- Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu, Sichuan, China
Objectives: The purpose of this paper is to provide a mini-review covering the recent progress in human and animal studies on local field potentials (LFPs) of major depressive disorder (MDD) and obsessive-compulsive disorder (OCD).
Materials and methods: PubMed and EMBASE were searched to identify related studies. Inclusion criteria were (1) reported the LFPs on OCD or MDD, (2) published in English, and (3) human or animal studies. Exclusion criteria were (1) review or meta-analysis or other literature types without original data and (2) conference abstract without full text. Descriptive synthesis of data was performed.
Results: Eight studies on LFPs of OCD containing 22 patients and 32 rats were included: seven were observational studies with no controls, and one animal study included a randomized and controlled phase. Ten studies on LFPs of MDD containing 71 patients and 52 rats were included: seven were observational studies with no controls, one study with control, and two animal studies included a randomized and controlled phase.
Conclusion: The available studies revealed that different frequency bands were associated with specific symptoms. Low frequency activity seemed to be closely related to OCD symptoms, whereas LFPs findings in patients with MDD were more complicated. However, limitations of recent studies restrict the drawing of definite conclusions. Combined with other measures such as Electroencephalogram, Electrocorticography, or Magnetoencephalography and long-term recordings in various physiological states (rest state, sleep state, task state) could help to improve the understanding of potential mechanisms.
Introduction
Major depressive disorder (MDD) is a common psychiatric disorders (1), characterized by symptoms including depressed mood or anhedonia (2), affecting approximately 6% of the population worldwide (3). About 30% of this disease becomes treatment-resistant depression (TRD) among patients with major depression.
It is estimated that 2–3% of the population suffer from obsessive–compulsive disorder (OCD) (4), characterized by distressing intrusive thoughts (obsessions) and often time consuming repetitive behaviors (compulsions) (5). Apart from obsessions and compulsions, substantial comorbidity, anxiety disorders, mood disorders, impulse-control disorders, and substance use disorders are common in OCD, with patients feeling confused in relationships and social functioning domains (6). However, 10% of patients are not relieved with sufficient drugs and long duration (7).
In 1999, Deep brain stimulation (DBS) targeting the anterior limb of the internal capsule (ALIC) was found to be beneficial for intractable OCD (8), and many reports about other targets of DBS in patients with OCD have been published. Moreover, DBS had been proven to produce clinical benefits in patients with TRD since 2005 (9). With direct access to the subcortical activity, local field potentials (LFPs) has provided valuable insight into disease mechanisms and cognitive processes. It is known that aberrant activity in cortico-basal ganglia-thalamo-cortical loops may be involved in the symptoms, but the specific relationship between LFPs and OCD is unclear. Likewise, the relationship between LFPs and MDD is unknown.
This review aimed to summarize the changes of LFPs in patients with OCD and MDD. In addition, we expect to point out how electrophysiology research in patients with DBS will continuously offer new information with mechanism when combined with LFPs.
Local field potentials
As an aspect of neural activity in the brain, LFPs are increasingly used as a reflection of the ongoing transmission through neural networks (10), which can be recorded using metal, glass electrodes, or silicon probes inserted in the deep brain areas during or after operation (11). In fact, LFPs have been neglected for a few decades because in vivo neurophysiological research focused mostly on isolating action potentials from individual neurons. They are of interest to researchers who study cortical function in recent years, because LFPs provide a unique window to integrative excitatory and inhibitory synaptic processes for neural population activity (12, 13).
According to the changes of frequency, LFP bands were defined as follows: delta (0–3 Hz), theta (4–7 Hz), alpha (8–12 Hz), beta (13–35 Hz), gamma (31–200 Hz), and high-frequency oscillation (>200 Hz) (14). Different frequency-band oscillations reflect diverse neural processing pathways and contributions of transmembrane current from cellular activities. Moreover, LFPs can provide stable signals for long-term chronic experiments or clinical applications.
Therefore, LFPs provide more chances to explore the electrophysiological mechanism of psychiatric disorders. It is known that the presence of abnormal beta bursts is significantly correlated with the degree of motor impairment and the severity of rigidity/bradykinesia in Parkinson’s disease (PD) (15). However, there are few reports on the electrophysiological characteristics of OCD and MDD.
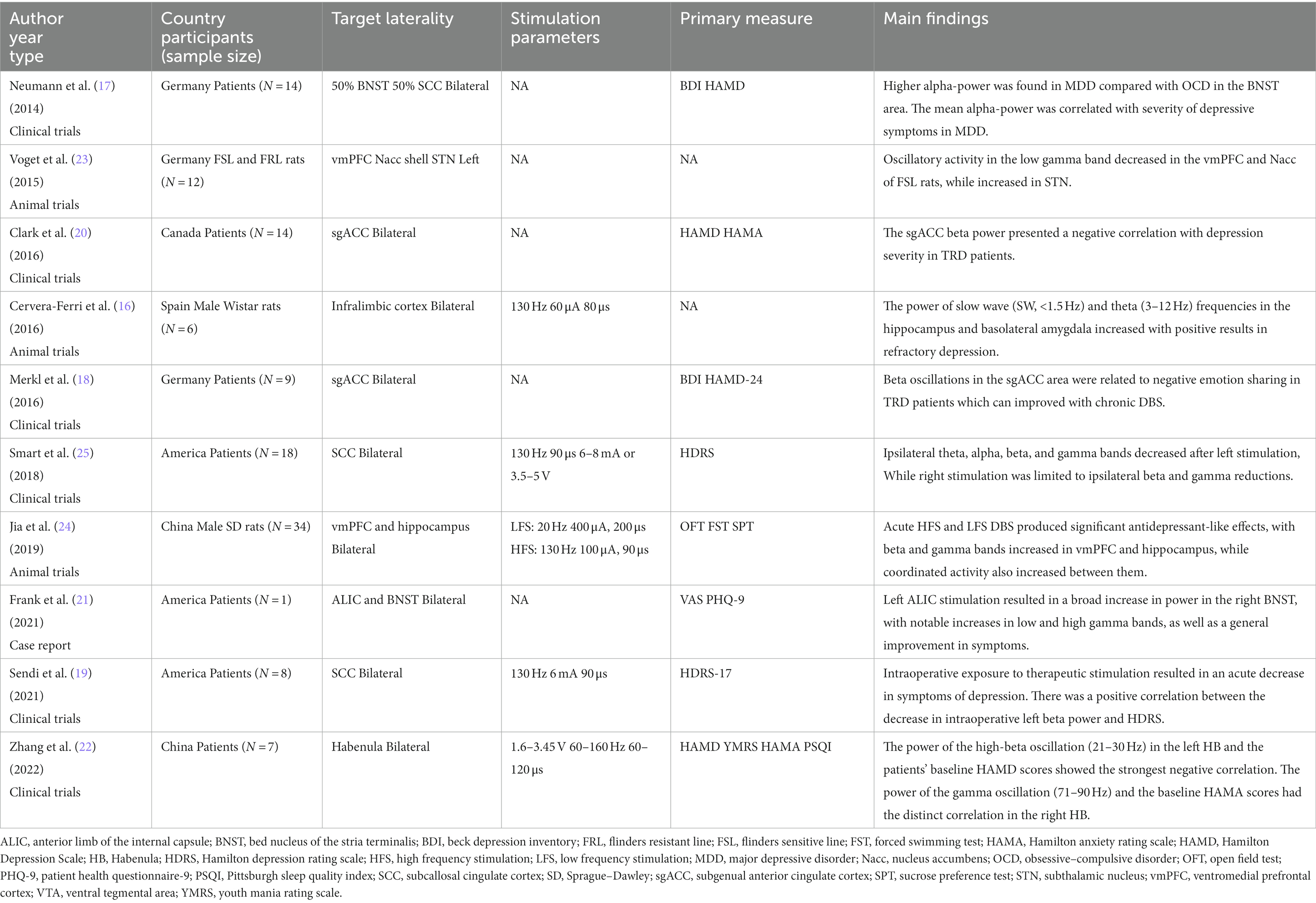
LFPs in MDD
Theta frequency
In a study by Cervera-Ferri et al., stimulating electrodes were implanted in the subgenual cingulate gyrus (SCG) in urethane-anesthetized rats bilaterally, while recording electrodes were placed at the dorsal hippocampus and basolateral amygdala. After 1 h stimulation of SCG, the power of slow wave (SW, <1.5 Hz) and theta (3–12 Hz) frequencies in the hippocampus and basolateral amygdala increased with positive results in refractory depression (16). However, the oscillatory changes detected might not be equivalent to those observed in the awake rats, which cannot reflect the real changes after long stimulation periods as they are obtained shortly after DBS offset.
Alpha frequency
Neumann et al. found that the increased alpha-activity in the bed nucleus of the stria terminalis (BNST) showed a positive correlation with the level of depression and aberrant alpha-band activity in MDD might be reduced by DBS (17).
Beta frequency
Merkl et al. evaluated the LFP changes during multifaceted empathy test in patients who received DBS in the subcallosal cingulate cortex (SCC). The study revealed that beta desynchronization for empathic involvement was associated with self-reported severity of depression (18). Moreover, early antidepressant response during intraoperative stimulation was linked with a decrease in beta power in eight patients who received SCC-DBS (19). Clark et al. also reported that beta power in subgenual anterior cingulate cortex (sgACC) was inversely related to HAMD score in MDD patients (20).
Gamma frequency
DBS in the left ALIC resulted in a broad power increase in the right BNST, including significant increases in low and high gamma power, and an overall improvement of symptoms (21). Zhang et al. reported seven patients underwent Habenula (HB)–DBS surgery and found the LFP asymmetry in the left and right HB. Specifically, the power of the high-beta oscillation (21–30 Hz) in the left HB presented the largest negative connection with the patients’ baseline HAMD scores. While the most distinct correlations occurs between the power of both the gamma oscillation (71–90 Hz) and the patients’ baseline HAMA scores in the right HB (22). Voget et al. compared the difference of LFP between the FSL rats and Flinders resistant line (FRL) rats under urethane anesthesia, and found decreased oscillatory activity in the low gamma band in vmPFC and nucleus accumbens(Nacc) of FSL rats, with increased activity in the subthalamic nucleus (STN) (23). In a rat model of depression, a unipolar stimulating electrode was inserted into right vmPFC, while recording electrodes were implanted bilaterally into vmPFC and hippocampus. After DBS treatment, LFP oscillations in beta and gamma bands increased in vmPFC and hippocampus, with increased coordinated activity between them (24). In 14 consecutive patients who underwent bilateral SCC-DBS lead implantation, Smart et al. found that asymmetric power spectral density changed after acute unilateral SCC stimulation. Left stimulation induced broadband ipsilateral decrease in theta, alpha, beta, and gamma bands and right stimulation effects were restricted to ipsilateral beta and gamma bands decrease (25). Low band frequencies, typically between 2 and 20 Hz, were observed in SCC and adjacent targets, evolving with stimulation (26).
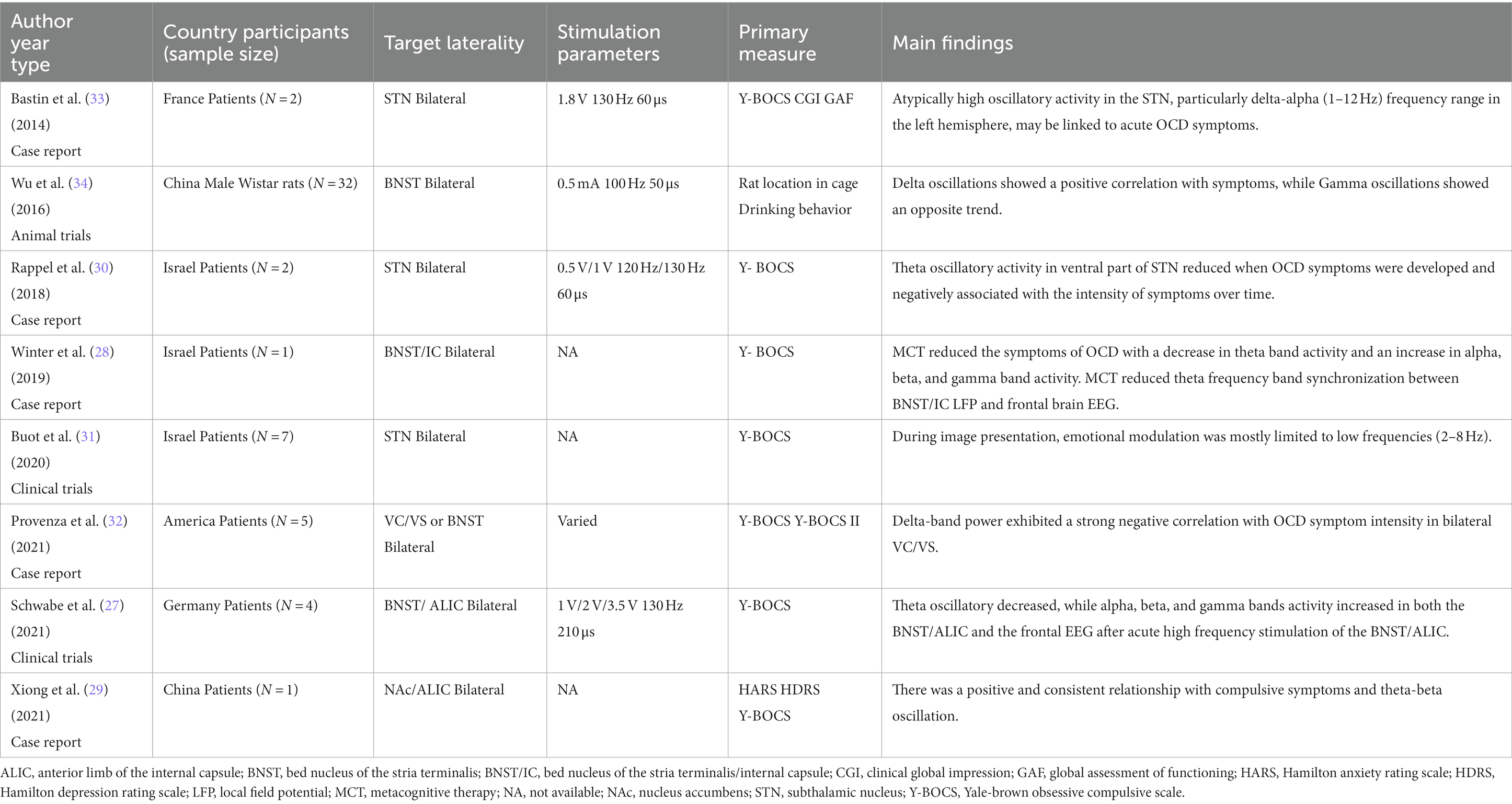
LFPs in OCD
Theta frequency
Acute high frequency stimulation reduced theta band activities, accompanied by other frequency bands increasing in BNST/ALIC and frontal cortex in four patients with OCD (27). In Winter et al.’s study, OCD symptoms subsided in one patient who received metacognitive therapy (MCT), with a decrease in theta band activity and an evident increase in alpha, beta, and gamma band activity in BNST/IC (28). Xiong et al. also reported a positive and consistent trend between clinical outcomes and theta-beta oscillation (29). Rappel et al. compared the LFPs between OCD and PD patients targeted in the subthalamic nucleus (STN), and they found that the ventral area of the STN displays distinct theta (6.5–8 Hz) oscillatory activity only in patients with OCD (30). Buot et al. recorded STN LFP activity while patients with OCD performed emotional categorization tasks, and discovered that modulations of STN theta band activity which related to emotional regulation were correlated with OCD symptoms severity (31).
Delta frequency
Delta band power showed a strong negative correlation with OCD symptom intensity in the bilateral ventral capsule/ventral striatum (VC/VS), when intracranial electrophysiological data of VC/VS and BNST were recorded in three patients with OCD both in the clinic and natural environments (32). Bastin et al. reported that acute OCD symptoms might be related to abnormally high oscillatory activity in STN, particularly in the left hemisphere and delta-alpha (1–12 Hz) frequency (33).
Other frequency
Wu et al. implanted electrodes bilaterally in the BNST and random control brain regions in 32 male Wistar rats and recorded corresponding LFPs during compulsive and noncompulsive behavior (34). During the initial phase of compulsion, delta oscillations increased, peaked during compulsion, and dropped afterwards. In contrast, gamma oscillations decreased before and during compulsion, and increased after compulsion. Beta oscillations increased when compulsion symptoms stopped. Furthermore, the percentage change of these bands during compulsion was strongly linked with the compulsive suppression effect of BNST electrical stimulation. Overall, few articles on LFP alterations in animals with OCD exist.
Discussion
Different neuronal oscillations have always been considered as brain organizers, coordinating brain areas into networks in the normal or disordered state (35–38). To our knowledge, this was the first review to investigate LFP oscillations in patients with OCD and MDD. In this review, low frequency bands, especially the theta band, appeared more positively involved in modulating psychiatric-condition-related networks in patients with OCD (Table 1). However, such involvement may also be observed in levodopa-induced dyskinesias, dystonia, Tourette’s syndrome, schizophrenia, and attention deficit hyperactivity disorder (27). Meanwhile, changes in other frequency bands have also been reported. Since frequency bands are interrelated and interact with each other in psychiatric-disorder-related networks, changes in one frequency band activity may also influence the activity of other frequency bands. Here, critical caution is needed when considering the theta band as a biomarker in patients with OCD.
In clinical studies, LFP findings in patients with MDD involved different frequency bands, unlike animal study observations. No clear conclusions could be drawn about the direction of the difference due to considerable inconsistencies between the study design and methodology. We concluded that the neuronal architecture of the mouse brain is different from that of the human. For example, the human hippocampus has a larger size and more neurons than rodents. Therefore, humans would have a longer period than animals to bring more cell assemblies to be linked synaptically in various brain processes such as memory and learning (39), showing lower frequency oscillations than rodents. Some researchers conjecture that the frequency of a neural oscillation has an inverse relationship with the size of a neuronal network (40–42).
The SCC LFP recordings in the bilateral hemispheres are asymmetric; left-sided stimulation resulted in widespread changes in the frequency bands, and the right-side stimulation effects were restricted to the beta and gamma bands (25). Similarly, the HB LFP recordings in the left hemisphere showed high-beta oscillation with depressive symptoms, while gamma oscillation was distinct in the right hemisphere (22). This lateralized alteration implied an asymmetric role of the bilateral hemispheres in modulating psychiatric-disorder-related networks, identical to the laterality of brain speech function.
However, current research has produced strikingly different results, as they were obtained through various targets and under different physiological states, which impeded arriving at definite conclusions. Furthermore, some studies did not use stimulation with the targets, and the stimulation parameters were not unified. As LFP is a partly ambiguous signal involving multiple neuronal processes, careful analytical modeling and empirical considerations are required to interpret the derived information.
Based on the findings, we conclude that synchronization in multiple bands, rather than just abnormalities in a single frequency band, may play an essential role in modulating OCD and MDD symptoms. In the future, we might consider modulating networks that support specific symptom patterns instead of focusing on a single, optimal gray matter target (43).
Current challenges and future perspective
Despite great potential, there are still limitations in the current study. Firstly, recent studies have focused on different targets or nuclei, but they can produce different oscillation bands, which precluded a quantitative synthesis of the outcome. Apart from frequency, the LFP signals contain multiple dimensions, including waveform, power, phase, entropy, and coupling (14); developing advanced algorithms could help retrieve more pertinent information (44). Secondly, LFP oscillations were recorded in different timelines, such as intraoperatively and before or after DBS stimulation (19, 45); therefore, comparisons could not be performed, more studies with recording in the same timeline are needed in the future. Finally, because of the small sample sizes of patients (Tables 1, 2), more studies are needed to confirm the validity and reliability of LFPs. In addition, LFPs data are mostly recorded in the resting state, with few reports on the task (46) or sleep state (32), making it difficult to fully reflect the mechanisms of MDD and OCD.
As LFPs represent the multiple neuronal processes, how to filter and interpret the LFPs is a challenge for current researchers. Combined with other measures such as Electroencephalogram, Electrocorticography, or Magnetoencephalography, LFPs may provide more cortical information (27, 47–50). Future research using long-term LFP recordings in various physiological states (rest state, sleep state, task state) could help to improve the understanding of potential mechanisms.
Conclusion
This mini-review was not comprehensive; however, it summarized the recent advances in LFPs of MDD and OCD. As mentioned above, LFPs provided more chances to understand the electrophysiological characteristics and explore the potential mechanisms. Low-frequency activity seemed closely related to OCD symptoms, whereas LFP findings in patients with MDD were complicated. While MDD in human had a close relationship with the alpha or beta frequency or gamma oscillations. All different frequency bands seemed to participate in MDD and OCD networks. In the future, other multiple measures with long-term LFP recordings in various physiological states (rest, sleep, and task states) could be applied in patients with OCD or MDD.
Author contributions
WZ, BX, and WW proposed the conception and design of the study. WZ and BX conducted the literature search, study screening, data extraction, and quality assessment. WZ, BX, YW, LX, and WW were involved in the analysis and interpretation of data. WZ and BX drafted the manuscript and all authors revised it critically under the guidance of WW. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the 135 Project of Outstanding Development of West China Hospital, Sichuan University (Grant number ZY2017307).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Malhi, GS, and Mann, JJ. Depression. Lancet. (2018) 392:2299–312. doi: 10.1016/S0140-6736(18)31948-2
2. Uher, R, Payne, JL, Pavlova, B, and Perlis, RH. Major depressive disorder in DSM-5: implications for clinical practice and research of changes from DSM-IV. Depress Anxiety. (2014) 31:459–71. doi: 10.1002/da.22217
3. Kessler, RC, and Bromet, EJ. The epidemiology of depression across cultures. Annu Rev Public Health. (2013) 34:119–38. doi: 10.1146/annurev-publhealth-031912-114409
4. Milad, MR, and Rauch, SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. (2012) 16:43–51. doi: 10.1016/j.tics.2011.11.003
5. Schüller, T, Gruendler, TOJ, Smith, EE, Baldermann, JC, Kohl, S, Fischer, AG, et al. Performance monitoring in obsessive–compulsive disorder: insights from internal capsule/nucleus accumbens deep brain stimulation. NeuroImage Clin. (2021) 31:102746. doi: 10.1016/j.nicl.2021.102746
6. Ruscio, AM, Stein, DJ, Chiu, WT, and Kessler, RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. (2010) 15:53–63. doi: 10.1038/mp.2008.94
7. Denys, D. Pharmacotherapy of obsessive-compulsive disorder and obsessive-compulsive spectrum disorders. Psychiatr Clin North Am. (2006) 29:553–84. doi: 10.1016/j.psc.2006.02.013
8. Nuttin, B, Cosyns, P, Demeulemeester, H, Gybels, J, and Meyerson, B. Electrical stimulation in anterior limbs of internal capsules in patients with obsessive-compulsive disorder. Lancet. (1999) 354:1526. doi: 10.1016/S0140-6736(99)02376-4
9. Mayberg, HS, Lozano, AM, Voon, V, Mcneely, HE, Seminowicz, D, Hamani, C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. (2005) 45:651–60. doi: 10.1016/j.neuron.2005.02.014
10. Herreras, O. Local field potentials: myths and misunderstandings. Front Neural Circuits. (2016) 10:101. doi: 10.3389/fncir.2016.00101
11. Buzsaki, G, Anastassiou, CA, and Koch, C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat Rev Neurosci. (2012) 13:407–20. doi: 10.1038/nrn3241
12. Brunel, N, and Wang, X-J. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J Neurophysiol. (2003) 90:415–30. doi: 10.1152/jn.01095.2002
13. Logothetis, NK. What we can do and what we cannot do with fMRI. Nature. (2008) 453:869–78. doi: 10.1038/nature06976
14. Yin, Z, Zhu, G, Zhao, B, Bai, Y, Jiang, Y, Neumann, WJ, et al. Local field potentials in Parkinson's disease: a frequency-based review. Neurobiol Dis. (2021) 155:105372. doi: 10.1016/j.nbd.2021.105372
15. Tinkhauser, G, Pogosyan, A, Tan, H, Herz, DM, Kuhn, AA, and Brown, P. Beta burst dynamics in Parkinson's disease OFF and ON dopaminergic medication. Brain. (2017) 140:2968–81. doi: 10.1093/brain/awx252
16. Cervera-Ferri, A, Teruel-Marti, V, Barcelo-Molina, M, Martinez-Ricos, J, Luque-Garcia, A, Martinez-Bellver, S, et al. Characterization of oscillatory changes in hippocampus and amygdala after deep brain stimulation of the infralimbic prefrontal cortex. Phys Rep. (2016) 4:e12854. doi: 10.14814/phy2.12854
17. Neumann, WJ, Huebl, J, Brücke, C, Gabriëls, L, Bajbouj, M, Merkl, A, et al. Different patterns of local field potentials from limbic DBS targets in patients with major depressive and obsessive compulsive disorder. Mol Psychiatry. (2014) 19:1186–92. doi: 10.1038/mp.2014.2
18. Merkl, A, Neumann, WJ, Huebl, J, Aust, S, Horn, A, Krauss, JK, et al. Modulation of Beta-band activity in the Subgenual anterior cingulate cortex during emotional empathy in treatment-resistant depression. Cereb Cortex. (2016) 26:2626–38. doi: 10.1093/cercor/bhv100
19. Sendi, MSE, Waters, AC, Tiruvadi, V, Riva-Posse, P, Crowell, A, Isbaine, F, et al. Intraoperative neural signals predict rapid antidepressant effects of deep brain stimulation. Transl Psychiatry. (2021) 11:551. doi: 10.1038/s41398-021-01669-0
20. Clark, DL, Brown, EC, Ramasubbu, R, and Kiss, ZHT. Intrinsic local Beta oscillations in the Subgenual cingulate relate to depressive symptoms in treatment-resistant depression. Biol Psychiatry. (2016) 80:e93–4. doi: 10.1016/j.biopsych.2016.02.032
21. Frank, AC, Scangos, KW, Larson, PS, Norbu, T, Lee, AT, and Lee, AM. Identification of a personalized intracranial biomarker of depression and response to DBS therapy. Brain Stimul. (2021) 14:1002–4. doi: 10.1016/j.brs.2021.06.009
22. Zhang, C, Zhang, Y, Luo, H, Xu, X, Yuan, TF, Li, D, et al. Bilateral Habenula deep brain stimulation for treatment-resistant depression: clinical findings and electrophysiological features. Transl Psychiatry. (2022) 12:52. doi: 10.1038/s41398-022-01818-z
23. Voget, M, Rummel, J, Avchalumov, Y, Sohr, R, Haumesser, JK, Rea, E, et al. Altered local field potential activity and serotonergic neurotransmission are further characteristics of the flinders sensitive line rat model of depression. Behav Brain Res. (2015) 291:299–305. doi: 10.1016/j.bbr.2015.05.027
24. Jia, L, Sun, Z, Shi, D, Wang, M, Jia, J, He, Y, et al. Effects of different patterns of electric stimulation of the ventromedial prefrontal cortex on hippocampal-prefrontal coherence in a rat model of depression. Behav Brain Res. (2019) 356:179–88. doi: 10.1016/j.bbr.2018.08.032
25. Smart, O, Choi, KS, Riva-Posse, P, Tiruvadi, V, Rajendra, J, Waters, AC, et al. Initial unilateral exposure to deep brain stimulation in treatment-resistant depression patients alters spectral power in the subcallosal cingulate. Front Comput Neurosci. (2018) 12:43. doi: 10.3389/fncom.2018.00043
26. Tiruvadi, V, Choi, KS, Gross, RE, Butera, R, Jirsa, V, and Mayberg, H. Dynamic oscillations evoked by subcallosal cingulate deep brain stimulation. Front Neurosci. (2022) 16:768355. doi: 10.3389/fnins.2022.768355
27. Schwabe, K, Alam, M, Saryyeva, A, Lutjens, G, Heissler, HE, Winter, L, et al. Oscillatory activity in the BNST/ALIC and the frontal cortex in OCD: acute effects of DBS. J Neural Transm (Vienna). (2021) 128:215–24. doi: 10.1007/s00702-020-02297-6
28. Winter, L, Alam, M, Heissler, HE, Saryyeva, A, Milakara, D, Jin, X, et al. Neurobiological mechanisms of metacognitive therapy - an experimental paradigm. Front Psychol. (2019) 10:660. doi: 10.3389/fpsyg.2019.00660
29. Xiong, B, Wen, R, Gao, Y, and Wang, W. Longitudinal changes of local field potential oscillations in nucleus accumbens and anterior limb of the internal capsule in obsessive-compulsive disorder. Biol Psychiatry. (2021). doi: 10.1016/j.biopsych.2021.06.004
30. Rappel, P, Marmor, O, Bick, AS, Arkadir, D, Linetsky, E, Castrioto, A, et al. Subthalamic theta activity: a novel human subcortical biomarker for obsessive compulsive disorder. Transl Psychiatry. (2018) 8:118. doi: 10.1038/s41398-018-0165-z
31. Buot, A, Karachi, C, Lau, B, Belaid, H, Fernandez-Vidal, S, Welter, ML, et al. Emotions modulate subthalamic nucleus activity: new evidence in obsessive-compulsive disorder and Parkinson's disease patients. Biol Psychiatry Cogn Neurosci Neuroimaging. (2021) 6:556–67. doi: 10.1016/j.bpsc.2020.08.002
32. Provenza, NR, Sheth, SA, Dastin-Van Rijn, EM, Mathura, RK, Ding, Y, Vogt, GS, et al. Long-term ecological assessment of intracranial electrophysiology synchronized to behavioral markers in obsessive-compulsive disorder. Nat Med. (2021) 27:2154–64. doi: 10.1038/s41591-021-01550-z
33. Bastin, J, Polosan, M, Piallat, B, Krack, P, Bougerol, T, Chabardes, S, et al. Changes of oscillatory activity in the subthalamic nucleus during obsessive-compulsive disorder symptoms: two case reports. Cortex. (2014) 60:145–50. doi: 10.1016/j.cortex.2013.12.007
34. Wu, H, Tambuyzer, T, Nica, I, Deprez, M, Van Kuyck, K, Aerts, JM, et al. Field potential oscillations in the bed nucleus of the stria terminalis correlate with compulsion in a rat model of obsessive-compulsive disorder. J Neurosci. (2016) 36:10050–9. doi: 10.1523/JNEUROSCI.1872-15.2016
35. Fries, P. Rhythms for cognition: communication through coherence. Neuron. (2015) 88:220–35. doi: 10.1016/j.neuron.2015.09.034
36. Torres-Herraez, A, Watson, TC, and Rondi-Reig, L. Delta oscillations coordinate intracerebellar and cerebello-hippocampal network dynamics during sleep. J Neurosci. (2022) 42:2268–81. doi: 10.1523/JNEUROSCI.1479-21.2021
37. Uhlhaas, PJ, and Singer, W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. (2010) 11:100–13. doi: 10.1038/nrn2774
38. Voytek, B, and Knight, RT. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biol Psychiatry. (2015) 77:1089–97. doi: 10.1016/j.biopsych.2015.04.016
39. Jacobs, J. Hippocampal theta oscillations are slower in humans than in rodents: implications for models of spatial navigation and memory. Philos Trans R Soc Lond Ser B Biol Sci. (2014) 369:20130304. doi: 10.1098/rstb.2013.0304
40. Buzsáki, G, and Draguhn, A. Neuronal oscillations in cortical networks. Science. (2004) 304:1926–9. doi: 10.1126/science.1099745
41. Steriade, M. Impact of network activities on neuronal properties in corticothalamic systems. J Neurophysiol. (2001) 86:1–39. doi: 10.1152/jn.2001.86.1.1
42. Csicsvari, J, Jamieson, B, Wise, KD, and Buzsáki, G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. (2003) 37:311–22. doi: 10.1016/S0896-6273(02)01169-8
43. Vlis, TAMB, Ackermans, L, Mulders, AEP, Vrij, CA, Schruers, K, Temel, Y, et al. Ventral capsule/ventral striatum stimulation in obsessive-compulsive disorder: toward a unified connectomic target for deep brain stimulation? Neuromodulation. (2020) 24:316–23. doi: 10.1111/ner.13339
44. Golshan, HM, Hebb, AO, and Mahoor, MH. LFP-net: a deep learning framework to recognize human behavioral activities using brain STN-LFP signals. J Neurosci Methods. (2020) 335:108621. doi: 10.1016/j.jneumeth.2020.108621
45. Bangel, KA, Bais, M, Eijsker, N, Schuurman, PR, Van Den Munckhof, P, Figee, M, et al. Acute effects of deep brain stimulation on brain function in obsessive-compulsive disorder. Clin Neurophysiol. (2023) 148:109–17. doi: 10.1016/j.clinph.2022.12.012
46. Fridgeirsson, EA, Bais, MN, Eijsker, N, Thomas, RM, Smit, DJA, Bergfeld, IO, et al. Patient specific intracranial neural signatures of obsessions and compulsions in the ventral striatum. J Neural Eng. (2023) 20:026008. doi: 10.1088/1741-2552/acbee1
47. Geraedts, VJ, Boon, LI, Marinus, J, Gouw, AA, Van Hilten, JJ, Stam, CJ, et al. Clinical correlates of quantitative EEG in Parkinson disease: a systematic review. Neurology. (2018) 91:871–83. doi: 10.1212/WNL.0000000000006473
48. Hagen, E, Naess, S, Ness, TV, and Einevoll, GT. Multimodal modeling of neural network activity: computing LFP, ECoG, EEG, and MEG signals with LFPy 2.0. Front Neuroinform. (2018) 12:92. doi: 10.3389/fninf.2018.00092
49. Oswal, A, Jha, A, Neal, S, Reid, A, Bradbury, D, Aston, P, et al. Analysis of simultaneous MEG and intracranial LFP recordings during deep brain stimulation: a protocol and experimental validation. J Neurosci Methods. (2016) 261:29–46. doi: 10.1016/j.jneumeth.2015.11.029
Keywords: deep brain stimulation, obsessive–compulsive disorder, major depressive disorder, local field potentials, mechanisms, frequency bands
Citation: Zhang W, Xiong B, Wu Y, Xiao L and Wang W (2023) Local field potentials in major depressive and obsessive-compulsive disorder: a frequency-based review. Front. Psychiatry 14:1080260. doi: 10.3389/fpsyt.2023.1080260
Edited by:
Dejan Georgiev, University Medical Centre, Ljubljana, SloveniaReviewed by:
Vineet Tiruvadi, HumeAI, United StatesCopyright © 2023 Zhang, Xiong, Wu, Xiao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang, d2Nuc3d3QDE2My5jb20=
†These authors share co-first authorship
 Wei Zhang
Wei Zhang Botao Xiong†
Botao Xiong† Wei Wang
Wei Wang