
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 02 June 2022
Sec. Schizophrenia
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.925007
 Qiang Wang1†
Qiang Wang1† Lingyun Zeng2†
Lingyun Zeng2† Wenjuan Hong1
Wenjuan Hong1 Mingying Luo3
Mingying Luo3 Nan Zhao1
Nan Zhao1 Xiaofen Hu1
Xiaofen Hu1 Meili Shi1
Meili Shi1 Jing Qiu1
Jing Qiu1 Yanmin Shen1
Yanmin Shen1 Xiuju Teng1
Xiuju Teng1 Haiying Min1*
Haiying Min1* Weiqing Liu1*
Weiqing Liu1*Studies have found that repetitive transcranial magnetic stimulation rTMS can produce antidepressant effects by affecting inflammatory cytokines in patients with depression, which plays a key role in the therapeutic mechanism of antidepressants. This study aimed to explore the changes in inflammatory cytokine levels in patients with depression after 4 weeks of rTMS treatment to determine the possible antidepressant mechanism of rTMS. This prospective, double-blind, pseudo-stimulus-controlled study was conducted, and a total of 57 patients with depression and 30 healthy controls were recruited. Patients were randomly divided into the active rTMS (n = 29) and sham rTMS groups (n = 28). The Hamilton Depression Scale was used to evaluate depressive symptoms and their severity. The serum levels of seven inflammatory cytokines were measured using enzyme-linked immunosorbent assay. Inflammatory cytokines include high-sensitivity C-reactive protein (CRP-hc); tumor necrosis factor (TNF-α); interferon (IFN-γ); interleukin-2 (IL-2); interleukin-4 (IL-4); interleukin-6 (IL-6); and interleukin-8 (IL-8). At baseline, TNF-α (F = 36.699, p < 0.001), IFN-γ (F = 8.907, p < 0.001), IL-4 (F = 66.256, p < 0.001), and IL-2 (F = 9.162, p < 0.001) levels in the depression group were significantly different from those of healthy controls. In the self-control analysis of the active rTMS group, the levels of IL-2 and CRP-hc increased significantly after 2 and 12 weeks of treatment. In the sham-rTMS group, IFN-γ increased after 2 and 12 weeks of treatment. Our results revealed that the changes in inflammatory cytokines after rTMS treatment showed different patterns compared to the sham group, suggesting that the antidepressant effect of rTMS may be related to changes in inflammatory cytokines.
Over the past two decades, there has been increasing evidence that depression is associated with a dysregulated immune system, including abnormalities in inflammatory markers (1). Compared with the healthy control (HC) group, the levels of pro-inflammatory cytokines and proteins in patients with acute phase depression were increased, and the results of the increase in interleukin (IL)-6, tumor necrosis factor (TNF)-α, and C-reactive protein (CRP) levels in the blood of patients with depression were relatively consistent (2). A recent meta-analysis of 82 studies, including 3,212 patients with depression and 2,798 HCs, showed that patients with depression had increased peripheral levels of IL-6, TNF-α, IL-10, soluble IL-2 receptor, chemokine ligand 2, IL-13, IL-18, IL-12, IL-1 receptor antagonist, and soluble TNF receptor 2, whereas interferon-gamma (IFN-γ) levels were decreased (3). In general, there is great heterogeneity in the data, which depends on the cytokine composition. This is at least in part due to the failure to consider the clinical course and course of disease, as well as the influence of potential confounding factors, such as comorbidity, drugs, fasting, smoking, and test methods (4–6).
Transcranial Magnetic Stimulation (TMS) is a non-invasive physical regulation method with antidepressant effects (7–10). It has been proven to have clinical application value for patients with depression (9, 11–13), including adolescents (14), the elderly (15, 16), patients with suicidal ideation (17) and patients with anxiety symptoms (18). These pulses can be transmitted at a high frequency (10–20 Hz) or low frequency (less than or equal to 1 Hz). Most clinical TMS treatments used to treat depression are usually performed at frequencies of 10–18 Hz (19, 20). In the early stages, there have been three large, multicenter, randomized pseudo-stimulus-controlled trials, including a total sample of 703 adult patients with major depression. One to four antidepressant treatments were ineffective in the enrolled patients. Two of these studies were industry-sponsored registered trials that led to Food and Drug Administration (FDA) approval of TMS treatment for depression in 2008 (10, 21). The evidence from all three trials was consistent, and TMS had statistically significant and clinically relevant benefits compared with pseudo-stimulation.
Few clinical studies have investigated the effect of rTMS on inflammatory cytokine levels in patients with depression. Langguth et al. (22) reported that peripheral levels of CRP and IL-6 increased after receiving 20-Hz rTMS in an elderly female patient with depression and rheumatoid arthritis, indicating that rTMS enhanced the patient’s peripheral inflammatory response. Zhao et al. (23) recruited 58 elderly patients with depression and 30 HCs. The peripheral levels of IL-1β and TNF were higher in the patient group than in the HC group at baseline. Compared with the non-rTMS treatment group, patients treated with 10-Hz rTMS for 4 weeks had IL-1β, and TNF in the peripheral blood levels decreased significantly.
However, the dynamic changes of inflammatory cytokine in depression before and after the TMS treatment are still largely unknown. The goal of this study was to explore the changes in inflammatory cytokine levels in drug-free patients with depression at recruitment and treated with antidepressants and 4 weeks of active/sham-rTMS treatment and to profile the inflammatory cytokine levels before (baseline) and after (week 2 and week 12) active/sham-rTMS treatment in patients with depression.
The study participants were drawn from a consecutive clinical sample of adult patients, aged 18–80 years, who presented at the Shanghai Pudong New Area Mental Health Center, Tongji University School of Medicine. The hospital’s Ethical Committee approved the study and it was performed according to the Declaration of Helsinki. All participants provided written informed consent during the recruitment stage of the study. Patients with depression were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5). The inclusion criteria were as follows: (1) age 18–80 years, (2) experienced an acute exacerbation of the symptoms of depression or had a baseline score of at least 14 points on the 24-item Hamilton Rating Scale for Depression (HAMD), (3) satisfied the DSM-5 criteria for depression based on the Structured Clinical Interview for DSM Disorders/Patient Edition, (4) having only one type of antidepressant during rTMS treatment and willing and capable of completing at least 20 sessions. None of the patients had a history of neurosurgery, seizures, head trauma, substance abuse or dependence, psychiatric or neurological disorders other than depression. Patients were excluded from the study if they have current inflammatory disorders, had received past rTMS treatment or other electromagnetic stimulations such as electroconvulsive therapy, ferromagnetic metallic implants, pacemakers.
We recruited 70 patients who were randomly and separately assigned to active (n = 36) or sham rTMS (n = 34) conditions. However, 13 patients (seven in the active-rTMS group and six in the sham-rTMS group) could not complete the study and withdrew their consent; thus, 57 patients (29 patients in the active-rTMS group and 28 patients in the sham-rTMS group) were finally included in the subsequent analysis. Independent, third-party assorted participants were enrolled into either the active or sham rTMS groups through computer-generated randomization numbers compiled through simple randomization. The clinical staff and patients were blinded to the assignment, except for one clinical technician who provided active or sham rTMS treatment according to the randomization numbers.
The primary outcome measure for the study was the score on the 24-item version of the HAMD (24). Seven factor scores including anxiety/somatization (items 10–13, 15, and 17), weight loss (item 16), cognitive disturbance (items 2, 3, 9, and 19–21), diurnal variation (item 18), retardation (items 1, 7, 8, and 14), sleep disturbance (items 4–6), and hopelessness (items 22–24) were calculated (25). Clinical assessment were administered at baseline (before rTMS treatment), mid-treatment (2 weeks), and 8 weeks after the last TMS session (12 weeks).
Patients were instructed to sit on a comfortable chair in a quiet room, and rTMS was delivered using a MagPro ×100 magnetic stimulator (Medtronic Co., Denmark) equipped with a butterfly coil. In the rTMS group, the stimulation was targeted to the position of the left unilateral dorsolateral prefrontal cortex, stimulated at a frequency of 10 Hz, power (intensity) level of 120% of the motor threshold, 80 trains, 30 pulses per train, 12-s intertrain-interval, 2,400 pulses per session, and five sessions per week. The same protocol was followed for the sham rTMS group using a fake coil that was unable to produce a magnetic field. The patients were blinded to the treatment received.
Venous blood samples were collected in anticoagulant-free tubes between 7 a.m. and 9 a.m. after an overnight fasting. The samples were kept at room temperature for 1 h, followed by centrifugation (3,000 g for 20 min at 4°C) for serum separation. Subsequently, the serum was separated and stored at –80°C until assayed. Serum levels of TNF-α, IFN-γ-, IL-4, IL-6, IL-8, IL-2, and CRP-hc for each sample were measured in duplicate using an enzyme-linked immunosorbent assay (ELISA) with a commercial human ELISA kit (Multisciences [Lianke] Biotech, Co., Ltd.), according to the manufacturer’s instructions. The concentrations of TNF-α, IFN-γ, IL-4, IL-6, IL-8, IL-2, and CRP-hc were expressed as picograms of protein per mL of serum. The intra- and inter-assay coefficients of variation were less than 9%.
The sociodemographic and clinical characteristics of our participants are presented as descriptive statistics, such as percentages and mean scores (standard deviation, SD) (Table 1). Analyses of variance (ANOVA) and χ2 tests were used to investigate differences in demographics, clinical variables, and antidepressant medications among the HC, active-rTMS, and sham-rTMS groups. The total and subscale HAMD scores before and after rTMS treatment were displayed and compared using an independent t-test (Table 2). Spearman correlation analysis was used to test the correlations between decreased HAMD scores and decreased cytokine levels. A box-plot diagram was created using GraphPad Prism software to analyze the differences in serum levels of TNF-α, IFN-γ, IL-4, IL-6, IL-8, IL-2, and CRP-hc at baseline in the active/sham-rTMS groups (Figure 1). The paired t-test was used for the self-controlled analysis of serum levels of inflammatory cytokines before and after treatment in the patient groups (Tables 3, 4).
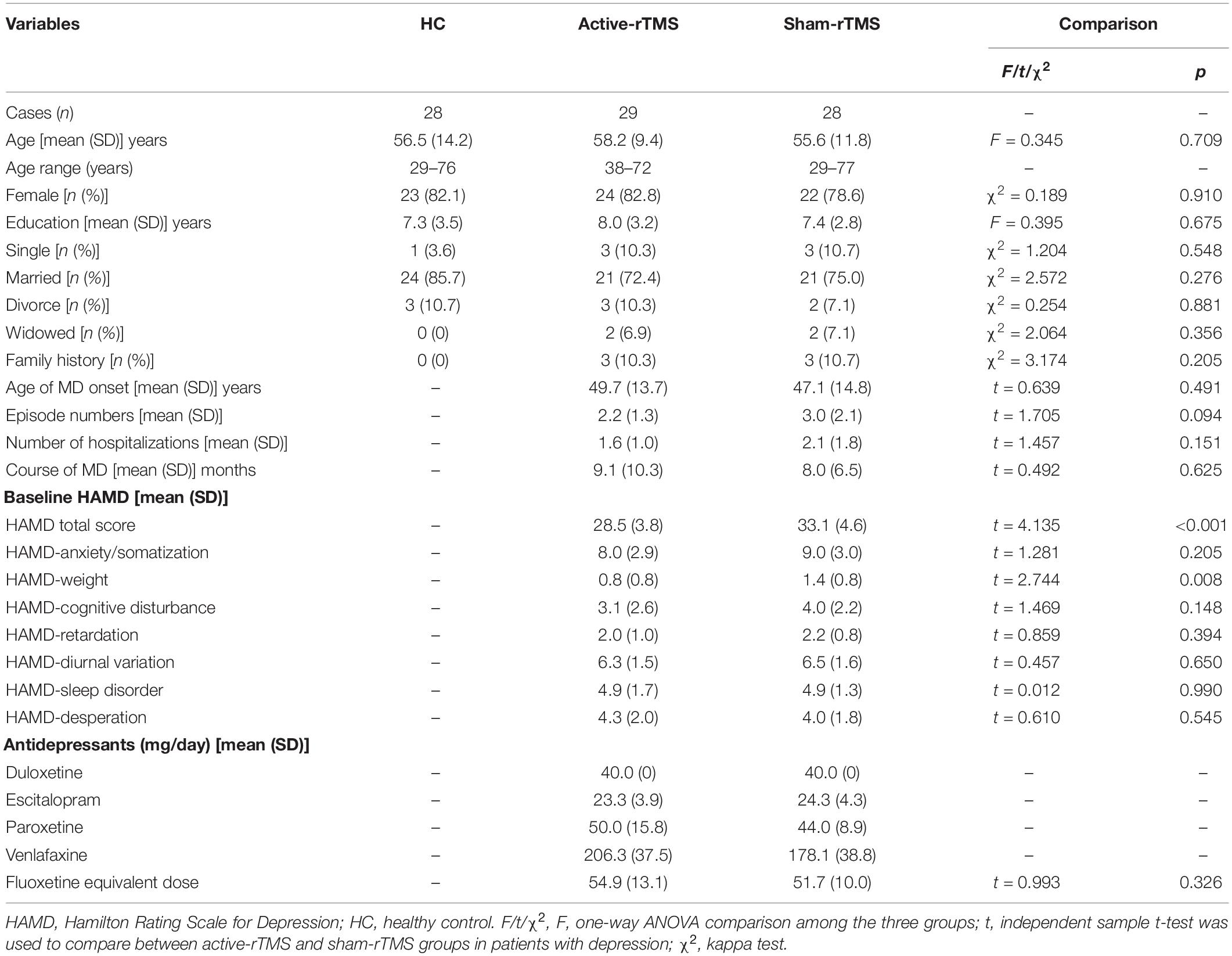
Table 1. Demographic and clinical variables, comparison among healthy controls (HC), active-rTMS, and sham-rTMS groups.
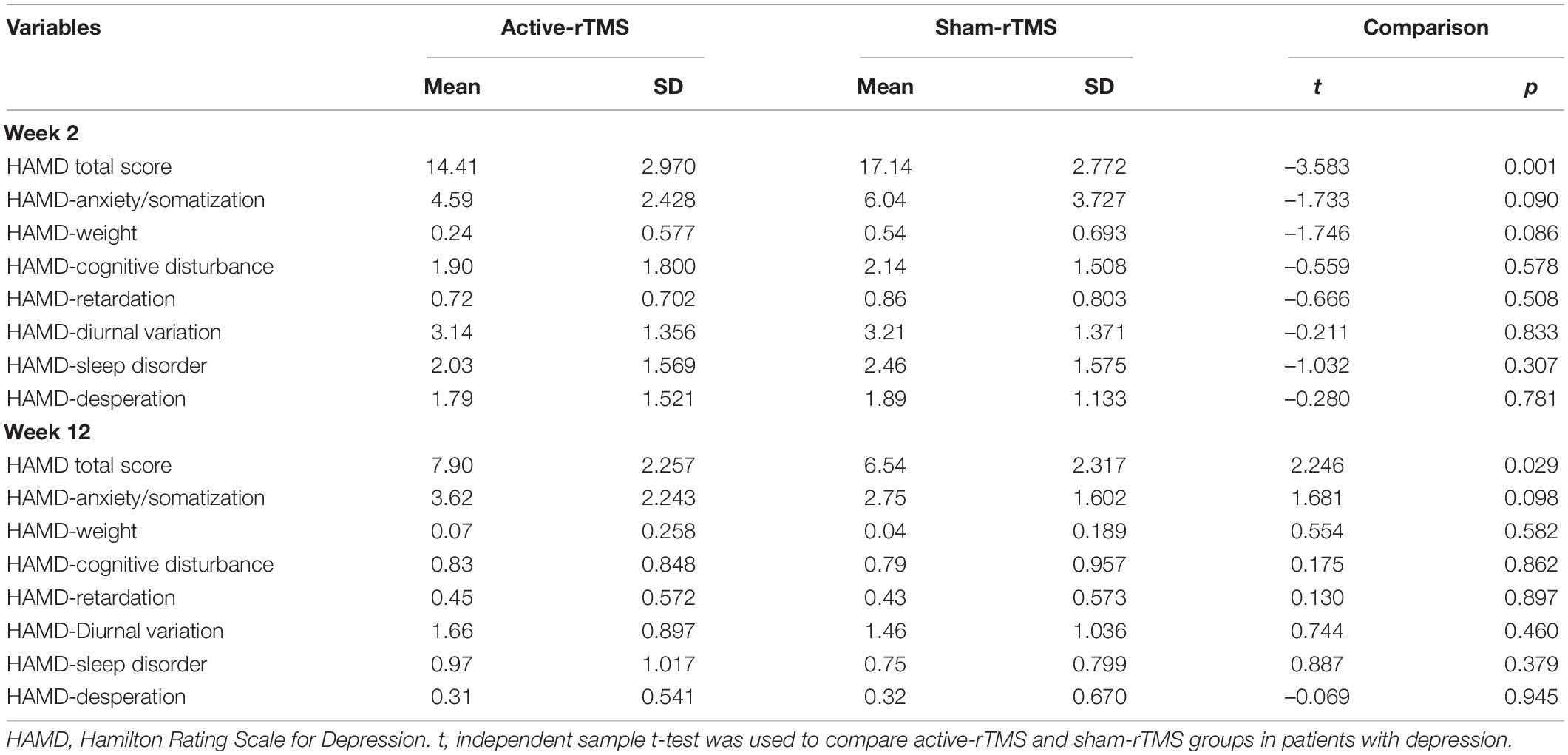
Table 2. Hamilton Rating Scale for depression (HAMD) scores at 2 and 12 weeks in the active-rTMS and sham-rTMS groups in patients with depression.
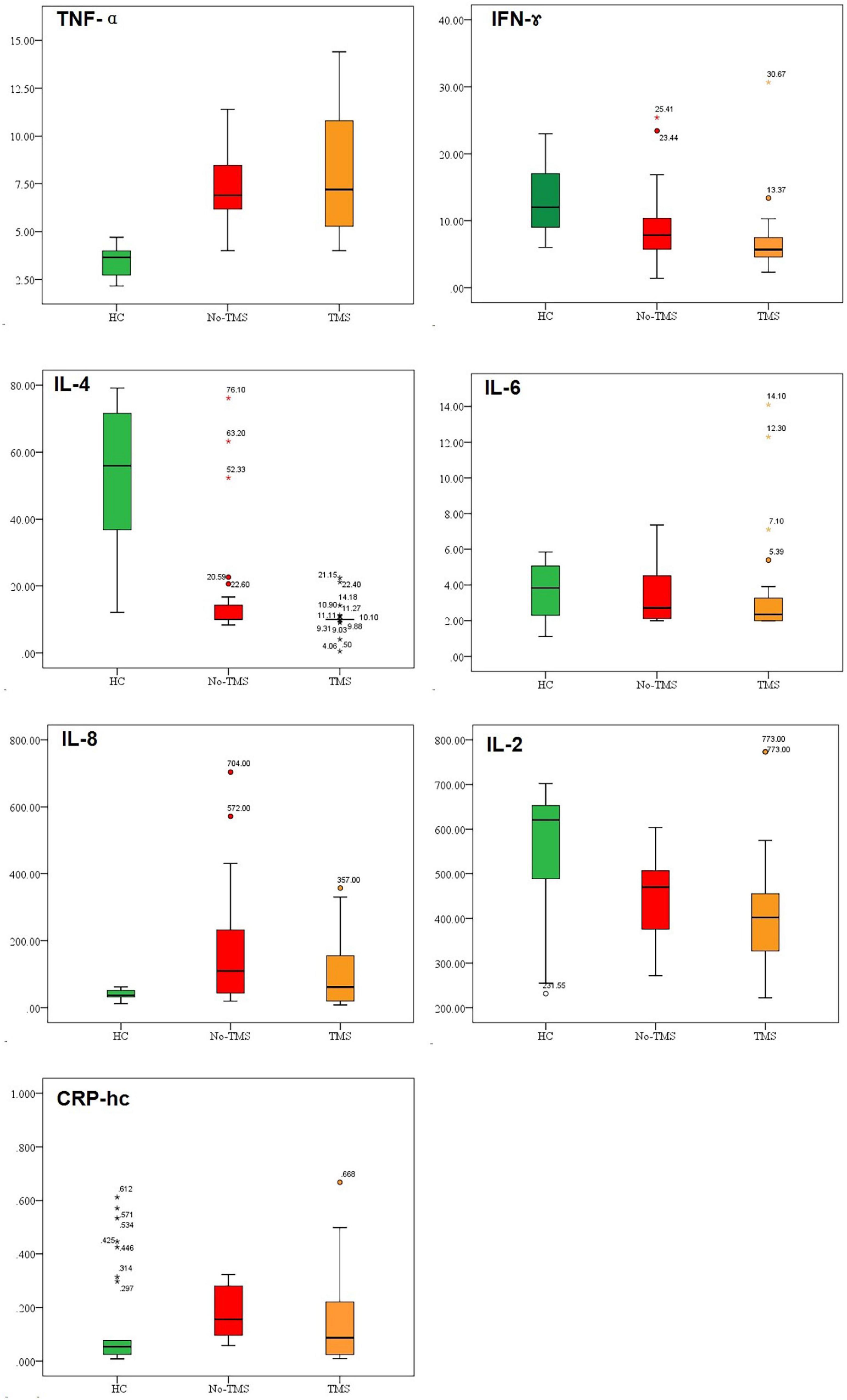
Figure 1. Baseline inflammatory cytokine levels in healthy controls (HC), active-rTMS (TMS), and sham-rTMS (no-TMS) groups in patients with depression.
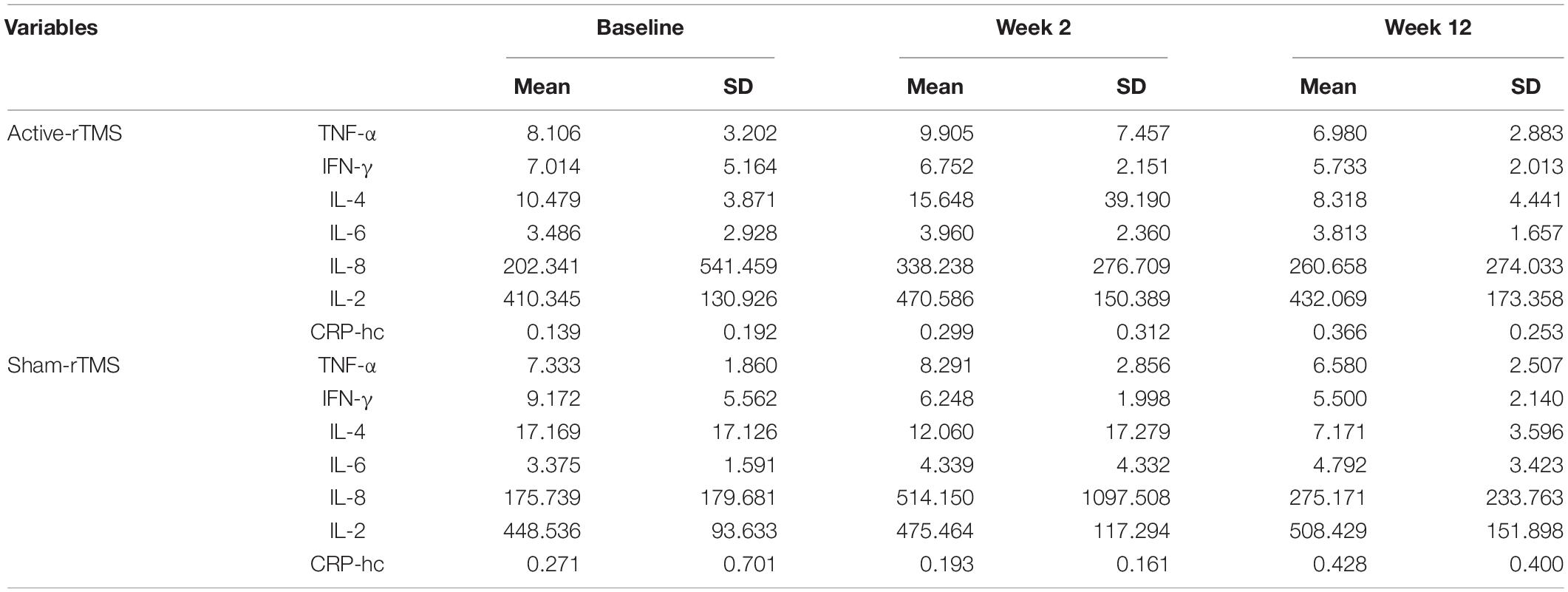
Table 3. The levels of inflammatory cytokines at baseline, 2 and 12 weeks in the active-rTMS and sham-rTMS groups in patients with depression.
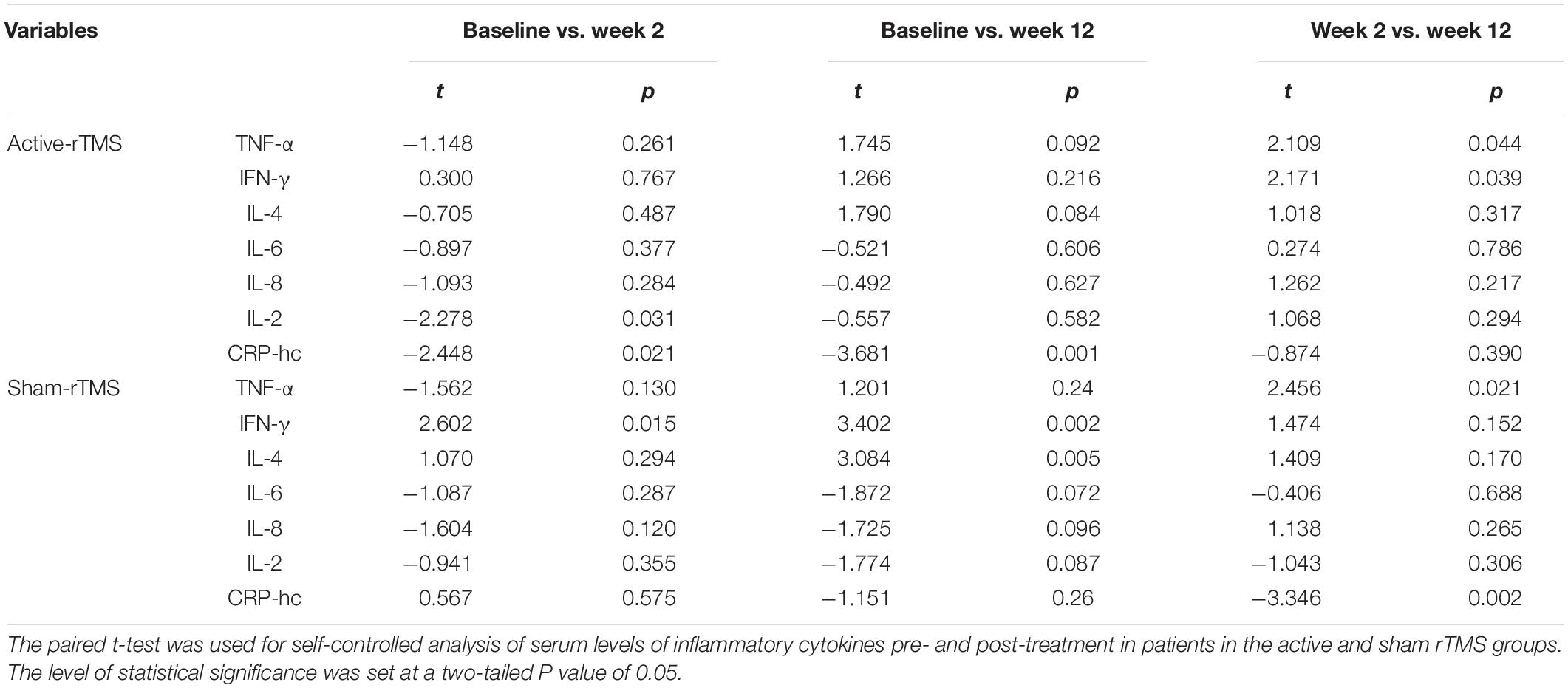
Table 4. Self-controlled comparisons for serum levels of inflammatory cytokines before and after treatment in patients with depression.
As shown in Table 1, there were no significant differences in baseline age, years of education, marital status, or family history among the active-rTMS, sham-rTMS, and HC groups. Compared with the sham rTMS group, the HAMD total score in the active-rTMS group was significantly lower than that in the pseudo-stimulation group. The main difference in each HAMD factor score is the body weight factor. There was no significant difference in the antidepressant dose between the active-rTMS and sham-rTMS groups. Table 1 lists the demographic and clinical characteristics and antidepressant exposure of the three groups.
As shown in Table 2, the HAMD total score at 2 weeks between the active-rTMS and sham-rTMS groups was statistically different. The total score on the 2-week HAMD in the active-rTMS group was significantly lower than that in the sham-rTMS group. At 12 weeks, the total HAMD score in the active rTMS group was significantly higher than that in the sham rTMS group.
As shown in Figure 1, the serum levels of TNF-α, IFN-γ, IL-4, and IL-2 at baseline in the HC, active rTMS, and sham rTMS groups were significantly different. The difference mainly lies in the fact that the baseline TNF-α level was significantly lower in the HC group than in the patients with depression in either the active-rTMS or sham-rTMS groups. The level of IFN-γ, IL-4, and IL-2 were significantly higher in HC group than that of the depression group.
The levels of several inflammatory cytokines in the active-rTMS and sham-rTMS groups changed significantly after 2 and 12 weeks of treatment (Table 3). In the self-control analysis of active-rTMS group, the levels of IL-2 and CRP-hc changed significantly after 2 weeks of treatment, the level of CRP-hc changed significantly after 12 weeks of treatment, and the level of TNF-α and IFN-γ changed significantly between week 2 and week 12. In the self-control analysis of sham-rTMS group, IFN-γ changed significantly after 12 weeks of treatment. The level of IFN-γ and IL-4 changed significantly, and the level of TNF-α and CRP-hc changed significantly between week 2 and week 12 (Table 4).
After the active or sham TMS treatment, the decrease in depressive symptoms of the patients (n = 57) were negatively correlated with the decreased levels of TNF- α after 2 weeks of treatment. While the decrease in depressive symptoms were negatively correlated with the decreased levels of IL-2 at 12 weeks. The changes of all the other inflammatory cytosines were not significantly correlated with the decreased depressive symptoms in the patients (Table 5).
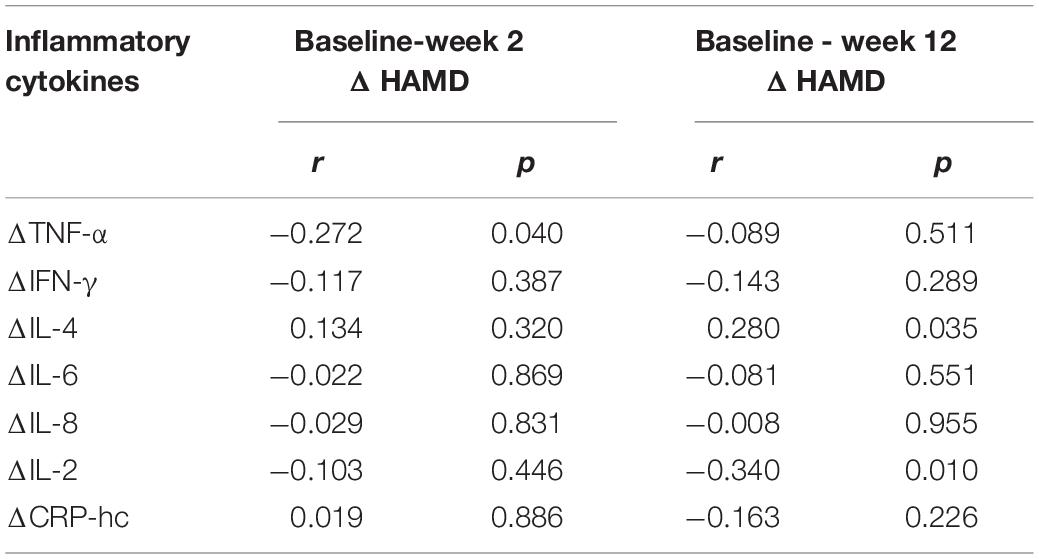
Table 5. Correlations between decreased HAMD score and decreased serum levels of inflammatory cytokines before and after TMS treatment in patients with depression.
In this study, we hypothesized that, in association with depressive symptom improvements, serum inflammatory cytokine levels in patients with depression would be changed after active rTMS treatment compared with the sham rTMS group. The main findings supported the assumption that the change patterns of inflammatory cytokine levels highly varied after rTMS treatment. In the active rTMS group, the serum levels of CRP-hc and IL-2 significantly changed at week 2, and the change last through the 12 weeks period, which were not seen in the sham-rTMS group, suggesting that rTMS can change serum inflammatory cytokine levels in patients with depression. Furthermore, the improvement of depressive symptoms in the patients were negatively correlated with the decreased levels of TNF-α during the TMS treatment at 2 weeks and negatively correlated with decreased IL-2 levels at 12 weeks after the treatment. Besides, the baseline TNF-α level was significantly higher and IFN-γ, IL-4, and IL-2 were lower in patients with depression compared with the HC group. After the to the best of our knowledge, this is the first study to examine dynamic changes in the levels of inflammatory cytokines following active rTMS and sham-rTMS in patients with depression.
This study found that the concentration of TNF-α in the depression group was significantly higher than that in the HC group, but there was no significant change after 2 and 12 weeks of antidepressant plus active or sham rTMS treatment. However, serum TNF-α levels in the active TMS group was significantly decreased from weeks 2 to weeks 12, indicating a dynamic effect of TMS on TNF-α levels at different treatment period. Interestingly, the early improvement of depressive symptoms among the patients was negatively correlated with the decrease of TNF-α levels, suggesting that early stage TMS treatment may have adverse effects of increasing TNF-α levels. At present, it is generally believed that TNF-α is an important pro-inflammatory cytokine, which has attracted much attention because it can cause inflammatory and apoptotic cell death and mediate the release of a variety of cytokines (26). Two mechanisms may link TNF-α to the pathophysiology of depression. First, TNF-α may regulate the activity of neuronal serotonin transporters and stimulate serotonin uptake (27). Second, TNF-α activates tryptophan- and serotonin-degrading enzyme indoleamine-2,3-dioxygenase, resulting in reduced availability of serotonin in patients with depression (28). From the perspective of the mechanism, it has been assumed that the increased production of TNF-α may play a pathogenic role in depression, and the TNF-α levels of patients with depression vary greatly in many clinical studies. TNF-α levels have been reported to be elevated in patients with depression (29–31), unchanged (32), and decreased (33). One possible explanation for this inconsistency is that different TNF-α expression patterns may occur in different clinical subtypes of depressive episodes (34, 35).
We found significant increases in CRP-hc levels after 2 weeks of TMS treatment and last through the whole 12 weeks of follow-up in the active-rTMS groups. CRP is a non-specific acute-phase protein, and its level increases in response to an inflammatory response. A meta-analysis of cross-sectional studies confirmed that the average concentrations of CRP in patients with depressive episodes were higher than those in the control group (36). Cheng et al. reported that in patients treated with antidepressants, baseline CRP levels were significantly correlated with treatment response at 2 weeks. Higher CRP levels were associated with poorer treatment outcomes. After 6 weeks, CRP levels in both treatment groups increased significantly (37). It is speculated that CRP may serve as a useful marker of inflammation in the antidepressant treatment of depression (38).
In this study, IFN-γ decreased after 2 and 12 weeks of antidepressant treatment with sham-rTMS and decreased from 2 to 12 weeks in the active-rTMS group. Chen et al. (39) found that the baseline IFN-γ levels in patients with depression were significantly higher than those in the control group. In the antidepressant treatment group, a decrease in IFN-γ levels was observed after 8 weeks of treatment. The decrease in IFN-γ level was not statistically significant in the remission group, while the decrease in IFN-γ levels was statistically significant in the non-remission group. Dahl et al. (40) found that, compared with HC, the IFN-γ level of untreated patients with depression was significantly higher. In the group of patients who achieved remission, IFN-γ levels decreased significantly compared to baseline levels. These results suggest that IFN-γ may be involved in the pathogenesis of depression and in the therapeutic mechanism of antidepressant treatment.
This study found that rTMS had an effect on various inflammatory factors during antidepressant treatment, which was significantly different from that in the sham-rTMS group. An interesting study by Zhao et al. (23) reported the effects of rTMS on IL-1β and TNF-α levels in elderly patients with refractory depression. In their study, serum levels of IL-1β and TNF-α were measured before the study, 48 h, and 1, 2, 3, and 4 weeks after the first TMS treatment. The levels of IL-1β and TNF-α gradually decreased and were significantly lower than those in the control group. Serum factors of healthy individuals were not affected by rTMS. The levels of IL-1β and TNF-α were positively correlated with the HAMD score. These results suggested that rTMS may reduce serum IL-1β and TNF-α levels during antidepressant treatment. Interestingly, our results showed that IL-2 levels in the depression patients was significantly decreased at the baseline and increased after 2 weeks of active TMS treatment. Furthermore, the long-term improvement of depressive symptoms in the patients was significantly correlated with the change of IL-2 levels, in supporting of the pathological role of IL-2 in the development and TMS treatment of depression.
The present study had a few limitations. First, the sample size of the diagnostic subgroup was small. The number of patients in each group was smaller after stratification according to active rTMS and sham rTMS. Another limitation is the uncertainty over how accurately serum inflammatory cytokine levels reflect levels in the brain. Third, due to ethical reasons, all the depressive patients in this study were administered by antidepressants besides the TMS or sham-TMS treatments, so the effects of antidepressants on serum cytosines could not be excluded. The current study relied only on the assessment of depressive symptoms, which is inadequate.
Despite these limitations, we performed a careful evaluation of depressive symptoms, as well as dynamic measuring of the serum inflammatory cytokines throughout the active/sham-rTMS treatment period, thus obtaining reliable results. This study preliminarily explored the characteristics of inflammatory cytokines in patients with depression and assessed the dynamic changes of these cytokines 2 and 12 weeks after active/sham-rTMS treatment. Our results revealed that there were significant changes in several inflammatory cytokines in patients with depression compared with HC, and the changes in inflammatory cytokines in the rTMS treatment group followed different patterns compared with the sham control group, suggesting that the antidepressant effect of rTMS is related to the changes in inflammatory cytokines.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical Committee of Shanghai Pudong New Area Mental Health Center. The patients/participants provided their written informed consent to participate in this study.
QW designed the study and drafted the manuscript. LZ conceived the analysis. WH, ML, NZ, and XH performed the analysis and interpretation of the results. MS, JQ, YS, and XT collected the data. HM and WL supervised the experiment and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81860253); Shanghai Pudong New Area Health Committee Disciplinary Leader Training Program (Grant No. PWRd2021-06); Pudong New Area of Science and Technology Development Fund (Grant No. PKJ2019-Y25); Shenzhen Science and Technology R&D Fund Project (Grant No. JCYJ20180306171033310); and Medical Discipline Construction Project of Pudong Health Committee of Shanghai (Grant No. PWYgy2021-02).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gibney SM, Drexhage HA. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J Neuroimmune Pharmacol. (2013) 8:900–20. doi: 10.1007/s11481-013-9462-8
2. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. (2009) 65:732–41. doi: 10.1016/j.biopsych.2008.11.029
3. Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand. (2017) 135:373–87. doi: 10.1111/acps.12698
4. Liu JJ, Wei YB, Strawbridge R, Bao Y, Chang S, Shi L, et al. Peripheral cytokine levels and response to antidepressant treatment in depression: a systematic review and meta-analysis. Mol Psychiatry. (2020) 25:339–50. doi: 10.1038/s41380-019-0474-5
5. Kohler CA, Freitas TH, Stubbs B, Maes M, Solmi M, Veronese N, et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol Neurobiol. (2018) 55:4195–206. doi: 10.1007/s12035-017-0632-1
6. Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. (2012) 139:230–9. doi: 10.1016/j.jad.2011.08.003
7. Burt T, Lisanby SH, Sackeim HA. Neuropsychiatric applications of transcranial magnetic stimulation: a meta analysis. Int J Neuropsychopharmacol. (2002) 5:73–103. doi: 10.1017/S1461145702002791
8. McNamara B, Ray JL, Arthurs OJ, Boniface S. Transcranial magnetic stimulation for depression and other psychiatric disorders. Psychol Med. (2001) 31:1141–6. doi: 10.1017/s0033291701004378
9. Herrmann LL, Ebmeier KP. Factors modifying the efficacy of transcranial magnetic stimulation in the treatment of depression: a review. J Clin Psychiatry. (2006) 67:1870–6. doi: 10.4088/jcp.v67n1206
10. O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. (2007) 62:1208–16.
11. Jorge RE, Moser DJ, Acion L, Robinson RG. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. (2008) 65:268–76.
12. Manes F, Jorge R, Morcuende M, Yamada T, Paradiso S, Robinson RG. A controlled study of repetitive transcranial magnetic stimulation as a treatment of depression in the elderly. Int Psychogeriatr. (2001) 13:225–31.
13. Mosimann UP, Schmitt W, Greenberg BD, Kosel M, Muri RM, Berkhoff M, et al. Repetitive transcranial magnetic stimulation: a putative add-on treatment for major depression in elderly patients. Psychiatry Res. (2004) 126:123–33. doi: 10.1016/j.psychres.2003.10.006
14. Zhang T, Zhu J, Xu L, Tang X, Cui H, Wei Y, et al. Add-on rTMS for the acute treatment of depressive symptoms is probably more effective in adolescents than in adults: evidence from real-world clinical practice. Brain Stimul. (2019) 12:103–9. doi: 10.1016/j.brs.2018.09.007
15. Zhang T, Sun W, Zhu J, Tang Y, Hui L, Zhou L, et al. Effect of adjunct repetitive transcranial magnetic stimulation in elderly patients with acute depressive episode: supporting evidence from a real-world observation. Am J Geriatr Psychiatry. (2019) 27:91–2. doi: 10.1016/j.jagp.2018.10.010
16. Qiao Y, Wang J, Zhu J, Cui H, Tang Y, Xu L, et al. Antidepressant effect of adjunct repetitive transcranial magnetic stimulation in inpatients 60 years and older. J ECT. (2020) 36:216–21. doi: 10.1097/YCT.0000000000000648
17. Zhang T, Zhu J, Wang J, Tang Y, Xu L, Tang X, et al. An open-label trial of adjuvant high-frequency left prefrontal repetitive transcranial magnetic stimulation for treating suicidal ideation in adolescents and adults with depression. J ECT. (2021) 37:140–6. doi: 10.1097/YCT.0000000000000739
18. Zhang L, Zhu J, Zhang T, Jia Q, Hui L, Zhu H, et al. Comparative efficacy of add-on rTMS in treating the somatic and psychic anxiety symptoms of depression comorbid with anxiety in adolescents, adults, and elderly patients-A real-world clinical application. J Affect Disord. (2020) 276:305–11. doi: 10.1016/j.jad.2020.05.151
19. George MS, Post RM. Daily left prefrontal repetitive transcranial magnetic stimulation for acute treatment of medication-resistant depression. Am J Psychiatry. (2011) 168:356–64. doi: 10.1176/appi.ajp.2010.10060864
20. George MS, Taylor JJ, Short EB. The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry. (2013) 26:13–8. doi: 10.1097/YCO.0b013e32835ab46d
21. Levkovitz Y, Isserles M, Padberg F, Lisanby SH, Bystritsky A, Xia G, et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry. (2015) 14:64–73. doi: 10.1002/wps.20199
22. Langguth B, Braun S, Aigner JM, Landgrebe M, Weinerth J, Hajak G, et al. Repetitive transcranial magnetic stimulation in a patient suffering from depression and rheumatoid arthritis: evidence for immunomodulatory effects. Neuro Endocrinol Lett. (2005) 26:314–6.
23. Zhao X, Li Y, Tian Q, Zhu B, Zhao Z. Repetitive transcranial magnetic stimulation increases serum brain-derived neurotrophic factor and decreases interleukin-1beta and tumor necrosis factor-alpha in elderly patients with refractory depression. J Int Med Res. (2019) 47:1848–55. doi: 10.1177/0300060518817417
25. Nicolini H, Bakish D, Duenas H, Spann M, Erickson J, Hallberg C, et al. Improvement of psychic and somatic symptoms in adult patients with generalized anxiety disorder: examination from a duloxetine, venlafaxine extended-release and placebo-controlled trial. Psychol Med. (2009) 39:267–76. doi: 10.1017/S0033291708003401
26. Ma K, Zhang H, Baloch Z. Pathogenetic and therapeutic applications of tumor necrosis factor-alpha (TNF-alpha) in major depressive disorder: a systematic review. Int J Mol Sci. (2016) 17:733. doi: 10.3390/ijms17050733
27. Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. (2006) 31:2121–31. doi: 10.1038/sj.npp.1301029
28. Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int J Neuropsychopharmacol. (2002) 5:375–88. doi: 10.1017/S1461145702003103
29. Himmerich H, Fulda S, Linseisen J, Seiler H, Wolfram G, Himmerich S, et al. Depression, comorbidities and the TNF-alpha system. Eur Psychiatry. (2008) 23:421–9. doi: 10.1016/j.eurpsy.2008.03.013
30. Yang K, Xie G, Zhang Z, Wang C, Li W, Zhou W, et al. Levels of serum interleukin (IL)-6, IL-1beta, tumour necrosis factor-alpha and leptin and their correlation in depression. Aust N Z J Psychiatry. (2007) 41:266–73. doi: 10.1080/00048670601057759
31. Yao L, Pan L, Qian M, Sun W, Gu C, Chen L, et al. Tumor necrosis factor-alpha variations in patients with major depressive disorder before and after antidepressant treatment. Front Psychiatry. (2020) 11:518837. doi: 10.3389/fpsyt.2020.518837
32. Brambilla F, Maggioni M. Blood levels of cytokines in elderly patients with major depressive disorder. Acta Psychiatr Scand. (1998) 97:309–13. doi: 10.1111/j.1600-0447.1998.tb10005.x
33. Brambilla F, Monteleone P, Maj M. Interleukin-1beta and tumor necrosis factor-alpha in children with major depressive disorder or dysthymia. J Affect Disord. (2004) 78:273–7. doi: 10.1016/S0165-0327(02)00315-4
34. Zhang T, Tang X, Li H, Woodberry KA, Kline ER, Xu L, et al. Clinical subtypes that predict conversion to psychosis: a canonical correlation analysis study from the ShangHai at risk for Psychosis program. Aust N Z J Psychiatry. (2019) 54:482–95. doi: 10.1177/0004867419872248
35. Zhang TH, Tang XC, Xu LH, Wei YY, Hu YG, Cui HR, et al. Imbalance model of heart rate variability and pulse wave velocity in psychotic and nonpsychotic disorders. Schizophr Bull. (2022) 48:154–65. doi: 10.1093/schbul/sbab080
36. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. (2009) 71:171–86. doi: 10.1097/PSY.0b013e3181907c1b
37. Chang HH, Lee IH, Gean PW, Lee SY, Chi MH, Yang YK, et al. Treatment response and cognitive impairment in major depression: association with C-reactive protein. Brain Behav Immun. (2012) 26:90–5. doi: 10.1016/j.bbi.2011.07.239
38. Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychol Med. (2019) 49:1958–70. doi: 10.1017/S0033291719001454
39. Chen CY, Yeh YW, Kuo SC, Liang CS, Ho PS, Huang CC, et al. Differences in immunomodulatory properties between venlafaxine and paroxetine in patients with major depressive disorder. Psychoneuroendocrinology. (2018) 87:108–18. doi: 10.1016/j.psyneuen.2017.10.009
Keywords: depression, repetitive transcranial magnetic stimulation, antidepressant effect, inflammatory cytokine, TNF-α, CRP-hc, IFN-γ
Citation: Wang Q, Zeng L, Hong W, Luo M, Zhao N, Hu X, Shi M, Qiu J, Shen Y, Teng X, Min H and Liu W (2022) Inflammatory Cytokines Changed in Patients With Depression Before and After Repetitive Transcranial Magnetic Stimulation Treatment. Front. Psychiatry 13:925007. doi: 10.3389/fpsyt.2022.925007
Received: 21 April 2022; Accepted: 16 May 2022;
Published: 02 June 2022.
Edited by:
Tianhong Zhang, Shanghai Jiao Tong University, ChinaReviewed by:
Maorong Hu, Nanchang University, ChinaCopyright © 2022 Wang, Zeng, Hong, Luo, Zhao, Hu, Shi, Qiu, Shen, Teng, Min and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiying Min, MTM2MjE2ODY2MzRAMTM5LmNvbQ==; Weiqing Liu, bGl1d3EyMDEwQGhvdG1haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.