
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry , 04 November 2022
Sec. Public Mental Health
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.984677
This article is part of the Research Topic Sport and Exercise: Challenges and Perspectives in Mental Health View all 11 articles
 Mengqi Yuan1,2†
Mengqi Yuan1,2† Hongyang Chen1,2†
Hongyang Chen1,2† Dongmei Chen2†
Dongmei Chen2† Donggui Wan2
Donggui Wan2 Fan Luo1,2
Fan Luo1,2 Chenyang Zhang1,2
Chenyang Zhang1,2 Yunxin Nan1,2
Yunxin Nan1,2 Xiaoning Bi3*
Xiaoning Bi3* Jing Liang3*
Jing Liang3*Background: Physical activity (PA) is considered a favorable preventive intervention for postpartum depression (PPD), but evidence defining a corresponding dose-response relationship is lacking. This meta-analysis was conducted to assess the protective effects of PA on PPD and define a potential dose-response relationship between them.
Methods: PubMed, Medline, Embase, and Web of Science were searched from 1968 to May 2022. Only randomized control trials (RCTs) and prospective studies were considered, and the PICOS tool was used to identify eligible articles based on the inclusion and exclusion criteria. Effect-size estimates were unified as odds ratio (OR) and 95% confidence interval (CI). We calculated the ORs and their 95% CI for studies that did not report them using the Practical Meta-Analysis Effect Size Calculator.
Results: A total of 23 studies were eligible, including 14 RCTs and 9 prospective cohort studies. The overall analysis showed a statistically significant positive association between PA and PPD prevention (adjusted OR = 0.73; 95% CI: 0.61–0.87; P < 0.001). Subgroup analyses indicated that studies conducted in Europe demonstrated a significant correlation between PA and reduced PPD risk (adjusted OR = 0.85, 95% CI: 0.76–0.95, P = 0.004). Concerning PA type, sports activity was associated with relieving PPD symptoms (adjusted OR = 0.89, 95% CI: 0.78 to 1.00, P < 0.001), while work (adjusted OR = 1.05, 95% CI: 0.37–2.97, P = 0.065) and household activities (adjusted OR = 1.16, 95% CI: 0.89–1.52, P = 0.986) contributed to a greater risk of PPD. Our dose-response analysis revealed a reverse J-shaped trend between ascending PA duration and PPD incidence.
Conclusion: This meta-analysis identified PA as a potential intervention to reduce the risk of PPD. The dose-response analysis revealed that at least 90 min of PA per week could efficiently decrease the risk of PPD.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022335731.
Postpartum depression (PPD) is a specific type of mental disorder that might occur up to 1 year after giving birth and affects 5–25% of new mothers (1). However, ~50% of the affected mothers remain undiagnosed by healthcare professionals (2). As a severe mental health issue that may severely affect women and their families worldwide, PPD might disrupt the mother-infant relationship and negatively impact the child's cognitive, behavioral, and social-emotional development in the long term (3–5). In severe cases, women with PPD may develop suicidal tendencies and even harm their babies (6–8). Many causes have been attributed to PPD, including physiological, situational, or social difficulties (9). The major predisposing factors include stressful life events, child-care stress, prenatal anxiety, prior episodes of PPD (10), marital conflicts, and single-parent households (11).
At present, among the many interventions recommended for treating PPD are antidepressant medications, cognitive behavioral therapy (CBT), psychosocial support through support groups, electroconvulsive therapies, and complementary therapy (11, 12). However, antidepressants might affect the infants' health via breastfeeding, and the other three therapies are usually expensive, time-consuming, and require a lot of energy that might not be at the disposal of the suffering mother. Thus, newer and more efficient primary PPD prevention strategies are required to improve the recovery process of these patients.
Complementary therapy has been recognized as an effective method for the prevention and treatment of chronic diseases (13). Nigella sativa, a kind of herb, is helpful in the treatment of asthma, liver and kidney disease, influenza, and gastrointestinal problems (14). Phytosterols, a group of natural compounds of plant-cell membranes, could be easily obtained from the diet and have been proven to decrease the risk of chronic diseases, such as cardiovascular diseases, obesity, and diabetes (15). Like other complementary therapies, physical activities (PA) have an advantage in improving the conditions of many chronic diseases, such as cognitive impairment, coronary artery disease, diabetes, and depression (16, 17).
The three main categories of PA during pregnancy include (1) physical exercise, a subset of planned, structured, and repetitive PA, such as aerobic exercise, stretching and breathing exercises, walking programs, strength exercises, pilates, and yoga; (2) housework PA, such as child care and household chores; and (3) work activities. The National Physical Activity Guidelines for Americans, released in 2008, encourages women to participate in 150–300 min of moderate-intensity aerobic activities per week during pregnancy and after delivery (18). In addition, the American College of Obstetricians and Gynecologists (ACOG) recommends 20–30 min of daily moderate-intensity PA to stay healthy during pregnancy (19). In 2014, a randomized controlled trial (RCT) conducted in America reported that 90 min of yoga per week could help reduce the risk of PPD (20), and in 2019, RCTs performed in Spain suggested that 180 min of exercise per week were needed to prevent PPD (21, 22). In contrast, a study from Northern Taiwan showed that depressive symptoms were not affected by the duration or frequency of PA (23). Thus, the optimal duration of PA that could help reduce the risk of PPD remains undetermined.
In this study, we conducted a meta-analysis to determine the effects of PA on PPD and to define the dose-response relationship between them to provide more detailed evidence for health authorities and pregnant women to reduce the risk of PPD.
This meta-analysis was conducted following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (24) and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) statement (25). Both the PRISMA and MOOSE checklists used in this study are shown in Supplementary Table 1. The study protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42022335731).
A literature search was performed from 1968 to May 2022 on PubMed, Medline, Embase, and Web of Science databases. The PICOS principle of this meta-analysis was as follows: (P) Population involving women who were pregnant or up to 1 year postpartum; (I) Intervention involving all kinds of physical activities; (C) Comparator including standard prenatal and postpartum care; (O) Outcomes dicussing the effect of PA on PPD symptoms; and (S) Study type including randomized controlled trials (RCTs) and prospective cohort studies. The detailed search terms are presented in the Supplementary Table 2.
Qualified articles were restricted to the following criteria: (1) study participants including women who were pregnant or up to 1 year postpartum; (2) endpoints including the effects of PA on PPD symptoms, identified via a structured clinical interview and a validated tool [e.g., Edinburgh Postnatal Depression Scale, EPDS (26)] or judged by a healthcare professional (e.g., general practitioner or psychiatrist); (3) study type including RCTs and prospective cohort studies; (4) intervention including any type of PA during or after pregnancy; and (5) language including studies published in English. Studies were excluded if they (1) did not contain data of interest for this meta-analysis; (2) were duplicated publications; (3) were reviews, case reports, or comments; or (4) contained animal or genomics experiments.
Endnote version 20 was used for literature management to file the literature search records. The study selection process, conducted by two authors (M. Y. and H. C.), was performed following these three steps: First, two authors screened the titles of all the recovered articles and removed duplicates. An article was included if at least one reviewer judged it qualified. If there were any doubts about the studies, they were included in the abstract review phase. Second, the selected studies were filtrated by reviewing their abstracts and reevaluated by the two independent reviewers according to the inclusion and exclusion criteria. Finally, using standardized eligibility criteria, the two independent reviewers assessed the full texts of potentially relevant studies. If the reviewers disagreed on any of the three steps, a third independent reviewer from our group intervened to make a judgment after mutual discussions.
The two reviewers (M. Y. and H. C) separately extracted the following data from the eligible studies: first author, year of publication, the country where the study was conducted, gender, sample size, study type, age of the population, PA type, frequency and duration of PA, PA intensity, and assessment of PPD.
The two authors independently evaluated all eligible studies for bias using the Risk of Bias in Non-Randomized Studies of Interventions (ROBINS-I) assessment tool (27), which contained seven different types of bias and divided each part into five grades of risk of bias (ROB). Eligible articles were classified into three levels based on the number of components for which high ROB potentially existed, namely, high risk (five or more), moderate risk (three or four), and low risk (two or less).
STATA software version 14.1 for Windows (Stata Corp, College Station, TX, USA) was used for data management and analysis. A two-sided P-value of < 0.05 was considered significantly different. The effect size of this meta-analysis was odds ratio (OR). For the studies that did not report ORs, we calculated their ORs and corresponding 95% confidence intervals (95% CI) using the Practical Meta-Analysis Effect Size Calculator (28). Generalized least squares regression (29, 30), which was proposed by Greenland and Longnecker, was used to estimate the dose-response relationship by summarizing dose-response data. Furthermore, the nonlinearity test between PA duration and risk of PPD was calculated using restricted cubic splines of exposure distribution with 3 knots (25, 50, and 75th percentiles).
The inconsistency index (I2) statistic was used to quantify potential heterogeneity among the studies. Obvious heterogeneity was determined for I2 ≥50%. Publication bias was assessed using Begg's funnel plots and Egger regression asymmetry tests. The trim-and-fill method was applied to reckon the number of missing studies in theory. Sensitivity analysis was performed to judge the solidity of the overall results by consecutively omitting the study.
To clarify the possible causes of between-study heterogeneity from clinical and methodological perspectives, prespecified subgroup analyses were performed based on the study region, the age of the participants, gestation age of the pregnant participants, activity duration for a week, PA types, PA intensity, PA timing, and study type.
A total of 1,591 articles were initially retrieved after screening the public database using the predefined retrieval code. Of them, 23 (14 RCTs and 9 prospective cohort studies) met the inclusion criteria of this study, comprising a total of 186,412 subjects. The detailed selection process is presented in Figure 1.
The baseline characteristics of the 23 (20–22, 28, 31–49) eligible studies are displayed in Table 1. Of them, 12 were conducted in Europe (21, 33, 36–38, 40, 41, 44, 45, 47, 48), 6 in America (20, 32, 34, 42, 43, 46), and 5 in Asia (28, 31, 35, 39, 49). Of the 14 RCT studies (20–22, 28, 31–40), 4,607 participants with 1,722 cases were selected in the meta-analysis. Among the 9 prospective cohort studies (41–49), 181,805 participants with 43,069 cases were analyzed.
The items of the ROB assessment of each study are listed in Supplementary Table 3. The ROB rating was low in twenty-two studies and moderate in one study. Regarding confounding bias, 14 articles were determined as low risk, 8 as moderate risk, and one as high risk. An assessment of bias of the selection identified only one article as moderate risk but 22 as low risk. Bias resulting from deviation from the intended interventions was low in all studies, as well as bias in outcome measurement and selection.
When the results of all eligible articles (Table 2) were synthesized, we found a statistically significant positive association between PA and reduction of PPD risk (adjusted OR = 0.73; 95% CI: 0.61–0.87; P < 0.001) (Figure 2).
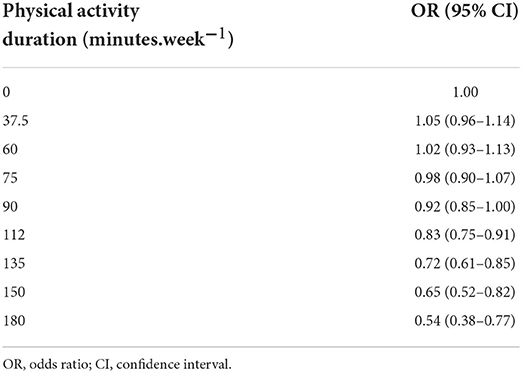
Table 2. The does-response analysis of physical activity duration and the risk of postpartum depression.
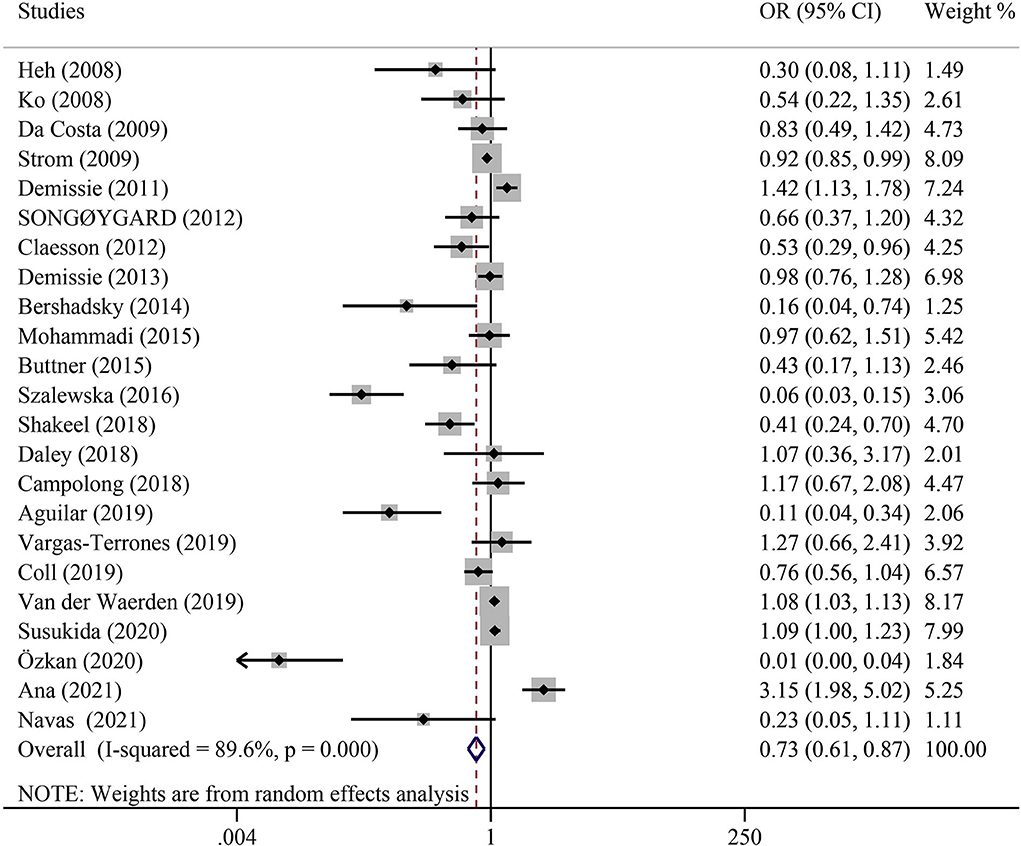
Figure 2. Overall analysis of the association between physical activity and postpartum depression with odds ratio (OR) and 95% confidence interval (CI).
Publication bias was assessed using Begg's funnel plot and Egger's test. The results showed that Begg's funnel plots were not perfectly symmetrical (Figure 3), Egger's test demonstrated potential publication bias (P = 0.014), and no potentially missing study was shown by a further filled funnel plot.
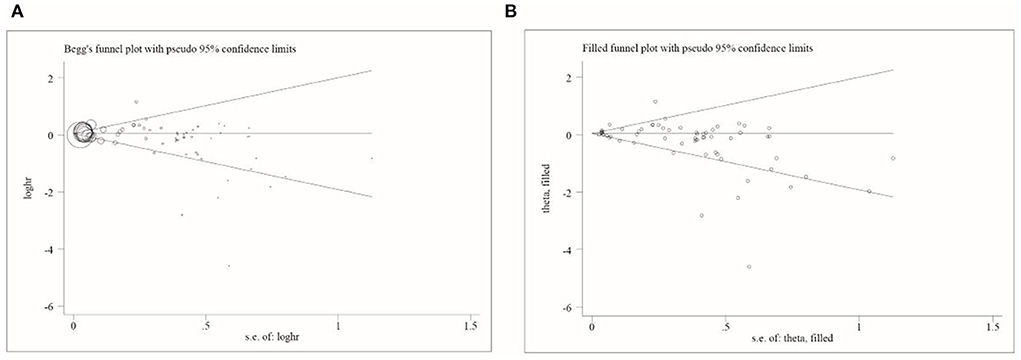
Figure 3. Begg's and filled funnel plots in included studies. (A) Begg's funnel plot and (B) filled funnel plot.
A sensitivity analysis was performed to assess the influence of individual studies on the pooled ORs. By omitting one study each time, the results revealed that the corresponding pooled ORs did not change, which confirmed the reliability of our results.
Our dose-response analysis of the relationship between PA duration and the risk of PPD demonstrated an inverted J-shaped trend (Figure 4). The results indicated that longer PA duration decreased the risk of PPD, and when the PA duration reached 90 min per week (adjusted OR = 0.92, 95% CI: 0.85–1.00), an efficient decrease in the risk of PPD was observed (Table 2).

Figure 4. The dose response analysis of physical activity duration and the risk of postpartum depression.
Subgroup analyses were performed to clarify the cause of between-study heterogeneity (Table 3). Significant heterogeneities were found in the study region, age, gestation age, PA duration, PA type, PA intensity, PA timing, and study type.
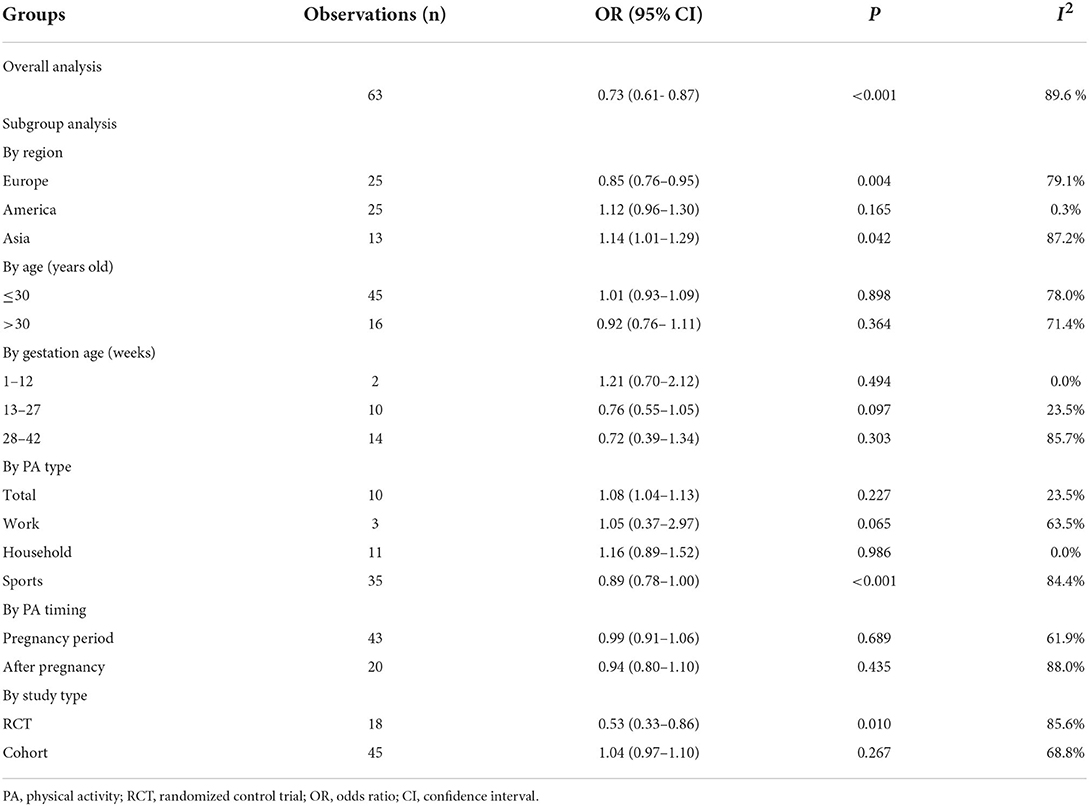
Table 3. Overall and subgroup analyses of the associations between physical activity and postpartum depression.
According to region, our results showed that studies conducted in Europe demonstrated a significant correlation between PA and reduced PPD risk (adjusted OR = 0.85, 95% CI: 0.76–0.95, P = 0.004), whereas studies conducted in America (adjusted OR = 1.12, 95% CI: 0.96–1.30, P = 0.165) and Asia (adjusted OR = 1.14, 95% CI: 1.01–1.29, P = 0.004) displayed a negative association between PA and decreasing PPD risk, with a statistical significance observed in the Asian subgroup.
According to the age of pregnant women, women older than 30 years demonstrated a downward trend of PPD (adjusted OR = 0.92, 95% CI: 0.76–1.11, P = 0.364), although the result was not statistically significant. In terms of gestation age of the pregnant participants, an obvious decrease in trend was observed for women in their second trimester (13–27 weeks) (adjusted OR = 0.76, 95% CI: 0.55–1.05, P = 0.097) and third trimester (28–42 weeks) (adjusted OR = 0.72, 95% CI: 0.39–1.34, P = 0.303).
Next, we divided PA into three major categories, namely, sports, work, and household activities. All kinds of physical exercises, such as yoga, walking, ball games, pilates, and other aerobics workouts, were included in sports activities. Work activities are the amount of muscle- and energy-demanding activities at work. All kinds of housework and childcare were included in household activities. According to our analysis of PA type, sports activities were associated with relieving PPD symptoms (adjusted OR = 0.89, 95% CI: 0.78–1.00, P < 0.001). However, work (adjusted OR = 1.05, 95% CI: 0.37–2.97, P = 0.065) and household activities (adjusted OR = 1.16, 95% CI: 0.89–1.52, P = 0.986) were identified as risk factors of PPD.
According to PA timing, PA during pregnancy (adjusted OR = 0.99, 95% CI: 0.91–1.06, P = 0.689) or after pregnancy (adjusted OR = 0.94, 95% CI: 0.80–1.10, P = 0.435) demonstrated a downward trend for PPD risk. Study type analysis showed that the results of the RCTs demonstrated a statistically significant association between PA and reduction of PPD risk (adjusted OR = 0.53, 95% CI: 0.33–0.86, P = 0.010). In contrast, cohort studies suggested that PA could be regarded as a risk factor for PPD (adjusted OR = 1.04, 95% CI: 0.97–1.10, P = 0.267), though the result was not statistically significant.
Based on our literature search and existing knowledge, the present study is the first to investigate the connection between PA and PPD through a dose-response meta-analysis. The main finding of this meta-analysis was that PA was positively associated with a reduced risk of PPD. Synchronously, sensitivity and subgroup analyses revealed a robust association between PA and PPD prevention. Moreover, the dose-response analysis showed a reduced risk of PPD with a longer PA duration and that at least 90 min of PA per week could efficiently prevent PPD.
Subgroup analysis of the study regions indicated that PA was not associated with a reduced risk of PPD in women from Asia, while contradictory results were observed for European women. Previous studies verified that societal differences could influence the incidence of PPD. European and Australian women were observed to have a lower risk of PPD, but women from Asia were osbserved to have higher risks (50, 51). PPD could be caused by diverse factors, including financial problems, cultural differences, and social status. In some Asian countries, such as China, Singapore, and Vietnam, “doing the month” is a tradition for new mothers to recover from the effects of pregnancy. However, such cultural practices might have two-sided effects as, despite providing physical comforts, they can also play a major role in interpersonal conflicts and emotional frustration (52). Existing evidence observed few psychological benefits for new mothers. In Singapore, a prospective cohort study involving 278 new mothers discovered that ~33% of the participants demonstrated negative experiences with such practices during confinement, which significantly contributed to their depression (53). Similarly, a cross-sectional survey including 506 Vietnamese women revealed that, despite following traditional practices, new mothers still suffered from depression symptoms (54). Based on these studies, it is indicated that the occurrence and progression of PPD might be multifactorial.
Different types of PA have different effects on the prevention of PPD. This meta-analysis proved that sports activities played an obvious role in the prevention of PPD, while work and household activities were potential risk factors. It also reported that extensive physical exercise could reduce the risk of depression in pregnant women. An RCT enrolling 57 postpartum women found that yoga was a promising prevention activity for PPD (34). In 2017, a meta-analysis of 13 RCTs demonstrated that aerobic exercise could reduce the risk of PPD (55). Alhough the mechanisms of sports having an impact in reducing PPD risk are not yet fully understood, several hypotheses have been pooled and proposed. From the perspective of biological mechanisms, it is hypothesized that sports could alleviate PPD by enhancing beta-endorphin levels, which are related to improved mood, euphoria, and passion, as well as reduced pain and increased levels of brain neurotransmitters, including dopamine and noradrenaline,which are associated with feelings of satisfaction and euphoria, respectively (56). The neurotrophic factor hypothesis suggests another possible mechanism for PPD reduction. As a necessary neurotrophic factor for normal neural structure development, synaptic transmission maturation, and maintenance, the brain-derived neurotrophic factor (BNDF) is at low levels in patients with depression. However, a study by Aguiar et al. reported that exercise could significantly increase the expression of BDNF mRNA and the level of BDNF in rats and enhance the synaptic plasticity of the hippocampus by increasing the level of BDNF (57). In 2009, Agarwal et al. found that exercise could reduce oxidative stress in rats, suggesting exercise as an influencing factor of oxidative stress, which was a primary cause of depression (58). However, work and household activities were considered strenuous and repetitive, countering the benefits of PA. In contrast, performing involuntary PAs such as work and household activities may be intense; therefore, it is easy to elevate depressive symptoms rather than alleviate them (59, 60).
The majority of the reviewed literature indicated that a greater amount of PA is likely to reduce the risk of depression, concordant with recommendations given in the national physical activity guidelines (i.e., 30 min of moderate-intensity physical activity on most, if not all, days of the week) (61), which indicated that benefits could also be observed for a lower amount of PA, i.e., at least 20–60 min PA/week (62, 63). However, the appropriate duration of PA for pregnant women remains undetermined. Our dose-response analysis revealed that at least 90 min of PA per week, except for household and work activities, could effectively reduce the risk of PPD and improve the mental wellbeing of women.
Previously, Nakamura and Waerden conducted a meta-analysis of 17 articles, including 6 RCTs and 11 observation studies, to assess the effects of PA during pregnancy on PPD (64). They reported that PA was a significant factor in reducing the risk of PPD. Based on their meta-analysis, we expanded the original literature to 23 articles and included only RCT and prospective cohort studies to increase the evidence level of our meta-analysis. To specifically quantify the effects of PA on PPD, the effect size was unified as OR, and a dose-response analysis was conducted, which showed that at least 90 min of PA per week could efficiently prevent PPD. Furthermore, the refinement of the PA type provided a more specific suggestion for new mothers.
There are some limitations to this study. First, only English-language articles were analyzed, possibly leading to a certain level of publication bias. Second, the error of dose-response analysis persisted and was inevitable in secondary analysis; however, the overall reverse J-shaped trend deserves further attention in the association of PA and the risk of PPD. Third, significant heterogeneity was observed in some subgroups, restricting the explanation of pooled effect-size estimates. Finally, since most of the original articles did not report detailed statistics on PA intensity, the relationship between PA intensity and PPD could not be explored in this meta-analysis, and further studies are still needed to fill this gap.
In summary, PA was identified as a potentially beneficial intervention to reduce the risk of PPD, even though no statistical significance was observed in this meta-analysis. Dose-response analysis between PA and the risk of PPD showed a reduced risk of PPD with a longer PA duration. Furthermore, 90 min of PA per week could efficiently reduce the risk of PPD.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Conceived and designed the experiments: DW, JL, and XB. Performed the experiments: MY, HC, DC, and CZ. Analyzed the data: MY, FL, XB, and HC. Contributed materials and analysis tools: MY, CZ, DC, FL, and YN. Wrote and revised the article: MY, JL, and DW. All authors read and approved the final manuscript before submission.
This work was funded by the Clinical Study on Prevention of Recurrence of Uterine Fibroids After Minimally Invasive Surgery by Integrated Traditional Chinese and Western Medicine (2022-NHLHCRF-LX-02-0116).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.984677/full#supplementary-material
1. O'Hara MW, McCabe JE. Postpartum depression: current status and future directions. Annu Rev Clin Psychol. (2013) 9:379–407. doi: 10.1146/annurev-clinpsy-050212-185612
2. Peindl KS, Wisner KL, Hanusa BH. Identifying depression in the first postpartum year: guidelines for office-based screening and referral. J Affect Disord. (2004) 80:37–44. doi: 10.1016/S0165-0327(03)00052-1
3. Field T. Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav Dev. (2010) 33:1–6. doi: 10.1016/j.infbeh.2009.10.005
4. Goodman JH. Women's attitudes, preferences, and perceived barriers to treatment for perinatal depression. Birth. (2009) 36:60–9. doi: 10.1111/j.1523-536X.2008.00296.x
5. Tronick E, Reck C. Infants of depressed mothers. Harv Rev Psychiatry. (2009) 17:147–56. doi: 10.1080/10673220902899714
6. Shi P, Ren H, Li H, Dai Q. Maternal depression and suicide at immediate prenatal and early postpartum periods and psychosocial risk factors. Psychiatry Res. (2018) 261:298–306. doi: 10.1016/j.psychres.2017.12.085
7. Do T, Hu Z, Otto J, Rohrbeck P. Depression and suicidality during the postpartum period after first time deliveries, active component service women and dependent spouses, U.S. Armed Forces, 2007–2012. MSMR. (2013) 20:2–7.
8. Collardeau F, Corbyn B, Abramowitz J, Janssen PA, Woody S, Fairbrother N. Maternal unwanted and intrusive thoughts of infant-related harm, obsessive-compulsive disorder and depression in the perinatal period: study protocol. BMC Psychiatry. (2019) 19:94. doi: 10.1186/s12888-019-2067-x
9. Fishel AH. Mental health disorders and substance abuse. Matern Women's Health Care. (2004) 2004:960–82.
10. Leopold KA ZL. Women's primary health grand rounds at the University of Michigan: postpartum depression. Female Patient Total Health Care Women Int. (1997) 22:12–30.
11. Andrews-Fike C. A Review of Postpartum Depression. Prim Care Companion J Clin Psychiatry. (1999) 1:9–14. doi: 10.4088/PCC.v01n0103
12. Appleby L, Warner R, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive-behavioural counselling in the treatment of postnatal depression. BMJ. (1997) 314:932–6. doi: 10.1136/bmj.314.7085.932
13. Falci L, Shi Z, Greenlee H. Multiple chronic conditions and use of complementary and alternative medicine among US adults: results from the 2012 national health interview survey. Prev Chronic Dis. (2016) 13:E61. doi: 10.5888/pcd13.150501
14. Hadi V, Pahlavani N, Malekahmadi M, Nattagh-Eshtivani E, Navashenaq JG, Hadi S, et al. Nigella sativa in controlling Type 2 diabetes, cardiovascular, and rheumatoid arthritis diseases: molecular aspects. J Res Med Sci. (2021) 26:20. doi: 10.4103/jrms.JRMS_236_20
15. Nattagh-Eshtivani E, Barghchi H, Pahlavani N, Barati M, Amiri Y, Fadel A, et al. Biological and pharmacological effects and nutritional impact of phytosterols: a comprehensive review. Phytother Res. (2022) 36:299–322. doi: 10.1002/ptr.7312
16. Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry. (2009) 31:306–15. doi: 10.1016/j.genhosppsych.2009.04.002
17. Lysy Z, Da Costa D, Dasgupta K. The association of physical activity and depression in Type 2 diabetes. Diabet Med. (2008) 25:1133–41. doi: 10.1111/j.1464-5491.2008.02545.x
18. US Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Washington, DC: US Department of Health and Human Services (2008).
19. ACOG Committee Opinion No. 650. Physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol. (2015) 126:e135–42. doi: 10.1097/AOG.0000000000001214
20. Bershadsky S, Trumpfheller L, Kimble HB, Pipaloff D, Yim IS. The effect of prenatal Hatha yoga on affect, cortisol and depressive symptoms. Complement Ther Clin Pract. (2014) 20:106–13. doi: 10.1016/j.ctcp.2014.01.002
21. Aguilar-Cordero MJ, Sánchez-García JC, Rodriguez-Blanque R, Sánchez-López AM, Mur-Villar N. Moderate physical activity in an aquatic environment during pregnancy (SWEP study) and its influence in preventing postpartum depression. J Am Psychiatr Nurses Assoc. (2019) 25:112–21. doi: 10.1177/1078390317753675
22. Vargas-Terrones M, Barakat R, Santacruz B, Fernandez-Buhigas I, Mottola MF. Physical exercise programme during pregnancy decreases perinatal depression risk: a randomised controlled trial. Br J Sports Med. (2019) 53:348–53. doi: 10.1136/bjsports-2017-098926
23. Chang SH, Chien NH, Chen MC. Regular exercise and depressive symptoms in community-dwelling elders in Northern Taiwan. J Nurs Res. (2016) 24:329–36. doi: 10.1097/JNR.0000000000000117
24. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
25. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. (2000) 283:2008–12. doi: 10.1001/jama.283.15.2008
26. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. (1987) 150:782–6. doi: 10.1192/bjp.150.6.782
27. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
28. Ko YL, Yang CL, Chiang LC. Effects of postpartum exercise program on fatigue and depression during “doing-the-month” period. J Nurs Res. (2008) 16:177–86. doi: 10.1097/01.jnr.0000387304.88998.0b
29. Greenland S LM. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. (1992) 135:1301–9. doi: 10.1093/oxfordjournals.aje.a116237
30. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. (2012) 175:66–73. doi: 10.1093/aje/kwr265
31. Heh SS, Huang LH, Ho SM, Fu YY, Wang LL. Effectiveness of an exercise support program in reducing the severity of postnatal depression in Taiwanese women. Birth. (2008) 35:60–5. doi: 10.1111/j.1523-536X.2007.00192.x
32. Da Costa D, Lowensteyn I, Abrahamowicz M, Ionescu-Ittu R, Dritsa M, Rippen N, et al. A randomized clinical trial of exercise to alleviate postpartum depressed mood. J Psychosom Obstet Gynaecol. (2009) 30:191–200. doi: 10.1080/01674820903212136
33. Songøygard KM, Stafne SN, Evensen KA, Salvesen K, Vik T, Mørkved S. Does exercise during pregnancy prevent postnatal depression? A randomized controlled trial. Acta Obstet Gynecol Scand. (2012) 91:62–7. doi: 10.1111/j.1600-0412.2011.01262.x
34. Buttner MM, Brock RL, O'Hara MW, Stuart S. Efficacy of yoga for depressed postpartum women: a randomized controlled trial. Complement Ther Clin Pract. (2015) 21:94–100. doi: 10.1016/j.ctcp.2015.03.003
35. Mohammadi F, Malakooti J, Babapoor J, Mohammad-Alizadeh-Charandabi S. The effect of a home-based exercise intervention on postnatal depression and fatigue: a randomized controlled trial. Int J Nurs Pract. (2015) 21:478–85. doi: 10.1111/ijn.12259
36. Daley A, Riaz M, Lewis S, Aveyard P, Coleman T, Manyonda I, et al. Physical activity for antenatal and postnatal depression in women attempting to quit smoking: randomised controlled trial. BMC Pregnancy Childbirth. (2018) 18:156. doi: 10.1186/s12884-018-1784-3
37. Coll CVN, Domingues MR, Stein A, da Silva BGC, Bassani DG, Hartwig FP, et al. Efficacy of regular exercise during pregnancy on the prevention of postpartum depression: the PAMELA randomized clinical trial. JAMA Netw Open. (2019) 2:e186861. doi: 10.1001/jamanetworkopen.2018.6861
38. Özkan SA, Kücükkelepce DS, Korkmaz B, Yilmaz G, Bozkurt MA. The effectiveness of an exercise intervention in reducing the severity of postpartum depression: a randomized controlled trial. Perspect Psychiatr Care. (2020) 56:844–50. doi: 10.1111/ppc.12500
39. Ana Y, Lewis MG, van Schayck OCP, Babu GR. Is physical activity in pregnancy associated with prenatal and postnatal depressive symptoms?: Results from MAASTHI cohort study in South India. J Psychosom Res. (2021) 144:110390. doi: 10.1016/j.jpsychores.2021.110390
40. Navas A, Carrascosa MDC, Artigues C, Ortas S, Portells E, Soler A, et al. Effectiveness of moderate-intensity aerobic water exercise during pregnancy on quality of life and postpartum depression: a multi-center, randomized controlled trial. J Clin Med. (2021) 10:2432. doi: 10.3390/jcm10112432
41. Strøm M, Mortensen EL, Halldorson TI, Osterdal ML, Olsen SF. Leisure-time physical activity in pregnancy and risk of postpartum depression: a prospective study in a large national birth cohort. J Clin Psychiatry. (2009) 70:1707–14. doi: 10.4088/JCP.09m05012blu
42. Demissie Z, Siega-Riz AM, Evenson KR, Herring AH, Dole N, Gaynes BN. Associations between physical activity and postpartum depressive symptoms. J Womens Health (Larchmt). (2011) 20:1025–34. doi: 10.1089/jwh.2010.2091
43. Demissie Z, Siega-Riz AM, Evenson KR, Herring AH, Dole N, Gaynes BN. Physical activity during pregnancy and postpartum depressive symptoms. Midwifery. (2013) 29:139–47. doi: 10.1016/j.midw.2011.12.006
44. Claesson IM, Klein S, Sydsjö G, Josefsson A. Physical activity and psychological well-being in obese pregnant and postpartum women attending a weight-gain restriction programme. Midwifery. (2014) 30:11–6. doi: 10.1016/j.midw.2012.11.006
45. Szalewska D, Skrzypkowska M. Physical activity patterns, depressive symptoms and awareness of cardiovascular risk factors in postpartum women. Ann Agric Environ Med. (2016) 23:502–5. doi: 10.5604/12321966.1219195
46. Campolong K, Jenkins S, Clark MM, Borowski K, Nelson N, Moore KM, et al. The association of exercise during pregnancy with trimester-specific and postpartum quality of life and depressive symptoms in a cohort of healthy pregnant women. Arch Womens Ment Health. (2018) 21:215–24. doi: 10.1007/s00737-017-0783-0
47. Shakeel N, Richardsen KR, Martinsen EW, Eberhard-Gran M, Slinning K, Jenum AK. Physical activity in pregnancy and postpartum depressive symptoms in a multiethnic cohort. J Affect Disord. (2018) 236:93–100. doi: 10.1016/j.jad.2018.04.081
48. van der Waerden J, Nakamura A, Pryor L, Charles MA, El-Khoury F, Dargent-Molina P. Domain-specific physical activity and sedentary behavior during pregnancy and postpartum depression risk in the French EDEN and ELFE cohorts. Prev Med. (2019) 121:33–9. doi: 10.1016/j.ypmed.2019.02.012
49. Susukida R, Usuda K, Hamazaki K, Tsuchida A, Matsumura K, Nishi D, et al. Association of prenatal psychological distress and postpartum depression with varying physical activity intensity: Japan Environment and Children's Study (JECS). Sci Rep. (2020) 10:6390. doi: 10.1038/s41598-020-63268-1
50. Huang YC, Mathers N. Postnatal depression – biological or cultural? A comparative study of postnatal women in the UK and Taiwan. J Adv Nurs. (2001) 33:279–87. doi: 10.1046/j.1365-2648.2001.01664.x
51. Affonso DD De AK, Horowitz JA, Mayberry LJ. An international study exploring levels of postpartum depressive symptomatology. J Psychosom Res. (2000) 49:207–16. doi: 10.1016/S0022-3999(00)00176-8
52. Klainin P, Arthur DG. Postpartum depression in Asian cultures: a literature review. Int J Nurs Stud. (2009) 46:1355–73. doi: 10.1016/j.ijnurstu.2009.02.012
53. Chee CY, Lee DT, Chong YS, Tan LK, Ng TP, Fones CS. Confinement and other psychosocial factors in perinatal depression: a transcultural study in Singapore. J Affect Disord. (2005) 89:157–66. doi: 10.1016/j.jad.2005.09.004
54. Fisher JR, Morrow MM, Ngoc NT, Anh LT. Prevalence, nature, severity and correlates of postpartum depressive symptoms in Vietnam. BJOG. (2004) 111:1353–60. doi: 10.1111/j.1471-0528.2004.00394.x
55. Pritchett RV, Daley AJ, Jolly K. Does aerobic exercise reduce postpartum depressive symptoms? a systematic review and meta-analysis. Br J Gen Pract. (2017) 67:e684–91. doi: 10.3399/bjgp17X692525
56. Schuch F, Vancampfort D, Firth J, Rosenbaum S, Ward P, Reichert T, et al. Physical activity and sedentary behavior in people with major depressive disorder: a systematic review and meta-analysis. J Affect Disord. (2017) 210:139–50. doi: 10.1016/j.jad.2016.10.050
57. Aguiar AS, Castro AA, Moreira EL, Glaser V, Santos AR, Tasca CI, et al. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev. (2011) 132:560–7. doi: 10.1016/j.mad.2011.09.005
58. Agarwal D, Haque M, Sriramula S, Mariappan N, Pariaut R, Francis J. Role of proinflammatory cytokines and redox homeostasis in exercise-induced delayed progression of hypertension in spontaneously hypertensive rats. Hypertension. (2009) 54:1393–400. doi: 10.1161/HYPERTENSIONAHA.109.135459
59. Abu-Omar K, Rutten A. Relation of leisure time, occupational, domestic, and commuting physical activity to health indicators in Europe. Prev Med. (2008) 47:319–23. doi: 10.1016/j.ypmed.2008.03.012
60. Jurakic D, Pedisic Z, Greblo Z. Physical activity in different domains and health-related quality of life: A population-based study. Qual Life Res. (2010) 19:1303–9. doi: 10.1007/s11136-010-9705-6
61. National Physical Activity Guidelines for Australians. Commonwealth Department of Health and Aged Care. Canberra: National Physical Activity Guidelines for Australians (1999).
62. Thirlaway K, Benton D. Participation in physical activity and cardiovascular fitness have different effects on mental health and mood. J Psychosom Res. (1992) 36:657–65. doi: 10.1016/0022-3999(92)90055-7
63. Annesi JJ, Gann S, Westcott WW. Preliminary evaluation of a 10-wk. resistance and cardiovascular exercise protocol on physiological and psychological measures for a sample of older women. Percept Mot Skills. (2004) 98:163–70. doi: 10.2466/pms.98.1.163-170
Keywords: physical activity, postpartum depression, mental-health, dose-response analysis, meta-analysis
Citation: Yuan M, Chen H, Chen D, Wan D, Luo F, Zhang C, Nan Y, Bi X and Liang J (2022) Effect of physical activity on prevention of postpartum depression: A dose-response meta-analysis of 186,412 women. Front. Psychiatry 13:984677. doi: 10.3389/fpsyt.2022.984677
Received: 02 July 2022; Accepted: 06 September 2022;
Published: 04 November 2022.
Edited by:
Thomas Wenzel, University of Vienna, AustriaReviewed by:
Hua Li, University of Saskatchewan, CanadaCopyright © 2022 Yuan, Chen, Chen, Wan, Luo, Zhang, Nan, Bi and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoning Bi, Yml4bjIwMDdAMTYzLmNvbQ==; Jing Liang, bGlhbmdqaW5nQHpyeWh5eS5jb20uY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.