
53. Waage, IM. Pulmonary embolism possibly associated with olanzapine treatment. BMJ. (2003) 327:1384–4. doi: 10.1136/bmj.327.7428.1384
54. Tse, L, Barr, A, Scarapicchia, V, and Vila-Rodriguez, F. Neuroleptic malignant syndrome: a review from a clinically oriented perspective. Curr Neuropharmacol. (2015) 13:395–406. doi: 10.2174/1570159X13999150424113345
55. Gurrera, RJ, Caroff, SN, Cohen, A, Carroll, BT, DeRoos, F, Francis, A, et al. An international consensus study of neuroleptic malignant syndrome diagnostic criteria using the Delphi method. J Clin Psychiatry. (2011) 72:1222–8. doi: 10.4088/JCP.10m06438
56. Strawn, JR, Keck, PE, and Caroff, SN. Neuroleptic malignant syndrome. Am J Psychiatr. (2007) 164:870–6. doi: 10.1176/ajp.2007.164.6.870
57. Kane, JM, Correll, CU, Delva, N, Gopal, S, Savitz, A, and Mathews, M. Low incidence of neuroleptic malignant syndrome associated with paliperidone palmitate long-acting injectable. J Clin Psychopharmacol. (2019) 39:180–2. doi: 10.1097/JCP.0000000000001019
58. Guinart, D, Taipale, H, Rubio, JM, Tanskanen, A, Correll, CU, Tiihonen, J, et al. Risk factors, incidence, and outcomes of neuroleptic malignant syndrome on long-acting injectable vs oral antipsychotics in a Nationwide schizophrenia cohort. Schizophr Bull. (2021) 47:1621–30. doi: 10.1093/schbul/sbab062
59. Guinart, D, Misawa, F, Rubio, JM, Pereira, J, Filippis, R, Gastaldon, C, et al. A systematic review and pooled, patient-level analysis of predictors of mortality in neuroleptic malignant syndrome. Acta Psychiatr Scand. (2021) 144:329–41. doi: 10.1111/acps.13359
60. Young, SL, Taylor, M, and Lawrie, SM. “First do no harm”. A systematic review of the prevalence and management of antipsychotic adverse effects. J Psychopharmacol. (2015) 29:353–62. doi: 10.1177/0269881114562090
61. Serretti, A, and Chiesa, A. A meta-analysis of sexual dysfunction in psychiatric patients taking antipsychotics. Int Clin Psychopharmacol. (2011) 26:130–40. doi: 10.1097/YIC.0b013e328341e434
62. Tewksbury, A, and Olander, A. Management of antipsychotic-induced hyperprolactinemia. Ment Health Clin. (2016) 6:185–90. doi: 10.9740/mhc.2016.07.185
63. Kelly, DL, Powell, MM, Wehring, HJ, Sayer, MA, Kearns, AM, Hackman, AL, et al. Adjunct aripiprazole reduces prolactin and prolactin-related adverse effects in premenopausal women with psychosis. J Clin Psychopharmacol. (2018) 38:317–26. doi: 10.1097/JCP.0000000000000898
64. Bebbington, PE, Angermeyer, M, Azorin, J-M, Marwaha, S, Marteau, F, and Toumi, M. Side-effects of antipsychotic medication and health-related quality of life in schizophrenia. Acta Psychiatr Scand. (2009) 119:22–8. doi: 10.1111/j.1600-0447.2008.01310.x
65. Rossi, G, Frediani, S, Rossi, R, and Rossi, A. Long-acting antipsychotic drugs for the treatment of schizophrenia: use in daily practice from naturalistic observations. BMC Psychiatry. (2012) 12:122. doi: 10.1186/1471-244X-12-122
66. Lytle, S, McVoy, M, and Sajatovic, M. Long-acting injectable antipsychotics in children and adolescents. J Child Adolesc Psychopharmacol. (2017) 27:2–9. doi: 10.1089/cap.2016.0055
67. Cicala, G, Barbieri, MA, Santoro, V, Tata, C, Colucci, PV, Vanadia, F, et al. Safety and tolerability of antipsychotic drugs in pediatric patients: data from a 1-year naturalistic study. Front Psychiatry. (2020) 11:1–9. doi: 10.3389/fpsyt.2020.00152
68. Gilday, EA, and Nasrallah, H. Clinical pharmacology of paliperidone palmitate a parenteral long-acting formulation for the treatment of schizophrenia. Rev Recent Clin Trials. (2012) 7:2–9. doi: 10.2174/157488712799363307
69. Feroz-Nainar, C, Selvaraj, P, and Roy, M. Risperidone induced oedema in a child with learning disability and autism. Autism. (2006) 10:308–10. doi: 10.1177/1362361306063302
70. Cicek, E, Cicek, IE, and Uguz, F. Bilateral pretibial edema associated with Paliperidone palmitate long-acting injectable: a case report. Clin Psychopharmacol Neurosci. (2017) 15:184–6. doi: 10.9758/cpn.2017.15.2.184
71. Zhang, L, Brown, SJ, Shan, Y, Lee, AM, Allen, JD, Eum, S, et al. Genetic polymorphisms and risperidone pharmacokinetics: a systematic review and meta-analysis. Pharmacother J Hum Pharmacol Drug Ther. (2020) 40:632–47. doi: 10.1002/phar.2434
72. Turkoz, I, Bossie, CA, Lindenmayer, J-P, Schooler, N, and Canuso, CM. Paliperidone ER and oral risperidone in patients with schizophrenia: a comparative database analysis. BMC Psychiatry. (2011) 11:21. doi: 10.1186/1471-244X-11-21
73. Huhn, M, Nikolakopoulou, A, Schneider-Thoma, J, Krause, M, Samara, M, Peter, N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. (2019) 394:939–51. doi: 10.1016/S0140-6736(19)31135-3
74. Schoretsanitis, G, Spina, E, Hiemke, C, and de Leon, J. A systematic review and combined analysis of therapeutic drug monitoring studies for long-acting paliperidone. Expert Rev Clin Pharmacol. (2018) 11:1237–53. doi: 10.1080/17512433.2018.1549489
75. Fernández-Miranda, JJ, Díaz-Fernández, S, de Berardis, D, and López-Muñoz, F. Paliperidone palmitate every three months (PP3M) 2-year treatment compliance, effectiveness and satisfaction compared with Paliperidone palmitate-monthly (PP1M) in people with severe schizophrenia. J Clin Med. (2021) 10:1408. doi: 10.3390/jcm10071408
76. Gastaldon, C, Arzenton, E, Raschi, E, Spigset, O, Papola, D, Ostuzzi, G, et al. Neonatal withdrawal syndrome following in utero exposure to antidepressants: a disproportionality analysis of VigiBase, the WHO spontaneous reporting database. Psychol Med. (2022):1–9. doi: 10.1017/S0033291722002859
Keywords: adverse drug reaction, antipsychotics, schizophrenia, pharmacovigilance, paliperidone palmitate, long-acting injectable, drug-induced reaction, EUDRAVigliance
Citation: Cicala G, de Filippis R, Barbieri MA, Cutroneo PM, De Fazio P, Schoretsanitis G and Spina E (2023) Tolerability profile of paliperidone palmitate formulations: A pharmacovigilance analysis of the EUDRAVigilance database. Front. Psychiatry. 14:1130636. doi: 10.3389/fpsyt.2023.1130636
Received: 23 December 2022; Accepted: 16 March 2023;
Published: 06 April 2023.
Edited by:
João Gama Marques, Centro Hospitalar Psiquiátrico de Lisboa, PortugalReviewed by:
Sofia Brissos, Centro Hospitalar Psiquiátrico de Lisboa, PortugalCopyright © 2023 Cicala, de Filippis, Barbieri, Cutroneo, De Fazio, Schoretsanitis and Spina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giuseppe Cicala, Z2NpY2FsYUB1bmltZS5pdA==
†ORCID: Renato de Filippis https://orcid.org/0000-0001-6928-1224
Pasquale De Fazio https://orcid.org/0000-0001-5375-3565
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.