- 1Department of Neurology, The First Hospital of Tsinghua University, Beijing, China
- 2Department of Neurology, China-Japan Friendship Hospital, Beijing, China
- 3Department of Nuclear Medicine, China-Japan Friendship Hospital, Beijing, China
- 4Department of Radiology, China-Japan Friendship Hospital, Beijing, China
Objective: The aim of the study was to investigate the clinical, neuropsychological, and regional cerebral blood flow (rCBF) perfusion changes in patients with neuropsychiatric symptoms caused by nitrous oxide (N2O) abuse.
Methods: A total of 16 patients with neuropsychiatric symptoms caused by nitrous oxide abuse were recruited for this study. The study was carried out in the withdrawal phase of N2O abuse. A 925–1110 MBq 99mTc-ECD was administered intravenously. SPECT/CT images were collected with a low-energy and high-resolution collimator. The region uptake statistics of different brain regions of interest between patients with N2O abuse and normal people of the databases for younger subjects from the Scenium DB Comparison software were calculated automatically.
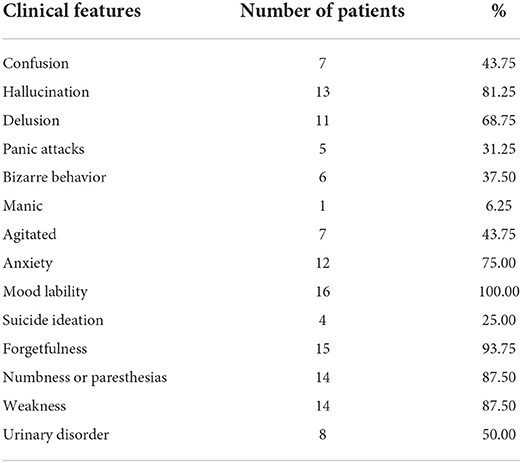
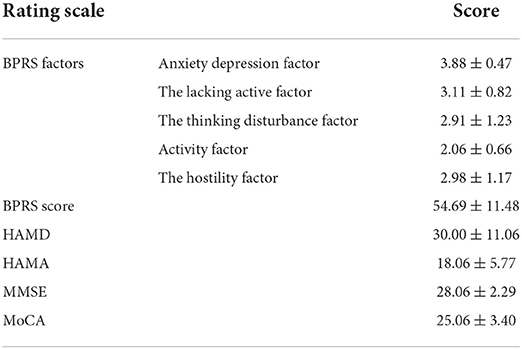
Results: The clinical manifestations of the 16 patients with neuropsychiatric symptoms were mood lability, anxiety, hallucination, delusion, agitation, confusion, and other psychiatric symptoms. In addition, 15 of the patients also complained of memory decline; 14 patients manifested numbness or paresthesia; 14 patients developed limb weakness, and their motor impairments were more severe in the lower limbs than in the upper limbs; and eight patients had urinary and defecation disturbances. In the neuropsychological examination, the BPRS score was 54.69 ± 11.48, the HAMD score was 30.00 ± 11.06, the HAMA score was 18.06 ± 5.77, the MMSE score was 28.06 ± 2.29, and the MoCA score was 25.06 ± 3.40. SPECT showed hypoperfusion in the frontal and temporal lobes, which is consistent with the clinical findings.
Conclusion: This was the first study to demonstrate the obvious effect of N2O abuse on CBF in patients with neuropsychiatric symptoms. CBF perfusion imaging is helpful to detect the changes in the local brain functional activity in patients with N2O abuse.
Introduction
The Global Drug Survey 2019, which was conducted in more than 30 countries, revealed that nitrous oxide (N2O) was the 10th most popular substance among the studied population (1). N2O abuse for recreation is increasingly popular among Chinese adolescents and young adults, which leads to neurological and psychiatric complications (2). Subacute combined degeneration and peripheral neuropathy are identified as the most common neurological manifestations (3, 4). However, psychiatric and cognitive impairments caused by N2O abuse have not received much attention, and only case reports have been reported in the literature (5, 6).
Numerous studies have demonstrated significant mental health and behavioral comorbidities among patients who are inhalant abusers. Inhalant abusers are more likely to have an episode of major depression (7) and suicidality (8) and are at an increased risk of drug abuse problems in future (9). Functional imaging has proven to be very sensitive in detecting cerebral blood flow and metabolism derangement early in the course of drug addiction (10). This work focuses on single-photon emission computed tomography (SPECT) findings in patients with neuropsychiatric symptoms caused by N2O abuse.
Methods
Subjects
A total of 16 patients who exhibited neuropsychiatric symptoms caused by N2O abuse between February 2018 and August 2020 were enrolled. The enrollment criteria for this study are as follows: (1) Patients with a history of N2O inhaling; (2) patients whose conditions comply with the diagnostic criteria of inhalant-related disorders, as coded according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-V); and (3) patients with a Brief Psychiatric Rating Scale (BPRS) score >35 points. The exclusion criteria of the study are as follows: patients with neuropsychiatric symptoms caused by other diseases or mental disorders.
This study was approved by the Ethics Committee of the China–Japan Friendship Hospital (trial number 2018-34-K25). Written informed consent was obtained from all the participants. This study was carried out in the withdrawal phase of N2O abuse.
Clinical data collection
All the patients underwent a standard neurologic examination conducted by a neurologist, who registered clinical data and performed neuroimaging studies and laboratory tests. The mental state examination and neuropsychological rating were assessed by a psychiatrist using the following measures: (1) Brief Psychiatric Rating Scale (BPRS), (2) Hamilton Depression Scale (HAMD), (3) Hamilton Anxiety Scale (HAMA), (4) Mini-Mental State Examination (MMSE), and (5) Montreal Cognitive Assessment (MoCA).
Single-photon emission computerized tomography (SPECT)
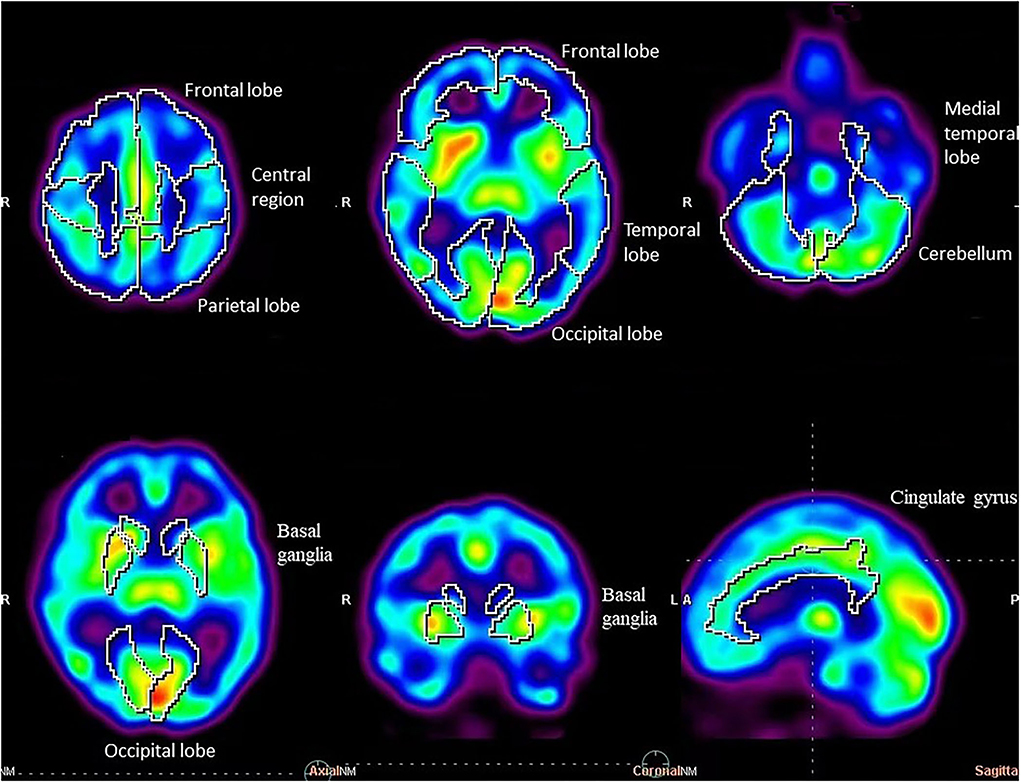
SPECT were performed using a conventional dual-head gamma camera system (Symbia T2, Siemens Medical Solutions, Germany) equipped with low-energy high-resolution parallel-hole collimators nearly at the same time as subjective assessment. All the patients were supplemented with methylcobalamin in hospitalization. Brain perfusion imaging was performed for 20 min, followed by an intravenous injection of a 925–1110 MBq 99mTc ethyl cysteinate dimer (ECD) (radiochemical purity >95%, HTA Co. Ltd, Beijing, China) in a dimly lit and quiet room with the patients' eyes closed. Projection images were acquired through a 20% window centered on the 140 keV peak. The scan time per view was 25 s, and the matrix was 256 × 256. Scenium DB Comparison software (from the Siemens symbia.net Neurology software package) was used for neurological evaluations with SPECT/CT imaging, enabling the comparison of functional studies (SPECT) of a specific patient to a database composed of scans from confirmed normal individuals. Each SPECT image was anatomically standardized using the MNI template provided by SPM. Then, the count of each voxel was standardized to the mean voxel count of the whole brain using proportional scaling. Quantitative parametric analysis was performed by database comparison software like Scenium (we choose the databases for younger subjects to analyze SPECT/CT imaging of patients with N2O abuse), which provides powerful quantification tools for the assessment of SPECT/CT, performs a voxel-by-voxel evaluation of the abnormal regions, and automatically identifies anatomical regions of interest (ROIs). The neurology workflow provides voxel-based statistics displayed as an image volume and calculates ROI statistics by comparing the corresponding estimated normal population mean with the value observed in the patient. Each SPECT image was segmented into 18 regions of interest (ROI) automatically and calculated by Scenium DB Comparison software. The statistical difference in the average uptake value of each brain region was calculated automatically.
The statistic was calculated by means of equation:
A statistic >1.68 indicated an increase in local CBF perfusion, while that < -1.68 indicated a decrease in local CBF perfusion. A statistic was normal between −1.68 and 1.68.
Statistical analysis
All continuous variables were presented as mean ± standard deviation. After SPECT image reconstruction, database comparison software was used for automatic processing and analysis. In total, 18 brain regions (e.g., basal ganglia, central region, cerebellum, cingulate gyrus, frontal lobe, medial temporal lobe, occipital lobe, parietal lobe, and temporal lobe) were calculated automatically. The paired sample t-test was performed on left and right brain regions. One-way ANOVA, followed by the least significant difference (LSD) test, was used to compare the averages and variances among different brain regions. The χ2 test was used to compare the changes in regional cerebral blood flow (rCBF) in different brain regions, and a P-value < 0.05 was considered statistically significant.
Results
Demographic and clinical features
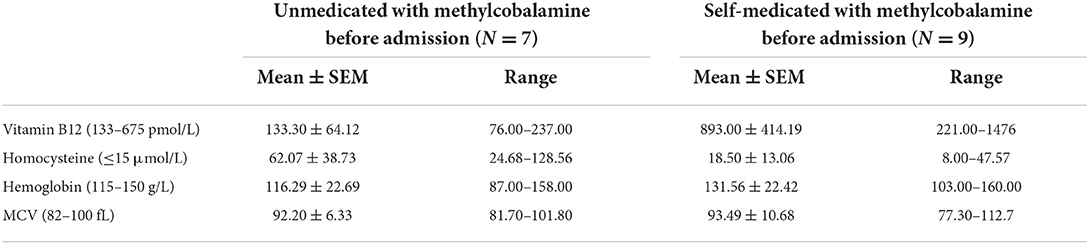
In this study, there were eight male and eight female patients with an age of onset ranging between 18 and 29 (mean age 21.56 ± 2.83) years old. The duration of N2O exposure varied from 3 to 36 (mean 17.88 ± 11.23) months. The course of disease varied from 0.5 to 4 (mean 1.81 ± 1.09) months. Nonmedical abuse of more than one substance was found in four patients. Abuse of both N2O and cannabinoids was found in cases 1, 7, 8, and 10. A total of eight patients self-medicated with methylcobalamin prior to their hospital admission.
Clinical features of N2O abuse resulting in neuropsychiatric disorders are provided in Table 1, including mood lability, anxiety, hallucination, delusion, agitation, confusion, and other psychiatric symptoms. In addition, 15 of the patients complained of memory decline; 14 patients manifested numbness or paresthesia; 14 patients developed limb weakness, with motor impairments found more severe in lower limbs than in upper limbs; and eight patients had urinary and defecation disturbances.
Laboratory findings are given in Table 2. Vitamin B12 levels were normal (893.00 ± 414.19 pmol/L) in the nine self-medicated patients with methylcobalamin before admission, and homocysteine levels were still high in four of these patients. Among the remaining nine patients who were unmedicated with methylcobalamin before admission, vitamin B12 levels (133.30 ± 64.12 pmol/L) were low in four patients and low to normal in the remaining three patients. Homocysteine levels were high in all the unmedicated patients (62.07 ± 38.73 μmol/L). Anemia was diagnosed in nine patients.
Neuropsychological rating scale
Data from the neuropsychological rating scale are listed in Table 3. The BPRS score was 54.69 ± 11.48 (anxiety depression factor score was 3.88 ± 0.47; the lacking active factor score was 3.11 ± 0.82; thinking disturbance factor score was 2.91 ± 1.23; activity factor score was 2.06 ± 0.66; the hostility factor score was 2.98 ± 1.17), self-knowledge impairment score was 2.88 ± 0.96, inability to work score was 4.25 ± 1.13, HAMD score was 30.00 ± 11.06, HAMA score was 18.06 ± 5.77, MMSE score was 28.06 ± 2.29 (two patients scored < 27), and MoCA score was 25.06 ± 3.40 (seven patients scored < 26).
SPECT studies
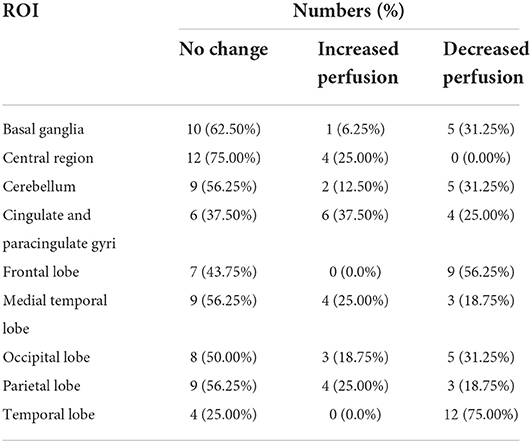
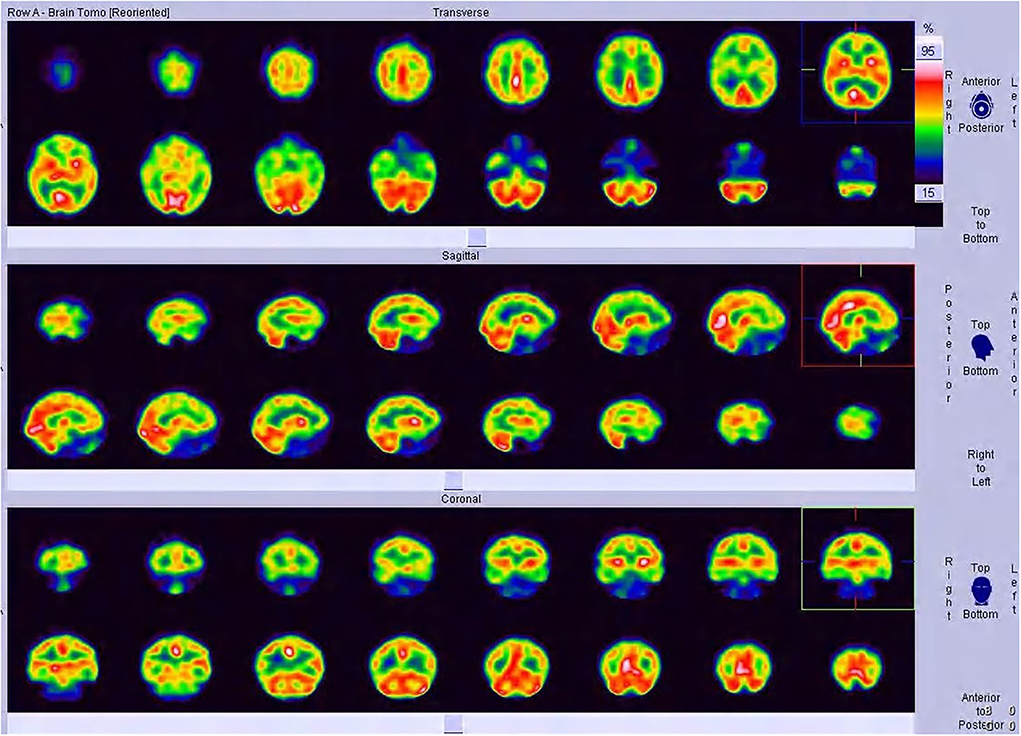
Regional uptake statistics of the 99mTC-ECD between patients and normal people of the same age-group from the background software database are given in Table 4. No statistical significance was found in the t-test results of left and right uptake statistics (P > 0.05). No significant difference was found between brain regions (F = 5.919, P < 0.01). Significant differences were identified in the changes in rCBF in different brain regions (P < 0.01). Although brain MRI was normal in all patients, SPECT showed hypoperfusion in the frontal lobe (9/16, 56.25%) and the temporal lobe (12/16, 75.00%), which is consistent with the clinical findings (Table 5, Figures 1, 2).
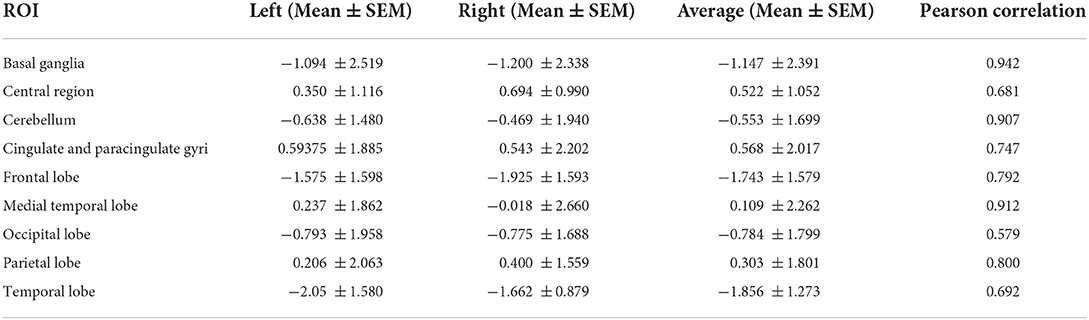
Table 4. Regional uptake statistics of 99mTC-ECD between patients and normal people of the same age group.
Discussion
N2O is colloquially known as “hippy crack” or “laughing gas.” It is increasingly taken for recreational purposes for its euphoric and relaxing effects and hallucinogenic properties. The abuse of N2O is a significant public health concern, which predominantly affects adolescents. Although the subacute combined degeneration of the spinal cord and peripheral neuropathy caused by N2O abuse are gradually concerned as the most common neurological damage (3, 4), psychiatric symptoms and cognitive dysfunction caused by N2O abuse have not received much attention (11, 12). All the patients enrolled in this study consumed large amounts of N2O for a long time before the onset of neuropsychiatric symptoms. Their psychiatric symptoms included emotional symptoms (e.g., mania, depression, anxiety, and fear), psychotic symptoms (hallucinations and delusions), personality changes, and impulsive and aggressive behavior, in line with previous reports (13). The anxiety depression factor score was the highest in the BPRS score, which was consistent with the HAMD and HAMA scores, suggesting that the patients must have symptoms of anxiety and depression. Thinking factors (e.g., disorder of concept, exaggeration, hallucinations, and abnormal thinking content) have high factor scores, indicating that patients with positive psychotic symptoms are prominent, and affect self-awareness and ability to work and study. The lack of vitality factor scores in the BPRS scores indicates that in addition to the positive symptoms, negative symptoms are also more prominent. This is not reported in previous studies. The relationship between negative symptoms and long-term prognosis and brain function needs further follow-up.
Of the 16 patients, 15 patients and their families complained of unresponsiveness and decreased memory, and seven of them had a MoCA score < 26, indicating that there was a decline in cognitive function (e.g., memory loss, inattention, and executive function decline). Both psychiatric symptoms and cognitive dysfunction result in the decline of work and life abilities.
Chronic N2O poisoning is related to the interference of vitamin B12, inactivates methionine synthase, interferes with myelin anabolic metabolism, and also results in the accumulation of homocysteine (14). High levels of homocysteine cause oxidative stress and mitochondrial dysfunction, leading to nerve demyelination (15). Homocysteine activates N-methyl-D-aspartate receptors on neuronal cell membranes and induces calcium ions to flow into neurons and mitochondrial calcium overload, eventually leading to neuronal necrosis and apoptosis (16). Of the 16 patients. 11 patients in this study had elevated serum homocysteine levels and only four of them had decreased levels of vitamin B12, which may be related to the nine patients who had self-medicated with methylcobalamin before their visit to our department. This result suggests that elevated serum levels of homocysteine is a more sensitive indicator than decreased serum levels of vitamin B12 for diagnosis (14).
Although the brain MRI of the patients enrolled in this study showed that the brain structure was normal, rCBF changes were different in different brain regions in this study. Local decreased or increased blood perfusion was observed in several brain regions, rather than diffuse decreased blood perfusion. The main findings were hypoperfusion in the frontal lobe and the temporal lobe, which is in accordance with the clinical manifestations of mental behavior abnormalities and cognitive functional decline caused by N2O abuse.
Similar to ketamine, N2O, as an NMDAR antagonist, can reduce the signaling of excitatory glutamate neurons, change the structure and function of hippocampal synapses, and cause learning and memory disorders. Short-term N2O exposure can cause reversible vacuolation of neurons, while long-term abuse can lead to neuron death (17). Psychiatric symptoms and cognitive decline in these patients may be dominated by this injury mechanism. Therefore, it is worth further studying the changes in neurotransmitters caused by N2O abuse and how they affect the learning and memory functions of adolescents.
In the treatment of patients with neuropsychiatric symptoms caused by N2O abuse, in addition to supplying vitamin B12 for nerve repair, we should also perform neuropsychological testing in time to strengthen interventions for mental symptoms and enhance patient compliance, which will not only improve the prognosis of patients but also help prevent relapse (18).
Previous studies have shown that N2O abuse may induce manic relapse in patients with mood disorders (19). The occurrence of psychiatric symptoms observed in this study is consistent with the clinical characteristics of substance-induced mental disorders, which indicate that N2O abuse can induce mental disorders. Future studies should expand the sample size to further explore the pathogenesis and disease characteristics of N2O-induced mental disorders.
As most of the patients reported initiation of N2O use in late adolescence or early adulthood, which involves risks to their still developing brain (20), education about N2O abuse is necessary to prevent impaired brain development. Follow-up and further investigation of the possible effects of N2O on brain development in young people will lead to meaningful prevention strategies.
The limitation of our study is the small sample size. We will continue to collect cases and expand the sample size to further explore the pathogenesis of N2O-induced neuropsychiatric damage.
Conclusion
This was the first study to demonstrate the obvious effect of N2O abuse on CBF in patients with neuropsychiatric symptoms. CBF perfusion imaging is helpful in detecting the changes in rCBF in patients with N2O abuse, thus indicating changes in local brain functional activity in an early stage.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The study involving human participants was approved by the Medical Ethics Committee of China–Japan Friendship Hospital. The trial number is: 2018-34-K25. Written informed consent was obtained from all the participants or his/her parents/legal representatives (for participant under 18 years old).
Author contributions
FL and CT: have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. LW, FL, and CT: study concept and design and study supervision. LW, QW, MD, and XD: acquisition of data. CT, RW, and ZL: neuropsychological rating. WH: magnetic resonance imaging. LY and YZ: SPECT/CT imaging. LW, QW, and FL: analysis and interpretation of data. LW and FL: drafting of the manuscript. LW: funding acquisition. All authors critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
This study was supported by research grants from the First Hospital of Tsinghua, University Pilot Funds (LH-02).
Acknowledgments
We are grateful to all the patients for their participation in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Barratta MJ, Hughes CE, Ferris JA, Global Drug Survey (2019). The GDS Core Research Team, London, UK. Available online at: https://www.globaldrugsurvey.com/wp-content/themes/globaldrugsurvey/results/GDS2019-Exec-Summary.pdf (accessed May 19, 2019).
2. Garakani A, Jaffe RJ, Savla D, Welch AK, Protin CA, Bryson EO, et al. Neurologic, psychiatric, and other medical manifestations of nitrous oxide abuse: a systematic review of the case literature. Am J Addict. (2016) 25:358–69. doi: 10.1111/ajad.12372
3. Alt RS, Morrissey RP, Gang MA, Hoffman RS, Schaumburg HH. Severe myeloneuropathy from acute high-dose nitrous oxide (N2O) abuse. J Emerg Med. (2011) 41:378–80. doi: 10.1016/j.jemermed.2010.04.020
4. Li HT, Chu CC, Chang KH, Liao MF, Chang HS, Kuo HC, et al. Clinical and electrodiagnostic characteristics of nitrous oxide-induced neuropathy in Taiwan. Clin Neurophysiol. (2016) 127:3288–93. doi: 10.1016/j.clinph.2016.08.005
5. Sethi NK, Mullin P, Torgovnick J, Capasso G. Nitrous oxide ‘whippit' abuse presenting with cobalmin responsive psychosis. J Med Toxicol. (2006) 2:714. doi: 10.1007/BF03161175
6. Chen T, Zhong N, Jiang H, Zhao M, Chen Z, Sun H. Neuropsychiatric symptoms induced by large doses of nitrous oxide inhalation: a case report. Shanghai Arch Psychiatry. (2018) 30:56–9. doi: 10.11919/j.issn.1002-0829.217084
7. Sakai JT, Hall SK, Mikulich-Gilbertson SK, Crowley TJ. Inhalant use, abuse, and dependence among adolescent patients: commonly comorbid problems. J Am Acad Child Adolesc Psychiatry. (2004) 43:1080. doi: 10.1097/01.chi.0000132813.44664.64
8. Freedenthal S, Vaughn MG, Jenson JM, Howard MO. Inhalant use and suicidality among incarcerated youth. Drug Alcohol Depend. (2007) 90:81–8. doi: 10.1016/j.drugalcdep.2007.02.021
9. Oussalah A, Julien M, Levy J, Hajjar O, Franczak C, Stephan C, et al. Global burden related to nitrous oxide exposure in medical and recreational settings: a systematic review and individual patient data meta-analysis. J Clin Med. (2019) 8:551–69. doi: 10.3390/jcm8040551
10. Quintana JC. Neuropsiquiatria: pet y spect. Revista Chilena de Radiología. (2019) 8:63–9. doi: 10.4067/S0717-93082002000200005
11. Lightfoot E, Brownlie D, Lightfoot J. Nitrous oxide toxicity: when laughing gas is no laughing matter—a discussion of two cases. Emerg Med Aust EMA. (2020) 32:710–1. doi: 10.1111/1742-6723.13538
12. Ehirim EM, Naughton DP, Petróczi A. No laughing matter: presence, consumption trends, drug awareness, and perceptions of “Hippy Crack” (nitrous oxide) among young adults in England. Front Psychiatry. (2018) 8:312. doi: 10.3389/fpsyt.2017.00312
13. Cousaert C, Heylens G, Audenaert K. Laughing gas abuse is no joke. An overview of the implications for psychiatric practice. Clin Neurol Neurosurg. (2013) 115:859–62. doi: 10.1016/j.clineuro.2013.04.004
14. Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. (2011) 301:1–8. doi: 10.1016/j.jns.2010.10.033
15. Shandal V, Luo JJ. Clinical manifestations of isolated elevated homocysteine induced peripheral neuropathy in adults. J Clin Neuromuscul Dis. (2016) 17:106–9. doi: 10.1097/CND.0000000000000108
16. Richardson KJ, Shelton KL. N-methyl-D-aspartate receptor channel blocker-like discriminative stimulus effects of nitrous oxide gas. J Pharmacol Exp Ther. (2015) 352:156–65. doi: 10.1124/jpet.114.218057
17. Jevtovic-Todorovic V, Beals J, Benshoff N, Olney JW. Prolonged exposure to inhalational anesthetic nitrous oxide kills neurons in adult rat brain. Neuroscience. (2003) 122:609–16. doi: 10.1016/j.neuroscience.2003.07.012
18. Kaar SJ, Ferris J, Waldron J, Devaney M, Ramsey J, Winstock AR. Up: the rise of nitrous oxide abuse. An international survey of contemporary nitrous oxide use. J Psychopharmacol. (2016) 30:395. doi: 10.1177/0269881116632375
19. Tym MK, Alexander J. Nitrous oxide induced manic relapse. Aust N Z J Psychiatry. (2011) 45:1002. doi: 10.3109/00048674.2011.580454
Keywords: neuropsychiatric symptoms, neuropsychological, regional cerebral blood flow, nitrous oxide abuse, SPECT
Citation: Wang L, Yin L, Wang Q, Wang R, Liu Z, Dong M, Duan X, Zheng Y, Hong W, Liu F and Tie C (2022) SPECT findings on neuropsychiatric symptoms caused by nitrous oxide abuse. Front. Psychiatry 13:980516. doi: 10.3389/fpsyt.2022.980516
Received: 28 June 2022; Accepted: 21 October 2022;
Published: 17 November 2022.
Edited by:
Yuka Kotozaki, Iwate Medical University, JapanReviewed by:
Richa Tripathi, All India Institute of Medical Sciences Gorakhpur, IndiaHiroshi Matsuda, Southern TOHOKU Research Institute for Neuroscience, Japan
Copyright © 2022 Wang, Yin, Wang, Wang, Liu, Dong, Duan, Zheng, Hong, Liu and Tie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Liu, ZmFuZ2xpdTIzQDE2My5jb20=; Changle Tie, dGllY2hhbmdsZUAxNjMuY29t
 Li Wang
Li Wang Lijie Yin
Lijie Yin Qian Wang1
Qian Wang1 Zunjing Liu
Zunjing Liu Xiaohui Duan
Xiaohui Duan Yumin Zheng
Yumin Zheng