
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 04 October 2022
Sec. Addictive Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.935491
This article is part of the Research Topic Addiction and the Brain: Current Knowledge, Methods, and Perspectives View all 14 articles
 Xiaorui Hu1†
Xiaorui Hu1† Tian Zhang2†
Tian Zhang2† Hongkun Ma3†
Hongkun Ma3† Xuhui Zhou4†
Xuhui Zhou4† Hongxuan Wang5†
Hongxuan Wang5† Xiaohong Wang1
Xiaohong Wang1 Chang Cheng1
Chang Cheng1 Yanfei Li2
Yanfei Li2 Ranran Duan2
Ranran Duan2 Bo Zhang1
Bo Zhang1 Huaizhi Wang1
Huaizhi Wang1 Jia Lu1
Jia Lu1 Chuanyi Kang1
Chuanyi Kang1 Na Zhao1
Na Zhao1 Yingjie Zhang6
Yingjie Zhang6 Lu Tian6
Lu Tian6 Jun Liu7
Jun Liu7 Jingjing Shi1
Jingjing Shi1 Zhe Wang7
Zhe Wang7 Xinxin Zhou7
Xinxin Zhou7 Shuang Zhu1
Shuang Zhu1 Qingxia Liu1
Qingxia Liu1 Xuemin Li1
Xuemin Li1 Honghui Wang1
Honghui Wang1 Mingxuan Nie1
Mingxuan Nie1 Mei Yang8
Mei Yang8 Jianzhong Yang9
Jianzhong Yang9 Yong Chi6
Yong Chi6 Xiaofeng Zhu10
Xiaofeng Zhu10 Jian Hu1
Jian Hu1 Yanjie Jia2*
Yanjie Jia2* Ying Peng5*
Ying Peng5* Lei Liu1*
Lei Liu1*Background: Alcohol dependence (AD) is a complex addictive disorder with a high relapse rate. Previous studies have shown that both repetitive transcranial magnetic stimulation (rTMS) and cognitive behavioral therapy (CBT) may be effective for AD, and we aim to explore more effective treatment options to reduce relapse rates for AD.
Materials and methods: A total of 263 AD patients were recruited. They were divided into six groups according to the location and the type of rTMS: left dorsolateral prefrontal cortex (DLPFC), right DLPFC, sham stimulation, and whether they received CBT treatment: with a fixed schedule (C1) and without a fixed plan (C0). There were included in sham rTMS + C0 group (n = 50), sham rTMS + C1 group (n = 37), right rTMS + C0 group (n = 45), right rTMS + C1 group (n = 42), left rTMS + C0 group (n = 49), left rTMS + C1 group (n = 40). We used obsessive compulsive drinking scale (OCDS), visual analogue scale (VAS), alcohol dependence scale (ADS), montreal cognitive assessment (MoCA), generalized anxiety disorder-7 (GAD-7), patient health questionnaire-9 items (PHQ-9), and Pittsburgh sleep quality index (PSQI) to assess alcohol cravings, alcohol dependence, cognition, anxiety, depression, and sleep quality. They were followed up and evaluated for relapse.
Results: The sham rTMS + C0 group relapse rate was significantly higher than the right rTMS + C1 group (P = 0.006), the left rTMS + C0 group (P = 0.031), the left rTMS + C1 group (P = 0.043). The right rTMS + C0 group showed significantly higher relapse rate compared to the right rTMS + C1 group (P = 0.046). There was no significant difference in relapse rates between other groups. The repeated-measures ANOVA showed an interaction effect between group and time was significant in the rate of patient health questionnaire-9 items (PHQ-9) scale reduction (P = 0.020). Logistic analysis indicated that smoking and alcohol consumption were independent determinants of relapse (P < 0.05). At 24 weeks of follow-up, Kaplan–Meier survival analysis reveal that there is statistically significant relapse rate between six groups (P = 0.025), left rTMS + C1 group has the best treatment effect for alcohol dependent patients. Cox regression analysis confirmed that current smoking, total cholesterol, and total bilirubin (TBIL) level were risk factors of relapse (P < 0.05).
Conclusion: This study is the first to suggest that the combination of rTMS and CBT may be a potentially effective treatment for reducing relapse.
It is currently widely recognized that alcoholism is a complex, multi-dimensional, and multifactorial disease. Notably, 9–17% of drinkers meet the diagnostic criteria for alcohol dependence (AD) (1). AD is a complex addictive disease. In general, addiction formation is closely related to biological factors, and the occurrence and development of addictive behaviors are related to psychological and social factors, such as parenting style (2), family relationships (3), and childhood sexual abuse (4). Because of long-term heavy drinking, the body gradually develops physical and psychological dependence on alcohol, damaging physical, mental, and social functions. AD in all countries in the world is a severe public health problem. With the development of the social economy, and the continuous improvement of people’s living standards, alcohol dependence prevalence is gradually rising. In 2018, the World Health Organization’s global status report on alcohol and health revealed approximately 283 million AD (accounting for 5.1% of the world’s 15 years old and older adults). The prevalence rates vary considerably between countries and are significantly influenced by drinking culture and social norms. Europe has the highest prevalence (8.8% of the adult population), followed by the United States (8.2% of the adult population), while the prevalence of alcohol dependence in China is 2.3% (5). AD is among the highest risk factors for shortening the life cycle and leads to more than 200 health conditions, with a high disease burden, high disability rate, and high mortality (6).
Early long-term AD treatment mainly included drug and behavioral therapy (7). However, these treatments are only moderately helpful, and more than 50% of treated patients relapse within 1 year (8). Even though research over the past 50 years has demonstrated that addiction is a brain disease, we still have no effective treatments based on neural circuits and specific neural targets that directly and specifically target AD. For the first time, a non-invasive neuroendocrine technique called transcranial magnetic stimulation (TMS) appears to be the primary physical therapy approach to fill this gap in developing AD treatment. Dr. Anthony Barker invented TMS technology in Sheffield, UK, in 1984 (9). TMS is based on electromagnetic induction, a brief focused electromagnetic pulse penetrating the skull without attenuation and stimulating the targeted brain region. Typically, the magnetic field is high enough to induce depolarization of neurons in the cortex region where the coils are located. This technique is called repetitive TMS (rTMS) when TMS pulses are transmitted continuously and repeatedly at a precise frequency. High-frequency repeat transcranial magnetic stimulation (HF-rTMS) (>5 Hz) promoted, while low frequency rTMS (<1 Hz) inhibited motor cortex excitability (10). rTMS can induce changes in brain function for a more extended period by modulating cortical excitability, neurotransmitters, and neuronal plasticity, reducing the desire for addictive substances, improving cognitive function, and ultimately reducing the relapse rate (11). It provides a new therapeutic advantage for alcohol dependence unmatched by pharmacotherapy.
Brodmann regions 9 and 46 are often known as the dorsolateral prefrontal cortex (DLPFC). DLPFC plays an essential role in decision making, reasoning, working memory, inhibition, and outcome prediction, plays a vital role in substance-related cravings, including alcohol, and has gained a recognized reputation as a successful treatment target for TMS in patients with depression (12). Previous studies have shown that rTMS, DLPFC was selected as the stimulation site to show promise for the reduction of craving and substance use in nicotine (13) and cocaine addiction (14), as well as in behavioral addictions (15), which are showing significant development potential. All these suggest that DLPFC may be an ideal target for TMS treatment of AD. However, the treatment duration, frequency of stimulation, and intensity of stimulation frequency of rTMS have not been determined. Also, it has not been clearly answered whether left, right, or bilateral stimulation is the most effective method (16–20).
Another critical aspect of TMS treatment for AD is combining it with existing behavioral therapies for AD. Ultimately, all treatments for AD need to emphasize changing behavior. Cognitive behavioral therapy (CBT) has become a front-line behavioral therapy for AD and other substance use disorders (SUDs) in recent years (21). Drawn up in the 1960s and 1970s in the United States, CBT is a time-limited, multistage intervention designed to address the cognitive, emotional, and environmental risks of drug use, identify and change unreasonable beliefs, and provide training in behavioral self-control skills. By overcoming the desire to seek out and consume alcohol and by dealing with situations that might trigger these desires, to improve patients’ psychological defense ability and build psychological defense mechanisms to help individuals achieve and maintain abstinence or reduce drinking cravings (22). A meta-analysis found that Internet-based alcohol interventions guided by health professionals were more effective than unguided (fully automated) interventions (23). However, therapist-guided interventions were not more effective than self-help interventions in the two most recent studies on internet-based cognitive behavioral therapy for alcohol (24, 25). Therefore, the trial hypothesizes that a therapist-guided, fixed-plan model CBT would be more effective in reducing relapse rates than simply providing a standardized basic interview of about 10 min without any therapeutic intervention.
A large part of the disease burden is due to the ongoing effects of alcohol on the central nervous system (26). As a central nervous system depressant, alcohol can, directly and indirectly, act on the central nervous system, leading to cognitive dysfunction (27). Cognitive impairment has increasingly become the focus of AD research. Depending on studies, 50–80% of AD patients have cognitive impairment (28). In addition, people with AD often experience an intense, uncontrollable desire for alcohol, also known as craving (29). Combining perceived desires with reduced cognitive control can lead to problems managing cravings, leading to relapse (30). AD is often associated with various psychiatric and social behavioral comorbidities, including severe sleep disorders, anxiety, and depression (31, 32).
Therefore, the biggest problem plaguing AD is its extraordinary relapse rate. There are limited treatment methods for AD relapse, and a high relapse rate will hinder the treatment effect. Depending on statistics, although the early treatment is beneficial, up to 85% of AD patients still drink again (33), significantly the highest within 6–12 months after treatment (34). This suggests a critical need to understand factors associated with relapse. The evidence is inconsistent regarding the potential impact of smoking on recovery from alcohol dependence. Although some studies have found that smoking negatively affects the treatment outcome of alcohol dependent patients (35), however, there is also evidence that smoking does not pose a risk to sobriety in AD (36). Variables associated with nicotine dependence may be predictors of future alcohol dependence (37). Alcohol biomarkers have become valuable tools for objectively assessing treatment outcomes (38). Routine blood tests may help predict the long-term development of alcohol withdrawal treatment and may become a more feasible and cost-effective method for assessing relapse risk (39). This study explored the relationship between pre-treatment predictors (demographics and laboratory tests), post-treatment predictors (rTMS and CBT), and relapse. We hypothesized that treatment-related variables would be best helpful in predicting the prognosis of alcohol dependent patients receiving treatment. Even though AD profoundly impacts individuals’ work, social life, and interpersonal relationships (40), the treatment rate of AD is extremely low, and the establishment of effective treatment is essential.
Although rTMS and CBT have positive effects on all dimensions of AD, there is currently more heterogeneity in the outcomes of rTMS and CBT for AD compared with pharmacological AD treatment. There are no studies on whether there is an advantage of combined treatment with CBT and rTMS. Therefore, we conducted a randomized, double-blind sham-controlled multicenter clinical trial in which sham rTMS, left DLPFC rTMS, right DLPFC rTMS, and combined with CBT were utilized to treat AD patients. The ADS, VAS, OCDS, PHQ-9, GAD-7, PSQI, and MoCA scales were regularly used to evaluate the patients’ alcohol dependence, drinking desire, cognitive function anxiety, depression, and sleep. And during the follow-up period, whether the patients relapsed were recorded by a self-assessment diary of alcohol consumption. The primary objectives of this study: assess the effectiveness of rTMS in combination with CBT for AD in reducing relapse and investigate the risk factors of relapse. The secondary objectives of this study: whether different treatment modalities improve anxiety, depression, cognitive function, and craving and indirectly reduce the rate of relapse to drinking, and provide new directions for AD treatment.
We selected patients with AD who were outpatients and inpatients at the First Affiliated Hospital of Harbin Medical University, Sun Yat-sen Memorial Hospital, the First Affiliated Hospital of Zhengzhou University, Mudanjiang Medical University, Beijing Anding Hospital Affiliated to Capital Medical University, Shenzhen Kangning Hospital, Hunan Provincial Brain Hospital, the Second Affiliated Hospital of Kunming Medical University from March 2019 to September 2021 as the study population. The Ethics Committee endorsed the study, and all participants obtained informed consent from themselves and signed informed consent. Supplementary Figure 1 shows the CONSORT diagram of the flow of participants through the trial.
Inclusion criteria: (1) 18–65 years old; (2) Meet the diagnostic criteria of DSM-IV alcohol dependence; (3) No history of neurological diseases or family history of mental disorders.
Exclusion criteria: (1) Clinical Institute Alcohol Withdrawal Syndrome Scale (CIWA-Ar) > 9 points in acute alcohol withdrawal reaction stage; (2) Severe neurological or mental diseases caused by other diseases other than chronic alcohol dependence, such as stroke, intracranial infection, brain tumors, schizophrenia, etc.; (3) Have experienced a traumatic brain injury or other brain tissue damage; (4) Is taking or has taken any other psychotropic drugs or is dependent on other drugs or other substances; (5) Contraindications of rTMS therapy: a. Acute infectious diseases; b. Presence of metallic foreign bodies in the skull; c. After craniotomy; d. Intracranial aneurysm or other vascular malformation; e. Epilepsy history; f. Severe cardiovascular disease, especially those with pacemakers or cardiac stents.
Experimental termination criteria: (1) Severe adverse reactions occurred during the study; (2) Subjects did not cooperate with treatment and had poor compliance.
We collected general clinical data: self-designed case report form, general physical examination, basic vital signs, hematology routine, and blood biochemistry test results. And during the follow-up period, whether the patients relapsed were recorded by a self-assessment diary of alcohol consumption. Based on the self-assessment diary of alcohol consumption, combined with regular telephone follow-up with family members at week 0, week 2, week 8, week 12, and week 24, and outpatient follow-up, to ensure the authenticity of the self-assessment diary of alcohol consumption.
(1) Self-designed case report form, including gender, age, drinking years, alcohol consumption, drinking type, frequency of alcohol consumption, and current smoking.
(2) General physical examination and basic vital signs, including body mass index, heart rate, systolic blood pressure, and diastolic blood pressure.
(3) Hematology routine including white blood cell, red-blood-cell, Platelets, and hemoglobin.
(4) Blood biochemistry, including fasting glucose, uric acid, serum creatinine, alanine transaminase, aspartate aminotransferase, gamma-glutamyl transpeptidase, total bilirubin, direct bilirubin, indirect bilirubin, Total cholesterol, Triglycerides, low-density lipoprotein, high-density lipoprotein.
(1) Repetitive transcranial magnetic stimulation procedure:
Treatment device: Use of “8” coil transcranial magnetic stimulation instrument (using the YiRuiDe® CCY-1 classic magnetic stimulator device; YiRuiDe Group, Wuhan, China).
Treatment duration: Starting at baseline, stimulated on five consecutive days (W1), suspended on weekends, treatment continued for five consecutive working days in the second week (W2), total of 10 sessions.
Treatment: The individual motor threshold (MT) for the right/left abductor pollicis brevis muscle was determined using single-pulse TMS in combination with motor-evoked potentials (MEP). The MT was considered the lowest intensity to induce a visual MEP on electromyography (EMG). A stimulation intensity of 110% of the subject’s resting MT was used for the study. a. Left DLPFC rTMS: the Left dorsolateral prefrontal cortex was selected as the stimulation site, and the location was determined by the international 10–20 electroencephalography system (the location of the left DLPFC corresponds to the F3). Treatment parameters were set (stimulation intensity: 110% threshold, stimulation frequency: high-frequency 10 Hz, train duration: 5 s, intertrain interval: 20 s, total trains per session: 30 trains, total 10 sessions). b. Right DLPFC rTMS: the Right dorsolateral prefrontal cortex was selected as the stimulation site. The international 10–20 electroencephalogram system was used for localization (the location of the right DLPFC corresponds to the F4). The treatment parameters were the same as the left rTMS group. c. Sham rTMS: The spiral edge was placed at the stimulation site, and the stimulation intensity was set to 0 or 1%. The other treatment parameters were fixed as above. All subjects were unaware of the type of stimulation they received; they wore earplugs. The study was conducted in conformity with the current safety guidelines (41).
(2) Cognitive behavioral therapy procedure:
Treatment duration: Starting at baseline, once a week for 8 weeks (W1–W8), for a total of eight sessions per subject.
Treatment: Cognitive behavioral therapy with a fixed plan is 60 min per session, and each session is divided into three phases preparation, work, and summary, each phase being 20 min. According to the abstinence treatment research paradigm (pre-action stage, planning stage, preparation stage, action stage, maintenance/consolidation stage, and termination/relapse stage), this study designed eight individual cognitive-behavioral therapy sessions with different themes. The eight themes were: a. Individual motivational feedback; b. Identification and handling of predisposing factors; c. Transformation of negative cognition; d. Negative emotion control and management; e. Enrichment of drink-refusal skills; f. Improvement of interpersonal relationships; g. Establishment of a recovery support system; h. Reduction of relapse risk. The CBT group, without a fixed plan, provided only about 10 min of a standardized basic interview without any therapeutic intervention. The CBT treatment protocol was designed with reference to the studies of Johansson M (42) and Magill M (43), with appropriate adaptations. One of the treatment regimens was randomly assigned to CBT with a fixed plan (C1); CBT without a fixed plan (C0).
(3) Treatment as usual:
All subjects received routine drug therapy with the same treatment period, and dose, including the use of mecobalamin and vitamin B to nourish nerves, antioxidant damage with vitamin C and vitamin E. Temporary short-term low-dose Benzodiazepines were given to patients when necessary.
(1) Randomization and double-blind method: a clinical research assistant who is not involved in other clinical treatment, scale, and outcome evaluation will automatically randomize subjects by the computer algorithm. Neither the subject nor the clinical investigator knew which treatment group the subject was assigned. TMS investigators, and CBT study personnel, are unaware of changes in subject outcomes.
(2) This study was divided into six groups:
a. TAU + sham rTMS + CBT without a fixed plan (sham rTMS + C0 group).
b. TAU + sham rTMS + CBT with a fixed plan (sham rTMS + C1 group).
c. TAU + right DLPFC rTMS + CBT without a fixed plan (right rTMS + C0 group).
d. TAU + right DLPFC rTMS + CBT with a fixed plan (right rTMS + C1 group).
e. TAU + left DLPFC rTMS + CBT without a fixed plan (left rTMS + C0 group).
f. TAU + left DLPFC rTMS + CBT with a fixed plan (left rTMS + C1 group).
The CIWA-Ar (44) assessment was performed at enrollment to exclude patients in the acute alcohol withdrawal phase. The obsessive-compulsive drinking scale (OCDS) (45) and visual analogue scale (VAS) (46) were administered to measure the severity of alcohol cravings. The alcohol dependence scale (ADS) (47) was used to assess the severity of alcohol dependence. Montreal Cognitive Assessment (MoCA) (48) was used to measure the overall cognitive level of the patients. Pittsburgh Sleep Quality Index (PSQI) (49) was utilized to evaluate the Sleep Quality of patients. Generalized anxiety disorder-7 (GAD-7) (50) was used to assess the severity of anxiety symptoms. Patient Health Questionnaire-9 items (PHQ-9) (51) assess the patient’s depressive symptoms. The above scales of OCDS, VAS, PSQI, GAD-7, PHQ-9 were evaluated at week 0, week 2, week 8, week 12, and week 24. MoCA was evaluated at week 0, week 8, week 12, and week 24. ADS was evaluated at week 0, week 12, and week 24.
The Kolmogorov–Smirnov test was used to test normal distribution. Continuous variables were expressed as the mean ± standard deviation. One-way analysis of variance (ANOVA) model was used to analyze the differences in the treatment effects of different treatment regimens on relapse in AD patients. Categorical variables were analyzed using the chi-squared test or Fisher’s exact test. Repeated measures ANOVA was used to explore the interaction of different treatment modalities with changes in the rate of scale score reduction over treatment time, using baseline period, 2 weeks, 2 months, 3 months, and 6 months of treatment scores as relevant measures. And further simple effect analysis was performed for a significant interaction effect between group and time. Binary logistic regression analysis was performed to identify independent variables influencing relapse. Relapse rate of six groups were compared using Kaplan–Meier survival analysis. Risk factors for the relapse rate were assessed using Cox regression model analysis. Statistical analyses were performed in SPSS-23 (IBM). Insert additional missing values using the maximum expected value method. Differences between the groups were considered statistically significant at P < 0.05.
A total of 297 subjects were included in this study, of which 34 were excluded according to the experimental termination criteria, and 263 subjects were finally included in the analysis. There were included in sham rTMS + C0 group (n = 50), sham rTMS + C1 group (n = 37), right rTMS + C0 group (n = 45), right rTMS + C1 group (n = 42), left rTMS + C0 group (n = 49), left rTMS + C1 group (n = 40). Table 1 shows the comparison of the baseline data between the six groups. Regarding demographic characteristics, laboratory tests scale, and assessment at baseline, there was no statistically significant difference.
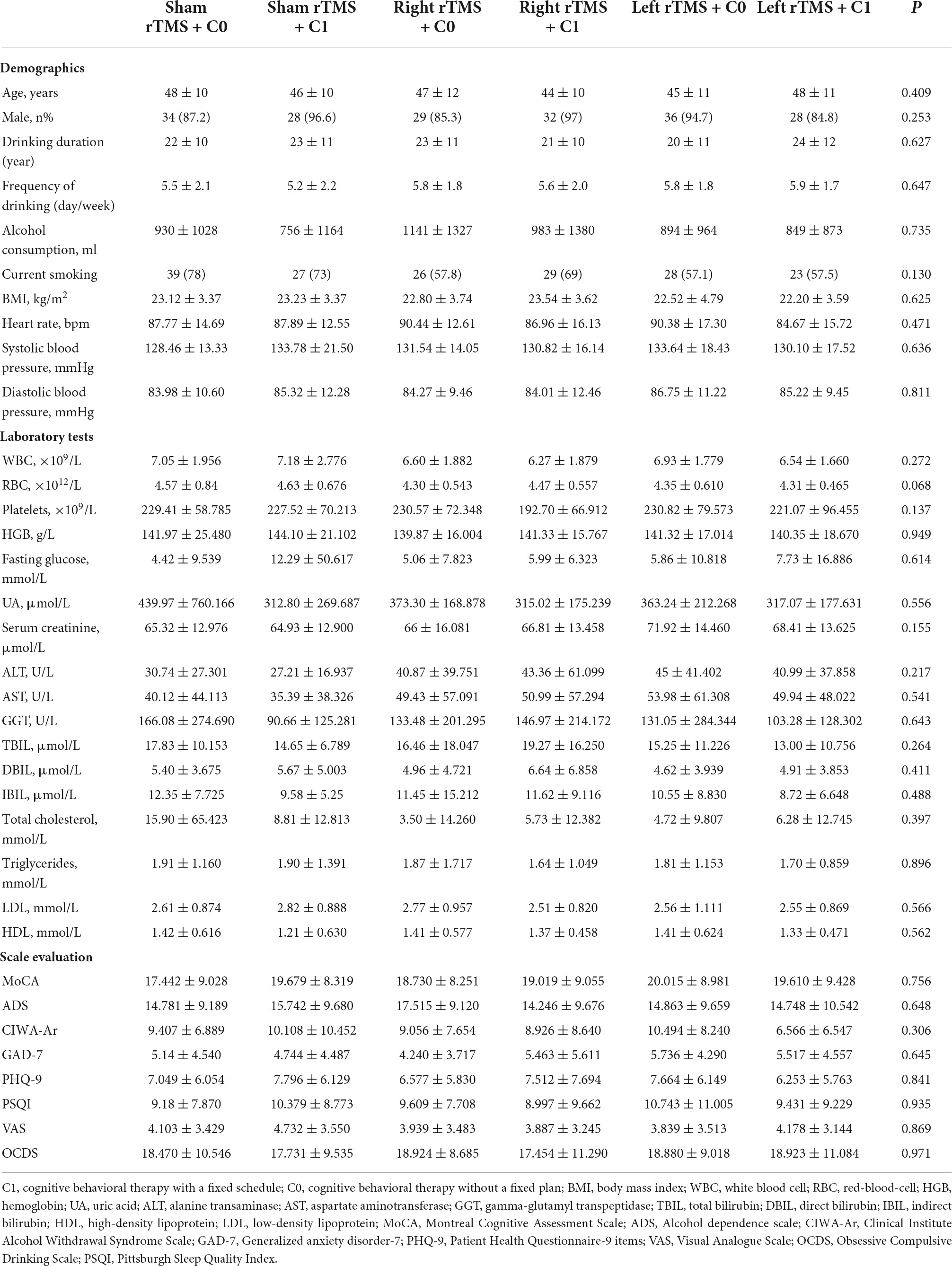
Table 1. Comparison of basic clinical information and laboratory test levels of patients recruited for the study.
The sham rTMS + C0 group relapse rate was significantly higher than the right rTMS + C1 group (P = 0.006), the left rTMS + C0 group (P = 0.031), the left rTMS + C1 group (P = 0.043); the right rTMS + C0 group relapse rate was significantly higher than the right rTMS + C1 group (P = 0.046) (Table 2). There was no significant difference in relapse rates between other groups.
For the reduction rates of GAD, MoCA, OCDS, and PSQI scores among the six groups of alcohol dependent patients, repeated measures ANOVA showed no main effect on the group but a significant main effect of time (Table 3). Also, there was a significant interaction effect between group and time in the rate of PHQ-9 scale score reduction rate (F = 3.001, P = 0.020), and both the main effect of group and the main effect of time were significant (F = 2.492, P = 0.032; F = 2.918, P = 0.037). Further simple effect analysis revealed that, at week 2 of follow-up, the right rTMS + C0 group had a significantly higher PHQ-9 scale score reduction rate than the sham rTMS + C1 group (P = 0.039); at week 2 of follow-up, the left rTMS + C0 group had a significantly higher PHQ-9 scale score reduction rate than the right rTMS + C0 group (P = 0.011); the right rTMS + C0 group had a significantly higher PHQ-9 scale score reduction rate at week 12 of follow-up than week 2 of follow-up (P = 0.046).
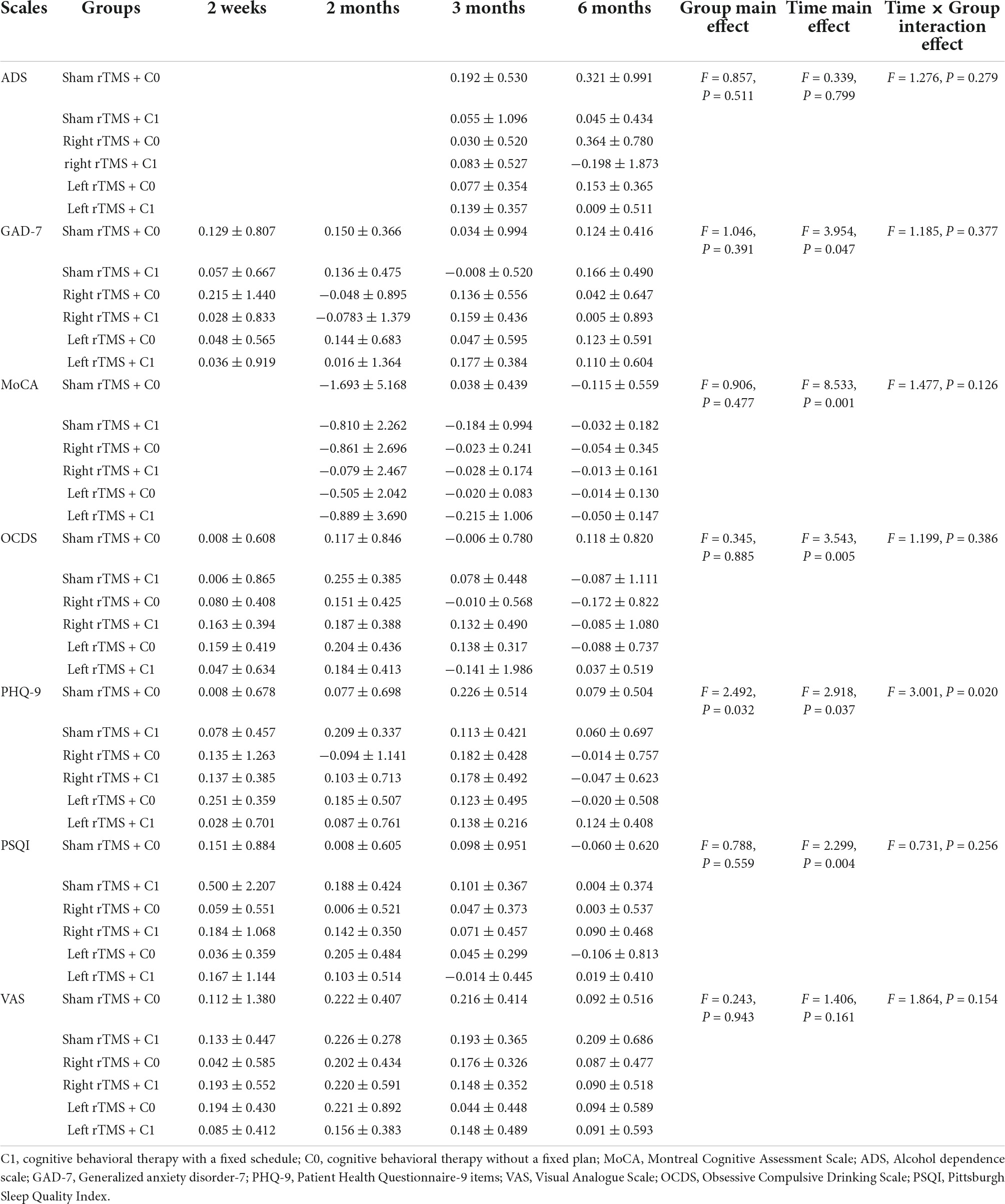
Table 3. Repeated measures ANOVA for the rate of scale score reduction in the six groups of patients after 2 weeks, 2 months, 3 months, and 6 months of follow-up.
Relapse was positively correlated with alcohol consumption (r = 0.186, P = 0.011), white blood cell (r = 0.182, P = 0.013), hemoglobin (r = 0.176, P = 0.017), current smoking (r = 0.170, P = 0.021), CBT (r = −0.169, P = 0.022). Binary logistic analysis indicated that current smoking (P = 0.038) and alcohol consumption (P = 0.009) was independent determinant of relapse (Table 4).
At 24 weeks of follow-up, Kaplan−Meier survival analysis reveals a statistically significant relapse rate between six groups (P = 0.025), left rTMS + C1 group has the best treatment effect for alcohol dependent patients (Supplementary Figure 2). Cox regression analysis showed that current smoking (β = 0.835, hazard ratio = 2.306, P = 0.045), total cholesterol (β = 0.006, hazard ratio = 1.006, P = 0.034), and TBIL (β = 0.025, hazard ratio = 1.025, P = 0.026) level were risk factors of relapse (Table 5).
Most importantly, let’s talk about the combination of rTMS and CBT. The effectiveness of CBT for AD has been demonstrated in an extensive review of psychosocial therapies (52, 53). The number of days of heavy drinking dropped significantly after CBT, according to new research (54). The combination of rTMS and CBT has been shown to be more effective than treatment strategies alone in patients with major depression (55), and a shorter course of treatment can achieve remission (56). To date, no studies have investigated the efficacy of this promising approach in AD. Our results are the first to show that rTMS combined with CBT is superior to rTMS alone in reducing the rate of relapse. At 24 weeks of follow-up, Kaplan−Meier survival analysis reveals a statistically significant relapse rate between six groups, left rTMS + C1 group has the best treatment effect for alcohol dependent patients. CBT has been proposed to function at the neural level through DLPFC (57). Therefore, stimulation of left DLPFC by rTMS may synergistically enhance the effect of CBT, inducing neural plasticity and making neural circuits recover faster (58). It is also hypothesized that CBT may provide a “foundation” for treatment to improve treatment retention and adherence and address other ancillary issues. So it makes more sense to combine these two approaches, making it possible to have more powerful stacking effects and more prolonged-lasting effects.
Anxiety and depression of AD patients may be aggravated due to decreased self-control, poor social support system, and deteriorating quality of life. At the same time, AD patients will show withdrawal symptoms such as anxiety and depression when they reduce or stop drinking, which will affect their abstinence compliance. Negative emotional states will affect and induce craving (59). Negative emotions and subjective stress levels are clearly predictors of relapse after AD treatment (60). In our study, the PHQ-9 assessed patients’ severity of depressive symptoms. Our study found that the PHQ-9 scale score reduction rate significantly affected treatment over time, as shown by the interaction effect. At week 2 of follow-up, the right rTMS + C0 group and the left rTMS + C0 group improved depressive symptoms are better. Previous studies have shown that high-frequency rTMS applied to left DLPFC and low-frequency rTMS applied to right DLPFC are an effective treatment for patients with major depression (61, 62). The above theories suggest that rTMS treatment of AD patients may reduce drinking cravings by improving depression, thereby reducing relapse to drinking.
Our study showed significant reductions in GAD, MoCA, OCDS, PSQI scales score reduction rate, improvements in anxiety, cognition, drinking cravings, and sleep in both the sham rTMS + C0 group, sham rTMS + C1 group, right rTMS + C0 group, right rTMS + C1 group, left rTMS + C0 group, and left rTMS + C1 group. There was continuous improvement at 2 weeks, 2 months, 3 months, and 6 months during the follow-up. However, there was no difference in treatment effect between these groups. In addition, we found an interesting finding that the sham rTMS group also improved sleep, anxiety and depression, cognition, drinking desire, and other aspects during 2 weeks, 2 months, 3 months, and 6 months follow-up. Drinking cravings are known to be sensitive to placebo (63), so it was first considered that sham rTMS might have a placebo effect. Second, neuroplasticity may be the most important mechanism in cognitive recovery (64), abstinence alone can restore cognitive impairment and brain abnormalities in some AD patients. Third, AD is seen as a symptom of a dysfunctional family system in which the alcohol dependent individual interacts with other family members. Family members and/or friends play a supportive and motivational role in AD, improve patients’ adherence to therapy, can prevent relapse, and are important in resolving conflicts caused by alcohol abuse (3).
Our research found that smoking was an independent factor influencing alcohol dependent relapse. Many studies support our results. Nearly half of alcohol dependent patients also smoked, and nicotine dependence was associated with a tremendous urge to drink, an increased risk of relapse after treatment, and more alcohol consumption at the time of relapse (65). Alcohol use disorder patients who actively smoke and quit smoking for fewer days before treatment have a significantly higher chance of relapse within 6 months. Implementing smoking cessation could reduce the risk of alcohol use disorder relapse (66). Our finding result may be that both alcohol and nicotine activate the opioid system in reward-related brain regions, leading to adaptive changes in opioid signaling after prolonged exposure. A previous finding suggests that nicotine can increase drinking activity by modulating μ receptor activity in the ventral tegmental area (67). Our study also showed that alcohol consumption was a risk factor for drinking again. A recent domestic survey showed that the daily alcohol consumption of patients in the relapse group was significantly higher than that of patients in the non-relapse group before withdrawal treatment and that high daily alcohol consumption was an independent risk factor for alcohol dependence on relapse (68). The reason may be that everyday heavy drinking increases the patient’s tolerance to alcohol and damages multiple body organs and systems. They were causing severe physical and psychological damage to the patient, leading to more pronounced withdrawal effects and increased psychological addiction, making the patient more susceptible to alcohol-related stimuli and relapse into drinking after withdrawal (69).
This study is the first to suggest that the combination of rTMS and CBT may be a potentially effective treatment for reducing relapse. Future research should focus on refining phenotypes to achieve personalized treatment approaches: Alcohol use disorders are complex and multifaceted disorders, and personalized treatment approaches may be the most effective to address this complexity. Given the role of individual differences in neuroregulatory effects and the high degree of heterogeneity in the AD population, the identification of phenotypes (including impaired cognitive function, craving, depressive and anxious mood, alcohol consumption, number of relapses, etc.) and individualized treatment options may be critical in the development of treatment for AD. It’s clear that the recovery process is not linear. In order to avoid relapse, much attention should be paid to the interplay between the aspects according to the bio-psycho-social model in the treatment for AD, as well as to increasing patients’ motivation to quit drinking. However, the present study has several limitations. Caution must be used in interpreting the current results, as the sample size for each AD group is not large and is an initial observation. Further studies with a larger sample are needed to replicate our results. In addition, given that one of the distinguishing features of CBT is its relative duration of effect, further follow-up can be extended to assess efficacy. Clinicians should also assess the lifestyle and family structure of the alcohol dependent patient and their role in the treatment process. Finally, although self-assessment diaries in reporting alcohol consumption are generally considered valid under certain conditions, self-assessment diaries are unreliable and inaccurate. In the future, we will further investigate sensitive and specific biological indicators of recent alcohol consumption as a secondary outcome measure to complement the self-reports obtained from patients.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of Harbin Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XH, LL, TZ, HM, XHZ, HXW, YP, and YJ contributed to the study conception and design. XH performed the statistical analysis and wrote the manuscript. All authors collected the data, commented on previous versions of the manuscript, and read and approved the final manuscript.
This study was supported by the National Key R&D Program of China (Nos. 2018YFC1314400 and 2018YFC1314402).
We would like to thank the National Key R&D Program of China for supporting this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.935491/full#supplementary-material
AD, alcohol dependence; rTMS, repetitive transcranial magnetic stimulation; CBT, cognitive behavioral therapy; DLPFC, dorsolateral prefrontal cortex; ADS, alcohol dependence scale; OCDS, obsessive compulsive drinking scale; VAS, visual analogue scale; MoCA, montreal cognitive assessment; PSQI, Pittsburgh sleep quality index; GAD-7, generalized anxiety disorder-7; PHQ-9, patient health questionnaire-9.
1. Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-Month Alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001-2002 to 2012-2013: Results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry. (2017) 74:911–23. doi: 10.1001/jamapsychiatry.2017.2161
2. Eun JD, Paksarian D, He J, Merikangas KR. Parenting style and mental disorders in a nationally representative sample of US adolescents. Soc Psychiatry Psychiatr Epidemiol. (2018) 53:11–20. doi: 10.1007/s00127-017-1435-4
3. Saatcioglu O, Erim R, Cakmak D. Role of family in alcohol and substance abuse. Psychiatry Clin Neurosci. (2006) 60:125–32. doi: 10.1111/j.1440-1819.2006.01476.x
4. Evans SM, Reed SC. Impulsivity and the effects of alcohol in women with a history of childhood sexual abuse: A pilot study. Exp Clin Psychopharmacol. (2021) 29:395–406. doi: 10.1037/pha0000419
5. Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction. (2018) 113:1905–26. doi: 10.1111/add.14234
6. Schuckit MA. Alcohol use disorders. Lancet. (2009) 373:492–501. doi: 10.1016/S0140-6736(09)60009-X
7. Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. (2008) 165:179–87. doi: 10.1176/appi.ajp.2007.06111851
8. Oudejans SCC, Schippers GM, Spits ME, Stollenga M, Brink W. Five years of ROM in substance abuse treatment centres in the Netherlands. Tijdschr Psychiatr. (2012) 54:185–90.
9. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. (1985) 1:1106–7. doi: 10.1016/s0140-6736(85)92413-4
10. Pascual-Leone A, Valls-Solé J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. (1994) 117:847–58. doi: 10.1093/brain/117.4.847
11. Diana M, Raij T, Melis M, Nummenmaa A, Leggio L, Bonci A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat Rev Neurosci. (2017) 18:685–93. doi: 10.1038/nrn.2017.113
12. Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neurosci Biobehav Rev. (2002) 26:631–64. doi: 10.1016/s0149-7634(02)00021-0
13. Li X, Hartwell KJ, Owens M, Lematty T, Borckardt JJ, Hanlon CA, et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry. (2013) 73:714–20. doi: 10.1016/j.biopsych.2013.01.003
14. Martinotti G, Pettorruso M, Montemitro C, Spagnolo PA, Martellucci CA, Carlo FD, et al. Repetitive transcranial magnetic stimulation in treatment-seeking subjects with cocaine use disorder: A randomized, double-blind, sham-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. (2022) 116:110513. doi: 10.1016/j.pnpbp.2022.110513
15. Pettorruso M, Di Giuda D, Martinotti G, Cocciolillo F, De Risio L, Montemitro C, et al. Dopaminergic and clinical correlates of high-frequency repetitive transcranial magnetic stimulation in gambling addiction: A SPECT case study. Addict Behav. (2019) 93:246–9. doi: 10.1016/j.addbeh.2019.02.013
16. Höppner J, Broese T, Wendler L, Berger C, Thome J. Repetitive transcranial magnetic stimulation (rTMS) for treatment of alcohol dependence. World J Biol Psychiatry. (2011) 1:57–62. doi: 10.3109/15622975.2011.598383
17. Mishra BR, Nizamie SH, Das B, Praharaj SK. Efficacy of repetitive transcranial magnetic stimulation in alcohol dependence: A sham-controlled study. Addiction. (2010) 105:49–55. doi: 10.1111/j.1360-0443.2009.02777.x
18. Felice AD, Bellamoli E, Formaggio E, Manganotti P, Masiero S, Cuoghi G, et al. Neurophysiological, psychological and behavioural correlates of rTMS treatment in alcohol dependence. Drug Alcohol Depend. (2016) 158:147–53. doi: 10.1016/j.drugalcdep.2015.11.018
19. Mishra BR, Praharaj SK, Katshu MZ, Sarkar S, Nizamie SH. Comparison of anticraving efficacy of right and left repetitive transcranial magnetic stimulation in alcohol dependence: A randomized double-blind study. J Neuropsychiatry Clin Neurosci. (2015) 27:e54–9. doi: 10.1176/appi.neuropsych.13010013
20. Herremans SC, Vanderhasselt M, Raedt RD, Baeken C. Reduced intra-individual reaction time variability during a Go—NoGo task in detoxified alcohol-dependent patients after one right-sided dorsolateral prefrontal HF-rTMS session. Alcohol Alcohol. (2013) 48:552–7. doi: 10.1093/alcalc/agt054
21. McCance-Katz EF. The substance abuse and mental health services administration (SAMHSA): New directions. Psychiatr Serv. (2018) 69:1046–8. doi: 10.1176/appi.ps.201800281
22. Carroll KM, Kiluk BD. Cognitive behavioral interventions for alcohol and drug use disorders: Through the stage model and back again. Psychol Addict Behav. (2017) 31:847–61. doi: 10.1037/adb0000311
23. Riper H, Hoogendoorn A, Cuijpers P, Karyotaki E, Boumparis N, Mira A, et al. Effectiveness and treatment moderators of internet interventions for adult problem drinking: An individual patient data meta-analysis of 19 randomised controlled trials. PLoS Med. (2018) 15:e1002714. doi: 10.1371/journal.pmed.1002714
24. Boß L, Lehr D, Schaub MP, Paz CR, Riper H, Berking M, et al. Efficacy of a web-based intervention with and without guidance for employees with risky drinking: Results of a three-arm randomized controlled trial. Addiction. (2018) 113:635–46. doi: 10.1111/add.14085
25. Sundström C, Eék N, Kraepelien M, Fahlke C, Gajecki M, Jakobson M, et al. High- versus low-intensity internet interventions for alcohol use disorders: Results of a three-armed randomized controlled superiority trial. Addiction. (2020) 115:863–74. doi: 10.1111/add.14871
26. Sachdeva A, Chandra M, Choudhary M, Dayal P, Anand KS. Alcohol-related dementia and neurocognitive impairment: A review study. Int J High Risk Behav Addict. (2016) 5:e27976. doi: 10.5812/ijhrba.27976
27. Schulte MHJ, Cousijn J, Uyl TE, Goudriaan AE, Brink W, Veltman DJ, et al. Recovery of neurocognitive functions following sustained abstinence after substance dependence and implications for treatment. Clin Psychol Rev. (2014) 34:531–50. doi: 10.1016/j.cpr.2014.08.002
28. Noël X, Brevers D, Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol (2013) 23:632–8. doi: 10.1016/j.conb.2013.01.018
29. Sinha R. The clinical neurobiology of drug craving. Curr Opin Neurobiol. (2013) 23:649–54. doi: 10.1016/j.conb.2013.05.001
30. Baler RD, Volkow ND. Drug addiction: The neurobiology of disrupted self-control. Trends Mol Med. (2006) 12:559–66. doi: 10.1016/j.molmed.2006.10.005
31. Lee EK, Douglass AB. Sleep in psychiatric disorders: Where are we now? Can J Psychiatry. (2010) 55:403–12. doi: 10.1177/070674371005500703
32. Suzuki S, Mell MM, O’Malley SS, Krystal JH, Anticevic A, Kober H. Regulation of craving and negative emotion in alcohol use disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. (2020) 5:239–50. doi: 10.1016/j.bpsc.2019.10.005
33. Boothby LA, Doering PL. Acamprosate for the treatment of alcohol dependence. Clin Ther. (2005) 27:695–714. doi: 10.1016/j.clinthera.2005.06.015
34. Sorg SF, Taylor MJ, Alhassoon OM, Gongvatana A, Theilmann RJ, Frank LR. Frontal white matter integrity predictors of adult alcohol treatment outcome. Biol Psychiatry. (2012) 71:262–8. doi: 10.1016/j.biopsych.2011.09.022
35. Weinberger AH, Platt J, Jiang B, Goodwin RD. Cigarette smoking and risk of alcohol use relapse among adults in recovery from alcohol use disorders. Alcohol Clin Exp Res. (2015) 39:1989–96. doi: 10.1111/acer.12840
36. Schmidt LG, Smolka MN. Results from two pharmacotherapy trials show alcoholic smokers were more severely alcohol dependent but less prone to relapse than alcoholic non-smokers. Alcohol Alcohol. (2007) 42:241–6. doi: 10.1093/alcalc/agm027
37. John U, Meyer C, Rumpf H, Hapke U. Probabilities of alcohol high-risk drinking, abuse or dependence estimated on grounds of tobacco smoking and nicotine dependence. Addiction. (2003) 98:805–14. doi: 10.1046/j.1360-0443.2003.00381.x
38. Helander A. Biological markers in alcoholism. J Neural Transm Suppl. (2003) 30:15–32. doi: 10.1007/978-3-7091-0541-2_2
39. Raabe F, Wagner E, Weiser J, Brechtel S, Popovic D, Adorjan K, et al. Classical blood biomarkers identify patients with higher risk for relapse 6 months after alcohol withdrawal treatment. Eur Arch Psychiatry Clin Neurosci. (2021) 271:891–902. doi: 10.1007/s00406-020-01153-8
40. Schmidt T, Roser P, Juckel G, Brüne M, Suchan B, Thoma P. Social cognition and social problem solving abilities in individuals with alcohol use disorder. J Clin Exp Neuropsychol. (2016) 38:974–90. doi: 10.1080/13803395.2016.1180346
41. Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. (2009) 120:2008–39. doi: 10.1016/j.clinph.2009.08.016
42. Johansson M, Berman AH, Sinadinovic K, Lindner P, Hermansson U, Andréasson S. Effects of internet-based cognitive behavioral therapy for harmful alcohol use and alcohol dependence as self-help or with therapist guidance: Three-armed randomized trial. J Med Internet Res. (2021) 23:e29666. doi: 10.2196/29666
43. Magill M, Tonigan JS, Kiluk B, Ray L, Walthers J, Carroll K. The search for mechanisms of cognitive behavioral therapy for alcohol or other drug use disorders: A systematic review. Behav Res Ther. (2020) 131:103648. doi: 10.1016/j.brat.2020.103648
44. Stuppaeck CH, Barnas C, Falk M, Guenther V, Hummer M, Oberbauer H, et al. Assessment of the alcohol withdrawal syndrome–validity and reliability of the translated and modified clinical institute withdrawal assessment for alcohol scale (CIWA-A). Addiction. (1994) 89:1287–92. doi: 10.1111/j.1360-0443.1994.tb03307.x
45. Anton RF. The obsessive compulsive drinking scale. Addiction. (2000) 2:S211–7. doi: 10.1080/09652140050111771
46. Flaudias V, Teisseidre F, Chazeron ID, Chalmeton M, Bertin C, Izaute M, et al. A multi-dimensional evaluation of craving and impulsivity among people admitted for alcohol-related problems in emergency department. Psychiatry Res. (2019) 272:569–71. doi: 10.1016/j.psychres.2018.12.118
47. Saxon AJS, Kivlahan DR, Doyle S, Donovan DM. Further validation of the alcohol dependence scale as an index of severity. J Stud Alcohol Drugs. (2007) 68:149–56. doi: 10.15288/jsad.2007.68.149
48. Rossetti HC, Lacritz LH, Cullum CM, Weiner MF. Normative data for the montreal cognitive assessment (MoCA) in a population-based sample. Neurology. (2011) 77:1272–5. doi: 10.1212/WNL.0b013e318230208a
49. Yi H, Shin K, Shin C. Development of the sleep quality scale. J Sleep Res. (2006) 15:309–16. doi: 10.1111/j.1365-2869.2006.00544.x
50. Kertz S, Bigda-Peyton J, Bjorgvinsson T. Validity of the generalized anxiety disorder-7 scale in an acute psychiatric sample. Clin Psychol Psychother. (2013) 20:456–64. doi: 10.1002/cpp.1802
51. Bélanger E, Thomas KS, Jones RN, Epstein-Lubow G, Mor V. Measurement validity of the patient-health questionnaire-9 in US nursing home residents. Int J Geriatr Psychiatry. (2019) 34:700–8. doi: 10.1002/gps.5074
52. Assanangkornchai S, Srisurapanont M. The treatment of alcohol dependence. Curr Opin Psychiatry. (2007) 20:222–7. doi: 10.1097/YCO.0b013e3280fa837d
53. Berglund M, Thelander S, Salaspuro M, Franck J, Andréasson S, Ojehagen A. Treatment of alcohol abuse: An evidence-based review. Alcohol Clin Exp Res. (2003) 27:1645–56. doi: 10.1097/01.ALC.0000090144.99832.19
54. Srivastava AB, Sanchez-Peña J, Levin FR, Levin JJ, Patel GH, Naqvi NH. Drinking reduction during cognitive behavioral therapy for alcohol use disorder is associated with a reduction in anterior insula-bed nucleus of the stria terminalis resting-state functional connectivity. Alcohol Clin Exp Res. (2021) 45:1596–606. doi: 10.1111/acer.14661
55. Donse L, Padberg F, Sack AT, Rush AJ, Arns M. Simultaneous rTMS and psychotherapy in major depressive disorder: Clinical outcomes and predictors from a large naturalistic study. Brain Stimul. (2018) 11:337–45. doi: 10.1016/j.brs.2017.11.004
56. Vedeniapin A, Cheng L, George MS. Feasibility of simultaneous cognitive behavioral therapy and left prefrontal rTMS for treatment resistant depression. Brain Stimul. (2010) 3:207–10. doi: 10.1016/j.brs.2010.03.005
57. Naqvi NH, Morgenstern J. Cognitive neuroscience approaches to understanding behavior change in alcohol use disorder treatments. Alcohol Res. (2015) 37:29–38.
58. Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trojanowski JQ, et al. Harnessing neuroplasticity for clinical applications. Brain. (2011) 134:1591–609. doi: 10.1093/brain/awr039
59. Seo D, Lacadie CM, Tuit K, Hong K, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. (2013) 70:727–39. doi: 10.1001/jamapsychiatry.2013.762
60. Potenza MN, Hong KA, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: Influences of sex and cocaine dependence. Am J Psychiatry. (2012) 169:406–14. doi: 10.1176/appi.ajp.2011.11020289
61. Fitzgerald PB, Daskalakis ZJ. A practical guide to the use of repetitive transcranial magnetic stimulation in the treatment of depression. Brain Stimul. (2012) 5:287–96. doi: 10.1016/j.brs.2011.03.006
62. Dell’Osso B, Oldani L, Camuri G, Dobrea C, Cremaschi L, Benatti B, et al. Augmentative repetitive transcranial magnetic stimulation (rTMS) in the acute treatment of poor responder depressed patients: A comparison study between high and low frequency stimulation. Eur Psychiatry. (2015) 30:271–6. doi: 10.1016/j.eurpsy.2014.12.001
63. Brunoni AR, Lopes M, Kaptchuk TJ, Fregni F. Placebo response of non-pharmacological and pharmacological trials in major depression: A systematic review and meta-analysis. PLoS One. (2009) 4:e4824. doi: 10.1371/journal.pone.0004824
64. Crews FT, Buckley T, Dodd PR, Ende G, Foley N, Harper C, et al. Alcoholic neurobiology: Changes in dependence and recovery. Alcohol Clin Exp Res. (2005) 29:1504–13. doi: 10.1097/01.alc.0000175013.50644.61
65. DeBaker M, Moen J, Robinson J, Robinson K, Lee A. Unequal interactions between alcohol and nicotine coconsumption: Suppression and enhancement of concurrent drug intake. Psychopharmacology. (2020) 237:967–78. doi: 10.1007/s00213-019-05426-6
66. Nguyen L, Durazzo T, Dwyer C, Rauch A, Humphreys K, Williams L, et al. Predicting relapse after alcohol use disorder treatment in a high-risk cohort: The roles of anhedonia and smoking. J Psychiatr Res. (2020) 126:1–7. doi: 10.1016/j.jpsychires.2020.04.003
67. Domi E, Xu L, Pätz M, Pätz A, Pätz G, Holm L, et al. Nicotine increases alcohol self-administration in male rats via a μ−opioid mechanism within the mesolimbic pathway. Br J Pharmacol. (2020) 177:4516–31. doi: 10.1111/bph.15210
68. Boschloo L, Vogelzangs N, Brink W, Smit J, Beekman A, Penninx B. Predictors of the 2-year recurrence and persistence of alcohol dependence. Addiction. (2012) 107:1639–40. doi: 10.1111/j.1360-0443.2012.03860.x
Keywords: alcohol dependence, repetitive transcranial magnetic stimulation, cognitive behavioral therapy, relapse, combination therapy
Citation: Hu X, Zhang T, Ma H, Zhou X, Wang H, Wang X, Cheng C, Li Y, Duan R, Zhang B, Wang H, Lu J, Kang C, Zhao N, Zhang Y, Tian L, Liu J, Shi J, Wang Z, Zhou X, Zhu S, Liu Q, Li X, Wang H, Nie M, Yang M, Yang J, Chi Y, Zhu X, Hu J, Jia Y, Peng Y and Liu L (2022) Repetitive transcranial magnetic stimulation combined with cognitive behavioral therapy treatment in alcohol-dependent patients: A randomized, double-blind sham-controlled multicenter clinical trial. Front. Psychiatry 13:935491. doi: 10.3389/fpsyt.2022.935491
Received: 04 May 2022; Accepted: 16 September 2022;
Published: 04 October 2022.
Edited by:
Giovanni Martinotti, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Debora Luciani, University of Studies G. d’Annunzio Chieti and Pescara, ItalyCopyright © 2022 Hu, Zhang, Ma, Zhou, Wang, Wang, Cheng, Li, Duan, Zhang, Wang, Lu, Kang, Zhao, Zhang, Tian, Liu, Shi, Wang, Zhou, Zhu, Liu, Li, Wang, Nie, Yang, Yang, Chi, Zhu, Hu, Jia, Peng and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Liu, bWFnZ2llLmxlaWxlaUAxNjMuY29t; Ying Peng, cGVuZ3kyQG1haWwuc3lzdS5lZHUuY24=; Yanjie Jia, amlheWFuamllMTk3MUB6enUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.