
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 30 March 2023
Sec. Neuroimaging
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1128808
This article is part of the Research Topic Social-Related Biomarkers and Potential Noninvasive Treatments for Sub-clinical and Clinical Emotional Disorders View all 5 articles
 Suming Zhang1,2†
Suming Zhang1,2† Bin Li3†
Bin Li3† Jiaxin Jiang3
Jiaxin Jiang3 Xinyu Hu1,2
Xinyu Hu1,2 Hailong Li1,2
Hailong Li1,2 Lingxiao Cao1,2
Lingxiao Cao1,2 Zilin Zhou1,2
Zilin Zhou1,2 Kaili Liang1,2
Kaili Liang1,2 Huan Zhou1,2
Huan Zhou1,2 Lianqing Zhang1,2
Lianqing Zhang1,2 Qiyong Gong1,4*
Qiyong Gong1,4* Xiaoqi Huang1,2*
Xiaoqi Huang1,2*Background: Although the specific role of the uncinate fasciculus (UF) in emotional processing in patients with obsessive–compulsive disorder (OCD) has been investigated, the exact focal abnormalities in the UF have not been identified. The aim of the current study was to identify focal abnormalities in the white matter (WM) microstructure of the UF and to determine the associations between clinical features and structural neural substrates.
Methods: In total, 71 drug-naïve patients with OCD and 81 age- and sex-matched healthy controls (HCs) were included. Automated fiber quantification (AFQ), a tract-based quantitative approach, was adopted to measure alterations in diffusion parameters, including fractional anisotropy (FA), mean diffusivity (MD), radial diffusivity (RD) and axial diffusivity (AD), along the trajectory of the UF. Additionally, we utilized partial correlation analyses to explore the relationship between the altered diffusion parameters and clinical characteristics.
Results: OCD patients showed significantly higher FA and lower RD at the level of the temporal and insular portions in the left UF than HCs. In the insular segments of the left UF, increased FA was positively correlated with the Hamilton Anxiety Scale (HAMA) score, while decreased RD was negatively correlated with the duration of illness.
Conclusion: We observed specific focal abnormalities in the left UF in adult patients with OCD. Correlations with measures of anxiety and duration of illness underscore the functional importance of the insular portion of left UF disturbance in OCD patients.
Obsessive–compulsive disorder (OCD) has a lifetime prevalence of 1–3% worldwide and is characterized by compulsions or obsessions that generate fear and anxiety (with or without autonomic symptoms) or doubts/uncertainty (1). The pathophysiological mechanism of OCD is unclear. In addition to the traditional cortico-striatal-thalamo-cortical (CSTC) circuit (2–4), studies have proven that the fronto-limbic system plays a vital role in emotional regulation among OCD patients (5, 6). Dysregulated fear and uncertainty intolerance aggravate obsession and are closely related to abnormalities of the fronto-limbic circuit in OCD (7).
The hook-shaped uncinate fasciculus (UF) is a white matter bundle belonging to the fronto-limbic circuit, which connects the lateral orbitofrontal cortex and the prefrontal cortex to the anterior temporal lobes and basolateral amygdala (8, 9). The UF plays a putative role in social emotional processing and episodic memory (10), which are impaired in OCD patients (11). Diffusion tensor imaging (DTI) allows the quantification of white matter microstructure and can reveal the brain substrates of pathologic alterations in structural connectivity (12). Examination of the abnormal microstructure of key white matter tracts in individuals with OCD can improve our understanding of the neural mechanisms that underlie this disorder.
Recent studies have elucidated abnormalities related to the UF using DTI techniques in OCD patients both at the group level (13–15) and at the individual level (16). At the group level, one study employing probabilistic tractography confirmed a decrease of fractional anisotropy (FA) in the right UF in OCD patients in addition to a significant correlation between axial diffusivity (AD) in the right UF and symptom severity (14). At the individual level, a multimodality study that combined whole-brain volumetry and diffusion tensor imaging used a support vector machine (SVM) to discriminate between OCD patients and HCs (16). The UF was identified as the region that contributed the most to this discriminatory ability (16). Taken together, this evidence indicates that the UF plays a potential role in the neuropathologic mechanisms of OCD.
Previous studies utilizing voxel-based analyses or tract-based spatial statistics (TBSS) revealed that the UF showed decreased integrity in OCD (14). Although those whole-brain studies provided evidence that some symptoms of OCD might be linked to the integrity of the UF, no previous study has provided a detailed description of the abnormalities along the trajectory of the UF in OCD patients. Thus, in the current study, we assessed the bilateral UF by quantifying diffusion parameters at multiple nodes along the tract’s trajectory rather than obtaining the mean value of the whole fiber using an automatic fiber quantification (AFQ) method similar to our previous study (17). We hypothesized that specific focal regions in the UF would be more vulnerable to microstructural dysconnectivity and correlated with symptom severity in patients with OCD.
The study was approved by the Institutional Reviews Board of West China Hospital, Sichuan University. After being informed of the nature and aims of the study, all subjects signed a consent form before participating in the study procedures.
Seventy-one adults with OCD (age range = 18–35 years, mean age = 30.14 years; male percentage = 60.83%) and 81 age- and sex-matched healthy adult controls (age range = 18–37 years, mean age = 29.76 years; male percentage = 58.33%) were included in this study. All patients were right handedness and native Han Chinese. Patients were recruited at the West China Hospital, Sichuan University. All OCD patients had never received medication or systematic psychotherapy before MRI data acquisition. We established a clinical diagnosis using the Structured Clinical Interview for DSM-IV Axis I disorders (SCID) (18), which was administered by two experienced clinical psychiatrists. We assessed the severity of OCD symptoms using the Yale-Brown Obsessive–Compulsive Scale (19). The 14-item Hamilton Anxiety Rating (HAMA) Scale (20) and 17-item Hamilton Depression Rating (HAMD) Scale (21) were used to assess accompanying anxiety and depression.
Healthy controls were recruited from the local area using poster advertisements and screened using the SCID (non-patient version) to confirm the current absence of psychiatric disorders, as well as the absence of a history of psychiatric disorders among their first-degree relatives.
The exclusion criteria, applied to both OCD patients and HCs, were as follows: age younger than 18 years or older than 60 years; history of a psychotic, affective, or anxiety disorder other than OCD, as determined with the SCID; a history of significant systemic illness, cardiovascular disease, or neurologic disorder; substance abuse or dependence; and pregnancy.
Images were acquired on a 3 T GE (EXCITE, General Electric) magnetic resonance imaging (MRI) system with an eight-channel phased array head coil. A single-shot spin echo planar imaging (EPI) sequence was acquired with 15 noncolinear directions (b = 1000 s/mm2) and one reference volume with b = 0 s/mm2. Repetition time (TR) = 12,000 ms, echo time (TE) = 70.8 ms, slice thickness = 3 mm (no slice gap), number of excitations = 2, matrix = 128*128, field-of-view (FOV) = 240 * 240 mm2, and voxel size = 1.875*1.875*3 mm3. DTI was performed using axial sections parallel to the anterior–posterior commissural line to cover the entire brain. As an anatomical reference for normalization, high-resolution T1-weighted images were acquired using a 3D spoiled gradient recalled (SPGR) sequence (TR/TE = 8.5/3.4 ms, 156 slices with thickness 1 mm, flip angle = 12°, matrix = 256 * 256, FOV = 240 * 240 mm2, and voxel size = 0.93 * 0.93 * 1 mm3).
DTI data were preprocessed by using the FMRIB Software Library (FSL) 6.0.1 All data were visually inspected for artifacts. Brain extraction was completed via FSL’s “BET” function (22) and correction for the effects of head motion and image distortions caused by eddy currents was performed using “EDDY” tool (23–26). FA images were created by fitting a tensor model to the raw diffusion data using the FMRIB Diffusion Toolbox (FDT) (27). The high-resolution T1-weighted brain structural images were coregistered into the averaged b0 images for each subject. We extracted the head motion parameters from DTI data to exclude subjects showing >2 mm displacement or translation in the X, Y, and Z directions or >2° rotation around the X, Y, Z axes (28, 29); the motion measurements did not differ between the OCD group and HC group (details in Supplementary Table S1).
Automated deterministic tractography of bilateral UF was performed according to standard protocols and pipelines, which are described by Yeatman in detail (30). Reconstruction of UF was completed via the open-source VISTASOFT package version 1.02 and AFQ toolkit package3 (version 1.2) (30).
First, whole-brain deterministic fiber tractography was estimated. The tracking algorithm started within a WM mask defined by voxels with FA values >3; then, continuing the path integration procedure, the fibers were traced in both directions along principal diffusion axes. Tracing was terminated when FA < 2 or the minimum angle between the last path segment and next step was >30. Fiber tract segmentation was performed using the waypoint ROI procedure, and fiber refinement was accomplished by comparing each candidate fiber to fiber tract probability maps. After the bilateral UF tracts were identified, the fiber points of each tract were resampled by using 100 equidistant points, and then the diffusion measurements of each participant, including the FA, MD, RD and AD, were extracted along the UF (15).
Two-sample t-tests were carried out to evaluate differences in DTI parameters (FA, MD, AD, and RD) between the OCD group and HC group at each node in the bilateral UF. Afterward, partial correlation analyses were further performed between symptom severity and DTI parameters (FA, MD, AD, and RD) extracted from significant nodes in the bilateral UF, with age and sex as covariates. Significant results were corrected for multiple comparisons using the false discovery rate (FDR corrected p-value < 0.05).
The demographic and clinical characteristics of the participants in this study are shown in Table 1.
In the point-wise comparison with HCs, significantly increased FA and reduced MD and RD were found at the level of the temporal portion (nodes 24–33) in the left UF tracts in OCD (p <0.05, FDR correction), while significantly increased FA and decreased RD were observed at the level of the insular segments (nodes 41–77) in the left UF tracts in OCD (p < 0.05, FDR correction; Figure 1).
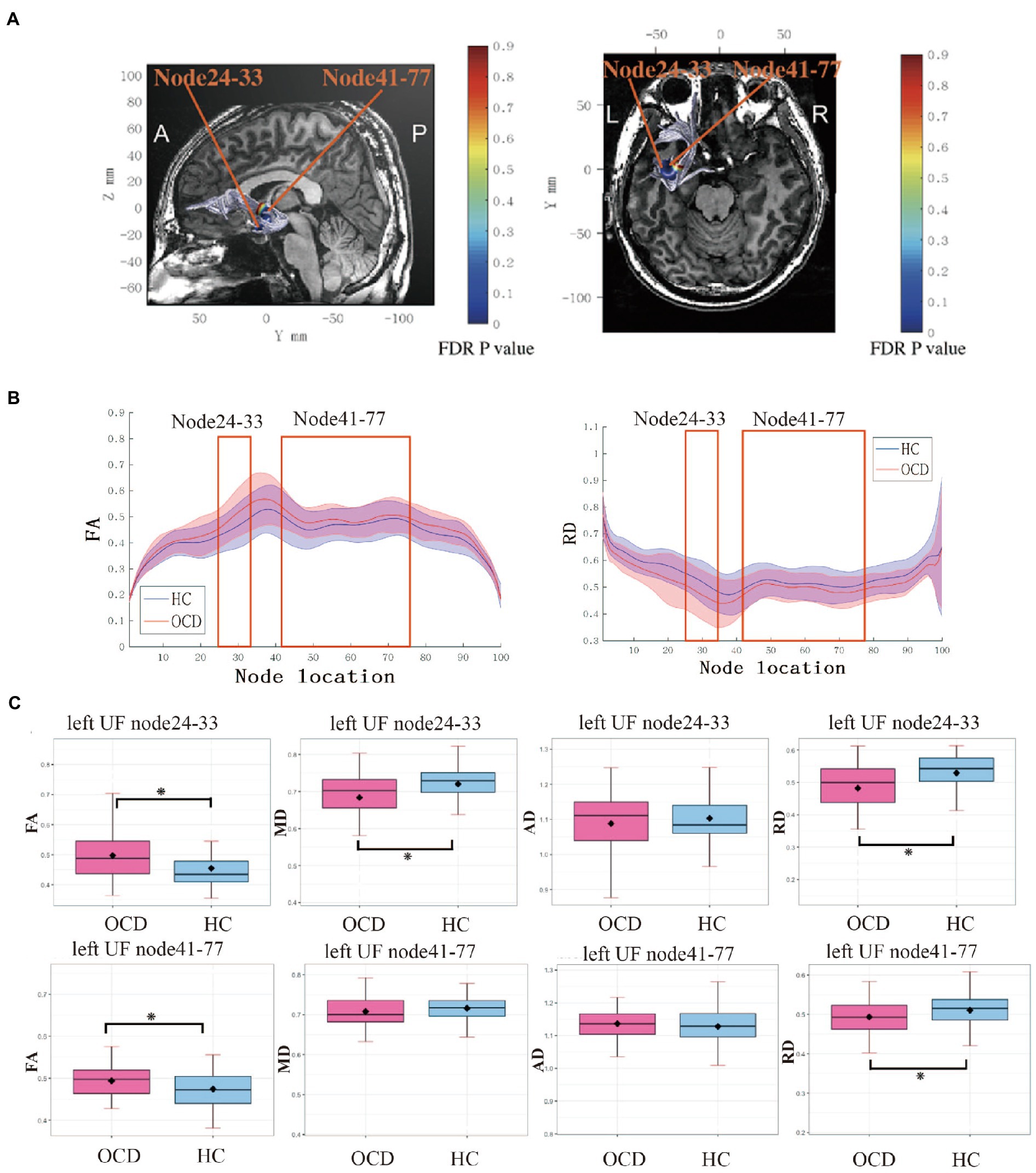
Figure 1. (A) Left UF tract with significant group differences (p < 0.05, FDR corrected) at two segments: temporal portion (node 24–33) and insular portion (node 41–77). (B) FA between nodes 1 and 100 for the left UF in OCD group and HC group. Solid lines represent the mean FA, and dotted lines denote the standard error of the mean. Consecutive nodes that showed significant differences are marked with red rectangles. (p < 0.05, FDR corrected). (C) Boxplots for FA, RD, MD, and AD in two significant segments between groups. Error bars represent standard deviation. UF, uncinate fasciculus; FA; fractional anisotropy; MD, mean diffusivity; AD, axial diffusivity, RD, radial diffusivity; OCD, obsessive–compulsive disorder; HC, healthy control.
In OCD patients, the mean FA of nodes 41–77 of the left UF correlated positively with HAMA scores (R = 0.251, p = 0.004). In addition, the mean RD of nodes 41–77 correlated negatively with the duration of illness (R = −0.26, p = 0.032; Figure 2).
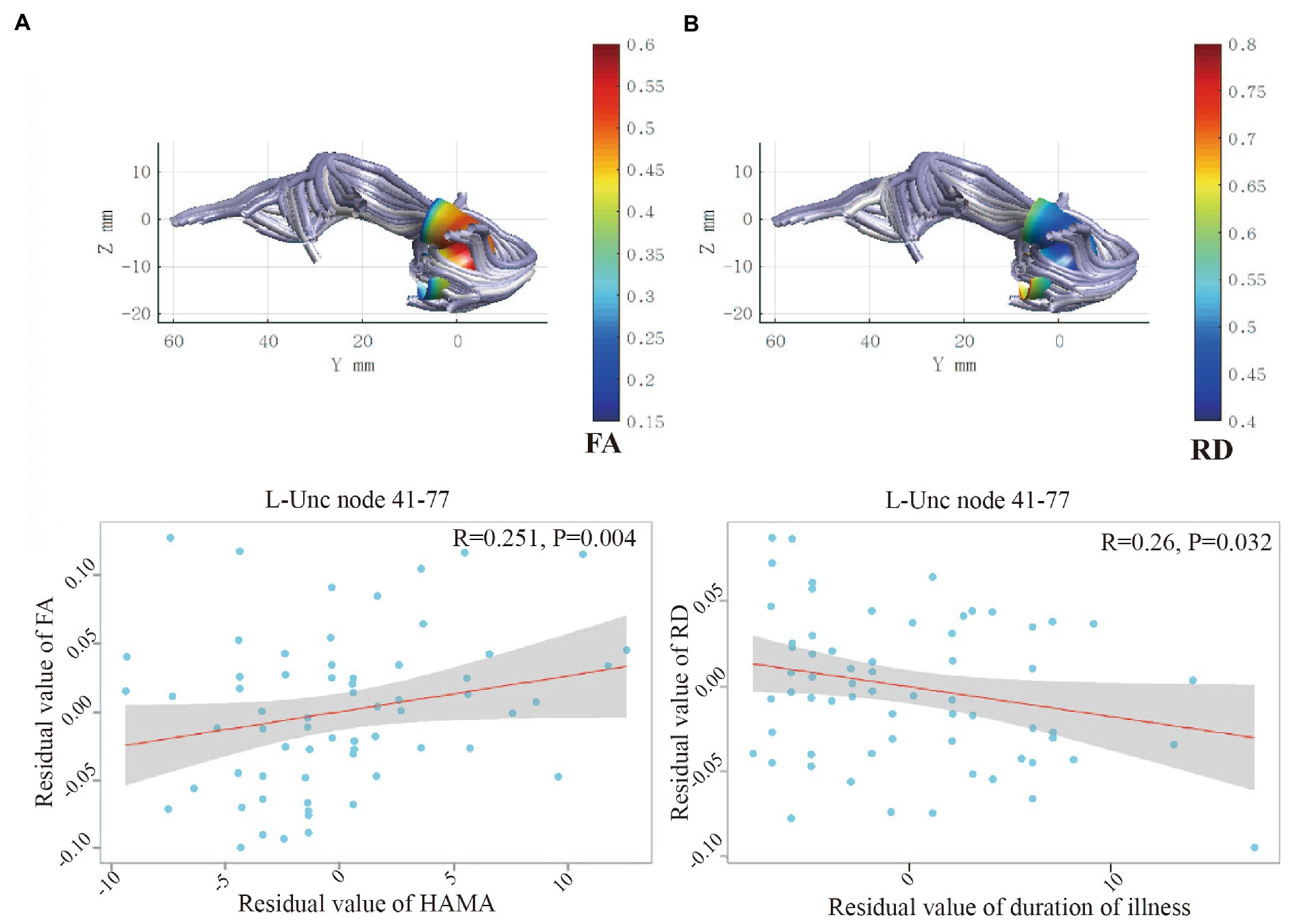
Figure 2. (A) Positive correlation between HAMA and FA for the node 41–77 of left UF. (B) Negative correlation between duration of illness and RD for the node 41–77 of left UF. UF, uncinate fasciculus; FA; fractional anisotropy; RD, radial diffusivity; OCD, obsessive–compulsive disorder; HC, healthy control; HAMA, Hamilton Anxiety Scale.
To our knowledge, this is the first study utilizing a tractography method to explore the focal abnormality of the UF in individuals with OCD. We found significantly higher FA along with lower RD at the level of the temporal portion and insular portion of the left UF in OCD patients than in HCs. Within the focal insular portion of the left UF, the increased FA difference showed a positive correlation with anxiety severity in individuals with OCD, whereas the decreased RD showed a negative correlation with the duration of illness. Taken together, these findings elucidated the focal structural connectivity of the left UF in neural mechanisms of OCD, which may help to innovate more targeted interventions for individuals with OCD in the future.
The UF connects the lateral orbitofrontal cortex and the prefrontal cortex to the anterior temporal lobes and basolateral amygdala (3, 4). The hook-shaped fiber plays a putative role in social emotional processing (5). Additionally, it is a key component of the fronto-limbic network, which plays a significant role in OCD pathophysiology (31). Evidence suggests that higher FA together with lower RD is related to increased myelination, dense axonal packing, white matter maturation, and neuronal remodeling (32–34). Our finding of higher FA suggests that OCD patients may exhibit greater fiber integrity in the insular portion and temporal portion of the left UF relative to HCs. Similar to the interpretation of Li et al., an increase in FA might be a compensatory process for neuronal injury in individuals with OCD (35). The negative correlation between decreased RD and the duration of illness indicated that flawed WM microstructure has predictive ability for the duration of illness. The longer the duration of illness, the lower the RD of the insular part of the left UF. Our finding is different from that of another TBSS study (36). That study reported higher RD in the whole left UF and no finding of an association with the duration of illness in patients with OCD.
Compared to previous studies that reported abnormalities in the entire uncinate fasciculus (13–15, 36), we demonstrated that higher FA and lower RD are focal in the insular portion and temporal portion of the left UF in individuals with OCD. Furthermore, increased FA in the insular portion of the left UF showed a positive correlation with anxiety severity in our study. It can be inferred that the more severe the anxiety symptoms are, the greater the remyelination that occurs in the insular portion of the left UF among OCD patients based on our findings. Evidence has shown that the insular lobe might be involved in affective processing (37). In particular, the insular cortex is believed to be involved in OCD (38–41). Our findings add evidence that abnormal fiber integrity in the insular portion of the left UF may underlie anxiety symptoms in individuals with OCD. Furthermore, decreased gray matter volume in the insular region has been reported in a voxel-based morphometric study among OCD patients (42). Thus, it may be inferred that greater myelination in the insular segment of the left UF, which increases the speed of information communication, is parallel to abnormal gray matter volume in the insular cortex area among OCD patients.
Our findings in the UF of OCD patients are different from those of previous diffusion MRI studies in OCD patients. In contrast to abnormal fiber integrity in the left UF in our findings, one former study reported a lower FA and higher RD of the right UF in OCD patients than in HCs (14, 36). We inferred that these differences are due to the analysis method and patient status. The TRActs Constrained by UnderLying Anatomy (TRACULA), which belongs to probabilistic tractography, was used in the former studies (14, 36), whereas deterministic tractography was utilized in our study. Another previous TBSS study demonstrated increased RD and MD in the bilateral UF in individuals with OCD (36), and some potential reasons for these differences in findings may be attributable to variations in the sample size, patient medication status and MRI scan parameters.
Our study had several limitations. First, we did not employ pediatric OCD patients in the current investigation. A previous publication identified distinct WM alterations in pediatric and adult OCD (43). Future studies involving pediatric OCD patients are needed to shed light on the neurodevelopmental alterations of WM in individuals with OCD. Second, since we included only medication-naïve patients with OCD, it remains to be explored whether our results could be generalized to larger OCD populations undergoing long-term treatment.
In summary, the present study revealed abnormal white matter changes along the left UF tract using a tract-wise approach in individuals with OCD. We demonstrated that altered fiber integrity is focal in the insular and temporal portions of the left UF. The increased FA and decreased RD of the insular part of the left UF are associated with anxiety and duration of illness, respectively. Our study suggests that the insular portion of the left UF plays an important role in OCD pathophysiology.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Institutional Review Board of the West China Hospital, Sichuan University. The patients/participants provided their written informed consent to participate in this study.
SZ, XiaoqiH, and QG designed the study. BL, JJ, and XinyuH acquired the data. SZ, KL, and LZ analyzed the data. SZ, BL, and XinyuH wrote the article. HL, LC, ZZ, HZ, and XiaoqiH reviewed the article. All authors contributed to the article and approved the submitted version.
The authors would like to thank their tutor and colleagues for their time and valuable help. This study was supported by the Clinical and Translational Research Fund of Chinese Academy of Medical Sciences (grant no. 2021-I2M-C&T-B-097), the Natural Science Foundation of Sichuan Province (grant no. 2022NSFSC0052), and the 1·3·5 Project for Disciplines of Excellence, West China Hospital of Sichuan University (grant no. ZYJC21041).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1128808/full#supplementary-material
1. Fontenelle, LF, Mendlowicz, MV, and Versiani, M. The descriptive epidemiology of obsessive–compulsive disorder. Prog Neuro-Psychopharmacol Biol Psychiatry. (2006) 30:327–37. doi: 10.1016/j.pnpbp.2005.11.001
2. Graybiel, AM, and Rauch, SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron. (2000) 28:343–7. doi: 10.1016/S0896-6273(00)00113-6
3. Aouizerate, B, Guehl, D, Cuny, E, Rougier, A, Bioulac, B, Tignol, J, et al. Pathophysiology of obsessive–compulsive disorder: a necessary link between phenomenology, neuropsychology, imagery and physiology. Prog Neurobiol. (2004) 72:195–221. doi: 10.1016/j.pneurobio.2004.02.004
4. Stein, DJ, Costa, DL, Lochner, C, Miguel, EC, Reddy, YJ, Shavitt, RG, et al. Obsessive–compulsive disorder. Nat Rev Dis Primers. (2019) 5:52. doi: 10.1038/s41572-019-0102-3
5. van Velzen, LS, de Wit, SJ, Ćurĉić-Blake, B, Cath, DC, de Vries, FE, Veltman, DJ, et al. Altered inhibition-related frontolimbic connectivity in obsessive–compulsive disorder. Hum Brain Mapp. (2015) 36:4064–75. doi: 10.1002/hbm.22898
6. Liu, J, Cao, L, Li, H, Gao, Y, Bu, X, Liang, K, et al. Abnormal resting-state functional connectivity in patients with obsessive-compulsive disorder: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2022) 135:104574. doi: 10.1016/j.neubiorev.2022.104574
7. van den Heuvel, OA, van Wingen, G, Soriano-Mas, C, Alonso, P, Chamberlain, SR, Nakamae, T, et al. Brain circuitry of compulsivity. Eur Neuropsychopharmacol. (2016) 26:810–27. doi: 10.1016/j.euroneuro.2015.12.005
8. Bhatia, K, Henderson, L, Yim, M, Hsu, E, and Dhaliwal, R. Diffusion tensor imaging investigation of Uncinate fasciculus anatomy in healthy controls: description of a Subgenual stem. Neuropsychobiology. (2017) 75:132–40. doi: 10.1159/000485111
9. Ghashghaei, H, Hilgetag, CC, and Barbas, H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage. (2007) 34:905–23. doi: 10.1016/j.neuroimage.2006.09.046
10. Von Der Heide, RJ, Skipper, LM, Klobusicky, E, and Olson, IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. (2013) 136:1692–707. doi: 10.1093/brain/awt094
11. Exner, C, Kohl, A, Zaudig, M, Langs, G, Lincoln, TM, and Rief, W. Metacognition and episodic memory in obsessive-compulsive disorder. J Anxiety Disord. (2009) 23:624–31. doi: 10.1016/j.janxdis.2009.01.010
12. Fields, RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. (2008) 31:361–70. doi: 10.1016/j.tins.2008.04.001
13. Zarei, M, Mataix-Cols, D, Heyman, I, Hough, M, Doherty, J, Burge, L, et al. Changes in gray matter volume and white matter microstructure in adolescents with obsessive-compulsive disorder. Biol Psychiatry. (2011) 70:1083–90. doi: 10.1016/j.biopsych.2011.06.032
14. He, X, Steinberg, E, Stefan, M, Fontaine, M, Simpson, HB, and Marsh, R. Altered frontal interhemispheric and fronto-limbic structural connectivity in unmedicated adults with obsessive-compulsive disorder. Hum Brain Mapp. (2018) 39:803–10. doi: 10.1002/hbm.23883
15. Lochner, C, Fouché, J-P, du Plessis, S, Spottiswoode, B, Seedat, S, Fineberg, N, et al. Evidence for fractional anisotropy and mean diffusivity white matter abnormalities in the internal capsule and cingulum in patients with obsessive–compulsive disorder. J Psychiatry Neurosci. (2012) 37:193–9. doi: 10.1503/jpn.110059
16. Zhou, C, Cheng, Y, Ping, L, Xu, J, Shen, Z, Jiang, L, et al. Support vector machine classification of obsessive-compulsive disorder based on whole-brain volumetry and diffusion tensor imaging. Front Psych. (2018) 9:524. doi: 10.3389/fpsyt.2018.00524
17. Bu, X, Yang, C, Liang, K, Lin, Q, Lu, L, Zhang, L, et al. Quantitative tractography reveals changes in the corticospinal tract in drug-naïve children with attention-deficit/hyperactivity disorder. J Psychiatry Neurosci. (2020) 45:134–41. doi: 10.1503/jpn.190024
18. First, MB, and Gibbon, M. “The structured clinical interview for DSM-IV axis I disorders (SCID-I) and the structured clinical interview for DSM-IV axis II disorders (SCID-II),” in Comprehensive Handbook of Psychological Assessment, Personality Assessment Vol. 2. eds. M. J. Hilsenroth and D. L. Segal (John Wiley & Sons Inc.) (2004) 134–143.
19. Goodman, WK, Price, LH, Rasmussen, SA, Mazure, C, Fleischmann, RL, Hill, CL, et al. The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Arch Gen Psychiatry. (1989) 46:1006–11. doi: 10.1001/archpsyc.1989.01810110048007
20. Hamilton, M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
21. Beauseigneur-Nuyts, T, Vallet, R, and Le Viet, M. Psychopharmacologic study of 8909 RP (Neuleptil) by Wittenborn’s psychiatric status rating scale. L’encephale. (1964) 53:405–14.
22. Smith, SM. Fast robust automated brain extraction. Hum Brain Mapp. (2002) 17:143–55. doi: 10.1002/hbm.10062
23. Andersson, JL, Graham, MS, Drobnjak, I, Zhang, H, and Campbell, J. Susceptibility-induced distortion that varies due to motion: correction in diffusion MR without acquiring additional data. NeuroImage. (2018) 171:277–95. doi: 10.1016/j.neuroimage.2017.12.040
24. Andersson, JL, Graham, MS, Drobnjak, I, Zhang, H, Filippini, N, and Bastiani, M. Towards a comprehensive framework for movement and distortion correction of diffusion MR images: within volume movement. NeuroImage. (2017) 152:450–66. doi: 10.1016/j.neuroimage.2017.02.085
25. Andersson, JL, Graham, MS, Zsoldos, E, and Sotiropoulos, SN. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. NeuroImage. (2016) 141:556–72. doi: 10.1016/j.neuroimage.2016.06.058
26. Andersson, JL, and Sotiropoulos, SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage. (2016) 125:1063–78. doi: 10.1016/j.neuroimage.2015.10.019
27. Basser, PJ, and Pierpaoli, C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. 1996. J Magn Reson. (2011) 213:560–70. doi: 10.1016/j.jmr.2011.09.022
28. Chang, YS, Owen, JP, Pojman, NJ, Thieu, T, Bukshpun, P, Wakahiro, ML, et al. White matter changes of neurite density and fiber orientation dispersion during human brain maturation. PLoS One. (2015) 10:e0123656. doi: 10.1371/journal.pone.0123656
29. Suo, X, Lei, D, Li, W, Sun, H, Qin, K, Yang, J, et al. Psychoradiological abnormalities in treatment-naive noncomorbid patients with posttraumatic stress disorder. Depress Anxiety. (2022) 39:83–91. doi: 10.1002/da.23226
30. Yeatman, JD, Dougherty, RF, Myall, NJ, Wandell, BA, and Feldman, HM. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One. (2012) 7:e49790. doi: 10.1371/journal.pone.0049790
31. Lee, KS, and Lee, SH. White matter-based structural brain network of anxiety. Adv Exp Med Biol. (2020) 1191:61–70. doi: 10.1007/978-981-32-9705-0_4
32. Beaulieu, C. The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed. (2002) 15:435–55. doi: 10.1002/nbm.782
33. Rosas, HD, Tuch, DS, Hevelone, ND, Zaleta, AK, Vangel, M, Hersch, SM, et al. Diffusion tensor imaging in presymptomatic and early Huntington’s disease: selective white matter pathology and its relationship to clinical measures. Mov Disord. (2006) 21:1317–25. doi: 10.1002/mds.20979
34. Tromp, D, and Scalars, D. How do they relate to brain structure. Winnower. (2016) 3:e146119. doi: 10.15200/winn.146119.94778
35. Li, F, Huang, X, Yang, Y, Li, B, Wu, Q, Zhang, T, et al. Microstructural brain abnormalities in patients with obsessive-compulsive disorder: diffusion-tensor MR imaging study at 3.0 T. Radiology. (2011) 260:216–23. doi: 10.1148/radiol.11101971
36. Bollettini, I, Mazza, MG, Muzzarelli, L, Dallaspezia, S, Poletti, S, Vai, B, et al. White matter alterations associate with onset symptom dimension in obsessive–compulsive disorder. Psychiatry Clin Neurosci. (2018) 72:13–27. doi: 10.1111/pcn.12563
37. Uddin, LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. (2015) 16:55–61. doi: 10.1038/nrn3857
38. Paulus, MP, and Stein, MB. An insular view of anxiety. Biol Psychiatry. (2006) 60:383–7. doi: 10.1016/j.biopsych.2006.03.042
39. Bu, X, Hu, X, Zhang, L, Li, B, Zhou, M, Lu, L, et al. Investigating the predictive value of different resting-state functional MRI parameters in obsessive-compulsive disorder. Transl Psychiatry. (2019) 9:17. doi: 10.1038/s41398-018-0362-9
40. Liu, J, Bu, X, Hu, X, Li, H, Cao, L, Gao, Y, et al. Temporal variability of regional intrinsic neural activity in drug-naive patients with obsessive-compulsive disorder. Hum Brain Mapp. (2021) 42:3792–803. doi: 10.1002/hbm.25465
41. Zhou, Z, Li, B, Jiang, J, Li, H, Cao, L, Zhang, S, et al. Abnormal resting-state functional connectivity of the insula in medication-free patients with obsessive-compulsive disorder. BMC Psychiatry. (2022) 22:742. doi: 10.1186/s12888-022-04341-z
42. Okada, K, Nakao, T, Sanematsu, H, Murayama, K, Honda, S, Tomita, M, et al. Biological heterogeneity of obsessive-compulsive disorder: a voxel-based morphometric study based on dimensional assessment. Psychiatry Clin Neurosci. (2015) 69:411–21. doi: 10.1111/pcn.12269
Keywords: obsessive–compulsive disorder, diffusion tensor imaging, automated fiber quantification, uncinate fasciculus, insula
Citation: Zhang S, Li B, Jiang J, Hu X, Li H, Cao L, Zhou Z, Liang K, Zhou H, Zhang L, Gong Q and Huang X (2023) Abnormal focal segments in left uncinate fasciculus in adults with obsessive–compulsive disorder. Front. Psychiatry. 14:1128808. doi: 10.3389/fpsyt.2023.1128808
Received: 21 December 2022; Accepted: 06 March 2023;
Published: 30 March 2023.
Edited by:
Jiaojian Wang, Kunming University of Science and Technology, ChinaCopyright © 2023 Zhang, Li, Jiang, Hu, Li, Cao, Zhou, Liang, Zhou, Zhang, Gong and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiyong Gong, cWl5b25nZ29uZ0BobXJyYy5vcmcuY24=; Xiaoqi Huang, anVsaWFuYWh1YW5nQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.