- 1Department of Medicine, University of British Columbia, Vancouver, BC, Canada
- 2MAPS Public Benefit Corporation, San Jose, CA, United States
- 3British Columbia Centre on Substance Use, Vancouver, BC, Canada
- 4Department of Health Sciences, Vancouver Island University, Nanaimo, BC, Canada
- 5MD Inc., Los Angeles, CA, United States
- 6Memorial University of Newfoundland, St. John’s, NL, Canada
Introduction: Increasing evidence demonstrates 3,4-methylenedioxymethamphetamine (MDMA)-assisted therapy (MDMA-AT) may be a safe and effective treatment for post-traumatic stress disorder (PTSD). There is growing interest in MDMA-AT to address a range of other health challenges. Chronic pain and PTSD are frequently comorbid, reciprocally interdependent conditions, though the possible role of MDMA-AT in treating chronic pain remains under-investigated. The present analysis examined the impact of manualized MDMA-AT on chronic pain severity among participants with PTSD who were enrolled in a Phase 2 clinical trial investigating MDMA-AT for PTSD (NCT03282123).
Materials and methods: Exploratory data from a subset of participants who completed chronic pain measures (n = 32) were drawn from a Phase 2 open-label study sponsored by the Multidisciplinary Association for Psychedelic Studies (MAPS). Multivariable analysis of variance (ANOVA) was utilized to compare pre- vs. post-treatment Chronic Pain Grade Scale (CPGS) values, adjusting for demographics (age, sex, and ethnicity). K-means clustering was then used to group the sample into three clusters to denote high (n = 9), medium (n = 11), and low (n = 12) baseline pain severity, and the same analysis was repeated for each cluster.
Results: Among the 32 participants included in this analysis, 59% (n = 19) were women, 72% (n = 23) were white, and median age was 38 years [interquartile range (IQR) = 31–47]. Overall, 84% (n = 27) reported having pain, and 75% (n = 24) reported disability associated with their pain. Significant reductions in CPGS subscales for pain intensity and disability score, and overall CPGS severity grade were observed among participants in the highest pain cluster (n = 9, p < 0.05), and for pain intensity in the medium pain cluster (n = 11, p < 0.05) post- vs. pre-treatment.
Discussion: Findings demonstrate a high prevalence of chronic pain in this sample of people with severe PTSD and that chronic pain scores among medium and high pain subgroups were significantly lower following MDMA-AT. While these data are preliminary, when considered alongside the frequency of comorbid chronic pain and PTSD and promising efficacy of MDMA-AT for treating PTSD, these findings encourage further research exploring the role of MDMA-AT for chronic pain.
Introduction
Increasing evidence demonstrates that MDMA-assisted therapy (MDMA-AT) may be a safe and effective treatment for post-traumatic stress disorder (PTSD) (1–4). To date, 11 blinded randomized, controlled Phase 2 and Phase 3 studies of MDMA-AT for PTSD have been published (1–5), and there is growing interest in MDMA-AT for addressing a range of physical and mental health challenges. For instance, pilot studies have explored the potential for MDMA-AT in end of life anxiety (6), social anxiety in autistic adults (7), and alcohol use disorder (8), and exploratory analysis has generated preliminary data for eating disorders (9, 10). However, the possible effect of MDMA-AT on chronic pain remains under-investigated.
Chronic pain is a common, complex disease affecting approximately one-fifth of Americans (11) and up to 40% of people worldwide according to some studies (12) with far-ranging impacts on individuals and society (13). Chronic pain, defined as consistent pain for at least 3 months (14), is a leading cause of disability and is associated with a reduced life expectancy (12). The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” and clearly denotes that the experience of pain is personal and influenced by biological, psychological, and social factors (15). Whereas opioid medications may be utilized to manage acute and/or severe pain such as postoperative and terminal cancer pain, they are largely inappropriate and ineffective to alleviate chronic non-cancer pain and moreover their use is associated with the development of problematic opioid use disorder (OUD) (16). Innovative approaches to managing chronic pain that reduce long term reliance on pharmaceutical interventions are needed. Current best practice guidelines for chronic pain management recommend multidisciplinary treatments that address biological, psychological and social factors that collectively influence a person’s experience of pain (17).
MDMA-AT is a novel intervention under investigation for PTSD that has been granted Breakthrough Therapy status for this indication by the United States’ Food and Drug Administration in 2017 (2). PTSD is a complex, difficult to treat, stress-related psychiatric condition with somatization symptoms that has a profound impact on individuals and society and for which novel treatments are needed (1, 18). In well-established protocols, MDMA-AT combines a program of manualized psychotherapy with administration of MDMA on up to three occasions across the course of treatment in a clinically monitored setting for PTSD (1, 5). Long term analysis of pooled data from recent phase 2 studies of MDMA-AT for chronic, treatment-resistant PTSD demonstrated resolution of PTSD diagnosis in 67% of participants at least 12 months later (3), and phase 3 data demonstrated clinically and statistically significant improvement in severe, chronic PTSD in the MDMA-AT group vs. placebo with therapy combined with an acceptable safety profile (5). MDMA-AT has not yet been investigated to treat chronic pain as an indication.
Chronic pain and PTSD are both challenging to treat, and each contributes to significant dysfunction across all aspects of one’s life despite current available treatments (19, 20). Chronic pain and PTSD are also frequently comorbid; two systematic reviews found prevalence rates of PTSD in clinical pain populations to range from 11.7 to 19.1% (21, 22), and in people with PTSD, prevalence estimates for chronic pain have been as high as 80%, as seen among military personnel (23–25). Both conditions are independently associated with OUD, yet when comorbid are associated with even higher odds of OUD than either condition alone (26). Those with comorbid pain and PTSD also experience greater pain, PTSD symptoms, depression, anxiety, and disability than those with only one of these conditions (27, 28). Chronic pain and PTSD have been described as reciprocally interdependent conditions, with similar maintenance mechanisms such as fear, avoidance, and catastrophizing (29, 30). One literature review notes, “the tight interdependence of symptoms that can be observed in both PTSD and chronic pain syndromes lends support to the idea that these disorders should be situated on the same level, that of a reactive disorder” (31). It has even been suggested that chronic pain might indeed be interpreted as a version of PTSD (32). The Mutual Maintenance Model suggests PTSD and chronic pain exacerbate one another through biases of attention and cognition that create heightened expectations, overestimations, and selective and negative interpretations of both pain-evoking and fear-evoking stimuli. Pain may also serve as a traumatic cue, leading to intrusive PTSD symptoms, and vice versa (33). The Shared Vulnerability Model posits that anxiety sensitivity and genetic predispositions related to the stress response increase the likelihood for certain individuals to develop both conditions (24).
Given the relationship and similarities between chronic pain and PTSD, and anticipated efficacy of MDMA-AT in treating PTSD, MDMA-AT may have potential as a treatment for chronic pain. This notion is further supported by evidence suggesting efficacy for psychotherapeutic approaches in chronic pain (34) and the established importance of biopsychosocial interventions for chronic pain, with MDMA-AT being at once a biological and psychological treatment, combining the pharmacological effects of MDMA with psychotherapy. However, there remains a lack of research and data on the effect of MDMA-AT on chronic pain conditions. The present study therefore aimed to explore the potential relationship between MDMA-AT and pain outcomes, drawing on exploratory pain data from a Phase 2 trial of MDMA-AT for severe PTSD (35).
Materials and methods
Data (December 2017 to August 2019) were drawn from a subset of participants enrolled in a multi-site Phase 2 open-label study known as MP16 (n = 33) conducted by MAPS Public Benefit Corporation (MAPS PBC) (35). This study served as a lead-in to Phase 3 studies investigating manualized MDMA-assisted therapy (MDMA-AT) to treat severe PTSD. The researchers designed the study to model the structure of planned Phase 3 trials, and to prepare and supervise sites planning to be part of the Phase 3 studies. The study took place across 12 sites in the United States and followed well-established protocols for MDMA-AT. Details and primary outcomes of the study have been previously described (1).
The primary outcome measure for the MP16 study was the Clinician Administered PTSD Scale according to DSM-5 (CAPS-5), a semi-structured interview addressing PTSD symptom clusters (36). Exploratory data were collected including participants’ Adverse Childhood Experiences (ACE) score at Baseline, and the Chronic Pain Grade Scale (CPGS) questionnaire at Baseline and Study Termination, which were of interest for the present analysis. Eligibility required confirmation of severe PTSD, defined as a CAPS-5 total severity score of 35 or greater. Participants could not have a diagnosis of substance use disorder within 60 days of screening, and psychiatric medications were tapered and discontinued prior to commencing study drug sessions. Participants were allowed to continue concomitant analgesic medications including non-steroidal anti-inflammatory drugs (NSAIDS), acetaminophen, gabapentin, and opiates limited to hydrocodone, morphine and codeine. Participants taking other opiates at enrollment were cross-tapered to an allowable opiate during the preparatory period. Concomitant use of cannabis was prohibited from enrollment to study termination. The protocol for MDMA-AT involved a series of therapy sessions delivered by two clinicians in a “co-therapy” dyad, and participant eligibility and screening were managed by a physician. MDMA administration occurred on three occasions and each session was 8-h in duration in the presence of the co-therapy dyad. These study drug sessions were spaced 3–5 weeks apart and were preceded and/or followed by three non-drug 90-min sessions that served to prepare for and/or facilitate psychotherapeutic integration of each study drug session. According to the flexible dosing schedule, in the first study drug session, all participants received an initial dose of 80 mg MDMA HCl, followed 1.5–2.5 h later by a supplemental dose of 40 mg MDMA HCl. In the second and/or third study drug sessions, participants received an increased dose of 120 mg MDMA HCl followed by a supplemental dose of 60 mg MDMA HCl, 1.5–2.5 h later. All participants followed this dose escalation. The supplemental dose was withheld by the investigator in two instances due to tachycardia and elevated blood pressure following the initial dose, though neither required further medical intervention (35).
The primary outcome for the present analysis was chronic pain, as measured by the CPGS questionnaire, which was administered twice, at Preparatory Session 3 (prior to the first MDMA session), and at study termination which occurred 10–14 weeks after the final MDMA session. Participants were asked to base their responses at study termination on the period since the end of treatment. Secondarily, we looked for associations between chronic pain, childhood adversity and PTSD severity at baseline.
The CPGS questionnaire is a 7-item self-report instrument that measures two dimensions of overall pain severity: pain intensity and pain-related disability, and is valid and reliable for measuring change in severity of chronic pain over time (37). Data encompass current pain, as well as past 6 months pain and pain-related disability and is suitable for use in all chronic pain conditions. Pain intensity and disability score subscales are combined with disability points score to assign a chronic pain severity grade (0-IV); individual subscales as well as overall severity grade have demonstrated validity and reliability (38) (for definitions of CPGS severity grade (0–IV), see Table 1). Demographic variables included age, sex, and ethnicity (white vs. other).
Statistical analysis
Statistical analyses were performed using R version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria). Bivariate linear and ordinal regression were used to estimate pre-treatment associations between CPGS values, and ACE score, CAPS-5 total severity, and demographic variables.
K-means clustering was used to cluster baseline pain into three groups to denote highest (cluster 1, n = 9), medium (cluster 2, n = 11), and lowest pain (cluster 3, n = 12) within the sample as measured using the CPGS subscale values of pain intensity, disability score, and the overall pain severity grade (39).
To explore the association between MDMA-AT and chronic pain, we compared pre- vs. post-treatment CPGS pain intensity, disability score, and composite severity grade for all participants (n = 32) using t-testing for bivariate association and two-way ANOVA for multivariable analysis, adjusting for demographics (sex, age, and ethnicity: white vs. non-white). We hypothesized that only participants reporting medium or high within-sample chronic pain at baseline would experience significant improvement post-treatment, therefore we repeated the same analysis for each CPGS cluster.
Results
Of the 33 participants enrolled in study MP16, 32 had pre/post CPGS data and were thus included in this analysis. Overall, 59% (n = 19) were female, 72% (n = 23) were white, and the median age was 38 [interquartile range (IQR) = 31–47] (Table 2). Of this sample, 84% (n = 27) reported having pain, and 75% (n = 24) reported disability associated with their pain. Participants median ACE score was 4 (IQR = 3–7). No strong associations were found between pre-treatment CPGS values and ACE score, CAPS-5 total severity, or demographics (Table 3).
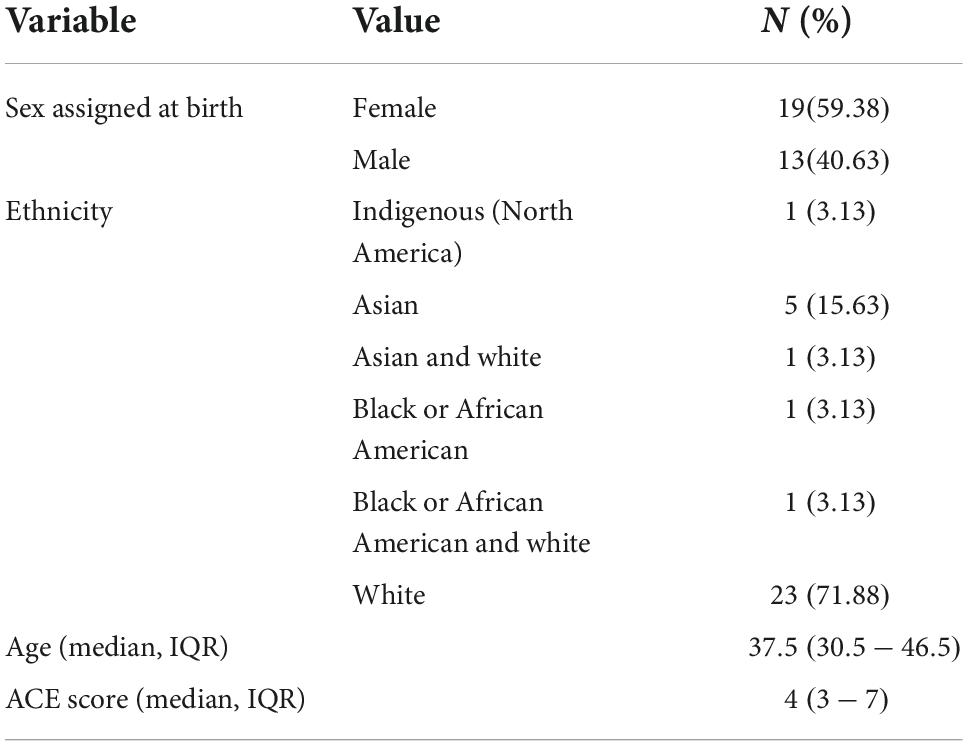
Table 2. Baseline demographic variables among participants receiving MDMA-assisted therapy for PTSD included in this analysis (n = 32).

Table 3. Bivariate linear and ordinal regression reveal no strong associations between pre-treatment CPG subscale values and pre-treatment CAPS-5 total severity, ACE score, or demographic variables.
In bivariate t-testing and ANOVA, among those in the highest pain cluster mean CPGS values (each of pain intensity, disability score, and severity grade) were significantly reduced post-intervention vs. baseline (n = 9, p < 0.05) (Tables 4–7). A significant reduction in pain intensity was observed in the medium pain cluster using ANOVA (n = 11, p = 0.020; p = 0.095 for t-test) and there was a trend to reduction for disability score and severity grade subscales in both bivariate and multivariable analysis (Tables 4, 8).
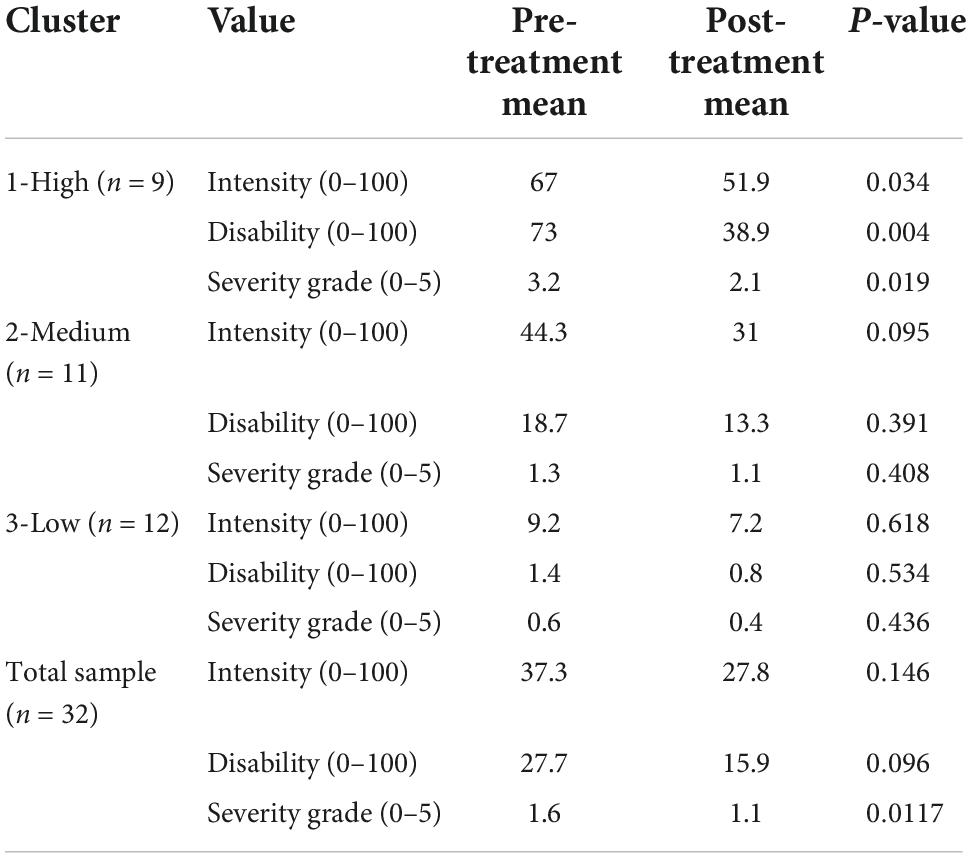
Table 4. Bivariate pre-treatment and post-treatment mean and p-values using t-testing for CPG pain intensity, disability score and the severity grade according to cluster groups (1-high n = 9, 2-medium n = 11, 3-low n = 12), and for the total sample (n = 32).
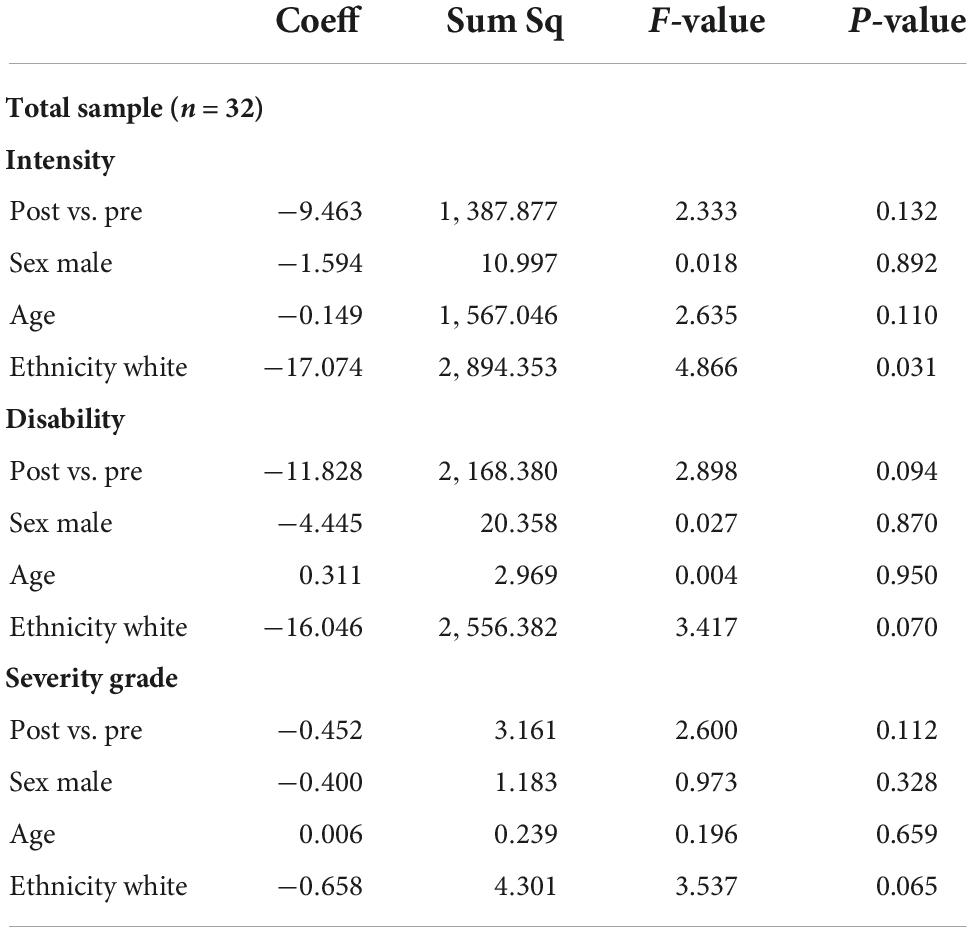
Table 5. Multivariable ANOVA, adjusted for demographics (sex male vs. female, age, ethnicity white vs. non-white) for the total sample (n = 32).
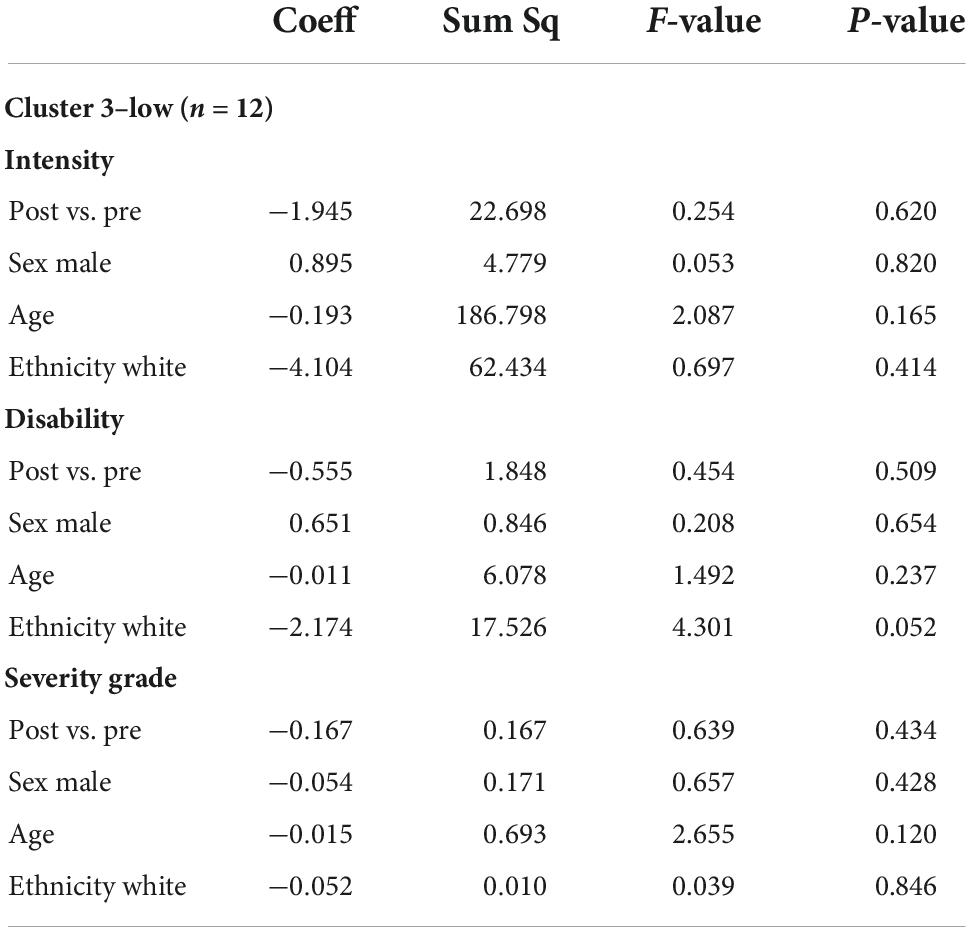
Table 6. Multivariable ANOVA, adjusted for demographics (sex male vs. female, age, ethnicity white vs. non-white) for cluster 3-low (n = 12).
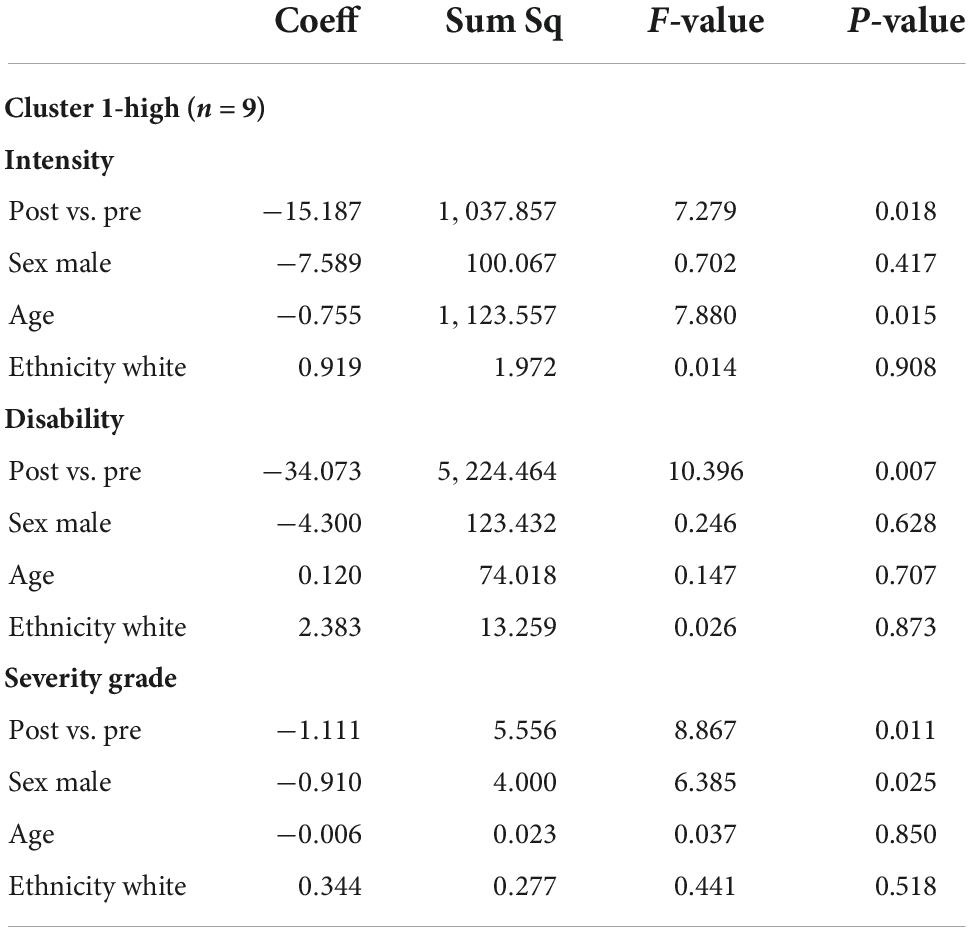
Table 7. Multivariable ANOVA, adjusted for demographics (sex male vs. female, age, Ethnicity white vs. non-white) for cluster 1-high (n = 9).
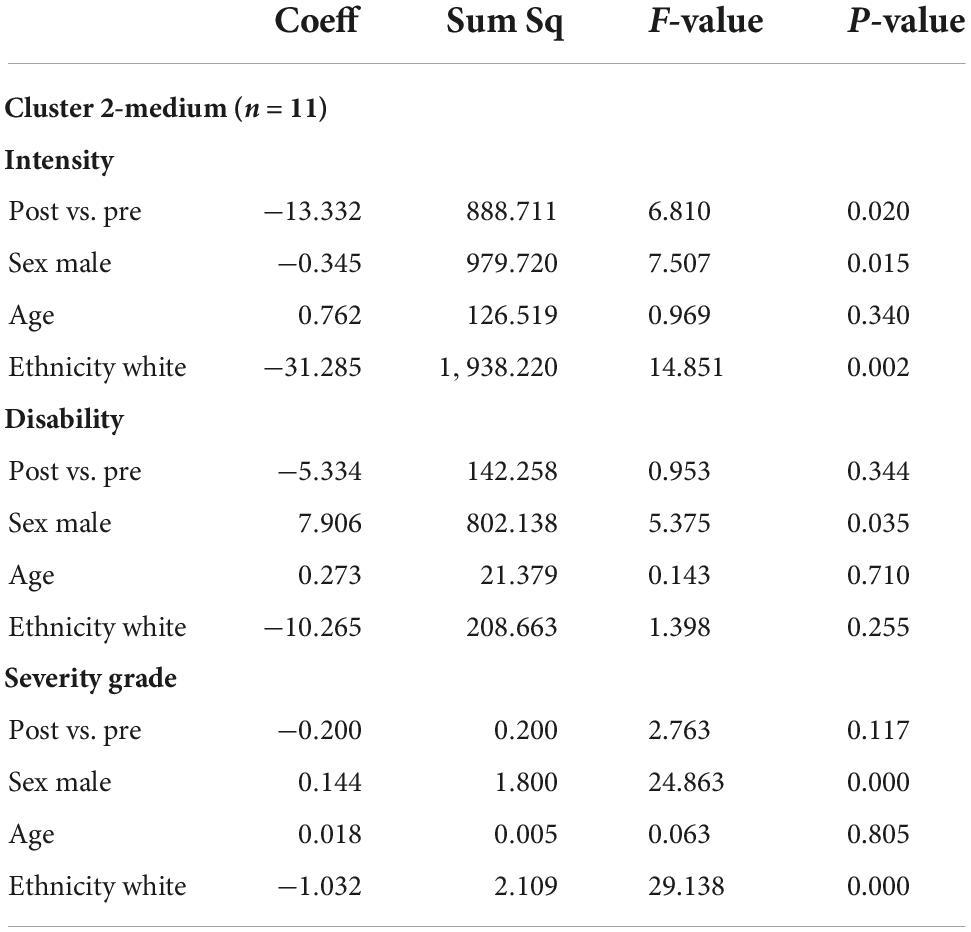
Table 8. Multivariable ANOVA, adjusted for demographics (sex male vs. female, age, ethnicity white vs. non-white) for the cluster 2-medium (n = 11).
Discussion
The present study found a high prevalence of pain among this sample of individuals with PTSD, in keeping with other published data demonstrating high rates of comorbid pain and PTSD (40). Statistically significant within subjects reductions in CPGS subscales including pain intensity, disability score, and composite chronic pain severity grade were observed following MDMA-AT among participants in the highest pain cluster (n = 9), and for pain intensity only in the medium pain cluster (n = 11), suggesting that MDMA-AT may be associated with reduction in chronic pain and pain-related disability. Significant reductions in pain and pain-related disability among participants in the highest pain cluster may be due to higher baseline pain values allowing greater margin for improvement; there may also be a mechanism related to an amygdala-based threat response to higher levels of pain being positively impacted by MDMA-AT, since overlapping brain areas have been shown to be active in both pain related threat perception, and anxiety and fear-based threat perception as typified by PTSD (32). Interestingly, among this sample baseline pain severity and PTSD severity were not positively correlated (Table 3). We speculate that differences in pain perception among individuals with PTSD symptoms may account for this finding; for example, high PTSD severity may distract from pain symptomatology, and vice versa, but this warrants further investigation. Previous research has demonstrated a unique and paradoxical pain perception pattern (hypo-responsiveness and hyper-sensitivity) in experimental and chronic pain among people with PTSD: PTSD-related dissociation was associated with higher pain threshold, whereas supra-threshold pain stimulus ratings were higher in those with PTSD in comparison to controls. Pain hyper-responsiveness was positively associated with anxiety sensitivity, but negatively correlated with dissociation levels (41). Anxiety and dissociation are intercorrelated in PTSD (41) yet their relationship to pain perception among people with PTSD has not been systematically studied and warrants further investigation. To our knowledge, this is the first study to demonstrate an association between MDMA-AT and reduced pain among individuals with PTSD.
Chronic pain is an international public health emergency for which new treatments are desperately needed (42, 43). While chronic pain is frequently comorbid with PTSD, few studies have examined treatments designed to effectively address both conditions concurrently (40), yet when outcomes are assessed related to psychological interventions for both disorders in individual studies, moderate effects for PTSD and small effects for pain emerge, suggesting transdiagnostic effects (44). International best practice recommendations involve understanding and addressing chronic pain within a biopsychosocial model, with personalized multidisciplinary treatment approaches that include both physical and psychological interventions (42, 43). Many chronic pain conditions, such as fibromyalgia, tension headache, and non-specific low back pain, involve alterations in central nervous system pain pathways leading to abnormal processing of pain signals, and are known collectively as “nociplastic” type pain. History of psychological, emotional, sexual, or physical abuse or a combination of these predispose to nociplastic pain (45). These conditions tend to respond poorly to physical treatment (medication or procedural interventions), and more readily to non-pharmacological approaches, such as educational, behavioral and psychotherapy interventions (46).
MDMA is a psychoactive compound that promotes serotonin release, and may exert therapeutic effects by enhancing fear memory extinction (47), promoting greater self-compassion (48), reducing self-criticism (48), reducing PTSD-related shame and anger (49) and causing acute prosocial and interpersonal effects that support the quality of the therapeutic alliance in psychotherapy (50). MDMA promotes oxytocin release, which helps increase interpersonal focus, feelings of interpersonal trust and social affiliation (51–53), and has been shown to reduce pain associated with social rejection (54). MDMA-AT has been associated with changes in personality persisting at two-month follow-up, notably increased personality trait Openness and reduction in Neuroticism (55). It has been suggested that MDMA may serve to acutely widen a “window of tolerance” in autonomic regulatory capacity which, combined with therapy, enables participants to approach and reprocess highly charged traumatic content without becoming overwhelmed or limited by hyperarousal or dissociative processes (5). MDMA may also re-open oxytocin-dependent “critical period” neuroplasticity in social learning that may also function to enhance or accelerate therapeutic change (56).
Given the high comorbidity of PTSD and chronic pain reflected in the present analysis, and the growing literature suggesting shared mechanisms both predisposing to and maintaining both conditions, it is conceivable that some of these proposed mechanisms explaining the efficacy of MDMA-AT for PTSD may be relevant for treating chronic pain. In particular, MDMA-AT effects related to enhanced fear extinction and approaching rather than avoiding negatively charged content may be relevant to fear and avoidance phenomena that are known to intensify and perpetuate chronic pain (32). Self-compassion, enhanced through MDMA, has also been linked with improved pain outcomes (57). Treatment with an existential focus may play an important role in chronic pain recovery (42, 58) particularly when combined with other psychological and pharmacological approaches (40) and has been shown to be effective in decreasing pain-related disability (59). MDMA-related improvements in intrapersonal attitudes including reduced self-criticism may impart benefit to chronic pain-associated existential challenges to related to identity, social roles, meaning and purpose, which are relevant yet underrepresented areas of treatment focus for chronic pain (40). Interpersonal effects that enhance therapeutic alliance may also be of relevance since psychological interventions for chronic pain require a strong therapeutic relationship to maximize benefit (12). Additionally, MDMA-enhanced perception of social connection and support may target chronic pain-related social isolation, a factor that exacerbates pain symptomatology, and when reduced, is associated with improvements in emotional and physical functioning among chronic pain patients (60). The potential for MDMA-AT to promote change in personality including reduced trait Neuroticism may also be of relevance, since neuroticism predicts poor adjustment to chronic pain (61).
Our data demonstrate a high median ACE score among this sample (4; IQR 3–7) and are consistent with previous research in the intersecting fields of chronic pain and trauma, that indicate an association with adverse childhood experiences (ACE) (13, 62). Stress response psychobiology is of particular relevance to this discussion. While short term stress responses support an individuals’ immediate survival, chronic adversity and/or repeated stressors, particularly during vulnerable developmental periods, can lead to long term changes to the structure and function of hormonal, neurological and psychological systems in ways that predispose to poor health outcomes (63–65), such as chronic pain. The capacity to tolerate increased levels of stress and to recover quickly, known as resilience, relies upon optimal environmental conditions whereby interacting psychobiological systems can develop ideal responses, and healthy attachment relationships are of particular importance (66). The potential of MDMA to re-open critical period neuroplasticity related to social reward learning, combined with interpersonal effects such as enhanced empathy and trust, may create optimal conditions within the MDMA-AT therapeutic relationship to improve resilience and thus create more favorable conditions for recovering from chronic pain and other chronic conditions that are negatively impacted by stress.
Strengths and limitations
K-means clustering provided a simple and efficient method to define groups according to pre-treatment CPGS values representing high, medium, and low within-sample pain values, which allowed for analysis using regression, t-testing and ANOVA for the whole sample and then separately for each of these stable clusters. The small sample size (n = 32) limited the power of these analyses, especially for the high pain cluster (n = 9) that demonstrated significant post-treatment reductions in pain values. Analysis was not conducted according to a hierarchical testing strategy, introducing the possibility for Type 1 error. Despite these limitations, participants in this sample were not subject to expectation bias regarding any positive impact of MDMA-AT on pain, since participants were recruited to the original study for experimental treatment of PTSD, whereas CPGS was administered and collected as exploratory data only. These data encourage further research with a larger sample in randomized, controlled trials in which the intervention is specifically investigated as a treatment for chronic pain.
In this sample, MDMA was administered 3 times according to a flexible dosing schedule; however, the analysis was not powered to detect differences in total MDMA dose given that the vast majority of participants received the escalation dose (120 mg HCl for the second and/or third dosing session) and supplemental dose of 40 or 60 mg HCl. Future studies could address exposure-response analyses on change in chronic pain.
While the CPGS is a valid and reliable instrument, recall bias may limit accuracy of self-report responses. Further, the CPGS version administered to participants included one question (#5) that differed from the published version of the measure: on the 10-point scale, 10 was defined for this analysis as 10 = Extreme Change, rather than 10 = Unable to Carry on Any Activities in response to the question In the past 6 months how much has pain interfered with your daily activities rated on 0-10 scale? Therefore, while participants were aware the 10-point scale asked for a rating of severity of pain interference with daily activities, they might have interpreted extreme change as still allowing for minimal activity, rather than absolute inability to carry on any activities. This could theoretically lead to false elevation of baseline and post-intervention disability score, which could likewise falsely elevate total severity score.
Associations between MDMA-AT and chronic pain may be influenced by confounding variables not examined in the present study, such as concurrent analgesic medications. Furthermore, participants in study MP16 were not selected for experiencing chronic pain, rather they were selected for having PTSD symptoms for at least 6 months, therefore some participants may have completed the questionnaire in reference to pain experienced for a shorter period. This limits generalizability of these exploratory data to true chronic pain conditions that are not comorbid with PTSD. In addition, different etiologies for pain could lead to differing pain or disability perceptions, or different secondary responses to the MDMA-AT for PTSD intervention. Within study population and within cluster distribution of pain by etiology could lead to further information and enhance future research protocols investigating MDMA-AT for chronic pain that include a mixed sample.
This study included predominantly white participants, which limits the generalizability of the findings to other populations. Not all people are affected by chronic pain equally; most data indicate higher prevalence in racialized and marginalized populations including African American and Indigenous people, women, and individuals from lower socioeconomic backgrounds (12). Further research should aim to increase diversity among research participants, as well as consider how chronic pain is experienced across the lifespan and across cultures.
Conclusion
These findings demonstrate a high prevalence of pain in this sample of participants with severe PTSD, and that pain intensity, disability, and a composite pain severity index grade among those with the highest pain were significantly lower following MDMA-AT. While these data are limited and primarily hypothesis generating, they suggest that MDMA-AT may be associated with reductions in pain and pain-related disability among individuals with PTSD. Despite being derived from an exploratory endpoint, these findings when considered alongside the promising efficacy of MDMA-AT for treating PTSD and the transdiagnostic similarities between PTSD and chronic pain, support advancement of research exploring the role of MDMA-AT as a novel intervention for chronic pain and PTSD/chronic pain co-morbidity.
Data availability statement
The datasets analyzed for this study were accessed by request to the MAPS Public Benefit Corporation (MAPS PBC). Restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are, however, available upon reasonable request and with the permission of MAPS at http://maps.org/datause. All requests for raw and analyzed data are promptly reviewed by MAPS PBC to verify if the request is subject to any confidentiality obligations. Patient-related data not included in this publication were generated as part of clinical trials and may be subject to patient confidentiality. Any data that can be shared may be released via a data use agreement.
Ethics statement
The MP16 clinical trial was reviewed and approved by the Western Copernicus Group Independent IRB (Research Triangle, NC, United States), Western IRB (Puyallup, WA, United States), University of California San Francisco Human Resource Protection Program IRB, University of Washington Human Subjects Division IRB, and University of British Columbia Providence Health Care Research Ethics Board. Participants provided their written and informed consent for participation in MP16.
Author contributions
BY-K: substantial contributions to the conception and design of study MP16. DC and EA: data analysis, plan conception, and design. DC: initial draft manuscript preparation. EN, DC, and EA: analysis and interpretation of the results. PK, WS, DL, and BY-K: review of data analysis and interpretation. DC, EA, PK, WS, DL, and BY-K: critical and final review of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
EA was supported by Michael Smith Foundation for Health Research and Canadian Institutes of Health Research (CIHR) postdoctoral awards. The clinical trial was sponsored by the Multidisciplinary Association for Psychedelic Studies (MAPS), a 501 (c) (3) non-profit organization. MAPS provided the MDMA and fully funded study MP16 from private donations. MAPS Public Benefit Corporation (MAPS PBC), wholly owned by MAPS, was the trial organizer.
Acknowledgments
We acknowledge the study sponsor MAPS and the MAPS Public Benefit Corporation for providing access to the data that were analyzed for this study, and in particular Julie Wang PhD, Data Science Manager and L.(Ilsa) Jerome Ph.D., Medical Coder, for their support. We express appreciation to all the participants, investigators, and research staff who made this work possible.
Conflict of interest
Author BY-K was employed by the company MAPS Public Benefit Corporation. Author WS was employed by Will Siu, MD Inc. Authors EA and DC were part-time consultants, and PK was a Clinical Advisor, to Numinus Wellness Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mithoefer MC, Feduccia AA, Jerome L, Mithoefer A, Wagner M, Walsh Z, et al. MDMA-assisted psychotherapy for treatment of PTSD: study design and rationale for phase 3 trials based on pooled analysis of six phase 2 randomized controlled trials. Psychopharmacology (Berl). (2019) 236:2735–45. doi: 10.1007/s00213-019-05249-5
2. Feduccia AA, Jerome L, Yazar-Klosinski B, Emerson A, Mithoefer MC, Doblin R. Breakthrough for trauma treatment: safety and efficacy of MDMA-assisted psychotherapy compared to paroxetine and sertraline. Front Psychiatry. (2019) 10:650. doi: 10.3389/fpsyt.2019.00650
3. Jerome L, Feduccia AA, Wang JB, Hamilton S, Yazar-Klosinski B, Emerson A, et al. Long-term follow-up outcomes of MDMA-assisted psychotherapy for treatment of PTSD: a longitudinal pooled analysis of six phase 2 trials. Psychopharmacology (Berl). (2020) 237:2485–97. doi: 10.1007/s00213-020-05548-2
4. Mithoefer MC, Mithoefer AT, Feduccia AA, Jerome L, Wagner M, Wymer J, et al. 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for post-traumatic stress disorder in military veterans, firefighters, and police officers: a randomised, double-blind, dose-response, phase 2 clinical trial. Lancet Psychiatry. (2018) 5:486–97. doi: 10.1016/S2215-0366(18)30135-4
5. Mitchell JM, Bogenschutz M, Lilienstein A, Harrison C, Kleiman S, Parker-Guilbert K, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. (2021) 27:1025–33. doi: 10.1038/s41591-021-01336-3
6. Wolfson PE, Andries J, Feduccia AA, Jerome L, Wang JB, Williams E, et al. MDMA-assisted psychotherapy for treatment of anxiety and other psychological distress related to life-threatening illnesses: a randomized pilot study. Sci Rep. (2020) 10:20442. doi: 10.1038/s41598-020-75706-1
7. Danforth AL, Grob CS, Struble C, Feduccia AA, Walker N, Jerome L, et al. Reduction in social anxiety after MDMA-assisted psychotherapy with autistic adults: a randomized, double-blind, placebo-controlled pilot study. Psychopharmacology (Berl). (2018) 235:3137–48. doi: 10.1007/s00213-018-5010-9
8. Sessa B, Sakal C, O’Brien S, Nutt D. First study of safety and tolerability of 3,4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy in patients with alcohol use disorder: preliminary data on the first four participants. BMJ Case Rep. (2019) 12:e230109. doi: 10.1136/bcr-2019-230109
9. Brewerton TD, Lafrance A, Mithoefer MC. The potential use of N-methyl-3,4-methylenedioxyamphetamine (MDMA) assisted psychotherapy in the treatment of eating disorders comorbid with PTSD. Med Hypotheses. (2020) 146:110367. doi: 10.1016/j.mehy.2020.110367
10. Mithoefer MC, Wagner MT, Mithoefer AT, Jerome L, Doblin R. The safety and efficacy of±3, 4-methylenedioxymethamphetamine-assisted psychotherapy in subjects with chronic, treatment-resistant posttraumatic stress disorder: the first randomized controlled pilot study. J Psychopharmacol. (2011) 25:439–52. doi: 10.1177/0269881110378371
11. Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults – United States, 2016. MMWR Morb Mortal Wkly Rep. (2018) 67:1001–6. doi: 10.15585/mmwr.mm6736a2
12. Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. (2021) 397:2082–97. doi: 10.1016/S0140-6736(21)00393-7
13. Mills SEE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. (2019) 123:e273–83. doi: 10.1016/j.bja.2019.03.023
14. Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. (2015) 156:1003–7. doi: 10.1097/j.pain.0000000000000160
15. IASP. Terminology. International Association for the Study of Pain. Washington, DC: IASP (2021).
16. Hunter P. New therapies to relieve pain: the search for more efficient and safer alternatives to opioid pain killers. EMBO Rep. (2018) 19:e46925. doi: 10.15252/embr.201846925
17. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. (2014) 69:119–30. doi: 10.1037/a0035514
18. Andreski P, Chilcoat H, Breslau N. Post-traumatic stress disorder and somatization symptoms: a prospective study. Psychiatry Res. (1998) 79:131–8. doi: 10.1016/S0165-1781(98)00026-2
19. Jellestad L, Vital NA, Malamud J, Taeymans J, Mueller-Pfeiffer C. Functional impairment in posttraumatic stress disorder: a systematic review and meta-analysis. J Psychiatr Res. (2021) 136:14–22. doi: 10.1016/j.jpsychires.2021.01.039
20. Balayan K, Kahloon M, Tobia G, Postolova A, Peek H, Akopyan A, et al. The impact of posttraumatic stress disorder on the quality of life: a systematic review. Int Neuropsychiatr Dis J. (2014) 2:214–33. doi: 10.9734/INDJ/2014/7649
21. Siqveland J, Hussain A, Lindstrøm JC, Ruud T, Hauff E. Prevalence of posttraumatic stress disorder in persons with chronic pain: a meta-analysis. Front Psychiatry. (2017) 8:164. doi: 10.3389/fpsyt.2017.00164
22. Fishbain DA, Pulikal A, Lewis JE, Gao J. Chronic pain types differ in their reported prevalence of post –traumatic stress disorder (PTSD) and there is consistent evidence that chronic pain is associated with PTSD: an evidence-based structured systematic review. Pain Med. (2017) 18:711–35. doi: 10.1093/pm/pnw065
23. Otis JD, Keane TM, Kerns RD, Monson C, Scioli E. The development of an integrated treatment for veterans with comorbid chronic pain and posttraumatic stress disorder. Pain Med. (2009) 10:1300–11. doi: 10.1111/j.1526-4637.2009.00715.x
24. Asmundson GJ, Coons MJ, Taylor S, Katz J. PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. Can J Psychiatry. (2002) 47:930–7. doi: 10.1177/070674370204701004
25. Otis JD, Keane TM, Kerns RD. An examination of the relationship between chronic pain and post-traumatic stress disorder. J Rehabil Res Dev. (2003) 40:397–405. doi: 10.1682/JRRD.2003.09.0397
26. Bilevicius E, Sommer JL, Asmundson GJG, El-Gabalawy R. Posttraumatic stress disorder and chronic pain are associated with opioid use disorder: results from a 2012-2013 American nationally representative survey. Drug Alcohol Depend. (2018) 188:119–25. doi: 10.1016/j.drugalcdep.2018.04.005
27. Jenewein J, Wittmann L, Moergeli H, Creutzig J, Schnyder U. Mutual influence of posttraumatic stress disorder symptoms and chronic pain among injured accident survivors: a longitudinal study. J Trauma Stress. (2009) 22:540–8. doi: 10.1002/jts.20453
28. Sullivan MJL, Thibault P, Simmonds MJ, Milioto M, Cantin A-P, Velly AM. Pain, perceived injustice and the persistence of post-traumatic stress symptoms during the course of rehabilitation for whiplash injuries. Pain. (2009) 145:325–31. doi: 10.1016/j.pain.2009.06.031
29. Kind S, Otis JD. The interaction between chronic pain and PTSD. Curr Pain Headache Rep. (2019) 23:91. doi: 10.1007/s11916-019-0828-3
30. Hooten WM. Chronic pain and mental health disorders: shared neural mechanisms, epidemiology, and treatment. Mayo Clin Proc. (2016) 91:955–70. doi: 10.1016/j.mayocp.2016.04.029
31. Brennstuhl MJ, Tarquinio C, Montel S. Chronic pain and PTSD: evolving views on their comorbidity. Perspect Psychiatr Care. (2015) 51:295–304. doi: 10.1111/ppc.12093
32. Elman I, Borsook D. Threat response system: parallel brain processes in pain vis-à-vis fear and anxiety. Front Psychiatry. (2018) 9:29. doi: 10.3389/fpsyt.2018.00029
33. Sharp TJ, Harvey AG. Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin Psychol Rev. (2001) 21:857–77. doi: 10.1016/S0272-7358(00)00071-4
34. Majeed MH, Ali AA, Sudak DM. Psychotherapeutic interventions for chronic pain: evidence, rationale, and advantages. Int J Psychiatry Med. (2019) 54:140–9. doi: 10.1177/0091217418791447
35. Wang JB, Lin J, Bedrosian L, Coker A, Jerome I, Feduccia A, et al. Scaling up: multisite open-label clinical trials of MDMA-assisted therapy for severe posttraumatic stress disorder. J Humanist Psychol. (2021). doi: 10.1177/00221678211023663 [Epub ahead of print].
36. Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, et al. The clinician-administered PTSD scale for DSM-5 (CAPS-5): development and initial psychometric evaluation in military veterans. Psychol Assess. (2018) 30:383–95. doi: 10.1037/pas0000486
37. Elliott AM, Smith BH, Smith CW, Chambers AW. Changes in chronic pain severity over time: the chronic pain grade as a valid measure. Pain. (2000) 88:303–8. doi: 10.1016/S0304-3959(00)00337-7
38. Smith BH, Penny KI, Purves AM, Munro C, Wilson B, Grimshaw J, et al. The chronic pain grade questionnaire: validation and reliability in postal research. Pain. (1997) 71:141–7. doi: 10.1016/S0304-3959(97)03347-2
39. Jin X, Han J. K-means clustering. In: Sammut C, Webb GI editors. Encyclopedia of Machine Learning. (Boston, MA: Springer) (2010). p. 563–4. doi: 10.1007/978-0-387-30164-8_425
40. Reed DE, Cobos B, Nabity P, Doolin J, McGeary DD. Chapter 15 – comorbid chronic pain and posttraumatic stress disorder: current knowledge, treatments, and future directions. In: Pangarkar S, Pham QG, Eapen BC editors. Pain Care Essentials and Innovations. (Amsterdam: Elsevier) (2021). p. 211–27. doi: 10.1016/B978-0-323-72216-2.00015-6
41. Defrin R, Schreiber S, Ginzburg K. Paradoxical pain perception in posttraumatic stress disorder: the unique role of anxiety and dissociation. J Pain. (2015) 16:961–70. doi: 10.1016/j.jpain.2015.06.010
42. Canadian Pain Task Force. Chronic Pain in Canada: laying a Foundation for Action. Ottawa, ON: Health Canada (2019). p. 190179
43. U. S. Department of Health and Human Services. Pain Management Best Practices Inter-Agency Task Force Report: updates, Gaps, Inconsistencies, and Recommendations. Washington, DC: U. S. Department of Health and Human Services (2019).
44. Goldstein E, McDonnell C, Atchley R, Dorado K, Bedford C, Brown RL, et al. The impact of psychological interventions on posttraumatic stress disorder and pain symptoms: a systematic review and meta-analysis. Clin J Pain. (2019) 35:703–12. doi: 10.1097/AJP.0000000000000730
45. Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. (2021) 397:2098–110. doi: 10.1016/S0140-6736(21)00392-5
46. Afari N, Ahumada SM, Wright LJ, Mostoufi S, Golnari G, Reis V, et al. Psychological trauma and functional somatic syndromes: a systematic review and meta-analysis. Psychosom Med. (2014) 76:2–11. doi: 10.1097/PSY.0000000000000010
47. Young MB, Andero R, Ressler KJ, Howell LL. 3,4-methylenedioxymethamphetamine facilitates fear extinction learning. Transl Psychiatry. (2015) 5:e634. doi: 10.1038/tp.2015.138
48. Kamboj SK, Kilford EJ, Minchin S, Moss A, Lawn W, Das RK, et al. Recreational 3,4-methylenedioxy-N-methylamphetamine (MDMA) or “ecstasy” and self-focused compassion: preliminary steps in the development of a therapeutic psychopharmacology of contemplative practices. J Psychopharmacol. (2015) 29:961–70. doi: 10.1177/0269881115587143
49. Dewey D, Schuldberg D, Madathil R. Do peritraumatic emotions differentially predict PTSD symptom clusters? Initial evidence for emotion specificity. Psychol Rep. (2014) 115:1–12. doi: 10.2466/16.02.PR0.115c11z7
50. Hysek CM, Schmid Y, Simmler LD, Domes G, Heinrichs M, Eisenegger C, et al. MDMA enhances emotional empathy and prosocial behavior. Soc Cogn Affect Neurosci. (2014) 9:1645–52. doi: 10.1093/scan/nst161
51. Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. (2005) 435:673–6. doi: 10.1038/nature03701
52. Bartz JA, Hollander E. The neuroscience of affiliation: forging links between basic and clinical research on neuropeptides and social behavior. Horm Behav. (2006) 50:518–28. doi: 10.1016/j.yhbeh.2006.06.018
53. Wagner AC. Couple therapy with MDMA—proposed pathways of action. Front Psychol. (2021) 12:733456. doi: 10.3389/fpsyg.2021.733456
54. Frye CG, Wardle MC, Norman GJ, de Wit H. MDMA decreases the effects of simulated social rejection. Pharmacol Biochem Behav. (2014) 117:1–6. doi: 10.1016/j.pbb.2013.11.030
55. Wagner MT, Mithoefer MC, Mithoefer AT, MacAulay RK, Jerome L, Yazar-Klosinski B, et al. Therapeutic effect of increased openness: investigating mechanism of action in MDMA-assisted psychotherapy. J Psychopharmacol. (2017) 31:967–74. doi: 10.1177/0269881117711712
56. Nardou R, Lewis EM, Rothhaas R, Xu R, Yang A, Boyden E, et al. Oxytocin-dependent reopening of a social reward learning critical period with MDMA. Nature. (2019) 569:116–20. doi: 10.1038/s41586-019-1075-9
57. Edwards KA, Pielech M, Hickman J, Ashworth J, Sowden G, Vowles KE. The relation of self-compassion to functioning among adults with chronic pain. Eur J Pain. (2019) 23:1538–47. doi: 10.1002/ejp.1429
58. McCabe R, Murray R, Austin P, Siddall P. Spiritual and existential factors predict pain relief in a pain management program with a meaning-based component. J Pain Manage. (2018) 11:163–70.
59. Gebler F, Maercker A. Effects of including an existential perspective in a cognitive-behavioral group program for chronic pain: a clinical trial with 6 months follow-up. J Humanist Psychol. (2014) 42:155–71. doi: 10.1080/08873267.2013.865188
60. Bannon S, Greenberg J, Mace RA, Locascio JJ, Vranceanu AM. The role of social isolation in physical and emotional outcomes among patients with chronic pain. Gen Hosp Psychiatry. (2021) 69:50–4. doi: 10.1016/j.genhosppsych.2021.01.009
61. Asghari A, Nicholas MK. Personality and pain-related beliefs/coping strategies: a prospective study. Clin J Pain. (2006) 22:10–8. doi: 10.1097/01.ajp.0000146218.31780.0b
62. Frewen P, Zhu J, Lanius R. Lifetime traumatic stressors and adverse childhood experiences uniquely predict concurrent PTSD, complex PTSD, and dissociative subtype of PTSD symptoms whereas recent adult non-traumatic stressors do not: results from an online survey study. Eur J Psychotraumatol. (2019) 10:1606625. doi: 10.1080/20008198.2019.1606625
63. Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. (2014) 94:1816–25. doi: 10.2522/ptj.20130597
64. McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. (2000) 886:172–89. doi: 10.1016/S0006-8993(00)02950-4
65. Bellis MDD, Zisk A. The biological effects of childhood trauma. Child Adolescent Psychiatric Clin. (2014) 23:185–222. doi: 10.1016/j.chc.2014.01.002
Keywords: MDMA-assisted therapy, chronic pain, MDMA, post-traumatic stress disorder, mental health
Citation: Christie D, Yazar-Klosinski B, Nosova E, Kryskow P, Siu W, Lessor D and Argento E (2022) MDMA-assisted therapy is associated with a reduction in chronic pain among people with post-traumatic stress disorder. Front. Psychiatry 13:939302. doi: 10.3389/fpsyt.2022.939302
Received: 09 May 2022; Accepted: 14 October 2022;
Published: 03 November 2022.
Edited by:
Peter Schuyler Hendricks, University of Alabama at Birmingham, United StatesReviewed by:
Yasmin Schmid, University of California, San Diego, United StatesNatalie Gukasyan, Johns Hopkins Medicine, United States
Brian Pilecki, Portland Psychotherapy Clinic, United States
Copyright © 2022 Christie, Yazar-Klosinski, Nosova, Kryskow, Siu, Lessor and Argento. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena Argento, YmNjc3UtZWFAYmNjc3UudWJjLmNh
†These authors share senior authorship
 Devon Christie
Devon Christie Berra Yazar-Klosinski2†
Berra Yazar-Klosinski2† Ekaterina Nosova
Ekaterina Nosova Pam Kryskow
Pam Kryskow Will Siu
Will Siu Danielle Lessor
Danielle Lessor Elena Argento
Elena Argento