
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 20 January 2023
Sec. Public Mental Health
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.947987
This article is part of the Research Topic Mental Health Promotion and Protection View all 34 articles
 Zhuo-Hui Huang1†
Zhuo-Hui Huang1† Fei Wang1†
Fei Wang1† Zi-Lang Chen2
Zi-Lang Chen2 Yao-Nan Xiao2
Yao-Nan Xiao2 Qian-Wen Wang3
Qian-Wen Wang3 Shi-Bin Wang1
Shi-Bin Wang1 Xiao-Yan He4
Xiao-Yan He4 Christine Migliorini5,6
Christine Migliorini5,6 Carol Harvey5,6
Carol Harvey5,6 Cai-Lan Hou1,7*
Cai-Lan Hou1,7*Objective: The consequences and impact of violent behavior in schizophrenia are often serious, and identification of risk factors is of great importance to achieve early identification and effective management.
Methods: This follow-up study sampled adult patients with schizophrenia in primary mental health care in a rural area of southern China, in which 491 participants completed a comprehensive questionnaire at baseline and the 2-year follow-up. Sociodemographic, clinical and psychological assessment data were collected from all participants. Paired sample T-Tests and the McNemar Test were performed to examine changes over the follow-up period. Generalized Estimating Equations (GEE) were used to analyze the risk factors for violent behavior.
Results: The results showed that about two in five community-dwelling patients with schizophrenia reported violent behavior in the past year. At follow-up, participants were significantly less employed, had more times of hospitalization, more psychotropic medication, and severer depressive symptoms, but had better health-related quality of life than at baseline. Use of clozapine and better insight into medication decreased the possibility of violent behavior, while more severe positive symptoms, insomnia, as well as use of second-generation antipsychotics other than clozapine, antidepressants and mood stabilizers increased the possibility of violent behavior.
Conclusions: Risk evaluation, prevention and management of violence in patients with schizophrenia are demanded in primary mental health care.
Violent behavior in patients with schizophrenia or other psychosis is a research interest dating back over six decades (1). Violence is a broad concept, including everything from verbal threats and hostility to homicide. Fazel et al. (2) defined violent behavior as homicide, assault, robbery, arson, any sexual offense, or illegal threats and harassment, while Rund's study focused on severe violence (3). The lack of clear operationalized definitions of violent behavior applied in different research has led to mixed results. In general, the causes of violent behavior are multifactorial, including individual as well as social or environmental factors, such as substance abuse, past violent victimization, violence in the surrounding environment and so on (3–5). Violence in psychiatric disorders might be a multidimensional issue which includes several subtypes with different psychopathological underpinnings, and is often linked to acute psychomotor agitation than a long-lasting state (48, 49).
Despite many inconsistent findings, it is generally agreed that there is a modest association between violence and schizophrenia or other psychosis (6, 7). Meta-analysis has revealed the odds of homicide being committed by individuals with psychosis was almost 20 times that of the general population irrespective of comorbid substance abuse (8). A further meta-analysis also demonstrated that schizophrenia spectrum disorders are associated with a heightened risk of violent offending (9), in which some studies estimated that up to 20% of patients with schizophrenia in the community will behave violently in a 6-month period (34).
The consequences of violent perpetration among people with schizophrenia or other psychosis are often very serious for the victims, the patient themselves and the wider community (3), which leads to serious public health concern and social problems (10). Furthermore, the high disease burden and economic impact associated with violence are also critically important. Senior et al. (11) noted that the estimated annual economic impact of violence perpetrated by people with severe mental illness was £2.5 billion in England and Wales in 2015 to 2016, or 5.3% of the total estimated societal cost of violence. Unfortunately, no relevant Chinese data of economic impact of violence perpetrated by patients with schizophrenia or other severe mental illness.
Therefore, exploration of risk factors for violent behavior in schizophrenia or other psychosis is of great importance, so as to achieve early identification and prevention. A previous review found that severe violence appears particularly associated with poor insight, high impulsivity, psychopathy, poor motor speed and global cognition (3). Fazel et al. (2) reported that the strongest predictors for violent offending within 12 months in patients with severe mental illness were conviction for previous violent crime (adjusted OR = 5.03) and male sex (adjusted OR = 2.32), and meanwhile the decline in probability of violent offending was linearly related to increasing age (adjusted OR = 0.63 per 10 years of age). Similarly, an Italian study showed that patients with severe mental illness with a history of violence had a greater frequency of lifetime domestic violence than those without a violence history (12).
However, many previous studies examining risk factors for violence in psychosis tend to be cross-sectional in design and conducted in well-developed regions (4, 12–14). Therefore, this study aimed to explore the socio-demographic features, clinical characteristics, and medication of patients with schizophrenia using a longitudinal design. We hypothesized that severer psychiatric symptoms, severer depressive symptoms, poorer insight into disease and insomnia will increase the possibility of violent behavior.
In particular, the design will enable the identification of risk factors for their violent behavior in a rural area of the middle-income country of China, thus enhancing the generalizability of the results to similar developing regions. A secondary aim of the study is to strengthen risk evaluation and guide prevention, as well as management, of potentially dangerous behaviors in patients with schizophrenia or other psychoses in this and similar settings.
This follow-up study was led by Guangdong Mental Health Center, Guangdong province in China, and The University of Melbourne. Ethical clearances were obtained from the above-mentioned institutions (Z2019-120 and 2021-20740-15415-3, respectively).
Study participants were all administered by, and recruited from Luoding city, which is an underdeveloped and rural area of Guangdong province in southern China, fitting the research interest of the present study. Also, Luoding is a city which can provide relatively good primary mental health services in Guangdong Province. In the present study, 21/63 townships with primary mental health care services in Luoding city were chosen by a random cluster sampling method, for which the randomized digital table was applied. All local patients with schizophrenia who presented to primary mental health care services were registered and managed in the Chinese National Psychiatric Management System (CNPMS). CNPMS was established to provide community follow-up management for severe mental illness, in which individuals with schizophrenia, schizoaffective disorder, paranoid psychosis, bipolar disorder, mental disorder related to epilepsy, and intellectual disability with psychotic symptoms were required to enroll.
First, the sampled schizophrenic patients from CNPMS were contacted by telephone to clarify the details of the research protocol. If they were willing to participate, both participants and their caregivers would took part in a 1-h face-to-face interview, which was conducted by one of three psychiatrists, each with no <3 years clinical and scientific research experience. After the interview, patients were included if they met the inclusion criteria (see below for more details) for the present study. At baseline, a total of 742 patients with schizophrenia were included. The baseline survey was conducted from October 2015 to January 2016 (15). 2 years later, researchers telephoned all the study participants and conducted face-to-face follow-up evaluations from November 2017 to January 2018, in which 491 participants completed the interview, and the remaining 251 participants refused follow-up. We only conducted one follow-up till now, yet there will be on-going follow-ups in the future.
Inclusion criteria for the participants were as follows: (a) aged 18 years or older; (b) diagnosed with schizophrenia (according to ICD-10) based on a review of the medical record and supplemented by clinical interview conducted by one psychiatrist; (c) capable of understanding and completing the interview according to the researchers. Exclusion criteria were having a history of significant head injury, seizures, cerebrovascular diseases and other neurological diseases.
Each eligible potential participant who was deemed competent to participate in an interview was informed of the purpose, significance, content (inclusion criteria, research procedures, etc.), benefit and confidentiality of the study, after which their written informed consent to participate in the study was obtained.
Basic sociodemographic information was collected for all study participants, including age, gender, education, marital status (married or unmarried) and occupational status (employed or unemployed); clinical features including first episode or not, age of onset of illness, number of lifetime hospitalizations, and psychotic medication usage were also collected. Additionally, gender, education, first episode or not, age of onset of illness and whether living with others were examined only at baseline, while the rest of the above variables were examined both at baseline and follow-up.
All the participants and their caregivers were asked whether the participants had engaged in any violent behavior toward others in the past 12 months. Violent behavior was evaluated by the psychiatrists, using a six-level risk assessment scale according to the National Standards for Basic Public Health Services (the Third Edition), which was issued by the Chinese National Health and Family Planning Commission (http://www.nhc.gov.cn/jws/s3578/201703/d20c37e23e1f4c7db7b8e25f34473e1b.shtml). According to the above regulations, the Chinese psychiatrists must apply this six-level risk assessment scale for the management and treatment in patients with severe mental disorders. Level 0: does not conform to any of the following level 1–5 behaviors; Level 1: verbal threats, shouting, but no beating people or smashing objects; Level 2: beating or smashing behavior against property, restricted to the home, can be persuaded to stop; Level 3: serious beating or smashing behavior against property, regardless of occasion, cannot be persuaded to stop; Level 4: continued beating or smashing behavior against property or people, regardless of occasion, cannot be persuaded to stop, including self-injury or suicidal behavior; Level 5: any act of violence against a person with a controlled dangerous weapon, or acts of arson or explosion, whether at home or in a public place. In this study, Level 0 was classified as no violent behavior, while levels 1–5 were classified as presence of violent behavior.
The Chinese version of the Brief Psychiatric Rating Scale (BPRS) was used to evaluate the severity of clinical symptoms. The results of total and sub-evaluation of BPRS were very consistent (r = 0.85–0.99, P < 0.01), and there was a high degree of consistency between the checks-recheck total scores within 3 days (r = 0.52, P < 0.01), which indicated good reliability. Additionally, there was a high positive correlation between the total score of BPRS and the clinician's judgment of the severity of the disease (r = 0.84, P < 0.01), and the efficacy assessed by BPRS was basically consistent with the clinical efficacy (r = 0.60, P < 0.01), which indicated good validity (16, 17). BPRS is a 7-point Likert-type clinician-administered scale, rated from no symptoms (1) to extremely severe (7), and higher scores indicate more severe symptoms.
The Chinese version of the Montgomery–Asberg Depression Rating Scale (MADRS) was used to measure depressive symptoms. The inter-rater reliability of Chinese version of MADRS was 0.954, the Cronbach α coefficient was 0.847, and the criterion related validity with HAMD total score and CGI-S were 0.853 and 0.672, respectively (both P < 0.01), indicating good reliability and validity (18, 19). MADRS is a 6-point Likert-type clinician-administered scale. The total score ranges from 0 to 60 points, and higher scores represent more severe depressive symptoms. Meanwhile, we employed the Chinese version of the 16-Item Quick Inventory of Depressive Symptomatology, self-report (QIDS-SR16) to enable the participants to rate their own depressive symptoms. The internal consistency (Cronbach's alpha) ranged from 0.73 to 0.82 for QIDS-SR at both the baseline and exit (6 weeks later). The QIDS-SR total scores were highly correlated with the HAMD total score at both baseline (r = 0.54, p < 0.01) and exit r = 0.72, p < 0.01, respectively) (20, 21). This scale consists of 16 items, each of them ranges from 0 to 3 points, and a higher total score indicates greater severity of depressive symptoms.
Insight was measured by the Chinese version of the Insight and Treatment Attitude Questionnaire (ITAQ). The retest reliability was 0.869 (p < 0.001), the inter-rater reliability was 0.80, the half-score reliability was 0.903, and the validity between clinical experience of the general evaluation results of insight and BPRS was 0.7075 and −0.40, respectively, indicating that ITAQ has good reliability and validity (22, 23). In the scale, five items assessed patients' awareness of illness, while 6 items assessed patients' awareness of the need for treatment. ITAQ is a clinician-administered scale, in which each item ranges from 0 to 2 points, and a higher total score means better insight.
Sleep status was also evaluated as sleep disturbance may also be relevant to violent behavior (24). We used an eight-question self-report scale to assess the sleep status of the participants over the past month, which is widely used in other Chinese studies (25, 26). The scale included four sub-domains: any type of insomnia [e.g., difficulty initiating sleep (Over the past month, have you had trouble falling asleep), difficulty maintaining sleep (Over the past month, have you had difficulty maintaining sleep for a long time or waking up from time to time?) and early morning wakening [For the past month, have you had trouble waking up in the middle of the night or waking up too early to go to sleep again]; whether insomnia requires treatment [Have you been using sleeping pills (medicines) for insomnia for the past 1 month?]; whether bothered by insomnia [Have you been bothered by insomnia for the past 1 month?]; whether insomnia affects life, work and study [Have you suffered from insomnia that affects life, work, and study for the past 1 month?
The Sheehan Disability Scale (SDS) with good reliability and validity (27) was used to evaluate functional impairment. The internal consistency reliability of the SDS is high, with coefficient alpha of 0.89 and the construct validity was substantiated in two ways (28). SDS is a self-report scale, including three sub-domains of functioning: work/school, social life and family life. The total score ranges from 0 to 30 points, and higher scores represent poorer functioning.
We used the Chinese brief version of the World Health Organization Quality of Life instrument (WHOQOL-BREF) to evaluate health-related quality of life. The scale has good internal consistency, discriminative validity and structural validity. There is a high correlation between the scores in each field of the WHOQOL-BREF and the scores in the corresponding fields of the WHOQOL-100 scale, in which the Pearson correlation coefficient is 0.89 (Social relationships domain) at the lowest and 0.95 (physical health domain) at the highest (29, 30). WHOQOL-BREF consists of 28 items divided into four sub-domains: physical health, psychological health, social relationships and environment. WHOQOL-BREF is a self-report scale, in which each item ranges from 1 to 5 points, and a higher total score means better quality of life.
Treatment satisfaction of the patients, their relatives and doctors were separately measured with a 7-point Likert-type self-report scale, in which the total score ranges from 1 to 7 points, representing the degree of satisfaction from extremely unsatisfactory to extremely satisfactory.
Reliability training was conducted by the three researchers prior to this study, in which 20 patients with schizophrenia were co-rated. The interrater reliability of the rating instruments yielded highly satisfactory agreement (intraclass correlation coefficients and kappa values >0.90).
Data were analyzed by IBM SPSS v26.0. The comparisons between the completed follow-up sample and the lost-to-follow-up sample on sociodemographic and clinical characteristics were performed by Pearson chi-square test and Independent-Samples T-Test as appropriate. McNemar Test and Paired-Samples T-Test were applied to analyze the differences concerning sociodemographic and clinical characteristics between the baseline group and the follow-up group in the 491 participants who completed the follow-up visit.
Subsequently, Generalized Estimating Equations (GEE) (31) were used to analyze the risk factors for violent behavior. GEE is mainly used for the analysis of repeated measurement data, in which there are more flexible observation time points. We conducted only one follow-up visit so far, yet there will be more follow-up visits in the future. Thus, GEE is more suitable for the statistical analysis in the present study. Risk factors were added as independent variables into the GEE analysis based on previous relevant studies, our clinical experience and the results of the bivariate analyses (see Table 2). More precisely, “Baseline variables” mean the variables which would not change over time, which include “Male, First-episode (or not), Education level at baseline” and so on. Besides, “Time-dependent variables” mean the variables which would change over time, including “Married, Employed, On FGAs” and so on. Additionally, in GEE analysis, variables with large correlations would not be selected as independent variables simultaneously. For example, “BPRS Positive, BPRS Negative and BPRS Affect” were chosen as independent variables, while “BPRS Total” not, as the latter was the sum of the former. The level of significance was established at 0.05 (two-tailed).
Effect size was calculated if the result was statistically significant, so as to consider whether the change was also clinically meaningful. Cohen's d value was calculated as the effect size when comparing two mean values, in which 0.2 represents a small effect size, 0.5 represents a medium effect size, and 0.8 represents a large effect size. Phi coefficient was calculated as the effect size when comparing two rates, in which 0.1 represents a small effect size, 0.3 represents a medium effect size, and 0.5 represents a large effect size.
There were 491 participants with schizophrenia who took part at both baseline and follow-up 2 years later, giving a response rate of 66.2% at follow-up. Those who dropped out were significantly more likely to be living with others (Phi = 0.07), and significantly less likely to be a current smoker (Phi = 0.08), taking clozapine (Phi = 0.11) and had a smaller number of hospitalizations across their lifetime (Cohen's d = 0.16): however, all four effect sizes were small or very small (see Table 1).
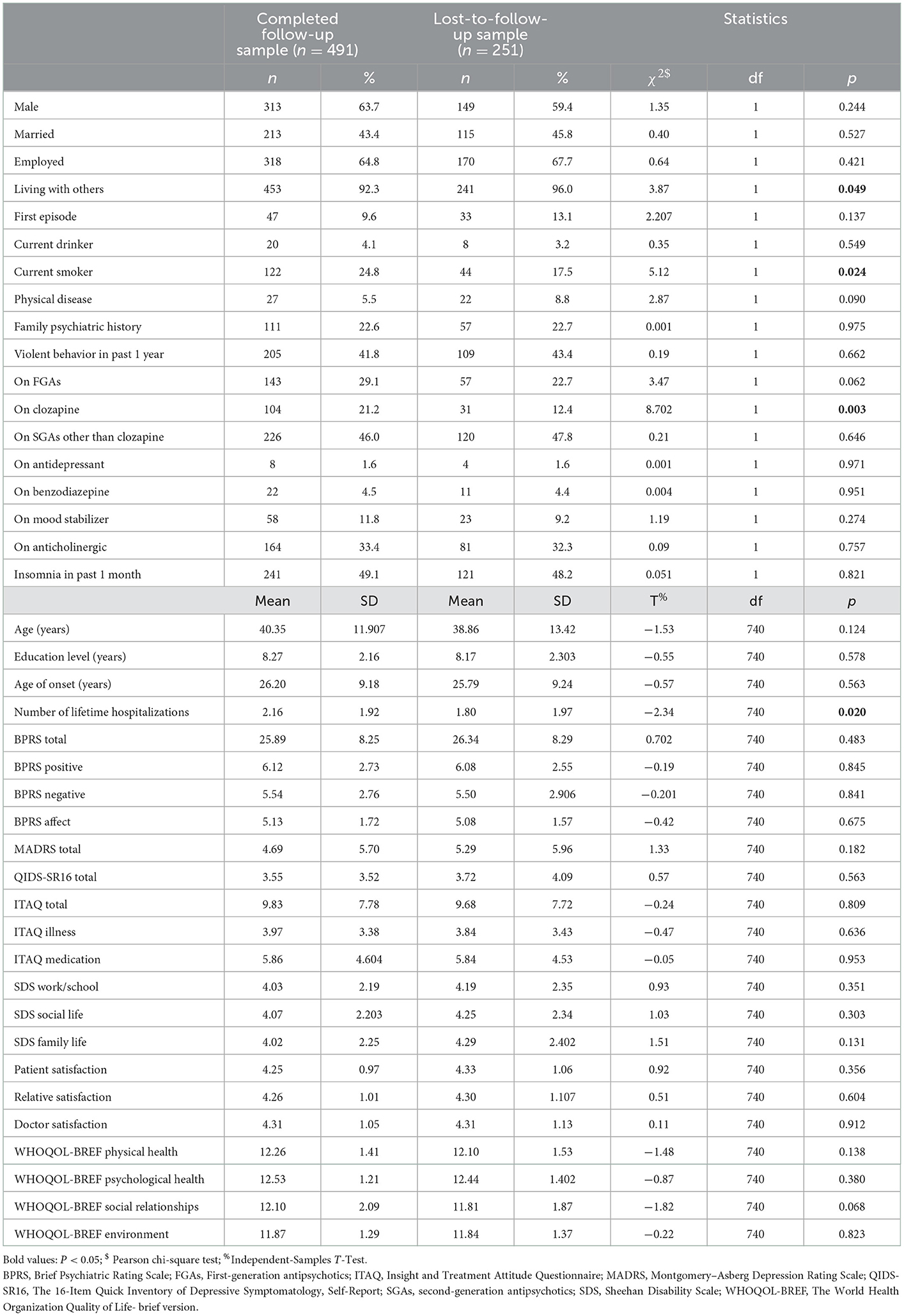
Table 1. Comparison of sociodemographic and clinical characteristics between the completed follow-up sample (n = 491) and the lost-to-follow-up sample (n=251).
At follow-up, participants were significantly less likely to be employed (Phi = 0.38) and experienced more hospitalization episodes over their lifetime (Cohen's d = 0.73) than at baseline. Compared with at baseline, participants were more likely to report taking clozapine (Phi = 0.25), second-generation antipsychotics (SGAs) other than clozapine (Phi = 0.44), antidepressants (Phi = 0.09), benzodiazepines (Phi = 0.26), mood stabilizers (Phi = 0.21) and anticholinergics (Phi = 0.13) after 2 years. Concerning medication dosage, participants reported taking significantly larger doses of clozapine (Cohen's d = 0.18) and SGAs other than clozapine (Cohen's d = 0.42) after 2 years in comparison with baseline (see Table 2).
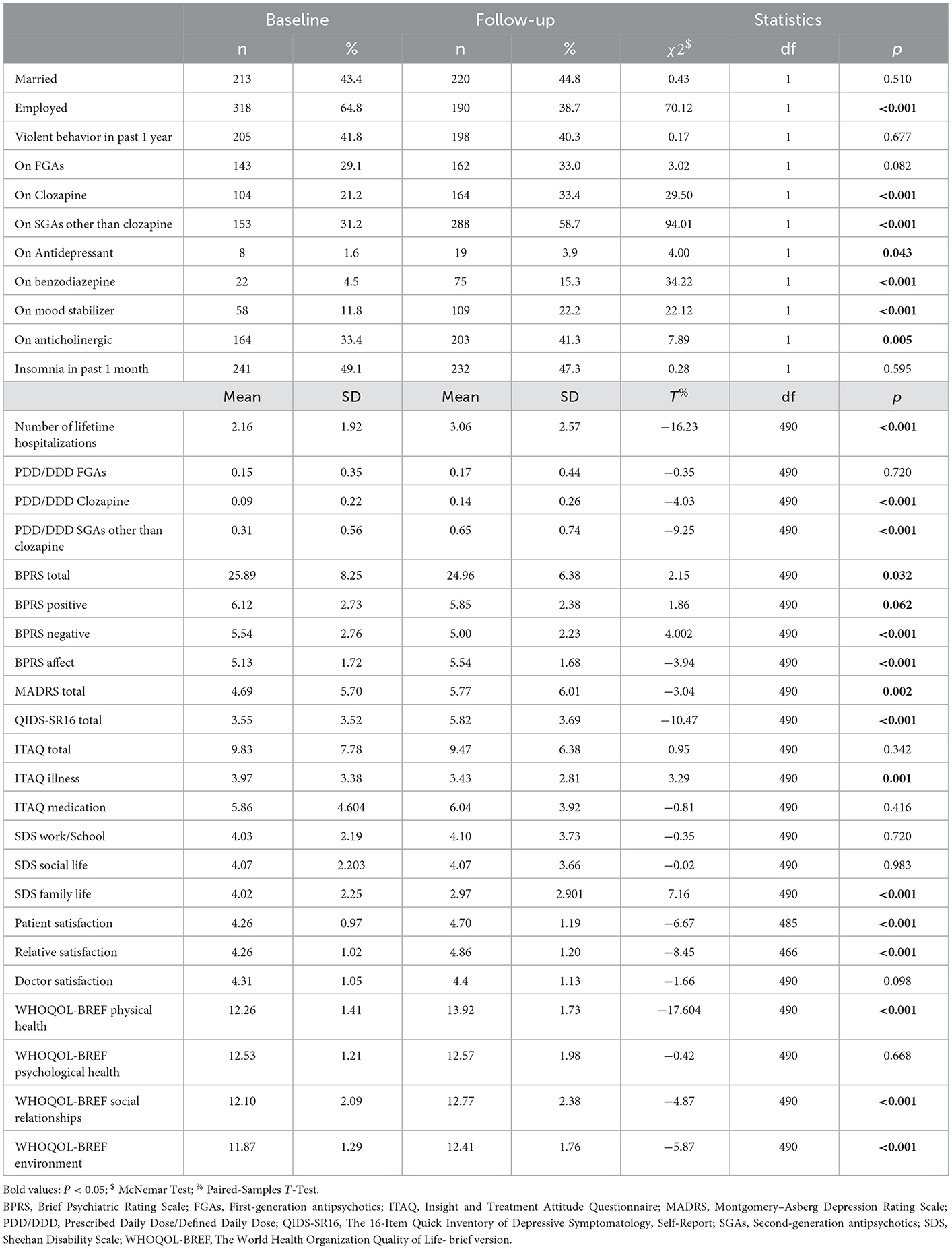
Table 2. Comparison of sociodemographic and clinical characteristics between baseline and 2-year follow-up in 491 patients with schizophrenia.
With regard to their mental health, participants scored significantly lower at follow-up in the BPRS Total (Cohen's d = 0.10), BPRS Positive (Cohen's d = 0.08) and BPRS Negative (Cohen's d = 0.18), indicating less general and positive as well as negative symptoms; whilst scoring higher in the BPRS Affect (Cohen's d = 0.18), MADRS Total (Cohen's d = 0.14) and QIDS-SR16 Total (Cohen's d = 0.47) than at baseline, indicating more affective symptoms, especially depressive symptoms. Furthermore, follow-up scores were lower in the ITAQ Illness subscale (Cohen's = 0.15) compared to baseline, indicating poorer insight into illness (see Table 2).
However, better patient satisfaction (Cohen's d = 0.30) and better relative satisfaction (Cohen's d = 0.39) regarding treatment were observed at follow-up. Scores at follow-up were lower in the SDS Family life domain (Cohen's d = 0.32) compared to baseline, indicating better family functioning. In addition, follow-up scores in the WHOQOL-BREF Physical health (Cohen's d = 0.79), WHOQOL-BREF Social relationships (Cohen's d = 0.22) and WHOQOL-BREF Environment (Cohen's d = 0.27) domains were higher in comparison with baseline, indicating better health-related quality of life (see Table 2).
GEE analysis suggested that for those on clozapine, the likelihood of violent behavior is decreased by 31% (OR = 0.69). Each additional point on ITAQ Medication also decreased the likelihood of violent behavior by 9.3% (OR = 0.907). In short, use of clozapine and better insight into the need for medication were the protective factors for violent behavior in participants with schizophrenia (see Table 3).
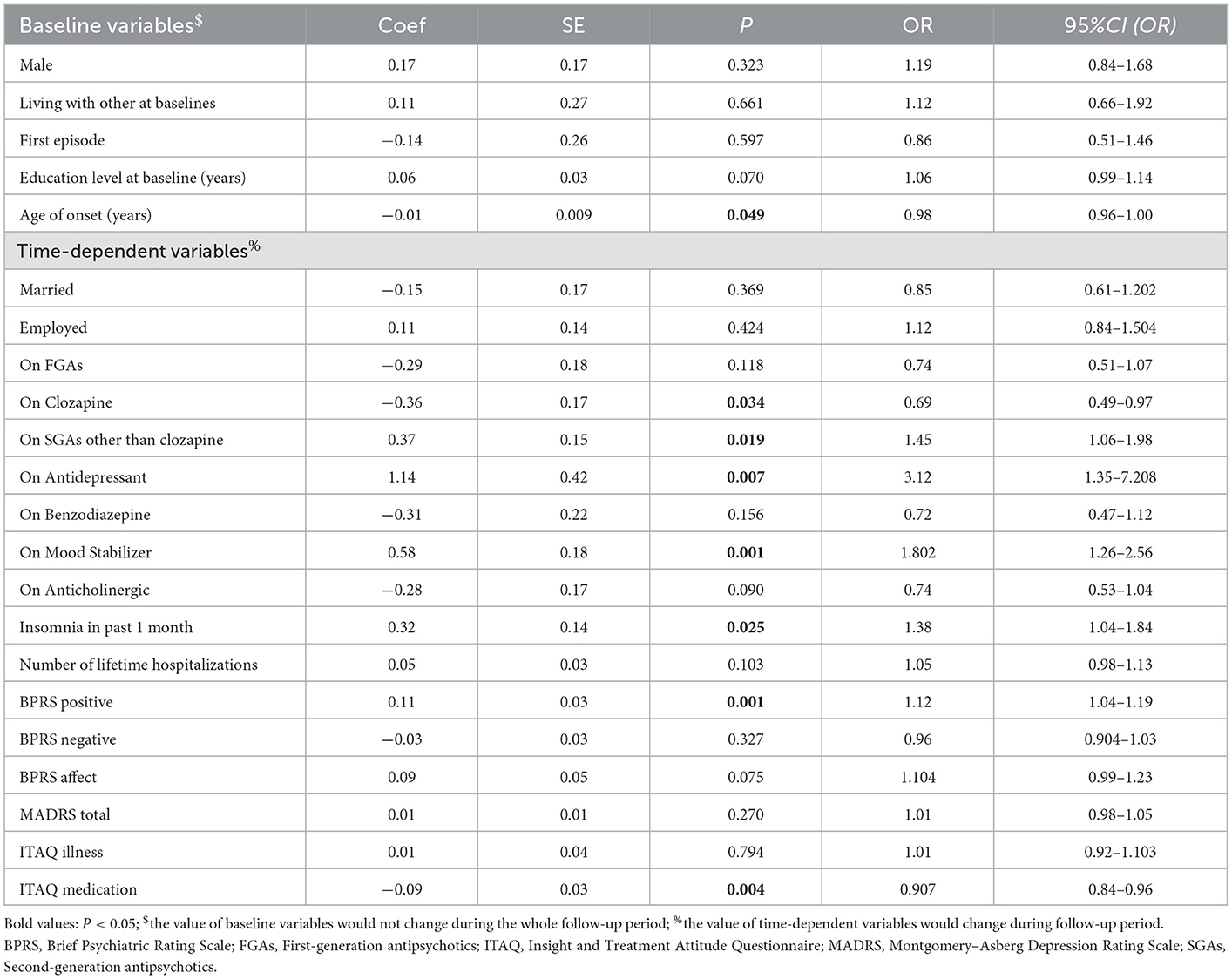
Table 3. Generalized Estimating Equations (GEE) results modeling risk factors associated with violent behavior in 491 patients with schizophrenia across 1 year.
In contrast, each additional point on BPRS Positive increases the likelihood of violent behavior by 12% (OR = 1.12), and for those with insomnia, the likelihood of violent behavior increases by 38% (OR = 1.38). Those on SGAs other than clozapine experienced a 45% increase in the odds of violent behavior (OR = 1.45), and those on antidepressants were more than three times as likely to report engaging in violent behavior (OR = 3.12), while those on mood stabilizers had an 80% increase in the odds of violent behavior (OR = 1.802). In short, higher positive symptoms, insomnia, as well as use of SGAs other than clozapine, use of antidepressants and use of mood stabilizers were the risk factors for violent behavior in these participants (see Table 3).
This study found that about two in five community-dwelling patients with schizophrenia reported violent behavior in the past year. Bivariate analysis showed that at follow-up, participants were significantly less employed, experienced more lifetime hospitalization episodes, reported taking more psychotropic medication, and experienced more depressive symptoms, while having better health-related quality of life (especially in physical health domain) than at baseline. GEE analysis showed that use of clozapine and better insight into medication decreased the possibility of violent behavior, while more severe positive symptoms, insomnia, as well as use of second- generation antipsychotics other than clozapine, antidepressants and mood stabilizers increased the probability of violent behavior.
The drop-out rate of the present study is approximately 33.8%, which is larger than the common acceptable value (< 20%) (32). We have tried many efforts to increase the response rate during follow-up period, still the drop-out rate was relatively high. Therefore, we made comparison on variables between the lost-to-follow-up sample and the completed follow-up sample, to find out whether there was large difference between these two samples. Fortunately, those who dropped out were significantly different from participants who completed follow-up in only 4/43 examined variables, and meanwhile the four effect sizes were all small or very small, which suggested those differences may not be clinically meaningful and that the completed follow-up sample may be broadly representative of the whole sample.
We have reported relatively high rates of violent behavior over the past year for the follow-up participants, whether at baseline (41.8%) or 2 years later (40.3%). Reported rates of violent behavior have differed across studies: for instance, a review involving 31 studies showed that outpatients with severe mental illness reported rates of 2%–13% of violent perpetration in the past 6 months to 3 years (33), while other studies reported rates of 14–20% (34–36). The above inconsistent findings may possibly be explained by differences in sample size, the research subjects of interest, the definition and the time period of violent behavior. After a 2-year follow-up period, more participants with schizophrenia were admitted to the hospital and reported taking more, and higher doses of psychotropic drugs, especially SGAs other than clozapine, than at baseline. This suggests worsening of their illness over time, requiring more medical services and medication. Additionally, at follow-up, participants had a higher and probably meaningful rate of unemployment than at baseline, which may be explained by their continuing hospitalizations or accumulated experiences of stigma and/or depression.
Depression is commonly seen in all stages of schizophrenia (37), and is associated with serious consequences in patients with schizophrenia (38). Interestingly, in our study, the scores of BPRS Affect, MADRS Total and QIDS-SR16 Total increased compared with baseline, although only the effect size of QIDS-SR16 Total was close to medium effect size. This might indicate that clinicians should pay more attention to the aggravation of depressive symptoms in patients with schizophrenia, especially considering that the QIDS-SR16 was the only self-report measure. Moreover, health-related quality of life (especially in the subdomains of physical health, social relationships and environment) increased in these participants with schizophrenia after 2 years, although only the effect size of WHOQOL-BREF Physical health is close to a large effect size. Our observed decrease in positive and negative symptoms, especially likely to be associated with increased usage of clozapine, may partly account for this. Similarly, Verma's study found that treatment with clozapine leads to improvement in core symptoms of schizophrenia and is also associated with significant improvement in the quality of life (39).
We applied GEE analysis to identify the risk factors for violent behavior in participants with schizophrenia. Our study indicated that more severe positive symptoms in participants with schizophrenia related to more violent behavior, which was similar to the finding of another study (40), whose results showed that the most predictive variables for violence among inpatients with schizophrenia are suspiciousness and hostility, more severe hallucinations, poor insight into delusions and the overall illness, and greater disorganization of thought processes (40).
Otherwise, any type of insomnia (including difficulty initiating sleep, difficulty maintaining sleep and early morning wakening) in the past month was also a risk factor, in line with a systematic review, which reported a relationship between interpersonal violence and a broad range of sleep disturbance, whereby 46 to 100% of respondents endorsed moderate to severe insomnia (24). Symptoms of sleep disruption can predict the onset of positive psychotic symptoms, such as paranoia and hallucinations. With sleep disturbances inextricably linked to increased severity of schizophrenia and worsening clinical outcomes, insomnia is an important therapeutic target within this patient population (5, 41).
Unlike many other studies, this study explored in depth the relationship between psychotropic drugs and violent behavior in participants with schizophrenia. We found that clozapine usage was a protective factor, while usage of SGAs other than clozapine was a risk factor. Whilst all SGAs are prescribed for severe and/or ongoing psychotic symptoms, it is recognized that clozapine has greater efficacy than other SGAs due to its high affinity for dopamine 4 vs. dopamine 2 receptors (42), which might explain our findings. Furthermore, clozapine has also shown the strongest evidence for treating acute violence in schizophrenia (43). Thus, our finding may indicate that clozapine should be preferred to reduce acute aggression and control persistent violence in patients with schizophrenia rather than other SGAs and FGAs (44), especially in refractory conditions (45).
Use of antidepressants by participants showed a 3-fold association with violent behavior. The previously described worsening of depressive symptoms in participants with schizophrenia after 2 years follow-up may explain the increase in antidepressant usage. It is well-known that irritability associated with depression and anxiety could culminate in aggression (46). There is reason to believe that the depression reported here is under-treated and hence irritability leads to aggression, thus increasing the risk for violent behavior in participants with schizophrenia.
Besides, we found that use of mood stabilizers was also a risk factor, as increased mood stabilizer usage itself may manifest because of a more serious disease condition. Compared with our study, previous studies discovered that lithium has been repeatedly shown to reduce irritability and incidents of aggression in bipolar disorder patients (45), while valproate promoted significant reductions in aggression across multiple diagnostic categories (47). It could also be possible that the mood disturbance was under-diagnosed and under-treated in this sample, therefore leading to inconsistent results compared to other studies.
The strengths of this study were as follows: (a) compared to the many cross-sectional studies published before, a follow-up study is a more robust design for this enquiry, in which we could explore the complete development process and some key turning points in the development process. (b) GEE analysis (rather than traditional logistic regression analysis) was applied to identify the risk factors for violent behavior, by which we could effectively explore the changes of sociodemographic and clinical characteristics during the follow-up period that affect the outcome (rather than just explore baseline or endpoint variables). (c) participants in this study were patients with schizophrenia managed in primary mental health care in an under-developed city in China, from which the research findings could be applied to other similar developing regions.
The limitations of this study were as followed: (a) attrition bias: the drop-out rate of this follow-up study is relatively high (about 33.8%), and we only included participants from one rural city (Luoding city), which may have impacted the generalizability of the findings. (b) Follow-up study is a kind of descriptive study, which can only propose etiological hypotheses and clues, rather than determine causal relationship. (c) we only assessed whether the study participants were engaged in violent behavior in the past year or not, rather than recorded different degrees of severity and frequency of violent behaviors, which prevents fine-grained analysis of differences in frequency and severity. In the future follow-up, we will perform stratified analysis to deeply explore the status and characteristics of violent behaviors. (d) stigma, discrimination, and response bias (such as social desirability) may have influenced the accuracy of the results, as some of the scales are self-report. Further, participants reporting to the interviewers with or without the presence of relatives may also influence the results. (e) only individual factors were explored in the present study, whilst social or environmental or system level factors were not included, which are also critical to the occurrence of violent behavior. (f) we only conducted one follow-up till now, which limited the findings of the study, yet there will be on-going follow-ups in the future.
To conclude, risk evaluation, prevention and management of violence are demanded as part of the completed patient assessment and treatment in primary psychiatric practice or other settings. Psychiatrists should pay more attention to the irritability associated with depression and mood disturbance and give priority to the usage of clozapine, as appropriate, in patients with schizophrenia with severe or treatment-refractory illness. They should also evaluate and treat any insomnia experienced by their patients. Otherwise, both primary care practitioners and families should also concern more on the above insomnia problem, and seek help from psychiatrists if necessary.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Clinical Research Ethics Committee of Guangdong Provincial People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Study design: C-LH, S-BW, and CH. Data collection and typing: Z-HH, FW, Q-WW, X-YH, Z-LC, and Y-NX. Analysis and interpretation of data and drafting of the manuscript: Z-HH, FW, and C-LH. Critical revision of the manuscript: C-LH, CH, CM, S-BW, Z-LC, and Y-NX. Approval of the final version for publication: All coauthors. All authors contributed to the article and approved the submitted version.
This work was supported by Guangdong Provincial Foundation for Basic and Applied Basic Research Natural Science Foundation (grant number: 2022A1515010619).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Brill HB, Malzberg. Statistical Report Based on the Arrest Records of 5, 354. Male Ex-Patients Released from New York State Mental Hospitals During the Period 1946–1948, Mental Hospital Service Supplement 153. Washington, DC: American Psychiatric Association (1962).
2. Fazel S, Wolf A, Larsson H, Lichtenstein P, Mallett S, Fanshawe TR, et al. Identification of low risk of violent crime in severe mental illness with a clinical prediction tool (Oxford Mental Illness and Violence tool [OxMIV]): a derivation and validation study. The Lancet Psychiatry. (2017) 4:461–8. doi: 10.1016/S2215-0366(17)30109-8
3. Rund BR, A. review of factors associated with severe violence in schizophrenia. Nord J Psychiatry. (2018) 72:561–71. doi: 10.1080/08039488.2018.1497199
4. Swanson JW, Swartz MS, Essock SM, Osher FC, Wagner HR, Goodman LA, et al. The social–environmental context of violent behavior in persons treated for severe mental illness. Am J Public Health. (2002) 92:1523–31. doi: 10.2105/AJPH.92.9.1523
5. Batalla-Martín D, Belzunegui-Eraso A, Miralles Garijo E, Martínez Martín E, Romaní Garcia R, Heras JS, et al. Insomnia in Schizophrenia patients: prevalence and quality of life. Int J Environ Res Public Health. (2020) 17:1350. doi: 10.3390/ijerph17041350
6. Kooyman I, Dean K, Harvey S, Walsh E. Outcomes of public concern in schizophrenia. Br J Psychiatry Suppl. (2007) 50:s29–36. doi: 10.1192/bjp.191.50.s29
7. Guo Y, Yang X, Wang D, Fan R, Liang Y, Wang R, et al. Prevalence of violence to others among individuals with schizophrenia in China: a systematic review and meta-analysis. Front Psychiatry. (2022) 13:939329. doi: 10.3389/fpsyt.2022.939329
8. Fazel S, Gulati G, Linsell L, Geddes JR, Grann M. Schizophrenia and violence: systematic review and meta-analysis. PLoS Med. (2009) 6:e1000120. doi: 10.1371/journal.pmed.1000120
9. Fazel S, Grann M. The population impact of severe mental illness on violent crime. Am J Psychiatry. (2006) 163:1397–403. doi: 10.1176/ajp.2006.163.8.1397
10. Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, et al. Rapid health transition in China, 1990–2010: findings from the global burden of disease study 2010. Lancet. (2013) 381:1987–2015. doi: 10.1016/S0140-6736(13)61097-1
11. Senior M, Fazel S, Tsiachristas A. Tsiachristas The economic impact of violence perpetration in severe mental illness: a retrospective, prevalence-based analysis in England and Wales. Lancet Public Health. (2020) 5:e99–e106. doi: 10.1016/S2468-2667(19)30245-2
12. Barlati S, Stefana A, Bartoli F, Bianconi G, Bulgari V, Candini V, et al. Violence risk and mental disorders (VIORMED-2): a prospective multicenter study in Italy. PLoS ONE. (2019) 14:4924. doi: 10.1371/journal.pone.0214924
13. Steadman HJ, Mulvey EP, Monahan J, Robbins PC, Appelbaum PS, Grisso T, et al. Violence by people discharged from acute psychiatric inpatient facilities and by others in the same neighborhoods. Arch Gen Psychiatry. (1998) 55:393–401. doi: 10.1001/archpsyc.55.5.393
14. Harford TC, Chen CM, Kerridge BT, Grant BF. Self- and other-directed forms of violence and their relationship with lifetime DSM-5 psychiatric disorders: results from the national epidemiologic survey on alcohol related conditions-III (NESARC-III). Psychiatry Res. (2018) 262:384–92. doi: 10.1016/j.psychres.2017.09.012
15. Wang QW, Hou CL, Wang SB, Huang ZH, Huang YH, Zhang JJ, et al. Frequency and correlates of violence against patients with schizophrenia living in rural China. BMC Psychiatry. (2020) 20:286. doi: 10.1186/s12888-020-02696-9
16. Zhang MY, Zhou TX, Tang H, Chi YF, Xia ML, Wang ZY. The application of translated brief psychiatric rating scale (BPRS) (1) reliability test (in Chinese). Chin J Nerv Mental Dis. (1983) 02:76–80.
17. Zhang MY, Zhou TX, Liang JH, Wang ZY, Tang YH, Chi YF, et al. The application of translated brief psychiatric rating scale (BPRS) (2) validity test. Chin J Nerv Mental Dis. (1984) 02:74–7.
18. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. (1979) 134:382–9. doi: 10.1192/bjp.134.4.382
19. Zhong BL, Wang Y, Chen HH, Wang XH. Study on the reliability and sensitivity of Montgomery-Asberg depression scale in patients with severe depression. Chin J Behav Med Brain Sci. (2011) 1:85–7. doi: 10.3760/cma.j.issn.1674-6554.2011.01.032
20. Liu J, Xiang YT, Wang G, Zhu XZ, Ungvari GS, Kilbourne AM, et al. Psychometric properties of the Chinese versions of the quick inventory of depressive symptomatology - clinician rating (C-QIDS-C) and self-report (C-QIDS-SR). J Affect Disord. (2013) 147:1–3. doi: 10.1016/j.jad.2012.08.035
21. Liu J, Xiang YT, Lei H, Wang Q, Wang G, Ungvari GS, et al. Guidance on the conversion of the Chinese versions of the quick inventory of depressive symptomatology-self-report (C-QIDS-SR) and the montgomery-asberg scale (C-MADRS) in Chinese patients with major depression. J Affect Disord. (2014) 154:530–3. doi: 10.1016/j.jad.2013.09.023
22. McEVOY JP, Aland JRJ, Wilson WH, Guy W, Hawkins L. Measuring chronic schizophrenic patients attitudes toward their illness and treatment. Hosp Commun Psychiatry. (1981) 32:856–58. doi: 10.1176/ps.32.12.856
23. Gao H, Yu XJ F. Lv. Reliability and validity of insight and treatment attitude questionnaire (ITAQ) Chinese Mental Health Journal. (1998) 02:3–5.
24. Gallegos AM, Trabold N, Cerulli C, Pigeon WR. Sleep and interpersonal violence: a systematic review. Trauma Violence Abuse. (2019) 1524838019852633. doi: 10.1177/1524838019852633
25. Xiang YT, Weng YZ, Leung CM, Tang WK, Lai KY, Ungvari GS. Prevalence and correlates of insomnia and its impact on quality of life in Chinese Schizophrenia patients. Sleep. (2009) 32:105–9.
26. Li Y, Hou CL, Ma XR, Zhong BL, Zang Y, Jia FJ, et al. Quality of life in Chinese patients with schizophrenia treated in primary care. Psychiatry Res. (2017) 254:80–4. doi: 10.1016/j.psychres.2017.04.049
27. Sheehan DV, Harnett-Sheehan K, Raj B. The measurement of disability. Int Clin Psychopharmacol. (1996) 11:89–95. doi: 10.1097/00004850-199606003-00015
28. Leon AC, Olfson M, Portera L, Farber L, Sheehan DV. Assessing psychiatric impairment in primary care with the sheehan disability scale. Int J Psychiatry Med. (1997) 27:93–105. doi: 10.2190/T8EM-C8YH-373N-1UWD
29. Group TW. Development of the World Health Organization WHOQOL-BREF quality of life assessment. Psychol Med. (1998) 28:551–8. doi: 10.1017/S0033291798006667
30. Hao YT, Fang JQ. The introduce and usage of WHOQOL instrument. Modern Rehabil. (2000) 08:1127–9. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2000&filename=XDKF200008001&uniplatform=NZKPT&v=r6FpOnzzqDhvOJfWuuNJZVUZbLOV7VX2LtTqc07zDN0WwRrCxABTMBwLb4ZUqVnW
31. Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. (1986) 42:121–30. doi: 10.2307/2531248
32. Jones K. Evidence based medicine—How to practice and teach EBM. BMJ. (1996) 313:1410. doi: 10.1136/bmj.313.7069.1410
33. Choe JY, Teplin LA, Abram KM. Perpetration of violence, violent victimization, and severe mental illness: balancing public health concerns. Psychiatric Serv. (2008) 59:153–64. doi: 10.1176/ps.2008.59.2.153
34. Swanson JW, Swartz MS, Van Dorn RA, Elbogen EB, Wagner HR, Rosenheck RA, et al. A national study of violent behavior in persons with schizophrenia. Arch Gen Psychiatry. (2006) 63:490–9. doi: 10.1001/archpsyc.63.5.490
35. Winsper C, Singh SP, Marwaha S, Amos T, Lester H, Everard L, et al. Pathways to violent behavior during first-episode psychosis: a report from the UK National EDEN study. JAMA Psychiatry. (2013) 70, 1287–1293. doi: 10.1001/jamapsychiatry.2013.2445
36. Chang Z, Larsson H, Lichtenstein P, Fazel S. Psychiatric disorders and violent reoffending: a national cohort study of convicted prisoners in Sweden. Lancet Psychiatry. (2015) 2:891–900. doi: 10.1016/S2215-0366(15)00234-5
37. van Rooijen G, Vermeulen JM, Ruhé HG, de Haan L. Treating depressive episodes or symptoms in patients with schizophrenia. CNS Spectr. (2019) 24:239–48. doi: 10.1017/S1092852917000554
38. Xu YM Li F, Liu XB, Zhong BL. Depressive symptoms in Chinese male inpatients with schizophrenia: prevalence and clinical correlates. Psychiatry Res. (2018) 264:380–4. doi: 10.1016/j.psychres.2018.04.016
39. Verma M, Grover S, Chakrabarti S, Chakrabarti. Effectiveness of clozapine on quality of life and functioning in patients with treatment-resistant schizophrenia. Nord J Psychiatry. (2021) 75:135–44. doi: 10.1080/08039488.2020.1811374
40. Buckley PF, Noffsinger SG, Smith DA, Hrouda DR, Knoll JL. Treatment of the psychotic patient who is violent. Psychiatr Clin North Am. (2003) 26:231–72. doi: 10.1016/S0193-953X(02)00029-1
41. Robertson I, Cheung A, Fan X. Insomnia in patients with schizophrenia: current understanding and treatment options. Prog Neuropsychopharmacol Biol Psychiatry. (2019) 92:235–42. doi: 10.1016/j.pnpbp.2019.01.016
42. El-Mallakh RS, McKenzie C. The dopamine D4/D2 receptor antagonist affinity ratio as a predictor of anti-aggression medication efficacy. Med Hypotheses. (2013) 80:530–3. doi: 10.1016/j.mehy.2012.10.014
43. Quinn J, Kolla NJ. From clozapine to cognitive remediation: a review of biological and psychosocial treatments for violence in Schizophrenia. Can J Psychiatry. (2017) 62:94–101. doi: 10.1177/0706743716656830
44. Krakowski MI, Czobor P, Citrome L, Bark N, Cooper TB. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. (2006) 63:622–9. doi: 10.1001/archpsyc.63.6.622
45. Fava M. Psychopharmacologic treatment of pathologic aggression. Psychiatr Clin North Am. (1997) 20:427–51. doi: 10.1016/S0193-953X(05)70321-X
46. Fava M, Rosenbaum JF, Pava JA, McCarthy MK, Steingard RJ, Bouffides E, et al. Anger attacks in unipolar depression, Part 1: clinical correlates and response to fluoxetine treatment. Am J Psychiatry. (1993) 150:1158–63. doi: 10.1176/ajp.150.8.1158
47. Lindenmayer JP, Kotsaftis A. Use of sodium valproate in violent and aggressive behaviors: a critical review. J Clin Psychiatry. (2000) 61:123–8. doi: 10.4088/JCP.v61n0207
48. De Berardis D, Fornaro M, Orsolini L, Iasevoli F, Tomasetti C, De Bartolomeis A. The role of inhaled loxapine in the treatment of acute agitation in patients with psychiatric disorders: a clinical review. Int J Mol Sci. (2017) 18:349. doi: 10.3390/ijms18020349
Keywords: violence, schizophrenia, risk factor, primary care, Generalized Estimating Equations
Citation: Huang Z-H, Wang F, Chen Z-L, Xiao Y-N, Wang Q-W, Wang S-B, He X-Y, Migliorini C, Harvey C and Hou C-L (2023) Risk factors for violent behaviors in patients with schizophrenia: 2-year follow-up study in primary mental health care in China. Front. Psychiatry 13:947987. doi: 10.3389/fpsyt.2022.947987
Received: 19 May 2022; Accepted: 30 December 2022;
Published: 20 January 2023.
Edited by:
Giorgio Di Lorenzo, University of Rome Tor Vergata, ItalyCopyright © 2023 Huang, Wang, Chen, Xiao, Wang, Wang, He, Migliorini, Harvey and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cai-Lan Hou,  aG91Y2wxOTc1QDE2My5jb20=
aG91Y2wxOTc1QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.