
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 08 February 2023
Sec. Adolescent and Young Adult Psychiatry
Volume 14 - 2023 | https://doi.org/10.3389/fpsyt.2023.1111754
This article is part of the Research Topic Exploration of Major Depressive Disorder among Children and Adolescents: From Pathogenesis to Intervention View all 15 articles
Objective: This systematic review of randomized controlled trials (RCTs) was conducted to explore the therapeutic effects and safety of active low-frequency repetitive transcranial magnetic stimulation (LF-rTMS) versus sham LF-rTMS in children and adolescent patients with first-episode and drug-naïve (FEDN) major depressive disorder (MDD).
Methods: A systematic literature search was performed, and data were extracted by two independent researchers. The coprimary outcomes were study-defined response and remission.
Results: A systematic search of the literature yielded 442 references, of which 3 RCTs (130 children and adolescents with FEDN MDD, 50.8% male, and mean age range from 14.5 to 17.5 years) met the inclusion criteria. Among the two RCTs (66.7%, 2/3) examining the effects of LF-rTMS on study-defined response and remission and cognitive function, active LF-rTMS was more efficacious than sham LF-rTMS in terms of study-defined response rate and cognitive function (all p < 0.05) but not regarding study-defined remission rate (all p > 0.05). No significant group differences were found with regard to adverse reactions. None of the included RCTs reported the dropout rate.
Conclusion: These findings preliminarily found that LF-rTMS could benefit children and adolescents with FEDN MDD in a relatively safe manner, although further studies are warranted.
Major depressive disorder (MDD), as a leading cause of global disease burden (1), affects approximately 5–15% of children and adolescents (2). Children and adolescents with MDD are usually related to school dropout, pregnancy/parenthood, and unemployment (3). Furthermore, individuals suffering from MDD in childhood and adolescence have a relatively high risk for chronic recurrence, suicide, and long-term psychosocial impairment in adulthood (2, 4–6). Therefore, improvements in treating MDD among children and adolescents should positively affect public health.
Initial treatment of children and adolescents with MDD may include a selective serotonin reuptake inhibitor (SSRI) or cognitive-behavioural therapy (CBT) (7). A Treatment for Adolescents with Depression Study (TADS) randomized controlled trial (RCT) found that CBT combined with fluoxetine provided a more favorable tradeoff between risk and benefit in adolescent patients with MDD than either treatment alone (7). However, up to 40% of adolescents suffering from MDD fail to respond to traditional treatment (8, 9). As a result, new and effective treatment approaches for MDD patients among children and adolescents are urgently needed.
Repetitive transcranial magnetic stimulation (rTMS), as a noninvasive brain stimulation, is gaining attention in treating adults suffering from various conditions, including MDD and obsessive–compulsive disorder (OCD) (10–12). Repetitive transcranial magnetic stimulation uses a magnetic field to stimulate the cortex and depression-related areas with electrical currents and alter dysfunctional brain patterns (13, 14). Numerous RCTs have demonstrated the therapeutic effects of rTMS in adult patients with treatment-refractory depression (TRD) (15, 16). The utility of rTMS for adult patients with MDD and OCD who did not respond to medications has been approved by the US FDA (9, 17). Accumulating evidences found that rTMS also could accelerate the rapidity of the antidepressant response in adult patients suffering from first-episode MDD (18, 19). Case reports/series (20–22) and observational studies (23–28) reported that rTMS appeared to be suitable for children and adolescents diagnosed with MDD. However, the findings of RCTs (29–31) examining the therapeutic effects and safety of active low-frequency rTMS (LF-rTMS) versus sham LF-rTMS for children and adolescents with first-episode and drug-naïve (FEDN) MDD have been inconsistent.
Therefore, the primary aim in this systematic review of RCTs was to investigate the therapeutic effects and safety of active LF-rTMS versus sham LF-rTMS for children and adolescents with FEDN MDD. We hypothesized that active LF-rTMS would be more efficacious than sham LF-rTMS in ameliorating depressive symptoms in FEDN MDD patients among children and adolescents.
To identify studies for inclusion in this systematic review, two researchers (ZJQ and XJL) independently searched Chinese Journal Net, WanFang databases, PsycINFO, Cochrane Library, PubMed, and EMBASE through November 4 2022. The search terms are listed in Appendix S1. Additionally, we manually searched reference lists of previous reviews (2, 9, 32) and the included RCTs (29–31) on active LF-rTMS versus sham LF-rTMS for children and adolescent patients with FEDN MDD.
In accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta Analyses) guidelines (33), we included studies that fulfilled the following PICOS criteria. Participants: Children (6–11 years) (34) and adolescents (12–25 years) (35) with a diagnosis of first-episode MDD who did not receive any antidepressant treatment. In line with the methodology of a recent systematic review (35), adolescents were defined as those who are 12–25 years old rather than 13–18 years old. Intervention versus Comparison: active LF-rTMS versus sham LF-rTMS. Outcomes: The coprimary outcomes were study-defined response (i.e., at least 50% reduction in Hamilton Depression Rating Scale (HAMD) scores) and remission (i.e., at least 75% reduction in HAMD scores). Additional outcomes were cognitive function, dropout rate, and adverse events. Study: Only published RCTs on active LF-rTMS versus sham LF-rTMS for children and adolescents (6–25 years) with FEDN MDD were eligible for inclusion. Studies focusing on active LF-rTMS versus antidepressants (36) or high-frequency rTMS (HF-rTMS) combined with antidepressants versus antidepressant monotherapy (14) were excluded. Review articles and case reports/series were also excluded.
Two independent researchers (ZJQ and XJL) performed the data extraction from each included RCT, and any disagreements were resolved by joint discussion. We extracted data using a standardized form including author, year of publication, study design, rTMS protocol, and primary and secondary outcomes. Additional data were requested by contacting the original study author(s), if necessary.
The quality of the RCTs was independently assessed by the same two researchers (ZJQ and XJL) using the Jadad scale (37) and the Cochrane risk of bias (38). As reported previously (39), RCTs were considered “high quality” when the Jadad score was ≥3.
Our initial search of the above English and Chinese databases retrieved 442 references (Figure 1). Finally, 3 RCTs (29–31) conducted in China met the inclusion criteria of this systematic review. The screening process for the literature is presented in Figure 1.
The participant characteristics and LF-rTMS parameters of the three included RCTs (29–31) are summarized in Table 1. The studies (n = 130) were conducted between 2015 and 2019, comparing active LF-rTMS (n = 65) and sham LF-rTMS (n = 65) for children and adolescents with FEDN MDD. Their mean ages ranged from 14.5 to 17.5 years, and more than half (50.7%) of the children and adolescents with FEDN MDD were male. The LF-rTMS treatment duration varied from 10 days (2 RCTs (29, 30)) to 20 days (1 RCT (31)). The detailed LF-rTMS protocol of each included RCT is summarized in Table 1.
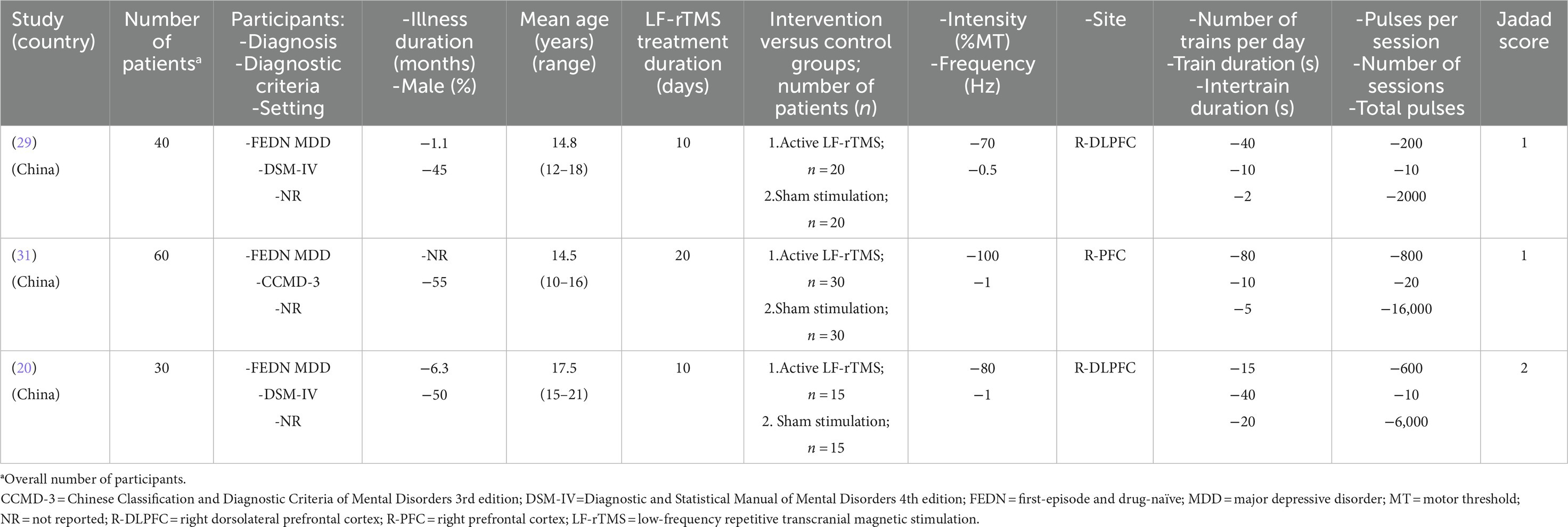
Table 1. Participant characteristics and low-frequency repetitive transcranial magnetic stimulation (LF-rTMS) parameters of each study included in this systematic review.
As shown in Table 1, the Jadad score ranged from 1 (2 RCTs (29, 31)) to 2 (1 RCT (30)); thus, none of the included RCTs fulfilled the criteria of high quality. All RCTs were rated as low risk regarding attrition and reporting bias according to the Cochrane risk of bias (Figure 2).
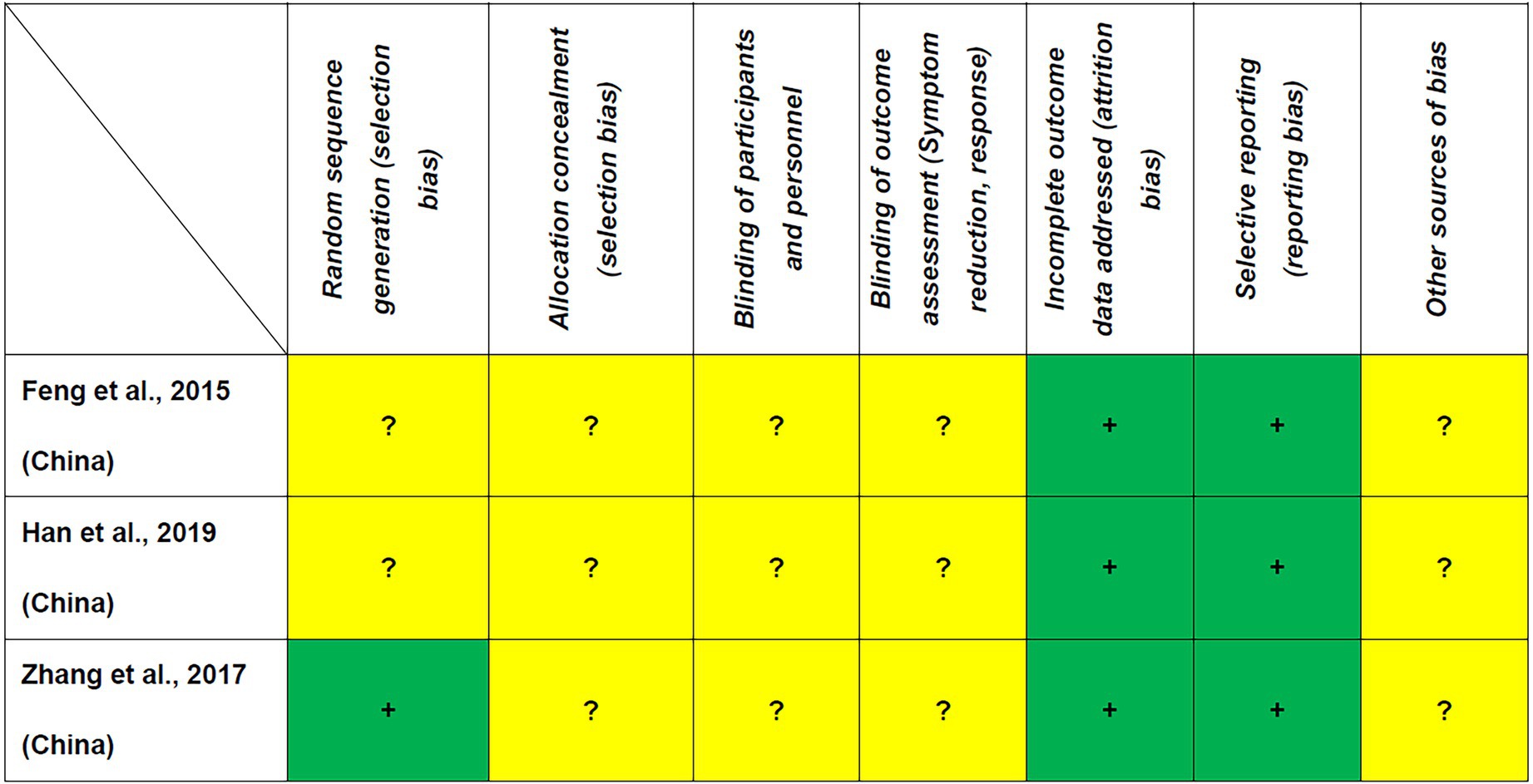
Figure 2. Cochrane risk of bias +: Low risk of bias, −: High risk of bias,?: Unclear risk of bias, nd: not determined.
Among the included three RCTs, one RCT (33.3%, 1/3) (29) did not report the rates of study-defined response and remission (Table 2) and found a significant superiority of active LF-rTMS over sham LF-rTMS in improving the depression subfactor scores of the Children’s depression inventory (CDI; all p < 0.05). In Han et al.’s study (31), a significant superiority of active LF-rTMS over sham LF-rTMS was found for study-defined response (40.0 vs. 13.3%; p < 0.05) between active LF-rTMS and sham LF-rTMS but not for study-defined remission (13.3 vs. 3.3%; p > 0.05). Similarly, a significant superiority of active LF-rTMS over sham LF-rTMS was found for study-defined response (46.7 vs. 0%, p < 0.05) but not for study-defined remission (0 vs. 0%, p > 0.05) in Zhang et al.’s study (30).
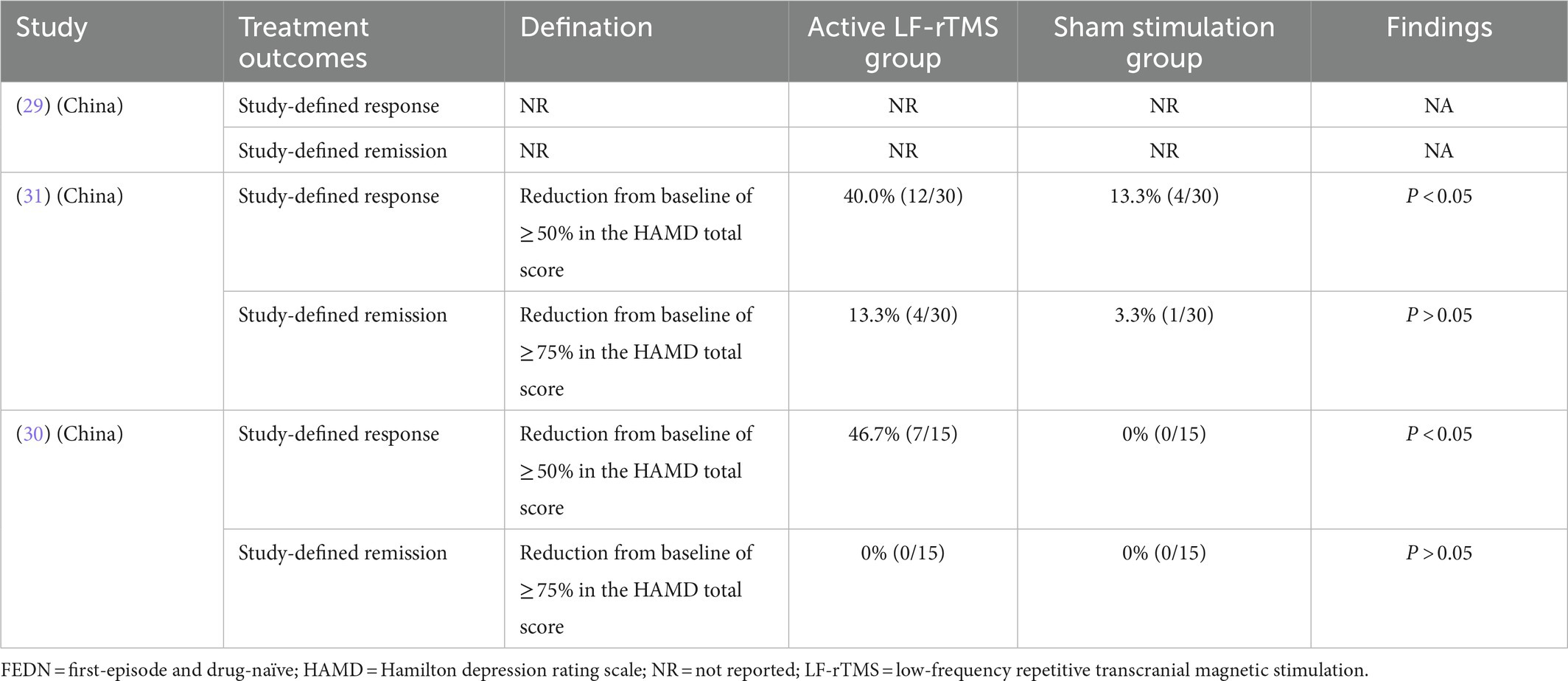
Table 2. Active versus sham LF-rTMS for adolescent patients with FEDN depression: study-defined response and remission.
Although 66.7% (2/3) of RCTs investigated the cognitive effects of active LF-rTMS versus sham LF-rTMS (Supplementary Table 1), their data measured by using different measures were not pooled. As shown in Supplementary Table 1, two RCTs consistently found that active LF-rTMS provided a significant improvement in cognitive function over sham LF-rTMS as measured by the Wisconsin Card Sorting Test (WCST) (31) and the cognitive subscale of HAMD (30), respectively.
As depicted in Supplementary Table 2, none of the included RCTs reported the dropout rate. Only one RCT (30) (33.3%, 1/3) reported adverse events, finding no significant difference regarding dizziness, nausea, or insomnia between the two groups (all p > 0.05; Supplementary Table 2).
To the best of our knowledge, this article is the first systematic review of RCTs to investigate the effectiveness and safety of active LF-rTMS versus sham LF-rTMS for children and adolescents (6–25 years) with FEDN MDD. Only three RCTs (29–31) involving 130 subjects with FEDN MDD among children and adolescents were included in this systematic review. The major findings of this systematic review were as follows: (1) active LF-rTMS was more efficacious than sham LF-rTMS in terms of the study-defined response rate and the improvement of cognitive function; and (2) there is a strong indication that LF-rTMS was relatively safe and well tolerated in subjects with FEDN MDD among children and adolescents, although better quality studies are warranted.
In this systematic review, LF-rTMS as a stand-alone treatment appears to be effective for children and adolescents with FEDN MDD, although long-term efficacy was not reported. A recent RCT (n = 103) examining the potential therapeutic role and safety of active LF-rTMS versus sham LF-rTMS for adolescents with TRD found that 41.7% responded, and 29.2% met the criteria of remission with active LF-rTMS (40). Numerous RCTs (14) and meta-analyses (2) found that rTMS as an adjunctive therapy is safe and effective in children and adolescents with MDD. Importantly, several recent studies found that LF-rTMS and antidepressants were equally efficacious in reducing depressive symptoms in children and adolescents with MDD (41). Taken together, these findings provide preliminary support for the utility of LF-rTMS in children and adolescents with MDD.
For other noninvasive brain stimulations, such as transcranial direct current stimulation (tDCS) (42–44) and electroconvulsive therapy (45, 46), another objective is to monitor the cognitive effects of rTMS. Consistent with previous meta-analyses focusing on adult patients with MDD (47, 48), this systematic review also found that a therapeutic rTMS course for child and adolescent patients with FEDN MDD may produce modest cognitive enhancing effects. A possible explanation is that cognitive effects were secondary to mood improvement (47). However, the WCST and the HAMD measure used in the included two RCTs (30, 31) do not appear to be suitable for evaluating cognitive performance in MDD. The Assessment of Neuropsychological Status (RBANS) (49) or the MATRICS Consensus Cognitive Battery (MCCB) (50) should be recommended to assess cognitive performance in individuals experiencing MDD in clinical trials. Thus, the cognitive effects of active LF-rTMS compared to sham LF-rTMS should be further examined in FEDN MDD patients among children and adolescents. A recent RCT found that rTMS and tDCS (rTMS-tDCS) than single-tDCS produced greater improvement in neuropsychiatric symptoms (51). As a type of noninvasive cranial electrical stimulation, transcranial alternating current stimulation (tACS) can significantly improve depressive symptoms in adults with FEDN MDD (52). However, there have been no head-to-head studies that compared rTMS either with tACS or tDCS in child and adolescent patients with FEDN MDD.
There are several limitations to this systematic review. First, data were not pooled due to the limited number of studies (3 RCTs) with the heterogeneity of significance between the studies. Second, the sample size (n = 130), ranging from 30 to 60, was relatively small. Third, the parameters of LF-rTMS used in the three included studies were varied. For example, the number of total pulses (Table 1) varied from 2,000 to 16,000, which may have resulted in different therapeutic effects and adverse effects. The optimal parameters of LF-rTMS as a stand-alone treatment for FEDN MDD patients among children and adolescents remain unclear. Interestingly, a recent RCT found a significant superiority of Stanford neuromodulation therapy (SNT), a neuroscience-informed accelerated intermittent theta-burst stimulation protocol (90,000 total pulses), in improving depressive symptoms in adults with TRD when compared to sham stimulation (53). Thus, the efficacy and the safety of SNT for patients with MDD among children and adolescents should be examined. Fourth, all 3 RCTs (29–31) included in this study were conducted in China and involved only Chinese children and adolescents. Thus, the findings of the present study could not be generalizable to children and adolescents in other countries. Finally, this systematic review has not been registered.
These findings preliminarily found that LF-rTMS could benefit children and adolescents with FEDN MDD in a relatively safe manner, although further studies are warranted.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
X-JL and Z-JQ selected studies and extracted the data. WZ reviewed all the data and helped mediate disagreements, wrote the first draft. All authors contributed to the article and approved the submitted version.
This study was funded by National Natural Science Foundation of China (82101609), Scientific Research Project of Guangzhou Bureau of Education (202032762), Science and Technology Program Project of Guangzhou (202102020658), Guangzhou Health Science and Technology Project (20211A011045), Guangzhou Science and Technology Project of Traditional Chinese Medicine and integrated traditional Chinese and Western medicine (20212A011018), China International Medical Exchange Foundation (Z-2018-35-2002), Guangzhou Clinical Characteristic Technology Project (2019TS67), Science and Technology Program Project of Guangzhou (202102020658) and Guangdong Hospital Association (2019ZD06). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2023.1111754/full#supplementary-material
2. Qiu, H, Liang, K, Lu, L, Gao, Y, Li, H, Hu, X, et al. Efficacy and safety of repetitive transcranial magnetic stimulation in children and adolescents with depression: a systematic review and preliminary meta-analysis. J Affect Disord. (2023) 320:305–12. doi: 10.1016/j.jad.2022.09.060
3. Clayborne, ZM, Varin, M, and Colman, I. Systematic review and meta-analysis: adolescent depression and long-term psychosocial outcomes. J Am Acad Child Adolesc Psychiatry. (2019) 58:72–9. doi: 10.1016/j.jaac.2018.07.896
4. Weersing, VR, Jeffreys, M, Do, MT, Schwartz, KT, and Bolano, C. Evidence base update of psychosocial treatments for child and adolescent depression. J Clin Child Adolesc Psychol. (2017) 46:11–43. doi: 10.1080/15374416.2016.1220310
5. Zisook, S, Lesser, I, Stewart, JW, Wisniewski, SR, Balasubramani, GK, Fava, M, et al. Effect of age at onset on the course of major depressive disorder. Am J Psychiatry. (2007) 164:1539–46. doi: 10.1176/appi.ajp.2007.06101757
6. Costello, EJ, Pine, DS, Hammen, C, March, JS, Plotsky, PM, Weissman, MM, et al. Development and natural history of mood disorders. Biol Psychiatry. (2002) 52:529–42. doi: 10.1016/s0006-3223(02)01372-0
7. March, J, Silva, S, Petrycki, S, Curry, J, Wells, K, Fairbank, J, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: treatment for adolescents with depression study (TADS) randomized controlled trial. JAMA. (2004) 292:807–20. doi: 10.1001/jama.292.7.807
8. Zhou, X, Michael, KD, Liu, Y, Del Giovane, C, Qin, B, Cohen, D, et al. Systematic review of management for treatment-resistant depression in adolescents. BMC Psychiatry. (2014) 14:340. doi: 10.1186/s12888-014-0340-6
9. Majumder, P, Balan, S, Gupta, V, Wadhwa, R, and Perera, TD. The safety and efficacy of repetitive transcranial magnetic stimulation in the treatment of major depression among children and adolescents: a systematic review. Cureus. (2021) 13:e14564. doi: 10.7759/cureus.14564
10. McClintock, SM, Reti, IM, Carpenter, LL, McDonald, WM, Dubin, M, Taylor, SF, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. (2018) 79:1–32. doi: 10.4088/JCP.16cs10905
11. Zheng, W, Zhang, XY, Xu, R, Huang, X, Zheng, YJ, Huang, XB, et al. Adjunctive accelerated repetitive transcranial magnetic stimulation for older patients with depression: a systematic review. Front Aging Neurosci. (2022) 14:1036676. doi: 10.3389/fnagi.2022.1036676
12. Carmi, L, Tendler, A, Bystritsky, A, Hollander, E, Blumberger, DM, Daskalakis, J, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. (2019) 176:931–8. doi: 10.1176/appi.ajp.2019.18101180
13. De Risio, L, Borgi, M, Pettorruso, M, Miuli, A, Ottomana, AM, Sociali, A, et al. Recovering from depression with repetitive transcranial magnetic stimulation (rTMS): a systematic review and meta-analysis of preclinical studies. Transl Psychiatry. (2020) 10:393. doi: 10.1038/s41398-020-01055-2
14. Chen, H, Hu, X, Gao, J, Han, H, Wang, X, and Xue, C. Early effects of repetitive transcranial magnetic stimulation combined with sertraline in adolescents with first-episode major depressive disorder. Front Psych. (2022) 13:853961. doi: 10.3389/fpsyt.2022.853961
15. Levkovitz, Y, Isserles, M, Padberg, F, Lisanby, SH, Bystritsky, A, Xia, G, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. (2015) 14:64–73. doi: 10.1002/wps.20199
16. George, MS, Lisanby, SH, Avery, D, McDonald, WM, Durkalski, V, Pavlicova, M, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. (2010) 67:507–16. doi: 10.1001/archgenpsychiatry.2010.46
17. Perera, T, George, MS, Grammer, G, Janicak, PG, Pascual-Leone, A, and Wirecki, TS. The clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. (2016) 9:336–46. doi: 10.1016/j.brs.2016.03.010
18. Huang, ML, Luo, BY, Hu, JB, Wang, SS, Zhou, WH, Wei, N, et al. Repetitive transcranial magnetic stimulation in combination with citalopram in young patients with first-episode major depressive disorder: a double-blind, randomized, sham-controlled trial. Aust N Z J Psychiatry. (2012) 46:257–64. doi: 10.1177/0004867411433216
19. Wang, YM, Li, N, Yang, LL, Song, M, Shi, L, Chen, WH, et al. Randomized controlled trial of repetitive transcranial magnetic stimulation combined with paroxetine for the treatment of patients with first-episode major depressive disorder. Psychiatry Res. (2017) 254:18–23. doi: 10.1016/j.psychres.2017.04.005
20. Pan, F, Li, D, Wang, X, Lu, S, Xu, Y, and Huang, M. Neuronavigation-guided high-dose repetitive transcranial magnetic stimulation for the treatment of depressive adolescents with suicidal ideation: a case series. Neuropsychiatr Dis Treat. (2018) 14:2675–9. doi: 10.2147/ndt.s176125
21. Yang, XR, Kirton, A, Wilkes, TC, Pradhan, S, Liu, I, Jaworska, N, et al. Glutamate alterations associated with transcranial magnetic stimulation in youth depression: a case series. J ECT. (2014) 30:242–7. doi: 10.1097/yct.0000000000000094
22. Coarkin, PE, Wall, CA, King, JD, Kozel, FA, and Daskalakis, ZJ. Pain during transcranial magnetic stimulation in youth. Innov Clin Neurosci. (2011) 8:18–23.
23. Bloch, Y, Grisaru, N, Harel, EV, Beitler, G, Faivel, N, Ratzoni, G, et al. Repetitive transcranial magnetic stimulation in the treatment of depression in adolescents: an open-label study. J ECT. (2008) 24:156–9. doi: 10.1097/YCT.0b013e318156aa49
24. Demirtas-Tatlidede, A, Mechanic-Hamilton, D, Press, DZ, Pearlman, C, Stern, WM, Thall, M, et al. An open-label, prospective study of repetitive transcranial magnetic stimulation (rTMS) in the long-term treatment of refractory depression: reproducibility and duration of the antidepressant effect in medication-free patients. J Clin Psychiatry. (2008) 69:930–4. doi: 10.4088/jcp.v69n0607
25. Croarkin, PE, Nakonezny, PA, Deng, ZD, Romanowicz, M, Voort, JLV, Camsari, DD, et al. High-frequency repetitive TMS for suicidal ideation in adolescents with depression. J Affect Disord. (2018) 239:282–90. doi: 10.1016/j.jad.2018.06.048
26. MacMaster, FP, Croarkin, PE, Wilkes, TC, McLellan, Q, Langevin, LM, Jaworska, N, et al. Repetitive transcranial magnetic stimulation in youth with treatment resistant major depression. Front Psych. (2019) 10:170. doi: 10.3389/fpsyt.2019.00170
27. Sonmez, AI, Kucuker, MU, Lewis, CP, Kolla, BP, Doruk Camsari, D, Vande Voort, JL, et al. Improvement in hypersomnia with high frequency repetitive transcranial magnetic stimulation in depressed adolescents: preliminary evidence from an open-label study. Prog Neuro-Psychopharmacol Biol Psychiatry. (2020) 97:109763. doi: 10.1016/j.pnpbp.2019.109763
28. Zhang, T, Zhu, J, Wang, J, Tang, Y, Xu, L, Tang, X, et al. An open-label trial of adjuvant high-frequency left prefrontal repetitive transcranial magnetic stimulation for treating suicidal ideation in adolescents and adults with depression. J ECT. (2021) 37:140–6. doi: 10.1097/yct.0000000000000739
29. Feng, H, Qin, GX, and Chen, J. Early intervention of repetitive low frequency transcranial magnetic stimulation in first episode children with depression (in Chinese). Chin J Rehab. (2015) 30:455–6. doi: 10.3870/zgkf.2015.06.018
30. Zhang, LL, Huang, SS, and Shao, XH. Treatment effects of low frequency repetitive transcranial magnetic stimulation(rTMS) on adolescent patients with first-episode depression: a controlled clinical study (in Chinese). J Neurosci Mental Health. (2017) 17:85–8. doi: 10.3969/j.issn.1009-6574.2017.02.003
31. Han, L, and Gao, C. Analysis on the effect of repeated low frequency transcranial magnetic stimulation on first-episode depression among children (in Chinese). Chin Primary Health Care. (2019) 33:72–3. doi: 10.3969/j.issn.1001-568X.2019.10.0025
32. Sigrist, C, Vöckel, J, MacMaster, FP, Farzan, F, Croarkin, PE, Galletly, C, et al. Transcranial magnetic stimulation in the treatment of adolescent depression: a systematic review and meta-analysis of aggregated and individual-patient data from uncontrolled studies. Eur Child Adolesc Psychiatry. (2022) 31:1501–25. doi: 10.1007/s00787-022-02021-7
33. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clin Res Ed). (2009) 339:b2535. doi: 10.1136/bmj.b2535
34. Zheng, W, Li, XB, Xiang, YQ, Zhong, BL, Chiu, HF, Ungvari, GS, et al. Aripiprazole for Tourette’s syndrome: a systematic review and meta-analysis. Hum Psychopharmacol. (2016) 31:11–8. doi: 10.1002/hup.2498
35. Hett, D, Rogers, J, Humpston, C, and Marwaha, S. Repetitive transcranial magnetic stimulation (rTMS) for the treatment of depression in adolescence: a systematic review. J Affect Disord. (2021) 278:460–9. doi: 10.1016/j.jad.2020.09.058
36. Feng, H, Xu, JJ, Qin, GX, Gan, JG, and Wang, TF. Effects of low-frequency repetitive transcranial magnetic stimulation on cognitive function and life ability of children with depressive disorder (in Chinese). Chin J Gen Pract. (2017) 15:289–91. doi: 10.16766/j.cnki.issn.1674-4152.2017.02.032
37. Jadad, AR, Moore, RA, Carroll, D, Jenkinson, C, Reynolds, DJ, Gavaghan, DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
38. Higgins, JP, Altman, DG, Gotzsche, PC, Juni, P, Moher, D, Oxman, AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed). (2011) 343:d5928. doi: 10.1136/bmj.d5928
39. Linde, K, Clausius, N, Ramirez, G, Melchart, D, Eitel, F, Hedges, LV, et al. Are the clinical effects of homeopathy placebo effects? A meta-analysis of placebo-controlled trials Lancet. (1997) 350:834–43.
40. Croarkin, PE, Elmaadawi, AZ, Aaronson, ST, Schrodt, GR Jr, Holbert, RC, Verdoliva, S, et al. Left prefrontal transcranial magnetic stimulation for treatment-resistant depression in adolescents: a double-blind, randomized, sham-controlled trial. Neuropsychopharmacology. (2021) 46:462–9. doi: 10.1038/s41386-020-00829-y
41. Feng, H, Xu, JJ, Qin, GX, Gan, JG, and Wang, TF. Effects of low-frequency repetitivetranscranial magnetic stimulation on cognitive function and life ability of children with depressive disorder (in Chinese). Chin J Gen Pract. (2017) 15:289–91. doi: 10.16766/j.cnki.issn.1674-4152.2017.02.032
42. Lefaucheur, JP, Antal, A, Ayache, SS, Benninger, DH, Brunelin, J, Cogiamanian, F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. (2017) 128:56–92. doi: 10.1016/j.clinph.2016.10.087
43. Sun, CH, Jiang, WL, Cai, DB, Wang, ZM, Sim, K, Ungvari, GS, et al. Adjunctive multi-session transcranial direct current stimulation for neurocognitive dysfunction in schizophrenia: a meta-analysis. Asian J Psychiatr. (2021) 66:102887. doi: 10.1016/j.ajp.2021.102887
44. Jiang, WL, Cai, DB, Sun, CH, Yin, F, Goerigk, S, Brunoni, AR, et al. Adjunctive tDCS for treatment-refractory auditory hallucinations in schizophrenia: a meta-analysis of randomized, double-blinded, sham-controlled studies. Asian J Psychiatr. (2022) 73:103100. doi: 10.1016/j.ajp.2022.103100
45. Wang, G, Zheng, W, Li, XB, Wang, SB, Cai, DB, Yang, XH, et al. ECT augmentation of clozapine for clozapine-resistant schizophrenia: a meta-analysis of randomized controlled trials. J Psychiatr Res. (2018) 105:23–32. doi: 10.1016/j.jpsychires.2018.08.002
46. Zheng, W, Tong, G, Ungvari, GS, Ng, CH, Chiu, HFK, Xiang, YQ, et al. Memory impairment following electroconvulsive therapy in Chinese patients with schizophrenia: meta-analysis of randomized controlled trials. Perspect Psychiatr Care. (2018) 54:107–14. doi: 10.1111/ppc.12206
47. Martin, DM, McClintock, SM, Forster, JJ, Lo, TY, and Loo, CK. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: a systematic review and meta-analysis of individual task effects. Depress Anxiety. (2017) 34:1029–39. doi: 10.1002/da.22658
48. Patel, R, Silla, F, Pierce, S, Theule, J, and Girard, TA. Cognitive functioning before and after repetitive transcranial magnetic stimulation (rTMS): a quantitative meta-analysis in healthy adults. Neuropsychologia. (2020) 141:107395. doi: 10.1016/j.neuropsychologia.2020.107395
49. Faust, K, Nelson, BD, Sarapas, C, and Pliskin, NH. Depression and performance on the repeatable battery for the assessment of neuropsychological status. Appl Neuropsychol Adult. (2017) 24:350–6. doi: 10.1080/23279095.2016.1185426
50. Lai, S, Zhong, S, Wang, Y, Zhang, Y, Xue, Y, Zhao, H, et al. The prevalence and characteristics of MCCB cognitive impairment in unmedicated patients with bipolar II depression and major depressive disorder. J Affect Disord. (2022) 310:369–76. doi: 10.1016/j.jad.2022.04.153
51. Hu, Y, Jia, Y, Sun, Y, Ding, Y, Huang, Z, Liu, C, et al. Efficacy and safety of simultaneous rTMS-tDCS over bilateral angular gyrus on neuropsychiatric symptoms in patients with moderate Alzheimer’s disease: a prospective, randomized, sham-controlled pilot study. Brain Stimul. (2022) 15:1530–7. doi: 10.1016/j.brs.2022.11.009
52. Wang, H, Wang, K, Xue, Q, Peng, M, Yin, L, Gu, X, et al. Transcranial alternating current stimulation for treating depression: a randomized controlled trial. Brain. (2022) 145:83–91. doi: 10.1093/brain/awab252
Keywords: rTMS, major depressive disorder, first episode, children, adolescents
Citation: Zheng W, Lan X-J, Qin Z-J, Yang X-H and Shi Z-M (2023) Low-frequency repetitive transcranial magnetic stimulation for children and adolescents with first-episode and drug-naïve major depressive disorder: A systematic review. Front. Psychiatry. 14:1111754. doi: 10.3389/fpsyt.2023.1111754
Received: 30 November 2022; Accepted: 20 January 2023;
Published: 08 February 2023.
Edited by:
Huanzhong Liu, Chaohu Hospital of Anhui Medical University, ChinaReviewed by:
Lei Xia, Chaohu Hospital of Anhui Medical University, ChinaCopyright © 2023 Zheng, Lan, Qin, Yang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zheng, ✉ emhlbmd3ZWkwNzAyQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.