
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Psychiatry, 15 July 2022
Sec. Molecular Psychiatry
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.928188
Cortisol is the main HPA axis hormone secreted by the adrenal cortex, and influences metabolism, cognition, and behavior. Recently, a plethora of studies have tried to confirm the correlation between peripheral cortisol and autism spectrum disorder (ASD). However, the results were controversial. We assessed the effects of peripheral cortisol on ASD in this study. The included studies were identified according to the inclusion and exclusion criteria. The pooled Hedges’ g and its 95% confidence interval were selected to evaluate the association between peripheral cortisol and ASD. Subgroup analyses, sensitivity analyses, meta-regression, and publication bias tests were also undertaken based on the obtained information. There were a total of twelve studies with 375 ASD patients and 335 controls included in our meta-analysis. Obvious heterogeneity across studies was found in the overall analysis. Peripheral cortisol levels were significantly elevated in ASD patients compared with controls in the absence of obvious heterogeneity. A single study did not influence the overall comparison results. Meta-regression analyses revealed that age and gender of the included subjects, sample size, and publication year did not moderate effects on the present results. These findings may provide us some targeted strategies to the diagnosis and treatment of ASD.
Autism spectrum disorder (ASD) is a group of neurodevelopmental disorders characterized by impaired social interaction and communication, restricted and repetitive patterns of behavior, interests, or activities according to the Diagnostic and Statistical Manual of Mental Disorders (DSM)-5. The cost of ASD to society is increasing worldwide and is more than $126 billion per year in the United States (1). Data from eleven sites (Arizona, Arkansas, California, Georgia, Maryland, Minnesota, Missouri, New Jersey, Tennessee, Utah, and Wisconsin) in the United States has showed that the overall ASD prevalence was 17.0 per 1,000 (one in 59) children aged 4 years (2). The rising prevalence of ASD is ascribed to complex interactions between genetic and environmental factors (3, 4). However, the exact etiology of ASD remains poorly understood despite of improvements in the diagnosis and treatment of ASD.
The hypothalamic-pituitary-adrenal (HPA) axis is one of the body’s main stress effector systems (5). A compelling body of evidence implicates that the activation of the HPA axis and pulsatile release of glucocorticoids play critical roles in response to physical or psychological stressors in physiological status (6). A growing literature has implicated that the HPA axis contributes to the development of psychiatry disorders, such as depression, anxiety, and PTSD (7–9). Animal studies suggested that the HPA axis dysfunction caused by environmental components could induce autism-like behaviors, and regulation of the HPA axis could alleviate autism-like behaviors (10). Clinical evidence also revealed that the related hormones of the HPA axis were obviously abnormal in ASD patients (11–13). The expression of HPA axis related genes, such as corticotropin-releasing hormone receptor-1 (Crhr1), and oxytocin receptors, were closely related to the development of autism-like behaviors (14, 15). It seems that the HPA axis plays a key role in the occurrence of ASD.
Cortisol is the main HPA axis hormone and the key glucocorticoid produced in response to stress, which has a variety of effects on cardiovascular function, immunity, metabolism, and neurobiology with being involved in several vital biological processes and interactions (16, 17). More and more evidence has shown that cortisol is implicated in emotional and cognitive development, and plays a crucial role in a series of psychiatric disorders (18–20). Cortisol was reported to regulate excitatory and inhibitory (E/I) neurotransmission and neurogenesis during the development of the central nervous system (CNS) (21). Recently, a large number of clinical studies have been performed to evaluate the association between the levels of cortisol in peripheral blood and ASD. However, the results were controversial (22, 23). Therefore, we conducted the present meta-analysis, and pooled the published data to confirm the association between peripheral cortisol and ASD, and strengthen the conclusions.
We systematically searched for the relevant literature up through December 21, 2021 in four databases (PubMed, Embase, Web of Science, and Cochrane databases). The retrieval combination of key words was as follows: “autism,” “autistic,” or “ASD”; and “glucocorticoid,” “corticosteroids,” “corticosterone,” “cortisol,” or “cort”; and “serum,” “plasma,” or “peripheral.” We also carried out a reference search on all retrieved records to identify available articles.
Our meta-analysis followed the guidelines recommended by the Preferred Reporting Items for Systematic Reviews and Meta-analysis (the PRISMA statement). Eligible studies must meet all of the following criteria: (1) clinical studies assessing the levels of cortisol in plasma or serum of ASD patients and controls; (2) the levels of cortisol presented as a mean with corresponding standard deviation (SD), or sample size and P-value; (3) studies in English. Studies met any of the following criteria were excluded: (1) studies with insufficient data such as concentration of cortisol or P-value; (2) animal study or in vitro study; (3) case reports, reviews, comments, or meeting abstracts. When some studies contained overlapping data, only the one with the largest sample size was included to calculate the pooled effect size (ES).
Two independent investigators screened the full text of the retrieved studies according to the inclusion and exclusion criteria, and extracted corresponding data into a standardized Excel spreadsheet. The following information and relevant data were extracted: author, publication year, country, diagnostic criteria, source of ASD patients, detection method for cortisol, sample type, age and gender of samples, sample size, mean, standard deviation, and P-value. If a consensus could not be reached, disagreement was resolved by discussion. Finally, data were rechecked by a third author for accuracy.
Hedges’ g is a more unbiased measurement calculated by sample sizes, mean cortisol concentrations, and corresponding SDs, or P-values. In our study, the pooled Hedges’ g and its corresponding 95% confidence interval (CI) were employed to evaluate the association between the levels of cortisol in peripheral blood and ASD. Heterogeneity test was conducted to assess heterogeneity across studies using the I2-value and Cochrane Q-test. A random-effect model was selected to pool the effect size when obvious heterogeneity across studies existed; otherwise, a fixed-effect model was selected. Sensitivity analyses were carried out by removing each study from the analysis in turn to explore the effect of a single study on the result directly. Publication bias was assessed by drawing a funnel plot qualitatively (the more symmetrical, the lower the risk of publication bias), and Egger’s test quantitatively. P < 0.05 was considered statistically significant in the present study.
To explore the source of heterogeneity across studies, subgroup analyses were performed based on different types of samples. A Galbraith plot was drawn to identify studies obviously deviated from the baseline. In addition, unrestricted maximum-likelihood random-effect meta-regressions of effect sizes were performed to verify whether some theoretical covariates, such as mean age, gender (male proportion) of ASD patients, sample size, and publication year, would serve as confounders that affect the association of peripheral cortisol and ASD in the present study. All statistical analyses were conducted using Comprehensive Meta-Analysis version 3 software and STATA15.0 software (StataCorp, College Station, TX, United States).
There were 485 studies (126 records from PubMed, 163 records from Embase, 180 records from Web of Science, and 16 records from Cochrane database) identified during the initial electronic search. After removing 191 duplications, there are two hundred and ninety-four studies remaining. There were 272 unrelated records excluded by reviewing titles or abstracts. Twenty-two records underwent further screening through full-text reading. Ten studies were removed for insufficient data, abstract, review, or overlapping data. At last, a total of twelve records (12 studies) met our inclusion criteria and were included in our study (22–33). The flow diagram for the study selection procedure is shown in Figure 1.
Of the twelve included studies, seven detected cortisol concentrations in serum, five in plasma. The studies were conducted in Turkey, Egypt, United States, Japan, Belgium, Finland, Israel, and Croatia. In most of the included studies, the diagnosis of ASD was based on the DSM. We noted that the detection method of peripheral cortisol differed from one another. Most of the included subjects were children, and some were adults. The characteristics of the included studies are presented in Table 1.
A random-effect model was selected to calculate the pooled Hedges’ g and its corresponding 95% confidence interval based on the extracted data from the twelve included studies for obvious heterogeneity across studies (I2 = 95.198, P < 0.001). The combined result of the overall comparison showed that there was no significant difference between the levels of cortisol in peripheral blood of different groups (Hedges’ g = −0.278, 95% CI: −1.026 to 0.470, P = 0.467) (Figure 2; Table 2).
Obvious heterogeneity may reduce the credibility of the pooled results. To avoid the impact of heterogeneity on the results, the source of heterogeneity across studies was investigated based on the available information. First, subgroup analyses were performed according to the extracted data. There was no significant difference in the levels of cortisol in serum between autistic patients and controls (Hedges’ g = −0.340, 95% CI: −1.449 to 0.769, P = 0.548) (Figure 3). Similarly, there was no significant difference in the levels of cortisol in plasma between autistic patients and controls (Hedges’ g = −0.192, 95% CI: −1.218 to 0.833, P = 0.713) (Figure 4). However, heterogeneity across studies was not changed obviously either in the serum subgroup (I2 = 96.549, P < 0.001) or the plasma subgroup (I2 = 92.754, P < 0.001). The types of samples may not significantly affect the source of heterogeneity.
A Galbraith plot was drawn to identify some specific studies, which may influence heterogeneity across studies. Three studies performed by Ćurin et al. (32), Hassan et al. (26), and Hamza et al. (22) were screened out using the Galbraith plot (Figure 5). These three studies were significantly deflected from the center line, and may be implicated with heterogeneity across studies. Therefore, we removed these three studies, and found that heterogeneity across studies was significantly decreased (I2 = 33.897, P = 0.147). We also noted that peripheral levels of cortisol were significantly higher in autistic patients compared with controls (Hedges’ g = 0.389, 95% CI: 0.190 to 0.588, P < 0.001) (Figure 6). These studies may be a source of heterogeneity across studies, and had a significant impact on the results (Table 2).
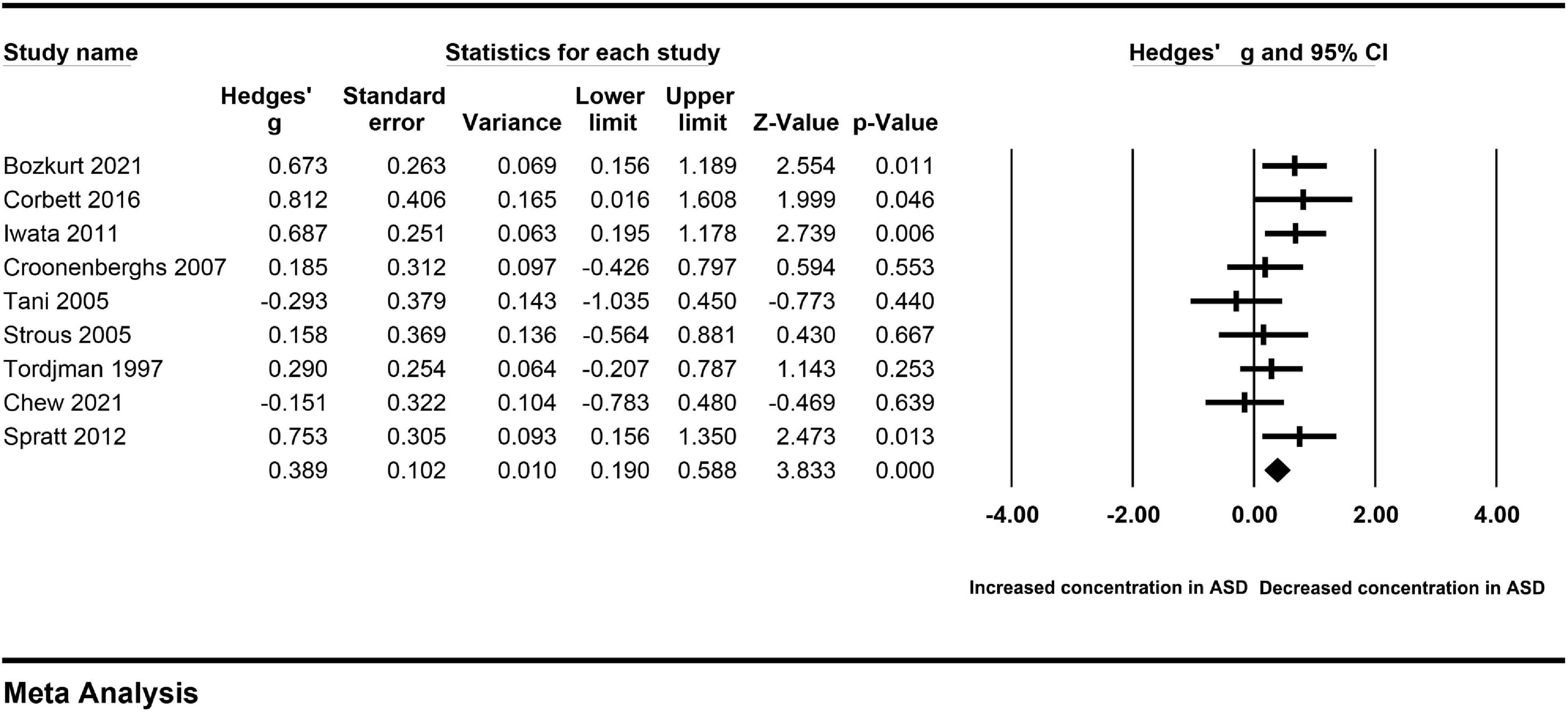
Figure 6. Forest plot for the fixed-effect meta-analysis after removing the studies outside the boundaries of the Galbraith plot.
Meta-regression analyses were conducted to evaluate the effects of some potential continuous variables (age, gender, sample size, and publication year) on the high levels of heterogeneity across studies. There were no significant associations between age, gender, sample size, or publication year and the pooled Hedges’ g, which meant that these potential continuous variables did not moderate effects on the outcomes in our meta-analysis (Figure 7).
To clarify the impact of a single included study on the pooled Hedges’ g, we carried out the sensitivity analysis by removing each included study from the analysis in turn. The association between peripheral cortisol and ASD changed little (Figure 8), suggesting that an individual study did not affect the results.
Visual inspection of a funnel plot suggested that no obvious publication bias was found in this meta-analysis (Figure 9). The results were further strengthened by the result of the Egger test (P = 0.156). Moreover, 46 missing studies were required to make the P-value less than 0.05, which was confirmed by the classic fail-safe N method. Therefore, publication bias did not affect our results significantly.
This meta-analysis comprehensively evaluates for the first time, to our knowledge, the available evidence regarding the relation of peripheral levels of cortisol to ASD. There were a total of twelve clinical studies included in our meta-analysis to make quantitative evaluation. We found that the levels of cortisol were not significantly increased in peripheral blood of autistic patients compared with controls. The sensitivity analysis was employed to verify whether a single study could affect the overall analysis. When we removed a single study from the overall analysis, the association between peripheral cortisol levels and ASD was not reversed. When there was obvious publication bias in a meta-analysis, the false-negative results were suppressed and the false-positive results could be magnified in this meta-analysis. No obvious publication bias was found in the study, which further confirmed that the results of our meta-analysis were unlikely to be induced by publication bias. More importantly, we noted that obvious heterogeneity across studies existed in the study, which may result in the evidence of relevance association between peripheral cortisol and ASD judged as only low confidence. Therefore, we should be cautious to explain the overall comparative results, and address the issues caused by heterogeneity across studies.
Subgroup analyses, a Galbraith plot, and meta-regression analyses were performed to identify the source of heterogeneity and some potential continuous variables moderating effects on the outcomes. For the limited information, we only carried out subgroup analyses based on different sample types, and found that the sample type was not the main source of heterogeneity across studies. The meta-regression analyses used in the present study confirmed that age, gender, sample size, and publication year may not play a role as moderators in the meta-analysis. Studies with more detailed information are required to explore the source of heterogeneity. The Galbraith plot identified three studies that may cause obvious heterogeneity across studies. After removing these three studies, heterogeneity across studies decreased obviously. Interestingly, peripheral cortisol levels were significantly elevated in ASD patients compared with controls. It means that peripheral cortisol may be associated with ASD. The result of overall analysis may be affected by heterogeneity. The result should be explained with great caution. Future population-based studies are urgently required to verify the effects of peripheral cortisol on ASD.
A clinical study has shown higher hair cortisol concentrations in autistic children with severe self-injurious behavior (34). Fetal cortisol exposure was verified to be a predictor of ASD (35). In addition, more clinical studies have indicated significantly elevated cortisol in peripheral blood of ASD patients (23, 28). What is the relationship between cortisol in peripheral blood and ASD? Early life events are known to greatly influence brain development, and HPA activity. A series of animal studies have suggested environmental factors, such as valproic acid (VPA), maternal immune activation, and maternal separation, resulted in the systemic stress reaction and autism-like behaviors (36–38). As we all know, chronic stress primarily promotes the release of excessive cortisol from the adrenal gland, which tends to activate microglia. Microglia was reported mediating synaptic pruning, which could affect excitation/inhibition imbalance in the CNS. In this way, peripheral cortisol may be partly responsible for the development of ASD (39). Early screening the levels of cortisol in peripheral blood of children may contribute to the diagnosis of ASD. The role of cortisol in the development of ASD, which may help us develop effective interventions on ASD, should be further verified by well-designed animal studies.
In our study, the established methods were used to leverage a large number of records identified from each database searched, and found significantly elevated peripheral cortisol in ASD patients compared with controls after removing three studies causing obvious heterogeneity across studies. Several limitations should be pointed out in our meta-analysis. First, the number of sample size and included studies was moderate, which may influence us to draw accurate conclusions and explore the source of heterogeneity. Second, we should pay more attention to the issues of heterogeneity in our study, although some studies have been found to affect heterogeneity. The inadequate information of included studies blocked our evaluation on some potential residual confounding factors. In addition, subgroup analyses were not fully carried out for the loss of information. Different detection methods investigating cortisol levels in peripheral blood may increase heterogeneity to some extent. Third, the causal relationship between peripheral cortisol and ASD still needs to be treated dialectically. We found that peripheral blood cortisol levels were significantly elevated in ASD patients in the absence of obvious heterogeneity. Whether increased cortisol was a cause of ASD should be further confirmed through more basic research. Finally, the cross-sectional studies could only focus on one point in time of the development of ASD, and the dynamic changes in ASD are still required to be further clarified. Similarly, the dynamic changes cortisol levels should be further monitored.
We found suggestive evidence that ASD was associated with a significantly increased cortisol in peripheral blood in the absence of obvious heterogeneity across studies. More well-designed studies are required for further confirm the roles of cortisol in ASD.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
JG and XF conceived the study and corrected the manuscript. JG, JZo, and LY collected the data and drafted the manuscript. JZh, LW, and TL corrected the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (No. 82071544), the pre-research funding from the Key Laboratory of Extreme Environment Medicine, and the Ministry of Education, China (PF-KL2020-005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zuvekas SH, Grosse SD, Lavelle TA, Maenner MJ, Dietz P, Ji X. Healthcare costs of pediatric autism spectrum disorder in the united states, 2003-2015. J Autism Dev Disord. (2021) 51:2950–8. doi: 10.1007/s10803-020-04704-z
2. Shaw KA, Maenner MJ, Bakian AV, Bilder DA, Durkin MS, Furnier SM, et al. Early identification of autism spectrum disorder among children aged 4 years – autism and developmental disabilities monitoring network, 11 sites, united states, 2018. MMWR Surveill Summ. (2021) 70:1–14. doi: 10.15585/mmwr.ss7010a1
3. Kong Y, Zhou W, Sun Z. Nuclear receptor corepressors in intellectual disability and autism. Mol Psychiatry. (2020) 25:2220–36. doi: 10.1038/s41380-020-0667-y
4. Rylaarsdam L, Guemez-Gamboa A. Genetic causes and modifiers of autism spectrum disorder. Front Cell Neurosci. (2019) 13:385. doi: 10.3389/fncel.2019.00385
5. Hardiman RL, Bratt A. Hypothalamic-pituitary-adrenal axis function in fragile x syndrome and its relationship to behaviour: a systematic review. Physiol Behav. (2016) 167:341–53. doi: 10.1016/j.physbeh.2016.09.030
6. Dempster KS, O’Leary DD, MacNeil AJ, Hodges GJ, Wade TJ. Linking the hemodynamic consequences of adverse childhood experiences to an altered hpa axis and acute stress response. Brain Behav Immun. (2021) 93:254–63. doi: 10.1016/j.bbi.2020.12.018
7. Sousa VC, Mantas I, Stroth N, Hager T, Pereira M, Jiang H, et al. P11 deficiency increases stress reactivity along with Hpa axis and autonomic hyperresponsiveness. Mol Psychiatry. (2021) 26:3253–65. doi: 10.1038/s41380-020-00887-0
8. Lin YL, Wei CW, Lerdall TA, Nhieu J, Wei LN. Crabp1 modulates Hpa Axis homeostasis and anxiety-like behaviors by altering Fkbp5 expression. Int J Mol Sci. (2021) 22:12240. doi: 10.3390/ijms222212240
9. Morris MC, Compas BE, Garber J. Relations among posttraumatic stress disorder, comorbid major depression, and HPA function: a systematic review and meta-analysis. Clin Psychol Rev. (2012) 32:301–15. doi: 10.1016/j.cpr.2012.02.002
10. Zhao X, Mohammed R, Tran H, Erickson M, Kentner AC. Poly (I:C)-induced maternal immune activation modifies ventral hippocampal regulation of stress reactivity: prevention by environmental enrichment. Brain Behav Immun. (2021) 95:203–15. doi: 10.1016/j.bbi.2021.03.018
11. Singh S, Yazdani U, Gadad B, Zaman S, Hynan LS, Roatch N, et al. Serum thyroid-stimulating hormone and interleukin-8 levels in boys with autism spectrum disorder. J Neuroinflammation. (2017) 14:113. doi: 10.1186/s12974-017-0888-4
12. Worsham W, Dalton S, Bilder DA. The prenatal hormone milieu in autism spectrum disorder. Front Psychiatry. (2021) 12:655438. doi: 10.3389/fpsyt.2021.655438
13. Ames JL, Windham GC, Lyall K, Pearl M, Kharrazi M, Yoshida CK, et al. Neonatal thyroid stimulating hormone and subsequent diagnosis of autism spectrum disorders and intellectual disability. Autism Res. (2020) 13:444–55. doi: 10.1002/aur.2247
14. Schaafsma SM, Gagnidze K, Reyes A, Norstedt N, Månsson K, Francis K, et al. Sex-specific gene-environment interactions underlying Asd-like behaviors. Proc Natl Acad Sci USA. (2017) 114:1383–8. doi: 10.1073/pnas.1619312114
15. Sanathara N, Alhassen L, Marmouzi I, Khoudari M, Phan J, Alhassen W, et al. Oxytocin-Mch circuit regulates monosynaptic inputs to Mch neurons and modulates social recognition memory. Neuropharmacology. (2021) 184:108423. doi: 10.1016/j.neuropharm.2020.108423
16. Lightman SL, Birnie MT, Conway-Campbell BL. Dynamics of Acth and cortisol secretion and implications for disease. Endocr Rev. (2020) 41:bnaa002. doi: 10.1210/endrev/bnaa002
17. Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. (1997) 20:78–84. doi: 10.1016/s0166-2236(96)10069-2
18. Bonnekoh LM, Seidenbecher S, Knigge K, Hünecke AK, Metzger CD, Tempelmann C, et al. Long-term cortisol stress response. in depression and comorbid anxiety is linked with reduced N-acetylaspartate in the anterior cingulate cortex. World J Biol Psychiatry. (2022) 1–35. doi: 10.1080/15622975.2022.2058084
19. Poole KL, Schmidt LA. Frontal brain delta-beta correlation, salivary cortisol, and social anxiety in children. J Child Psychol Psychiatry. (2019) 60:646–54. doi: 10.1111/jcpp.13016
20. O’Donovan A, Hughes BM, Slavich GM, Lynch L, Cronin MT, O’Farrelly C, et al. Clinical anxiety, cortisol and interleukin-6: evidence for specificity in emotion-biology relationships. Brain Behav Immun. (2010) 24:1074–7. doi: 10.1016/j.bbi.2010.03.003
21. Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, et al. Prenatal developmental origins of behavior and mental health: the influence of maternal stress in pregnancy. Neurosci Biobehav Rev. (2020) 117:26–64. doi: 10.1016/j.neubiorev.2017.07.003
22. Hamza RT, Hewedi DH, Ismail MA. Basal and adrenocorticotropic hormone stimulated plasma cortisol levels among egyptian autistic children: relation to disease severity. Ital J Pediatr. (2010) 36:71. doi: 10.1186/1824-7288-36-71
23. Spratt EG, Nicholas JS, Brady KT, Carpenter LA, Hatcher CR, Meekins KA, et al. Enhanced cortisol response to stress in children in autism. J Autism Dev Disord. (2012) 42:75–81. doi: 10.1007/s10803-011-1214-0
24. Chew L, Sun KL, Sun W, Wang Z, Rajadas J, Flores RE, et al. Association of serum allopregnanolone with restricted and repetitive behaviors in adult males with autism. Psychoneuroendocrinology. (2021) 123:105039. doi: 10.1016/j.psyneuen.2020.105039
25. Bozkurt H, Şimşek Ş, Şahin S. Elevated levels of cortisol, brain-derived neurotropic factor and tissue plasminogen activator in male children with autism spectrum disorder. Autism Res. (2021) 14:2078–84. doi: 10.1002/aur.2582
26. Hassan MH, Desoky T, Sakhr HM, Gabra RH, Bakri AH. Possible metabolic alterations among autistic male children: clinical and biochemical approaches. J Mol Neurosci. (2019) 67:204–16. doi: 10.1007/s12031-018-1225-9
27. Corbett BA, Bales KL, Swain D, Sanders K, Weinstein TAR, Muglia LJ. Comparing oxytocin and cortisol regulation in a double-blind, placebo-controlled, hydrocortisone challenge pilot study in children with autism and typical development. J Neurodev Disord. (2016) 8:32. doi: 10.1186/s11689-016-9165-6
28. Iwata K, Matsuzaki H, Miyachi T, Shimmura C, Suda S, Tsuchiya KJ, et al. Investigation of the serum levels of anterior pituitary hormones in male children with autism. Mol Autism. (2011) 2:16. doi: 10.1186/2040-2392-2-16
29. Croonenberghs J, Wauters A, Deboutte D, Verkerk R, Scharpe S, Maes M. Centrat serotonergic hypofunction in autism: results of the 5-hydroxy-tryptophan challenge test. Neuro Endocrinol Lett. (2007) 28:449–55.
30. Tani P, Lindberg N, Matto V, Appelberg B, Wendt TNV, von Wendt L, et al. Higher plasma acth levels in adults with asperger syndrome. J Psychosom Res. (2005) 58:533–6. doi: 10.1016/j.jpsychores.2004.12.004
31. Strous RD, Golubchik P, Maayan R, Mozes T, Tuati-Werner D, Weizman A, et al. Lowered Dhea-S plasma levels in adult individuals with autistic disorder. Eur Neuropsychopharmacol. (2005) 15:305–9. doi: 10.1016/j.euroneuro.2004.12.004
32. Ćurin JM, Terzić J, Petković ZB, Zekan L, Terzić IM, Šušnjara IM. Lower cortisol and higher Acth levels in individuals with autism. J Autism Dev Disord. (2003) 33:443–8. doi: 10.1023/A:1025019030121
33. Tordjman S, McBride PA, Hertzig ME, Snow ME, Thompson SM, Cohen DJ, et al. Plasma B -endorphin, adrenocorticotropin hormone, and cortisol in autism. J Child Psychol Psychiatry. (1997) 38:705–15. doi: 10.1111/j.1469-7610.1997.tb01697.x
34. Courtemanche AB, Black WR, Meyer JS. Hair cortisol and self-injurious behavior among children with autism spectrum disorder. Am J Intellect Dev Disabil. (2021) 126:158–66. doi: 10.1352/1944-7558-126.2.158
35. Ram S, Howland MA, Sandman CA, Davis EP, Glynn LM. Prenatal risk for Asd: Fetal cortisol exposure predicts child autism-spectrum disorder symptoms. Clin Psychol Sci. (2019) 7:349–61. doi: 10.1177/2167702618811079
36. Vakili Shahrbabaki SS, Jonaidi H, Sheibani V, Bashiri H. Early postnatal handling alters social behavior, learning, and memory of pre– and postnatal Vpa-induced rat models of autism in a context-based manner. Physiol Behav. (2022) 249:113739. doi: 10.1016/j.physbeh.2022.113739
37. Delorme TC, Srivastava LK, Cermakian N. Altered circadian rhythms in a mouse model of neurodevelopmental disorders based on prenatal maternal immune activation. Brain Behav Immun. (2021) 93:119–31. doi: 10.1016/j.bbi.2020.12.030
38. Mansouri M, Pouretemad H, Roghani M, Wegener G, Ardalan M. Autistic-like behaviours and associated brain structural plasticity are modulated by oxytocin in maternally separated rats. Behav Brain Res. (2020) 393:112756. doi: 10.1016/j.bbr.2020.112756
Keywords: cortisol, autism spectrum disorder, serum, plasma, association
Citation: Gao J, Zou J, Yang L, Zhao J, Wang L, Liu T and Fan X (2022) Alteration of peripheral cortisol and autism spectrum disorder: A meta-analysis. Front. Psychiatry 13:928188. doi: 10.3389/fpsyt.2022.928188
Received: 25 April 2022; Accepted: 27 June 2022;
Published: 15 July 2022.
Edited by:
Stefania Schiavone, University of Foggia, ItalyReviewed by:
Roberto Keller, ASL Città di Torino, ItalyCopyright © 2022 Gao, Zou, Yang, Zhao, Wang, Liu and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junwei Gao, bnV0ZGdqd0AxNjMuY29t; Xiaotang Fan, ZmFueGlhb3RhbmcyMDA1QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.