
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry , 20 March 2023
Sec. Social Neuroscience
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1129252
 Clare M. Eddy1,2*
Clare M. Eddy1,2*The Reading the Mind in the Eyes test (RMET) is a widely applied test of social cognition, based on mental state judgments in response to photographs of human eyes, which can elicit impairment in patients with numerous psychiatric and neurological disorders. However, interpretation of task performance is limited without the use of appropriate control tasks. In addition to a matched task requiring age judgments of the RMET stimuli, it was recently shown that a mental state judgment task of comparable difficulty, could be developed using photographs of domestic cat eyes. The current study aimed to further develop a Non-human Animal RMET (NARMET) by testing additional stimuli in the form of photographs of domestic dog eyes. A variety of additional tasks were used alongside the eyes test stimuli in a large sample of healthy young adults, to explore how alexithymia, schizotypal features, and autistic tendencies may differentially influence mental state attribution in response to cat, dog, and human eyes test stimuli. The resulting NARMET features both cat and dog trials, depicting a similar range of complex mental states to the human RMET. It shows favorable psychometric properties as well as being well matched to the RMET in terms of linguistic variables, length and difficulty. However, reading measures predicted performance on the RMET, but not on the NARMET. Although further testing is required in samples with a higher proportion of males, future application of the NARMET in neuropsychiatric populations exhibiting cognitive and behavioral difficulties could offer enhanced assessment of social cognitive skills.
The Reading the Mind in the Eyes test (RMET) is a widely applied test of social cognition, involving selection of complex mental states to match photographs of the human eye region (1). Use of this task has revealed impairments in numerous clinical populations including those with a diagnosis of schizophrenia (2, 3), Huntington’s disease (4), and brain injury (5). Performance on the RMET is thought to be related to empathy (1, 6) and alexithymia (7), in addition to schizotypal personality (8, 9), and autistic spectrum features (6, 10, 11). However, the exact skills underlying performance on the task are still debated. Some scholars describe the task as involving emotion recognition (12) while others believe it measures abstract mentalizing about mental states (1). To gain insight into the reasons for any performance deficits, it is important to consider the use of matched control tasks, such as the age judgment eyes task (9, 13, 14).
In one recent study, it was shown that a counterpart measure of comparable difficulty to the human RMET could be developed using photographs of domestic cat eyes (9). Cats were selected because they familiar to humans, exhibit a similar layout of facial features, and an abundance of images were available online. It is known that humans attribute human-like personality traits such as curious, playful, and serious to their pet cat (15). There was a high level of consensus in relation to the attribution of secondary emotions on the cat eyes task (9), which followed the format of the human RMET, and comprised 18 photographs of the cat eye region surrounded by the same forced-choice complex mental state terms featured in the original task (e.g., preoccupied, decisive, and tentative). Performance on the RMET and cat eyes task was highly correlated, but while RMET accuracy was predicted by working memory, schizotypal personality and measures of empathy toward humans, cat eyes test responses were not, suggesting that stimuli featuring human versus non-human animals may be differentially impacted by psychopathology. The current study aimed to further develop a non-human animal version of the RMET.
It was previously found that higher accuracy on both the RMET and cat eyes test was positively associated with ratings of liking dogs on a simple animal preference questionnaire (9). Given this finding and the proposed existence of “cat” people and “dog” people (16), it was decided to expand the stimulus set to also include dog eyes, as in addition to adding variety, dogs should be familiar to test subjects, and work in a similar way to cats. As well as having have many of the same muscles that produce facial expressions in humans (17), dogs are adept at interacting with humans, and react to human affective states (18, 19). It has even been suggested that dogs seem to use facial movements (e.g., brow lift, jaw drop, and tongue show) specifically when their owner is able to view these cues (20). Personality judgments about dogs and humans have been shown to reach similar levels of agreement and consistency (21), projection of self-views onto dogs appears no more likely than onto other humans (22), and some traits attributed to dogs appear to reflect a consistent personality or temperament (23). While it is important to note the potential differences between personality traits, emotions and non-emotional mental states, humans generally agree that dogs can experience emotions (24), including secondary emotions e.g., jealousy and guilt (25). Basic emotional expressions in dogs are reported by 65–100% of dog owners (25, 26), and are reported to be slightly more frequent in dogs than cats (25). It was therefore expected that a similar degree of consensus would be found for newly created dog eyes test stimuli as was found for the cat eyes test (9).
A variety of additional tasks were used alongside the eyes test stimuli to explore whether psychological factors may differentially influence performance across the human and non-human animal versions of the tasks. Because vocabulary and verbal fluency can be related to RMET performance (27, 28), measures of reading were included. Previously identified associations with empathy were further explored by including measures associated with emotion processing (alexithymia; emotion contagion; mood). Measures of anthropomorphism, and motivation associated with understanding mental states were also included because participants were asked to attribute mental states to non-human animals. Scales for autistic features, schizotypal personality characteristics, and social anhedonia were included, to test for differential relationships with social cognition in relation to human versus non-human stimuli. Based on previous findings (9), it was predicted that responses to all types of eyes stimuli would correlate with aspects of empathy, but that performance on the human and animal stimuli would be differentially predicted by measures associated with psychopathology, with more relationships of this nature being expected for the human RMET.
After ethical permission was granted for the study by University of Birmingham, 210 undergraduate students without any current psychiatric or neurological diagnoses, or cat/dog phobia, volunteered to participate for course credit. Five had missing data and 1 demonstrated below chance performance on the eyes tests. A further three participants had incomplete data on a few questionnaires but were included after imputation of missing values based on group mean (29). The final sample consisted of 204 participants (189 females; 15 males), of mean age 19.81 ± 0.93 years, median = 19.7; range = 18.26–23.95 (age was not normally distributed, 95% CI: 19.68, 19.94).
Participants completed the tasks individually in a lab at the University. They provided demographical information (gender, date of birth, year of study), and completed the Hospital Anxiety, and Depression Scale (HADS), Test of Irregular Word Reading Efficiency (TIWRE), and Test of Word Reading Efficiency (TOWRE). Participants then completed the three computerized eyes tasks, which were presented using Presentation (Neurobehavioral Systems) software, after being provided with Baron-Cohen et al.’s glossary of mental state terms. The order of presentation of each stimuli set (human RMET, cats, and dogs) was counterbalanced across participants. These were followed by computerized questionnaires: Interpersonal Reactivity Index (IRI), Toronto Alexithymia Scale (TAS-20), Revised Social Anhedonia Scale (rSAS), Autism Spectrum Quotient (AQ50), Emotional Contagion Scale (ECS), Individual Differences in Anthropomorphism Questionnaire (IDAQ), Mind Reading Motivation scale (MRM), and Oxford-Liverpool Inventory of Feelings and Experiences (O-Life).
Participants read out loud 108 regular words and then 39 irregular words (30, 31) with no time limit. TOWRE includes part A (sight reading efficiency) and part B (phonemic decoding efficiency). Errors and time taken were recorded.
The IRI (32, 33) contains 4 subscales each with 7 items. Perspective taking (PT) assesses the tendency to adopt other people’s points of view, and empathic concern (EC), addresses feelings of warmth, and consideration toward others. High scores for personal distress (PD) indicate more feelings of negative emotion when around other people in distress and the fantasy subscale measures the propensity to imagine and relate to characters in books and films. Participants respond on a 5 point Likert scale based on how well each item describes them. Some items are reverse scored, and total score ranges from 0 to 112.
The TAS assesses alexithymia and has good reliability and construct validity (34–36). There are three subscales: difficulty identifying feelings (DIF e.g., “I have feelings that I can’t quite identify”); difficulty describing feelings (DDF e.g., “It is difficult for me to find the right word for my feelings”), and externally oriented thinking (EOT e.g., “I prefer to just let things happen rather than to understand why they turned out that way”). Some items are reverse scored. Scores can range from 20 to 100, with a cut of at 61 being proposed to identify alexithymic individuals.
The revised SAS (37) contains 40 items and assesses social withdrawal and lack of pleasure from social relationships e.g., “A car ride is much more enjoyable if someone is with me”; “Having close friends is not as important as some people say.” A cut-off of 12 or over has been proposed (38) with higher scores indicating more social anhedonia. Some items are reverse scored.
This self-report measure (39) contains 30 items in total and is used to assess anthropomorphic tendencies whereby participants are asked to rate how much they believe a non-human entity (e.g., wind; robot etc.) possesses human characteristics on a scale of 0 (not at all) to 10 (very much). For example, items include “To what extent does the average computer have a mind of its own?” and “To what extent does the average reptile have consciousness?” The two subscales (each 15 items) measure anthropomorphic (mental state related) and non-anthropomorphic attribution (attributions related to clearly observable or functional aspects of a stimulus).
This scale (40, 41) contains 15 items and is used to assess individual differences in susceptibility to feeling the emotions exhibited by other people, including love, happiness, fear, anger, and sadness.
This self-report measure (42) was developed to assess anxiety (7 items) and depression (7 items) that a person is experiencing.
This scale assesses tendencies toward thinking about mental states rather than ability (43). The developers showed it can be associated with teamwork, is stable over time, and goes beyond trait empathy.
This scale (44) assesses unusual experiences, cognitive disorganization, introvertive anhedonia, and impulsive non-conformity. Psychometric testing indicated high internal consistency (45) and test-retest reliability (46).
The AQ (47) consists of 50 statements, each of which is in a forced choice format with 4 ratings ranging from “definitely agree” to “definitely disagree.” Neurotypical individuals would agree with half of the statements and disagree with half. The statements are related to five different domains relevant to autistic traits: social skills; communication skills; imagination; attention to detail; and attention switching/tolerance of change. Statements answered in a fashion associated with autistic tendencies score a point. A score of 32 or more is thought to be indicative of high autistic traits.
The RMET (1, human eyes) contains 36 test trials plus one practice trial. Stimuli consist of photographs of the human eye region, surrounded by four mental state terms (e.g., terrified, upset, arrogant, and annoyed). Instructions require the participant to consider these terms and select the word they think is most appropriate to describe what the person in the photograph is thinking or feeling. Correct answers provided by Baron-Cohen et al. (47) were determined based on consensus across expert judges. Evidence of task validity comes from correlations of other measures of mental state, reasoning and recognition such as the faux pas test (48), and the ability of this task to identify individuals with known deficits in social cognition (49). The RMET has good test-retest stability as shown over 1 year in a non-clinical sample (50).
The development of the cats eyes test was described previously (9). Trials were designed to match corresponding RMET trials, and the best subset of trials (n = 18) was selected to match to the RMET for difficulty, and to achieve sufficient reliability and internal consistency. Correct answers were based on consensus (majority response for each item in the current study were no different to our previous study). The dog eyes test stimuli were developed for the current study following the same process as for the cats eyes stimuli i.e., 36 images (plus 1 for practice) without re-use restrictions were selected from the internet, based on visual similarity to the human stimuli (e.g., eye shape, direction of gaze), with a view to matching each individual RMET trial. Each eyes test commenced with onscreen instructions to pick “the word that best describes what the person/cat/dog in the image is thinking or feeling.” Images were approximately 28 cm × 9 cm high (24″ monitor; resolution 1,024 × 768), with response options in Arial 22 point (approximately 1 cm high) outside the corners of the image, mapped to the numeric keypad which was used to respond (1, 3, 7, 9). The first trial began after the participant pressed the space bar. There was no time limit, and a valid button response initiated the next trial.
Cat stimuli were validated using two separate samples in a previous study (9). The current paper presents additional analysis involving the cats stimuli (18 trials), and reports data on the dog eyes test stimuli for the first time. As with the cat eyes test, accuracy was determined based on consensus (initial sample majority responses) for the dog stimuli. 475 trials were removed (1.1% of 44,064 trials in total: 36 × 6 × 204) of data due to responses less than 200 ms, or more than mean + 3 times SD per condition. After determining the correct answers and the best subset of 18 dogs eyes test trials to match the RMET for accuracy, this accuracy match was tested again in a second sample (n = 228), as was done previously (9) for the cat stimuli. Further comparisons of accuracy and reaction times were made across the three sets of eyes stimuli. Reliability was also compared for human, cat, and dog stimuli, before investigating relationships between eyes test performance, and other measures. Normality was tested using Shapiro-Wilk, and a Box-Cox transformation was performed (λ = 2), consistent with our previous study that developed the cat stimuli (9).
Accuracy scores (%) and mean reaction times (RT; seconds) for the human (72.61 ± 0.12; RT 3.56 ± 1.07), cat (71.81 ± 0.14; RT 3.29 ± 1.07), and dog (72.53 ± 0.15; RT 3.36 ± 1.02) stimuli were well matched. Mean accuracy for all cats plus all dogs was 72.17 ± 0.15, RT 3.33 ± 1.05 s. Descriptive statistics for the full set of measures can be found in Supplementary Table 1. Few participants within the sample exhibited scores above cut-offs for scales such as the AQ50 (n = 4) and TAS-20 (n = 20), although more participants scored above the cut-off for the rSAS (n = 44).
Correlations between the eyes tests were strong and positive, suggestive of convergent validity: dogs and cats: r = 0.423, p < 0.0001; cats and human RMET: r = 0.483, p < 0.0001; dogs and human RMET: r = 0.468, p < 0.0001. Pairwise t-tests indicated no significant differences between any two stimulus sets [dogs and cats: t(203) = −0.873, p = 0.384; cats and human RMET: t(203)-0.465, p = 0.642; dogs and human RMET: t(203) = 0.508, p = 0.612]. Good performers on one type of eyes stimuli tended to score highly on the other types. Eyes stimuli score distributions and correlation plots are shown in Figure 1.
Participants took slightly longer to respond to human RMET trials in comparison to cat [t(203) = −5.334, p < 0.001] and dog trials [t(203) = −4.214, p < 0.001]. There was no difference between cat and dog trials [t(203) = 1.057, p = 0.212]. In real terms, this amounts to just a few more seconds required to complete the entire human RMET in comparison to the entire set of cats plus dogs (Supplementary Table 1).
Split half reliability (internal consistency) was 0.67 for the human RMET, 0.54 for cats and 0.59 for dogs; while Fleiss’ Kappa for inter-rater agreement was: human RMET = 0.44; cats = 0.39; dogs = 0.44 [fair agreement = 0.21–0.40; moderate agreement = 0.40–0.60; e.g., (51)].
To further test reliability of the newest stimuli i.e., the dog eyes test, extra data was collected for this task plus the human RMET in an additional sample of 228 undergraduate students (58 males and 170 females; mean age 19.87 years ± 1.04, median = 19.69, range = 18.26–24.07; age was not normally distributed, 95% CI: 19.59, 20.05). Accuracy for the dogs eyes stimuli was 72.45% (SE = 0.93%), and for the human RMET was 72.86% (SE = 0.76%), with no significant difference for accuracy [paired t(227) = 0.450, p = 0.650].
Correlations were calculated for each eyes stimulus set and all other measures (TOWRE A and B, TIWRE, HADS anxiety and depression subscales, the four IRI subscales, the three TAS subscales, the four O-LIFE subscales, the two IDAQ subscales, and total scores for the MRM, SAS-r, AQ50, and ECS). Stepwise linear regression models were then run with the score for each eyes stimuli set as DV, and scores for any correlated variables (Table 1) as IVs.
The best models were identified based on highest R2-values with all predictors making a significant contribution to the model. Human RMET [F(3,200) = 9.736, p < 0.001, adj R2 = 0.114] scores were predicted by TAS EOT (β = −0.208, t = −3.097, p = 0.002), TOWRE-A (β = 0.196, t = 2.964, p = 0.003), and IRI fantasy scores (β = 0.187, t = 2.782, p = 0.006). There were two predictors in the best model for the cat eyes stimuli [F(2,201) = 8.648, p < 0.001, adj R2 = 0.070] which were TAS EOT (β = −0.158, t = −2.197, p = 0.029) and MRM scores (β = −0.186, t = 2.586, p = 0.010), and for the dog stimuli set [F(2,201) = 7.797, p < 0.001, adj R2 = 0.063], which were EOT (β = −0.188, t = 2.744, p = 0.007), and IDAQ non-anthropomorphic attribution (β = 0.171, t = 2.499, p = 0.013) scores.
Combining the 18 trial cats eyes test with the set of 18 dog eyes trials creates the Non-human Animal Reading the Mind in the Eyes Test (NARMET: Figure 2), which is the same length as the original 36 trial human RMET. Predictors of NARMET total scores were the predictors for cat and dog stimuli combined [F(3,200) = 9.474, p < 0.001, adj R2 = 0.111], i.e., TAS EOT (β = −0.181, t = 2.568, p = 0.011), MRM (β = −0.167, t = 2.305, p = 0.022), and IDAQ non-anthropomorphic attributions (β = 0.154, t = 2.235, p = 0.027).
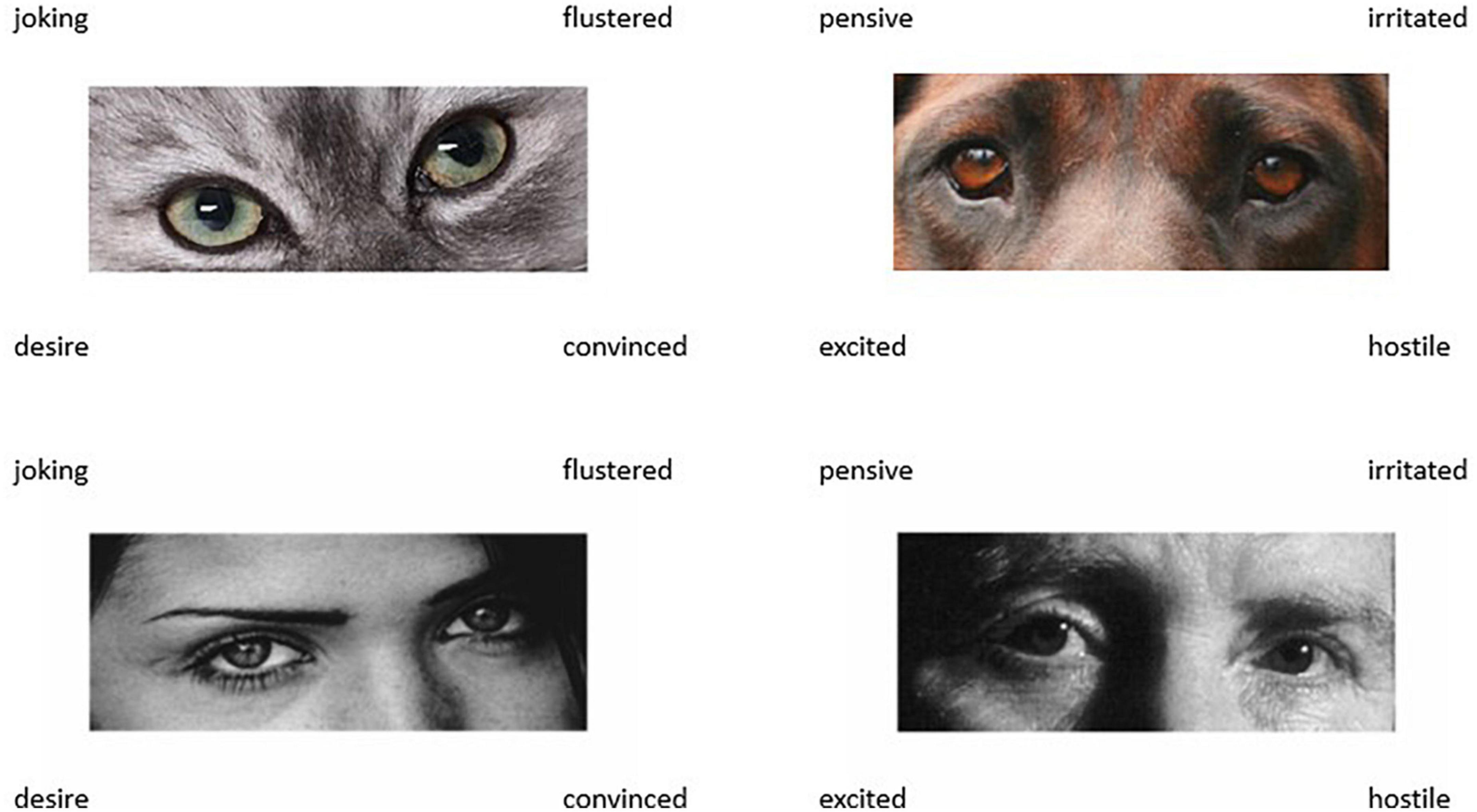
Figure 2. Example trials from the NARMET (upper) as compared to the RMET. Images were available without reuse restrictions. NARMET images adapted from https://pxhere.com/en/photo/979960, animals.com/animals/bavarian-mountain-hound/pictures/, and RMET images from https://www.autismresearchcentre.com/tests/eyes-test-adult/, licensed under CC0, CC BY-SA 3.0 and CC0 respectively.
The correct answers for the NARMET and the RMET (see Supplementary Table 2) were compared in terms of word length, frequency, valence, and concreteness using ratings from the English Lexicon Project (51). Ratings were available for all correct answers except fantasizing, which appears once in both the RMET and NARMET. Some other words were only available as verbs (e.g., insist versus insisting and regret versus regretful). Mean word length (RMET: 8.65; NARMET: 8.46), frequency (RMET: 7.51; NARMET: 7.46), and concreteness (RMET: 2.10; NARMET: 2.13) did not significantly differ between the RMET and NARMET. There was a slightly greater proportion of correct answers with a more obvious negative valence on the NARMET (RMET 4.89; NARMET: 4.30). Although the forced choice option combinations used in the RMET are also used in the NARMET, the number of unique correct answers after refinement is a little lower for the NARMET (RMET: 92%; NARMET: 69%). Finally, when considering other visual characteristics, there is also a difference in terms of the amount of stimuli depicting direct eye gaze (RMET: 66%; NARMET: 47%). Although these small differences are present they did not result in performance differences across cat, dog and human stimuli.
Building on the development of the cat eyes test (9), this study presents the new Non-Human Animal RMET. Together, the cat and dog stimuli comprise a 36 item test which can be used as a comparison measure alongside the original 36 item human RMET (1). The NARMET contains a similar range of complex mental states to the RMET such as suspicious, tentative, regretful, and defiant. Mental state judgments for NARMET stimuli are valid given that there was no significant differences in respondent accuracy compared to the standard RMET. This initial assessment of the NARMET indicates favorable psychometric properties in addition to demonstrating that the task is well matched to the RMET in terms of linguistic variables, plus overall difficulty.
Given that it was possible to create the NARMET, this may tell us something about the processes involved in these eyes tasks. Individuals appear to share a common interpretation of a fairly complex level of psychological state as reflected in the eyes of both humans and at least domesticated non-human animals such as cats and dogs. This ability seems unlikely to be a specialist skill, according to the degree of consensus seen across a few different samples during the development of the NARMET. It is still debated whether the RMET measures Theory of Mind, or emotion/mental state recognition, with difficulties posed by either interpretation. For example, Theory of Mind is often a more appropriate label when the cues are more abstract than visual (e.g., hearing a story, considering abstract mental states, like beliefs). On the other hand, visual cues may be more likely to prompt a recognition matching process. The issue with this latter interpretation is that we do not know whether the mental states that are being attributed to the eyes stimuli are indeed correct, and this is the case for both the RMET and NARMET stimuli, as correct responses are simply based on consensus. In the current study, response times were recorded, although most studies do not record this data. It took little more than 3 s of presentation time to per trial to elicit an accurate response, and this was consistent for both the human and non-human animal trials. This implies a fast and automatic underlying process, and perhaps reliance on a more instinctive mirroring type mechanism versus a more reasoned abstract perspective taking process, as the latter would likely take more time due to careful evaluation of each of the verbal labels in turn. Performance on the NARMET suggests that there are some fairly reliable visual cues (e.g., appearance of a low brow, gaze direction, and intensity) that individuals may use to draw conclusions about likely mental state, and that these cues may be more crude, and/or widely perceptible, than previously thought.
Although the range of mental states thought to be attributable to the cats and dogs was slightly less wide ranging than for humans, it was still quite extensive. This aligns with research showing that humans can attribute secondary emotions such as jealousy and guilt to dogs (25). While some studies have suggested that anthropomorphic judgments made by humans may be quite inaccurate [e.g., the guilty dog look: (52)], NARMET stimuli judgments were not related to mental state attributions on the anthropomorphism measure. Previous studies have shown that healthy participants activate many of the same brain areas (i.e., prefrontal and anterior temporal) when passively viewing humans, dogs, and primates (53) and when processing the expressions or biological motion of humans and animal faces including those of cats and dogs (54–57). However, there are potential differences in visual attention (58) and the neural correlates of face processing (59) when comparing human and non-human animals in children diagnosed with autistic spectrum disorders. Future fMRI studies may shed further light into any differences in the mental state attribution process across human and non-human eyes stimuli by using the NARMET alongside the RMET and the previously developed Age Eyes Test (9, 13, 14).
Mental state attribution in relation to human, cat and dog stimuli were all negatively associated with externally oriented thinking. When cats and dogs were combined into a single task, NARMET scores were predicted by mind reading motivation and non-anthropomorphic attributions, while human RMET performance was predicted by fantasy and word reading efficiency. Therefore, while NARMET performance may be more clearly associated with motivational factors, it is perhaps less susceptible to confounds related to abstraction, language or verbal skills, which can influence RMET performance (60). It may also avoid some of the cultural drawbacks [e.g., (61)] of the RMET. Further investigation is needed into its psychometric properties in additional samples, especially given the significant limitations such as the high majority of females, in addition to e.g., less than 2% of the sample showing any evidence of autistic tendencies. While some studies have found an effect of gender on RMET performance [e.g., (62–64)], others have not (9, 65, 66). Either way, future studies involving clinical or more diverse groups may identify specific predictors of performance on the NARMET, or on its two (cat and dog) subtests.
In conclusion, using the NARMET alongside the RMET (1), and the Age Eyes Test (9, 13, 14) could allow for the testing of double-dissociations based on making equivalent mental state judgments about different (human versus non-human animal) stimuli, versus different judgments (mental state versus physical state) about the same stimuli. It is anticipated that future studies employing this combination of tasks in developmental and clinical contexts could help to control for some performance confounds, offering enhanced assessment of social cognitive skills and unique insight into the processes involved in mental state attribution based on facial cues.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the University of Birmingham Research Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
CE conceived and designed the study, prepared materials for the data collection, performed statistical analysis, interpreted the findings, and wrote the first and final draft of the manuscript.
We thank the Dr. Peter Hansen (University of Birmingham) supported the data collection and contributed to aspects of the statistical analysis.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1129252/full#supplementary-material
1. Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “reading the mind in the eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. (2001) 42:241–51. doi: 10.1111/1469-7610.00715
2. Balogh N, Egerházi A, Berecz R, Csukly G. Investigating the state-like and trait-like characters of social cognition in schizophrenia: a short term follow-up study. Schizophr Res. (2014) 159:499–505. doi: 10.1016/j.schres.2014.08.027
3. Fernandes J, Cajão R, Lopes R, Jerónimo R, Barahona-Corrêa J. Social cognition in schizophrenia and autism spectrum disorders: a systematic review and meta-analysis of direct comparisons. Front Psychiatry. (2018) 9:504. doi: 10.3389/fpsyt.2018.00504
4. Eddy C, Sira Mahalingappa S, Rickards H. Is Huntington’s disease associated with deficits in theory of mind? Acta Neurol Scand. (2012) 126:376–83. doi: 10.1111/j.1600-0404.2012.01659.x
5. Dal Monte O, Schintu S, Pardini M, Berti A, Wassermann E, Grafman J, et al. The left inferior frontal gyrus is crucial for reading the mind in the eyes: brain lesion evidence. Cortex. (2014) 58:9–17. doi: 10.1016/j.cortex.2014.05.002
6. Nahal P, Hurd P, Read S, Crespi B. Cognitive empathy as imagination: evidence from reading the mind in the eyes in autism and schizotypy. Front Psychiatry. (2021) 12:665721. doi: 10.3389/fpsyt.2021.665721
7. Lyvers M, Kohlsdorf S, Edwards M, Thorberg F. Alexithymia and mood: recognition of emotion in self and others. Am J Psychol. (2017) 130:83–92. doi: 10.5406/amerjpsyc.130.1.0083
8. Irani F, Platek S, Panyavin I, Calkins M, Kohler C, Siegel S, et al. Self-face recognition and theory of mind in patients with schizophrenia and first-degree relatives. Schizophr Res. (2006) 88:151–60. doi: 10.1016/j.schres.2006.07.016
9. Eddy C, Hansen P. Predictors of performance on the reading the mind in the eyes test. PLoS One. (2020) 15:e0235529. doi: 10.1371/journal.pone.0235529
10. Peñuelas-Calvo I, Sareen A, Sevilla-Llewellyn-Jones J, Fernández-Berrocal P. The “reading the mind in the eyes” test in autism-spectrum disorders comparison with healthy controls: a systematic review and meta-analysis. J Autism Dev Disord. (2019) 49:1048–61. doi: 10.1007/s10803-018-3814-4
11. Hadjikhani N, Galazka M, Kenet T, Joseph R, Åsberg Johnels J. Discrepancy between high non-verbal intelligence and low accuracy at reading emotional expressions in the eyes reflects the magnitude of social-emotional difficulties in autism. Eur Arch Psychiatry Clin Neurosci. (2022). doi: 10.1007/s00406-022-01471-z [Epub ahead of print].
12. Oakley B, Brewer R, Bird G, Catmur C. Theory of mind is not theory of emotion: a cautionary note on the reading the mind in the eyes test. J Abnorm Psychol. (2016) 125:818–23. doi: 10.1037/abn0000182
13. Eddy C, Cavanna A, Hansen P. Empathy and aversion: the neural signature of mentalizing in Tourette syndrome. Psychol Med. (2017) 47:507–17. doi: 10.1017/S0033291716002725
14. Eddy C, Rickards H, Hansen P. Through your eyes or mine? The neural correlates of mental state recognition in Huntington’s disease. Hum Brain Mapp. (2018) 39:1354–66. doi: 10.1002/hbm.23923
15. Bennett P, Rutter N, Woodhead J, Howell T. Assessment of domestic cat personality, as perceived by 416 owners, suggests six dimensions. Behav Processes. (2017) 141(Pt 3):273–83. doi: 10.1016/j.beproc.2017.02.020
16. Gosling S, Sandy C, Potter J. Personalities of self-identified “dog people” and “cat people”. Anthrozoös. (2010) 23:213–22. doi: 10.2752/175303710X12750451258850
17. Schirmer A, Seow C, Penney T. Humans process dog and human facial affect in similar ways. PLoS One. (2013) 8:e74591. doi: 10.1371/journal.pone.0074591
18. Hare B, Brown M, Williamson C, Tomasello M. The domestication of social cognition in dogs. Science. (2002) 298:1634. doi: 10.1126/science.1072702
19. Albuquerque A, Guo K, Wilkinson A, Sawalli C, Otta E, Mills D. Dogs recognise dog and human emotions. Biol Lett. (2015) 12:20150883. doi: 10.1098/rsbl.2015.0883
20. Kaminski J, Hynds J, Morris P, Waller B. Human attention affects facial expressions in domestic dogs. Sci Rep. (2017) 7:12914. doi: 10.1038/s41598-017-12781-x
21. Gosling S, Kwan V, John O. A dog’s got personality: a cross-species comparative approach to personality judgments in dogs and humans. J Pers Soc Psychol. (2003) 85:1161–9. doi: 10.1037/0022-3514.85.6.1161
22. Kwan V, Gosling S, John O. Anthropomorphism as a special case of social perception: a cross-species social relations model analysis of humans and dogs. Soc Cogn. (2008) 26:129–42. doi: 10.1521/soco.2008.26.2.129
23. Fratkin J, Sinn D, Patall E, Gosling S. Personality consistency in dogs: a meta-analysis. PLoS One. (2013) 8:e54907. doi: 10.1371/journal.pone.0054907
24. Walker J, McGrath N, Handel I, Waran N, Phillips C. Does owning a companion animal influence the belief that animals experience emotions such as grief? Anim Welf. (2014) 23:71–9. doi: 10.7120/09627286.23.1.071
25. Morris P, Doe C, Godsell E. Secondary emotions in non-primate species? Behavioural reports and subjective claims by animal owners. Cogn Emot. (2008) 22:3–20. doi: 10.1080/02699930701273716
26. Morris P, Knight S, Lesley S. Belief in animal mind: does familiarity with animals influence beliefs about animal emotions? Soc Anim. (2012) 20:211–24. doi: 10.1163/15685306-12341234
27. Olderbak S, Wilhelm O, Olaru G, Geiger M, Brenneman M, Roberts R. A psychometric analysis of the reading the mind in the eyes test: toward a brief form for research and applied settings. Front Psychol. (2015) 6:1503. doi: 10.3389/fpsyg.2015.01503
28. Eddy C, Sira Mahalingappa S, Rickards H. Putting things into perspective: the nature and impact of theory of mind impairment in Huntington’s disease. Eur Arch Psychiatry Clin Neurosci. (2014) 264:697–705. doi: 10.1007/s00406-014-0498-4
29. Graham J. Missing data analysis: making it work in the real world. Ann Rev Psychol. (2009) 60:549–76. doi: 10.1146/annurev.psych.58.110405.085530
30. Torgesen J, Wagner R, Rashotte C. Test of Word Reading Efficiency (TOWRE). Austin, TX: Pro-Ed (1999).
31. Reynolds C, Kamphaus R. Test of Irregular Word Reading Efficiency. Lutz, FL: Psychological Assessment Resources Inc (2007).
32. Davis M. A multidimensional approach to individual differences in empathy. JSAS Catalog Select Doc Psychol. (1980) 10:85.
33. Davis M. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Social Psychol. (1983) 44:113–26. doi: 10.1037/0022-3514.44.1.113
34. Bagby R, Parker J, Taylor G. The twenty-item Toronto Alexithymia Scale: I. Item selection and cross-validation of the factor structure. J Psychosom Res. (1994) 38:23–32. doi: 10.1016/0022-3999(94)90005-1
35. Bagby R, Taylor G, Parker J. The twenty-item Toronto Alexithymia Scale: II. Convergent, discriminant, and concurrent validity. J Psychosom Res. (1994) 38:33–40. doi: 10.1016/0022-3999(94)90006-X
36. Taylor G, Bagby R, Ryan D, Parker J, Doody K, Keefe P. Criterion validity of the Toronto Alexithymia Scale. Psychosom Med. (1988) 50:500–9. doi: 10.1097/00006842-198809000-00006
37. Eckblad M, Chapman L, Chapman J, Mishlove M. The Revised Social Anhedonia Scale. Unpublished Test. (1982).
38. Assouly-Besse F, Dollfus S, Petit M. Traduction française des questionnaires d’anhédonie sociale et physique de Chapman: validation de la traduction française à partir de témoins et de patients schizophrènes. Encephale. (1995) 21:273–84.
39. Waytz A, Cacioppo J, Epley N. Who sees human? The stability and importance of individual differences in anthropomorphism. Perspect Psychol Sci. (2010) 5:219–32. doi: 10.1177/1745691610369336
40. Doherty R, Orimoto L, Singelis T, Hatfield I, Hebb J. Emotional contagion: gender and occupational differences. Psychol Women Q. (1995) 19:355–71. doi: 10.1111/j.1471-6402.1995.tb00080.x
41. Doherty R. The emotional contagion scale: a measure of individual differences. J Nonv Behav. (1997) 21:131–54. doi: 10.1037/t07987-000
42. Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatr Scand. (1983) 67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x
43. Carpenter J, Green M, Vacharkulksmsuk T. Beyond perspective-taking: mind-reading motivation. Motiv Emot. (2016) 40:358–74. doi: 10.1007/s11031-016-9544-z
44. Mason O, Claridge G. The Oxford-Liverpool inventory of feelings and experiences (O-LIFE): further description and extended norms. Schizophr Res. (2006) 82:203–11. doi: 10.1016/j.schres.2005.12.845
45. Mason O, Claridge G, Jackson M. New scales for the assessment of schizotypy. Pers Individ Differ. (1995) 18:7–13. doi: 10.1016/0191-8869(94)00132-C
46. Burch G, Steel C, Hemsley D. Oxford-Liverpool inventory of feelings and experiences: reliability in an experimental population. Br J Clin Psychol. (1998) 37:107–8. doi: 10.1111/j.2044-8260.1998.tb01284.x
47. Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. (2001) 31:5–17. doi: 10.1023/A:1005653411471
48. Ferguson F, Austin E. Associations of trait and ability emotional intelligence with performance on theory of mind tasks in an adult sample. Pers Individ Differ. (2010) 49:414–8. doi: 10.1016/j.paid.2010.04.009
49. Baron-Cohen S, Jolliffe T, Mortimore C, Robertson M. Another advanced test of theory of mind: evidence from very high functioning adults with autism or Asperger syndrome. J Child Psychol Psychiatry. (1997) 38:813–22. doi: 10.1111/j.1469-7610.1997.tb01599.x
50. Fernández-Abascal E, Cabello R, Fernández-Berrocal P, Baron-Cohen S. Test–retest reliability of the “reading the mind in the eyes” test: a one-year follow-up study. Mol Autism. (2013) 4:1–6. doi: 10.1186/2040-2392-4-33
51. Elexicon. Elexicon. Available online at: https://elexicon.wustl.edu/ (accessed October 2020).
52. Horowitz A. Disambiguating the “guilty look”: salient prompts to a familiar dog behaviour. Behav Processes. (2009) 81:447–52. doi: 10.1016/j.beproc.2009.03.014
53. Spunt R, Ellsworth E, Adolphs R. The neural basis of understanding the expression of the emotions in man and animals. Soc Cogn Affect Neurosci. (2017) 12:95–105. doi: 10.1093/scan/nsw161
54. Blonder L, Smith C, Davis C, Kesler-West M, Garrity T, Avison M, et al. Regional brain response to faces of humans and dogs. Brain Res Cogn Brain Res. (2004) 20:384–94. doi: 10.1016/j.cogbrainres.2004.03.020
55. Franklin R Jr, Nelson A, Baker M, Beeney J, Vescio T, Lenz-Watson A, et al. Neural responses to perceiving suffering in humans and animals. Soc Neurosci. (2013) 8:217–27. doi: 10.1080/17470919.2013.763852
56. Kanwisher N, Stanley D, Harris A. The fusiform face area is selective for faces not animals. Neuroreport. (1999) 10:183–7. doi: 10.1097/00001756-199901180-00035
57. Tong F, Nakayama K, Moscovitch M, Weinrib O, Kanwisher N. Response properties of the human fusiform face area. Cogn Neuropsychol. (2000) 17:257–79. doi: 10.1080/026432900380607
58. Valiyamattam G, Katti H, Chaganti V, O’Haire M, Sachdeva V. Do animals engage greater social attention in autism? An eye tracking analysis. Front Psychol. (2020) 11:727. doi: 10.3389/fpsyg.2020.00727
59. Whyte E, Behrmann M, Minshew N, Garcia N, Scherf K. Animal, but not human, faces engage the distributed face network in adolescents with autism. Dev Sci. (2016) 19:306–17. doi: 10.1111/desc.12305
60. Pavlova M, Sokolov A. Reading language of the eyes. Neurosci Biobehav Rev. (2022) 140:104755. doi: 10.1016/j.neubiorev.2022.104755
61. Van Staden J, Callaghan C. An evaluation of the reading the mind in the eyes test’s psychometric properties and scores in South Africa-cultural implications. Psychol Res. (2022) 86:2289–300. doi: 10.1007/s00426-021-01539-w
62. Kirkland R, Peterson E, Baker C, Miller S, Pulos S. Meta-analysis reveals adult female superiority in “reading the mind in the eyes” test. North Am J Psychol. (2013) 15:121–46.
63. Isernia S, Sokolov A, Fallgatter A, Pavlova M. Untangling the ties between social cognition and body motion: gender impact. Front Psychol. (2020) 11:128. doi: 10.3389/fpsyg.2020.00128
64. Pavlova M, Sokolov A. Reading covered faces. Cereb Cortex. (2022) 32:249–65. doi: 10.1093/cercor/bhab311
65. Baron-Cohen S, Ring H, Chitnis X, Wheelwright S, Gregory L, Williams S, et al. fMRI of parents of children with Asperger syndrome: a pilot study. Brain Cogn. (2006) 61:122–30. doi: 10.1016/j.bandc.2005.12.011
Keywords: alexithymia, animal images, assessment, emotion, empathy, measures, social cognition
Citation: Eddy CM (2023) The non-human animal reading the mind in the eyes test (NARMET): A new measure for the assessment of social cognition. Front. Psychiatry 14:1129252. doi: 10.3389/fpsyt.2023.1129252
Received: 21 December 2022; Accepted: 28 February 2023;
Published: 20 March 2023.
Edited by:
Gabriela Rosenblau, George Washington University, United StatesReviewed by:
Marina A. Pavlova, University Hospital Tübingen, GermanyCopyright © 2023 Eddy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clare M. Eddy, Y2xhcmUuZWRkeTFAbmhzLm5ldA==, Yy5lZGR5QGJoYW0uYWMudWs=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.