- 1Center for Genomic Medicine, Desert Research Institute, Reno, NV, United States
- 2School of Public Health, University of Nevada, Reno, NV, United States
- 3Renown Health, Reno, NV, United States
In this research, we examine and identify the implications of Adverse Childhood Experiences (ACEs) on a range of health outcomes, with particular focus on a number of mental health disorders. Many previous studies observed that traumatic childhood events are linked to long-term adult diseases using the standard Adverse Childhood Experience Questionnaire. The study cohort was derived from the Healthy Nevada Project, a volunteer-based population health study in which each adult participant is invited to take a retrospective questionnaire that includes the Adverse Childhood Experience Questionnaire, the 12-item Short Form Survey measuring quality of life, and self-reported incidence of nine mental disorders. Using participant’s cross-referenced electronic health records, a phenome-wide association analysis of 1,703 phenotypes and the incidence of ACEs examined links between traumatic events in childhood and adult disease. These analyses showed that many mental disorders were significantly associated with ACEs in a dose-response manner. Similarly, a dose response between ACEs and obesity, chronic pain, migraine, and other physical phenotypes was identified. An examination of the prevalence of self-reported mental disorders and incidence of ACEs showed a positive relationship. Furthermore, participants with less adverse childhood events experienced a higher quality of life, both physically and mentally. The whole-phenotype approach confirms that ACEs are linked with many negative adult physical and mental health outcomes. With the nationwide prevalence of ACEs as high as 67%, these findings suggest a need for new public health resources: ACE-specific interventions and early childhood screenings.
Introduction
Childhood adversity and trauma have long been associated with greater risk of adult physical and mental health outcomes (1–35). Adverse Childhood Experiences (ACEs) are defined as traumatic events and unsafe environments occurring in children before the age of 18 (3). ACEs include the incidence of emotional, physical, sexual maltreatment, neglect, substance abuse within the household, mental illness in the household, violence, and incarceration of a member in the household (3). These experiences may include unsafe housing, violence, lack of education, or access to food, which are categories of Social Determinants of Health (SDOH) defined by the Center for Disease Control (CDC). SDOH factors have been shown to have strong impact on individuals’ physical health and quality of life: importantly, studies indicate that SDOH alone, as well as when paired with genetics, underlie health and wellbeing (36).
Numerous national studies indicate the seriousness of ACEs with prevalence as high as 67% of adults experiencing at least one ACE and 16% experiencing at least four ACEs. The CDC reports that at least five of the top ten leading causes of death are associated with ACEs (18, 19, 21, 37). Other research highlights the implications and consequences of ACEs, including risk for chronic disease such as asthma, cancer, diabetes, heart disease, inflammatory disorders, obesity and others (4, 6, 9, 10, 18, 19, 21, 38). The relationship between ACEs and mental illness is also known (32, 39–44): relationships with mental disorders such as adult depression, anxiety, eating disorders, schizophrenia, substance abuse, suicide, among others have been clearly established (23–25, 30, 39, 42, 44).
The Healthy Nevada Project (HNP) is an all-comers, adult population health and genomics study based in Northern Nevada. The study also collects self-reported survey information on ACEs and other SDOH. Nevada has a high prevalence of adults with mental illness and poor access to care (e.g., care1) and the problem is more acute in children2. The state of Nevada has structural healthcare delivery problems and is at or near the bottom in public health funding (45), resulting in an increase in preventable deaths (46). As access to care, and the social and structural determinants of health are all linked to health outcomes, there are unique opportunities in the HNP cohort for studying problems beyond population genomics. Here, a three-pronged approach to study the effects of ACEs is used: we first observe the distribution of ACEs in the cohort with respect to sociodemographic characteristics, as well as self-reported mental health conditions. A more comprehensive examination between ACE exposure and a wide spectrum of mental and physical health outcomes is performed with phenome-wide association analyses (PheWAS): first using ACEs as a predictor in a dose-response style and secondly as a binary case vs. control covariate. Additionally, the SF12-v2® quality of life metric is used to point out quality of life differences between participants with high ACE exposure and those with none. Although we use a different approach compared to previous studies, the HNP cohort follows outcomes published since the original study in 1998 (3).
Materials and methods
Data disclosure statement
In order to minimize the chance of individual reidentification for participants in this study, a subset of the phenotype data and summary statistics of phenome-wide association results are available at https://doi.org/10.5061/dryad.73n5tb30g. For data derived from electronic health records (EHR), all HIPAA regulations were followed, including removing individuals with an age greater than 88 years old. For further information, please see our full data availability statement below.
The Renown electronic health records database
The Renown Health EHR system was instantiated in 2007 on the EPIC system (EPIC System Corporation, Verona, WI, United States), and contains lab results, diagnosis codes (ICD9/ICD10), and demographic information of approximately 1.6 million hospital patient visits from 2005 to the present date.
IRB and informed consent
This study was conducted under a human subject protocol approved by the University of Nevada Institutional Review Board under project #1106618-15. Participants in the Healthy Nevada Project undergo written and informed consent to having genetic information associated with (EHR) in a deidentified manner. Inclusion criteria are individuals older than 18 years who can complete the consent process either online or in person at a study location. A copy of the consent can be found at https://healthynv.org/about/consent/. Patient identifiers are not incorporated into the research EHR: the EHR and genetic data are linked in a separate environment via a unique identifier as approved by the IRB.
The Healthy Nevada Project cohort
The Healthy Nevada Project (HNP), is a volunteer-based population health study that was formed in 2016 (38, 47–51). As of May 2022, the HNP includes 45,421 whole-exome sequenced participants who are cross-referenced with up to sixteen years of EHR. The HNP also includes a voluntary, retrospective Social Health Determinants Questionnaire containing mental health, social health, and ACE-related questions. More than 18,000 participant responses to the survey have been collected and collated representing approximately 5% of the population of the Reno/Sparks metropolitan area where the study is focused.
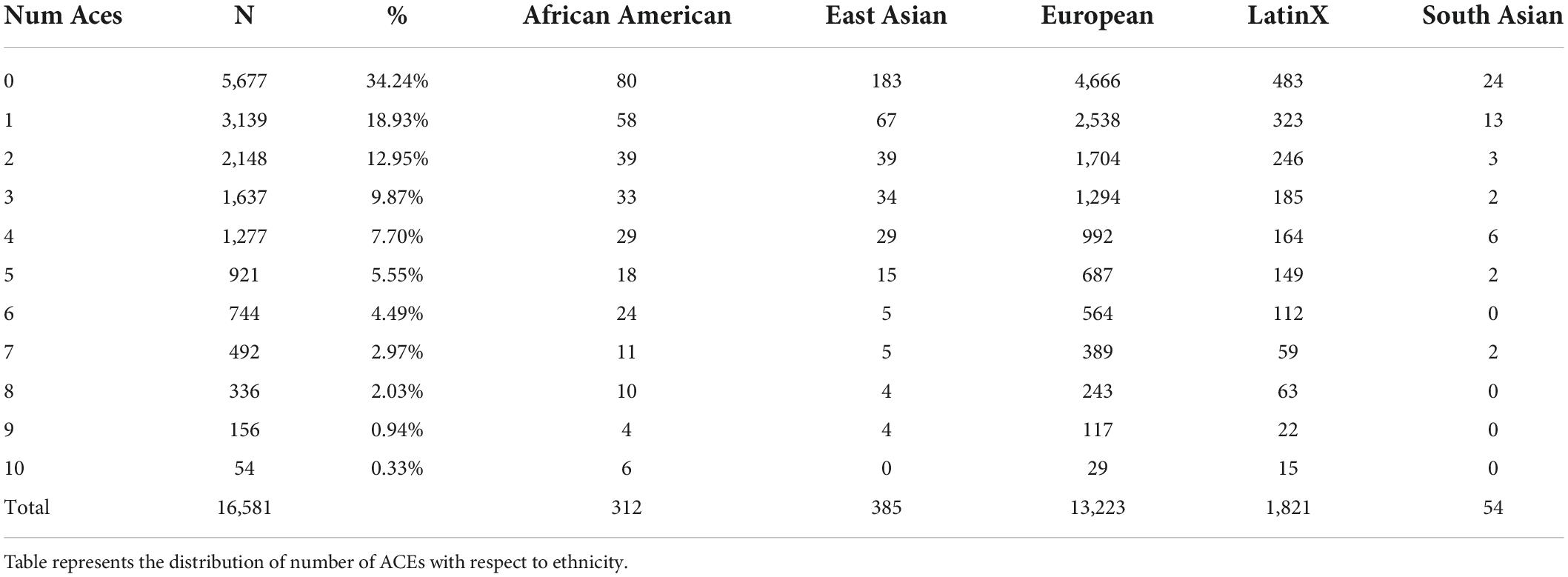
This research focuses on the 16,581 HNP participants with self-reported survey responses to the ten adverse childhood events questions. We refer to this as the HNPACE cohort. Demographics are included in Table 1 and Supplementary Figure 1. Reported ethnicity was based on the genetic admixture from Exome + sequencing of all HNP participants (38).
Social Health Determinants Questionnaire
All consenting HNP participants are invited to complete the HNP Social Health Determinants Questionnaire. The survey is voluntary and confidential bound through an NIH Certificate of Confidentiality. It consists of 103 multi-part sociodemographic-related questions including ACEs, other traumatic events, education level, household income, substance abuse, drug use, mental illnesses, and other behavioral patterns.
The HNP ACE questionnaire follows the ten questions from the standard ACE instrument, covering physical, sexual, and emotional abuse, physical and emotional neglect, and household dysfunction, mental illness, incarceration, intimate partner violence, substance abuse, and divorce in the home (3, 18, 19, 52). A standard ACE questionnaire based on the original version can be found here: https://elcentro.sonhs.miami.edu/research/measures-library/aces/aces_eng.doc.docx. The survey was administered to adult participants on the Survey Monkey platform3, based on retrospective recall of experiences in the first 18 years of life.
The HNP questionnaire also includes the option of self-reporting any of nine mental illnesses/disorders: Attention Deficit/Hyperactivity Disorder (ADHD), Anxiety, Bipolar Disorder (BP), Depression, Eating Disorder (ED), Obsessive-Compulsive Disorder (OCD), Post-Traumatic Stress Disorder (PTSD), Schizophrenia, and Substance Use Disorder. The wording of the questionnaire for self-reporting the first eight mental conditions is: “Have you ever been diagnosed with, or treated for, any of the conditions.” Substance use disorder is assessed by the question “Substance use disorder: have you ever been addicted to any substance”; for example: nicotine, alcohol, marijuana, cocaine, methamphetamine, LSD/Magic Mushrooms, Ecstasy, prescription stimulants, or painkillers not as prescribed, opium. Possible answers are “Yes” or “No” in both cases.
ACE cohorts and ACE score
Each of the ten ACEs was self-reported as “Yes” or “No” for each of ten types of events, with unanswered questions left as “NA.” Any participant answering at least one of the ten ACE questions was included in this study; i.e., participants were excluded if all ACE questions were left unanswered. The ACE score was computed as the sum of affirmative responses a participant reported (21, 32, 53, 54). A participant with two ACEs, for example, encountered two different types of ACEs at least one time each by the age of 18. Although the score does not measure event frequency within ACE type, it represents the accumulation of different types of ACEs. For this study, we define the HNPACE case cohort as participants with four or more ACEs, and the control cohort as those with no ACEs. This definition is similar to those utilized by Merrick, Jones, and Godoy (18, 19, 55). As reported in (38), question non-response was low: 517 (0.31%) of the questions were unanswered, stemming from 384 participants. The ACE score was computed for these 384 individuals, as all had at least one ACE. Note that ACE subcohorts are defined differently per study. There is no rigid consensus in the literature to define ACE subgroups. For ease of discussion, let ACEi denote the group of participants who experience i ACEs. Jones and Godoy only examine ACE4+ and ACE0 (19, 55); Merrick separates participants into ACE1, ACE2–3, ACE4+ (18), and Gilbert divides the study participants into ACE1–3, ACE4–6, ACE7–9 (53). Both Felitti and Dube examine ACE1, ACE2, ACE3 and ACE4+ (3, 4). Although the definition of ACE subcohorts is dependent on the sample size, sample demographics, study hypotheses, and other factors, the group “high ACE exposure” is most often constructed as ACE4+.
Phenome-wide association studies
Phenome-wide association studies (PheWAS) examine and identify associations between a large number of disease phenotypes and a specific genotype or phenotype of interest. Here we used the PheWAS approach to identify associations between 1,703 possible disease phenotypes in the HNPACE and the number of ACEs. Disease phenotypes were based on participants’ recorded EHR ICD codes, which were then aggregated and converted into 1,703 individual phenotype groups (“phecodes”) using the R package “PheWAS” as described in Carroll and Denny (56). Only the phenotype groups that included at least 20 cases were used for downstream analyses, following Carroll’s protocol (56).
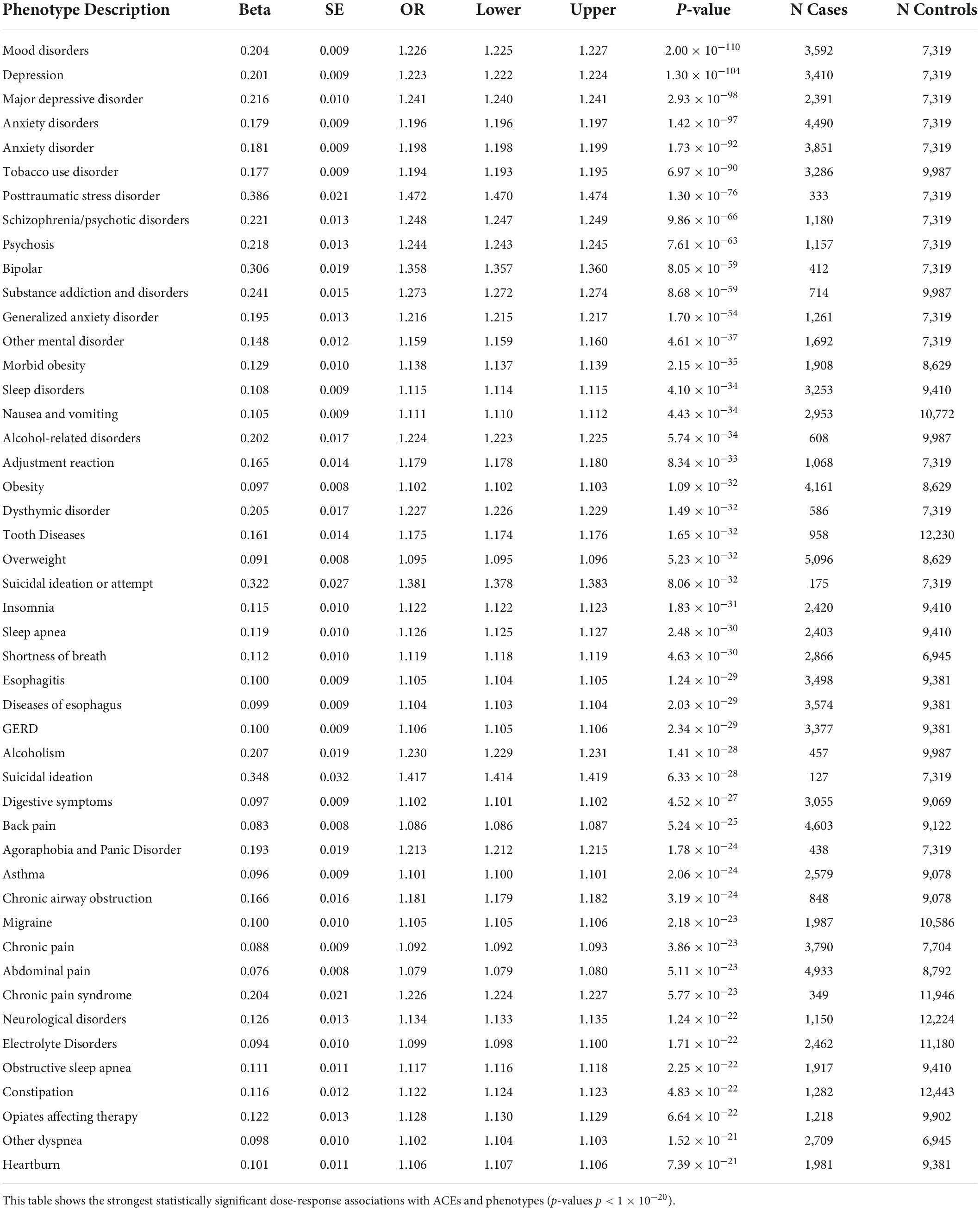
The first PheWAS used the ACE score to assess a dose-response relationship with each of 1,447 disease groups. Specifically, a logistic regression of disease status versus the ACE score was performed for each of the 1,447 phenotypes with more than 20 members, controlling for age and sex. The raw p-values for each of the 1,447 associations are shown in Supplementary Table 1; a Bonferroni correction for the number of comparisons yields α = 3.5 × 10–5 (0.05/1,447). This value is represented by a horizontal red line in Figure 1. Note that only associations with p-value p < 1 × 10–20 were annotated for ease of viewing.
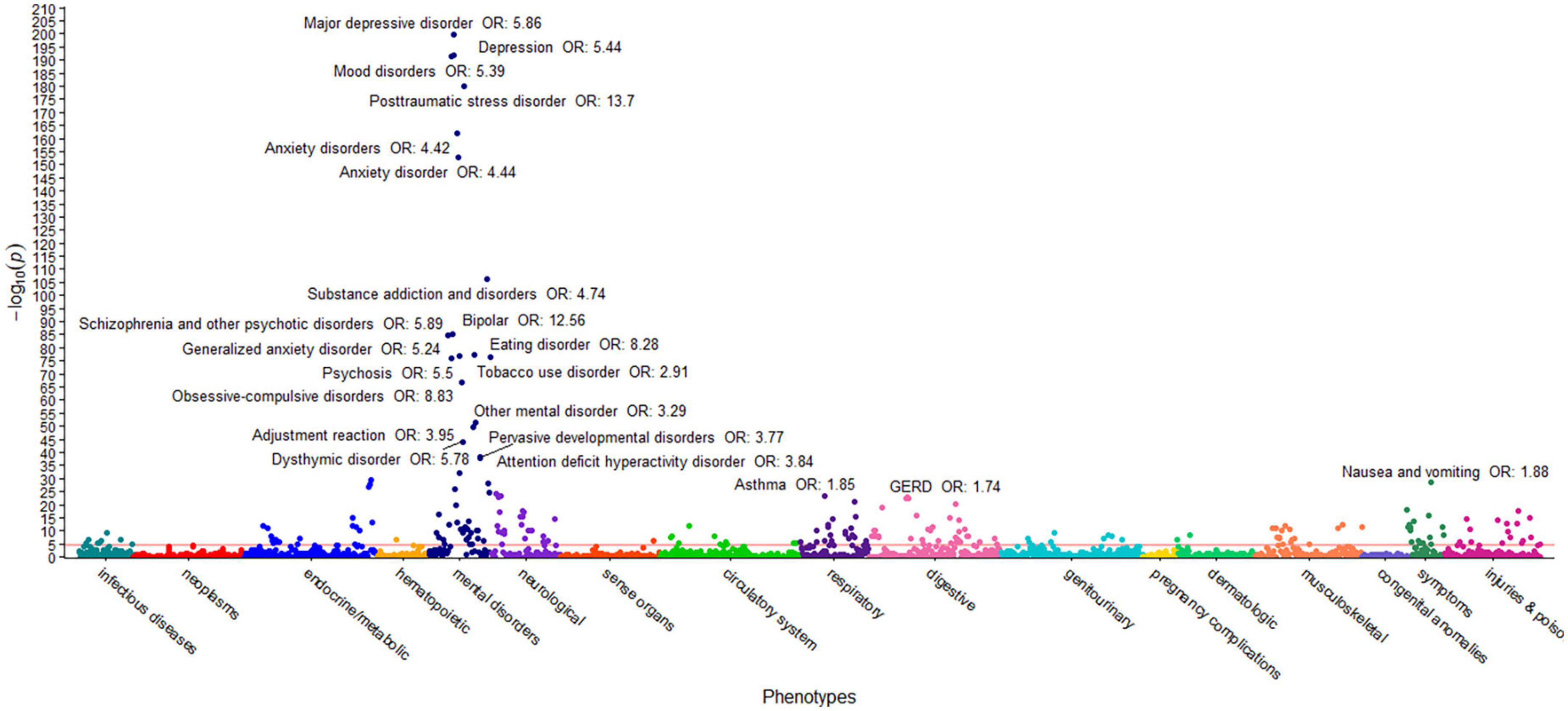
Figure 1. ACEs PheWAS Dose-response ICD10 only. This figure illustrates the results of 1,447 individual associations between the ACE score and the incidence of phenotype group (phecode) of the HNPACE cohort. Covariates included in the models are sex and age at genotyping. Each point denotes the p-value of association of that phenotype. The red horizontal line represents the Bonferroni-corrected significance level of α = 3.5 × 10–5. Only associations with p < 1 × 10–20 are annotated for ease of viewing.
The second PheWAS used the ACE case/control status as a predictor for disease. Specifically, a logistic regression of disease state versus ACE case/control status was performed for each phenotype group with more than 20 members, controlling for age and sex. There were 1,703 associations conducted. Results are shown in Supplementary Table 2 in which all p-values are raw. A Bonferroni correction yields a significance threshold of α = 2.9 × 10–5. Again, this value is denoted in Supplementary Figure 2 as a red horizontal line. For ease of viewing, only associations yielding a p-value p < 1 × 10–20 were annotated.
As many of the questionnaire participants had cross-referenced EHR data (N = 13,728, 83%), we performed a supplementary set of PheWAS that added the ICD diagnosis codes of the nine mental illnesses for participants who were not diagnosed in the Renown Healthcare system. The details of this analysis and its results are included in the Supplementary material.
The SF-12 v2® short form survey
The HNP survey recorded responses to the short form SF-12 questions. The SF-12 questions are a shorter one-page version of the SF-36, consisting of 12 of its 36 questions and adaptable to a diverse set of populations (57). Survey responses to SF-12v2 questions were used to generate the mental composite score (MCS) and physical composite score (PCS) as is standard (58). Scores greater than 50 represent a better quality of life compared to the average. The HNP SF-12 survey contained only 1,122 missing answers (0.56%) based on 686 individuals who failed to answer one or more mental health survey questions. Thus, 686 SF-12 agglomerative scores were not computable, and these participants were excluded from further study, leaving 15,895 participants for analyses including both ACE and SF-12 scores (Supplementary Figure 1).
Statistical analysis
All hypothesis tests were two-sided. All single hypothesis tests were tested at the α = 0.05 significance level. The word “significant” corresponds to a statistically significant result. The “PheWAS” package v. 0.99.5.5 in R was used for both phenome-wide association studies. As SF-12 scores were negatively skewed, either square root function transforms were used with Student t-tests, or Mann–Whitney tests were used with means and medians reported, medians reported.
Results
Distribution of ACEs in the HNPACE
Results of the 16,581 participants responding to the ACE survey are shown in Table 1: 65.8% of adults recalled at least one type of ACE in childhood, and 24.1% endured four or more different types of ACEs. This distribution is very similar to other studies (3, 12, 19). When compared to other ancestries, Europeans generally were less likely to incur more than four ACEs than African American or LatinX participants. The number of ACE cases was 3,980 and the number of ACE controls was 5,677. Other demographic details of the 16,581 self-reporting participants are presented in Supplementary Table 3, showing that participants with college education have a notably lower prevalence of ACEs than the general cohort. Supplementary Table 4 demonstrates that females incur a higher number of ACEs than males. Supplementary Table 5 shows the percentage of each ACE group in the respective income bracket. Of interest is the lowest income bracket, which shows the starkest increase in participants with greater ACE exposure.
Distribution of mental health and ACEs in the HNPACE
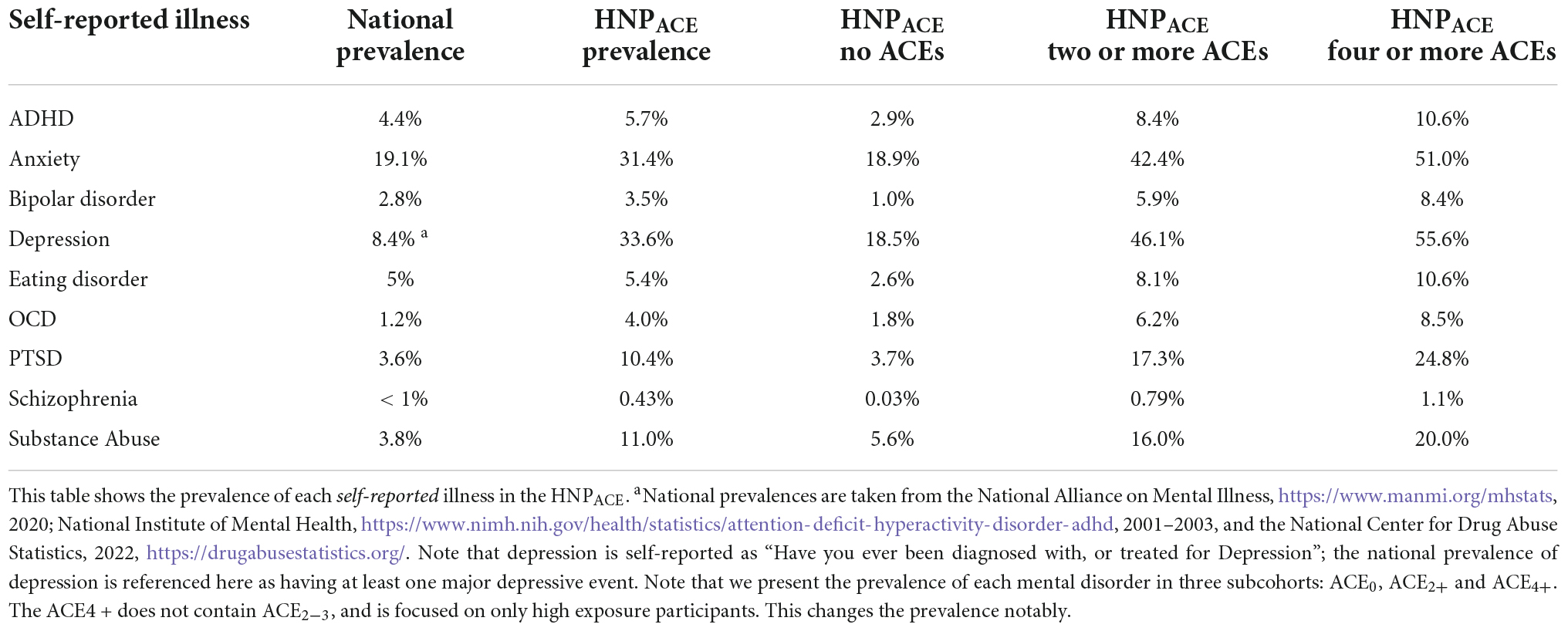
The distribution of self-reported mental illnesses alongside ACE exposures is shown in Table 2. Note that the HNPACE results stem strictly from the volunteer questionnaire, whereas the national prevalence is based on clinical diagnoses, specific patient characteristics, and their diagnosis codes.
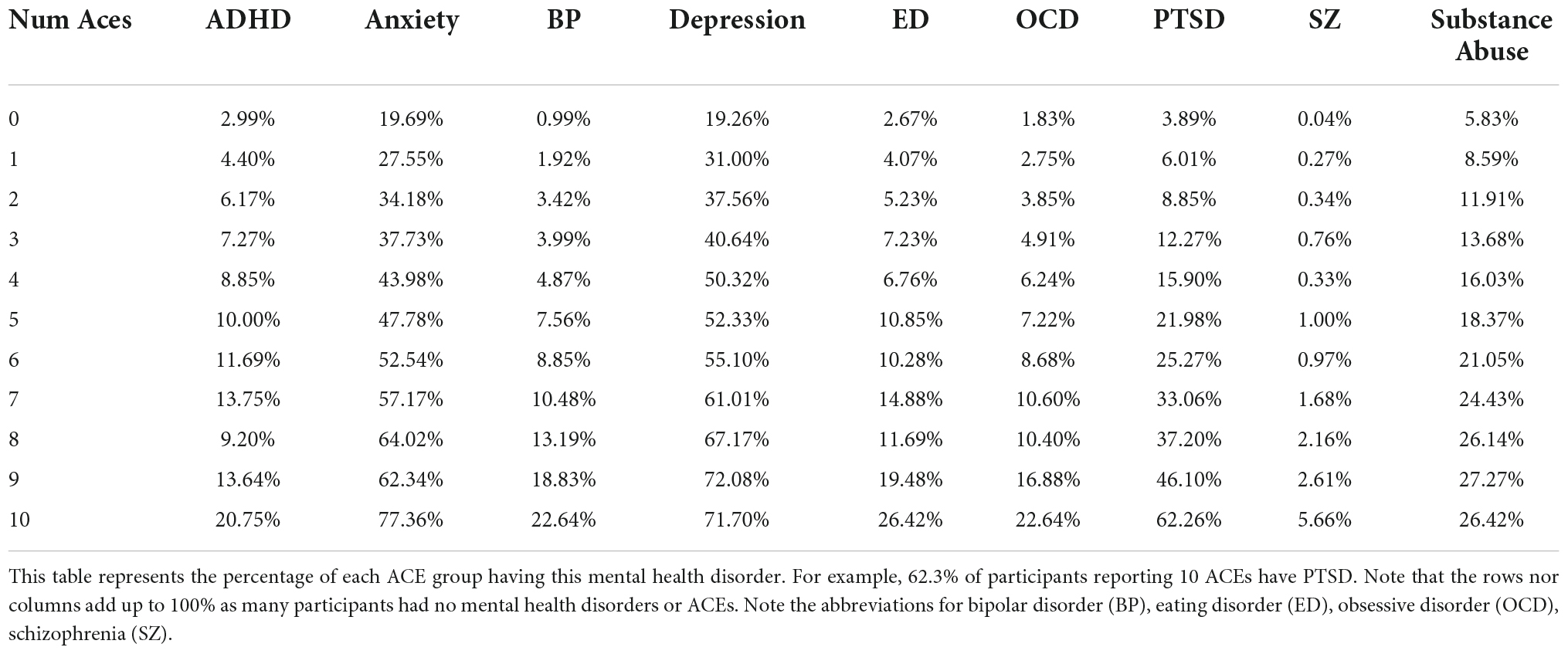
Table 3 outlines that some mental disorders occur at a higher percentage in participants with a higher number of ACEs. For example, in Table 3, 62% of participants with ten ACEs reported as having been diagnosed with PTSD, compared to 3.9% in the ACE controls. In fact, this same trend is shown clearly for bipolar disorder, eating disorder, OCD, PTSD, schizophrenia and substance abuse in this table. Furthermore, Supplementary Table 6 shows an interesting trend: participants who have experienced ACEs show a general increasing trend in the number of concurrent self-reported mental conditions. In other words, if ACEi is the group of people reporting i ACEs, as i increases, the percentage of mental illnesses reported by ACEi increases in general. The trend is more noticeable with i ≥ 3, as well as in the categories with at least three mental illnesses reported.
Phenome-wide association studies
The first PheWAS examined associations between the ACE score and the incidence of 1,447 medical phenotypes. The thirteen most statistically significant associations were with mental disorders, with effect sizes ranging from 0.15 (SE = 0.012) (other mental disorder) to 0.39 (SE = 0.021) (PTSD); thus every additional type of ACE experienced corresponds to an increase in the odds of the diseases 16% and 47%, respectively. Effect sizes of associations with suicide attempt were 0.32 to 0.35, meaning that for each type of ACE endured, the odds of the participant making a suicide attempt increase by 33% on average, with p-values ranging from p = 8.1 × 10–32 to p = 3.91 × 10–18. These values are reported in Supplementary Table 1, with the strongest associations (p < 1 × 10–20) shown in Table 4.
The second case/control PheWAS examined which EHR phenotype groups associate with high ACE exposure (four or more ACEs) when compared to no ACE exposure. Of the 1,703 phenotype groups containing more than 20 participants, 237 were statistically significant after using the Bonferroni correction (Supplementary Figure 2), with very similar results as the dose-response PheWAS (Supplementary Table 2). Analyses for the phenome-wide analyses which included both ICD codes and self-reported illnesses were performed identically, and results are shown in Supplementary Tables 7–9 and Supplementary Figures 3, 4.
SF-12 quality of life scores in ACE cases and controls
We observed similar patterns as those in Ware (58), although the means were consistently lower. Physical Functioning and Bodily Pain presented the greatest correlation (0.710). Clear differences were identified in the PCS and MCS between ACE cases and ACE controls. The mean PCS scores were computed as 47.9 for cases and 51.1 for controls; a t-test on the transformed values yields p < 2.2 × 10–16, SE = 0.022. Similarly, the mean MCS of cases and controls was 45.8 and 52.7, respectively, with p < 2.2 × 10–16, SE = 0.022. Scores for participants suffering from self-reported mental health disorders were also significantly lower than those without a self-reported mental health diagnosis (Supplementary Table 10).
Discussion
This study observes the distribution of ACEs in an all-comers retrospective survey of almost 17,000 participants. The all-comers population of Healthy Nevada Project comprises an over-representation of female, highly educated, white, and middle-to-high-income individuals, relative to the overall population of the Renown Health service area in Nevada. Despite this, with and without respect to biological sex, ancestry, and income, the distribution of HNP ACEs follow many previous studies, e.g., (3, 18, 19). Here, we focus on the profound effect that childhood trauma has on adult mental health.
We believe our study is novel in its broad examination of all mental diseases with a focus on nine specific disorders as shown in Supplementary Table 9. By analyzing a broad spectrum of mental health outcomes in a single population cohort, we were able to better assess the relative magnitudes of specific mental health burdens likely attributable to high ACEs in our cohort. Unsurprisingly, PheWAS results demonstrate strong associations between ACEs and mental health disorders overall, as expected based on prior research (22, 26, 30, 34, 42, 44, 59). These associations between ACEs and mental health mirror other trauma studies, in which disaster survivors and refugees also demonstrate increased prevalence of anxiety, depression and PTSD due to traumatic past experiences (60–62). Together these results indicate that previous trauma is clearly a major factor in mental health outcomes.
Our study subsequently identified pronounced differences in SF-12 quality of life metrics between the ACE cases and controls, with the two metrics for cases notably under the national mean of 50, while ACE control cohort metrics were above the national mean. Furthermore, we identified significant SF-12 score differences between participants with and without mental disorders (Supplementary Table 10). As annual costs in North America stemming from the incurrence of ACEs was estimated in Bellis et al. to be $748 billion (59), these stark differences in quality of life provide additional evidence that ACEs can be costly both in body and mind.
This study, focused in Northern Nevada, is a window into deeply rooted problems of public health in the United States. A valid concern is whether or not screening for ACEs and studying the long-term health impact of ACEs will bring us any closer to addressing and reducing their health consequences [e.g., (63)] particularly in states such as Nevada with low public health infrastructure. We found a profound association between the number of ACEs endured and increases in suicide attempt. Suicide ideation, attempt, and completion are all on the rise in youth (64). Thus, a practical intervention for individuals with high ACEs may be more focused mental health screening and suicide prevention programs with an important caveat that post hoc interventions have been shown to be largely ineffective. For example, Heymann et al. (65) showed no reduction in intimate partner violence (ACEs: abuse in the household) for couples undergoing Couple Commitment and Relationship Enhancement (Couple CARE) for parents of newborns (65). There is a heritability in ACEs either from genetics but more likely from environmental or learned behavior. One area where prevention has shown encouraging results is related to ACEs neglect and specifically food insecurity; numerous studies have shown relieving this has positive impacts on health (e.g., food reducing diabetes risk and healthcare costs) (66). Thus ACE-specific interventions may begin in an area of public health investment given these successes. Recently, the HNP has started working with a Federally Qualified Health Center (FQHC) and a behavioral health and addiction center to focus on preventing ACE occurrences with the overall goal to reduce its prevalence in our community.
Based on the results in this study, ACEs are associated with a number of physical and mental health burdens. However, it is well known that many of these have genetic components (38, 48, 49, 67). Both components must be included to produce a reliable risk assessment for health outcomes. Previous research by Schlauch et al. used an interaction model to examine the effect of ACEs and genetics on BMI and found the phenotypic effect of ACEs be strengthened for those carrying specific germline genetic variations (38). In order to better understand the overall effects of ACEs, particularly with severe mental disorders, this suggests that future studies may want to include phenotypic associations with ACEs, as well as GxE interactions to identify missing phenotypic variability that is often unaccounted for in standard analyses.
Limitations
There are several limitations we can note about our approach. Firstly, only nine mental conditions were self-reported by participants, and as many of these disorders were not diagnosed in the EHR, the second supplementary PheWAS analysis based on ICD codes and the self-reported disorders was biased for these nine conditions. The preliminary PheWAS, however, does not include self-reported mental illnesses, so it is also not quite accurate, although each PheWAS independently shows that ACEs are strongly predictive of mental illness. The sample size of the cohort is somewhat prohibitive when studying rarer disorders and/or high ACE exposure; the HNP is continuously growing, thus this restriction will likely be relieved.
Data availability statement
The data analyzed in this study are subject to the following licenses/restrictions: These data are available to qualified researchers upon reasonable request and with permission of the Center for Genomic Medicine. EHR data for the Healthy Nevada Project cohort are subject to HIPAA and other privacy and compliance restrictions. Phenotype data for each de-identified participant will be made available on https://doi.org/10.5061/dryad.73n5tb30g, with a small subset of data removed to comply with HIPAA requirements. The Center for Genomic Medicine encourages and collaborates with scientific researchers on an individual basis. Examples of restrictions that will be considered in requests to data access include but are not limited to: (1) Whether the request comes from an academic institution in good standing and will collaborate with our team to protect the privacy of the participants and the security of the data requested. (2) Type and amount of data requested. (3) Feasibility of the research suggested. (4) Amount of resource allocation for the IHI and Renown Hospital required to support the collaboration. Any correspondence and data availability requests should be addressed to Sm9lLkdyenltc2tpQGRyaS5lZHU= and Q3JhaWcuS3VnbGVyQGRyaS5lZHU=.
Ethics statement
The studies involving human participants were reviewed and approved by University of Nevada Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
KS processed and analyzed the data and wrote the manuscript. RR performed data analysis and wrote the manuscript. IN acquired and processed Social Health Determinants Questionnaire data. SK provided critical manuscript review. JG conceived and procured funding for the Healthy Nevada project and this study, had full access to all data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors provided input on the manuscript.
Funding
Research support was provided by Renown Health, the Renown Health Foundation, and the Stacie Mathewson Behavioral Health and Addiction Institute. The funders of this study did not contribute to the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
We would like to thank the participants of the Healthy Nevada Project. We would also like to thank the Healthy Nevada Project team, Renown Health, Helix, Stacie Mathewson, and the Nevada Governor’s Office of Economic Development.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.984366/full#supplementary-material
Footnotes
- ^ https://www.mhanational.org/issues/ranking-states
- ^ https://www.mhanational.org/issues/2021/mental-health-america-youth-data
- ^ www.SurveyMonkey.com
References
1. Bethell CD, Newacheck P, Hawes E, Halfon N. Adverse childhood experiences: assessing the impact on health and school engagement and the mitigating role of resilience. Health Aff. (2014) 33:2106–15. doi: 10.1377/hlthaff.2014.0914
2. Bethell C, Jones J, Gombojav N, Linkenbach J, Sege R. Positive childhood experiences and adult mental and relational health in a statewide sample: associations across adverse childhood experiences levels. JAMA Pediatr. (2019) 173:e193007. doi: 10.1001/jamapediatrics.2019.3007
3. Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) Study. Am J Prev Med. (1998) 14:245–58. doi: 10.1016/s0749-3797(98)00017-8
4. Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. (2003) 37:268–77. doi: 10.1016/s0091-7435(03)00123-3
5. Lehman BJ, Taylor SE, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to blood pressure in the CARDIA Study. Health Psychol. (2009) 28:338–46. doi: 10.1037/a0013785
6. Danese A, Tan M. Childhood maltreatment and obesity: systematic review and meta-analysis. Mol Psychiatry. (2014) 19:544–54. doi: 10.1038/mp.2013.54
7. Shin SH, Miller DP. A longitudinal examination of childhood maltreatment and adolescent obesity: results from the National Longitudinal Study of Adolescent Health (AddHealth) Study. Child Abuse Negl. (2012) 36:84–94. doi: 10.1016/j.chiabu.2011.08.007
8. Gouin J-P, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser JK. Childhood abuse and inflammatory responses to daily stressors. Ann Behav Med. (2012) 44:287–92. doi: 10.1007/s12160-012-9386-1
9. Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. (2007) 104:1319–24. doi: 10.1073/pnas.0610362104
10. Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med. (2009) 163:1135–43. doi: 10.1001/archpediatrics.2009.214
11. Pace TWW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. (2006) 163:1630–3. doi: 10.1176/ajp.2006.163.9.1630
12. Lin JE, Neylan TC, Epel E, O’Donovan A. Associations of childhood adversity and adulthood trauma with C-reactive protein: a cross-sectional population-based study. Brain Behav Immun. (2016) 53:105–12. doi: 10.1016/j.bbi.2015.11.015
13. Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Mol Psychiatry. (2016) 21:642–9. doi: 10.1038/mp.2015.67
14. Ehrlich KB, Ross KM, Chen E, Miller GE. Testing the biological embedding hypothesis: is early life adversity associated with a later proinflammatory phenotype? Dev Psychopathol. (2016) 28:1273–83. doi: 10.1017/S0954579416000845
15. Elwenspoek MMC, Kuehn A, Muller CP, Turner JD. The effects of early life adversity on the immune system. Psychoneuroendocrinology. (2017) 82:140–54. doi: 10.1016/j.psyneuen.2017.05.012
16. Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Ann Epidemiol. (2006) 16:91–104. doi: 10.1016/j.annepidem.2005.06.053
17. Tamayo T, Christian H, Rathmann W. Impact of early psychosocial factors (childhood socioeconomic factors and adversities) on future risk of type 2 diabetes, metabolic disturbances and obesity: a systematic review. BMC Public Health. (2010) 10:525. doi: 10.1186/1471-2458-10-525
18. Merrick MT, Ford DC, Ports KA, Guinn AS, Chen J, Klevens J, et al. Vital signs: estimated proportion of adult health problems attributable to adverse childhood experiences and implications for prevention - 25 States, 2015-2017. MMWR Morb Mortal Wkly Rep. (2019) 68:999–1005. doi: 10.15585/mmwr.mm6844e1
19. Jones CM, Merrick MT, Houry DE. Identifying and preventing adverse childhood experiences: implications for clinical practice. JAMA. (2020) 323:25–6.
20. McCrory E, De Brito SA, Viding E. The impact of childhood maltreatment: a review of neurobiological and genetic factors. Front Psychiatry. (2011) 2:48. doi: 10.3389/fpsyt.2011.00048
21. Park Y-M, Shekhtman T, Kelsoe JR. Interaction between adverse childhood experiences and polygenic risk in patients with bipolar disorder. Transl Psychiatry. (2020) 10:326. doi: 10.1038/s41398-020-01010-1
22. Afifi TO, Enns MW, Cox BJ, Asmundson GJG, Stein MB, Sareen J. Population attributable fractions of psychiatric disorders and suicide ideation and attempts associated with adverse childhood experiences. Am J Public Health. (2008) 98:946–52. doi: 10.2105/AJPH.2007.120253
23. Dube SR, Anda RF, Felitti VJ, Chapman DP, Williamson DF, Giles WH. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life spanfindings from the adverse Childhood Experiences Study. JAMA. (2001) 286:3089–96. doi: 10.1001/jama.286.24.3089
24. Joiner TE, Sachs-Ericsson NJ, Wingate LR, Brown JS, Anestis MD, Selby EA. Childhood physical and sexual abuse and lifetime number of suicide attempts: a persistent and theoretically important relationship. Behav Res Ther. (2007) 45:539–47. doi: 10.1016/j.brat.2006.04.007
25. Ports KA, Merrick MT, Stone DM, Wilkins NJ, Reed J, Ebin J, et al. Adverse childhood experiences and suicide risk: toward comprehensive prevention. Am J Prev Med. (2017) 53:400–3. doi: 10.1016/j.amepre.2017.03.015
26. Briggs ES, Price IR. The relationship between adverse childhood experience and obsessive-compulsive symptoms and beliefs: the role of anxiety, depression, and experiential avoidance. J Anxiety Disord. (2009) 23:1037–46. doi: 10.1016/j.janxdis.2009.07.004
27. Caslini M, Bartoli F, Crocamo C, Dakanalis A, Clerici M, Carrà G. Disentangling the association between child abuse and eating disorders: a systematic review and meta-analysis. Psychosom Med. (2016) 78:79–90. doi: 10.1097/PSY.0000000000000233
28. Isohookana R, Marttunen M, Hakko H, Riipinen P, Riala K. The impact of adverse childhood experiences on obesity and unhealthy weight control behaviors among adolescents. Compr Psychiatry. (2016) 71:17–24. doi: 10.1016/j.comppsych.2016.08.002
29. Guillaume S, Jaussent I, Maimoun L, Ryst A, Seneque M, Villain L, et al. Associations between adverse childhood experiences and clinical characteristics of eating disorders. Sci Rep. (2016) 6:35761–7. doi: 10.1038/srep35761
30. Brockie TN, Dana-Sacco G, Wallen GR, Wilcox HC, Campbell JC. The relationship of adverse childhood experiences to PTSD, depression, poly-drug use and suicide attempt in reservation-based native american adolescents and young adults. Am J Community Psychol. (2015) 55:411–21. doi: 10.1007/s10464-015-9721-3
31. Carvalho Fernando S, Beblo T, Schlosser N, Terfehr K, Otte C, Löwe B, et al. The impact of self-reported childhood trauma on emotion regulation in borderline personality disorder and major depression. J Trauma Dissociation. (2014) 15:384–401. doi: 10.1080/15299732.2013.863262
32. Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. (2004) 82:217–25. doi: 10.1016/j.jad.2003.12.013
33. Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the Adverse Childhood Experiences Study. Pediatrics. (2003) 111:564–72.
34. Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. (2003) 160:1453–60. doi: 10.1176/appi.ajp.160.8.1453
35. Trotta A, Murray RM, Fisher HL. The impact of childhood adversity on the persistence of psychotic symptoms: a systematic review and meta-analysis. Psychol Med. (2015) 45:2481–98. doi: 10.1017/S0033291715000574
36. Cockerham WC, Hamby BW, Oates GR. The social determinants of chronic disease. Am J Prev Med. (2017) 52:S5–12. doi: 10.1016/j.amepre.2016.09.010
37. Merrick MT, Ford DC, Ports KA, Guinn AS. Prevalence of adverse childhood experiences from the 2011-2014 behavioral risk factor surveillance system in 23 States. JAMA Pediatr. (2018) 172:1038–44. doi: 10.1001/jamapediatrics.2018.2537
38. Schlauch KA, Read RW, Neveux I, Lipp B, Slonim A, Grzymski JJ. The impact of ACEs on BMI: an investigation of the genotype-environment effects of BMI. Front Genet. (2022) 13:816660. doi: 10.3389/fgene.2022.816660
39. Copeland WE, Shanahan L, Hinesley J, Chan RF, Aberg KA, Fairbank JA, et al. Association of childhood trauma exposure with adult psychiatric disorders and functional outcomes. JAMA Netw Open. (2018) 1:e184493. doi: 10.1001/jamanetworkopen.2018.4493
40. Hayashi Y, Okamoto Y, Takagaki K, Okada G, Toki S, Inoue T, et al. Direct and indirect influences of childhood abuse on depression symptoms in patients with major depressive disorder. BMC Psychiatry. (2015) 15:244. doi: 10.1186/s12888-015-0636-1
41. Polanco-Roman L, Alvarez K, Corbeil T, Scorza P, Wall M, Gould MS, et al. Association of childhood adversities with suicide ideation and attempts in puerto rican young adults. JAMA Psychiatry. (2021) 78:896–902. doi: 10.1001/jamapsychiatry.2021.0480
42. Prokopez CR, Vallejos M, Farinola R, Alberio G, Caporusso GB, Cozzarin LG, et al. The history of multiple adverse childhood experiences in patients with schizophrenia is associated with more severe symptomatology and suicidal behavior with gender-specific characteristics. Psychiatry Res. (2020) 293:113411. doi: 10.1016/j.psychres.2020.113411
43. Schilling EA, Aseltine RH, Gore S. Adverse childhood experiences and mental health in young adults: a longitudinal survey. BMC Public Health. (2007) 7:30. doi: 10.1186/1471-2458-7-30
44. Vallejos M, Cesoni OM, Farinola R, Bertone MS, Prokopez CR. Adverse childhood experiences among men with schizophrenia. Psychiatr Q. (2017) 88:665–73. doi: 10.1007/s11126-016-9487-2
45. Mays GP, Mamaril CB. Public health spending and medicare resource use: a longitudinal analysis of U.S. Communities. Health Serv Res. (2017) 52(Suppl. 2):2357–77. doi: 10.1111/1475-6773.12785
46. Mays GP, Smith SA. Evidence links increases in public health spending to declines in preventable deaths. Health Aff. (2011) 30:1585–93. doi: 10.1377/hlthaff.2011.0196
47. Read RW, Schlauch KA, Elhanan G, Metcalf WJ, Slonim AD, Aweti R, et al. GWAS and PheWAS of red blood cell components in a Northern Nevadan cohort. PLoS One. (2019) 14:e0218078. doi: 10.1371/journal.pone.0218078
48. Schlauch KA, Read RW, Lombardi VC, Elhanan G, Metcalf WJ, Slonim AD. A comprehensive genome-wide and phenome-wide examination of BMI and obesity in a Northern Nevadan Cohort. G3. (2020) 10:645–64. doi: 10.1534/g3.119.400910
49. Read RW, Schlauch KA, Lombardi VC, Cirulli ET, Washington NL, Lu JT, et al. Genome-wide identification of rare and common variants driving triglyceride levels in a nevada population. Front Genet. (2021) 12:639418. doi: 10.3389/fgene.2021.639418
50. Cirulli ET, White S, Read RW, Elhanan G, Metcalf WJ, Tanudjaja F, et al. Genome-wide rare variant analysis for thousands of phenotypes in over 70,000 exomes from two cohorts. Nat Commun. (2020) 11:542–510. doi: 10.1038/s41467-020-14288-y
51. Grzymski JJ, Elhanan G, Morales Rosado JA, Smith E, Schlauch KA, Read R, et al. Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat Med. (2020) 26:1235–9.
52. Shonkoff JP. Capitalizing on advances in science to reduce the health consequences of early childhood adversity. JAMA Pediatr. (2016) 170:1003–7. doi: 10.1001/jamapediatrics.2016.1559
53. Gilbert LK, Breiding MJ, Merrick MT, Thompson WW, Ford DC, Dhingra SS, et al. Childhood adversity and adult chronic disease: an update from ten states and the District of Columbia, 2010. Am J Prev Med. (2015) 48:345–9. doi: 10.1016/j.amepre.2014.09.006
54. Dube SR, Fairweather D, Pearson WS, Felitti VJ, Anda RF, Croft JB. Cumulative childhood stress and autoimmune diseases in adults. Psychosom Med. (2009) 71:243–50. doi: 10.1097/PSY.0b013e3181907888
55. Godoy LC, Frankfurter C, Cooper M, Lay C, Maunder R, Farkouh ME. Association of adverse childhood experiences with cardiovascular disease later in life: a review. JAMA Cardiol. (2021) 6:228–35.
56. Carroll RJ, Bastarache L, Denny JC. R PheWAS: data analysis and plotting tools for phenome-wide association studies in the R environment. Bioinformatics. (2014) 30:2375–6. doi: 10.1093/bioinformatics/btu197
57. Ware JE. Health Assessment Lab. How to Score Version 2 of the SF-12 Health Survey (with a Supplement Documenting Version 1). Lincoln, RI: QualityMetric Incorporated (2002).
58. Ware J, Kosinski M, Keller S. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. Boston, MA: New England Medical Center (1998).
59. Bellis MA, Hughes K, Ford K, Ramos Rodriguez G, Sethi D, Passmore J. Life course health consequences and associated annual costs of adverse childhood experiences across Europe and North America: a systematic review and meta-analysis. Lancet Public Health. (2019) 4:e517–28. doi: 10.1016/S2468-2667(19)30145-8
60. Makwana N. Disaster and its impact on mental health: a narrative review. J Fam Med Prim Care. (2019) 8:3090–5. doi: 10.4103/jfmpc.jfmpc_893_19
61. Thoresen S, Birkeland MS, Arnberg FK, Wentzel-Larsen T, Blix I. Long-term mental health and social support in victims of disaster: comparison with a general population sample. BJPsych Open. (2019) 5:e2. doi: 10.1192/bjo.2018.74
62. Blackmore R, Boyle JA, Fazel M, Ranasinha S, Gray KM, Fitzgerald G, et al. The prevalence of mental illness in refugees and asylum seekers: a systematic review and meta-analysis. PLoS Med. (2020) 17:e1003337. doi: 10.1371/journal.pmed.1003337
63. Campbell TL. Screening for adverse childhood experiences (ACEs) in primary care: a cautionary note. JAMA. (2020) 323:2379–80. doi: 10.1001/jama.2020.4365
64. Ivey-Stephenson AZ, Demissie Z, Crosby AE, Stone DM, Gaylor E, Wilkins N, et al. Suicidal ideation and behaviors among high school students - youth risk behavior survey, United States, 2019. MMWR Suppl. (2020) 69:47–55. doi: 10.15585/mmwr.su6901a6
65. Heyman RE, Slep AMS, Lorber MF, Mitnick DM, Xu S, Baucom KJW, et al. A randomized, controlled trial of the impact of the couple CARE for parents of newborns program on the prevention of intimate partner violence and relationship problems. Prev Sci. (2019) 20:620–31. doi: 10.1007/s11121-018-0961-y
66. Cheyne K, Smith M, Felter EM, Orozco M, Steiner EA, Park Y, et al. Food bank-based diabetes prevention intervention to address food security, dietary intake, and physical activity in a food-insecure cohort at high risk for diabetes. Prev Chronic Dis. (2020) 17:E04. doi: 10.5888/pcd17.190210
Keywords: SF-12 quality of life metric, adverse childhood experiences, phenome-wide association study, public mental health, social determinants of health
Citation: Schlauch KA, Read RW, Koning SM, Neveux I and Grzymski JJ (2022) Using phenome-wide association studies and the SF-12 quality of life metric to identify profound consequences of adverse childhood experiences on adult mental and physical health in a Northern Nevadan population. Front. Psychiatry 13:984366. doi: 10.3389/fpsyt.2022.984366
Received: 01 July 2022; Accepted: 15 September 2022;
Published: 06 October 2022.
Edited by:
Barna Konkoly-Thege, Waypoint Center for Mental Health Care, CanadaReviewed by:
Mehmet Karadağ, University of Gaziantep, TurkeyMahlagha Dehghan, Kerman University of Medical Sciences, Iran
Copyright © 2022 Schlauch, Read, Koning, Neveux and Grzymski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joseph J. Grzymski, am9lZ0BkcmkuZWR1
 Karen A. Schlauch
Karen A. Schlauch Robert W. Read
Robert W. Read Stephanie M. Koning
Stephanie M. Koning Iva Neveux
Iva Neveux Joseph J. Grzymski
Joseph J. Grzymski