
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 02 February 2023
Sec. Schizophrenia
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1124691
This article is part of the Research Topic Safety and Side Effects of Psychotropic Medications, Volume II View all 11 articles
Background: One of the most frequent side effects of atypical antipsychotics is hyperprolactinemia (HPRL), and metformin or aripiprazole co-prescription is regarded as an effective therapy option for reducing prolactin (PRL) levels. However, whether either of the two drugs can reduce PRL levels in patients with long-term hospitalized chronic schizophrenia with co-morbid type 2 diabetes (T2DM) has not been adequately reported.
Methods: In our study, long-term hospitalized chronic schizophrenia patients with co-T2DM who were prescribed olanzapine or risperidone as the primary antipsychotic medication were enrolled. A total of 197 of these cases with co-prescribed aripiprazole were set up as the study group (co-Ari group), and the other 204 cases without co-prescribed aripiprazole were set up as the control group (non-Ari group). The two groups’ variations in each target parameter were compared, and the variables affecting PRL levels were examined.
Results: Compared to the non-Ari group, fasting blood glucose (FBG), blood uric acid (UA), total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C) levels were significantly higher in the co-Ari group, but there was no difference in PRL levels. Co-prescribing aripiprazole had no impact on PRL levels in all patients with co-T2DM, and aripiprazole dose had no impact on PRL levels in the clinical subgroup of the co-Ari group.
Conclusion: Aripiprazole not only worsened the severity of index disturbances associated to metabolism in long-term hospitalized chronic schizophrenia patients with co-T2DM on metformin-based hypoglycemic medications but also failed to lower PRL levels.
The most significant and fundamental form of treatment for schizophrenia is antipsychotic medication, as we all know. And one of the most prevalent and common adverse drug reactions to antipsychotic medications is hyperprolactinemia (HPRL) (1), but it is believed to have decreased with the broad and extensive clinical prescribing of second-generation antipsychotics as opposed to traditional antipsychotics (2). However, the real clinical situation may not be as optimistic as perceived. According to a significant Chinese study, the prevalence of HPRL in hospitalized schizophrenia patients is up to 61.3% and is similar in men and women (3). Among the many atypical antipsychotics, risperidone is considered to have one of the most significant effects on elevating prolactin (PRL) levels in psychiatric patients, and the PRL- increasing pharmacological effects of olanzapine should not be underestimated (1). A study from the Chinese province of Taiwan found that olanzapine can cause high levels of PRL (up to 51.6%) (4). What’s more, risperidone and olanzapine are two of the top-ranked antipsychotic drugs in terms of prescription rates, according to reports of prescribing patterns for psychiatric patients from around the world, including China (5–8). It is therefore not surprising to observe that HPRL brought on by atypical antipsychotics, such as risperidone and olanzapine, continue to be widespread and require proper clinical attention.
Long-term exposure to excessive PRL levels is frequently linked to an increased risk of sexual dysfunction (9), weight gain (10), cardiovascular disease (11), osteoporosis (12), and even cancer (13). In order to decrease the risk of adverse events and accidents in psychiatric patients and to promote patient compliance with treatment, it is vital for psychiatrists to find effective ways to diminish or neutralize antipsychotic-induced HPRL. In clinical practice, metformin is a frequently used hypoglycemic drug that has been shown to have pharmacological effects in attenuating antipsychotic-induced HPRL (14), but the specific mechanism of action is uncertain. It has been found that metformin can cross the blood-brain barrier and appears at higher levels in the pituitary gland than in other brain tissues (15). This suggests that metformin may have a local interaction with PRL-secreting cells in the pituitary gland to suppress elevated PRL levels. Distinguishing from metformin, as a novel antipsychotic with “PRL-sparing” effects (16, 17), aripiprazole has an partial agonistic effect on D2, as well as a partial agonistic effect on 5-HT1A and/or an antagonistic effect on 5-HT2A that exerts a PRL-lowering effect (18, 19). As a result, co-prescription of metformin or aripiprazole is considered an effective treatment option to reduce or alleviate HPRL caused by psychiatric drugs (20, 21). Unfortunately, the results of these studies or the specific recommendations given are for the general population with schizophrenia rather than for the group of long-term hospitalized chronic schizophrenia people with co-type 2 diabetes (T2DM).
We discovered by chance that aripiprazole did not lower PRL levels in a subclinical group of schizophrenic patients with co-T2DM in our earlier study when we examined the factors influencing PRL levels in chronically schizophrenic patients with long-term hospitalization for co-T2DM (22). However, due to the study’s limited sample size of aripiprazole-prescribed cases, statistical efficacy was compromised, leaving the conclusions without any useful advice for clinical action. The purpose of this study was to clarify the specific effects of aripiprazole on the PRL levels in co-T2DM schizophrenic patients, to fill in the gaps of the aforementioned study, to provide a reasonable and effective approach to the specific prescription, and to increase the sample size of this clinical subgroup.
A total of 197 schizophrenic patients with long-term hospitalization and co-T2DM who were hospitalized at Wuhan Mental Health Center and Suzhou Guangji Hospital from June 2015 to August 2022 with co-prescription of aripiprazole were included as the study group in this study.
(1) Meet the criteria for the diagnosis of schizophrenia in the QQInternational Classification of Diseases 10th Revision (ICD-10).
(2) Age range of 25–70 years old, male and female cannot be restricted.
(3) The duration of psychiatric illness was 6 years or more, and there were no adjustments in the dose or type of antipsychotic medication in the 2 months before collection of the target data.
(4) The duration of continuous uninterrupted inpatient treatment is not less than 2 years.
(5) The antipsychotic prescribed during hospitalization was olanzapine in combination with aripiprazole or risperidone in combination with aripiprazole, and the doses of the three antipsychotics involved were not limited.
(6) All enrolled patients were co-T2DM and were treated with oral hypoglycemic agents for glycemic control. Additionally, diabetes mellitus did not last less than 1 year.
Exclude bipolar disorder, major depressive disorder, personality disorders, psychiatric disorders due to epilepsy, intellectual developmental disorders, psychiatric disorders due to somatic disorders, and other psychiatric disorders other than schizophrenia. Those with type 1 diabetes (T1DM) and T2DM who need extra exogenous insulin for glycemic control should be excluded. Patients with severe physical comorbidities were also disqualified, including those with cerebrovascular disease, severe heart disease, somatic dysfunction, and other conditions that limit free movement and affect executive function, as well as those with polycystic ovary syndrome and pituitary tumors that affect PRL levels.
In the course of gathering study group cases, we included 204 schizophrenic patients with co-T2DM as the control group, which had the same inclusion and exclusion criteria as the study group, except that aripiprazole was not prescribed.
This study was reviewed and approved by the Ethics Committee of Wuhan Mental Health Center.
The study design was a two-center retrospective case-control study. We studied long-term hospitalized chronic schizophrenic patients with co-T2DM, with the group of patients co-prescribed with aripiprazole as the study group (co-Ari group) and the group of patients not co-prescribed with aripiprazole as the control group (non-Ari group), comparing the differences between the two clinical subgroups in terms of common demographic data and general clinical data, especially in terms of PRL levels. The factors influencing the PRL levels in the study group were also analyzed.
We extracted demographic information and general clinical data from the electronic case systems of the two centers for cases meeting the inclusion criteria for the study and control groups, including age, gender, educational background, body weight (BW), abdominal circumference (AC), body mass index (BMI), duration of psychiatric illness and age of onset, length of stay in the hospital, antipsychotic type and dose, glucose-lowering drug type and dose, fasting blood glucose (FBG), renal function [namely: blood urea nitrogen (BUN); blood creatinine (CRE); blood uric acid (UA)], blood lipids [namely: total cholesterol (TC); triglyceride (TG); low-density lipoprotein cholesterol (LDL-C); high-DL-C (HDL-C)], and PRL. The above indicators were recorded in a self-made spreadsheet.
We defined patients with continuous and uninterrupted hospitalization of greater than or equal to 2 years from the first day of the current hospitalization as long-term hospitalized patients. The time point at which the target parameters were tested and extracted for all samples was required to no history of antipsychotic drug type and dose adjustment and exogenous insulin supplementation in the 2 months before that time. All parameters involved that require the testing of venous blood were measured using morning fasting venous blood as the specimen. BW, AC, BMI were also measured and calculated values obtained in the morning fasting state.
The obtained continuous variables that fit the normal distribution were expressed as mean and standard deviation, and categorical variables were expressed as counts (percentages). First, we used independent samples t-tests or chi-square tests to compare the differences between the study and control groups for each target parameter. Secondly, we used Pearson’s correlation analysis to obtain parameters related to PRL levels for all included samples (a total of 401 participants). Thirdly, a multiple linear regression model was constructed to analyze the factors influencing PRL levels in the total sample size. Finally, a second multiple linear regression model was constructed for the study group sample to analyze the factors influencing PRL levels in this clinical subgroup. All P-values were two-tailed, and the significance level was <0.05. Statistical analyses were performed using SPSS 27 (SPSS, Inc., Chicago, IL, USA).
In patients prescribed olanzapine, the average daily drug dose of olanzapine was (12.86 ± 5.42) mg, and the average daily drug dose of risperidone was (4.10 ± 1.38) mg. The average daily dose of aripiprazole in the co-Ari group was (13.96 ± 3.75) mg. FBG, UA, TC, TG, and LDL-C levels were significantly higher in the study group compared to the control group (t = 2.00, p = 0.046; t = 2.17, p = 0.030; t = 2.50, p = 0.013; t = 3.81, p < 0.001; respectively), but there was no difference in PRL levels (t = 0.56, p = 0.579) (Table 1).
For all included co-T2DM schizophrenia patients, age (r = 0.01, p = 0.017), schizophrenia duration (r = 0.17, p = 0.001), prescription olanzapine (r = 0.11, p = 0.035), prescription risperidone (r = −0.03, p = 0.035), and LDL-C levels (r = 0.27, p < 0.001) were positively associated with PRL levels, while, prescription metformin (r = −0.11, p = 0.048), prescription aripiprazole (r = −0.01, p = 0.009), FBG levels (r = −0.09, p = 0.041), and UA levels (r = −0.14, p = 0.007) were positively associated with PRL levels (Table 2).
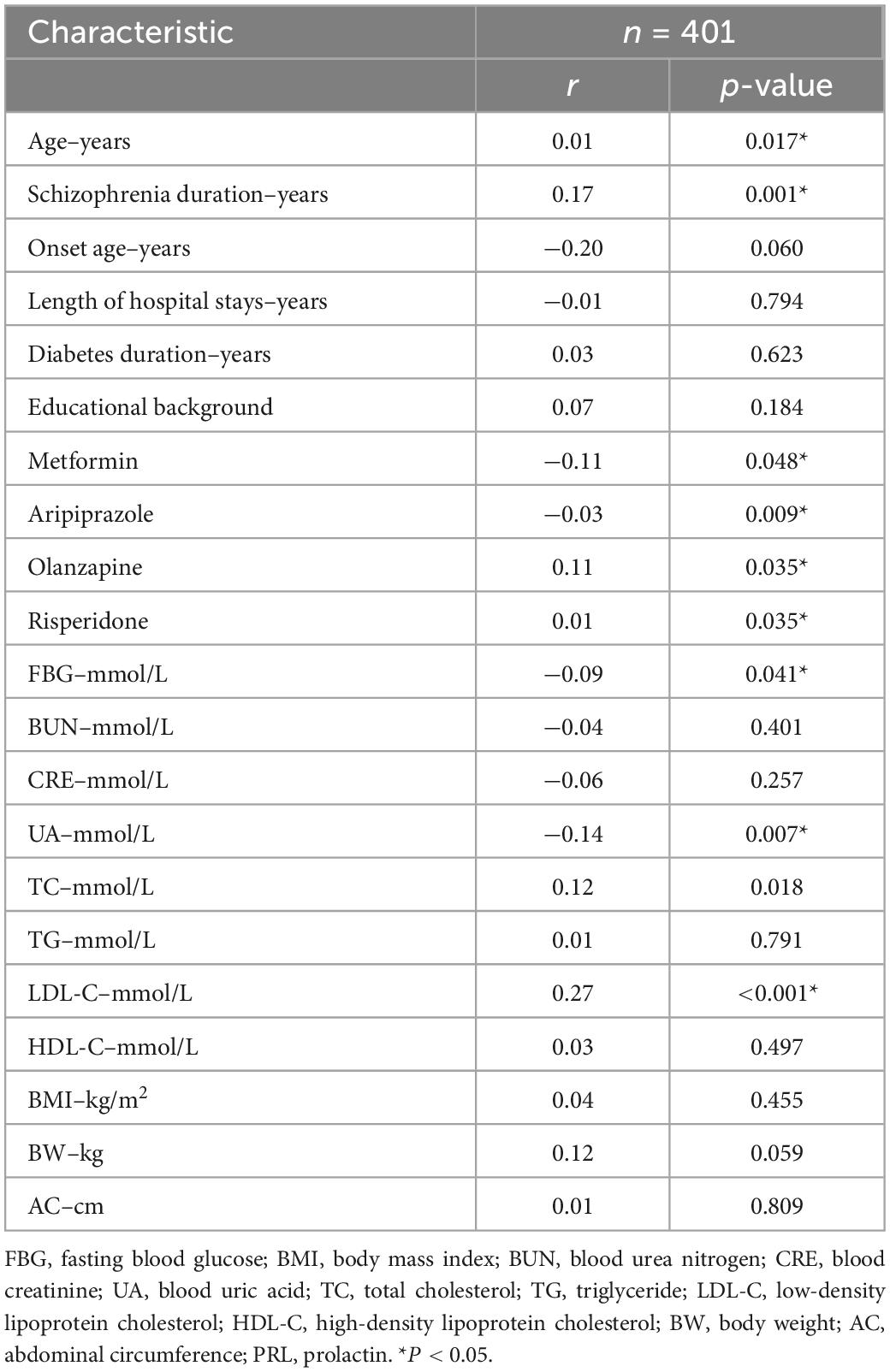
Table 2. Factors associated with PRL levels in patients with schizophrenia co-type 2 diabetes (T2DM).
We constructed a multivariate linear model with PRL level as the dependent variable and the above clinical parameters associated with PRL level as independent variables. The three variables involved in the model, aripiprazole, olanzapine, and risperidone, were defined as dichotomous variables (0 = prescribed, 1 = unprescribed). Age (B = −0.61, t = −3.64, p = 0.021), prescription metformin (B = −0.12, t = −1.04, p = 0.017), and FBG (B = −1.71, t = −3.42, p = 0.001) levels were protective factors for HPRL, whereas prescription olanzapine (B = 2.11, t = 1.05, p = 0.036) and risperidone (B = 7.21, t = 2.72, p = 0.007) were risk factors for HPRL (Table 3).
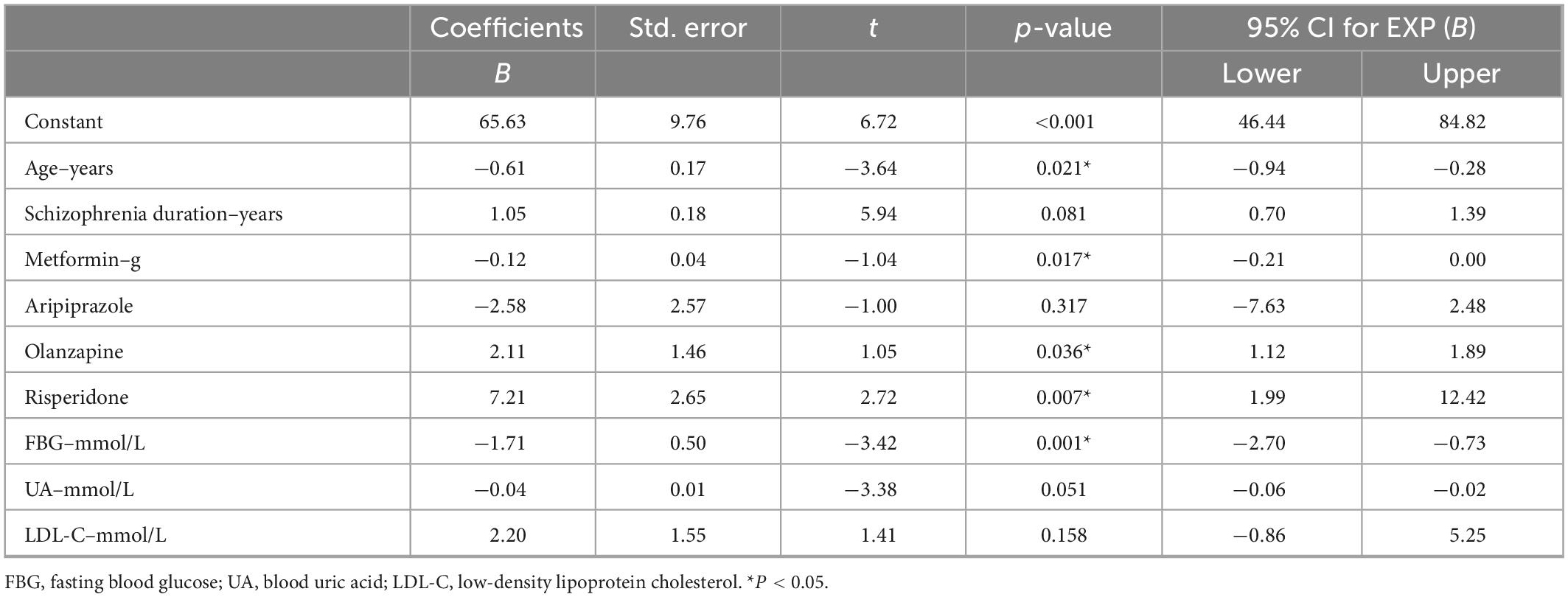
Table 3. Influencing factors of prolactin (PRL) levels in include patients with type 2 diabetes (T2DM): multiple linear regression model.
In the clinical subgroup co-prescribed with aripiprazole, we constructed a multiple linear regression model again with PRL levels as the dependent variable and parameters associated with PRL levels as independent variables. Age (B = −0.95, t = −2.93, p = 0.014), prescription metformin (B = −0.11, t = −0.89, p = 0.015), FBG (B = −3.63, t = −3.71, p < 0.001) levels, and UA (B = −0.07, t = −3.18, p = 0.002) levels were protective factors for HPRL, whereas prescription risperidone (B = 2.32, t = 3.23, p = 0.032) were risk factors for HPRL (Table 4).
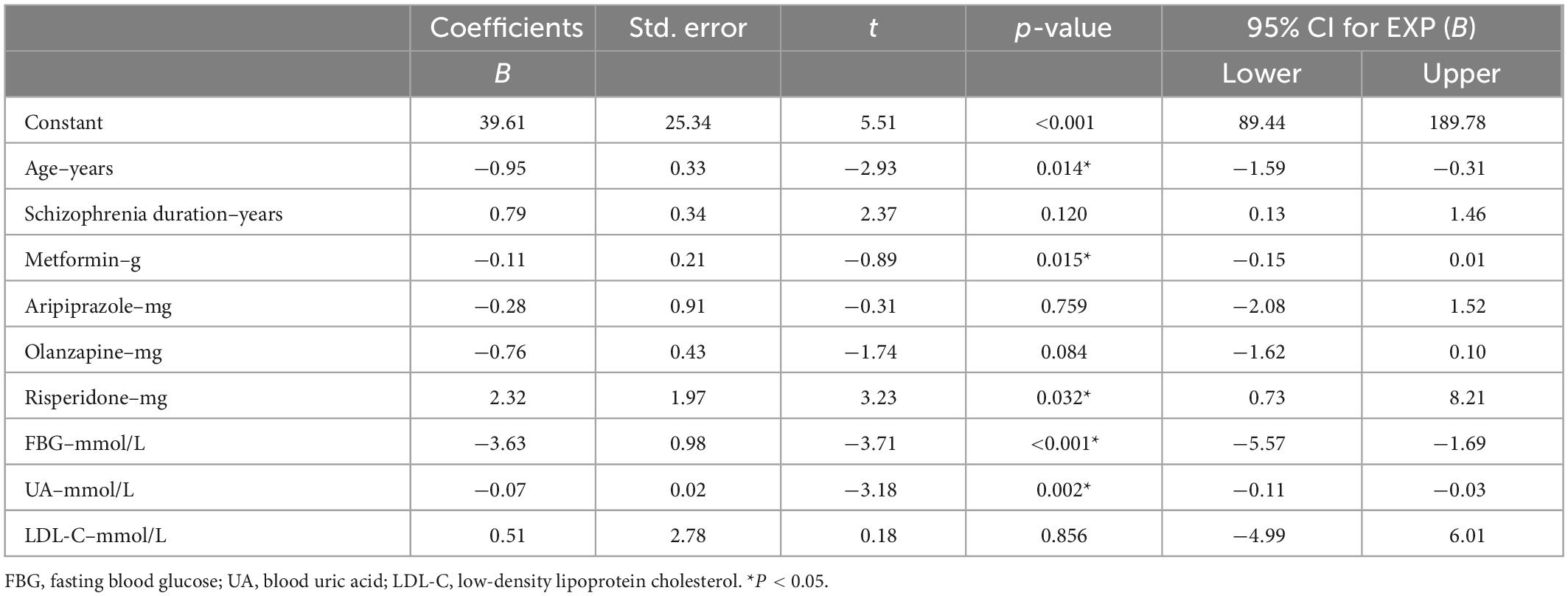
Table 4. Influencing factors of prolactin (PRL) levels in clinical subgroups prescribed aripiprazole: multiple linear regression model.
According to us, this may be the only study to date to clarify whether aripiprazole can reduce PRL levels in schizophrenia patients with co-T2DM. The main findings of this study were that co-prescribing aripiprazole not only had no clinical value in reducing PRL levels in the target population of our study but also increased the severity of metabolic disorders compared to the clinical subgroup without co-prescribing aripiprazole. Our secondary findings also include: (1) in the group of schizophrenic patients with co-T2DM, higher age, prescription metformin, and higher FBG levels were protective factors for HPRL, and prescription olanzapine and risperidone were risk factors for HPRL, but aripiprazole did not affect PRL levels. (2) In the subclinical group with co-prescribed aripiprazole, higher age, metformin dose, and higher FBG and UA levels were protective factors for HPRL, and risperidone dose was a risk factor for HPRL, but aripiprazole dose had also no effect on PRL levels.
There are many clinical studies on the effectiveness of aripiprazole in reducing antipsychotic-induced HPRL (23–25). In China, it is relatively uniform and widely accepted that co-prescribing aripiprazole at doses less than 5 mg is the most effective and optimal prescribing regimen (14, 26). However, one meta-analysis gave differing conclusions, such as Zhang et al. (27) who included 53 randomized controlled double-blind studies and found that either adjuvant less than 5 mg or greater than 10 mg of aripiprazole was the best regimen to control antipsychotic-induced HPRL. In contrast to the above reports, a study from India found that the percentage reduction in PRL levels did not correlate with the specific dose of aripiprazole (28). A multicenter, open-label, prospective study from Korea also reported that administration of the maximum dose of co-prescribed aripiprazole (30 mg/day) similarly achieved a reduction in antipsychotic-induced HPRL (29). These above studies may suggest that the PRL-sparing effect of aripiprazole may not be dependent on the dose of the drug. Although there is a wide range of opinions about which dose of aripiprazole is optimal for improving HPRL, the conclusion that aripiprazole can reduce antipsychotic-induced HPRL is relatively uniform and clear. Puzzlingly, our findings all differ from the above studies in that we found no actual clinical value of co-prescribing aripiprazole for lowering PRL levels in the schizophrenia group with co-morbid T2DM. We speculate that this may be related to the more specific study population we enrolled in or the narrower range of aripiprazole doses (10–20 mg/day) that the study population was prescribed.
As a basic and primary therapeutic agent for T2DM, metformin also unsurprisingly showed a large prescription rate for controlling patients’ blood glucose levels in the schizophrenia group included in our study, while its other significant pharmacological effect is its use for controlling antipsychotic-induced HPRL (14, 20, 27). There is similar controversy and uncertainty regarding the optimal dose of metformin for the treatment of antipsychotic-induced HPRL. The expert consensus from China and clinical studies from Poland both conclude that high doses of metformin (2.55–3.0 g/day) are effective in reducing antipsychotic-derived HPRL (14, 30), but this dose exceeds the maximum daily dose limit (maximum 2 g/day) given in the metformin instructions, which may introduce other metformin-derived adverse drug reactions and ethical issues, and therefore should not be used as a routine clinical treatment regimen. A meta-analysis reported that metformin doses below 1 g/day were also effective in reducing PRL levels in patients with HPRL induced by atypical antipsychotics (27). In our study, the conventional metformin dose (1–2 g/day) used to treat diabetes mellitus in schizophrenia with co-morbid T2DM also had the same function of lowering PRL levels and showed a negative dose-dependence of metformin dose and PRL levels. Whether the reason for this phenomenon is related to the co-prescription of aripiprazole, resulting in a dose shift of metformin to lower PRL levels, is a question that deserves further investigation. And whether aripiprazole is competing with metformin for the failure of targets that inhibit the synthesis and/or release of PRL and thus losing its function in reducing the utility of PRL levels is likewise a question that needs to be further answered.
In the present study, although the function of lowering PRL levels was lost, the co-prescription of aripiprazole exacerbated the severity of abnormal metabolic markers in the included patients, although aripiprazole is considered to be one of the antipsychotics with the least metabolic adverse effects (31–33). In contrast to the more common extrapyramidal adverse effects of first-generation antipsychotics, atypical antipsychotics exhibit more prominent abnormalities in metabolic indicators (34). A study from Hong Kong, China, reported that the combination of multiple antipsychotics increased the risk of abnormal metabolic parameters associated with cardiovascular disease in patients with schizophrenia spectrum disorders (35). Another study reported a higher incidence of metabolic syndrome and lipid markers of insulin resistance in patients receiving antipsychotic polypharmacy compared to those receiving antipsychotic monotherapy (36). In contrast to our study, the participants we included were co-prescribed aripiprazole, which is thought to have no or minimal effect on metabolic indices, and two studies even reported that adjunctive use of 5–20 mg/day of aripiprazole improved metabolic disturbances, while the original atypical antipsychotic dose was maintained (37–40). However, a review of systematic reviews concludes that this possible protective effect of aripiprazole needs to be further elaborated by more robust studies (41), because longer-term observations and studies have found that the severity of metabolic adverse effects of aripiprazole is not superior to that of antipsychotics such as risperidone and quetiapine (42, 43). This is consistent with the results of our study, which found that long-term hospitalized chronic schizophrenia patients co-prescribed with aripiprazole exhibited metabolic abnormalities of even worse severity.
In the secondary findings, we found that older age was a protective factor for HPRL, and one study also found that antipsychotic-derived high levels of PRL decline with age in patients with schizophrenia (44), which may be attributed to the fact that the gonads shrink with age. Higher FBG levels were also a protective factor for HPRL, which is inconsistent with previous findings (45), and in our opinion may be related to the more aggressive addition of glucose-lowering agents represented by metformin for those patients with poorly controlled blood glucose levels. Risperidone remained an important contributor to HPRL, which was the same as the previous findings (1). And higher UA levels were a protective factor for HPRL, which was consistent with our previous report (22).
In conclusion, aripiprazole not only worsened the severity of index disturbances associated to metabolism in long-term hospitalized chronic schizophrenia patients with co-T2DM on metformin-based hypoglycemic medications but also failed to lower PRL levels.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was reviewed and approved by the Ethics Committee of Wuhan Mental Health Center. Written informed consent to participate was not required in accordance with institutional requirements and legislation.
JM and YG made substantial contributions to conception and design of the study. XL and XS drafted the manuscript. LL had polished and re-edited the language and logic of the manuscript. KZ and YL were responsible for setting up and complement and modify the contents of the manuscript. JM gave final approval of the version to be published. All authors contributed to the article and approved the submitted version.
This study was funded by the scientific research project of the Wuhan Municipal Health Commission (WX19Y12 to JM).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhu Y, Zhang C, Siafis S, Zhuo K, Zhu D, Wu H, et al. Prolactin levels influenced by antipsychotic drugs in schizophrenia: a systematic review and network meta-analysis. Schizophr Res. (2021) 237:20–5. doi: 10.1016/j.schres.2021.08.013
2. Wong-Anuchit C. Clinical management of antipsychotic-induced hyperprolactinemia. Perspect Psychiatr Care. (2016) 52:145–52. doi: 10.1111/ppc.12111
3. An FR, Yang R, Wang ZM, Ungvari GS, Ng CH, Chiu HF, et al. Hyperprolactinemia, prolactin-related side effects and quality of life in Chinese psychiatric patients. Compr Psychiatry. (2016) 71:71–6. doi: 10.1016/j.comppsych.2016.08.009
4. Wu TH, Lin CH, Goh KK, Chen CY, Chen CH, Lane HY, et al. The relationships between hyperprolactinemia, metabolic disturbance, and sexual dysfunction in patients with schizophrenia under olanzapine treatment. Front Pharmacol. (2021) 12:718800. doi: 10.3389/fphar.2021.718800
5. Wang J, Jiang F, Zhang Y, Cotes RO, Yang Y, Liu Z, et al. Patterns of antipsychotic prescriptions in patients with schizophrenia in China: a national survey. Asian J Psychiatr. (2021) 62:102742. doi: 10.1016/j.ajp.2021.102742
6. Ashong S, Kretchy IA, Afrane B, de-Graft Aikins A. Patterns of prescription of psychotropic medications and their adherence among patients with schizophrenia in two psychiatric hospitals in Accra, Ghana: a cross-sectional survey. Psychiatry J. (2018) 2018:9850594. doi: 10.1155/2018/9850594
7. Rolland B, Dalon F, Gauthier N, Nourredine M, Bérard M, Carton L, et al. Antipsychotic prescribing practices in real-life (Appreal Study): findings from the French National Healthcare System Database (2007-2017). Front Psychiatry. (2022) 13:1021780. doi: 10.3389/fpsyt.2022.1021780
8. Grover S, Avasthi A, Sinha V, Lakdawala B, Bathla M, Sethi S, et al. Indian psychiatric society multicentric study: prescription patterns of psychotropics in India. Indian J Psychiatry. (2014) 56:253–64. doi: 10.4103/0019-5545.140632
9. Del Cacho N, Vila-Badia R, Butjosa A, Cuadras D, Rubio-Abadal E, Rodriguez-Montes MJ, et al. Sexual dysfunction in drug- naïve first episode nonaffective psychosis patients. relationship with prolactin and psychotic symptoms. Gender differences. Psychiatry Res. (2020) 289:112985. doi: 10.1016/j.psychres.2020.112985
10. Sobrinho LG, Horseman ND. Prolactin and human weight disturbances: a puzzling and neglected association. Rev Endocr Metab Disord. (2019) 20:197–206. doi: 10.1007/s11154-019-09503-1
11. Therkelsen KE, Abraham TM, Pedley A, Massaro JM, Sutherland P, Hoffmann U, et al. Association between prolactin and incidence of cardiovascular risk factors in the Framingham Heart Study. J Am Heart Assoc. (2016) 5:e002640. doi: 10.1161/jaha.115.002640
12. De Hert M, Detraux J, Stubbs B. Relationship between antipsychotic medication, serum prolactin levels and osteoporosis/osteoporotic fractures in patients with schizophrenia: a critical literature review. Expert Opin Drug Saf. (2016) 15:809–23. doi: 10.1517/14740338.2016.1167873
13. Froes Brandao D, Strasser-Weippl K, Goss PE. Prolactin and breast cancer: the need to avoid undertreatment of serious psychiatric illnesses in breast cancer patients: a review. Cancer. (2016) 122:184–8. doi: 10.1002/cncr.29714
14. Chinese Society of Neuroscience, Schizophrenia Clinical Research Alliance of Basic and Clinical Branch of Psychiatry. Consensus on the management of antipsychotic-induced hyperprolactinemia. Chinese J Psychiatry. (2021) 54:163–9. doi: 10.3760/cma.j.cn113661-20201219-00514
15. Łabuzek K, Suchy D, Gabryel B, Bielecka A, Liber S, Okopień B. Quantification of metformin by the Hplc method in brain regions, cerebrospinal fluid and plasma of rats treated with lipopolysaccharide. Pharmacol Rep. (2010) 62:956–65. doi: 10.1016/s1734-1140(10)70357-1
16. Naber D, Lambert M. Aripiprazole: a new atypical antipsychotic with a different pharmacological mechanism. Prog Neuropsychopharmacol Biol Psychiatry. (2004) 28:1213–9. doi: 10.1016/j.pnpbp.2004.06.020
17. Peuskens J, Pani L, Detraux J, De Hert M. The effects of novel and newly approved antipsychotics on serum prolactin levels: a comprehensive review. CNS Drugs. (2014) 28:421–53. doi: 10.1007/s40263-014-0157-3
18. Urban JD, Vargas GA, von Zastrow M, Mailman RB. Aripiprazole has functionally selective actions at dopamine D2 receptor-mediated signaling pathways. Neuropsychopharmacology. (2007) 32:67–77. doi: 10.1038/sj.npp.1301071
19. Di Sciascio G, Riva MA. Aripiprazole: from pharmacological profile to clinical use. Neuropsychiatr Dis Treat. (2015) 11:2635–47. doi: 10.2147/ndt.S88117
20. Bo QJ, Wang ZM, Li XB, Ma X, Wang CY, de Leon J. Adjunctive metformin for antipsychotic-induced hyperprolactinemia: a systematic review. Psychiatry Res. (2016) 237:257–63. doi: 10.1016/j.psychres.2016.01.031
21. Labad J, Montalvo I, González-Rodríguez A, García-Rizo C, Crespo-Facorro B, Monreal JA, et al. Pharmacological treatment strategies for lowering prolactin in people with a psychotic disorder and hyperprolactinaemia: a systematic review and meta-analysis. Schizophr Res. (2020) 222:88–96. doi: 10.1016/j.schres.2020.04.031
22. Zhu J, Wang H, Huang S, Zhang Y, Liu X, Li Y, et al. Factors influencing prolactin levels in chronic long-term hospitalized schizophrenic patients with co-morbid type 2 diabetes mellitus. Front Psychiatry. (2022) 13:1034004. doi: 10.3389/fpsyt.2022.1034004
23. Jen YW, Hwang TJ, Chan HY, Hsieh MH, Liu CC, Liu CM, et al. Abnormally low prolactin levels in schizophrenia patients after switching to aripiprazole in a randomized trial: a biomarker for rebound in psychotic symptoms? BMC Psychiatry. (2020) 20:552. doi: 10.1186/s12888-020-02957-7
24. Qiao Y, Yang F, Li C, Guo Q, Wen H, Zhu S, et al. Add-on effects of a low-dose aripiprazole in resolving hyperprolactinemia induced by risperidone or paliperidone. Psychiatry Res. (2016) 237:83–9. doi: 10.1016/j.psychres.2015.12.033
25. Byerly MJ, Marcus RN, Tran QV, Eudicone JM, Whitehead R, Baker RA. Effects of aripiprazole on prolactin levels in subjects with schizophrenia during cross-titration with risperidone or olanzapine: analysis of a randomized, open-label study. Schizophr Res. (2009) 107:218–22. doi: 10.1016/j.schres.2008.09.019
26. Li X, Tang Y, Wang C. Adjunctive aripiprazole versus placebo for antipsychotic-induced hyperprolactinemia: meta-analysis of randomized controlled trials. PLoS One. (2013) 8:e70179. doi: 10.1371/journal.pone.0070179
27. Zhang L, Qi H, Xie YY, Zheng W, Liu XH, Cai DB, et al. Efficacy and safety of adjunctive aripiprazole, metformin, and paeoniae-glycyrrhiza decoction for antipsychotic-induced hyperprolactinemia: a network meta-analysis of randomized controlled trials. Front Psychiatry. (2021) 12:728204. doi: 10.3389/fpsyt.2021.728204
28. Raveendranthan D, Rao NP, Rao MG, Mangot AG, Varambally S, Kesavan M, et al. Add-on aripiprazole for atypical antipsychotic-induced, clinically significant hyperprolactinemia. Indian J Psychol Med. (2018) 40:38–40. doi: 10.4103/ijpsym.Ijpsym_147_17
29. Yoon HW, Lee JS, Park SJ, Lee SK, Choi WJ, Kim TY, et al. Comparing the effectiveness and safety of the addition of and switching to aripiprazole for resolving antipsychotic-induced hyperprolactinemia: a multicenter, open-label, prospective study. Clin Neuropharmacol. (2016) 39:288–94. doi: 10.1097/wnf.0000000000000175
30. Krysiak R, Kowalcze K, Szkrobka W, Okopien B. The effect of metformin on prolactin levels in patients with drug-induced hyperprolactinemia. Eur J Intern Med. (2016) 30:94–8. doi: 10.1016/j.ejim.2016.01.015
31. Zhang Y, Wang Q, Reynolds GP, Yue W, Deng W, Yan H, et al. Metabolic effects of 7 antipsychotics on patients with schizophrenia: a short-term, randomized, open-label, multicenter, pharmacologic trial. J Clin Psychiatry. (2020) 81:19m12785. doi: 10.4088/JCP.19m12785
32. Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. (2020) 7:64–77. doi: 10.1016/s2215-0366(19)30416-x
33. Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. (2019) 394:939–51. doi: 10.1016/s0140-6736(19)31135-3
34. Hammoudeh S, Al Lawati H, Ghuloum S, Iram H, Yehya A, Becetti I, et al. Risk factors of metabolic syndrome among patients receiving antipsychotics: a retrospective study. Community Ment Health J. (2020) 56:760–70. doi: 10.1007/s10597-019-00537-y
35. Bressington D, Mui J, Tse ML, Gray R, Cheung EF, Chien WT. Cardiometabolic health, prescribed antipsychotics and health-related quality of life in people with schizophrenia-spectrum disorders: a cross-sectional study. BMC Psychiatry. (2016) 16:411. doi: 10.1186/s12888-016-1121-1
36. Correll CU, Frederickson AM, Kane JM, Manu P. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. (2007) 89:91–100. doi: 10.1016/j.schres.2006.08.017
37. Wang LJ, Ree SC, Huang YS, Hsiao CC, Chen CK. Adjunctive effects of aripiprazole on metabolic profiles: comparison of patients treated with olanzapine to patients treated with other atypical antipsychotic drugs. Prog Neuropsychopharmacol Biol Psychiatry. (2013) 40:260–6. doi: 10.1016/j.pnpbp.2012.10.010
38. Gupta B, Chee KS, Neo LQ, Tang C, Hariram J, Tan GC, et al. Effect of aripiprazole as an adjunct to atypical antipsychotics on weight and metabolic profile: a 12-week open-label trial. Ther Adv Psychopharmacol. (2021) 11:20451253211046765. doi: 10.1177/20451253211046765
39. Galling B, Roldán A, Rietschel L, Hagi K, Walyzada F, Zheng W, et al. Safety and tolerability of antipsychotic co-treatment in patients with schizophrenia: results from a systematic review and meta-analysis of randomized controlled trials. Expert Opin Drug Saf. (2016) 15:591–612. doi: 10.1517/14740338.2016.1165668
40. Fleischhacker WW, Heikkinen ME, Olié JP, Landsberg W, Dewaele P, McQuade RD, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. (2010) 13:1115–25. doi: 10.1017/s1461145710000490
41. Ijaz S, Bolea B, Davies S, Savović J, Richards A, Sullivan S, et al. Antipsychotic polypharmacy and metabolic syndrome in schizophrenia: a review of systematic reviews. BMC Psychiatry. (2018) 18:275. doi: 10.1186/s12888-018-1848-y
42. Vázquez-Bourgon J, Ortiz-García de la Foz V, Gómez-Revuelta M, Mayoral-van Son J, Juncal-Ruiz M, Garrido-Torres N, et al. Aripiprazole and risperidone present comparable long-term metabolic profiles: data from a pragmatic randomized controlled trial in drug-naïve first-episode psychosis. Int J Neuropsychopharmacol. (2022) 25:795–806. doi: 10.1093/ijnp/pyac033
43. Vázquez-Bourgon J, Pérez-Iglesias R, Ortiz-García de la Foz V, Suárez Pinilla P, Díaz Martínez Á, Crespo-Facorro B. Long-term metabolic effects of aripiprazole, ziprasidone and quetiapine: a pragmatic clinical trial in drug-naïve patients with a first-episode of non-affective psychosis. Psychopharmacology. (2018) 235:245–55. doi: 10.1007/s00213-017-4763-x
44. Montgomery J, Winterbottom E, Jessani M, Kohegyi E, Fulmer J, Seamonds B, et al. Prevalence of hyperprolactinemia in schizophrenia: association with typical and atypical antipsychotic treatment. J Clin Psychiatry. (2004) 65:1491–8. doi: 10.4088/jcp.v65n1108
Keywords: schizophrenia, aripiprazole, prolactin, type 2 diabetes, long-term hospitalized, chronic
Citation: Liu X, Sun X, Li L, Zeng K, Li Y, Gao Y and Ma J (2023) Co-prescription of aripiprazole on prolactin levels in long-term hospitalized chronic schizophrenic patients with co-morbid type 2 diabetes: A retrospective clinical study. Front. Psychiatry 14:1124691. doi: 10.3389/fpsyt.2023.1124691
Received: 15 December 2022; Accepted: 20 January 2023;
Published: 02 February 2023.
Edited by:
Mohammadreza Shalbafan, Iran University of Medical Sciences, IranReviewed by:
Masahiro Banno, Seichiryo Hospital, JapanCopyright © 2023 Liu, Sun, Li, Zeng, Li, Gao and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yujun Gao,  Z2FveXVqdW4xOTgyMDIxNEAxNjMuY29t; Jun Ma,
Z2FveXVqdW4xOTgyMDIxNEAxNjMuY29t; Jun Ma,  bWFqdW4wMzEzQG1zbi5jbg==
bWFqdW4wMzEzQG1zbi5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.