
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Psychiatry , 15 June 2022
Sec. Psychopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fpsyt.2022.917361
This article is part of the Research Topic Safety and Side Effects of Psychotropic Medications View all 10 articles
Background: Real world evidence about antipsychotics focuses on rehospitalization. Modeling the time course of pharmacotherapy would show patients' adherence to medications and physicians' adherence to medication guidelines. We aimed to calculate the cumulative time spent in second generation antipsychotics (SGAs), gaps, antipsychotic polypharmacy, and clozapine in discharged schizophrenia patients.
Methods: Hospitalization and pharmacy dispensing data from 2008–2018 in Manitoba, Saskatchewan, and British Columbia were linked and an electronic cohort (N = 2,997) was created (mean follow-up: 49 months, SD = 38). Cohort members were required to have a minimum of 6 weeks medicated with aripiprazole, olanzapine, paliperidone, quetiapine, risperidone, or ziprasidone.
Results: The multistate model predicted that schizophrenia patients accumulated 44 months in SGA monotherapy, 4 months in polypharmacy, 11 months in medication gaps and 17 days in clozapine over a 5-year period. The majority of transitions were between SGA and medication gap. Accumulated time in medication gaps was seven times as much as in clozapine. Each 10% delay in SGA initiation post-discharge was associated with a 2, 1, and 6% higher risk for polypharmacy (95% CI: 1.01–1.02), gap (95% CI: 1.01–1.01), and clozapine (95% CI: 1.04–1.08), respectively.
Interpretation: Schizophrenia patients accumulated more time unmedicated and in polypharmacy compared to clozapine. Either treatment guidelines for schizophrenia are not followed, or real-world challenges hamper their implementation.
Pharmacotherapy remains the mainstay of treatment for schizophrenia (1). Yet with few specific treatment guidelines, prescribers necessarily rely upon clinical acumen in collaboration with patients. High-quality evidence for first-episode psychosis is even scarcer, as ethical considerations discourage placebo-controlled trials (1).
An exception is clozapine for treatment-resistant schizophrenia, which continues to be strongly supported (1, 2). There is also evidence to suggest clozapine for patients with prominent negative symptoms (3, 4), aggression (5), suicidality (6), and comorbid substance use (7). However, clozapine is prescribed less frequently than would be expected by its indications (2, 8, 9), suggesting either an implementation failure or treatment guidelines that overestimate real-world effectiveness. Real world studies on clozapine tend to focus on more severely ill patients, so it is hard to estimate at what point it is introduced in treatment (10).
According to the Maudsley Treatment Review and Assessment Team (TREAT), various practical considerations contribute to the under-prescribing (or delayed initiation) of clozapine (11). Among these are: identifying treatment refractory patients, ascertaining if previous treatment was adequate, establishing the patient's willingness to engage, and weighing the risks vs. benefits of clozapine for each patient (11). These steps require close coordination among different care providers. Patients on their part may refuse blood tests, and this can delay clozapine initiation (12). There are also barriers on the side of clinicians such as fear of serious side-effects, the burden of constant monitoring, and a self-reported lack of competence (12). Among patients who have agreed to clozapine treatment, several reasons are given for discontinuing: intolerable side effects, non-compliance with blood monitoring, and dysfunctional beliefs about clozapine treatment (13).
Advanced statistical techniques such as network meta-analysis have recently been used to rank large numbers of antipsychotics in terms of efficacy and tolerability/safety with data from clinical trials (3, 14). It remains less clear if these rankings inform clinical practice. The extensive selection criteria found in most trials is a constraint to the generalizability of the findings, thereby making it necessary to examine real-world data (15).
Here, we analyzed prescription and hospitalization data for patients with schizophrenia from three Canadian provinces, with the following objectives:
1. Estimate the dispensing frequency of several second-generation antipsychotics (SGA).
2. Estimate the prevalence of (i) initial SGA, (ii) polypharmacy, (iii) unmedicated treatment gaps, (iv) clozapine, (v) rehospitalization, as well as the cumulative time spent and total visits (or returns) to each state.
3. Calculate the probability of transitions between states and how these are affected by socio-demographic characteristics.
One-on-one interviews with six persons with schizophrenia and a mental health nurse were held to elicit their lived experience and inform our research questions. After obtaining the results, one patient provided feedback. Recruiting patients, a vulnerable group, was approved by the university ethics board and all participants gave their informed consent and received a small honorarium. Our research was conducted in accordance with the Helsinki declaration of respect for patients.
This was a retrospective study of administrative data about hospitalizations and pharmacy dispensing data in British Columbia, Manitoba, and Saskatchewan from 2008–2018. Hospitalization data consisted of emergency room visits captured in the National Ambulatory Care Reporting System (NACRS) and inpatient stays in the Discharge Abstract Database (DAD). Psychotropic medications were obtained from the National Prescription Drug Utilization Information System Database (NPDUIS)—a Canada-wide register of publicly funded medications dispensed by pharmacies in the community. Despite this national scope, the provincial (territorial) programs only cover low income people and seniors, except for the provinces in this study. Hospitalization and medication files were provided separately by the Canadian Institute of Health Information (CIHI). We linked these files using a unique person identifier provided by CIHI. Our data captured all mental-health related visits and >85% of all psychotropic medications. The exceptions were medications used in hospital and those covered by federal programs. Also, patients emigrating from the three Canadian provinces may have had their records truncated.
Patients in an electronic register differ importantly from those enrolled in randomized clinical trials. Trial participants satisfy explicit inclusion and exclusion criteria, thereby ensuring that they are a homogenous group. In contrast, patients in our registries only had the common characteristic of visiting the hospital for a mental condition and obtaining medications from a pharmacy. As such, our first task was to identify a more-or-less homogenous cohort in diagnosis and severity of illness. Accordingly, we formed a cohort of patients using their first hospital visit as an index date and applied the following criteria:
1. Schizophrenia diagnosis (ICD-10 codes: F20.1–F20.9).
2. Adult onset (i.e., aged 19+ at index hospital visit, allowing for the possibility that the true illness onset may have been 18 or younger).
3. Medicated for at least 6 weeks with one of the following SGAs after the hospital visit: aripiprazole, olanzapine, paliperidone, quetiapine, risperidone, and ziprasidone.
We further applied the following criteria in order to capture patients with less serious presentations (i.e., excluding resistant or seriously ill patients):
4. First hospital visit did not contain an ICD code for intentional self-injury or poisoning (X60–X84).
5. Patient was not already on SGA polypharmacy (i.e., not medication resistant).
6. Patient was not already on clozapine or a less-commonly dispensed SGA.
Applying these criteria to the 7,897 patients with a schizophrenia diagnosis at the index visit yielded a cohort of 2,997 people (Figure 1). The socio-demographic characteristics of these people at their index visit are summarized in Table 1. For each cohort member, SGA refills (and rehospitalization, if applicable) were followed until February 2019 or loss to follow-up, whichever came first. Cohort members had a mean follow-up time of 49 months (SD = 38) and this did not differ by province.
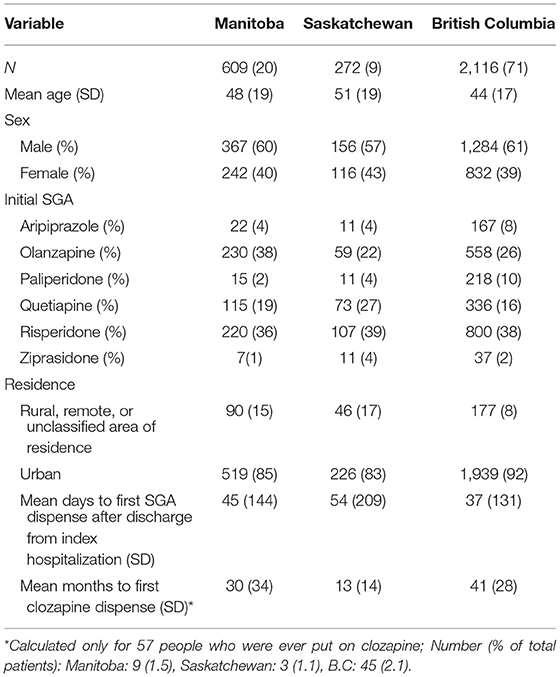
Table 1. Demographic profile of schizophrenia patients at index hospitalization in three Canadian provinces.
Each person in the cohort had a longitudinal record of hospitalizations and medication refills. We sorted these records from earliest to most recent. We were interested in four transient states: (1) initial antipsychotic, (2) polypharmacy, (3) unmedicated (treatment gaps), and (4) clozapine. Each person had to have spent at least 6 weeks on SGA monotherapy. This was based on the guideline that response to treatment (if it happens at all) can typically be observed within the first 6–8 weeks (16). The terminal state was rehospitalization. From SGA monotherapy, each person could progress to any of the transient States (2–4), with repeated visits. Follow-up ended when a person was rehospitalized, the person ran out of data, or the cut-off of CIHI data was reached. In the latter two cases, the person was considered lost to follow-up. The assignment of states, especially gaps and polypharmacy (discussed below), was greatly facilitated by the Stata newspell package (17).
A patient was assigned to the polypharmacy state if two or more psychotropic medications overlapped during follow-up. These psychotropic medications were not limited to the six SGAs in State 1 but included asenapine, chlorpromazine, flupentixol, fluphenazine, haloperidol, levomepromazine, lithium, loxapine, lurasidone, perphenazine, pimozide, pipotiazine, prochlorperazine, sulpiride, trifluoroperazine, and zuclopenthixol. If clozapine overlapped with another medication, this period was assigned to clozapine. Rehospitalization in this study was defined as a hospital visit associated with a mental health condition (ICD F code) or self-harm (ICD X60–X84).
Since all hospitalizations and all psychotropic refills were captured, the follow-up period for each person could be classified into the five states above, subject to the limitation that we did not have quantity and dose at each refill. For this reason, we could not be sure that a person possessed adequate medication during the period between refills, or if there was a gap for which a person was unmedicated. People filled prescriptions at irregular intervals. According to a paper about estimating medication adherence, the grace period between consecutive refills, in various studies, ranges from 15 to 120 days (18). Based on typical prescribing practices, we fixed the grace period at 30 days. Treatment gap was therefore operationally defined as the period starting from day 31 to the date of the subsequent refill (Supplementary Figure S1).
We fitted a multistate survival model (MSM) using the R package msm (19). This was based on several considerations: (1) The msm package is capable of handling continuous (vs. discrete) time transitions. (2) It handles intermittently observed events (i.e., refills). (3) The msm package allows for cyclic transitions. A MSM is comparable to a simple survival model except that the hazard is calculated for transitions between transient states (i.e., SGA, polypharmacy, gap, and clozapine) and from transient states to the absorbing state (rehospitalization). For a schematic of the states and transitions in our model, please refer to Supplementary Figure S2. We had four candidate predictor variables: age at index hospitalization, the interval between hospital discharge and the first pharmacy dispense date, rural/remote/unclassified area of residence vs. urban, and gender.
As Table 2 shows, the proportion of schizophrenia patients who were on an SGA was close to 70% at up to 5 years. However, at any single time except at baseline, 16–20% of patients had a gap in treatment and 5–7% were on polypharmacy. The total proportion of patients who received clozapine (whether by itself or with another antipsychotic) never reached 3% over the entire follow-up (n = 57 people). Close to 5% of remaining cohort members were rehospitalized at 5 years.
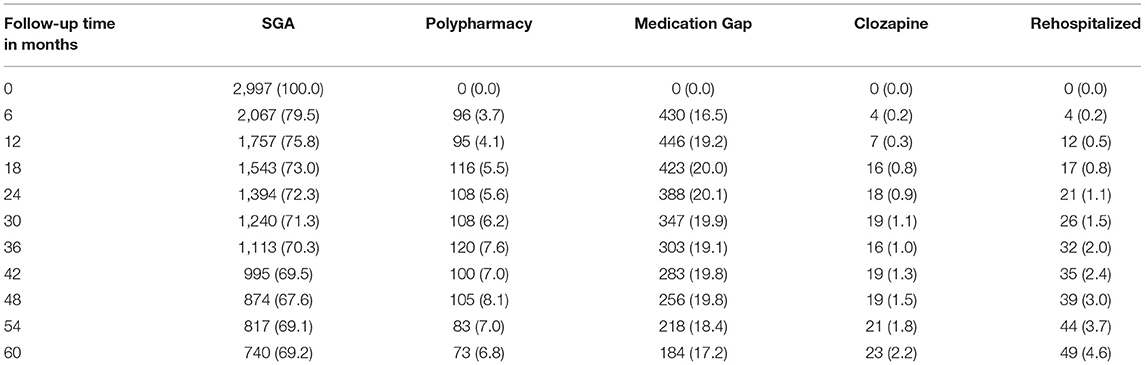
Table 2. Number (%) of patients in a stage by follow-up time (months). The denominator is the total number of patients remaining in the cohort at a given month.
Over a 5-year (60-month) period, the accumulated time spent in each of the states were: 44 months in initial SGA, 4 months in polypharmacy, 11 months in medication gaps, and 17 days in clozapine. On average, patients had 10 spells of SGA monotherapy, 1 spell of polypharmacy, 10 untreated spells and <1 clozapine spell. SGA monotherapy and gaps tend to cycle back-and-forth as shown in Supplementary Figure S3.
At 3 months, there was an 18% percent chance of a gap in treatment given that one was on an SGA. Given that a patient has a medication gap, returning to an SGA was most likely but there was a 22% chance of continuing to be unmedicated. People on clozapine had a 73% chance of remaining on clozapine. The probability of rehospitalization at 3 months was close to zero (Table 3).
The transition probabilities including loss to follow-up for the entire follow-up period is depicted in Supplement Figure S3. Over the entire follow-up period, only 57 patients (1.9%) ever received clozapine and 64 people (2.1%) were rehospitalized.
Here we focus on how socio-demographic characteristics modify the transitions from SGA, since this is the initial state. See Supplementary Table S1 for the complete results. Each 10% increase after age 19 represented a 3, 2, and 7% risk reduction for polypharmacy, gap, and clozapine, respectively. Each 10% delay in first SGA dispense after discharge was associated with a 2, 1, and 6% higher risk for polypharmacy, gap, and clozapine, respectively. Living in a non-urban area was associated with a 33% lower risk of polypharmacy, a 22% higher risk of a medication gap, and 27% lower risk of being treated with clozapine. Female patients, compared to males had a 20% higher risk of transitioning to polypharmacy from an initial SGA. Female patients on polypharmacy were 88% more likely to have a medication gap. None of the variables predicted rehospitalization from any state.
We had two main findings: (i) schizophrenia patients accumulated substantial periods without medication (treatment gaps), and (ii) patients are on polypharmacy 7 times as long as they are on clozapine.
Although we did not have access to the reasons for treatment gaps, we offer two possibilities. First, patients may have recovered from their illness so as not to require medication, but relapsed sometime later. Up to 20% of schizophrenia patients only experience a single episode as noted in the Canadian Schizophrenia Guidelines (1). This is also consistent with unmedicated rates in naturalistic longitudinal studies in the US (20) and Finland (21). In these studies, ongoing medication was associated with disease severity, and most of the unmedicated patients were clinically stable (20, 21). Therefore, some treatment gaps may reflect periods of recovery and prudent prescribing on the part of physicians.
Alternatively, patients may have needed medications but stopped taking them because of tolerability, logistical, or financial reasons. This fits with the 22% higher risk of treatment gaps among non-urban dwellers from an initial SGA and a 77% higher risk of a gap from polypharmacy (Table 4). Adherence to medications is influenced by patient insight into their illness, perceived efficacy of the medication, family support, and the availability of case managers (22, 23). Patients in non-urban areas may have more limited contact with physicians, community health managers, and health services in general.
Regarding affordability, it is estimated that 10% of Canadians cannot afford out-of-pocket medication expenses (24). Up to 80% of people with schizophrenia are unemployed (25), so it is possible that some medication gaps are a result of poverty. Unlike universal healthcare coverage, prescription medications are left for provincial governments to decide (26). According to the schizophrenia patients we interviewed, affordability was not a problem because clozapine was paid for by the Saskatchewan provincial government. However, they were generally unable to join the job market—even if they wanted—because their income would count against what they receive from the government.
Although the prevalence of polypharmacy was lower than other studies (27, 28), patients in this cohort were still 7 times as likely to be on polypharmacy as on clozapine. This likely reflects the extent that clozapine is underutilized. Notably, a Canadian study noted that polypharmacy is more prevalent than any antipsychotic monotherapy (29). In the US, <5% of schizophrenia patients were treated with clozapine in 2008 (30)—still higher than the 2% of patients in this cohort. By comparison, up to 50% of schizophrenia patients are on polypharmacy (31) despite the lack of compelling evidence for its efficacy (32). A high polypharmacy rate therefore may therefore represent a gap between treatment guidelines and implementation.
The single biggest barrier to clozapine utilization is probably administrative burden associated with patient monitoring (33). A mental health nurse we interviewed stated that a brand name manufacturer of clozapine relieves some of the administrative burdens by monitoring the patients' blood test results for signs of neutropenia. Patient refusal to initiate (or continue) clozapine treatment may have also contributed to underutilization (12, 34). The three provinces varied significantly with regard to clozapine initiation, with British Columbia waiting more than 3 years on average. By comparison, a study of outpatient schizophrenia patients in Manitoba reported that clozapine was initiated at 8.9 years for males and 7.7 years for females, on average (35). Two-thirds of them had been on 3 or more antipsychotics before the switch to clozapine (35). Delayed initiation of clozapine treatment—perhaps by lingering in mono- or polypharmacy—tends to reduce its efficacy (36).
With evidence-based guidelines being recommendatory, they cannot compel physicians and patients to use clozapine. However, a softer approach that eases administrative burden, provides logistical support, and alleviates patient and clinician concerns has been recommended (37). There is a debate about the ethics of offering monetary rewards for adherence to medications, and a few randomized control studies have been undertaken (38, 39). But the possibility that such an incentive is tantamount to coercion has made it a contentious topic (40).
The finding that older people were at lower risk of polypharmacy, unmedicated periods, and clozapine could mean that they had later onset of the disease. Younger age of onset tends to be associated with a worse prognosis (41). The finding that delay to the first pharmacy claim from hospital discharge increases the risk for polypharmacy, unmedicated periods, and clozapine treatment has implications for the provision of health services. Transitioning from hospital to the community is known to be a vulnerable period in which medication compliance is paramount (42, 43). The use of depot antipsychotics and intensive community treatment programs may improve adherence and prognosis (44–46).
Female patients had higher risk of polypharmacy. Similar results have been reported elsewhere (28, 47, 48) but not invariably (49, 50). Schizophrenia has earlier onset in men than in women and earlier onset is associated with a more severe course of illness (5). Some have reported that women are more adherent to treatment than men (5–7), so we are unsure if this finding reflects a gender difference per se or confounding by other variables.
Our findings are subject to several limitations. We did not know the true age of schizophrenia onset and used age at index hospitalization as a proxy. Given the mean age at index hospitalization (>40 years), it is highly improbable that patients in the cohort were first-onset cases. Since they probably lived with schizophrenia for a decade or more, it is more surprising that clozapine was dispensed to so few and so late in the treatment. Unfortunately, the data cannot distinguish if this is a result of under-prescribing or patients refusing to accept clozapine medication—something that needs to be further studied. Our data sources did not contain clinical notes and symptoms, so we did not have indications for the antipsychotic prescriptions, and the reasons for polypharmacy. Likewise, we could not examine the effect of gaps and polypharmacy on symptoms. Some periods classified as polypharmacy may have been gradual transitions from one SGA to another. Earlier Canadian studies have estimated the prevalence of polypharmacy upon hospital discharge at 19 to 40 percent (29, 51, 52). These are consistent with our finding that polypharmacy is more prevalent than treatment with clozapine. Finally, our data did not include mortality—this may have contributed to the low re-hospitalization rate.
The data used in this paper were acquired from the Canadian Institute of Health Information, with the agreement of the provinces of British Columbia, Saskatchewan, and Manitoba. The terms of the agreement prohibit the sharing of data without the prior consent of the provinces. Requests to access these datasets should be directed to Smriti Fernandez, c21mZXJuYW5kZXpAY2loaS5jYQ==, www.cihi.ca.
LB, GM, and RJL contributed to the design of the study. LB and AS performed the statistical analysis. EP wrote the introduction and discussion sections. EP and LB presented preliminary results at a meeting and gathered comments. LB wrote the methods and results sections. SH, EP, RL, KJ, and GM contributed to the interpretation of results. All authors contributed to the submitted version. LB, GM, KJ, and RJL acquired funding and the data. All authors contributed to the article and approved the submitted version.
This work received funding from the Saskatchewan Center for Patient Oriented Research through a postdoctoral fellowship to Dr. Arash Shamloo.
LB received a research grant from AA Pharma. AA Pharma did not have a role in the design, data collection, analysis, interpretation, and writing of the paper.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We acknowledge the patients who participated in the interviews. We are grateful for the information and support provided by Ms. Darcie Nelson, RN regarding the clozapine clinic at Prince Albert, SK. We acknowledge Dr. Donna Malcolm for valuable comments on an early draft.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2022.917361/full#supplementary-material
1. Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M. Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatry. (2017) 62:604–16. doi: 10.1177/0706743717720448
2. Kane JM, Agid O, Baldwin ML, Howes O, Lindenmayer JP, Marder S, et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry. (2019) 80:18com12123. doi: 10.4088/JCP.18com12123
3. Huhn M, Nikolakopoulou A, Schneider-Thoma J, Krause M, Samara M, Peter N, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Focus. (2020) 18:443–55. doi: 10.1176/appi.focus.18306
4. Siskind D, McCartney L, Goldschlager R, Kisely S. Clozapine v. First- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. (2016) 209:385–92. doi: 10.1192/bjp.bp.115.177261
5. Frogley C, Taylor D, Dickens G, Picchioni M. A systematic review of the evidence of clozapine's anti-aggressive effects. Int J Neuropsychopharmacol. (2012) 15:1351–71. doi: 10.1017/S146114571100201X
6. Hennen J, Baldessarini RJ. Suicidal risk during treatment with clozapine: a meta-analysis. Schizophr Res. (2005) 73:139–45. doi: 10.1016/j.schres.2004.05.015
7. Krause M, Huhn M, Schneider-Thoma J, Bighelli I, Gutsmiedl K, Leucht S. Efficacy, acceptability and tolerability of antipsychotics in patients with schizophrenia and comorbid substance use. A systematic review and meta-analysis. Eur Neuropsychopharmacol. (2019) 29:32–45. doi: 10.1016/j.euroneuro.2018.11.1105
8. Rubio JM, Kane JM. How and when to use clozapine. Acta Psychiatr Scand. (2020) 141:178–89. doi: 10.1111/acps.13111
9. Whiskey E, Barnard A, Oloyede E, Dzahini O, Taylor DM, Shergill SS. An evaluation of the variation and underuse of clozapine in the United Kingdom. Acta Psychiatr Scand. (2021) 143:339–47. doi: 10.1111/acps.13280
10. Masuda T, Misawa F, Takase M, Kane JM, Correll CU. Association with hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies. JAMA Psychiatry. (2019) 76:1052–62. doi: 10.1001/jamapsychiatry.2019.1702
11. Beck K, McCutcheon R, Bloomfield MA, Gaughran F, Reis Marques T, MacCabe J, et al. The practical management of refractory schizophrenia—the Maudsley treatment review and assessment team service approach. Acta Psychiatr Scand. (2014) 130:427–38. doi: 10.1111/acps.12327
12. Farooq S, Choudry A, Cohen D, Naeem F, Ayub M. Barriers to using clozapine in treatment-resistant schizophrenia: systematic review. BJPsych Bull. (2019) 43:8–16. doi: 10.1192/bjb.2018.67
13. Legge SE, Hamshere M, Hayes RD, Downs J, O'Donovan MC, Owen MJ, et al. Reasons for discontinuing clozapine: A cohort study of patients commencing treatment. Schizophr Res. (2016) 174:113–9. doi: 10.1016/j.schres.2016.05.002
14. Zhao YJ, Lin L, Teng M, Khoo AL, Soh LB, Furukawa TA, et al. Long-term antipsychotic treatment in schizophrenia: systematic review and network meta-analysis of randomised controlled trials. BJPsych Open. (2016) 2:59–66. doi: 10.1192/bjpo.bp.115.002576
15. Leucht S, Heres S, Hamann J, Kane JM. Methodological issues in current antipsychotic drug trials. Schizophr Bull. (2008) 34:275–85. doi: 10.1093/schbul/sbm159
16. Lally J, MacCabe JH. Antipsychotic medication in schizophrenia: a review. Br Med Bull. (2015) 114:169–79. doi: 10.1093/bmb/ldv017
17. Kroger H. newspell: Easy management of complex spell data. Stata J. (2015) 15:155–72. doi: 10.1177/1536867X1501500110
18. Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. (2005) 11:453–60.
19. Jackson CH. Multi-state models for panel data: the msm package for R. J Stat Softw. (2011) 38:1–28. doi: 10.18637/jss.v038.i08
20. Harrow M, Jobe TH, Faull RN. Do all schizophrenia patients need antipsychotic treatment continuously throughout their lifetime? A 20-year longitudinal study. Psychol Med. (2012) 42:2145–55. doi: 10.1017/S0033291712000220
21. Moilanen J, Haapea M, Miettunen J, Jaaskelainen E, Veijola J, Isohanni M, et al. Characteristics of subjects with schizophrenia spectrum disorder with and without antipsychotic medication—a 10-year follow-up of the Northern Finland 1966 birth cohort study. Eur Psychiatry. (2013) 28:53–8. doi: 10.1016/j.eurpsy.2011.06.009
22. Zygmunt A, Olfson M, Boyer CA, Mechanic D. Interventions to improve medication adherence in schizophrenia. Am J Psychiatry. (2002) 159:1653–64. doi: 10.1176/appi.ajp.159.10.1653
23. Barkhof E, Meijer CJ, de Sonneville LM, Linszen DH, de Haan L. Interventions to improve adherence to antipsychotic medication in patients with schizophrenia—a review of the past decade. Eur Psychiatry. (2012) 27:9–18. doi: 10.1016/j.eurpsy.2011.02.005
24. Law MR, Cheng L, Dhalla IA, Heard D, Morgan SG. The effect of cost on adherence to prescription medications in Canada. CMAJ. (2012) 184:297–302. doi: 10.1503/cmaj.111270
25. Marwaha S, Johnson S. Schizophrenia and employment—a review. Soc Psychiatry Psychiatr Epidemiol. (2004) 39:337–49. doi: 10.1007/s00127-004-0762-4
26. Morgan SG, Law M, Daw JR, Abraham L, Martin D. Estimated cost of universal public coverage of prescription drugs in Canada. CMAJ. (2015) 187:491–7. doi: 10.1503/cmaj.141564
27. Gallego JA, Bonetti J, Zhang J, Kane JM, Correll CU. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. (2012) 138:18–28. doi: 10.1016/j.schres.2012.03.018
28. Sneider B, Pristed SG, Correll CU, Nielsen J. Frequency and correlates of antipsychotic polypharmacy among patients with schizophrenia in Denmark: a nation-wide pharmacoepidemiological study. Eur Neuropsychopharmacol. (2015) 25:1669–76. doi: 10.1016/j.euroneuro.2015.04.027
29. Procyshyn RM, Honer WG, Wu TK, Ko RW, McIsaac SA, Young AH, et al. Persistent antipsychotic polypharmacy and excessive dosing in the community psychiatric treatment setting: a review of medication profiles in 435 Canadian outpatients. J Clin Psychiatry. (2010) 71:566–73. doi: 10.4088/JCP.08m04912gre
30. Meltzer HY. Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses. (2012) 6:134–44. doi: 10.3371/CSRP.6.3.5
31. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. (2011) 25:383–99. doi: 10.2165/11587810-000000000-00000
32. Buchanan RW, Kreyenbuhl J, Kelly DL, Noel JM, Boggs DL, Fischer BA, et al. The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. (2010) 36:71–93. doi: 10.1093/schbul/sbp116
33. Kelly DL, Freudenreich O, Sayer MA, Love RC. Addressing barriers to clozapine underutilization: a national effort. Psychiatr Serv. (2018) 69:224–7. doi: 10.1176/appi.ps.201700162
34. Gee SH, Shergill SS, Taylor DM. Patient attitudes to clozapine initiation. Int Clin Psychopharmacol. (2017) 32:337–42. doi: 10.1097/YIC.0000000000000188
35. Alessi-Severini S, Le Dorze JA, Nguyen D, Honcharik P, Eleff M. Clozapine prescribing in a Canadian outpatient population. PLoS ONE. (2013) 8:e83539. doi: 10.1371/journal.pone.0083539
36. Kohler-Forsberg O, Horsdal HT, Legge SE, MacCabe JH, Gasse C. Predictors of nonhospitalization and functional response in clozapine treatment: a nationwide, population-based cohort study. J Clin Psychopharmacol. (2017) 37:148–54. doi: 10.1097/JCP.0000000000000649
37. John AP, Ko EKF, Dominic A. Delayed initiation of clozapine continues to be a substantial clinical concern. Can J Psychiatry. (2018) 63:526–31. doi: 10.1177/0706743718772522
38. Noordraven EL, Wierdsma AI, Blanken P, Bloemendaal AF, Staring AB, Mulder CL. Financial incentives for improving adherence to maintenance treatment in patients with psychotic disorders (money for medication): a multicentre, open-label, randomised controlled trial. Lancet Psychiatry. (2017) 4:199–207. doi: 10.1016/S2215-0366(17)30045-7
39. Priebe S, Yeeles K, Bremner S, Lauber C, Eldridge S, Ashby D, et al. Effectiveness of financial incentives to improve adherence to maintenance treatment with antipsychotics: cluster randomised controlled trial. BMJ. (2013) 347:f5847. doi: 10.1136/bmj.f5847
40. Priebe S, Sinclair J, Burton A, Marougka S, Larsen J, Firn M, et al. Acceptability of offering financial incentives to achieve medication adherence in patients with severe mental illness: a focus group study. J Med Ethics. (2010) 36:463–8. doi: 10.1136/jme.2009.035071
41. Immonen J, Jaaskelainen E, Korpela H, Miettunen J. Age at onset and the outcomes of schizophrenia: a systematic review and meta-analysis. Early Interv Psychiatry. (2017) 11:453–60. doi: 10.1111/eip.12412
42. Chung DT, Ryan CJ, Hadzi-Pavlovic D, Singh SP, Stanton C, Large MM. Suicide rates after discharge from psychiatric facilities: a systematic review and meta-analysis. JAMA Psychiatry. (2017) 74:694–702. doi: 10.1001/jamapsychiatry.2017.1044
43. Schennach R, Obermeier M, Meyer S, Jager M, Schmauss M, Laux G, et al. Predictors of relapse in the year after hospital discharge among patients with schizophrenia. Psychiatr Serv. (2012) 63:87–90. doi: 10.1176/appi.ps.201100084
44. Addington D, Anderson E, Kelly M, Lesage A, Summerville C. Canadian practice guidelines for comprehensive community treatment for schizophrenia and schizophrenia spectrum disorders. Can J Psychiatry. (2017) 62:662–72. doi: 10.1177/0706743717719900
45. Tiihonen J, Haukka J, Taylor M, Haddad PM, Patel MX, Korhonen P, et al. Nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. (2011) 168:603–9. doi: 10.1176/appi.ajp.2011.10081224
46. Tomita A, Herman DB. The role of a critical time intervention on the experience of continuity of care among persons with severe mental illness after hospital discharge. J Nerv Ment Dis. (2015) 203:65–70. doi: 10.1097/NMD.0000000000000224
47. Maxwell CJ, Mondor L, Pefoyo Kone AJ, Hogan DB, Wodchis WP. Sex differences in multimorbidity and polypharmacy trends: a repeated cross-sectional study of older adults in Ontario, Canada. PLoS ONE. (2021) 16:e0250567. doi: 10.1371/journal.pone.0250567
48. Sun F, Stock EM, Copeland LA, Zeber JE, Ahmedani BK, Morissette SB. Polypharmacy with antipsychotic drugs in patients with schizophrenia: trends in multiple health care systems. Am J Health Syst Pharm. (2014) 71:728–38. doi: 10.2146/ajhp130471
49. Fleischhacker WW, Uchida H. Critical review of antipsychotic polypharmacy in the treatment of schizophrenia. Int J Neuropsychopharmacol. (2014) 17:1083–93. doi: 10.1017/S1461145712000399
50. Jaracz J, Tetera-Rudnicka E, Kujath D, Raczynska A, Stoszek S, Czernas W, et al. The prevalence of antipsychotic polypharmacy in schizophrenic patients discharged from psychiatric units in Poland. Pharmacol Rep. (2014) 66:613–7. doi: 10.1016/j.pharep.2014.02.024
51. Procyshyn RM, Thompson B. Patterns of antipsychotic utilization in a tertiary care psychiatric institution. Pharmacopsychiatry. (2004) 37:12–7. doi: 10.1055/s-2004-815469
Keywords: polypharmacy, real world evidence (RWE), treatment guideline implementation, antipsychotics, schizophenia, adherence - compliance - persistance
Citation: Peters E, Shamloo A, Lodhi RJ, Marcoux G, Jackson K, Halayka S and Balbuena L (2022) Medication Gaps and Antipsychotic Polypharmacy in Previously Hospitalized Schizophrenia Patients: An Electronic Cohort Study in Three Canadian Provinces. Front. Psychiatry 13:917361. doi: 10.3389/fpsyt.2022.917361
Received: 11 April 2022; Accepted: 26 May 2022;
Published: 15 June 2022.
Edited by:
Marijn Lijffijt, Baylor College of Medicine, United StatesReviewed by:
Paroma Mitra, New York University, United StatesCopyright © 2022 Peters, Shamloo, Lodhi, Marcoux, Jackson, Halayka and Balbuena. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lloyd Balbuena, bGxveWQuYmFsYnVlbmFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.