- 1West China School of Nursing, Department of Respiratory and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China
- 2Sleep Medicine Center, Mental Health Center, Translational Neuroscience Center, West China Hospital, Sichuan University, Chengdu, China
- 3Ward 3, Department of Tuberculosis, The Fourth People Hospital of Nanning, Guangxi Zhuang Autonomous Region, Nanning, China
Background: Depression and anxiety are major psychological issues among patients with tuberculosis (TB) owing to chronic and complex treatments, have been reported to be closely correlated with immune and inflammation. However, the association of peripheral immune-inflammatory characteristics with depression/anxiety symptoms in in-patients with TB has rarely been reported.
Methods: A cross-sectional study of 338 in-patients with TB from 3 hospitals in China were enrolled to investigate their depression and anxiety status by using the nine-item Patient Health Questionnaire (PHQ-9) and seven-item Generalized Anxiety Disorder Scale (GAD-7). Participants were divided into groups based on their PHQ-9 and GAD-7 scores, and differences in demography and immune-inflammatory characteristics were studied. Logistic analysis was performed to explore factors related to depression and anxiety symptoms.
Results: Depression and anxiety prevalence among patients with TB was 47.9 and 42.6%, respectively. Furthermore, 38.5% of patients reported a comorbidity of depression and anxiety symptoms. The counts of CD3, CD4, CD8, and lymphocytes decreased, whereas those of neutrophils, platelets, and peripheral blood cells and their derived indices increased among TB patients with depression or anxiety in comparison with those without symptoms (p < 0.05). In addition, increasing age, lower income (monthly income ≤ 3,000 yuan), divorced or widowed, drug resistance, and higher systemic immune inflammation index (SII) were significantly associated with depression or anxiety symptoms (p < 0.05).
Conclusion: Approximately half of the patients with TB suffered from depression or/and anxiety symptoms. Patients with depression or anxiety present worse cell immune status and stronger inflammatory responses compared to those without symptoms. We emphasized the importance of paying attention to the dysfunction of immune-inflammation process of TB patients with depression or anxiety symptoms. Especially, SII has a potential application value in guiding the evaluation of TB-related depression or anxiety owing to its easily accessibility and being economical.
Introduction
Tuberculosis (TB) is a chronic inflammatory disease with highly contagious nature (1). It is the primary cause of death due to single pathogen infection, with a mortality rate of 15% in 2020 (2). Several challenges such as long treatment periods, great side effects, and drug resistance lead to a high prevalence of mental disorders in TB treatment. Previous studies have demonstrated that more than half of the patients with TB possibly suffer from depression and anxiety (3). TB with comorbidity of mental disorders is more prone to poor medication adherence and delayed treatment (4), resulting in higher drug resistance, risk of treatment failure, mortality, and transmission (5, 6). Therefore, the mental health of TB patients should be emphasized.
Having TB affects the human immune system as it can disarm the host immune responses, manifested by abnormalities in the number and function of antigen-specific T-cells (7, 8). Protection against Mycobacterium tuberculosis (Mtb) is primarily dependent on cell-mediated immunity, particularly that conferred by CD4 and CD8 T cell subsets (8). An obvious decrease of percentage or absolute counts of CD3, CD4, and CD8 T cells in the peripheral blood of patients with TB indicates significant immunosuppression or immune deterioration which is closely related to the severity of TB illness (9). CD4 and CD8 were detected to be lower among college students with anxiety and depression than in those without symptoms, indicating lower concentrations of immune parameters are associated with depression and anxiety symptoms among healthy adults (10).
Accumulating evidence suggests that immune system play an important role in pathophysiology of psychiatric disorders, involving altering immune cell and inflammatory cytokines, neurotransmitter metabolism, neuroendocrine function, neural plasticity, or reactive oxygen species (11). Inflammation is considered a central component of the innate immune response (12). Numerous studies have shown that many psychiatric disorders, such as depression and anxiety, are closely related to inflammation (13, 14). Pro-inflammatory cytokines in infectious diseases can induce behavioral symptoms referred to as sickness behavior, which is accompanied by depression, anxiety or sleep disorder (15). Because of their value and convenience, peripheral blood cell count-derived indices such as neutrophil/lymphocyte ratio (NLR), monocyte/lymphocyte ratio (MLR), platelets/lymphocyte ratio (PLR), and the systemic immune-inflammation index (SII) have been recognized as biomarkers to assess inflammatory sensitivity in recent years (16). Significant research has established these ratios as severity markers in many psychiatric disorders (17), and these indices have been linked to morbidity, mortality, and prognosis in psychiatric and non-psychiatric diseases such as diabetes mellitus and cancers (18–20).
Although several surveys have reported immune-inflammatory abnormality in patients with TB (21, 22), its relationship with mental and emotional health has not been determined completely. Thus, this study aimed to investigate the immune-inflammatory characteristics of TB patients with depression and anxiety.
Materials and methods
Study design and settings
A cross-sectional clinical study was conducted at West China Hospital, Guangyuan Mental Health Center, and the Fourth People's Hospital of Guangxi in China from November 2021 to February 2022 using a convenience sampling method. A self-designed demographic questionnaire was used to collect demographic information. Appropriate clinical scoring instruments were used to evaluate their depression and anxiety symptoms. Fasting venous blood samples were collected on the next morning of admission to further have immune and inflammatory test. The Biomedical Research Ethics Committee of West China Hospital, Sichuan University approved this study.
Participants
Inclusion criteria for the study were as follows: (1) confirmed and highly suspected TB patients who have been diagnosed in accordance with national TB program guidelines (23, 24); (2) those who could complete the questionnaire with their communication and cognitive skills; and (3) those who voluntarily participated in the study. Patients with severe somatic disorders, including but not limited to tumors, critical infections, immune diseases; or confirmed psychiatry disorders; or those took on special drugs, such as psychotropic or immune-altering medications, were excluded.
Measurements
Clinical evaluation
An electronic structured questionnaire was used to collect information containing age, gender, height, weight, education years, marital status, income per month, duration of illness, and drug resistance status. Drug resistance was defined as drug susceptibility testing showed that Mtb were resistant to at least one first-line anti-TB drug such as isoniazid, rifampicin.
Evaluation of depression and anxiety symptoms
The nine-item Patient Health Questionnaire (PHQ-9) and seven-item Generalized Anxiety Disorder Scale (GAD-7), which are validated and widely applied self-report scales were used to assess depression and anxiety, with an excellent Cronbach's alpha of 0.901 and 0.938, respectively, in the present study. The optimal and most common cut-point of PHQ-9 and GAD-7 used for screening for depressive and anxiety disorders was 10 or above (25, 26). Hence, PHQ-9 and GAD-7 scores above 10 were considered a sign of depression or anxiety in the present study (27).
Immune-inflammatory parameters
Data of immune-inflammatory parameters were derived from the hospital information system containing diagnosis, prescriptions, laboratory tests, including T cell subsets, and blood routine examinations. The immune biomarkers, including the CD3, CD4, CD8 counts and the CD4/CD8 ratio. The parameters from blood routine test were used to calculate the inflammatory indicators, including neutrophil/lymphocyte ratio (NLR), monocyte/lymphocyte ratio (MLR), platelets/lymphocyte ratio (PLR), and the systemic immune-inflammation index (SII, SII = platelets X neutrophils/lymphocytes) (28).
Statistical analysis
Continuous variables were expressed as means ± standard deviation (SD) for normally distributed data or median (interquartile range, IQR) for non-normal distributed data, and group comparisons were performed using the Independent sample's t-test or Mann-Whitney U-test, as appropriate. Categorical variables were expressed as frequencies and percentages, and group differences were examined using the Chi square test. A logistic regression analysis was conducted to identify risk factors for depression and anxiety. A statistically significant p-value of < 0.05 was used. For statistical analyses, SPSS (version 21.0) was used.
Results
Prevalence and demographic characteristics
A total of 350 TB patients were recruited to complete a questionnaire. Of these, 12 questionnaires were deleted due to logical errors or a large number of missing data, with a participation rate of 96.6%. Finally, 338 participants were enrolled with a mean age of 53.8 ± 18.2 years (range: 14–89), comprising 213 men (63%) and 125 women (37%). The average PHQ-9 and GAD-7 scores were 11.7 ± 6.5 and 10.0 ± 5.8, respectively. Using a cut-off score of 10 points, 162 (47.9%) patients exhibited depression scores >10, while 144 (42.6%) patients exhibited anxiety scores >10. In addition, 130 patients (38.5%) exhibited depression and anxiety scores >10. Considering the sample size in the depression only (N = 30) or anxiety only (N = 14) groups was insufficient for informative analyses, patients were divided into two groups based on PHQ-9 and GAD-7 scores, including those with depression or anxiety (DA) and those without depression or anxiety (DA) (neither depression nor anxiety, NDA).
We first analyzed age, education, body mass index (BMI), sex, marital status, monthly income, duration of illness, and whether there was drug resistance. Except for sex and BMI, there were significant statistical differences between the two groups in all variables. The age of participants in group DA was significantly older than those in group NDA (p < 0.05). The education years of participants in group DA were significantly fewer than those in group NDA (p < 0.05). The present study also indicated a higher depression or anxiety prevalence among patients with TB who were divorced or widowed, with lower income (monthly income ≤ 3,000 yuan), who were suffering from illness for >12 months, or who were drug-resistant. The details were presented in Table 1.
The immune-inflammatory characteristics
We secondly analyzed T cell subsets, inflammatory cell counts and their derived indices of the participants. Significant statistical differences were detected in the counts of CD3, CD4, CD8, neutrophil, platelets, lymphocyte, and derived indices between the two groups. The counts of CD3, CD4, CD8, and lymphocytes in the DA group were significantly lower than that in the NDA group (p < 0.05). The DA group had significantly higher counts of neutrophils, platelets, and derived indices (NLR, MLR, PLR, and SII), as well as a higher CD4/CD8 ratio than the NDA group (p < 0.05). However, no significant differences in monocyte counts were found. The parameters were shown in Table 2.
Logistic regression analysis of associated factors in TB patients with depression or anxiety
Logistics regression analysis indicated that increasing age, absence of spouse (divorced or widowed), lower income (monthly income ≤ 3,000 yuan), drug resistance, and higher SII were significantly correlated to the depression and/or anxiety symptoms (p < 0.05) (see Table 3).
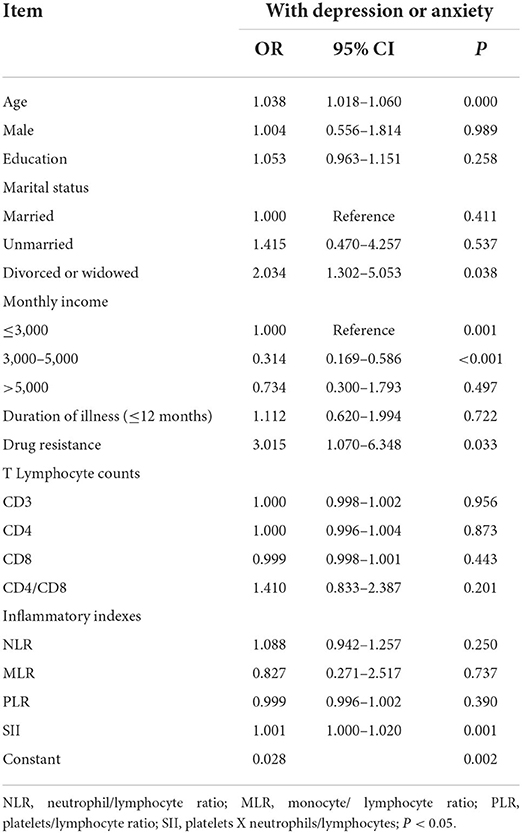
Table 3. Logistic regression analysis of associated factors in tuberculosis patients with depression or anxiety.
Discussion
In the present study, more than half (52.1%) of the patients showed depression or/and anxiety to varying degrees, similar to most studies with a prevalence ranging from 40 to 60% (29–31); however, < 60–80% of multidrug-resistant and extensively drug-resistant TB (32). Previous research has shown that Mtb infection and mental illness can interact, with an underlying biopsychosocial mechanism that comprises biological factors like the inflammatory cascade and psychosocial factors like perceived stigma (33). Infection-triggered perturbation of the immune system could induce psychopathology, promoting susceptibility to psychiatric symptoms (13). A higher incidence of drug resistance was reported among patients with depression or anxiety compared to those without the symptoms (10.8 vs. 1.9%). Moreover, 38.5% of patients reported depression and anxiety symptoms as comorbidity, in line with other studies reporting a co-occurrence prevalence of 20–50% or above (26, 34, 35). The complex conditions in a large number of critical patients was a possible cause of the high comorbidity of depression and anxiety.
In logistic regression analysis, increasing age, absence of spouse (divorced or widowed), lower income (monthly income ≤ 3,000 yuan), and drug resistance were predictors for developing depression and/or anxiety in tuberculosis patients. TB was more prevalent in older people and there was a progressive increase in the notification rate and mortality with age (36, 37). An increase in depression or anxiety among the elderly is possibly owing to their poorer adaptability, physical deterioration, higher frailty, and decreased social activities. Moreover, divorced or widowed individuals have a greater susceptibility to psychological issues, potentially because this population has experienced more stressful life events and lacks the companionship, comfort, and support from their spouses. Previous studies have also concluded that psychological distress was common in older adults, with ~30% of this population experiencing depressive or anxiety symptoms (38), and the percentage was higher in divorced or widowed population than those married (39). Poverty is both a risk factor and a consequence of TB. Another risk factor for developing depression or anxiety was lower-income ( ≤ 3,000 yuan), potentially due to a lack of welfare security and poorer social support. Consistent with prior studies conducted in China, income and marital status were still key predictors of depression in patients with TB after the socio-demographic variables were controlled (40). In addition, patients with drug resistance need a more complicated regimen along with more critical side effects, longer and worse treatment, and higher costs than those without resistance; these may further contribute to the development of depression and anxiety.
Further, when TB patients with depression and/or anxiety were compared to those without symptoms, they presented lower cell immune function and a stronger inflammatory response. Effective cell-mediated immunity is essential for the control of Mtb infection, determining whether the bacterial infection will be controlled or develop into a serious disease. Antigen-specific CD4 T cell response is the primary constituent in the host adaptive immune response against Mtb, and CD8 T cells directly eradicate mycobacteria and lyse infected host cells to generate cytokines like IFN-γ against Mtb (41). Chronic stress and Mtb infection have been considered to reduce immunity, such as decreased peripheral lymphocyte amount and impaired neutrophil phagocytosis. Moreover, the decreased CD4 and CD8 lymphocyte counts have been recognized to be tightly related to depressive and anxiety severity (10). The possible pathological mechanism is that mitochondrial fission caused by stress leading to purine metabolic disturbance in peripheral CD4 T cells can induce anxiety-like behaviors, indicating that CD4 T cells play an essential role in mediating mood disorders (42). In line with this, our study found that CD3, CD4, and CD8 levels were lower in patients with depression and/or anxiety than in those without symptoms, possibly involving in change of psychoneuroimmunology.
Pro-inflammatory factors released by peripheral immunocytes (e.g., lymphocytes, monocytes, neutrophils) can drive neuroinflammation by activating astrocytes and microglia, thus modulating the brain areas participating in regulating mood. Typically, they also reduce the intracerebral monoamine contents, activate neuroendocrine responses, promote excitotoxicity (elevated glutamate contents) and impair brain plasticity (43). An increased count of neutrophils represents inflammatory response intensity, while the decreased count of lymphocytes indicates the impairment of the immune system. Platelets contribute to coagulation and preserve various pro-inflammatory molecules modulating the immune and inflammatory responses (19). The derived indices of peripheral cells especially SII can provide a more comprehensive and stable inflammation assessment since it add platelet counts to NLR formulae to reflect different immune pathways and is less influenced by mixed conditions (44). In general, higher SII reveals enhanced inflammatory response and decreased immune response in patients. Previous studies have confirmed that the NLP and platelet counts were significantly elevated in the active TB group (45, 46). Prior studies demonstrate that SII is in a positive relationship with follow-up depression and anxiety scores (13), and the reduction degree of SII between admission and discharge forecasts the relief of depression among coronavirus disease survivors (47). In a study by HU, increased SII is significantly correlated with post-stroke depression and possibly provides several prognostic clues for early discovering post-stroke depression (44). In TB patients with mood problems, we found elevated peripheral blood inflammatory cells and their count-derived indices (NLR, MLR, PLR, and SII). Furthermore, higher SII was also a predictor of depression and anxiety in TB patients, highlighting more consistent associations of inflammation with mental disorders. The blood routine test is a must-check examination for all hospitalized patients. Peripheral blood cell count-derived indices, particularly SII show the prospect of being widely applied in clinical practice because of their convenience, economy and easily accessibility.
The present study has some limitations. First, the study is cross-sectional, which does not support an explanation for causality. Additionally, no distinction was made in TB type, therefore, potential inaccuracies could exist in data reporting. Lastly, analyzing the same indicators again at different times and exploring the correlation with depression or anxiety levels can improve the accuracy of matching markers.
Conclusion
The study reports depression and anxiety are prevalent in in-patients with TB. Close attention to the screening and treatment for psychological stress are important to patients with TB owing to its negatively impact on outcomes. We also found lower and higher concentrations of immune and inflammatory parameters in TB patients with depression or anxiety. The dysfunction of the immune-inflammation process among patients with depression or anxiety is stressed; particularly SII has a potential application value in guiding the evaluation and management of TB-related depression or anxiety. However, the time relationship between the indicators and psychopathology should be evaluated, and the true clinical effect of such markers should be confirmed.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by the Biomedical Research Ethics Committee of West China Hospital, Sichuan University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
XL and XT designed and supervised the study. XB, HL, and QY all participated in data collection. RR, YZ, and JH provided guidance on study design and methods. XL contributed to data analysis and writing the manuscript. LT and XT assisted in this article revision and writing. All authors contributed substantially to the study design, data interpretation, and the writing of the manuscript. All authors read and approved the final version of the article.
Funding
This work was supported by the Ministry of Science and Technology of the People's Republic of China (2021ZD0201900) and the National Natural Science Foundation of China (82120108002 and U21A20335).
Acknowledgments
The authors would like to acknowledge the volunteers who participated in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
TB, tuberculosis; PHQ-9, nine-item Patient Health Questionnaire; GAD-7, seven-item Generalized Anxiety Disorder Scale; Mtb, Mycobacterium tuberculosis; SII, systemic immune-inflammation index; NLR, neutrophil/lymphocyte ratio; MLR, monocyte/lymphocyte ratio; PLR, platelets/lymphocyte ratio; SD, standard deviations; DA, depression or anxiety; NDA, neither depression nor anxiety; BMI, body mass index.
References
1. Mushiroda T, Yanai H, Yoshiyama T, Sasaki Y, Okumura M, Ogata H, et al. Development of a prediction system for anti-tuberculosis drug-induced liver injury in Japanese patients. Hum Genome Var. (2016) 3:16014. doi: 10.1038/hgv.2016.14
2. World Health Organization. Global Tuberculosis Report 2021. (2021). Available online at: https://www.who.int/publications/digital/global-tuberculosis-report-2021 (accessed October 14, 2021).
3. Mohammedhussein M, Alenko A, Tessema W, Mamaru A. Prevalence and associated factors of depression and anxiety among patients with pulmonary tuberculosis attending treatment at public health facilities in Southwest Ethiopia. Neuropsychiatr Dis Treat. (2020) 16:1095–104. doi: 10.2147/NDT.S249431
4. Molebatsi K, Wang Q, Dima M, Ho-Foster A, Modongo C, Zetola N, et al. Depression and delayed tuberculosis treatment initiation among newly diagnosed patients in Botswana. Glob Public Health. (2021) 16:1088–98. doi: 10.1080/17441692.2020.1826049
5. Zhang K, Wang X, Tu J, Rong H, Werz O, Chen X. The interplay between depression and tuberculosis. J Leukoc Biol. (2019) 106:749–57. doi: 10.1002/JLB.MR0119-023R
6. Rouf A, Masoodi MA, Dar MM, Khan SMS, Bilquise R. Depression among tuberculosis patients and its association with treatment outcomes in district Srinagar. J Clin Tuberc Other Mycobact Dis. (2021) 25:100281. doi: 10.1016/j.jctube.2021.100281
7. Andrzej P, Marianne J, Markus S, Martin ER, Gunilla K. Tuberculosis and HIV co-infection. PLoS Pathog. (2012) 8:e1002464. doi: 10.1371/journal.ppat.1002464
8. Deveci F, Akbulut HH, Celik I, Muz MH, Ilhan F. Lymphocyte subpopulations in pulmonary tuberculosis patients. Mediators Inflamm. (2006) 2006:89070. doi: 10.1155/MI/2006/89070
9. Editorial Editorial Board of Chinese Journal of Antituberculosis Basic Basic and Clinical Speciality Academic Department of Tuberculosis Control Branch Branch of China International Exchange and Promotive Association for Medical and Health Care. Expert consensus on detection and clinical application of peripheral blood lymphocyte subsets in patients with tuberculosis. Chin J Antitubercul. (2020) 42:1009–16. doi: 10.3969/j.issn.1000-6621.2020.10.001
10. Balderas-Vazquez CL, Bernal-Morales B, Garcia-Montalvo EA, Vega L, Herrera-Huerta EV, Rodríguez-Landa JF, et al. Association between socio-affective symptoms and glutathione and CD4 and CD8 lymphocytes in college students. Front Psychol. (2021) 12:666347. doi: 10.3389/fpsyg.2021.666347
11. Meng G, Wang L, Wang X, Chi VTQ, Zhang Q, Liu L, et al. Association between neutrophil to lymphocyte ratio and depressive symptoms among Chinese adults: a population study from the Tclsih Cohort Study. Psychoneuroendocrinology. (2019) 103:76–82. doi: 10.1016/j.psyneuen.2019.01.007
12. Gao S, Quick C, Guasch-Ferre M, Zhuo Z, Hutchinson JM, Su L, et al. The association between inflammatory and oxidative stress biomarkers and plasma metabolites in a longitudinal study of healthy male welders. J Inflamm Res. (2021) 14:2825–39. doi: 10.2147/JIR.S316262
13. Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. (2020) 89:594–600. doi: 10.1016/j.bbi.2020.07.037
14. Renna ME, O'Toole MS, Spaeth PE, Lekander M, Mennin DS. The association between anxiety, traumatic stress, and obsessive-compulsive disorders and chronic inflammation: a systematic review and meta-analysis. Depress Anxiety. (2018) 35:1081–94. doi: 10.1002/da.22790
15. Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. (2001) 933:222–34. doi: 10.1111/j.1749-6632.2001.tb05827.x
16. Zulfic Z, Weickert CS, Weickert TW, Liu D, Myles N, Galletly C. Neutrophil-lymphocyte ratio - a simple, accessible measure of inflammation, morbidity and prognosis in psychiatric disorders? Australas Psychiatry. (2020) 28:454–8. doi: 10.1177/1039856220908172
17. Mazza MG, Lucchi S, Tringali AGM, Rossetti A, Botti ER, Clerici M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. (2018) 84(Pt A):229–36. doi: 10.1016/j.pnpbp.2018.03.012
18. Bartlett EK, Flynn JR, Panageas KS, Ferraro RA, Sta Cruz JM, Postow MA, et al. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with Pd-1 inhibitor monotherapy. Cancer. (2020) 126:76–85. doi: 10.1002/cncr.32506
19. Dionisie V, Filip GA, Manea MC, Movileanu RC, Moisa E, Manea M, et al. Neutrophil-to-lymphocyte ratio, a novel inflammatory marker, as a predictor of bipolar type in depressed patients: a quest for biological markers. J Clin Med. (2021) 10:1924. doi: 10.3390/jcm10091924
20. Wang JR, Chen Z, Yang K, Yang HJ, Tao WY, Li YP, et al. Association between neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and diabetic retinopathy among diabetic patients without a related family history. Diabetol Metab Syndr. (2020) 12:55. doi: 10.1186/s13098-020-00562-y
21. Díaz A, Bongiovanni B, D'Attilio L, Santucci N, Dídoli G, Fernández RDV, et al. The clinical recovery of tuberculosis patients undergoing specific treatment is associated with changes in the immune and neuroendocrine responses. Pathog Dis. (2017) 75:10. doi: 10.1093/femspd/ftx087
22. Afzal N, Javed K, Saleem uz Z, Mumtaz A, Hussain S, Tahir R, et al. Percentage of CD4+ and CD8+ T-lymphocytes in blood of tuberculosis patients. J Ayub Med Coll Abbottabad. (2010) 22:182–6.
23. Chen X, Wu R, Xu J, Wang J, Gao M, Chen Y, et al. Prevalence and associated factors of psychological distress in tuberculosis patients in Northeast China: a cross-sectional study. BMC Infect Dis. (2021) 21:563. doi: 10.1186/s12879-021-06284-4
24. Bresenham D, Kipp AM, Medina-Marino A. Quantification and correlates of tuberculosis stigma along the tuberculosis testing and treatment cascades in South Africa: a cross-sectional study. Infect Dis Poverty. (2020) 9:145. doi: 10.1186/s40249-020-00762-8
25. Kroenke K, Spitzer RL, Williams JB, Löwe B. The patient health questionnaire somatic, anxiety, and depressive symptom scales: a systematic review. Gen Hosp Psychiatry. (2010) 32:345–59. doi: 10.1016/j.genhosppsych.2010.03.006
26. Kroenke K, Wu J, Yu Z, Bair MJ, Kean J, Stump T, et al. Patient health questionnaire anxiety and depression scale: initial validation in three clinical trials. Psychosom Med. (2016) 78:716–27. doi: 10.1097/PSY.0000000000000322
27. Febi AR, Manu MK, Mohapatra AK, Praharaj SK, Guddattu V. Psychological stress and health-related quality of life among tuberculosis patients: a prospective cohort study. ERJ Open Res. (2021) 7:00251-2021. doi: 10.1183/23120541.00251-2021
28. Marazziti D, Torrigiani S, Carbone MG, Mucci F, Flamini W, Ivaldi T, et al. Neutrophil/lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in mood disorders. Curr Med Chem. (2022) 29:5758–81. doi: 10.2174/0929867328666210922160116
29. Duko B, Gebeyehu A, Ayano G. Prevalence and correlates of depression and anxiety among patients with tuberculosis at Wolaitasodo University Hospital and Sodo Health Center, Wolaitasodo, South Ethiopia, Cross Sectional Study. BMC Psychiatry. (2015) 15:214. doi: 10.1186/s12888-015-0598-3
30. Sunjaya DK, Paskaria C, Pramayanti M, Herawati DMD, Parwati I. The magnitude of anxiety and depressive symptoms among tuberculosis patients in community health centers setting during the peak of COVID-19 pandemic. J Multidiscip Healthc. (2022) 15:755–64. doi: 10.2147/JMDH.S359530
31. Duko B, Bedaso A, Ayano G. The prevalence of depression among patients with tuberculosis: a systematic review and meta-analysis. Ann Gen Psychiatry. (2020) 19:30. doi: 10.1186/s12991-020-00281-8
32. Srinivasan G, Chaturvedi D, Verma D, Pal H, Khatoon H, Yadav D, et al. Prevalence of depression and anxiety among drug resistant tuberculosis: a study in North India. Indian J Tuberc. (2021) 68:457–63. doi: 10.1016/j.ijtb.2021.04.010
33. Chandra M, Rana P, Chandra K, Arora VK. Tuberculosis - depression syndemic: a public health challenge. Indian J Tuberc. (2019) 66:197–202. doi: 10.1016/j.ijtb.2019.02.007
34. Wang XB, Li XL, Zhang Q, Zhang J, Chen HY, Xu WY, et al. A survey of anxiety and depressive symptoms in pulmonary tuberculosis patients with and without tracheobronchial tuberculosis. Front Psychiatry. (2018) 9:308. doi: 10.3389/fpsyt.2018.00308
35. Liu K, Zhang Y, Qu S, Yang W, Guo L, Zhang L. Prevalence and correlates of anxiety and depressive symptoms in patients with and without multi-drug resistant pulmonary tuberculosis in China. Front Psychiatry. (2021) 12:674891. doi: 10.3389/fpsyt.2021.674891
36. Yew WW, Yoshiyama T, Leung CC, Chan DP. Epidemiological, clinical and mechanistic perspectives of tuberculosis in older people. Respirology. (2018) 23:567–75. doi: 10.1111/resp.13303
37. Caraux-Paz P, Diamantis S, de Wazières B, Gallien S. Tuberculosis in the elderly. J Clin Med. (2021) 10:5888. doi: 10.3390/jcm10245888
38. Yan Y, Du X, Lai L, Ren Z, Li H. Prevalence of depressive and anxiety symptoms among Chinese older adults during the COVID-19 pandemic: a systematic review and meta-analysis. J Geriatr Psychiatry Neurol. (2022) 35:182–95. doi: 10.1177/08919887221078556
39. Li D, Zhang DJ, Shao JJ, Qi XD, Tian L. A meta-analysis of the prevalence of depressive symptoms in Chinese older adults. Arch Gerontol Geriatr. (2014) 58:1–9. doi: 10.1016/j.archger.2013.07.016
40. Fang XH, Wu Q, Tao SS, Xu ZW, Zou YF, Ma DC, et al. Social support and depression among pulmonary tuberculosis patients in Anhui, China. J Multidiscip Healthc. (2022) 15:595–603. doi: 10.2147/JMDH.S356160
41. Qin S, Chen R, Jiang Y, Zhu H, Chen L, Chen Y, et al. Multifunctional T cell response in active pulmonary tuberculosis patients. Int Immunopharmacol. (2021) 99:107898. doi: 10.1016/j.intimp.2021.107898
42. Fan KQ, Li YY, Wang HL, Mao XT, Guo JX, Wang F, et al. Stress-induced metabolic disorder in peripheral CD4(+) T cells leads to anxiety-like behavior. Cell. (2019) 179:864–79.e19. doi: 10.1016/j.cell.2019.10.001
43. Rhie SJ, Jung EY, Shim I. The role of neuroinflammation on pathogenesis of affective disorders. J Exerc Rehabil. (2020) 16:2–9. doi: 10.12965/jer.2040016.008
44. Hu J, Wang L, Fan K, Ren W, Wang Q, Ruan Y, et al. The association between systemic inflammatory markers and post-stroke depression: a prospective stroke cohort. Clin Interv Aging. (2021) 16:1231–9. doi: 10.2147/CIA.S314131
45. Tang P, Liang E, Zhang X, Feng Y, Song H, Xu J, et al. Prevalence and risk factors of subclinical tuberculosis in a low-incidence setting in China. Front Microbiol. (2021) 12:731532. doi: 10.3389/fmicb.2021.731532
46. Sahin F, Yazar E, Yildiz P. Prominent features of platelet count, plateletcrit, mean platelet volume and platelet distribution width in pulmonary tuberculosis. Multidiscip Respir Med. (2012) 7:38. doi: 10.4081/mrm.2012.625
Keywords: tuberculosis, depression, anxiety, immune, inflammation
Citation: Liu X, Bai X, Ren R, Tan L, Zhang Y, Lan H, Yang Q, He J and Tang X (2022) Association between depression or anxiety symptoms and immune-inflammatory characteristics in in-patients with tuberculosis: A cross-sectional study. Front. Psychiatry 13:985823. doi: 10.3389/fpsyt.2022.985823
Received: 04 July 2022; Accepted: 03 October 2022;
Published: 20 October 2022.
Edited by:
S. M. Yasir Arafat, Enam Medical College, BangladeshReviewed by:
Isela Juarez-Rojop, Universidad Juárez Autónoma de Tabasco, MexicoVictor Lasebikan, University of Ibadan, Nigeria
Copyright © 2022 Liu, Bai, Ren, Tan, Zhang, Lan, Yang, He and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangdong Tang, MjM3MjU2NDYxM0BxcS5jb20=
 Xiangmin Liu
Xiangmin Liu Xinyu Bai1
Xinyu Bai1 Rong Ren
Rong Ren Ye Zhang
Ye Zhang Xiangdong Tang
Xiangdong Tang
