
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Psychiatry, 16 February 2023
Sec. Anxiety and Stress Disorders
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1118836
Objective: Previous studies have demonstrated an association between anxiety and metabolic syndrome (MetS). However, the association is still controversial. This updated meta-analysis aimed to reanalyze the association between anxiety and MetS.
Methods: We comprehensively searched PubMed, Embase and Web of Science for all related studies published before January 23, 2023. Observational studies that informed effect size with 95% confidence interval (CI) for the association between anxiety and MetS were included. According to heterogeneity between studies, fixed or random effects models were applied to calculate the pooled effect size. Publication bias was examined by funnel plots.
Results: The research included 24 cross-sectional studies: 20 studies used MetS as the dependent variable with a pooled OR of 1.07 (95% CI: 1.01–1.13) and four studies used anxiety as the dependent variable with a pooled OR of 1.14 (95% CI: 1.07–1.23). Three cohort studies were found: two studies detected the association of baseline anxiety with the risk of MetS, one of the studies demonstrated a significant association, but a similar result was not found in another study; one study showed no significant association between baseline MetS and the risk of anxiety.
Conclusion: Cross-sectional studies indicated an association between anxiety and MetS. The results from cohort studies are still inconsistent and limited. More large-scale prospective studies are needed to further reveal the causal relationship of anxiety with MetS.
Metabolic syndrome (MetS) is an assemblage of metabolic abnormalities, characterized by obesity, increased fasting blood glucose, dyslipidemia and hypertension (1). MetS constitutes growing health problems due to it contributes to the increased risk of cardiovascular disease (CVD) and type 2 diabetes mellitus (T2DM) (2, 3). The prevalence of MetS has increased dramatically worldwide. It has reported that the prevalence of MetS among adults in the US between 2011 and 2016 was 34.7%, and the prevalence significantly increased with advancing age (4). In the past two decades, the prevalence of MetS has increased rapidly from 13.7 to 31.1% in China (5).
Previous studies indicated a mutual relationship between physical illnesses (i.e., CVD, MetS) and mental disorders (6, 7). Anxiety disorders are one of the most common mental disorders with onset usually in childhood (8, 9). The lifetime prevalence of anxiety disorders is 5–25%, and the 12 months prevalence is 3.0–19.0% (10). Previous studies indicated that the prevalence of MetS among people with anxiety ranges from 30 to 40% (11, 12). The prevalence of anxiety was approximately 10% higher among people with MetS compared with those without MetS (13). However, the association between MetS and anxiety is still inconsistent due to the differences in diagnostic criteria for anxiety and MetS, study sample size, and study design (12, 14, 15).
Our previous systematic review and meta-analysis suggested that anxiety was positively associated with MetS (16). Since our last meta-analysis, there are nine studies on the association between anxiety and MetS have been published (12, 14, 15, 17–22). Therefore, it is necessary to update the review to obtain current evidence based on the newly published research. In addition, the previous study was limited to exploring the association between anxiety and MetS, the effect of different study outcomes on this association was not explored. So, we performed an updated meta-analysis to derive a more comprehensive and reliable estimation of the association.
The present study followed the Meta-analyses and Systematic Review of Observational Studies in Epidemiology (MOOSE) and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, Supplementary Table 1) guidelines (23, 24). We performed systematic literature searches in PubMed, Embase and Web of Science for all related studies published before January 23, 2023. Two authors (SJ and YCH) independently conducted the search using MeSH terms and free terms for “anxiety” and “metabolic syndrome.” An example search strategy for the three databases is listed in Supplementary Table 2. All eligible studies were limited to those published in English. Manual searches of the reference lists of the included studies were performed.
After we searched the literature, titles, abstracts and full-text articles were reviewed by two independent authors (SJ and YCH) to identify studies that met the inclusion criteria. Any discrepancies were resolved by consensus. Articles were considered for inclusion according to the following criteria: (1) observational study design (including cross-sectional study, cohort study or case-control study); (2) studies that measured MetS using International Diabetes Federation (IDF), National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) or World Health Organization (WHO) criteria; and (3) studies presenting the effect size of the association between anxiety and MetS [odds ratio (OR), relative risk (RR), or hazard ratio (HR) with 95% confidence interval (CI)] or sufficient data to estimate it. Reviews, conference abstracts, letters to editors and unpublished studies not meeting the criteria above were excluded. Studies with low quality scores were excluded from further analysis. If more than one eligible publication was conducted within the same population, only the most relevant study was chosen.
The following general characteristics of the eligible studies were collected by two authors: study characteristics (first author, country, year of publication, study design etc.), follow-up time for cohort studies, population, sample size, age, sex, anxiety measurement, definition of MetS, statistical results (unadjusted or adjusted OR, RR, or HR with its 95% CI) and adjustment for potential confounding factors. The Agency for Healthcare Research and Quality (AHRQ) and Newcastle-Ottawa scale (NOS) criteria were used to evaluate the quality of the included cross-sectional studies and cohort studies, respectively. The AHRQ criteria was scored as follows: low quality = 0–3; moderate quality = 4–7; high quality = 8–11 (25). According to the NOS, the included studies were defined as low quality (1–3 stars), moderate quality (4–6 stars) and high quality (7–9 stars) (26). Methodologically, low quality studies tend to exaggerate the overall estimate and may lead to incorrect inferences (27). Therefore, we removed the studies with low quality. Two independent authors (SJ and YCH) performed the data extraction and quality assessment. Any disagreement was resolved through negotiation until an agreement was reached.
The included studies with effect sizes (ORs, RRs, or HRs) for the association between anxiety disorders and MetS were synthesized into pooled ORs and RRs with corresponding 95% CIs. Heterogeneity among the included studies was detected with the Q test and the I2 statistic. A random or fixed effects model was used depending on heterogeneity among studies. I2 > 50% indicates heterogeneity among studies, and random effects model was adopted as the pooling method; otherwise, we used a fixed effects model to pool effect sizes (28). Subgroup analyses were performed to explore the heterogeneity across studies based on anxiety assessment, MetS diagnosis criteria, country, sample size, and study quality. Sensitivity analyses removing one individual study each time were investigated to evaluate the stability of the pooled results. In addition, publication bias was evaluated by funnel plot symmetry, Egger’s and Begg’s tests. Data analyses were performed using STATA/MP 16.0. In the bilateral situation, P < 0.05 was considered to be statistically significant. However, P < 0.1 illustrated heterogeneity among studies in the Q test (29).
A flowchart of the literature screening process is presented in Figure 1. We searched three additional literatures manually. A total of 27 studies ultimately met the inclusion criteria (12, 14, 15, 17–22, 30–47). There were six high quality studies and 21 moderate quality studies. Information about the quality assessment of eligible studies can be obtained from Supplementary Tables 4, 5.
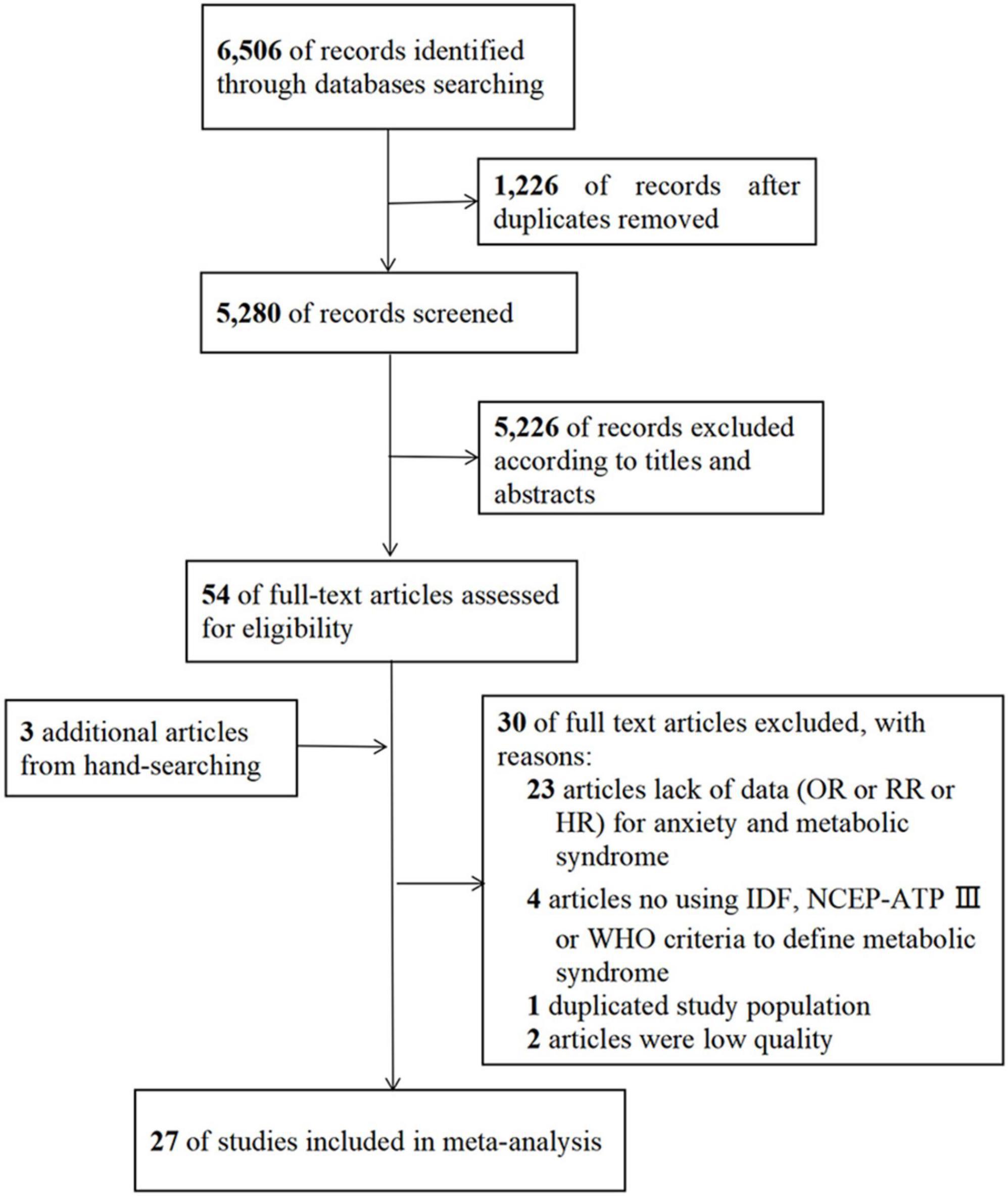
Figure 1. Flowchart of the literature search and study selection. HR, hazard ratio; IDF, International Diabetes Federation; NCEP-ATP III, National Cholesterol Education Program-Adult Treatment Panel III; OR, odds ratio; RR, relative risk; WHO, World Health Organization.
The characteristics of the 24 cross-sectional studies are presented in Supplementary Table 3. Twenty studies reported MetS as the dependent variable (12, 14, 15, 17, 18, 30–37, 39, 40, 43–47) and four studies reported anxiety as the dependent variable (19, 20, 22, 41). Twenty-four studies used two methods to diagnose anxiety, seven studies used structured diagnostic interviews, and 17 studies used self-report symptom scales. NCEP-ATP III (n = 19) and IDF (n = 5) were used to define MetS. The 24 studies were conducted in fifteen different countries (i.e., America, Brazil, Finland, China, France, Germany, Greece, Iran, Italy, Japan, Lithuania, Netherlands, Norway, Switzerland, and Thailand).
Supplementary Table 3 displays the characteristics of the cohort studies. Three cohort studies were included (21, 38, 42), and two reported the association of baseline anxiety status with the risk of MetS. In contrast, another study reported that baseline MetS status predicted the risk of anxiety. The follow-up duration in cohort studies varied from 1 to 15 years.
The 24 cross-sectional studies included in the present analysis comprised 80,466 subjects. We calculated the pooled OR with MetS as the dependent variable using the random effects model given that considerable heterogeneity was noted (I2 = 51.3%, PQ = 0.004). The pooled results of random effects analysis showed that patients with anxiety had a higher risk of MetS than people without anxiety, with a pooled OR of 1.07 (95% CI: 1.01–1.13) (Figure 2).
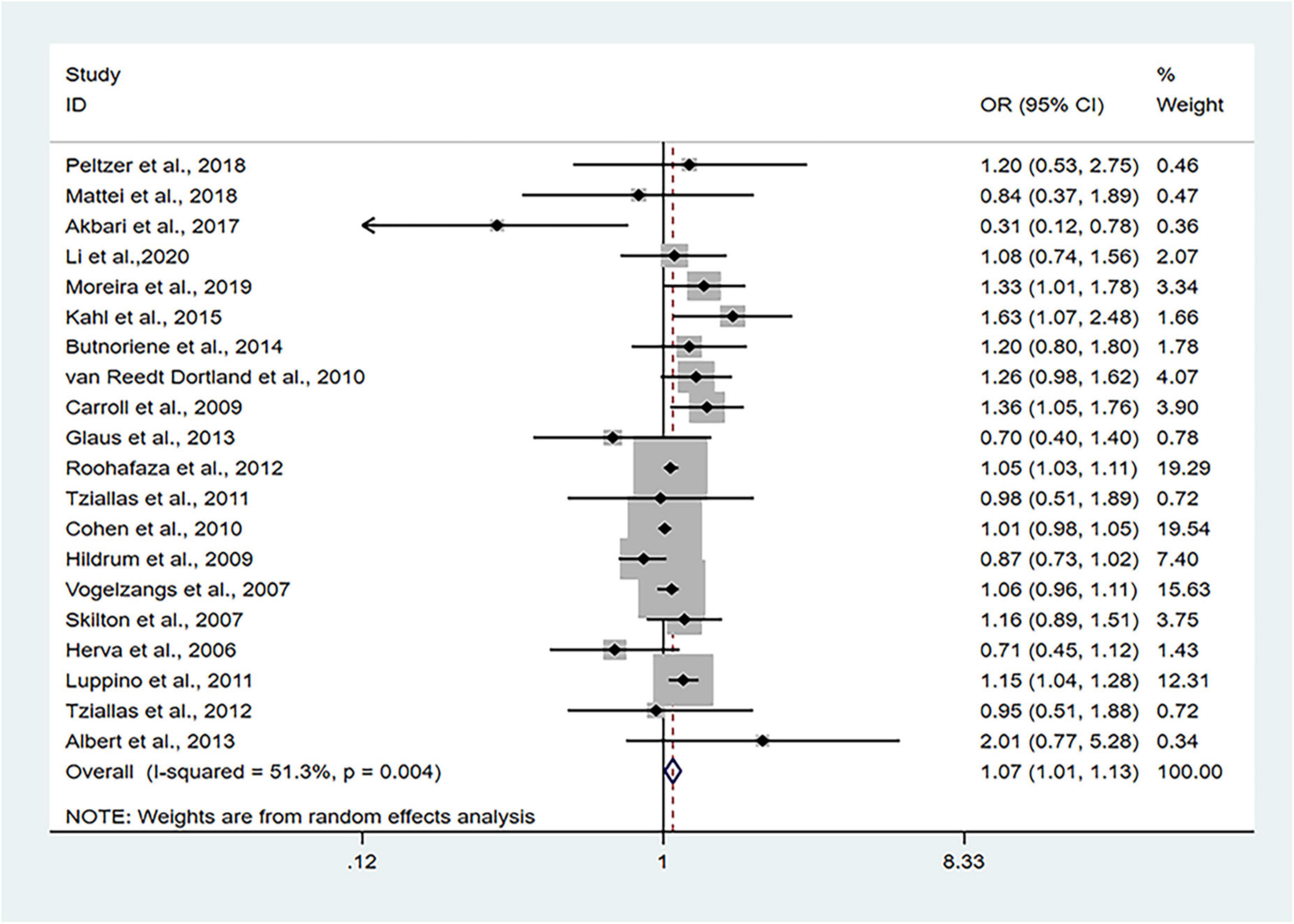
Figure 2. Forest plots of the association of anxiety with MetS among cross-sectional studies with MetS as the dependent variable. CI, confidence interval; MetS, metabolic syndrome; OR, odds ratio.
To investigate the factors affecting heterogeneity, subgroup analyses according to anxiety assessment, MetS diagnosis criteria, country, sample size and study quality were performed (Table 1). On the basis of anxiety assessment, we found that using structured clinical interviews tended to report a stronger association than using self-reported symptom scales. The pooled ORs of the two assessment methods were 1.29 (95% CI: 1.13–1.48, I2 = 4.02%) and 1.04 (95% CI: 0.99–1.09, I2 = 43.60%), respectively. Based on MetS diagnostic criteria, the NCEP-ATP III criteria expressed a stronger association between anxiety and MetS (OR: 1.09, 95% CI: 1.03–1.16, I2 = 51.33%). The IDF criteria presented a negative but non-significant association (OR: 0.89, 95% CI: 0.76–1.04, I2 = 0%). The P-values of the chi-square test among the two subgroups above indicated statistical significance (Table 1). Differences in the diagnosis of anxiety and MetS might be the source of heterogeneity. The heterogeneity in other subgroups was not significant, which indicated that these factors were not the source of heterogeneity.
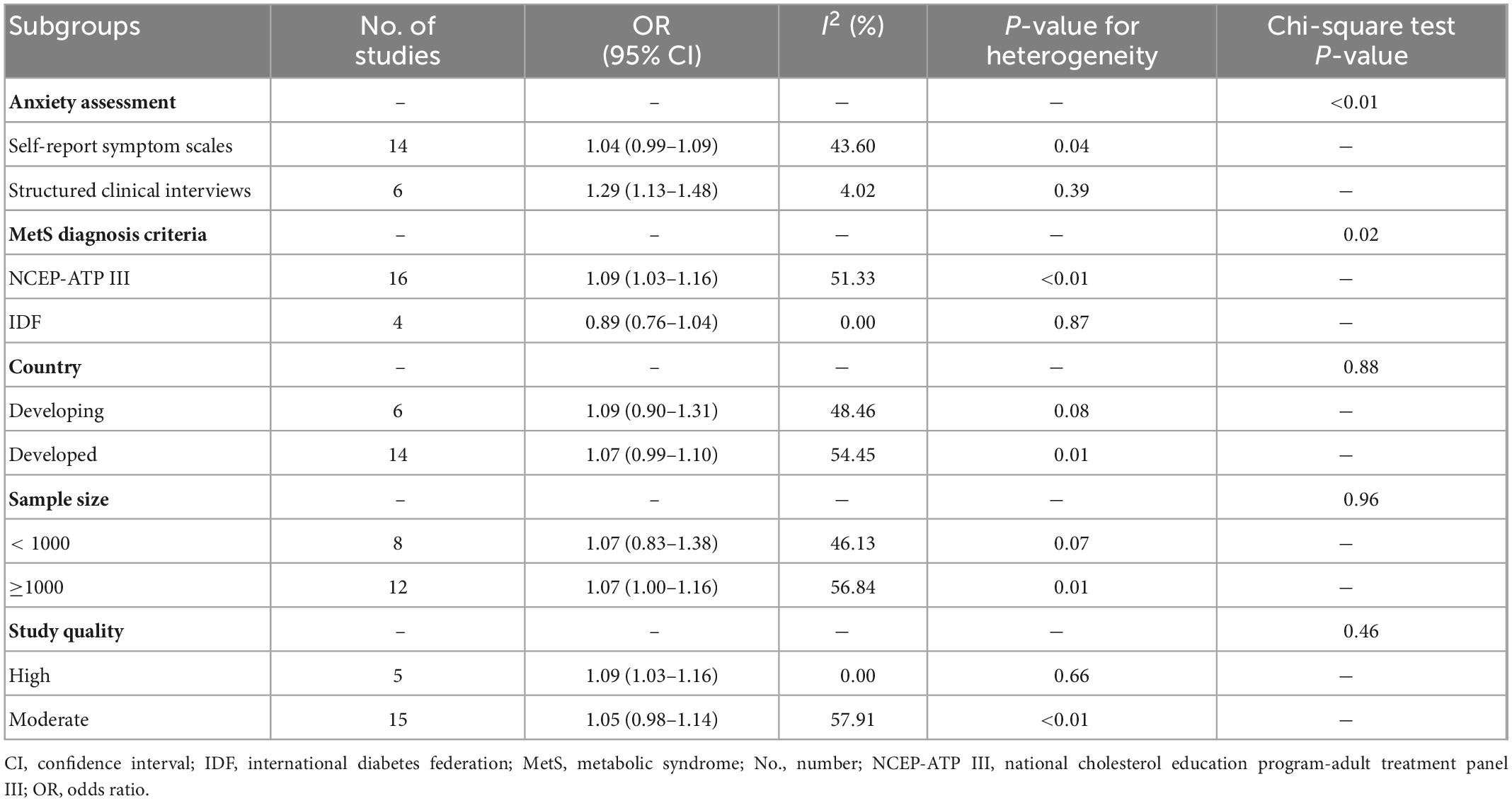
Table 1. Results of subgroup analysis stratified by anxiety assessment, metabolic syndrome (MetS) diagnosis criteria, country, sample size and study quality.
Sensitivity analyses that eliminated each study in turn were performed to examine whether individual studies affected the pooled results. Sensitivity analyses illustrated that there was no substantial modification in the results after removing any of the studies (varied from 1.00 to 1.17) (Figure 3A). Results of Begg’s test (P = 0.50) and Egger’s test (P = 0.51) did not observe publication bias. Nor did the funnel plot exhibit obvious asymmetry (Figure 4), suggesting no publication bias in our analysis.
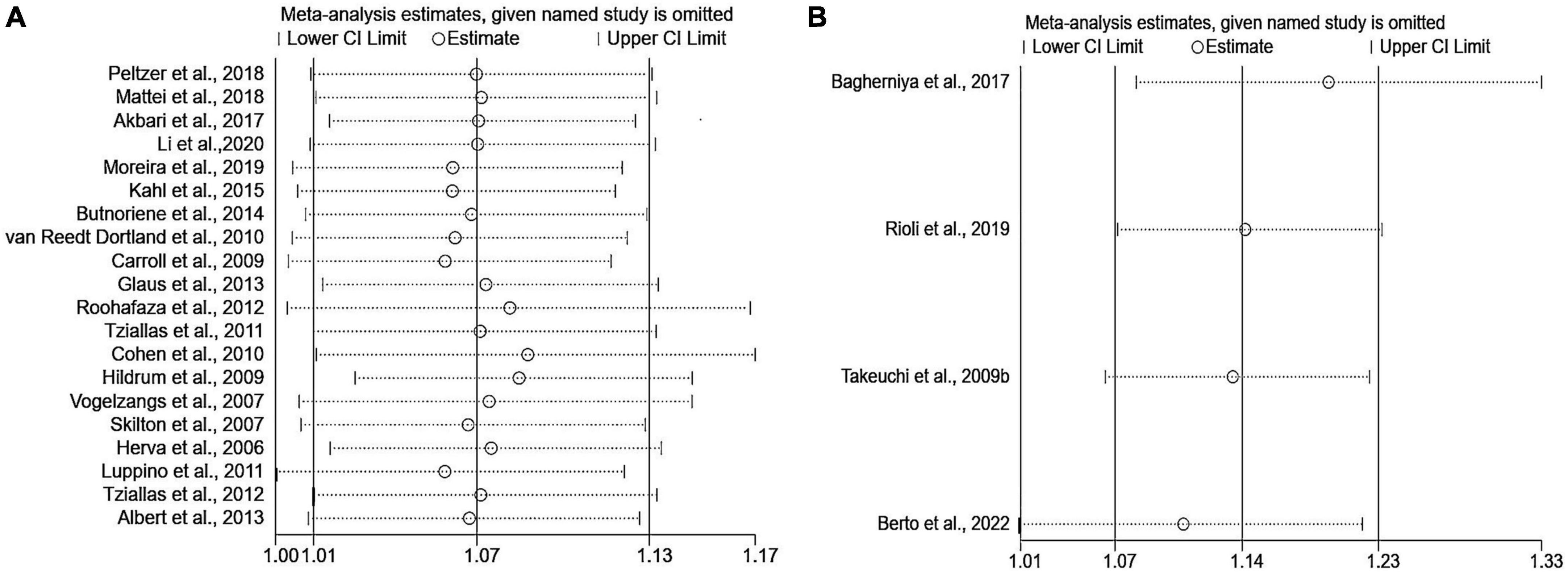
Figure 3. Sensitivity analyses of the association of anxiety with MetS. (A) Metabolic syndrome as the dependent variable; (B) anxiety as the dependent variable. MetS: metabolic syndrome.
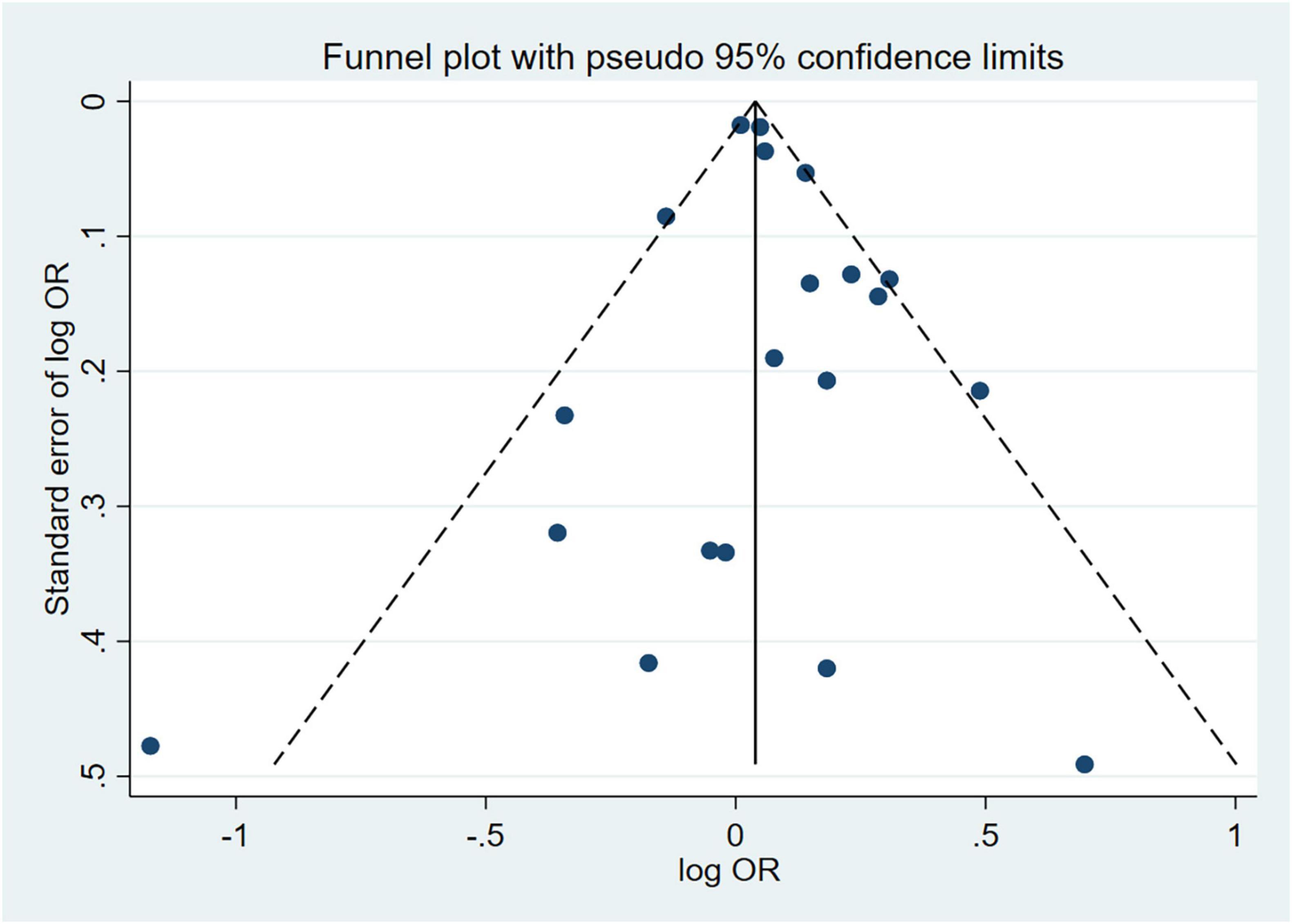
Figure 4. Funnel plot of effect estimates for 20 cross-sectional studies with MetS as the dependent variable assessing the association between anxiety and MetS (P = 0.51 by Egger’s test and P = 0.50 by Begg’s test). MetS, metabolic syndrome; OR, odds ratio.
The pooled OR with anxiety as the dependent variable is presented in Figure 5. The results showed that individuals with MetS expressed significantly higher risks of anxiety than people without MetS, with no heterogeneity (OR: 1.14, 95% CI: 1.07–1.23; I2 = 0.0%). The sensitivity analysis is presented in Figure 3B, and the results showed that no individual study significantly influenced the pooled OR.
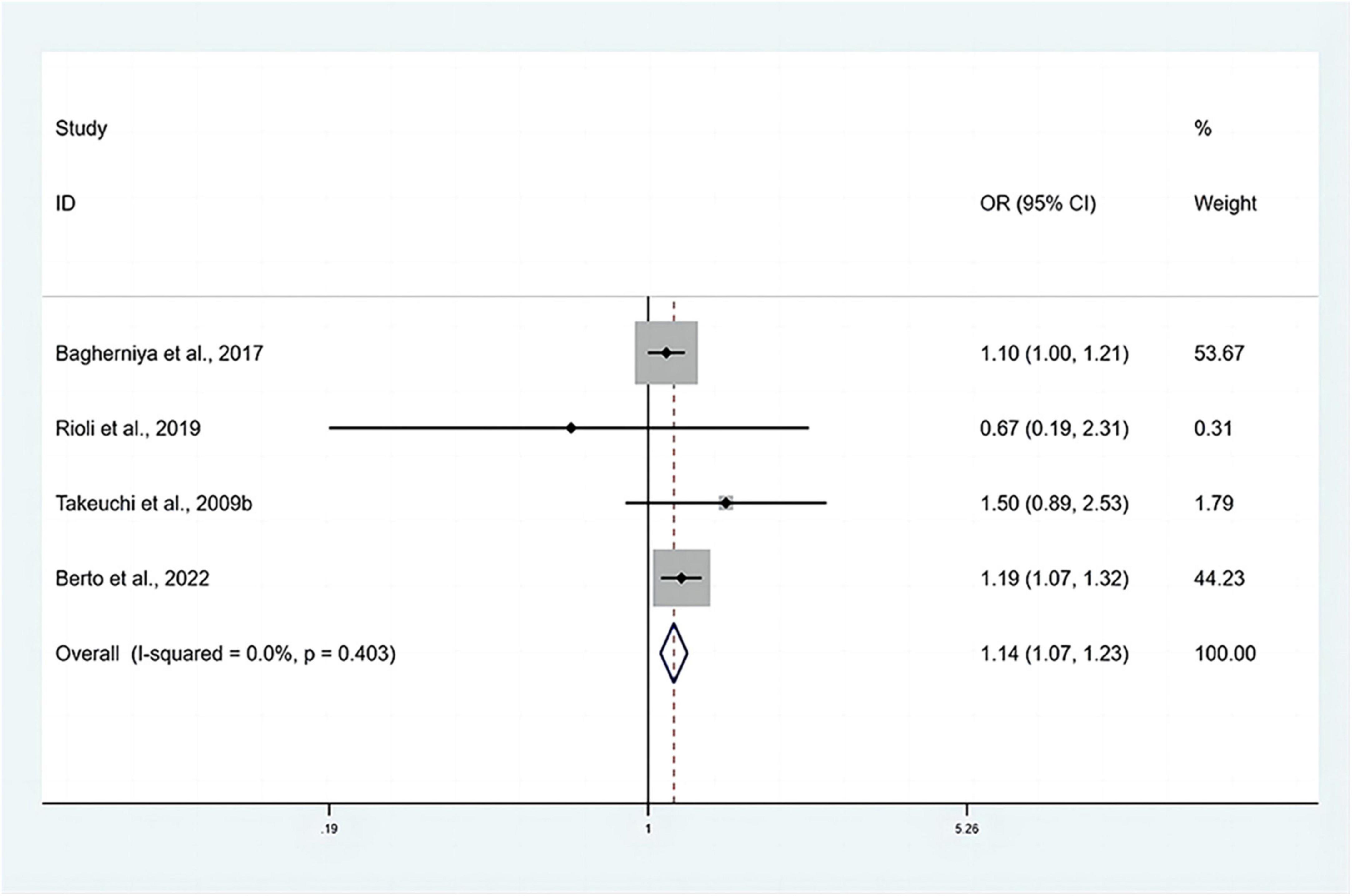
Figure 5. Forest plots of the association of anxiety with MetS among cross-sectional studies with anxiety as the dependent variable. CI, confidence interval; MetS, metabolic syndrome; OR, odds ratio.
Two cohort studies explored the association between baseline anxiety status and future risk of MetS. One prospective cohort study, which included 432 participants with a 15 years follow-up, used IDF, NCEP-ATP III, and WHO criteria to diagnose MetS. The assessment of anxiety was performed using the Spielberger Trait Anxiety Questionnaire (38). The adjusted RRs were 1.04 (95% CI: 0.87–1.25), 1.07 (95% CI: 0.87–1.32), and 1.08 (95% CI: 0.76–1.54) for the IDF, NCEP-ATP III and WHO criteria, respectively. The other study was a retrospective cohort study designed by Lubas et al. (21) with an average of 3.9 years of follow-up. A total of 3,267 participants were included in this study, 809 of whom were diagnosed with MetS according to the NCEP-ATP III criteria. Anxiety disorder was evaluated using the Brief Symptom Inventory-18 (BSI-18). The adjusted RR was 1.34 (95% CI: 1.12–1.59). The quality scores of the two studies were both of seven (Supplementary Table 5), and the characteristics of the two studies are presented in Supplementary Table 3.
One cohort study investigated the association between baseline MetS status and the future risk of anxiety with a total sample size of 956. The characteristics of the study are shown in Supplementary Table 3. The follow-up time of this study was 1 year, and the quality score was six (Supplementary Table 5) (42). MetS was assessed according to the IDF criteria. The profile of mood states (POMS) was used to define anxiety. The RR of the association was 0.7 (95% CI: 0.35–1.41).
This updated systematic review and meta-analysis has consistently confirmed the association between anxiety and MetS using the data from cross-sectional studies with anxiety and MetS as dependent variables, respectively. Subgroup analysis including anxiety assessment, MetS diagnosis criteria, country, study sample size and study quality showed that the diagnostic criteria for anxiety and MetS could influence the strength of the association.
The final pooled OR based on the cross-sectional studies was 1.07 (95% CI: 1.01–1.13) for MetS as the dependent variable and 1.14 (95% CI: 1.07–1.23) for anxiety as the dependent variable. Our findings are consistent with other studies included in this study that prior anxiety increased the risk of MetS (12, 31, 36, 37, 39). The subgroup analyses according to different diagnostic criteria for anxiety suggested that the association was stronger for anxiety assessed by structured clinical interviews compared with the self-reported symptom scale. A previous study suggested that anxiety, which could coexist with one or more mental disorders, may have the same symptoms as other psychiatric disorders (48). This may result in the inclusion of individuals who do not meet the formal criteria for the Diagnostic and Statistical Manual of Mental Disorders (DSM) diagnosis. The accuracy of structured clinical interviews in diagnosing anxiety may explain the higher association between anxiety and metabolic syndrome.
We observed that the result of the NCEP-ATP III criteria was similar to the main results. The IDF criteria indicated a non-significant association between anxiety and MetS. Considering that the IDF criteria focused on abdominal obesity and its mandatory obesity thresholds and diagnostic segmentation points of metabolic abnormalities are lower than those recommended by NCEP-ATP III criteria, it is expected that the IDF criteria would detect a higher proportion of patients with MetS (49), which may explain the difference between the two diagnostic criteria. We also observed that the result of studies with sample sizes greater than 1,000 was similar to the main results. However, the result from studies with a small sample size was insignificant. In the subgroup analyses of anxiety and MetS risk according to study quality, we observed that the increased risk reported in high quality studies was similar to that reported in the overall analysis, while a non-significant increase in risk was observed for moderate quality studies. However, the difference between high and moderate quality was insignificant, indicating that quality was not the main source of the overall heterogeneity.
According to the pooled results of the cross-sectional studies included, the causality of this association could not be assessed. If anxiety occurs earlier than MetS, the following pathophysiological mechanisms of anxiety may contribute to MetS. First, anxiety is correlated with sympathetic nervous system excitation, which will lead to the contraction of systemic arterioles and increased peripheral vascular resistance that cause the elevation of blood pressure. Increased secretion of catecholamines from the adrenal glands could also increase blood pressure (50–52). Second, the evidence suggested that anxiety could enhance the excitability of the hypothalamic-pituitary-adrenocortical (HPA) axis, which will increase the secretion of cortisol (53–55). The chronically high concentration of cortisol inhibits the pro-glycemic uptake function of insulin and causes the increase of blood glucose. In addition, cortisol has a strong aggregation effect on adipose tissue, and the abnormal increase of cortisol may cause visceral fat deposition, leading to obesity and dyslipidemia (56, 57). Third, anxiety could promote the inflammatory response of patients, and some inflammatory factors, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), are risk factors for insulin resistance, which could contribute to insulin resistance, thereby promoting the prevalence of MetS (58, 59).
If MetS occurs prior to anxiety, the pathophysiologic mechanisms may be attributed to insulin resistance. Insulin, as a key hormone in regulating carbohydrate metabolism, not only promotes glucose uptake and glucose oxidation but is also essential for maintaining normal brain function (60). It has been suggested that total cerebral blood flow which maintains the normal function of brain tissue was 15% lower in patients with MetS (61). There is evidence indicated that peripheral insulin resistance could lead to down-regulation of insulin receptor activity in brain tissue and decrease the level of insulin entering brain tissue, which may result in low metabolism of brain tissue and abnormalities of different functional areas of the brain (62, 63). The frontal lobe, as an important functional area of emotion regulation, is often affected and ultimately contributes to the occurrence of anxiety (64). Furthermore, insulin resistance in brain tissue may cause structural and functional damage in the hippocampus, resulting in hyperfunction of the HPA axis (65). The hyperfunction of the HPA axis contributes to the disturbance of cortisol rhythm and the imbalance of salt and glucocorticoids in the brain, which leads to abnormal levels of 5-hydroxytryptamine (5-HT). Dysfunction of the 5-HT system also promotes the occurrence of anxiety (66). Finally, prospective studies have demonstrated that insulin resistance and impaired glucose tolerance are related to somnipathy, which is one of the causes of anxiety (67–69).
The association between anxiety and MetS could also be attributed to other factors. Emotional eating is an emotional response led by negative stimuli, such as feeling stressed or anxious (70). In the context of acute psychosocial stress, cortisol levels which may influence eating behavior were higher in patients with anxiety, and high cortisol levels could contribute to binge eating (71).
The findings of this meta-analysis are of important clinical significance. Anxiety has been documented to contribute to the risk of CVD in initially healthy people (72, 73). MetS is not only a risk factor for T2DM and CVD but also related to anxiety (19, 74). For patients with anxiety, the risk factors for MetS should be routinely checked. Proper lifestyle modification and pharmaceutical therapy should be proposed if patients are at high risk of adiposity, hyperglycemia and CVD. In addition, for patients with MetS, appropriate attention should be given to their psychological condition rather than limiting the treatment to physical disease. For patients already suffering from anxiety, psychological treatment or pharmacological treatment could be used to reduce mental and physical symptoms, and improve their social and interpersonal relationships (75).
Previous studies have demonstrated that psychotropic medications could impact cardiovascular health and increase MetS risk in psychiatric patients (76, 77). It has been widely reported that second-generation antipsychotics (SGAs), including antidepressants and anxiolytics, are associated with weight gain, lipid disorder and dysregulation of glucose (78). Antipsychotics also have effects on the central nervous system, inflammatory response, and cellular metabolism (79). Therefore, providing medication guidance for patients with anxiety, which could minimize the risk of MetS and prevent iatrogenic MetS, is of significant interest.
This meta-analysis has several strengths. To our knowledge, this is the first meta-analysis that evaluates the association between anxiety and MetS using data from both cross-sectional and cohort studies with anxiety and MetS as dependent variables, respectively. Moreover, we conducted comprehensive subgroup analyses to reveal the heterogeneity and indicated that the diagnostic criteria for anxiety and MetS could influence the strength of the association. However, the limitations of this study merit consideration. First, the meta-analysis involved mainly cross-sectional studies, the cause-and-effect relationship of anxiety with MetS cannot be proven. Second, studies with anxiety as the dependent variable were limited, more studies are recommended to assess the impact of MetS on anxiety. Third, our study focused on the overall association between anxiety and MetS, further study could concentrate on the association between specific MetS components and anxiety.
This updated systematic review and meta-analysis added the evidence of the association between anxiety and MetS. Psychologists treating individuals with anxiety should detect changes in metabolic components, and physicians dealing with MetS patients should be aware of their possible predisposition to anxiety. As most of the studies included in the current study were cross-sectional studies, no conclusions on causal inference could be reached. Therefore, more prospective studies, particularly long-term follow-up and large-scale cohort studies, are required to explain the causal relationship of anxiety with MetS.
FT and GD conceived the study protocol. SJ and YCh conducted the literature search and selection, data extraction and analysis, and drafted the manuscript. All authors participated in the discussions and interpretation of the results and approved the final version of the manuscript.
This study was supported by the National Natural Science Foundation of China (71804093), Academic Promotion Programme of Shandong First Medical University (2019LJ005 and 2019QL013), and Academic Promotion Programme of School of Public Health, Shandong First Medical University (GW202218).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1118836/full#supplementary-material
1. Eckel R, Alberti K, Grundy S, Zimmet P. The metabolic syndrome. Lancet. (2010) 375:181–3. doi: 10.1016/S0140-6736(09)61794-3
2. Després J, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. (2006) 444:881–7. doi: 10.1038/nature05488
3. Ford E, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. (2008) 31:1898–904. doi: 10.2337/dc08-0423
4. Hirode G, Wong R. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. (2020) 323:2526–8. doi: 10.1001/jama.2020.4501
5. Yao F, Bo Y, Zhao L, Li Y, Ju L, Fang H, et al. Prevalence and influencing factors of metabolic syndrome among adults in China from 2015 to 2017. Nutrients. (2021) 13:1–10. doi: 10.3390/nu13124475
6. Moran T. Anxiety and working memory capacity: a meta-analysis and narrative review. Psychol Bull. (2016) 142:831–64. doi: 10.1037/bul0000051
7. Pitman A, Suleman S, Hyde N, Hodgkiss A. Depression and anxiety in patients with cancer. BMJ. (2018) 361:1–6. doi: 10.1136/bmj.k1415
9. Mazzone C, Pati D, Michaelides M, DiBerto J, Fox J, Tipton G, et al. Acute engagement of Gq-mediated signaling in the bed nucleus of the stria terminalis induces anxiety-like behavior. Mol Psychiatry. (2018) 23:143–53. doi: 10.1038/mp.2016.218
10. Alonso J, Liu Z, Evans-Lacko S, Sadikova E, Sampson N, Chatterji S, et al. Treatment gap for anxiety disorders is global: results of the world mental health surveys in 21 countries. Depress Anxiety. (2018) 35:1–24. doi: 10.1002/da.22711
11. Skogberg N, Castaneda A, Agyemang C, Lilja E. The association of depressive and anxiety symptoms with the metabolic syndrome and its components among Russian, Somali, and Kurdish origin adults in Finland: a population-based study. J Psychosom Res. (2022) 159:110944. doi: 10.1016/j.jpsychores.2022.110944
12. Moreira F, Jansen K, Cardoso T, Mondin T, Magalhães PV, Kapczinski F, et al. Metabolic syndrome and psychiatric disorders: a population-based study. Rev Bras Psiquiatr. (2019) 41:38–43. doi: 10.1590/1516-4446-2017-2328
13. Butnoriene J, Steibliene V, Saudargiene A, Bunevicius A. Does presence of metabolic syndrome impact anxiety and depressive disorder screening results in middle aged and elderly individuals? A population based study. BMC Psychiatry. (2018) 18:5. doi: 10.1186/s12888-017-1576-8
14. Akbari H, Nizal S, Aria H, Noor D. Anxiety but not depression is associated with metabolic syndrome: the Isfahan Healthy Heart Program. J Res Med Sci. (2017) 24:1–6.
15. Li R, Zhang L, Luo H, Lei Y, Zeng L, Zhu J, et al. Subclinical hypothyroidism and anxiety may contribute to metabolic syndrome in Sichuan of China: a hospital-based population study. Sci Rep. (2020) 10:2261. doi: 10.1038/s41598-020-58973-w
16. Tang F, Wang G, Lian Y. Association between anxiety and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Psychoneuroendocrinology. (2017) 77:112–21. doi: 10.1016/j.psyneuen.2016.11.025
17. Peltzer K, Pengpid S. Relationship between depression, generalized anxiety, and metabolic syndrome among Buddhist temples population in Nakhon Pathom-Thailand. Iran J Psychiatry Behav Sci. (2018) 12:4. doi: 10.5812/ijpbs.60829
18. Mattei G, Padula M, Rioli G, Arginelli L, Bursi R, Bursi S, et al. Metabolic syndrome, anxiety and depression in a sample of Italian primary care patients. J Nerv Ment Dis. (2018) 206:316–24. doi: 10.1097/NMD.0000000000000807
19. Bagherniya M, Khayyatzadeh S, Avan A, Safarian M, Nematy M, Ferns G, et al. Metabolic syndrome and its components are related to psychological disorders: a population based study. Diabetes Metab Syndr Clin Res Rev. (2017) 11:S561–6. doi: 10.1016/j.dsx.2017.04.005
20. Rioli G, Tassi S, Mattei G, Ferrari S, Galeazzi G, Mancini S, et al. The association between symptoms of anxiety, depression, and cardiovascular risk factors results from an Italian cross-sectional study. J Nerv Ment Dis. (2019) 207:340–7. doi: 10.1097/NMD.0000000000000969
21. Lubas M, Wang M, Jefferies J, Ness K, Ehrhardt M, Krull K, et al. The contribution of stress and distress to cardiovascular health in adult survivors of childhood cancer. Cancer Epidemiol Biomarkers Prev. (2021) 30:286–94. doi: 10.1158/1055-9965.EPI-20-1183
22. Berto L, Suemoto C, Moreno A, Fonseca M, Nunes M, Molina M, et al. Increased prevalence of depression and anxiety among subjects with metabolic syndrome in the Brazilian longitudinal study of adult health (ELSA-Brasil). J Acad Consult Psychiatry. (2022) 63:529–38. doi: 10.1016/j.jaclp.2022.06.001
23. Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
24. Stroup D, Berlin J, Morton S, Olkin I, Williamson G, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of observational studies in epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
25. Hu J, Dong Y, Chen X, Liu Y, Ma D, Liu X, et al. Prevalence of suicide attempts among Chinese adolescents: a meta-analysis of cross-sectional studies. Compr Psychiatry. (2015) 61:78–89. doi: 10.1016/j.comppsych.2015.05.001
26. Zhang X, Sun Z, Zhou A, Tao L, Chen Y, Shi X, et al. Association between infections and risk of ankylosing spondylitis: a systematic review and meta-analysis. Front Immunol. (2021) 12:768741. doi: 10.3389/fimmu.2021.768741
27. Khan K, Daya S, Jadad A. The importance of quality of primary studies in producing unbiased systematic reviews. Arch Intern Med. (1996) 156:661–6. doi: 10.1001/archinte.156.6.661
28. Du X, Gu J. The efficacy and safety of parecoxib for reducing pain and opioid consumption following total knee arthroplasty: a meta-analysis of randomized controlled trials. Int J Surg. (2018) 59:67–74. doi: 10.1016/j.ijsu.2018.09.017
29. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. London: Cochrane (2021).
30. Butnoriene J, Bunevicius A, Norkus A, Bunevicius R. Depression but not anxiety is associated with metabolic syndrome in primary care based community sample. Psychoneuroendocrinology. (2014) 40:269–76. doi: 10.1016/j.psyneuen.2013.11.002
31. Carroll D, Phillips A, Thomas G, Gale C, Deary I, Batty G. Generalized Anxiety disorder is associated with metabolic syndrome in the vietnam experience study. Biol Psychiatry. (2009) 66:91–3. doi: 10.1016/j.biopsych.2009.02.020
32. Cohen B, Panguluri P, Na B. Psychological risk factors and the metabolic syndrome in patients with coronary heart disease: findings from the Heart and Soul Study. Psychiatry Res. (2010) 23:133–7. doi: 10.1016/j.psychres.2009.02.004
33. Glaus J, Vandeleur C, Gholam-Rezaee M, Castelao E, Perrin M, Rothen S, et al. Atypical depression and alcohol misuse are related to the cardiovascular risk in the general population. Acta Psychiatr Scand. (2013) 128:282–93. doi: 10.1111/acps.12057
34. Herva A, Räsänen P, Miettunen J, Timonen M, Läksy K, Veijola J, et al. Co-occurrence of metabolic syndrome with depression and anxiety in young adults: the Northern Finland 1966 Birth Cohort Study. Psychosom Med. (2006) 68:213–6. doi: 10.1097/01.psy.0000203172.02305.ea
35. Hildrum B, Mykletun A, Midthjell K, Ismail K, Dahl A. No association of depression and anxiety with the metabolic syndrome: the Norwegian HUNT study. Acta Psychiatr Scand. (2009) 120:14–22. doi: 10.1111/j.1600-0447.2008.01315.x
36. Kahl K, Schweiger U, Correll C, Müller C, Busch M, Bauer M, et al. Depression, anxiety disorders, and metabolic syndrome in a population at risk for type 2 diabetes mellitus. Brain Behav. (2015) 5:7. doi: 10.1002/brb3.306
37. Luppino F, Van Reedt Dortland A, Wardenaar K, Bouvy P, Giltay E, Zitman F, et al. Symptom dimensions of depression and anxiety and the metabolic syndrome. Psychosom Med. (2011) 73:257–64. doi: 10.1097/PSY.0b013e31820a59c0
38. Räikköonen K, Matthews K, Kuller L. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: a comparison of world health organization, adult treatment panel III, and international diabetes foundation definitions. Diabetes Care. (2007) 30:872–7. doi: 10.2337/dc06-1857
39. Roohafza H, Sadeghi M, Talaei M, Pourmoghaddas Z, Sarrafzadegan N. Psychological status and quality of life in relation to the metabolic syndrome: Isfahan cohort study. Int J Endocrinol. (2012) 2012:10–5. doi: 10.1155/2012/380902
40. Skilton M, Moulin P, Terra J, Bonnet F. Associations between anxiety, depression, and the metabolic syndrome. Biol Psychiatry. (2007) 62:1251–7. doi: 10.1016/j.biopsych.2007.01.012
41. Takeuchi T, Nakao M, Nomura K, Yano E. Association of metabolic syndrome with depression and anxiety in Japanese men. Diabetes Metab. (2009) 35:32–6. doi: 10.1016/j.diabet.2008.06.006
42. Takeuchi T, Nakao M, Nomura K, Inoue M, Tsurugano S, Shinozaki Y, et al. Association of the metabolic syndrome with depression and anxiety in Japanese men: a 1-year cohort study. Diabetes Metab Res Rev. (2009) 25:762–7. doi: 10.1002/dmrr.1041
43. Tziallas D, Kostapanos M, Skapinakis P, Milionis H, Athanasiou T, S Elisaf M, et al. The association between type D personality and the metabolic syndrome: a cross-sectional study in a university-based outpatient lipid clinic. BMC Res Notes. (2011) 4:105. doi: 10.1186/1756-0500-4-105
44. Tziallas D, Kastanioti C, Kostapanos M, Skapinakis P, Elisaf M, Mavreas V. The impact of the metabolic syndrome on health-related quality of life: a cross-sectional study in Greece. Eur J Cardiovasc Nurs. (2012) 11:297–303. doi: 10.1016/j.ejcnurse.2011.02.004
45. Van Reedt Dortland A, Giltay E, Van Veen T, Zitman F, Penninx B. Metabolic syndrome abnormalities are associated with severity of anxiety and depression and with tricyclic antidepressant use. Acta Psychiatr Scand. (2010) 122:30–9. doi: 10.1111/j.1600-0447.2010.01565.x
46. Vogelzangs N, Beekman A, Kritchevsky S, Newman A, Pahor M, Yaffe K, et al. Psychosocial risk factors and the metabolic syndrome in elderly persons: findings from the health, aging and body composition study. J Gerontol Ser A Biol Sci Med Sci. (2007) 62:563–9. doi: 10.1093/gerona/62.5.563
47. Albert U, Aguglia A, Chiarle A, Bogetto F, Maina G. Metabolic syndrome and obsessive-compulsive disorder: a naturalistic Italian study. Gen Hosp Psychiatry. (2013) 35:154–9. doi: 10.1016/j.genhosppsych.2012.10.004
48. Saha S, Lim C, Cannon D, Burton L, Bremner M, Cosgrove P, et al. Co-morbidity between mood and anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. (2021) 38:286–306. doi: 10.1002/da.23113
49. Ko G, Cockram C, Chow C, Yeung V, Chan W, So W, et al. Metabolic syndrome by the international diabetes federation definition in Hong Kong Chinese. Diabetes Res Clin Pract. (2006) 73:58–64. doi: 10.1016/j.diabres.2005.11.009
50. Byrd J, Brook R. Anxiety in the “age of hypertension”. Curr Hypertens Rep. (2014) 16:486. doi: 10.1007/s11906-014-0486-0
51. Kuo W, Bratzke L, Oakley L, Kuo F, Wang H, Brown R. The association between psychological stress and metabolic syndrome: a systematic review and meta-analysis. Obes Rev. (2019) 20:1651–64. doi: 10.1111/obr.12915
52. Tank A, Wong D. Peripheral and central effects of circulating catecholamines. Compr Physiol. (2015) 5:1–15. doi: 10.1002/cphy.c140007
53. Fiksdal A, Hanlin L, Kuras Y, Gianferante D, Chen X, Thoma MV, et al. Associations between symptoms of depression and anxiety and cortisol responses to and recovery from acute stress. Psychoneuroendocrinology (2019) 102:44–52. doi: 10.1016/j.psyneuen.2018.11.035
54. Tafet G, Nemeroff C. Pharmacological treatment of anxiety disorders: the role of the HPA axis. Front Psychiatry. (2020) 11:443. doi: 10.3389/fpsyt.2020.00443
55. Jacobson L. Hypothalamic-pituitary-adrenocortical axis: neuropsychiatric aspects. Compr Physiol. (2014) 4:715–38. doi: 10.1002/cphy.c130036
56. Heraclides A, Chandola T, Witte D, Brunner E. Psychosocial stress at work doubles the risk of type 2 diabetes in middle-aged women: evidence from the Whitehall II study. Diabetes Care. (2009) 32:2230–5. doi: 10.2337/dc09-0132
57. Russell G, Lightman S. The human stress response. Nat Rev Endocrinol. (2019) 15:525–34. doi: 10.1038/s41574-019-0228-0
58. Weisberg S, McCann D, Desai M, Rosenbaum M, Leibel R, Ferrante A. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. (2003) 112:1796–808. doi: 10.1172/JCI200319246
59. Michopoulos V, Powers A, Gillespie C, Ressler K, Jovanovic T. Inflammation in fear-and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology. (2017) 42:254–70. doi: 10.1038/npp.2016.146
60. Espeland M, Bryan R, Goveas J, Robinson J, Siddiqui M, Liu S, et al. Influence of type 2 diabetes on brain volumes and changes in brain volumes: results from the Women’s Health Initiative Magnetic Resonance Imaging Studies. Diabetes Care. (2013) 36:90–7. doi: 10.2337/dc12-0555
61. Birdsill A, Carlsson C, Willette A, Johnson S, Xu G, Oh J, et al. Low cerebral blood flow is associated with lower memory function in metabolic syndrome. Obes. (2013) 21:1313–20. doi: 10.1002/oby.20170
62. Hahn C, Scott D, Xu X, Roda M, Gregory A, Wells J, et al. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer’s disease. JAMA Neurol. (2015) 1:1–21.
63. Talbot K, Wang H, Kazi H, Han L, Bakshi K, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. (2012) 122:1316–38. doi: 10.1172/JCI59903
64. Likhtik E, Stujenske J, Topiwala M, Harris A, Gordon J. Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat Neurosci. (2014) 17:106–13. doi: 10.1038/nn.3582
65. Anacker C, Scholz J, O’Donnell K, Allemang-Grand R, Diorio J, Bagot R, et al. Neuroanatomic differences associated with stress susceptibility and resilience. Biol Psychiatry. (2016) 79:840–9. doi: 10.1016/j.biopsych.2015.08.009
66. Ressler K, Nemeroff C. Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety. (2000) 12(Suppl. 1):2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.3.CO;2-W
67. Balkau B, Vol S, Loko S, Andriamboavonjy T, Lantieri O, Gusto G, et al. High baseline insulin levels associated with 6-year incident observed sleep apnea. Diabetes Care. (2010) 33:1044–9. doi: 10.2337/dc09-1901
68. Jordan Gaines P, Alexandros N, Vgontzas M, Julio Fernandez-Mendoza P, Edward O, Bixler P. Obstructive sleep apnea and the metabolic syndrome: the road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep Med Rev. (2018) 176:139–48. doi: 10.1016/j.smrv.2018.08.009
69. Strand L, Carnethon M, Biggs M, Djoussé L, Kaplan R, Siscovick D, et al. Sleep disturbances and glucose metabolism in older adults: the cardiovascular health study. Diabetes Care. (2015) 38:2050–8. doi: 10.2337/dc15-0137
70. Macht M, Simons G. Emotions and eating in everyday life. Appetite. (2000) 35:65–71. doi: 10.1006/appe.2000.0325
71. Chang R, Cerit H, Hye T, Durham E, Aizley H, Boukezzi S, et al. Stress-induced alterations in HPA-axis reactivity and mesolimbic reward activation in individuals with emotional eating. Appetite. (2022) 168:105707. doi: 10.1016/j.appet.2021.105707
72. Batelaan N, Seldenrijk A, Bot M, Van Balkom A, Penninx B. Anxiety and new onset of cardiovascular disease: critical review and meta-analysis. Br J Psychiatry. (2016) 208:223–31. doi: 10.1192/bjp.bp.114.156554
73. Roest A, Martens E, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease. A meta-analysis. J Am Coll Cardiol. (2010) 56:38–46. doi: 10.1016/j.jacc.2010.03.034
74. Lee M, Han K, Kim M, Koh E, Kim E, Nam G, et al. Combinations of metabolic syndrome components and the risk of type 2 diabetes mellitus: a nationwide cohort study. Diabetes Res Clin Pract. (2020) 165:108237. doi: 10.1016/j.diabres.2020.108237
75. Penninx B, Pine D, Holmes E, Reif A. Anxiety disorders. Lancet. (2021) 397:914–27. doi: 10.1016/S0140-6736(21)00359-7
76. Mitchell A, Vancampfort D, Sweers K, Van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders-a systematic review and meta-analysis. Schizophr Bull. (2013) 39:306–18. doi: 10.1093/schbul/sbr148
77. Penninx B, Lange S. Metabolic syndrome in psychiatric patients: overview, mechanisms, and implications. Dialogues Clin Neurosci. (2018) 20:63–73. doi: 10.31887/DCNS.2018.20.1/bpenninx
78. Koro C, Fedder D, L’Italien G, Weiss S, Magder L, Kreyenbuhl J, et al. Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study. BMJ. (2002) 325:243. doi: 10.1136/bmj.325.7358.243
Keywords: anxiety, metabolic syndrome, cross-sectional study, cohort study, meta-analysis
Citation: Ji S, Chen Y, Zhou Y, Cao Y, Li X, Ding G and Tang F (2023) Association between anxiety and metabolic syndrome: An updated systematic review and meta-analysis. Front. Psychiatry 14:1118836. doi: 10.3389/fpsyt.2023.1118836
Received: 08 December 2022; Accepted: 30 January 2023;
Published: 16 February 2023.
Edited by:
Anna Rita Atti, University of Bologna, ItalyReviewed by:
Sitong Chen, Victoria University, AustraliaCopyright © 2023 Ji, Chen, Zhou, Cao, Li, Ding and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Tang,  dGFuZ2ZhbmdzZHVAZ21haWwuY29t; Guoyong Ding,
dGFuZ2ZhbmdzZHVAZ21haWwuY29t; Guoyong Ding,  ZGd5LTE1M0AxNjMuY29t
ZGd5LTE1M0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.