- 1Department of Psychiatry, The Fourth People's Hospital of Chengdu, Chengdu, China
- 2The Clinical Hospital of Chengdu Brain Science Institute, MOE Key Lab for Neuroinformation, University of Electronic Science and Technology of China, Chengdu, China
- 3School of Life Science and Technology, University of Electronic Science and Technology of China, Chengdu, China
- 4CAS Key Laboratory of Mental Health, Institute of Psychology, Chinese Academy of Sciences, Beijing, China
- 5Department of Psychiatry, Chongqing Mental Health Center, Chongqing, China
- 6Department of Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China
- 7Department of Psychiatry, Guangdong Engineering Technology Research Center for Translational Medicine of Mental Disorders, Guangzhou, China
Background: Sex differences may be presented in the clinical features or symptoms of schizophrenia patients but also affect the occurrence of hospital-acquired pneumonia (HAP). Modified electroconvulsive therapy (mECT) is a common treatment method for schizophrenia, used in combination with antipsychotics. This retrospective research explores the sex difference in HAP affecting patients with schizophrenia who have received mECT treatment during hospitalization.
Methods: We included schizophrenia inpatients treated with mECT and antipsychotics between January 2015 and April 2022. Blood-related and demographic data collected on admission were analyzed. Influencing factors of HAP in male and female groups were assessed separately.
Results: A total of 951 schizophrenia patients treated with mECT were enrolled in the study, including 375 males and 576 females, of which 62 patients experienced HAP during hospitalization. The risk period of HAP in these patients was found to be the first day after each mECT treatment and the first three sessions of mECT treatment. Statistically significant differences in the incidence of HAP were identified in male vs. female groups, with an incidence in men about 2.3 times higher than that in women (P < 0.001). Lower total cholesterol (Z = −2.147, P = 0.032) and the use of anti-parkinsonian drugs (χ2 = 17.973, P < 0.001) were found to be independent risk factors of HAP in male patients, while lower lymphocyte count (Z = −2.408, P = 0.016), hypertension (χ2 = 9.096, P = 0.003), and use of sedative-hypnotic drugs (χ2 = 13.636, P < 0.001) were identified in female patients.
Conclusion: Influencing factors of HAP in schizophrenia patients treated with mECT have gender differences. The first day after each mECT treatment and the first three sessions of mECT treatment were identified to have the greatest risk for HAP development. Therefore, it would be imperative to monitor clinical management and medications during this period according to these gender differences.
1. Introduction
Schizophrenia (SCZ) is a common severe psychiatric disorder and usually manifests clinically with psychotic symptoms such as hallucinations, delusions, emotional indifference, and cognitive dysfunction (1, 2). According to a 2018 World Health Organization report, more than 20 million people are already living with SCZ worldwide, and China accounts for about half of this population (3). SCZ is characterized by high incidence, high disability, and a low cure rate, with a lifetime incidence of about 1% (4). As such, the disease brings heavy psychological and economic burdens to patient families and broader society (5, 6) and has become a major societal challenge (4).
Gender differences may exist in the clinical symptoms of schizophrenia patients; for example, the age of onset for men may be a 3-year younger than that for women (7, 8). The correlation between non-social functioning and objective social cognition in men may be much stronger than in women (9), whereas hostile bias correlates with verbal fluency found in women (10). Women are good at processing speed and verbal situational memory, but men are good at visual working memory (11). There are also sex differences in the cognitive correlates of first-episode schizophrenia (12), i.e., working memory and executive function were correlated to onset age, negative symptoms were associated with memory or working memory in women, whereas processing speed was correlated with antipsychotic dosage in men. Antipsychotics may induce gender differences in extrapyramidal and anticholinergic responses, sexual problems, and subjective tolerance (13). However, no sex differences in the efficacy of amisulpride or risperidone medication were found in elderly schizophrenia patients (14). Although women may be more prone to gain weight from the antipsychotic medication (15), women may have a better prognosis than men (16). According to the current pathology of schizophrenia, these gender differences may be related to gene expression (17), the mechanism of the microbiota-brain-gut axis (18), differences in brain structure and function (19), or even sociocultural (20). However, these hypotheses are inconsistent to a large extent, so the mechanisms underlying the clinical characterization induced by gender differences need further investigation.
Electroconvulsive therapy (ECT) is widely used, particularly in patients with refractory schizophrenia (21–24). To avoid the generalized convulsions triggered by ECT treatment and the related fear of convulsions (25), modified ECT (mECT) was developed. Due to the use of anesthetic and muscle relaxants before the mECT treatment (26, 27), the risk of post-treatment infections may be increased (28). Prior research on ECT has shown that the incidence of pneumonia infection is 3.8 per 10,000 ECT treatments (29). Hospital-acquired pneumonia (HAP) is a leading cause of morbidity and mortality, and the incidence of HAP remains high, but effective treatment is usually lacking (30). Sex differences in the incidence of HAP exist (31), with men being a risk factor for HAP (32, 33), but a lower incidence of non-ventilator HAP in women (34). Some pathologies may be explained by these differences induced by genders, such as immune response to the virus (24), diabetes (35), or chronic obstructive pulmonary disease (COPD) (36). However, there are few studies on gender differences in risk factors related to hospital-acquired pneumonia (HAP) in SCZ patients with mECT around the world. Therefore, this paper aims to analyze the risk factors associated with the development of HAP in SCZ patients who have received mECT in recent years, explore the possible pathogenesis of HAP caused by gender differences, and provide a basis for guiding clinical management and improving the quality of patient treatment.
2. Materials and methods
2.1. Patients
This retrospective study included inpatients with schizophrenia admitted between January 2015 and April 2022. Patients met the diagnosis criteria of schizophrenia according to the International Classification of Diseases-10 (ICD-10) and received mECT treatment during their hospitalization. The diagnosis of HAP required all the following criteria: new lung infiltrates on chest imaging, respiratory decline, fever, and productive cough (37). Patients with infections within 48 h of hospitalization were excluded. This study was approved by the Ethics Committee of the Fourth People's Hospital of Chengdu.
Patient information collected included name, age, gender, as well as the status of diabetes mellitus, hypertension, epilepsy, or substance dependence (smoking or drinking), and excluded patients with comorbid cardiovascular disease. Blood samples were collected on admission for routine biochemical testing (white blood cells, red blood cells, platelets, lipids, glucose, blood proteins, etc.). Other medications of patients receiving mECT at the time of hospitalization were recorded, such as sedative-hypnotic drugs (SHD), antidepressant drugs (ADD), anti-anxiety drugs (AAD), antimanic drugs (AMD), anti-epileptic drugs (AED), anti-parkinsonian drugs (APD), and other neurological drugs. mECT-related conditions only for patients with HAP, such as the days from the first mECT to HAP (dfE2H) occurrence, the numbers of mECT treatments before HAP (nE2H) occurrence, and the days from the last mECT treatment to HAP (dlE2H) occurrence.
2.2. mECT parameters
The ECT Instrument was Thymatron System IV (Somatics, LLC, 149 Amityville Street Islip Terrace, NY 11752 USA). Electrode placement was a bilateral temporal energization mode. The electrical stimulation procedure was LOW 0.5: Frequency: 10–70 Hz; Pulse width: 0.5 ms; Duration: ≤ 8s; Waveform: bipolar, brief pulsed, square wave. Stimulus intensity (power): “Age mode” is used. For the first treatment session, the power is set to age × 80% for younger than 30 and age × 100% for older than 50; The power of follow-up treatment increased by 1–5% depending on the seizure index. Convulsions quality assessments: EEG Endpoint was 20–60 s; Average Seizure Energy Index was over 5,000; Postictal Suppression Index was over 80%.
2.3. Statistical analysis
Software SPSS 26 (IBM Corporation, New Orchard Road, Armonk, NY 10504, USA) was used for statistical calculations. These patients were divided into two groups by gender, with subgroups of HAP and non-HAP. Then the statistical analysis was conducted as follows: First, a general linear model (univariate model) was used for assessing the interaction effect of gender vs. other factors on HAP. Second, influencing factors for HAP men and women were analyzed separately. The χ2 test was used for categorical variables. Continuous variables were first tested for normality by Kolmogorov-Smirnov; if variables conformed to a normal distribution, t-testing was used, while non-normality variables were tested by non-parametric (Mann-Whitney) test. Since the included data were almost all non-normal variables, Spearman correlation analysis was used. Third, binary logistic regression modeling was later used for risk factors analysis. Finally, statistical calibration was performed using the Bonferroni method (P < 0.05/31 ≈ 0.0016). Continuous variables were expressed as mean ± standard deviation (x̄ ± std.), and P < 0.05 was considered to be statistically significant.
3. Results
3.1. General characteristics of included SCZ patients
A total of 951 inpatients, aged between 14 and 71 years old, were included. Of the 951 inpatients, 375 were male, and 576 were female, with mean ages of 32.57 ± 11.93 years and 36.85 ± 13.96 years, respectively, and males being significantly younger than females (t = 5.053, P < 0.001). 62 inpatients were HAP, with 37 in men and 25 in women. Covariance analysis results indicate interaction between sex and epilepsy, TC, anti-depressant drugs, anti-epileptic drugs, anti-parkinsonian drugs, and other neuron drugs (all Ps < 0.05), as shown in Table 1. Only epilepsy passed the Bonferroni test.
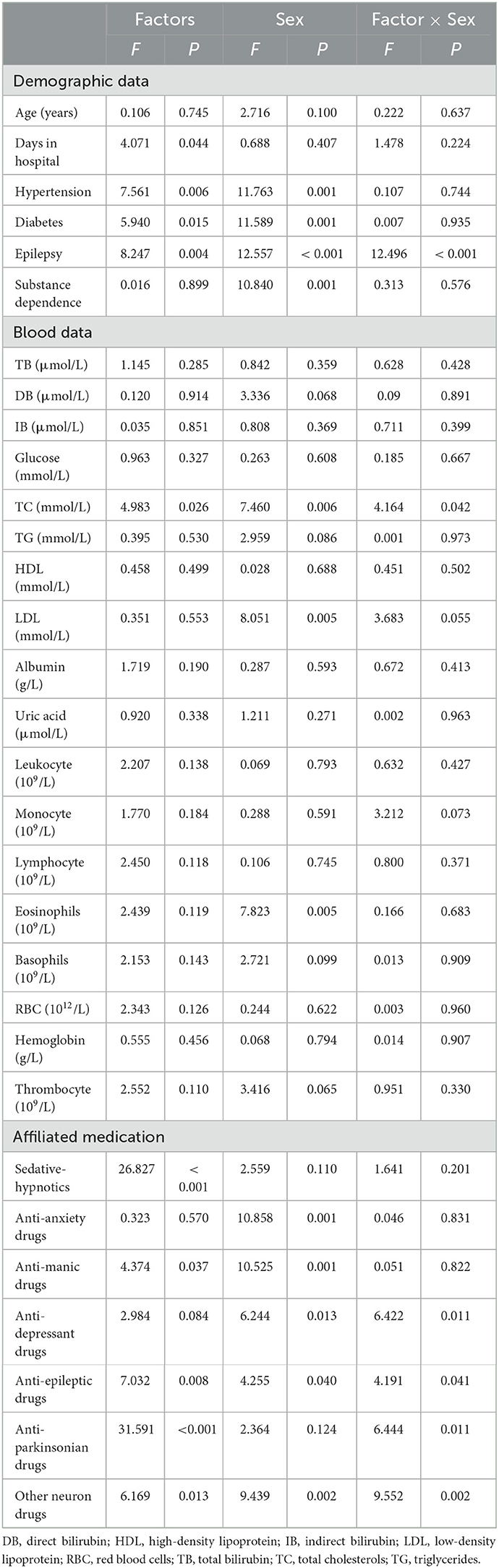
Table 1. Interaction effect between influencing factors and sex on hospital-acquired pneumonia (HAP).
3.2. HAP occurrence in patients receiving mECT
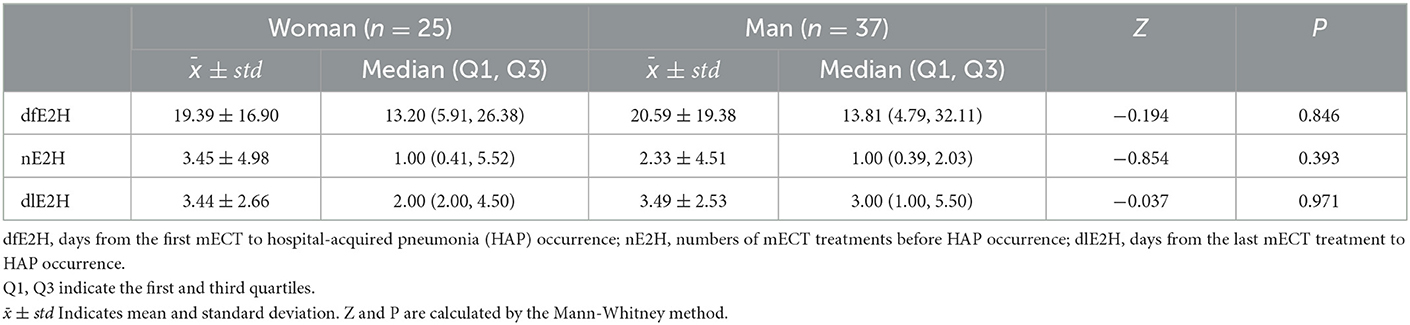
HAP occurred in 62 of the 951 study subjects, with an incidence of 6.52%. Patients developed HAP 20.10 ± 18.29 days after admission, received about 3.47 ± 2.57 sessions of mECT treatment before HAP, and HAP occurred approximately 2.78 ± 4.70 days after the last MECT treatment. The prevalence of HAP was significantly higher in men than in women (37/375:25/576 = 9.87%:4.34% ≈ 2.3, χ2 = 11.382, P < 0.001). There was no statistically significant difference between men and women in dfE2H, nE2H, or dlE2H, as shown in Table 2.
3.3. Risk factors for HAP in male SCZ patients receiving mECT
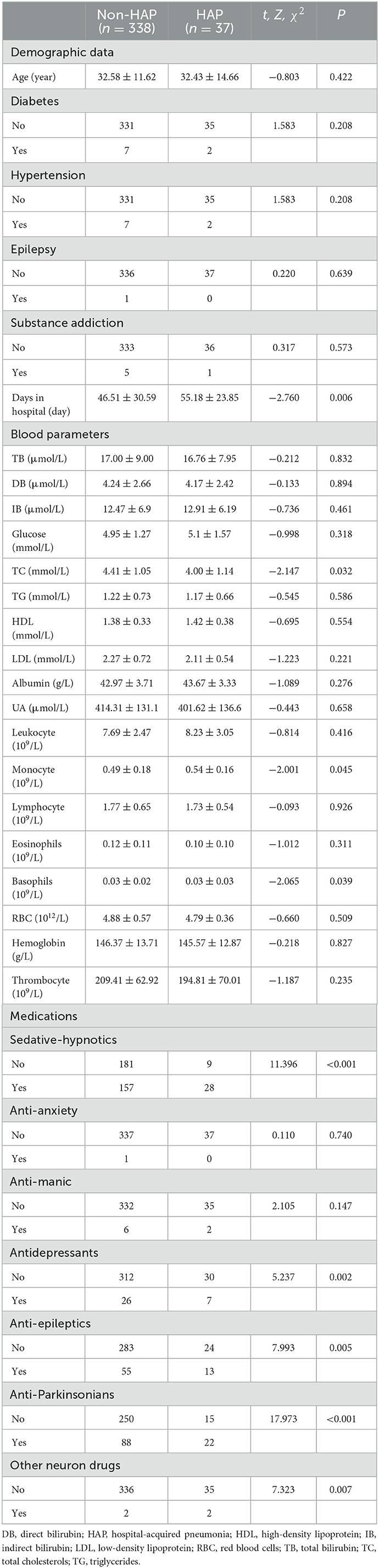
In Table 3, hospitalized days were significantly higher in the HAP group, as compared to the non-HAP group (Z = −2.760, P = 0.006). Total cholesterol (mmol/L) was significantly lower in the HAP group as compared to the non-HAP group (Z = −2.147, P = 0.032), while monocyte count was significantly higher (Z = −2.001, P = 0.045). There was a statistically significant difference in basophils between the HAP and non-HAP group (Z = −2.065, P = 0.039). There were also statistically significant increases in HAP incidence found in patients who had taken any medications of SHD (χ2 = 11.396, P < 0.001), ADD (χ2 = 5.237, P = 0.002), AED (χ2 = 7.993, P = 0.005), APD (χ2 = 17.973, P < 0.001), or other neurological drugs (χ2 = 7.323, P = 0.007), though only SHD and APD passed the Bonferroni test.
There were correlations between HAP and days in hospital (rs = 0.143, P = 0.006), total cholesterol (rs = −0.111, P = 0.032), monocyte count (rs = 0.103, P = 0.045), SHD (rs = 0.174, P = 0.001), ADD (rs = 0.118, P = 0.022), AED (rs = 0.146, P = 0.005), APD (rs = 0.219, P < 0.001), and other neurological drugs (rs = 0.140, P = 0.007) by Spearman test. After input of these variables into the logistic regression equation, only total cholesterol [Beta = −0.373, Wald = 3.920, P = 0.048, Exp(B) = 0.688 (0.476, 0.996)] and APD [Beta = 1.366, Wald = 14.84, P < 0.001, Exp(B) = 3.920 (1.925, 7.981)] survived.
3.4. Risk factors for HAP in female SCZ patients receiving mECT
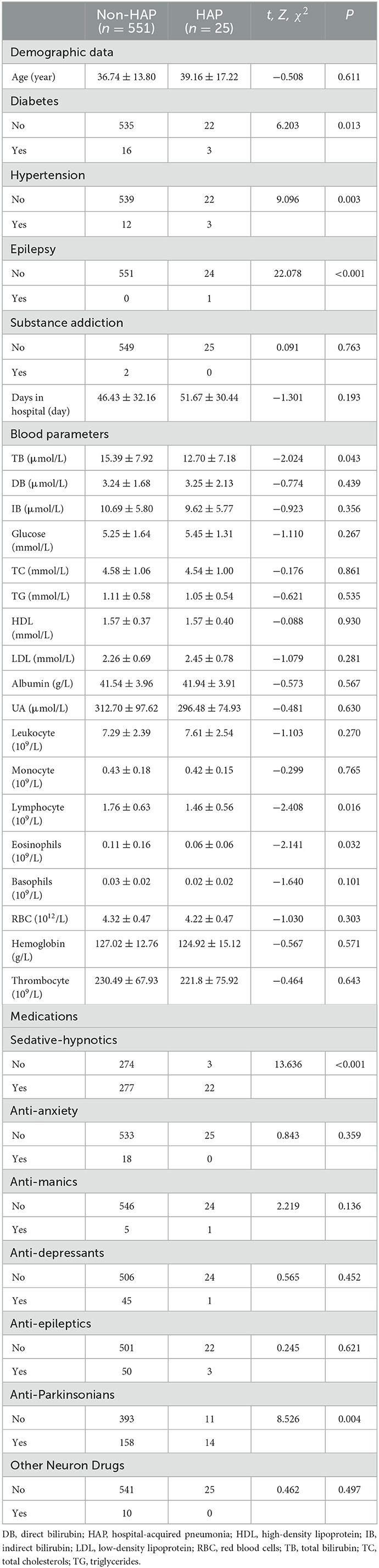
In Table 4, statistically significant higher incidences of diabetes (χ2 = 6.203, P = 0.013), hypertension (χ2 = 9.096, P = 0.003), epilepsy (χ2 = 5.643, P < 0.001), lymphocyte count (Z = −2.408, P = 0.016), eosinophil count (Z = −2.141, P = 0.032), SHD (χ2 = 13.636, P < 0.001), and APD (χ2 = 8.526, P = 0.004) were identified in the HAP group, as compared to the non-HAP group, while lower levels of total bilirubin (Z = −2.024, P = 0.043) on admission were found in the HAP group. Only variables of epilepsy and SHD passed the Bonferroni test.
Spearman testing showed that HAP is correlated with diabetes (rs = 0.104, P = 0.013), hypertension (rs = 0.126, P = 0.003), epilepsy (rs = 0.196, P < 0.001), total bilirubin (rs = −0.084, P = 0.043), lymphocyte count (rs = −0.100, P = 0.016), eosinophil count (rs = −0.089, P = 0.032), SHD (rs = 0.154, P < 0.001), and APD (rs = 0.122, P = 0.003). After input of these variables into the logistic regression equation, only lymphocyte count [Beta = 1.702, Wald = 5.432, P = 0.020, Exp(B) = 5.483 (1.311, 22.937)], hypertension [Beta = −0.835, Wald = 4.764, P = 0.029, Exp(B) = 0.434 (0.205, 0.918)], and SHD [Beta = 2.287, Wald = 9.387, P = 0.002, Exp(B) = 9.847 (2.280, 42.536)] survived.
4. Discussion
To the best of our knowledge, this is the first study to investigate the risk factors of HAP in patients with mECT treatment. We found that the incidence of HAP in mECT patients was 6.52%, greater than the 1.80% previously reported by Han et al. in patients with schizophrenia spectrum disorder (38), but slightly less than the 7.8% reported in a study of elderly SCZ patients (age >50) by Yang et al. (39).
ECT treatment improves the structure and function of the hippocampus and insula in SCZ patients and regulates the function of the prefrontal and thalamic striatum of the default network (40, 41). Animal experiments have also shown that ECT attenuates microglia and astrocyte proliferation, thus improving schizophrenic behavior (42). However, ECT treatment may also induce acute immunoinflammatory responses, such as elevated plasma cortisol and levels of interleukin-1 or−6; lowered levels of blood tumor necrosis factor alpha (TNF-α) and interleukin-6 in long-term treatment (43); or even detrimentally alter blood parameters (44), leading to a decrease in immunity. Schizophrenia itself is also a risk factor for the development of pneumonia (45), thus ECT may increase the risk of HAP in patients, and our prior work (46) had found that mECT may be a risk factor influencing the occurrence of HAP. Further, we wanted to find whether there were gender differences in HAP, and the discussion of the results was divided into the following four parts.
4.1. mECT induced increased incidence of HAP
Our results showed that among HAP patients who underwent mECT, there was no statistical difference between men and women in the three indicators: days from first mECT treatment to HAP occurrence (dfE2H), the numbers of mECT treatments before HAP occurrence (nE2H), and days from last mECT treatment to HAP occurrence (dlE2H). However, the results showed that the median number of days from the last mECT treatment to HAP was 1 day in both men and women, indicating that all patients had a very high risk of developing HAP within 1 day after receiving mECT treatment. In addition, the risk of HAP within the first three mECT treatments was quite high in men and women, respectively, which may be due to the fact that patients need to gradually adapt to the clinical symptoms that may occur after mECT and ignore the possible risk of HAP. Therefore, healthcare providers should be on alert the first day after each mECT treatment, and special attention should be paid to clinical care after the first 3 sessions of mECT treatment.
Interestingly, we found that male patients with SCZ are more prone to HAP than women, and the risk factors predisposing both groups to HAP also differ from each other.
4.2. Risk factors for male mECT patients
The prevalence of HAP in male mECT patients in this study was significantly higher than that in women (male incidence ~2.3 times that of females). This increased prevalence may be related to lifestyle habits of male patients, such as smoking, alcohol abuse, low weight, frequent contact with children, and poor oral hygiene, which are all risk factors for HAP (47). In addition, this study found that lower total cholesterol prior to hospital admission and APD medication in hospitalization may be risk factors for the development of HAP in men.
Our results showed that male SCZ patients treated with mECT with lower total cholesterol levels at admission were more likely to develop HAP, and logistic regression analysis showed that low levels of total cholesterol might be an independent risk factor for HAP in SCZ patients treated with mECT. Cholesterol is widely distributed in many tissues of the body, especially the brain and neural tissues, and may be associated with a variety of diseases (48), and even mental status or personality changes (49). Importantly, cholesterol has an important role in coronavirus entry, membrane fusion, and pathological syncytium formation. 25-hydroxycholesterol (25HC) is one of the metabolites of cholesterol, and 25HC inhibits coronavirus infection by blocking membrane fusion (50), so lower total cholesterol levels may lead to lower 25HC levels, increasing the chance of coronavirus infection in SCZ patients and potentially explaining the correlation between viral infection and lower cholesterol levels (51, 52). Lower total cholesterol may also be a factor of increased short-term mortality in elderly patients with community-acquired pneumonia (CAP) (53). In addition, men may be more sensitive to low levels of cholesterol (54), which accounts for the fact that men with low total cholesterol levels were more likely to develop HAP in our study, whereas women showed no statistical difference in HAP based on the level of total cholesterol.
APDs are used to improve Parkinsonian-like symptoms and extrapyramidal effects in schizophrenic patients. Our results show that male mECT SCZ patients using APDs during hospitalization were more likely to develop HAP, and logistic regression analysis showed that APD use is an independent risk factor for the incidence of HAP. Relatedly, it has been shown that anticholinergic drugs may increase the risk of HAP (55); despite some studies showing that amantadine can be used to prevent pneumonia (56), the presence of drowsiness, falls, and skin problems (57) may in turn lead to an increased risk of developing HAP in patients.
4.3. Risk factors for female mECT patients
Risk factors for HAP in women are hypertension, low lymphocyte count on admission, and use of SHDs during hospitalization. There was no statistically significant difference in the prevalence of hypertension between men and women in patients treated with mECT (9/375:15/576, χ2 = 0.038, P = 0.844), however, SCZ patients with comorbid hypertension in women had a higher risk of HAP, compared to men. ECT treatment may not result in significant changes in blood pressure in hypertensive patients (58), however, patients with comorbidities such as chronic renal insufficiency or diabetes mellitus are more likely to be affected by COVID-19, potentially fatally (59–61). Therefore, for female SCZ patients with comorbid diseases such as hypertension, clinical management should be particularly strengthened during hospitalization to reduce the risk of HAP and improve the quality of patient survival.
Lymphocytes have an important role in immune regulation and can be involved in the pathogenesis of respiratory diseases such as pneumonia, infections, asthma, and acute respiratory distress syndrome (62). The lower lymphocyte count in female mECT patients suggests that immunity may be reduced, potentially increasing the incidence of HAP.
Benzodiazepines drugs (BZD) are often used in sedation-hypnosis and can modulate peripheral γ-aminobutyric acid (GABA) type A on macrophages and increase the incidence of infection by inhibiting proinflammatory cytokines (63). Experiments performed on mice have further shown that benzodiazepines enhance GABA signaling, leading to increased mortality from pneumonia (64). In addition, certain meta-analyses have shown an increased risk of pneumonia with recent or current exposure to benzodiazepines (65, 66), which is consistent with our findings. Therefore, BZDs should be used with caution in SCZ patients receiving mECT.
4.4. Limitations
Although mECT is used as a routine treatment for patients with schizophrenia, the number of patients receiving it remains low, which may stem from the fear of electrical stimulation. Since the outbreak of COVID-19, more standardized clinical management and increased awareness of personal protection implemented by medical institutions have led to a decrease in the number of patients with mECT experiencing HAP; therefore, random errors induced by small sample size may have a greater impact on the statistical analysis. Incomplete demographic indicators for some patients, such as height, weight, and education, as well as the lack of cognitive assessment of patients with schizophrenia during data collection, prevented our results from fully reflecting the full picture of patients. Data from an individual psychiatric hospital is another limitation, as regional differences, local culture, economic conditions, and ethnic groups of patients may also influence our results. Therefore, these risk factors deserve to be investigated as a prospective study with a larger patient sample size and collaboration of multiple clinical centers.
5. Conclusions
Among schizophrenia patients treated with mECT, men were more likely to develop HAP than women. Schizophrenia patients were at very high risk of developing HAP within the first day after each mECT treatment or in the first three sessions of mECT treatment. Lower levels of total cholesterol and use of anti-parkinsonian drugs were identified as independent risk factors of HAP in male patients, while hypertension, lower lymphocyte count on admission, and use of sedative-hypnotic drugs in hospitalization were identified as independent risk factors in female patients. These gender-based differences may be due to differences in physiological immune function, lifestyle habits, and the complicated nature of schizophrenia itself. Our results may help guide future clinical management and care of patients with SCZ, and help elucidate the potential direction of follow-up studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Fourth People's Hospital of Chengdu. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
MY: conception, writing—original draft preparation, and funding acquisition. YY, DK and MX: data curation. LL: validation. XH: resources. GZ and XZ: writing—review. YH: writing—review and editing. YT: project administration and funding acquisition. ZL: writing—review, supervision, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by National Natural Science Foundation of China (62073058), Chengdu Science and Technology Bureau (2022-YF05-01867-SN), Chengdu Municipal Health Commission (2021057), the Science and Technology Plan Project of Guangdong Province (2019B030316001), Guangzhou Municipal Key Discipline in Medicine (2021–2023), Open Project Program of State Key Laboratory of Virtual Reality Technology and Systems, Beihang University (No. VRLAB2022 B02), and Shanghai Key Laboratory of Psychotic Disorders Open Grant (21-K03).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Keeley JW, Gaebel W. Symptom rating scales for schizophrenia and other primary psychotic disorders in ICD-11. Epidemiol Psychiatr Sci. (2018) 27:219–24. doi: 10.1017/S2045796017000270
2. Tandon R, Gaebel W, Barch DM, Bustillo J, Gur RE, Heckers S, et al. Definition and description of schizophrenia in the DSM-5. Schizophr Res. (2013) 150:3–10. doi: 10.1016/j.schres.2013.05.028
3. James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global regional and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
4. Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, et al. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr Bull. (2018) 44:1195–203. doi: 10.1093/schbul/sby058
5. Peng MM, Xing J, Tang X, Wu Q, Wei D, Ran MS. Disease-related risk factors for caregiver burden among family caregivers of persons with schizophrenia: a systematic review and meta-analysis. Int J Environ Res Public Health. (2022) 19:1862. doi: 10.3390/ijerph19031862
6. Weber S, Scott JG, Chatterton ML. Healthcare costs and resource use associated with negative symptoms of schizophrenia: a systematic literature review. Schizophr Res. (2022) 241:251–9. doi: 10.1016/j.schres.2022.01.051
7. Hafner Han der Heiden W. Epidemiology of schizophrenia. Can J Psychiatry. (1997) 42:139–51. doi: 10.1177/070674379704200204
8. Adachi N, Hara T, Oana Y, Matsuura M, Okubo Y, Akanuma N, et al. Difference in age of onset of psychosis between epilepsy and schizophrenia. Epilepsy Res. (2008) 78:201–6. doi: 10.1016/j.eplepsyres.2007.12.001
9. Ferrer-Quintero M, Green MF, Horan WP, Penn DL, Kern RS, Lee J. The effect of sex on social cognition and functioning in schizophrenia. NPJ Schizophr. (2021) 7:57. doi: 10.1038/s41537-021-00188-7
10. Kubota R, Okubo R, Ikezawa S, Matsui M, Adachi L, Wada A, et al. Sex differences in social cognition and association of social cognition and neurocognition in early course schizophrenia. Front Psychol. (2022) 13:867468. doi: 10.3389/fpsyg.2022.867468
11. Torniainen M, Suvisaari J, Partonen T, Castaneda AE, Kuha A, Perala J, et al. Sex differences in cognition among persons with schizophrenia and healthy first-degree relatives. Psychiatry Res. (2011) 188:7–12. doi: 10.1016/j.psychres.2010.11.009
12. Li AWY, Hui CLM, Lee EHM, Chang WC, Chan SKW, Chen EYH. Gender differences in correlates of cognition in first-episode psychosis. Psychiatry Res. (2019) 271:412–20. doi: 10.1016/j.psychres.2018.12.011
13. Barbui C, Nose M, Bindman J, Schene A, Becker T, Mazzi MA, et al. Sex differences in the subjective tolerability of antipsychotic drugs. J Clin Psychopharmacol. (2005) 25:521–6. doi: 10.1097/01.jcp.0000185423.15891.02
14. Riedel M, Eich FX, Moller HJ. A pilot study of the safety and efficacy of amisulpride and risperidone in elderly psychotic patients. Eur Psychiatry. (2009) 24:149–53. doi: 10.1016/j.eurpsy.2008.10.005
15. Seeman MV. Schizophrenia: women bear a disproportionate toll of antipsychotic side effects. J Am Psychiatr Nurses Assoc. (2010) 16:21–9. doi: 10.1177/1078390309350918
16. Grossman LS, Harrow M, Rosen C, Faull R, Strauss GP. Sex differences in schizophrenia and other psychotic disorders: a 20-year longitudinal study of psychosis and recovery. Compr Psychiatry. (2008) 49:523–9. doi: 10.1016/j.comppsych.2008.03.004
17. Hoffman GE, Ma Y, Montgomery KS, Bendl J, Jaiswal MK, Kozlenkov A, et al. Sex differences in the human brain transcriptome of cases with schizophrenia. Biol Psychiatry. (2022) 91:92–101. doi: 10.1016/j.biopsych.2021.03.020
18. Shobeiri P, Kalantari A, Teixeira AL, Rezaei N. Shedding light on biological sex differences and microbiota-gut-brain axis: a comprehensive review of its roles in neuropsychiatric disorders. Biol Sex Differ. (2022) 13:12. doi: 10.1186/s13293-022-00422-6
19. Rivera-Garcia MT, McCane AM, Chowdhury TG, Wallin-Miller KG, Moghaddam B. Sex and strain differences in dynamic and static properties of the mesolimbic dopamine system. Neuropsychopharmacology. (2020) 45:2079–86. doi: 10.1038/s41386-020-0765-1
20. Lewine R. At issue: sex and gender in schizophrenia. Schizophr Bull. (2004) 30:755–62. doi: 10.1093/oxfordjournals.schbul.a007128
21. Sinclair DJM, Zhao S, Qi F, Nyakyoma K, Kwong JSW, Adams CE. Electroconvulsive therapy for treatment-resistant schizophrenia. Schizophr Bull. (2019) 45:730–2. doi: 10.1093/schbul/sbz037
22. Chan CYW, Abdin E, Seow E, Subramaniam M, Liu J, Peh CX, et al. Clinical effectiveness and speed of response of electroconvulsive therapy in treatment-resistant schizophrenia. Psychiatry Clin Neurosci. (2019) 73:416–22. doi: 10.1111/pcn.12855
23. Purohith AN, Chatorikar SA, Praharaj SK, Bhandary RP, Sharma P. Efficacy and safety of maintenance electroconvulsive therapy (M-ECT) in treatment-resistant schizophrenia: a case series. Asian J Psychiatr. (2022) 73:103132. doi: 10.1016/j.ajp.2022.103132
24. Kellner CH, Obbels J, Sienaert P. When to consider electroconvulsive therapy (ECT). Acta Psychiatr Scand. (2020) 141:304–15. doi: 10.1111/acps.13134
25. Lava-Parmele S, Lava C, Parmele JB. The historical struggles of modified electroconvulsive therapy: how anesthesia came to the rescue. J Anesth Hist. (2021) 7:17–25. doi: 10.1016/j.janh.2021.03.001
26. Soehle M, Bochem J, Kayser S, Weyerhauser J, Valero R. Challenges and pitfalls in anesthesia for electroconvulsive therapy. Best Pract Res Clin Anaesthesiol. (2021) 35:181–9. doi: 10.1016/j.bpa.2020.12.012
27. Bryson EO, Aloysi AS, Farber KG, Kellner CH. Individualized anesthetic management for patients undergoing electroconvulsive therapy: a review of current practice. Anesth Analg. (2017) 124:1943–56. doi: 10.1213/ANE.0000000000001873
28. Kuehnert MJ, Webb RM, Jochimsen EM, Hancock GA, Arduino MJ, Hand S, et al. Staphylococcus aureus bloodstream infections among patients undergoing electroconvulsive therapy traced to breaks in infection control and possible extrinsic contamination by propofol. Anesth Analg. (1997) 85:420–5. doi: 10.1097/00000539-199708000-00031
29. Blumberger DM, Seitz DP, Herrmann N, Kirkham JG, Ng R, Reimer C, et al. Low medical morbidity and mortality after acute courses of electroconvulsive therapy in a population-based sample. Acta Psychiatr Scand. (2017) 136:583–93. doi: 10.1111/acps.12815
30. Roquilly A, Torres A, Villadangos JA, Netea MG, Dickson R, Becher B, et al. Pathophysiological role of respiratory dysbiosis in hospital-acquired pneumonia. Lancet Respir Med. (2019) 7:710–20. doi: 10.1016/S2213-2600(19)30140-7
31. Jiao J, Yang XY Li Z, Zhao YW, Cao J, Li FF, et al. Incidence and related factors for hospital-acquired pneumonia among older bedridden patients in china: a hospital-based multicenter registry data based study. Front Public Health. (2019) 7:221. doi: 10.3389/fpubh.2019.00221
32. Kim BG, Kang M, Lim J, Lee J, Kang D, Kim M, et al. Comprehensive risk assessment for hospital-acquired pneumonia: sociodemographic, clinical, and hospital environmental factors associated with the incidence of hospital-acquired pneumonia. BMC Pulm Med. (2022) 22:21. doi: 10.1186/s12890-021-01816-9
33. Goncalves-Pereira J, Mergulhao P, Nunes B, Froes F. Incidence and impact of hospital-acquired pneumonia: a portuguese nationwide four-year study. J Hosp Infect. (2021) 112:1–5. doi: 10.1016/j.jhin.2021.03.012
34. Lopez-de-Andres A, Albaladejo-Vicente R, de Miguel-Diez J, Hernandez-Barrera V, Ji Z, Zamorano-Leon JJ, et al. Gender differences in incidence and in-hospital outcomes of community-acquired, ventilator-associated and nonventilator hospital-acquired pneumonia in Spain. Int J Clin Pract. (2021) 75:e13762. doi: 10.1111/ijcp.13762
35. Lopez-de-Andres A, Lopez-Herranz M, Hernandez-Barrera V, de-Miguel-Diez J, de-Miguel-Yanes JM, Carabantes-Alarcon D, et al. Sex differences in hospital-acquired pneumonia among patients with type 2 diabetes mellitus patients: retrospective cohort study using hospital discharge data in Spain (2016-2019). Int J Environ Res Public Health. (2021) 18:12645. doi: 10.3390/ijerph182312645
36. de-Miguel-Diez J, Jimenez-Garcia R, Hernandez-Barrera V, de-Miguel-Yanes JM, Carabantes-Alarcon D, Lopez-de-Andres A. Assessing the impact of gender and COPD on the incidence and mortality of hospital-acquired pneumonia. A retrospective cohort study using the Spanish National Discharge Database (2016–2019). J Clin Med. (2021) 10:e5453. doi: 10.3390/jcm10225453
37. Modi AR, Kovacs CS. Hospital-acquired and ventilator-associated pneumonia: diagnosis, management, and prevention. Cleve Clin J Med. (2020) 87:633–9. doi: 10.3949/ccjm.87a.19117
38. Han J, Lv Z, Shen M, Wan Q, Xiao L, Wang G. Risk factors for hospital-acquired pneumonia among inpatients with mental disorders in a large mental health center within a tertiary general hospital. Am J Infect Control. (2022). doi: 10.1016/j.ajic.2022.06.014
39. Yang M, Li Q, Wang C, Li L, Xu M, Yan F, et al. Influencing factors of hospital-acquired pneumonia infection in the middle-aged and elderly patients with schizophrenia. Front Psychiatry. (2021) 12:746791. doi: 10.3389/fpsyt.2021.746791
40. Moon SY, Kim M, Lho SK, Oh S, Kim SH, Kwon JS. Systematic review of the neural effect of electroconvulsive therapy in patients with schizophrenia: hippocampus and insula as the key regions of modulation. Psychiatry Investig. (2021) 18:486–99. doi: 10.30773/pi.2020.0438
41. Thomann PA, Wolf RC, Nolte HM, Hirjak D, Hofer S, Seidl U, et al. Neuromodulation in response to electroconvulsive therapy in schizophrenia and major depression. Brain Stimul. (2017) 10:637–44. doi: 10.1016/j.brs.2017.01.578
42. Limoa E, Hashioka S, Miyaoka T, Tsuchie K, Arauchi R, Azis IA, et al. Electroconvulsive shock attenuated microgliosis and astrogliosis in the hippocampus and ameliorated schizophrenia-like behavior of gunn rat. J Neuroinflammation. (2016) 13:230. doi: 10.1186/s12974-016-0688-2
43. Yrondi A, Sporer M, Peran P, Schmitt L, Arbus C, Sauvaget A. Electroconvulsive therapy, depression, the immune system and inflammation: a systematic review. Brain Stimul. (2018) 11:29–51. doi: 10.1016/j.brs.2017.10.013
44. Chaturvedi S, Chadda RK, Rusia U, Jain N. Effect of electroconvulsive therapy on hematological parameters. Psychiatry Res. (2001) 104:265–8. doi: 10.1016/s0165-1781(01)00303-1
45. Karaoulanis SE, Christodoulou NG. Do patients with schizophrenia have higher infection and mortality rates due to COVID-19? A systematic review. Psychiatriki. (2021) 32:219–23. doi: 10.22365/jpsych.2021.027
46. Yang Y, Mi Y, Di K, Wei C, Guocheng Z, Xi T, et al. Non-antipsychotic medicines and modified electroconvulsive therapy are risk factors for hospital-acquired pneumonia in schizophrenia patients. Front Psychiatry. (2023). doi: 10.3389/fpsyt.2022.1071079
47. Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. (2013) 68:1057–65. doi: 10.1136/thoraxjnl-2013-204282
48. Song Y, Liu J, Zhao K, Gao L, Zhao J. Cholesterol-induced toxicity: an integrated view of the role of cholesterol in multiple diseases. Cell Metab. (2021) 33:1911–25. doi: 10.1016/j.cmet.2021.09.001
49. Boston PF, Dursun SM, Reveley MA. Cholesterol and mental disorder. Br J Psychiatry. (1996) 169:682–9. doi: 10.1192/bjp.169.6.682
50. Zang R, Case JB, Yutuc E, Ma X, Shen S, Gomez Castro MF, et al. Cholesterol 25-hydroxylase suppresses SARS-CoV-2 replication by blocking membrane fusion. Proc Natl Acad Sci USA. (2020) 117:32105–13. doi: 10.1073/pnas.2012197117
51. Dai J, Wang H, Liao Y, Tan L, Sun Y, Song C, et al. Coronavirus infection and cholesterol metabolism. Front Immunol. (2022) 13:791267. doi: 10.3389/fimmu.2022.791267
52. Zinellu A, Paliogiannis P, Fois AG, Solidoro P, Carru C, Mangoni AA. Cholesterol and triglyceride concentrations, COVID-19 severity, and mortality: a systematic review and meta-analysis with meta-regression. Front Public Health. (2021) 9:705916. doi: 10.3389/fpubh.2021.705916
53. Ko SH, Lee JS, Kim SK, Jeong KY. Serum cholesterol as a predictor of mortality among the elderly patients with pneumonia in the emergency department. Am J Emerg Med. (2021) 45:404–9. doi: 10.1016/j.ajem.2020.09.012
54. Tomson-Johanson K, Harro J. Low cholesterol, impulsivity and violence revisited. Curr Opin Endocrinol Diabetes Obes. (2018) 25:103–7. doi: 10.1097/MED.0000000000000395
55. Lee CY, Cheng YD, Cheng WY, Tsai TH, Huang KH. The prevalence of anticholinergic drugs and correlation with pneumonia in elderly patients: a population-based study in Taiwan. Int J Environ Res Public Health. (2020) 17:6260. doi: 10.3390/ijerph17176260
56. Lehnert R, Pletz M, Reuss A, Schaberg T. Antiviral medications in seasonal and pandemic influenza. Dtsch Arztebl Int. (2016) 113:799–807. doi: 10.3238/arztebl.2016.0799
57. Mohammad Zadeh N, Mashinchi Asl NS, Forouharnejad K, Ghadimi K, Parsa S, Mohammadi S, et al. Mechanism and adverse effects of COVID-19 drugs: a basic review. Int J Physiol Pathophysiol Pharmacol. (2021) 13:102–9.
58. Albin SM, Stevens SR, Rasmussen KG. Blood pressure before and after electroconvulsive therapy in hypertensive and nonhypertensive patients. J ECT. (2007) 23:9–10. doi: 10.1097/01.yct.0000263252.75926.98
59. Huang S, Wang J, Liu F, Liu J, Cao G, Yang C, et al. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. (2020) 43:824–31. doi: 10.1038/s41440-020-0485-2
60. Petrakis V, Panagopoulos P, Papazoglou D, Papanas N. Diabetes mellitus and hypertension as major risk factors of mortality from COVID-19 pneumonia. Exp Clin Endocrinol Diabetes. (2022) 130:205–6. doi: 10.1055/a-1325-0381
61. Denova-Gutierrez E, Lopez-Gatell H, Alomia-Zegarra JL, Lopez-Ridaura R, Zaragoza-Jimenez CA, Dyer-Leal DD, et al. The association of obesity, type 2 diabetes, and hypertension with severe coronavirus disease 2019 on admission among Mexican patients. Obesity (Silver Spring). (2020) 28:1826–32. doi: 10.1002/oby.22946
62. Deng Z, Zheng Y, Cai P, Zheng Z. The role of B and T lymphocyte attenuator in respiratory system diseases. Front Immunol. (2021) 12:635623. doi: 10.3389/fimmu.2021.635623
63. Skicki E, Morgan M, Brown C, Bradburn E, Rogers F. Benzodiazepines increase the likelihood of both infectious and thrombotic complications. J Trauma Acute Care Surg. (2021) 91:206–11. doi: 10.1097/TA.0000000000003134
64. Sanders RD, Godlee A, Fujimori T, Goulding J, Xin G, Salek-Ardakani S, et al. Benzodiazepine augmented gamma-amino-butyric acid signaling increases mortality from pneumonia in mice. Crit Care Med. (2013) 41:1627–36. doi: 10.1097/CCM.0b013e31827c0c8d
65. Rajamaki B, Hartikainen S, Tolppanen AM. Psychotropic drug-associated pneumonia in older adults. Drugs Aging. (2020) 37:241–61. doi: 10.1007/s40266-020-00754-1
Keywords: sex difference, modified electroconvulsive therapy, schizophrenia, hospital-acquired pneumonia, non-antipsychotics
Citation: Yang M, Yang Y, Liu L, Kong D, Xu M, Huang X, Luo C, Zhao G, Zhang X, Huang Y, Tu Y and Li Z (2023) Sex differences in factors influencing hospital-acquired pneumonia in schizophrenia patients receiving modified electroconvulsive therapy. Front. Psychiatry 14:1127262. doi: 10.3389/fpsyt.2023.1127262
Received: 19 December 2022; Accepted: 30 January 2023;
Published: 14 February 2023.
Edited by:
Tianhong Zhang, Shanghai Jiao Tong University, ChinaCopyright © 2023 Yang, Yang, Liu, Kong, Xu, Huang, Luo, Zhao, Zhang, Huang, Tu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunzhong Tu,  Z3owMjB0eXpAMTI2LmNvbQ==; Zezhi Li,
Z3owMjB0eXpAMTI2LmNvbQ==; Zezhi Li,  YmlvbHBzeWNoaWF0cnlAMTI2LmNvbQ==
YmlvbHBzeWNoaWF0cnlAMTI2LmNvbQ==
 Mi Yang
Mi Yang Yan Yang1
Yan Yang1 Xiangyang Zhang
Xiangyang Zhang Zezhi Li
Zezhi Li