- 1Department of Nursing, Sichuan Vocational College of Health and Rehabilitation, Zigong, China
- 2Zigong Affiliated Hospital of Southwest Medical University, Zigong Psychiatric Research Center, Zigong, China
- 3Psychiatric Hospital of Ziyang, Ziyang, China
Aim: Here, we investigate the relationship between mid-upper arm circumference (MUAC) and calf circumference (CC) screening indicators of sarcopenia and the risk of pneumonia in stable patients diagnosed with schizophrenia.
Method: The study is prospective and includes inpatients with schizophrenia from two mental health centers in Western China. The studied screening indicators, MUAC and CC were assessed in standing patients. The relationship between MUAC and CC as sarcopenia screening indicators with the risk of pneumonia in patients with schizophrenia was analyzed by performing a statistical logistic regression analysis.
Result: For this study, 339 patients with schizophrenia, aged 50 years and over were recruited. Moreover, four patients with pneumonia that occurred within 1 week of the relapse of schizophrenia were excluded. As a result, only 335 patients were included in the analysis. Pneumonia has been reported in 82 (24.5%) of all included patients with schizophrenia. Our data analysis confirmed that in the male patients, the higher CC was associated with a lower risk of pneumonia (odds ratio [OR] = 0.751, 95% CI: 0.635–0.889). We have divided men into two cohorts following the values of CC. Our analysis further showed that the patients with CC ≥ 34 cm had a lower risk of pneumonia in men (OR = 0.36, 95% CI: 0.163–0.795).
Conclusion: We demonstrate that CC is associated with pneumonia risk in stable men with schizophrenia.
Introduction
Sarcopenia, also called muscle atrophy, is an aging-associated muscle debilitation and decline in the overall body performance with age (1). Data show that the increased risk of pneumonia is associated with sarcopenia in patients with alcoholic hepatitis, esophageal cancer surgery, colorectal cancer surgery, and older age (2–5).
People's prejudice against patients with schizophrenia, so the social acceptance is low, it is not easy to return to society (6), and schizophrenia has a genetic susceptibility (7). Some families also have mental patients, there is no extra staff and financial care for patients with schizophrenia, and it is difficult to return to the family. Therefore, many patients with schizophrenia can only stay in the ward even after they recover. People with schizophrenia live in a relatively closed environment for a long time, have reduced physical activity, and have a relatively simple diet, which may lead to an increased risk of sarcopenia (8). Interestingly, some authors reported the presence of a similar cytokine (Interleukin-6 [IL-6]) in both patients with sarcopenia and schizophrenia (9). In addition, pneumonia was reported to be significantly higher in patients with schizophrenia than in the general population (10). Combining the above studies, we consider that pneumonia in schizophrenia may be associated with sarcopenia. Therefore, the detection of sarcopenia in patients with schizophrenia is very important in the course of its treatment.
In clinical practice, decreased muscle mass is diagnosed by magnetic resonance imaging (MRI), computed tomography (CT), dual-energy X-ray absorptiometry (DXA), and bioelectrical impedance analysis (BIA) (1). Some of these methods are expensive, some are radioactive, and some are interfering with food intake and the general patient's health status (11). Therefore, the search for inexpensive, safe, and food- and general-health status independent methods for early sarcopenia diagnosis to predict pneumonia is of great interest. Studies have found that mid-upper arm circumference (MUAC) and calf circumference (CC) can be used as simple proxy screening for sarcopenia (12–14).
However, the relationship between MUAC and CC (as surrogate indicators for sarcopenia) and the risk of pneumonia in stable patients with schizophrenia was not yet well-established. Therefore, we explore the association among MUAC, CC, and pneumonia in stable schizophrenia. We hypothesized that the lower values of patients' MUAC and CC indicators were linked with an increased risk of pneumonia in stable schizophrenia.
Methods
Study design and patients' characteristics
Inpatients diagnosed with stable schizophrenia from two mental health centers in Western China were enrolled in the study and established the cohort of sarcopenia. Baseline patients' data were collected from 1 September to 30 September 2020. Additionally, we gathered clinical data from patients with pneumonia diagnosed and treated at two mental health centers between October 2020 and October 2021. Our baseline inclusion and exclusion criteria for this study were based on a previous article (14). At the same time, during our follow-up period, if pneumonia occurred within 1 week after the change of condition, we excluded it.
The study was performed following the Declaration of Helsinki and was legitimated by the Institutional Review Board (IRB) of the two mental health centers in Western China (IRB number: 20191001; zjsjsbyy-kyxm-2019-2). All patients or their legitimate custodians signed an informed consent form.
Anthropometric measurements
Both MUAC and CC as screening indicators of sarcopenia were measured while the patients were upright. At this point, tape measures at the midpoint between the lateral projection of the scapula acromion and the lower border of the olecranon were performed with the arm flexed at 90 degrees. To measure CC, we measured the maximum circumference of the lower leg. Both indicators were calculated to the closest decimal point in centimeters. The two measurements on the leading lateral were averaged. All measurements were performed by trained professionals from the medical centers where the study was executed. The patients were divided into 2 groups according to the CC cut-off value recommended by the Asian Working Group for Sarcopenia (AWGS) 2019 diagnostic consensus, and sarcopenia was considered to be less than 34 cm in men and 33 cm in women (1). Since MUAC has no recommended cut-off value for the consensus, no grouping was performed (1).
Outcome indicator
Pneumonia was diagnosed by a clinician and was treated with drugs, mainly antibiotics. In the current study, only patients who developed pneumonia for the first time during follow-up were enrolled. Each patient had a chest radiograph or/and CT scan. Pneumonia was defined as an acute infection of the lung parenchyma, associated with acute infiltrates on chest radiograph or CT, accompanied by the prevalence of two or more symptoms, such as increased body temperature (≥38°C), hypothermia (<36°C), chills, sweating, novel cough, or respiratory tract change in color of discharge, chest discomfort, or difficulty in breathing (15).
Covariates
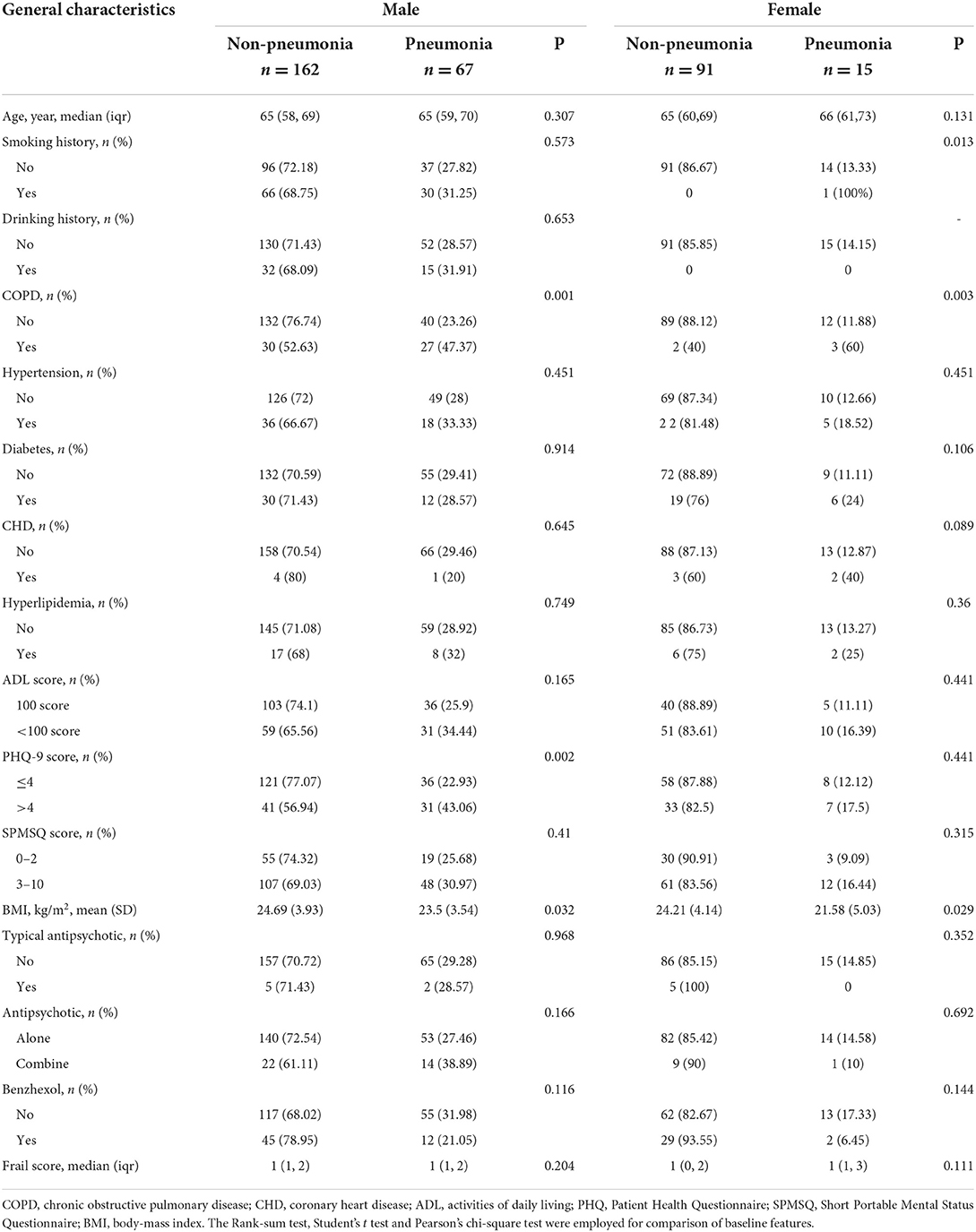
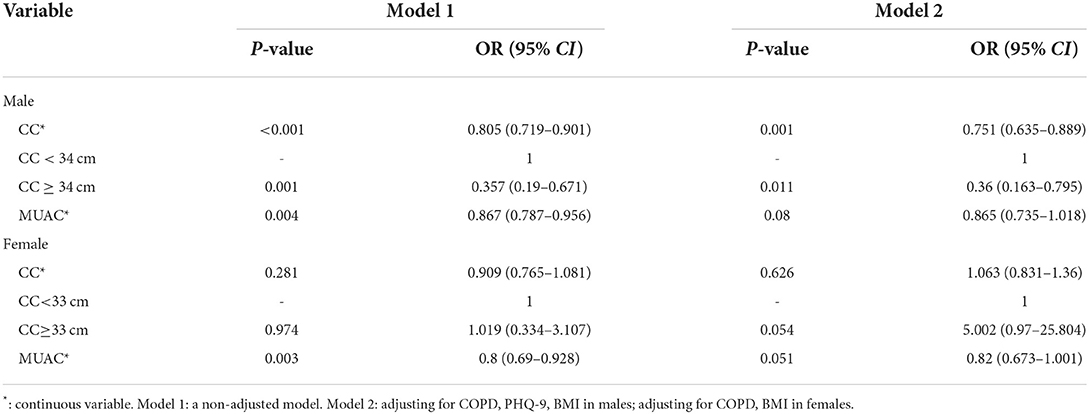
Covariate data were collected from patients diagnosed with schizophrenia who had been hospitalized for more than 6 months in the hospital through an electronic system to find out the corresponding ward area. After that, their basic medical data were collected face-to-face according to the inclusion criteria. We collected the baseline data of each patient, including age, sex, weight, height, smoking, and drinking history. All reported chronic illnesses, such as diabetes, increased blood pressure, coronary heart disease (CHD), chronic obstructive pulmonary disease (COPD), and hyperlipidemia were also recorded and were assessed as covariates in the analysis. In addition, the Activities of Daily Living (ADL) scores, based on the ADL assessment (16), Patient Health Questionnaire-9 (PHQ-9) depression scores, based on the PHQ-9 (17), Short Portable Mental Status Questionnaire (SPMSQ) cognitive function marks, based on the SPMSQ measurement (18), frail score (19), and antipsychotic (typical antipsychotic and drug kinds), and benzhexol as covariates were included in the study. Diabetes, hypertension, CHD, COPD, and hyperlipidemia as comorbidities met the respective diagnostic criteria (20). In this study, a single factor <0.05 was included in the model correction. We constructed two models: Model 1: a non-adjusted model and Model 2: adjusted for covariates, such as COPD, PHQ-9, and Body-Mass Index (BMI) in men and COPD and BMI in women. Although there were differences in the distribution of smoking history among women, the frequency of occurrence was too small to be corrected for inclusion.
Statistical analyses
The SPSS 25.0 software was applied for the statistical analyses of all gathered patients' data. The values of p less than 0.05 were assigned as the threshold for significance and all tests were two-sided. The ordinarily dispersed results were represented as mean ± standard deviation (SD), otherwise, the median (quartile) was provided. The Rank-sum test, Student's t-test, and Pearson's chi-square test were employed for the comparison of baseline features. The logistic regression analysis determined the relationship between MUAC and CC values with the risk of pneumonia in patients with schizophrenia.
Results
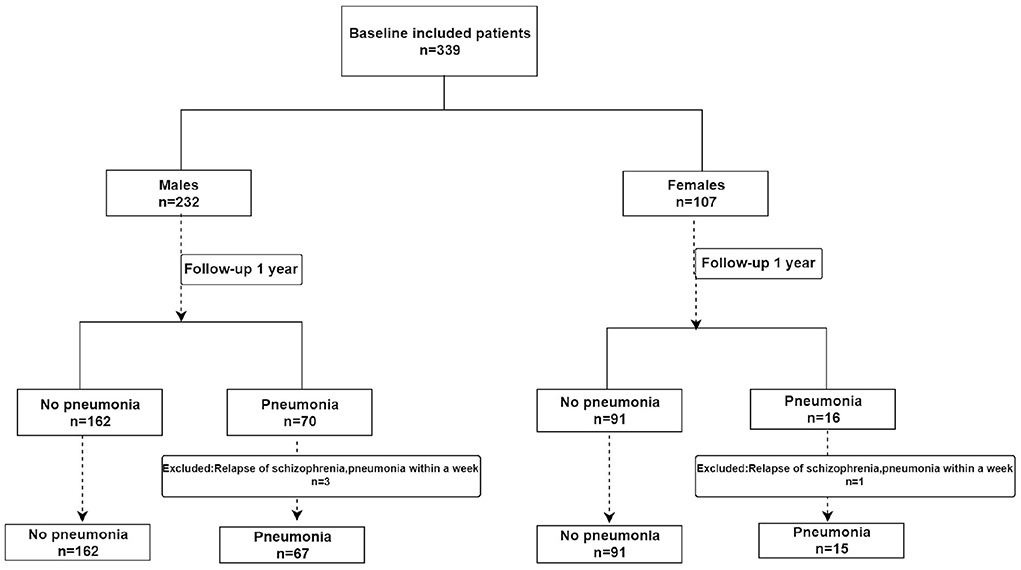
The patients with schizophrenia enrolled in the study included 232 men and 107 women aged 50 years and over. During the follow-up, four patients with pneumonia that occurred within 1 week of the relapse of schizophrenia were excluded. As a result, only 335 patients were included in the analysis (Figure 1). Pneumonia has been reported in 82 (24.5%) of the included schizophrenic patients. In the male patients with schizophrenia, the differences in COPD, PHQ-9 scores, and BMI between pneumonia and non-pneumonia groups were statistically significant. In the female patients, the smoking history, COPD, and BMI were statistically significant among patients with pneumonia and non-pneumonia (Table 1).
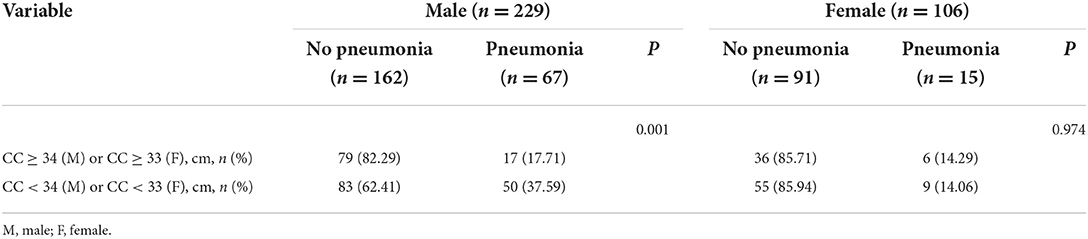
Among the male patients with schizophrenia, those with pneumonia had lower CC and MUAC measurements when compared with the non-pneumonia ones. The statistical analysis of the measured CC values showed p < 0.001, while for MUAC values p was equal to 0.003 (Figure 2). When all male patients with schizophrenia were divided into two categories according to the values of CC (34 cm), the detected prevalence of pneumonia was higher among those with CC <34 cm than with CC ≥ 34 cm (37.59% vs. 17.71%, p = 0.001). These data are presented in Table 2. Among the female patients with schizophrenia, the MUAC measurements in the patients with pneumonia were smaller than that of non-pneumonia ones (p = 0.001, Figure 3). However, the differences in the measured CC values in pneumonia and non-pneumonia female patients with schizophrenia were not statistically significant (Figure 3 and Table 2).
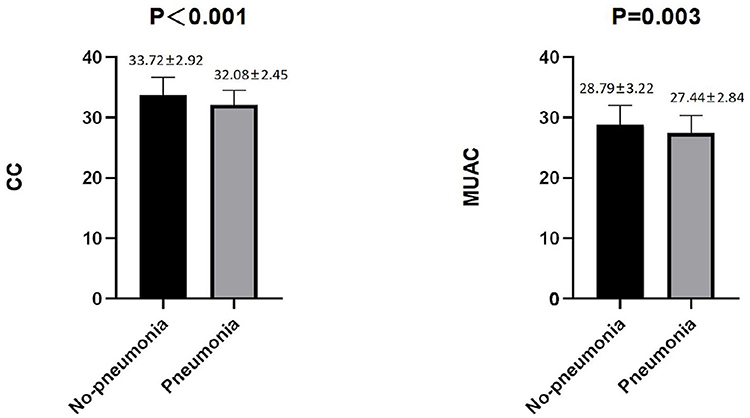
Figure 2. Differences in CC and MUAC between pneumonia and non-pneumonia in males. The Student's t-test was employed for comparison of two groups.
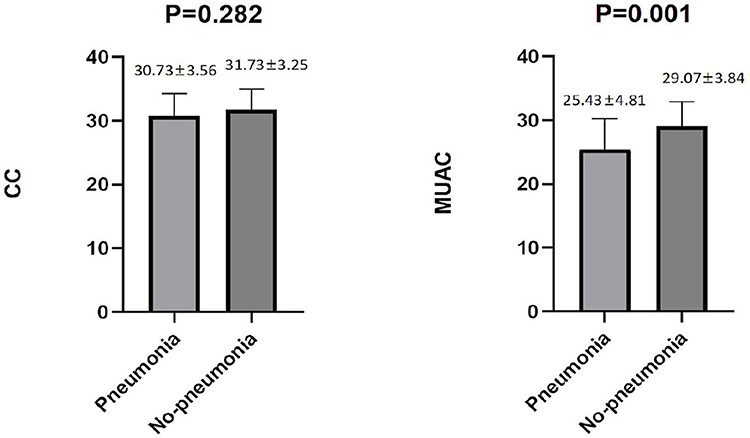
Figure 3. Differences in CC and MUAC between pneumonia and non-pneumonia in females. The Student's t-test was employed for comparison of two groups.
We found that in male patients, higher CC and MUAC scores in patients with schizophrenia were associated with a lower risk of pneumonia (CC: odds ratio [OR] = 0.805, 95% CI: 0.719–0.901; MUAC: OR = 0.867, 95% CI: 0.787–0.956; Table 3). After adjusting for some confounding factors, CC remained a protective factor for pneumonia (OR = 0.751, 95% CI: 0.635–0.889; Table 3). We further divided all male patients with schizophrenia into two categories taking into account 34 cm as the cut-off value for CC and found that patients with CC ≥ 34 cm had a lower risk of pneumonia (OR = 0.357, 95% CI: 0.19–0.671; Table 3). We adjusted the model for certain confounding factors, and the risk of pneumonia appeared reduced in individuals with CC ≥ 34 cm (OR = 0.36, 95% CI: 0.163–0.795; Table 3). Among women with schizophrenia, higher MUAC was associated with a lower risk of pneumonia (OR = 0.8, 95% CI: 0.69–0.928; Table 3). After adjusting for certain confounders, we found that MUAC was not associated with higher pneumonia risk (Table 3).
Discussion
Our study found that higher CC was associated with a lower risk of pneumonia in men with schizophrenia. To our knowledge, this investigation is the first to explore the relationship between two common sarcopenia screening tools, MUAC and CC, and the risk of pneumonia in patients with stable schizophrenia. The study further enables clinicians to assess the risk of pneumonia in stable male patients with schizophrenia with simple and reproducible anthropometric measures. It further enhances dynamic assessment during disease intervention. This is an advantage of the study as in clinical practice, the benefits of introducing CC measurements come from their simplicity and rapidity, as well as their economical and non-invasive nature.
In our study, CC, one of the screening markers for sarcopenia, was associated with pneumonia risk in patients with schizophrenia. People with sarcopenia have reduced interleukin-15, which has an effect on the immune system, especially on natural killer cells (21). In addition, the phosphoinositide 3-kinase (PI3K)-Akt pathway in patients with sarcopenia is also affected, thereby affecting part of the function of neutrophils (22). On the other hand, people with sarcopenia have reduced muscle strength throughout the body, including respiratory muscles, which is one of the risk factors for pneumonia because they are unable to cough effectively to clear their airways (23). Finally, patients with low muscle mass generally have poorer oral hygiene (24). The study by Juthani-Mehta et al. showed that impaired oral hygiene is one of the risk factors for pneumonia (25). That may explain the increased risk of pneumonia in patients with sarcopenia. But our results were meaningful only in men, not women. We believe that this may be due to higher leg fat mass in women than in men (26) and therefore, the association between CC and muscle mass in women is not as strong as in men (27). Additionally, Weiss et al. found a different degree of association between muscle sonography and CC between the two sexes (0.8 in men and 0.76 in women) (28).
MUAC is recommended as a screening marker for the nutritional status and sarcopenia, which is associated with muscle mass and subcutaneous fat, is less affected by fluid retention, and is easy to perform at no additional cost (13, 14). However, in our study, MUAC was not associated with the risk of pneumonia, and the same results were obtained in the study by Yardimci et al. (29). We speculate that it may be related to less muscle mass in the upper limbs than in the lower limbs. Ma et al. found that MUAC is one of the independent predictors of in-hospital mortality in older patients with pneumonia (30). However, our study has a short follow-up time and collected fewer death data, and it is not yet possible to confirm whether MUAC is associated with death in patients with stable schizophrenia. There is currently an ongoing follow-up.
The incidence of pneumonia in patients with schizophrenia was 24.5% in our study. Chou et al. conducted a 9-year follow-up study in Taiwan and found that 10.26% of patients with schizophrenia developed pneumonia (31). The mean age of the patients in their study was 40.62 years, while the patients in our study were all 50 years of age and older. We guess that the reason for the higher incidence of pneumonia in our study may be the advanced patients' age under our investigation.
The current study has certain limitations too. First, we did not collect information on the severity of pneumonia and there is currently little follow-up information on the patients with death data to further investigate the association of MUAC and CC with pneumonia severity and death. However, the follow-up studies continue and this will address this issue in the future. Second, the cohort of this study was small, including only a small number of patients with schizophrenia in Western China, so the results cannot be generalized to other races and countries, and validation with a larger cohort and a larger population is strongly needed. Third, we did not perform a categorical analysis of MUAC due to the lack of recommended cut-off values for MUAC in current guidelines. Therefore, a recommendation for further research is generally a huge need.
Conclusion
Our study has demonstrated that lower CC scores were associated with increased pneumonia risk in stable patients with schizophrenia in men, but not in women. Therefore, we recommend that in men with stable schizophrenia, CC can be applied as a solid screening marker for sarcopenia to identify patients who may be at risk for pneumonia.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Zigong Medical Foundation (IRB number: 20191001) and the Psychiatric Hospital of Ziyang (IRB number: zjsjsbyy-kyxm-2019-2). The patients/participants provided their written informed consent to participate in this study.
Author contributions
SR, SH, MC, TZ, QL, and XC: study concept and design. MC, TZ, and QL: acquisition of data. SR, SH, and XC: analysis and interpretation of data and drafting of the manuscript. XC: critical revision of the manuscript for important intellectual content. All authors of this manuscript have fully contributed to the manuscript and approved the final manuscript.
Funding
This work was funded by the Psychiatric Hospital of Ziyang Support Project (Project No. Zysjsbyy-kyxm-2022-6) and the 2021 Key Science and Technology Plan of Zigong City (Project No. 2021YXY12). The sponsors did not participate in the design, methods, data collection, analysis, or preparation of this manuscript.
Acknowledgments
We thank all personnel for their contribution to the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012
2. Wang PY, Xu LD, Chen XK, Xu L, Yu YK, Zhang RX, et al. Sarcopenia and short-term outcomes after esophagectomy: a meta-analysis. Ann Surg Oncol. (2020) 27:3041–51. doi: 10.1245/s10434-020-08236-9
3. Al-Azzawi Y, Albo B, Fasullo M, Coukos J, Watts GJ, Tai R, et al. Sarcopenia is associated with longer hospital stay and multiorgan dysfunction in alcoholic hepatitis. Eur J Gastroenterol Hepatol. (2020) 32:733–8. doi: 10.1097/MEG.0000000000001583
4. Aro R, Mäkäräinen-Uhlbäck E, Ämmälä N, Rautio T, Ohtonen P, Saarnio J, et al. The impact of sarcopenia and myosteatosis on postoperative outcomes and 5-year survival in curatively operated colorectal cancer patients - A retrospective register study. Eur J Surg Oncol. (2020) 46:1656–62. doi: 10.1016/j.ejso.2020.03.206
5. Altuna-Venegas S, Aliaga-Vega R, Maguiña JL, Parodi JF, Runzer-Colmenares FM. Risk of community-acquired pneumonia in older adults with sarcopenia of a hospital from Callao, Peru 2010-2015. Arch Gerontol Geriatr. (2019) 82:100–5. doi: 10.1016/j.archger.2019.01.008
6. Serafini G, Pompili M, Haghighat R, Pucci D, Pastina M, Lester D, et al. Stigmatization of schizophrenia as perceived by nurses, medical doctors, medical students and patients. J Psychiatr Ment Health Nurs. (2011) 18:576–85. doi: 10.1111/j.1365-2850.2011.01706.x
7. Fusar-Poli P, Smieskova R, Serafini G, Politi P, Borgwardt S. Neuroanatomical markers of genetic liability to psychosis and first episode psychosis: a voxelwise meta-analytical comparison. World J Biol Psychiatry. (2014) 15:219–28. doi: 10.3109/15622975.2011.630408
8. Kirwan R, McCullough D, Butler T, Perez de Heredia F, Davies IG, Stewart C. Sarcopenia during COVID-19 lockdown restrictions: long-term health effects of short-term muscle loss. Geroscience. (2020) 42:1547–78. doi: 10.1007/s11357-020-00272-3
9. Lacina L, Brábek J, Král V, Kodet O, Smetana K. Interleukin-6: a molecule with complex biological impact in cancer. Histol Histopathol. (2019) 34:125–36. doi: 10.14670/HH-18-033
10. Suetani S, Honarparvar F, Siskind D, Hindley G, Veronese N, Vancampfort D, et al. Increased rates of respiratory disease in schizophrenia: A systematic review and meta-analysis including 619,214 individuals with schizophrenia and 52,159,551 controls. Schizophr Res. (2021) 237:131–40. doi: 10.1016/j.schres.2021.08.022
11. Tosato M, Marzetti E, Cesari M, Savera G, Miller RR, Bernabei R, et al. Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin Exp Res. (2017) 29:19–27. doi: 10.1007/s40520-016-0717-0
12. Endo K, Sato T, Kakisaka K, Takikawa Y. Calf and arm circumference as simple markers for screening sarcopenia in patients with chronic liver disease. Hepatol Res. (2021) 51:176–89. doi: 10.1111/hepr.13589
13. Hu FJ, Liu H, Liu XL, Jia SL, Hou LS, Xia X, et al. Mid-upper arm circumference as an alternative screening instrument to appendicular skeletal muscle mass index for diagnosing sarcopenia. Clin Interv Aging. (2021) 16:1095–104. doi: 10.2147/CIA.S311081
14. Chen M, Lei X, Zhu T, Li Q, Chen X. Evaluation of the accuracy of six simple screening tools for sarcopenia in schizophrenic patients. J Nutr Health Aging. (2022) 26:571–5. doi: 10.1007/s12603-022-1799-3
15. Phua J, See KC, Chan YH, Widjaja LS, Aung NW, Ngerng WJ, et al. Validation and clinical implications of the IDSA/ATS minor criteria for severe community-acquired pneumonia. Thorax. (2009) 64:598–603. doi: 10.1136/thx.2009.113795
16. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. (1963) 185:914–9. doi: 10.1001/jama.1963.03060120024016
17. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
18. Pfeiffer E, A. short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. (1975) 23:433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x
19. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. (2019) 394:1376–86. doi: 10.1016/S0140-6736(19)31785-4
21. Lutz CT, Quinn LS. Sarcopenia, obesity, and natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging. (2012) 4:535–46. doi: 10.18632/aging.100482
22. Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res Rev. (2017) 36:1–10. doi: 10.1016/j.arr.2017.01.006
23. Okazaki T, Suzukamo Y, Miyatake M, Komatsu R, Yaekashiwa M, Nihei M, et al. Respiratory muscle weakness as a risk factor for pneumonia in older people. Gerontology. (2021) 67:581–90. doi: 10.1159/000514007
24. Solemdal K, Sandvik L, Møinichen-Berstad C, Skog K, Willumsen T, Mowe M. Association between oral health and body cell mass in hospitalised elderly. Gerodontology. (2012) 29:e1038–44. doi: 10.1111/j.1741-2358.2011.00607.x
25. Juthani-Mehta M, De Rekeneire N, Allore H, Chen S, O'Leary JR, Bauer DC, et al. Modifiable risk factors for pneumonia requiring hospitalization of community-dwelling older adults: the Health, Aging, and Body Composition Study. J Am Geriatr Soc. (2013) 61:1111–8. doi: 10.1111/jgs.12325
26. Nindl BC, Scoville CR, Sheehan KM, Leone CD, Mello RP. Gender differences in regional body composition and somatotrophic influences of IGF-I and leptin. J Appl Physiol. (2002) 92:1611–8. doi: 10.1152/japplphysiol.00892.2001
27. Kawakami R, Murakami H, Sanada K, Tanaka N, Sawada SS, Tabata I, et al. Calf circumference as a surrogate marker of muscle mass for diagnosing sarcopenia in Japanese men and women. Geriatr Gerontol Int. (2015) 15:969–76. doi: 10.1111/ggi.12377
28. Weiss LW, Clark FC. Ultrasonic protocols for separately measuring subcutaneous fat and skeletal muscle thickness in the calf area. Phys Ther. (1985) 65:477–81. doi: 10.1093/ptj/65.4.477
29. Yardimci B, Aksoy SM, Ozkaya I, Demir T, Tezcan G, Kaptanoglu AY. Anthropometric measurements may be informative for nursing home-acquired pneumonia. Pak J Med Sci. (2016) 32:694–9. doi: 10.12669/pjms.323.9635
30. Ma HM, Tang WH, Woo J. Predictors of in-hospital mortality of older patients admitted for community-acquired pneumonia. Age Ageing. (2011) 40:736–41. doi: 10.1093/ageing/afr087
Keywords: MUAC, mid-upper arm circumference, CC, calf circumference, schizophrenia, pneumonia
Citation: Ren S, Huang S, Chen M, Zhu T, Li Q and Chen X (2022) Association between the mid-upper arm circumference (MUAC) and calf circumference (CC) screening indicators of sarcopenia with the risk of pneumonia in stable patients diagnosed with schizophrenia. Front. Psychiatry 13:931933. doi: 10.3389/fpsyt.2022.931933
Received: 29 April 2022; Accepted: 02 August 2022;
Published: 26 August 2022.
Edited by:
Luca De Peri, Cantonal Sociopsychiatric Organization, SwitzerlandReviewed by:
Te-Jen Lai, Chung Shan Medical University, TaiwanFrancesco Monaco, Azienda Sanitaria Locale Salerno, Italy
Gianluca Serafini, San Martino Hospital (IRCCS), Italy
Copyright © 2022 Ren, Huang, Chen, Zhu, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Chen, Mzc5NTMxNzIyQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Silan Ren1†
Silan Ren1† Sha Huang
Sha Huang Xiaoyan Chen
Xiaoyan Chen