
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Psychiatry , 26 October 2022
Sec. Psychopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.972420
This article is part of the Research Topic Women in Psychiatry 2023: Psychopharmacology View all 7 articles
Background: Carriers of the 22q11.2 deletion syndrome (22q11DS) have an enhanced risk of developing psychotic disorders. Full-blown psychosis is typically diagnosed by late adolescence/adulthood. However, cognitive decline is already apparent as early as childhood. Recent findings in mice show that antipsychotic medication administered during adolescence has a long-lasting neuroprotective effect. These findings offer promising evidence for implementing preventive treatment in humans at risk for psychosis.
Methods: We conducted a 12-week double-blind randomized controlled clinical trial with individuals with 22q11DS. Recruitment difficulties resulted in a final sample size of 13 participants (n = 6 treated with antipsychotics and n = 7 receiving placebo). We examined the response to treatment and assessed its short- and long-term effects on psychotic symptomatology using the Structured Interview for Psychosis-Risk Syndromes (SIPS) and cognitive measures.
Results: First, two treated participants discontinued treatment after experiencing adverse events. Second, treated participants showed a short-term improvement in 33.3% of the SIPS items, mainly those targeting negative symptoms. Third, reliable improvements in at least one measure of working memory and attention were respectively found in 83.3 and 66.7% of treated participants.
Conclusion: This is the first double-blind study to investigate the potential neuroprotective effect of antipsychotics in humans at risk for psychosis. Our preliminary results suggest that antipsychotic treatment may prevent long-term deterioration in clinical symptoms and cognitive skills. Yet, given the limited sample size, our findings need to be replicated in larger samples. To do so, future studies may rather adopt open-label or retrospective designs to ensure sufficient power.
Clinical trial registration: [www.ClinicalTrials.gov], identifier [NCT04639960].
22q11.2 deletion syndrome (22q11DS) is a neurogenetic disorder which prevalence reaches as high as 1 in 2148 livebirths (1). The condition is associated with a 41% increased risk of developing schizophrenia by adulthood (2). The presentation of psychosis in 22q11DS is comparable to idiopathic psychosis, where its onset is understood as the last stage of an atypical neurodevelopment (3, 4). Studying 22q11DS therefore represents a unique opportunity to identify specific markers for the emergence of psychosis (5). One of the first apparent manifestations of psychosis lies in the cognitive development (6). In both deleted and non-deleted individuals, a global deterioration in reasoning skills [as measured by intellectual quotient (IQ) points] has been identified in early adolescence, prior the onset of psychotic symptoms (7–9). Moreover, such cognitive deficits are underpinned by several structural brain alterations which also precede psychosis onset affecting both cortical (10, 11) and subcortical structures (12, 13). These abnormalities are associated with developmental alterations in high-frequency oscillations, particularly in the gamma-band range [30–100 Hz (14, 15)], which synchronization allows for flexible communication between cortical regions (16).
In the last decades, a multitude of studies has allowed to better define the neuroanatomical and neuropsychological phenotypes in deleted individuals with psychosis. Yet currently, one major challenge is to develop early neuroprotective interventions to prevent the cerebral and cognitive deteriorations which predate psychosis (17). A recent study has highlighted the promising neuroprotective effect of selective serotonin reuptake inhibitors (SSRI) treatment in children and adolescents with 22q11DS (18). Participants treated with SSRIs showed improved IQ scores and increased cortical thickness in the frontal lobe and the hippocampus, which were more pronounced as treatment started early, suggesting the potential benefit to intervene during the critical developmental window of adolescence. Furthermore, a combination of SSRIs and atypical antipsychotics revealed stronger effects on cognitive and brain outcomes. However, the specific contribution of antipsychotics as a potential neuroprotective therapy is yet to be investigated in 22q11DS.
Recently, antipsychotic treatment has begun to attract attention as a promising neuroprotective intervention in 22q11DS. First evidence arose from one study in LgDel+/-mice, the homologous mice model of 22q11.2 deletion (19). The authors discovered deficits in parvalbumin interneurons (PVI) in LgDel+/-mice, which have a crucial role in the generation of gamma oscillations and in cognitive functioning (20). Interestingly, the severity of these deficits drastically depended on the developmental stage. While PVI hypofunction was restricted to the CA1 and subiculum regions of the hippocampus during early adolescence, it spread to medial prefrontal cortex (mPFC), basolatereal amygdala, and dorsal striatum from late adolescence onwards. By adulthood, these cerebral alterations became more pronounced and were associated with reduced power in the high-gamma range, along with impaired cognitive flexibility and episodic memory. To counteract this deterioration, the authors repeatedly administered a D2 receptor antagonist (risperidone) in ventral hippocampus or mPFC. Strikingly, their findings indicate a long-lasting rescue of PVI only during a specific time-window in late adolescence, which prevented cognitive and gamma-band deficits in LgDel+/- mice. As risperidone is a commonly used atypical antipsychotic, these results have important clinical implications for individuals with 22q11DS. When taken as a preventive treatment, antipsychotics may represent a promising intervention for counteracting the developmental alterations associated with the syndrome.
Antipsychotics are widely prescribed in deletion carriers to treat psychotic symptoms. The evidence for their effectiveness and safety in 22q11DS is yet limited. In a retrospective study (21), comparable rates of adverse events and improvement were showed between deleted and non-deleted individuals with psychosis. The authors concluded that antipsychotics are both effective and relatively safe in the management of psychotic symptoms. Nevertheless, their conclusion is limited by a small sample size, which is neither representative of the 22q11DS population nor comparable to the more substantial samples used in studies with non-deleted individuals. Moreover, as the adverse effects of antipsychotics are common manifestations observed in 22q11DS (e.g., weight gain, cardiac side effects; 22), no definite conclusions can be drawn regarding the safety and the tolerability of antipsychotics in 22q11DS.
In the present study, our aim was twofold. Firstly, to examine the response to atypical antipsychotic treatment (risperidone) in deletion carriers. Secondly, to investigate the short- and long-term effects of risperidone on psychotic symptoms and cognitive functions. Based on encouraging results on mice, we expected to find favorable effects of risperidone treatment on cognitive skills in deletion carriers. To test this hypothesis, we conducted a double-blind randomized controlled clinical trial (DBRCT) in deletion carriers. However, conducting a rigorous study with individuals affected by a relatively rare genetic condition proved to be tremendously challenging. Given the potential benefits for the families of individuals with 22q11DS, we have persevered in our efforts and carried out this study for 3 years and 7 months. Despite our strong commitment, we terminated this clinical trial, mainly due to challenges encountered in the recruitment process which resulted in a limited sample size. Nonetheless, given the paucity of pharmacological trials in 22q11DS, we present here novel and valuable evidence for the effects of risperidone on psychotic symptoms and cognitive functions in deletion carriers.
Participants were recruited from the longitudinal cohort of 22q11DS patients based in Geneva (see flow diagram in Figure 1). Sixteen participants (5 females) with 22q11DS were enrolled in this study and had a deletion confirmed by quantitative fluorescent polymerase chain reaction (QF-PCR). They were recruited from September 29th 2017 to May 1st 2021. Written informed consent based on protocols approved by the Swiss Ethical Committee of Geneva (CCER) and the Swiss Agency for Therapeutic Products (Swissmedic) was obtained from participants or parents (for participants younger than 18 years). Inclusion and exclusion criteria are available in Supplementary material.
Among the total sample, one participant was excluded due to long QTc (>460 ms) measured at baseline. Moreover, two participants discontinued the study. The first after experiencing a prolonged QTc interval (+103 ms) at Day 7. The second after experiencing malignant hyperthermia (body temperature increase of 1.3°C and trembling) at Day 25. For both cases, breaking the blind revealed active treatment, and complications resolved after discontinuation, without sequelae. The final sample included 6 participants in the risperidone group and 7 participants in the placebo group. Reasons for non-participation included lack of time to commit to the study, reluctance to take a preventive psychotropic treatment, and lack of evidence in humans with 22q11DS.
This study entailed three assessments (Supplementary Figure 2), conducted by a trained psychologist in Geneva. The first assessment (baseline) occurred 1 month before the treatment phase. A month later, treatment was introduced for a duration of 12 weeks. It took place under increased monitoring, with regular contacts organized between the examiner, the participant, and their caregivers. Frequent temperature checks and the completion of an electrocardiogram (ECG) at beginning of treatment were requested. The second and third visits occurred 7 days and 6 months after the end of treatment, and aimed to assess short- and long-term effects, respectively.
Participants were randomized to receive either placebo or risperidone. Treatment allocation was randomized by the pharmacy service of the Lausanne University Hospital (CHUV), such that investigators and participants were blind to the medication. Regarding dosage, three weight categories were defined (see Supplementary Table 1). Treatment was introduced and interrupted progressively. Unblinding procedure was performed after the third visit of the last enrolled participant.
First, we investigated safety and tolerance to treatment by assessing reported side effects. Second, we evaluated the presence and severity of psychotic symptoms. Third, we examined the effects of treatment on several cognitive domains. The study protocol is comparable to that of one of our previous studies of Maeder et al. (22).
Two ECGs were obtained: the first at baseline, and the second between Days 4 and 7 of treatment by the patient’s referring physician. We verified that no elongation of QTc (>30 ms) occurred after treatment onset.
A homemade questionnaire was sent to the participant and their caregivers on days of increasing or decreasing increments in dosage. We collected information regarding administration of treatment, temperature measurement and side effects. The evaluation of side effects was inspired by the Barkley Side Effect Rating Scale (23), with seven categories being investigated (cardiovascular, gastrointestinal, psychiatric, mood, neurologic, sleep disturbances, and other side effects). Severity of side effects was measured on a 5-intensity scale (1 = mild to 5 = severe).
We assessed the presence and the severity of psychotic symptoms at each visit using the Structured Interview for Psychosis-Risk Syndromes (SIPS), a clinical tool validated in the 22q11DS population (24, 25). The SIPS is comprised of symptoms belonging to four subscales (positive, negative, disorganization, and general), rated on a 7-severity scale (0 = absent to 6 = severe and psychotic).
We administered several cognitive tasks at each visit targeting the following domains: planning, initiation, processing speed, updating, inhibition, cognitive flexibility, attention, and (verbal and nonverbal) learning and long-term memory. A description of each measure is available in Supplementary Table 2.
Prior to the beginning of recruitment, a power analysis showed that a minimal sample size of n = 26 was needed to obtain 0.8 power, p-value of 0.05 and 0.5 mean effect size. Given our sample size of 13 participants, no statistical analysis could be performed to detect group differences. Considering our limited sample size and the within-group heterogeneity observed in cognitive measures (see Supplementary Figure 1), we opted for individual analyses. We assessed intra-individual change in clinical and cognitive measures after risperidone or placebo. We used the Reliable Change Index (RCI) to quantify the magnitude of short- and long-term change and determine whether it is clinically reliable or not (26). Using the Jacobson-Truax Method, we calculated the RCI formula for each variable, as follows:
where X1 represents the participant’s score at the first assessment (baseline), X2 represents the participant’s score at the second or third assessment (to assess short- or long-term change, respectively), and SEdiff is the standard error of difference between the two time-points. Reliable change is achieved if the index exceeds the threshold of ±1.96 (two-tailed 95% confidence interval). Beyond this threshold, the change is considered to not be only due to measurement error, and indicates either a reliable improvement or a reliable deterioration. Percentages were then computed based on measures that showed reliable change.
Findings regarding safety and tolerability of treatment are shown on Supplementary Table 3 (see Supplementary Table 4 for the placebo sample). The ECGs completed during treatment revealed no significant increase (>30 ms) in QTc intervals. Temperature checks showed slight decreases and increases (<1°C).
The distribution of side effects in each group appears in Figure 2. Most participants from the treated group (66.67%) reported at least one side effect during treatment. The majority of side effects (40%) was reported when dosage increased. The most prevalent side effects affected the gastrointestinal and neurologic systems. Among treated participants, half experienced gastrointestinal disturbances, with stomachache of mild intensity being the most represented (66.67%). Two participants experienced neurologic side effects when dosage increased: one reported finger paresthesia and the other, headache. Regarding other categories, moderate sleep disturbances were reported once (16.67%) and mild hot flashes (with no associated fever) were observed twice by the same participant.
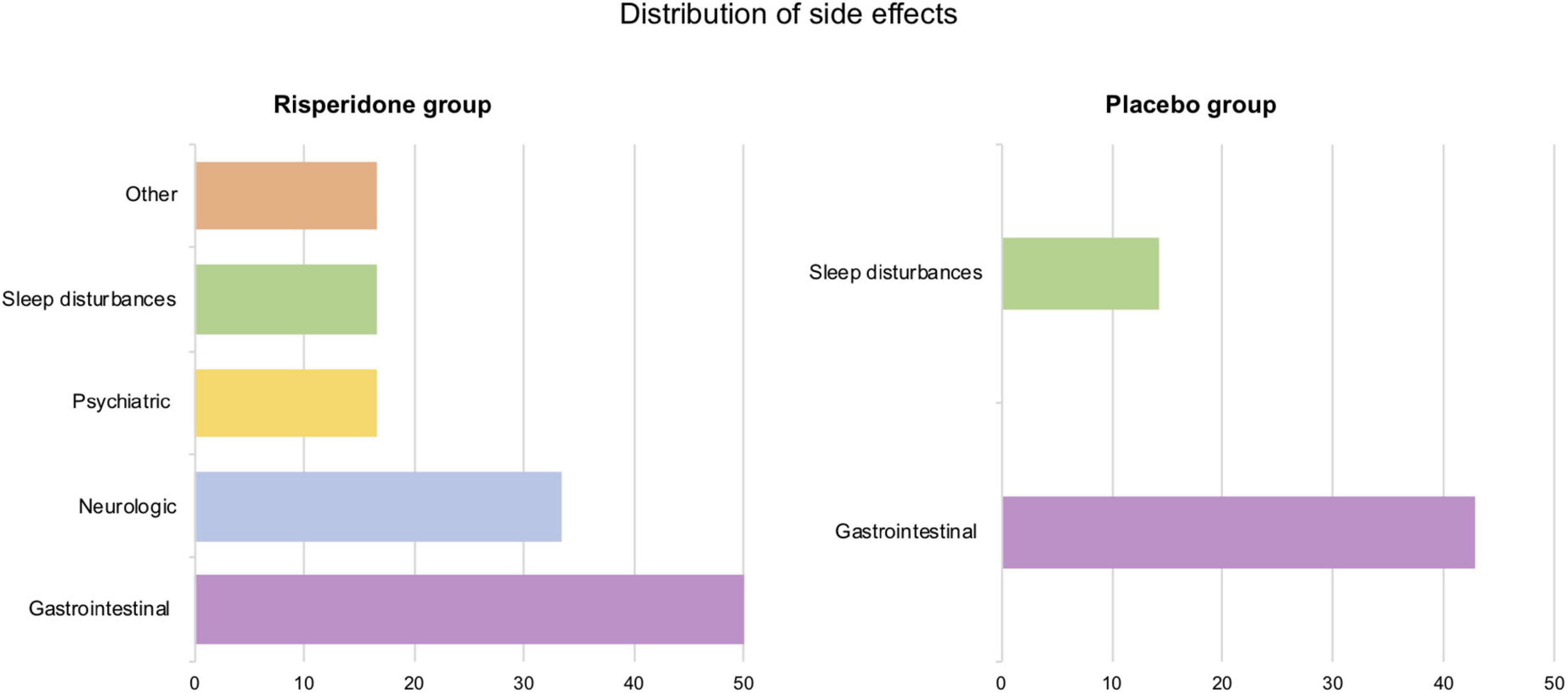
Figure 2. Distribution of side effects by category (in percentages) in the risperidone and the placebo groups.
The placebo group also reported several side effects (42.86%). Gastrointestinal disturbances were the most represented (42.86%) as well, both at the beginning and the end of the treatment phase. We observed a slight prolongment in QTc in two participants, yet as no functional complaint was associated, treatment was continued in agreement with their respective referring physicians.
Results regarding the SIPS are in Supplementary Table 5 for the treated group and in Supplementary Table 6 for the placebo group. We first examined the severity scores of each subscale at baseline. The treated group tended to display greater mean severity in the positive subscale (m = 5.17) compared to the placebo group (m = 1.57), meaning that the potential for improvement in positive symptoms was lower in the placebo group. Comparable scores between groups were observed for the negative (m = 11 and m = 10.86, for the risperidone and placebo groups, respectively), disorganization (m = 4.17 and m = 4.43), and general subscales (m = 2.83 and m = 2.43).
First, when considering the nineteen symptoms of the SIPS 1 week after treatment ended, a reliable improvement was found in 8.77% of the symptoms in treated participants (vs. 6.02% in non-treated participants). We observed a reliable deterioration in 1.75% of symptoms in the treated group (vs. 3.01% in the placebo group). Second, when only considering the four subscale scores, a reliable improvement of 33.3% was revealed in the treated group (vs. 14.29% in the placebo group). A reliable short-term deterioration was revealed in one treated participant (vs. none was found in the placebo group).
All treated participants showed improved psychotic symptomatology in at least one of the four subscale scores, mostly in negative (50%) and positive (33.3%) symptoms. In the placebo group, three participants (42.86%) showed short-term improvements, in the positive and the general subscales, to the same extent (28.57%). The proportion of short-term improvements for both groups and on each subscale score appears in Figure 3.
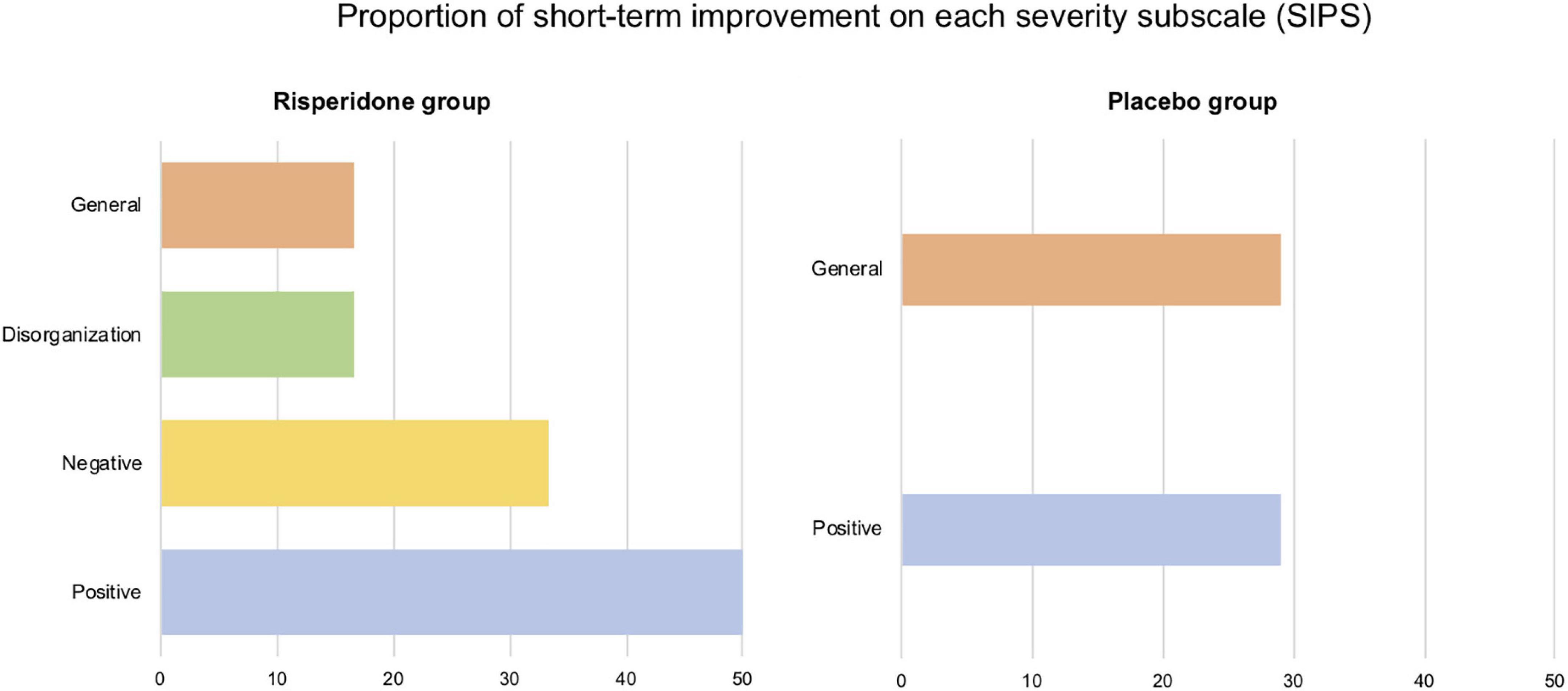
Figure 3. Proportion of short-term improvements on each severity subscale (positive, negative, disorganization, and general) of the Structured Interview for Psychosis-Risk Syndromes (SIPS). All (6/6) participants of the risperidone group displayed one improved score in at least one severity scale. In the placebo group, this proportion is 42.86% (3/7 participants).
Six months after risperidone treatment, 9.65% of symptoms showed a reliable improvement (vs. 5.26% in the placebo group). A reliable deterioration was revealed in 0.88% of symptoms (vs. 9.02% in the untreated group). Regarding subscale scores, we observed an improvement of 12.5% in treated participants (vs. 3.57% in untreated participants). No long-term deterioration was observed in the treated group while a deterioration of 3.57% was observed in the placebo group.
More precisely, in the treated group, the proportion of long-term improvement was driven by Participant 6 only, who showed reliable decreases in severity of the negative, disorganization and general subscale scores. In the placebo group, improvements in subscale scores were also focused on one participant (Participant 2), who showed improvements in general symptomatology only.
Our findings regarding all cognitive measures are shown in Figure 4 (for further details see Supplementary Table 7 for the risperidone group and Supplementary Table 8 for the placebo group).

Figure 4. Short- and long-term effects treatment on all cognitive measures in the risperidone and placebo groups. Reliable change (i.e., improvements and deteriorations) is achieved if the Reliable Change Index (RCI) exceeds the threshold of ±1.96 (two-tailed 95% confidence interval).
We observed a short-term improvement in 7.29% of cognitive measures in the treated group, compared to 6.70% in the placebo group. Conversely, a reliable deterioration was noted in 6.77% of cognitive measures in the treated group (vs. 9.38% in the placebo group). In the treated group, most improved measures in the short-term follow-up occurred in two domains, namely working memory (WM; 50%) and attention (28.57%), both detailed below.
Reliable long-term improvements were found in 11.46% of measures in the treated group (vs. 6.25% in the untreated group). We observed reliable deteriorations in 3.65% of cognitive measures in the treated group, compared to 9.38% in the untreated group. Most improvements in the treated group were again found in the domains of attention (40.91%) and WM (31.82%).
Most treated participants (83.3%) showed a reliable improvement (both short- and long-term) in at least one WM measure. In the placebo group, short- and long-term improvements are, respectively observed in 14.29 and 42.86% of the sample.
Regarding short-term effects, 29.17% of WM measures were improved in the risperidone group (vs. 3.57% in the placebo group). For long-term effects, an identical proportion of improvement was found in WM measures in the treated group (vs. 10.71% in the placebo group). Yet, we observed a short-term reliable deterioration in 16.67% of WM measures whereas no short-term deterioration was noted in the placebo group. Reliable long-term deteriorations in the treated group affected 8.33% of WM measures, which all occurred in Participant 5. No reliable long-term deterioration was observed in the placebo group.
More than half (66.67%) of the treated group showed improvements in at least one attentional measure (vs. 42.87% in the placebo group). These proportions were the same in both short- and long-term comparisons. Note that Participant 6 (placebo group) started taking methylphenidate (MPH) just before the third visit. As MPH was found to have a favorable effect on attention and inhibition in 22q11DS (22), careful attention must be paid when examining long-term effects.
Regarding short-term effects, 7.41% of attentional measures were improved in the treated group (vs. 6.35% in the non-treated group). For long-term effects, the treated group showed an improvement in 16.67% of measures (vs. 11.11% in the placebo group). Reliable short-term deteriorations were observed in 7.41% of attentional measures in the treated group and were less prevalent in the placebo group (3.1%). Yet, in the risperidone and the placebo groups, we respectively, noted a decrease and an increase in long-term deteriorations (3.70 and 11.11%).
To our knowledge, this is the first study to investigate the effectiveness of risperidone in individuals with 22q11DS using a DBRCT design. We first aimed at examining the response to risperidone treatment. Second, we aimed at exploring short- and long-term effects of risperidone on psychotic symptoms and cognitive functions.
To assess response to risperidone in 22q11DS, we repeatedly documented changes in cardiac rhythm and body temperature, along with side effects over the course of treatment. In total, 2/8 participants enrolled in the treated group discontinued the study due to cardiac rhythm disorder and malignant hyperthermia, respectively listed as occasional (≥1/1000 to <1/100) and rare (≥1/10,000 to <1/1000) side effects of risperidone.1 Yet, a recent systematic review did not report these adverse events as being related to risperidone treatment in 22q11DS de Boer et al. (27). Conversely, the authors reported movement disorders, seizures, and weight gain as the main adverse effects of risperidone in 22q11DS, which were not observed in our sample. While this difference is likely due to our small sample size or/and to the short period of treatment, these findings suggest the wide array of adverse experiences associated with risperidone. Moreover, the high prevalence of mild to moderate side effects underline the importance of raising awareness on the great heterogeneity of risperidone response among deletion carriers. Although our limited sample size does not allow generalizations on the safety of antipsychotics in 22q11DS, our results support the implementation of an increased treatment monitoring, especially the use of ECG to monitor QTc interval. Indeed, one of the main risk factors of QTc prolongation include cardiovascular anomalies (28, 29) which reach a prevalence as high as 75% in the 22q11DS population (30, 31).
The presence and severity of psychotic symptoms was assessed with the SIPS at each visit. We found greater short-term improvements in subscale scores in the treated group compared to the placebo group, mainly in negative symptoms (particularly avolition). This finding was unexpected, as antipsychotics are reported to have limited effects on primary negative symptoms (32), which are especially observed in 22q11DS. Indeed, Schneider et al. (33) found elevated rates of negative symptoms (73.9%) which severity predicted psychosis-risk and correlated with low global functioning. Regarding long-term effects, we observed that most improvements in subscale scores were not maintained 6 months after treatment, except for Participant 6 who showed increased improvements over time. Although these effects seem mostly transient, future studies will have to further investigate the effects of preventive antipsychotic treatment on negative symptoms in 22q11DS given the potential clinical implications.
Regarding short-term deteriorations, lower rates were found in the treated group compared to the placebo group when considering all symptoms. Interestingly, the rates of deterioration tended to decrease over time in the treated group, while they tended to increase in the placebo group. The lack of worsening symptoms over time in treated participants is especially striking as they experienced some attenuated symptoms at baseline. Indeed, this finding goes against the neurodevelopmental trajectory of schizophrenia, characterized by a progression from prodromal state to psychosis (3). Therefore, while this result needs to be replicated with larger sample size, it suggests that early antipsychotic intervention may reduce or prevent clinical deterioration.
We assessed the effects of risperidone on cognitive functions using several measures. Both groups showed comparable improvements a week after interrupting treatment. Yet, 6 months later, beneficial effects seemed to increase in the treated group while they seemed stable in the placebo group. While a learning effect may be present for some short-term results given the proximity of assessments, it is likely that this effect was reduced for long-term results. Interestingly, these findings mirror those on psychotic symptomatology, indicating a decreasing number of cognitive and clinical deteriorations over time in the treated group.
Moreover, WM and attention were the domains with the most improvements in treated participants. Strikingly, deficits in both domains characterize the cognitive profile of both 22q11DS (6, 34) and idiopathic schizophrenia (3, 35). Our findings are supported by previous studies in non-deleted individuals with psychosis which investigated the effects of antipsychotics on both verbal and spatial components of WM. One previous study (36) found that administering risperidone to chronic patients with schizophrenia led to reliable improvements in verbal WM performance after 4 and 8 weeks of treatment compared to patients administered other atypical antipsychotics. In another study (37), the authors conducted a DBRCT to compare the effects of risperidone and clozapine on spatial WM in patients with moderate treatment-resistant schizophrenia. After 17 and 29 weeks of treatment, reliable improvements in spatial WM performance were found in patients treated with risperidone, whereas with clozapine, performance decreased over time. The authors hypothesized that risperidone induced a beneficial effect on WM through its increased dopamine activity in the PFC, whereas antipsychotics with a strong anticholinergic effect like clozapine are associated with cognitive deficits in patients with schizophrenia (38). Previous studies indicate favorable effects of risperidone on WM in patients with chronic or treatment-resistant schizophrenia. Our findings yet seem to indicate that such improvements may already be observed in earlier stages of psychosis, i.e., in at-risk patients. In addition, the same rate of improvements was found at long-term follow-up, suggesting the potential prolonged effects of treatment.
Compared to WM, short-term improvements in attentional skills were less marked in the treated group. This is in line with a previous review reporting less consistent effects of risperidone on attention compared to WM (39). After 6 months, we found greater improvements and less deteriorations in attentional measures compared to the non-treated group. However, based on the scores from the Conners’ Continuous Performance Test 3rd edition [(40); Supplementary Table 2], we could not distinguish any specific effects on attention measures (e.g., on inattention, impulsivity, vigilance, or sustained attention). Yet, our preliminary results may pave the way for further research on the effects of risperidone on attention in 22q11DS, given the increased prevalence and the persistence of ADHD in this population (2, 41, 42).
The main limitation of this study is our small sample size, which prevented statistical group comparisons. While DBRCTs are recognized as the gold-standard for studying treatment effectiveness, they appear as unsuitable in a rare condition like 22q11DS. Future studies may adopt less sophisticated designs (e.g., retrospective studies) to enable sufficient power and the formulation of practical therapeutic guidelines.
Another important limitation is that we could not control for the effect of concomitant psychotropic medication, taken by 5/13 participants (2/6 and 3/7 in the treated and placebo groups, respectively). As SSRIs and MPH significantly improve cognitive functions in 22q11DS (18, 22), concurrent treatment likely had an effect on our findings. The presence of multiple treatments that could interfere with the outcome measures stresses the need for larger datasets and alternative study designs.
Our study offers encouraging evidence on the neuroprotective effects of preventive risperidone treatment, with deletion carriers showing less long-term deteriorations in cognitive functions and psychotic symptoms. Improvements were targeted at negative symptoms, WM, and attention, all identified as being altered in 22q11DS. Therefore, our study adds to emerging evidence that early pharmacological interventions may prevent the typical deteriorations in patients at-risk for psychosis.
The datasets presented in this article are not readily available because they contain information that could compromise the privacy of research participants. Requests to access the datasets should be directed to corresponding author.
The studies involving human participants were reviewed and approved by the Swiss Ethical Committee of Geneva (CCER). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
CL, JM, VM, MS, and SE: investigation, writing – review, and editing. CL, JM, VM, and SE: data acquisition. CL: formal analysis and writing – original draft. All authors contributed to the article and approved the submitted version.
This work was supported by the Swiss National Science Foundation (grant #320030_179404) and the National Center of Competence in Research (NCCR) “Synapsy–The Synaptic Bases of Mental Diseases” (grant #51NF40-185897) to SE. MS was supported by the SNSF (grant #PZ00P1_174206).
We express our gratitude to the families who participated in this study. We thank Léa Chambaz, Virginie Pouillard, and Eval Micol for their help in recruitment, as well as Laura Abdili, Giulia Binarelli, Farnaz Delavari, Marina Goncalves, Marc Jeanneret, Fiona Journal, Léa Moreau, Justine Quiblier, and Corrado Sandini for their help in data collection. We also thank Pico Caroni for providing valuable advice on the design of this study.
Author SE offered Scientific Advice for the Company Servier Laboratory. He also offered invited presentations for TAKEDA, and for Salmon Pharma.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.972420/full#supplementary-material
1. Blagojevic C, Heung T, Theriault M, Tomita-Mitchell A, Chakraborty P, Kernohan K, et al. Estimate of the contemporary live-birth prevalence of recurrent 22q11.2 deletions: a cross-sectional analysis from population-based newborn screening. CMAJ Open. (2021) 9:E802–9. doi: 10.9778/cmajo.20200294
2. Schneider M, Debbané M, Bassett AS, Chow EWC, Fung WLA, van den Bree MBM, et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the international consortium on brain and behavior in 22q11.2 deletion syndrome. Am J Psychiatry. (2014) 171:627–39. doi: 10.1176/appi.ajp.2013.13070864
4. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. (2012) 17:1228–38. doi: 10.1038/mp.2012.23
5. Tang SX, Moore TM, Calkins ME, Yi JJ, Savitt A, Kohler CG, et al. The psychosis spectrum in 22q11.2 Deletion syndrome is comparable to that of nondeleted youths. Biol Psychiatry. (2017) 82:17–25. doi: 10.1016/j.biopsych.2016.08.034
6. Lewandowski KE, Shashi V, Berry PM, Kwapil TR. Schizophrenic-like neurocognitive deficits in children and adolescents with 22q11 deletion syndrome. Am J Med Genet. (2007) 144B:27–36. doi: 10.1002/ajmg.b.30379
7. Mollon J, David AS, Zammit S, Lewis G, Reichenberg A. Course of cognitive development from infancy to early adulthood in the psychosis spectrum. JAMA Psychiatry. (2018) 75:270. doi: 10.1001/jamapsychiatry.2017.4327
8. Valli I, Tognin S, Fusar-Poli P, Mechelli A. Episodic memory dysfunction in individuals at high-risk of psychosis: a systematic review of neuropsychological and neurofunctional studies. Curr Pharm Des. (2012) 18:443–58. doi: 10.2174/138161212799316271
9. Vorstman JAS, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, et al. Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry. (2015) 72:377. doi: 10.1001/jamapsychiatry.2014.2671
10. Schaer M, Debbané M, Bach Cuadra M, Ottet MC, Glaser B, Thiran JP, et al. Deviant trajectories of cortical maturation in 22q11.2 deletion syndrome (22q11DS): a cross-sectional and longitudinal study. Schizophr Res. (2009) 115:182–90. doi: 10.1016/j.schres.2009.09.016
11. Bagautdinova J, Zöller D, Schaer M, Padula MC, Mancini V, Schneider M, et al. Altered cortical thickness development in 22q11.2 deletion syndrome and association with psychotic symptoms. Mol Psychiatry. (2021) 26:7671–8. doi: 10.1038/s41380-021-01209-8
12. Mancini V, Sandini C, Padula MC, Zöller D, Schneider M, Schaer M, et al. Positive psychotic symptoms are associated with divergent developmental trajectories of hippocampal volume during late adolescence in patients with 22q11DS. Mol Psychiatry. (2019) 25:2844–59. doi: 10.1038/s41380-019-0443-z
13. Mancini V, Zöller D, Schneider M, Schaer M, Eliez S. Abnormal development and dysconnectivity of distinct thalamic nuclei in patients with 22q11.2 deletion syndrome experiencing auditory hallucinations. Biol Psychiatry Cogn Neurosci Neuroimaging. (2020) 5:875–90. doi: 10.1016/j.bpsc.2020.04.015
14. Mancini V, Rochas V, Seeber M, Grent-‘t-Jong T, Rihs TA, Latrèche C, et al. Oscillatory neural signatures of visual perception across developmental stages in individuals with 22q11.2 deletion syndrome. Biol Psychiatry. (2022) 92:407–18. doi: 10.1016/j.biopsych.2022.02.961
15. Mancini V, Rochas V, Seeber M, Roehri N, Rihs TA, Ferat V, et al. Aberrant developmental patterns of gamma-band response and long-range communication disruption in youths with 22q11.2 deletion syndrome. Am J Psychiatry. (2022) 179:204–15. doi: 10.1176/appi.ajp.2021.21020190
16. Fries P. Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu Rev Neurosci. (2009) 32:209–24. doi: 10.1146/annurev.neuro.051508.135603
17. Patel PK, Leathem LD, Currin DL, Karlsgodt KH. Adolescent neurodevelopment and vulnerability to psychosis. Biol Psychiatry. (2021) 89:184–93. doi: 10.1016/j.biopsych.2020.06.028
18. Mancini V, Maeder J, Bortolin K, Schneider M, Schaer M, Eliez S. Long-term effects of early treatment with SSRIs on cognition and brain development in individuals with 22q11.2 deletion syndrome. Transl Psychiatry. (2021) 11:336. doi: 10.1038/s41398-021-01456-x
19. Mukherjee A, Carvalho F, Eliez S, Caroni P. Long-lasting rescue of network and cognitive dysfunction in a genetic schizophrenia model. Cell. (2019) 178:1387–402.e14. doi: 10.1016/j.cell.2019.07.023
20. Dienel SJ, Lewis DA. Alterations in cortical interneurons and cognitive function in schizophrenia. Neurobiol Dis. (2019) 131:104208. doi: 10.1016/j.nbd.2018.06.020
21. Dori N, Green T, Weizman A, Gothelf D. The effectiveness and safety of antipsychotic and antidepressant medications in individuals with 22q11.2 deletion syndrome. J Child Adolesc Psychopharmacol. (2017) 27:83–90. doi: 10.1089/cap.2014.0075
22. Maeder J, Mancini V, Sandini C, Journal F, Schneider M, Kliegel M, et al. Selective effects of methylphenidate on attention and inhibition in 22q11.2 deletion syndrome: results from a clinical trial. Int J Neuropsychopharmacol. (2021) 25:215–25. doi: 10.1093/ijnp/pyab057
23. Barkley RA, Fischer M, Newby RF, Breen MJ. Development of a multimethod clinical protocol for assessing stimulant drug response in children with attention deficit disorder. J Clin Child Psychol. (1988) 17:14–24. doi: 10.1207/s15374424jccp1701_3
24. Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. (2003) 29:703–15. doi: 10.1093/oxfordjournals.schbul.a007040
25. Tang SX, Yi JJ, Moore TM, Calkins ME, Kohler CG, Whinna DA, et al. Subthreshold psychotic symptoms in 22q11.2 deletion syndrome. J Am Acad Child Adolesc Psychiatry. (2014) 53:991–1000.e2. doi: 10.1016/j.jaac.2014.05.009
26. Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. (1991) 59:12–9. doi: 10.1037/0022-006X.59.1.12
27. de Boer J, Boot E, van Gils L, van Amelsvoort T, Zinkstok J. Adverse effects of antipsychotic medication in patients with 22q11.2 deletion syndrome: a systematic review. Am J Med Genet A. (2019) 179:2292–306. doi: 10.1002/ajmg.a.61324
28. Jensen KG, Juul K, Fink-Jensen A, Correll CU, Pagsberg AK. Corrected QT changes during antipsychotic treatment of children and adolescents: a systematic review and meta-analysis of clinical trials. J Am Acad Child Adolesc Psychiatry. (2015) 54:25–36. doi: 10.1016/j.jaac.2014.10.002
29. Mosheva M, Korotkin L, Gur RE, Weizman A, Gothelf D. Effectiveness and side effects of psychopharmacotherapy in individuals with 22q11.2 deletion syndrome with comorbid psychiatric disorders: a systematic review. Eur Child Adolesc Psychiatry. (2020) 29:1035–48. doi: 10.1007/s00787-019-01326-4
30. McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JAS, et al. 22q11.2 deletion syndrome. Nat Rev Dis Primers. (2015) 1:15071. doi: 10.1038/nrdp.2015.71
31. Goldmuntz E. 22q11.2 deletion syndrome and congenital heart disease. Am J Med Genet. (2020) 184:64–72. doi: 10.1002/ajmg.c.31774
32. Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. (2020) 16:519-34. doi: 10.2147/NDT.S225643
33. Schneider M, Armando M, Schultze-Lutter F, Pontillo M, Vicari S, Debbané M, et al. Prevalence, course and psychosis-predictive value of negative symptoms in 22q11.2 deletion syndrome. Schizophr Res. (2019) 206:386–93. doi: 10.1016/j.schres.2018.10.014
34. Maeder, J, Zuber S, Schneider M, Kliegel M, Eliez S. Age-related improvements in executive functions and focal attention in 22q11.2 deletion syndrome vary across domain and task. J Int Neuropsychol Soc. (2021) 28:337–50. doi: 10.1017/S135561772100059X
35. Forbes NF, Carrick LA, McIntosh AM, Lawrie SM. Working memory in schizophrenia: a meta-analysis. Psychol Med. (2009) 39:889–905. doi: 10.1017/S0033291708004558
36. Mori K, Nagao M, Yamashita H, Morinobu S, Yamawaki S. Effect of switching to atypical antipsychotics on memory in patients with chronic schizophrenia. Prog Neuro Psychopharmacol Biol Psychiatry. (2004) 28:659–65. doi: 10.1016/j.pnpbp.2004.01.019
37. McGurk SR, Carter C, Goldman R, Green MF, Marder SR, Xie H, et al. The effects of clozapine and risperidone on spatial working memory in schizophrenia. Am J Psychiatry. (2005) 162:1013–6. doi: 10.1176/appi.ajp.162.5.1013
38. Joshi YB, Thomas ML, Braff DL, Green MF, Gur RC, Gur RE, et al. Anticholinergic medication burden–associated cognitive impairment in schizophrenia. Am J Psychiatry. (2021) 178:838–47. doi: 10.1176/appi.ajp.2020.20081212
39. Lee J, Park S. Effects of antipsychotic drugs on memory and attention in schizophrenia. Psychiatry Investig. (2006) 3:55–65.
40. Conners CK, Mhs Staff. Conners Continuous Performance Test 3rd edition: Technical manual. North Tonawanda, NY: Multi-Health Systems (2014).
41. Antshel KM, Hendricks K, Shprintzen R, Fremont W, Higgins AM, Faraone SV, et al. The longitudinal course of attention deficit/hyperactivity disorder in velo-cardio-facial syndrome. J Pediatrics. (2013) 163:187–93.e1. doi: 10.1016/j.jpeds.2012.12.026
Keywords: 22q11 deletion syndrome, psychotic disorders, neuroprotective effect, cognition, antipsychotic treatment
Citation: Latrèche C, Maeder J, Mancini V, Schneider M and Eliez S (2022) Effects of risperidone on psychotic symptoms and cognitive functions in 22q11.2 deletion syndrome: Results from a clinical trial. Front. Psychiatry 13:972420. doi: 10.3389/fpsyt.2022.972420
Received: 18 June 2022; Accepted: 11 October 2022;
Published: 26 October 2022.
Edited by:
Marijn Lijffijt, Baylor College of Medicine, United StatesReviewed by:
Reiji Yoshimura, University of Occupational and Environmental Health Japan, JapanCopyright © 2022 Latrèche, Maeder, Mancini, Schneider and Eliez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caren Latrèche, Y2FyZW4ubGF0cmVjaGVAdW5pZ2UuY2g=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.